Abstract
Background
Early transmitral velocity / tissue Doppler mitral annular early diastolic velocity (E/Ea) has been correlated with pulmonary capillary wedge pressure (PCWP) in a wide variety of cardiac conditions. The objective of this study was to determine the reliability of mitral E/Ea for predicting PCWP in patients admitted for advanced decompensated heart failure (ADHF).
Methods and Results
Prospective consecutive patients with ADHF (ejection fraction [EF] ≤30%, NYHA class III-IV symptoms) underwent simultaneous echocardiographic and hemodynamic evaluation on admission and after 48 hours of intensive medical therapy. A total of 106 patients were included (mean age 57 ±12 years, EF 24 ±8%, PCWP 21 ±7 mmHg, mitral E/Ea 20 ±12). There was a lack of correlation between mitral E/Ea and PCWP, particularly in those with larger LV volumes, more impaired cardiac indices, and the presence of cardiac resynchronization therapy. Overall, mitral E/Ea was similar among patients with PCWP > and ≤ 18 mmHg, and sensitivity and specificity for mitral E/Ea > 15 to identify a PCWP > 18 mmHg was 66% and 50%, respectively. Contrary to prior reports, we did not observe any direct association between changes in PCWP and changes in mitral E/Ea.
Conclusion
In decompensated patients with advanced systolic heart failure, tissue Doppler derived mitral E/Ea may not be as reliable in predicting intracardiac filling pressures, particularly in those with larger LV volumes, more impaired cardiac indices, and the presence of cardiac resynchronization therapy.
Keywords: heart failure, hemodynamics, diastole, remodeling, echocardiography
INTRODUCTION
Invasively measured pulmonary capillary wedge pressure (PCWP) has been widely used as a surrogate for left ventricular (LV) filling pressure, and is directly associated with functional capacity and prognosis in patients with heart failure (1,2,3). However, given the cost, potential complications, and the lack of demonstrable benefits in routine use, hemodynamic assessment via pulmonary artery catheters has decreased substantially over the last decade (4,5,6).
Conventional echocardiography plays a critical role in the management of heart failure as it serves as a non-invasive bedside tool to determine abnormalities in cardiac structure and performance. Transmitral flow velocity curves and other Doppler variables have been utilized as non-invasive estimates of intracardiac filling pressures, albeit with limitations. In particular, the early transmitral velocity / tissue Doppler mitral annular early diastolic velocity (E/Ea) ratio has been shown to correlate with PCWP in a wide range of cardiac patients (7,8,9,10,11,12,13). However, while some smaller studies have included patients with depressed LV systolic function, none has included patients admitted with advanced heart failure and extensive reverse remodeling (14,15,16,17,18,19). Therefore, the primary goal of our study was to examine the relationship between mitral E/Ea and hemodynamic measurements in patients with advanced decompensated heart failure (ADHF) – a patient cohort wherein hemodynamic assessment is often considered. We further aimed to explore the potential clinical utility of serial mitral E/Ea assessment in estimating changes in intracardiac filling pressures in response to intensive medical therapy in the ADHF setting.
METHODS
Study Population
We prospectively enrolled consecutive patients, aged 18 years or older, with symptomatic chronic (>6 months) heart failure, who underwent a right heart catheterization (RHC) due to concerns regarding hemodynamic derangements at the Cleveland Clinic heart failure intensive care unit between September 15, 2006 and October 15, 2007. The inclusion criteria included: 1) markedly impaired systolic left ventricular (LV) function defined by LV ejection fraction (LVEF) ≤ 30%; and 2) New York Heart Association class III-IV symptoms. Exclusion criteria included: 1) patients on artificial ventilation; 2) status post aortic- and/or mitral valve repair or prosthesis; 3) status post cardiac transplantation. A previous cardiac resynchronization therapy with defibrillator (CRT-D) implant was not an exclusion criterium, and all data provided in the results for patients previously implanted with a CRT-D device are with the device ON. The Cleveland Clinic Institutional Review Board approved this research project, and informed consent was prospectively obtained in all subjects.
Hemodynamic Study Design
Hemodynamic and echocardiographic data were simultaneously collected at baseline (within 12 hours of admission) and at follow-up (after 48 hours of intensive medical therapy) if the pulmonary artery catheter was still in place. Hemodynamic data including systemic blood pressure, central venous pressure (CVP), and PCWP (wedge position was verified by fluoroscopy and phasic changes in pressure waveforms), represent the average of 5 cycles, and with balanced transducers (0 level at the mid-axillary line). The CVP and PCWP were assessed at end-expiration with a balloon-tipped catheter at steady state with the patient in a supine position by an investigator unaware of the echocardiographic measurements. Cardiac index (CI) was determined using the Fick equation through sampling of a mixed central venous blood gas taken in the pulmonary artery while assuming standard metabolic rates.
The hemodynamic goals and pharmacologic approach to intravenous therapy in the specialized heart failure intensive care unit have been previously described (20). Briefly, optimal hemodynamic response was defined as a decrease in PCWP ≤ 18 mmHg, decrease in CVP to ≤ 8 mmHg and improvement in CI to ≥ 2.2 l/min/m2, while maintaining mean arterial pressure > 65 mmHg. In order to achieve the hemodynamic goals, most patients were treated with intravenous loop diuretics in combination with vasodilators and/or inotropic agents while continuing or intensifying previous therapies with angiotensin converting enzyme inhibitors, anti-adrenergic blockers, aldosterone receptor antagonists, and other vasodilators as indicated and as tolerated.
Transthoracic Echocardiography
A comprehensive two-dimensional echocardiographic exam dedicated for research was performed with a commercially available system (Vingmed, System Seven, General Electric, USA) by a single American Society of Echocardiography Registered Diagnostic Cardiac Sonographer (AB). Images were acquired in the left lateral decubitus position using a phased array transducer in the standard parasternal and apical views. Standard two-dimensional and Doppler data, triggered to the QRS complex, were digitally stored in a cine-loop format.
Echocardiographic Analysis
The analysis was performed offline by two independent investigators experienced with echocardiographic measurements, blinded to hemodynamic data at the time of analysis. All reported echocardiographic measurements were averaged from three consecutive cycles. Left ventricular volumes, left ventricular ejection fraction, mitral regurgitation, and left atrial maximum volume were assessed as recommended by the American Society of Echocardiography (21). Mitral inflow was analyzed for peak E (early diastolic) and peak A (late diastolic) velocities, E/A ratio, and deceleration time (DT) of E velocity. Ea septal and lateral mitral annulus velocities were measured, and the dimensionless ratio mitral E/Ea for the septal and lateral annulus was calculated (7,8,15). Inter-ventricular mechanical dyssynchrony (VMD) was assessed as the difference between the pre-ejection intervals from QRS onset to the beginning of ventricular ejection at the pulmonic and aortic valve levels using pulsed-wave Doppler and intra-VMD by the opposing wall time-to-peak myocardial velocity intervals in a 4-segment model using color tissue-Doppler imaging.
To ensure optimal accuracy of mitral E/Ea in patients with advanced heart failure and possible regional wall motion abnormalities, all analysis provided are based on the mitral E/Ea ratio, computed from the average of the septal and lateral Ea (16). Pulsed wave and not color TDI was used, since temporal resolution is higher with pulsed wave TDI. Second, low gain and filter settings were applied so the onset of mitral Ea could be reliably identified. Third, the scale was adjusted as needed to range from −15 to 20 to +15 to 20 cm/s, and the sweep speed was set at 100 mm/s to achieve the optimal spectral display of myocardial velocities. Finally, identical R-R intervals (<5 ms) were chosen, to minimize potential differences in diastolic time intervals and subsequent differences in interpretation at slightly different R-R cycle lengths.
Statistical Analysis
All data are expressed as mean ± standard deviation for continuous data and as a ratio for categorical data. Univariate comparisons of these variables were performed between baseline and follow-up variables, and between patients with and without previous CRT-D implantation. A paired and unpaired t-test for continuous data and a Spearman correlation coefficient was used for appropriate comparisons. Receiver-operating characteristic (ROC) curves were constructed to determine optimal sensitivity and specificity for predicting PCWP >18 mm Hg using mitral E/Ea. A PCWP of 18 mmHg was chosen as the cut-off value, since this was the target according to treatment protocol. Based on previous studies, a cut-off value PCWP of 15 mmHg was analyzed also. Statistical significance was set at a two-tailed probability level of less than 0.05. All analyses were performed using SPSS for Windows, release 13.0 (SPSS Inc., Chicago, Illinois). The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Patient Characteristics
A total of 110 patients met eligibility criteria during the study period of which 4 patients refused to be enrolled. Baseline characteristics and treatment of the final 106 patients enrolled during admission are summarized in Table 1. All patients (93% of whom were in sinus rhythm at the time of exam, 7% in atrial fibrillation with an additional 14% having a history of atrial fibrillation) were classified as NYHA class III–IV, with a mean LVEF of 24 ±8%. The median length of intensive medical therapy in the heart failure intensive care unit was 3.5 days. Fifty-one patients (49%) had a CRT-D device at the time of inclusion in our study with overall comparable baseline characteristics (except QRS width) and treatment patterns compared to patients without previous CRT-D implantation.
Table 1.
Demographics and Vital Statistics
| All Patients (n = 106) |
Patients without CRT-D (n = 55) |
Patients with CRT-D (n = 51) |
|
|---|---|---|---|
| Baseline Characteristics | |||
| Age (y) | 57 ±12 | 56 ±13 | 59 ±12 |
| Men (%) | 76 | 72 | 78 |
| Weight (kg) | 81 ±22 | 82 ±22 | 78 ±22 |
| QRS width (msec) | 141 ±34 | 123 ±29 | 160 ±29* |
| Hypertension (%) | 58 | 56 | 60 |
| Hyperlipidemia (%) | 59 | 59 | 63 |
| Diabetes (%) | 32 | 34 | 27 |
| Ischemic etiology (%) | 42 | 44 | 39 |
| LV Ejection Fraction (%) | 24 ±8 | 25 ±8 | 24 ±9 |
| Hemoglobin (g/dl) | 11.7 ±2 | 11.7 ±2 | 11.8 ±2 |
| Creatinine (mg/dl) | 1.8 ±1.1 | 1.7 ±0.7 | 1.9 ±1.3 |
| BNP (pg/ml) | 1710 ±1406 | 1628 ±1247 | 1734 ±1386 |
| Medical Treatment During Admission (%) | |||
| Beta Blockers | 58 | 54 | 62 |
| ACE inhibitors / ARB | 54 | 56 | 52 |
| Spironolactone | 44 | 39 | 48 |
| Loop Diuretic | 85 | 84 | 78 |
| Digoxin | 22 | 17 | 24 |
| Hydralazine | 38 | 40 | 35 |
| Isosorbide dinitrate | 37 | 37 | 37 |
| Inotropic Drugs | 35 | 38 | 32 |
| Nitroprusside | 41 | 44 | 40 |
Values are mean ± SD or n (%).
Abbreviations: CRT-D: cardiac resynchronization therapy with defibrillator, ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker.
p<0.01 for comparison between patients with and without CRT-D
Hemodynamic and Echocardiographic Measurements
Adequate mitral inflow, tissue Doppler signals and hemodynamic variables were obtained in all patients. Table 2 presents the hemodynamic and echocardiographic measurements at the time of the baseline assessment for all patients, stratified according to the presence or absence of CRT-D implantation. Overall, both patients groups had similar baseline hemodynamic derangements except for a higher heart rate in patients without CRT-D. Compared to those without CRT-D, patients with previous CRT-D implant had larger LV end-diastolic volumes, and longer mitral deceleration times. Mitral Ea and E/Ea ratios for different annular regions were similar between those with versus without CRT-D. In addition, septal and lateral Ea for ischemic and idiopathic dilated cardiomyopathy patients were also similar (5.1 ±3.2 vs 5.1 ±2.4 cm/sec, p = 0.9 and 7.5 ±5.7 vs 7.3 ±3.4 cm/sec, p = 0.8 respectively). Overall, 22% of patients had more than moderate mitral regurgitation at baseline. Compared to patients with mild or moderate mitral regurgitation, these patients had a non-significant trend towards higher PCWP and higher mitral E/Ea. Finally, inter-VMD and intra-VMD was only 19±26 and 36±38 ms respectively.
Table 2.
Initial Hemodynamic and Echocardiographic Measurements
| All Patients (n = 106) |
Patients without CRT-D (n = 55) |
Patients with CRT-D (n = 51) |
|
|---|---|---|---|
| Body Mass Index (kg/m2) | 27 ±8 | 28 ±6 | 27 ±6 |
| Heart Rate (bpm) | 75 ±29 | 84 ±24 | 67 ±32* |
| MAP (mmHg) | 75 ±11 | 76 ±13 | 74 ±9 |
| CVP (mmHg) | 12 ±7 | 13 ±6 | 11 ±8 |
| MPA (mmHg) | 34 ±11 | 34 ±11 | 36 ±10 |
| PCWP (mmHg) | 21 ±7 | 21 ±6 | 20 ±7 |
| CI (l/min.m2) | 2.1 ±0.7 | 2.0 ±0.7 | 2.1 ±0.8 |
| LV mass (g) | 376 ±134 | 367 ±130 | 395 ±145 |
| LV mass index (g/m2) | 183 ±63 | 177 ±59 | 196 ±68 |
| Left atrial volume (ml) | 92 ±38 | 89 ±39 | 96 ±38 |
| Left atrial volume index (ml/m2) | 45 ±16 | 43 ±17 | 47 ±14 |
| LVEDV (ml) | 252 ±114 | 210 ±85 | 294 ±124* |
| LVEDV index (ml/m2) | 124 ±57 | 104 ±45 | 142 ±62* |
| Mitral E velocity (cm/s) | 96 ±28 | 97 ±25 | 96 ±32 |
| Mitral A velocity (cm/s) | 43 ±14 | 45 ±16 | 42 ±20 |
| Mitral E/A | 2.4 ±0.9 | 2.5±0.9 | 2.3 ±0.7 |
| Mitral DT (ms) | 150 ±45 | 136 ±38 | 164 ±46* |
| Mitral Ea septal annulus (cm/s) | 5.1 ±2.8 | 4.9 ±2.1 | 5.2 ±3.2 |
| Mitral E/Ea septal annulus | 23 ±12 | 24 ±11 | 22 ±13 |
| Mitral Ea lateral annulus (cm/s) | 7.4 ±4.6 | 7.2 ±3.1 | 7.5 ±5.8 |
| Mitral E/Ea lateral annulus | 17 ±11 | 17 ±10 | 17 ±12 |
| Mitral Ea average (cm/s) | 6.1 ±3.6 | 5.9 ±2.5 | 6.3 ±4.4 |
| Mitral E/Ea average | 20 ±12 | 21 ±11 | 19 ±12 |
Values are mean ± SD.
Abbreviations: CRT-D: cardiac resynchronization therapy with defibrillator, MAP: mean systemic arterial pressure, CVP: central venous pressure, MPA: mean pulmonary artery pressure, PCWP: pulmonary capillary wedge pressure, CI: cardiac index, LV: left ventricle, EDV: end-diastolic volume, DT: deceleration time.
p<0.01 for comparison between patients with and without CRT-D
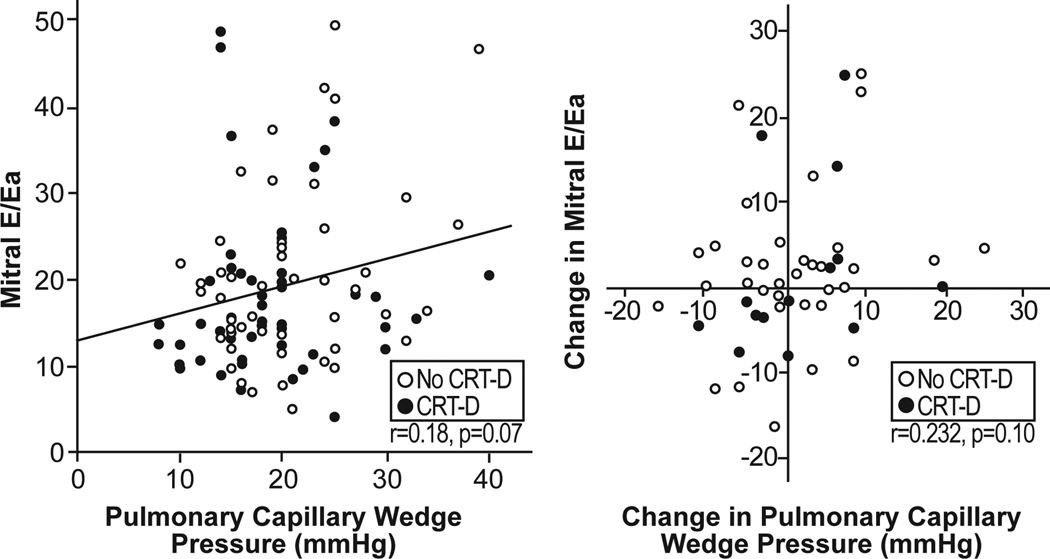
The relationships between PCWP and different Doppler variables are shown in Table 3. There was a weak but statistically significant negative correlation between PCWP and mitral DT as well as a weak positive correlation between PCWP and mitral E velocity, only in patients without previous CRT-D implantation. However, no correlation was observed between PCWP and mitral Ea in the septal annulus, lateral annulus, or when averaged over both annular regions. Furthermore, the ratio mitral E/Ea (using all definitions for Ea just mentioned) showed no significant correlation with PCWP. As illustrated in Figure 1, even an elevated mitral E/Ea could be associated with a relatively low PCWP and vice versa. Interpretable pulmonary vein (PV) Doppler tracings were obtained in 66% of patients at baseline (Systolic PV: 33 ±8 cm/s, diastolic PV: 56 ±19 cm/s and Atrial PV: 27 ±9 cm/s). However, no significant correlation could be detected among PV doppler tracings and PCWP (r for systolic PV: −0.01, diastolic PV: 0.1, systolic/diastolic PV: −0.04)
Table 3.
Correlation Coefficients of Echocardiographic Variables with PCWP
| All Patients (n = 106) |
Patients without CRT-D (n = 55) |
Patients with CRT-D (n = 51) |
|
|---|---|---|---|
| LV mass | 0.01 | −0.11 | 0.22 |
| LVEDV (ml) | −0.07 | −0.12 | 0.03 |
| Left atrial volume (ml) | 0.04 | 0.05 | −0.09 |
| E velocity (cm/s) | 0.28* | 0.29* | 0.17 |
| E/A | 0.24 | 0.47* | −0.06 |
| DT (ms) | −0.27* | −0.25* | −0.21 |
| Ea septal annulus (cm/s) | 0.06 | 0.03 | 0.16 |
| E/Ea septal annulus | 0.18 | 0.27 | 0.10 |
| Ea lateral annulus (cm/s) | 0.03 | −0.05 | 0.13 |
| E/Ea lateral annulus | 0.14 | 0.18 | 0.12 |
| Ea average (cm/s) | 0.02 | −0.06 | 0.11 |
| E/Ea average | 0.18 | 0.23 | 0.11 |
Abbreviations: CRT-D: cardiac resynchronization therapy with defibrillator, LV: left ventricle, DT: deceleration time.
p ≤ 0.01
Figure 1.
Relation between mitral E/Ea and pulmonary capillary wedge pressure at baseline (Upper panel) and relation between changes (baseline-follow-up) in mitral E/Ea and changes (baseline-follow-up) in pulmonary capillary wedge pressure (Lower panel)
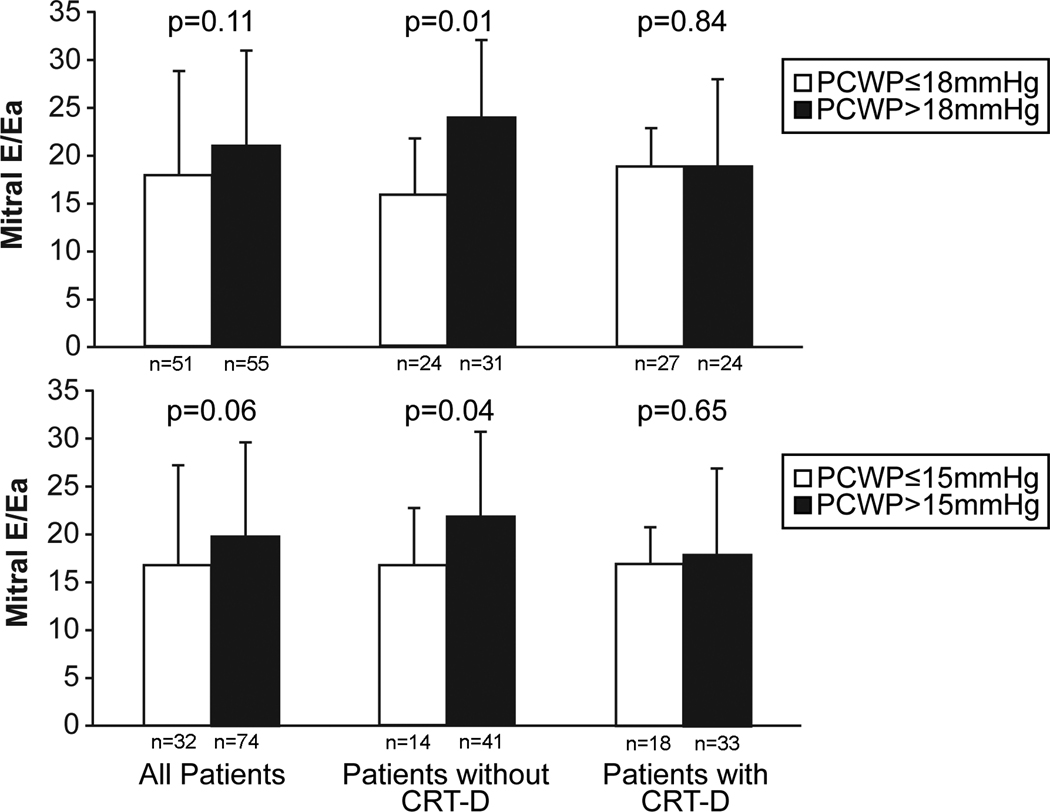
Only 53% of patients with a PCWP > 18 mmHg had a mitral E/Ea ratio > 15. Figure 2 compares mean mitral E/Ea values in patients with PCWP > 15 or 18 mmHg versus ≤ 15 or 18 mmHg and stratified by the presence or absence of previous CRT-D implant. Overall, averaged mitral E/Ea was similar among patients with normal versus elevated PCWP. Only patients without previous CRT-D implant had a modestly (but significantly) higher mitral E/Ea when PCWP > 15 or 18 mmHg.
Figure 2.
Mitral E/Ea in all patients and stratified to previous CRT-D implantation or not.
Clinical Accuracy of Mitral E/Ea ratio to Predict Pulmonary Capillary Wedge Pressure
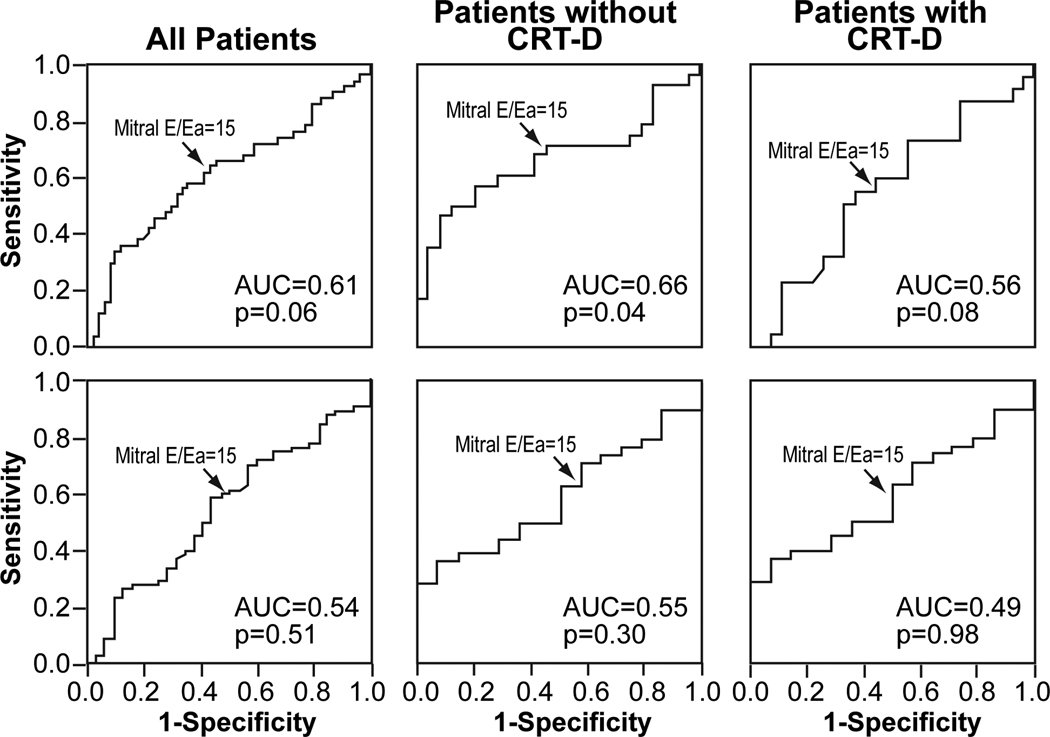
As illustrated in Figure 3, sensitivity and specificity for mitral E/Ea > 15 to identify a PCWP > 18 mmHg was 66% and 50%. The predictive value was similar when patients with atrial fibrillation were excluded from the analysis. However, a mitral E/Ea > 15 provided better accuracy in predicting PCWP > 18 mmHg in patients without previous CRT-D implantation (sensitivity 72%, specificity 54%) than in patients with previous CRT-D implantation (sensitivity 59%, specificity 52%). Sensitivity and specificities of mitral E/Ea > 15 to predict a PCWP > 15 mmHg in patients without previous CRT-D implantation (sensitivity 63%, specificity 57%) and in patients with previous CRT-D implantation (sensitivity 58%, specificity 50%) was poorer.
Figure 3.
Receiver operator characteristic curves for the prediction of pulmonary capillary wedge pressure > 18 mmHg (upper panel) and > 15 mmHg (lower panel) for mitral E/Ea.
To further analyze the potential importance of a cutoff value for mitral E/Ea, patients were divided into 3 groups. Of the patients with mitral E/Ea < 8, E/Ea 8–15 and E/Ea > 15, average PCWP were similar (19±4, 19±7, 20±6 mmHg respectively). We also tested a previously derived equation (PCWP = 2 + 1.3 mitral E/Ea) to predict measured PCWP in our study cohort but no correlation was observed (r = 0.03, p = ns).
Relation of Mitral E/Ea with Cardiac Structure and Performance
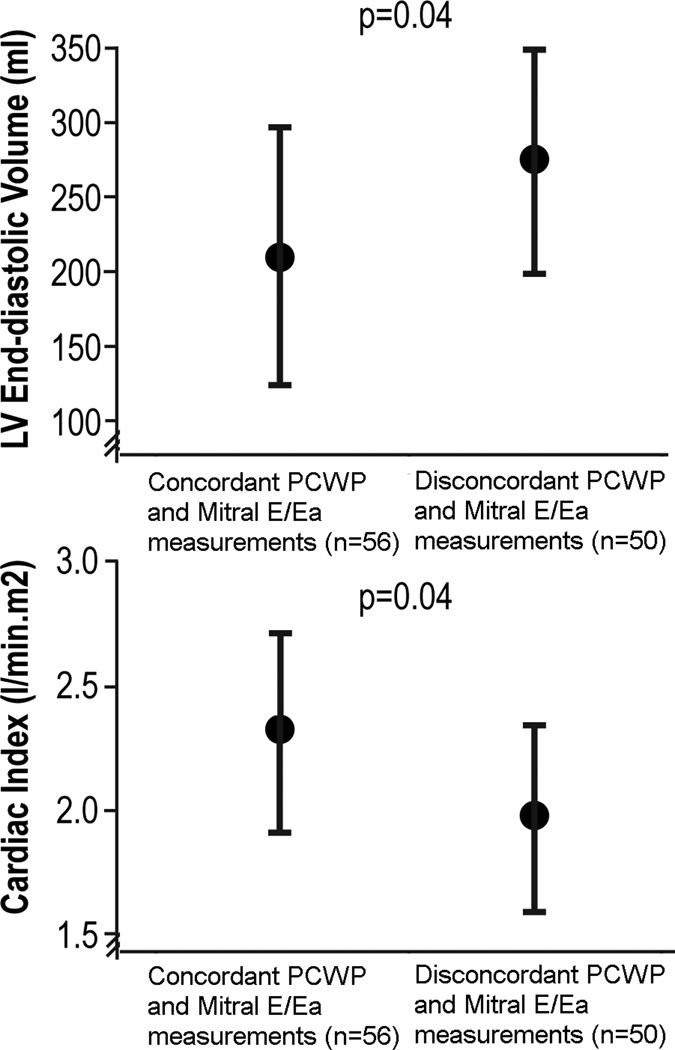
In order to better understand the lack of correlation between mitral E/Ea and PCWP, echocardiographic and hemodynamic variables were compared among 2 groups according to presence or absence of concordant mitral E/Ea > 15 and PCWP > 18 mmHg (Figure 4). Interestingly, the only variables that demonstrated statistically significant differences between concordant versus discordant mitral E/Ea-PCWP measurements were LV end-diastolic volume and cardiac index. In particular, those with discordant mitral E/Ea-PCWP measurements had significantly larger LV volumes and lower cardiac indices. Left atrial volume or severity of mitral regurgitation did not differ among the groups.
Figure 4.
Relation between mitral E/Ea and pulmonary capillary wedge pressure (PCWP) to LV end-diastolic volume and cardiac index. The p-value represents t-test between concordant and disconcordant PCWP and mitral E/Ea. Error bars represent standard deviation.
Follow-Up Measures
Fifty-one (49%) patients underwent also simultaneous Doppler and hemodynamic measurements at follow-up. The absolute change in mean PCWP levels ranged from −24 to + 16 mmHg. No correlation between absolute change in PCWP and change in mitral E/Ea was observed (Figure 1).
DISCUSSION
In the present study, we report for the first time the reliability of the mitral E/Ea ratio to estimate PCWP in a large, well-characterized “cold and wet” patient population admitted with decompensation from advanced systolic heart failure (LV ejection fraction ≤30%). Using simultaneously measured echocardiographic and invasive hemodynamic variables, we found the predictive value of baseline mitral E/Ea in estimating PCWP in this population to be less robust than previously reported, especially in patients with cardiac resynchronization therapy. Furthermore, we were unable to identify any reliable direct correlation between changes in mitral E/Ea and PCWP. We further explored this complex relationship and observed the discordance of mitral E/Ea and PCWP to be linked to larger left ventricular dimensions, more impaired cardiac output, and the presence of cardiac resynchronization therapy. With increasing acceptance of mitral E/Ea as a surrogate measure of diastolic function and as a reliable estimate of intracardiac filling pressures, our observations provide an important refinement in the clinical interpretation of the mitral E/Ea ratio as it applies to patient populations where confounders such as alterations in myocardial structure, severity of systolic dysfunction, or the presence of synchronized pacing may influence its predictive value. Our data also caution the use of serial mitral E/Ea assessment for titration of diuretic therapy in such conditions.
Conventional Doppler recording of mitral inflow velocities and pulmonary vein velocities have shown to be useful in estimating PCWP though significant difficulties arise with alterations in loading conditions, mitral valve disease, aging, tachycardia and atrial fibrillation (22,23,24,25,26). Although LV filling is initiated and enhanced by augmentable myocardial relaxation in healthy individuals, it is driven by a high filling pressure in patients with heart failure because myocardial relaxation is reduced (27). Our data corroborate these findings, since we too found modest relations between PCWP and mitral inflow velocities and deceleration times in our study cohort. This relatively low correlation is an unexpected finding, probably attributable to the confounding effects of LV relaxation, LV stiffness, left atrial pressure, mitral valve function, and annular recoil in this advanced heart failure population which will impact mitral inflow velocities and deceleration times to a greater extent than in normal or less advanced heart failure patients. As a result, the mitral E and DT which are recorded early diastole are only a very rough estimate of PCWP. To better account for relaxation, mitral annular velocity Ea has been shown to be not as dependent on pressure gradients as blood flow (27,28,29). As a consequence, the ratio of mitral E/Ea, which can be easily measured by standard equipment without extensive post-processing, has been proposed as a surrogate for PCWP in patients with a variety of cardiac abnormalities including diastolic heart failure, mitral valve disease, hypertrophic cardiomyopathy, atrial fibrillation and sinus tachycardia (7,8,9,10,11,12,13). The increasing acceptance is reflected by the endorsement of mitral E/Ea as a marker for LV filling pressure by a consensus statement from the European Society of Cardiology on the assessment of diastolic function (30).
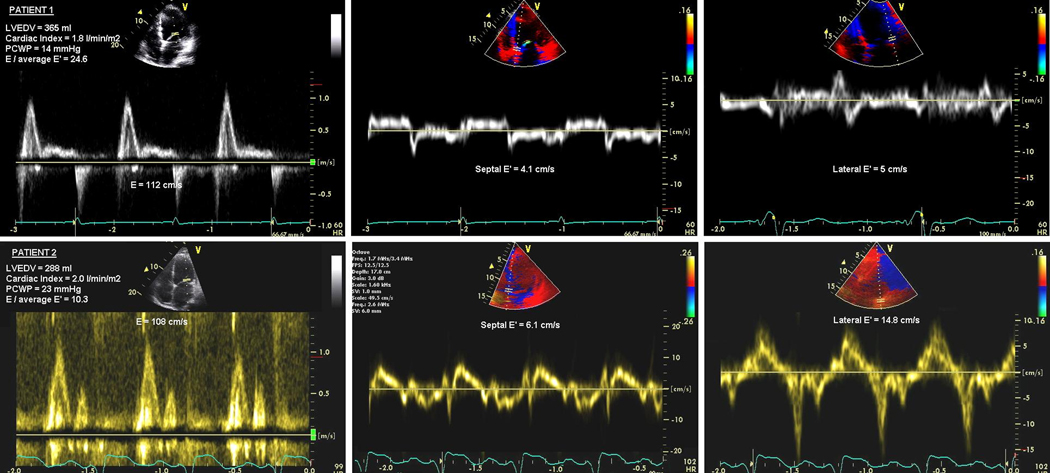
The sensitivity and specificity for previously described cutoff value of mitral E/Ea > 15 to detect elevated PCWP was far lower in our study population than described in prior studies, especially in those patients receiving cardiac resynchronization therapy. However, it is important to emphasize that the profile of our patient population has important differences from several published reports that advocated mitral E/Ea for estimation of LV filling pressures in patients with depressed systolic function (14,15,16,17,18,19). Not only was our sample size much larger, our patient population by design experienced worsening of their clinical status just preceding evaluation and had significantly more cardiac dysfunction and LV remodeling (mean LVEF 24 ±7%, mean CI 2.1 ±0.7 l/min/m2, mean LVEDV 263 ±117 ml) than prior studies. We also describe for the first time the impact of pacing on the accuracy of mitral E/Ea, as shown by the poorer correlation of this measure with PCWP in patients with biventricular pacing, though even without pacing we failed to find a robust relationship. To better illustrate the potential confounding aspects of this analysis, consider the two patients in Figure 5. The top panel is from a patient with dilated cardiomyopathy presenting with low-output in whom a high mitral E/Ea (27.3 for septal Ea, 22.4 for lateral Ea, and 24.6 for the mean) is seen with a relatively low PCWP of 14 mmHg. In contrast, the lower panel shows very elevated PCWP (23 mmHg) in a patient with ischemic cardiomyopathy, cardiac resynchronization therapy, low-output and relatively modest mitral E/Ea elevations (17.7 for septal Ea, 7.3 for lateral Ea, and 10.3 for the mean).
Figure 5.
Example of two patients with discordant pulmonary capillary wedge pressure (PCWP) and mitral E/Ea.
Upper panel shows patient with low PCWP and high mitral E/Ea and lower panel shows patient with high PCWP and low mitral E/Ea.
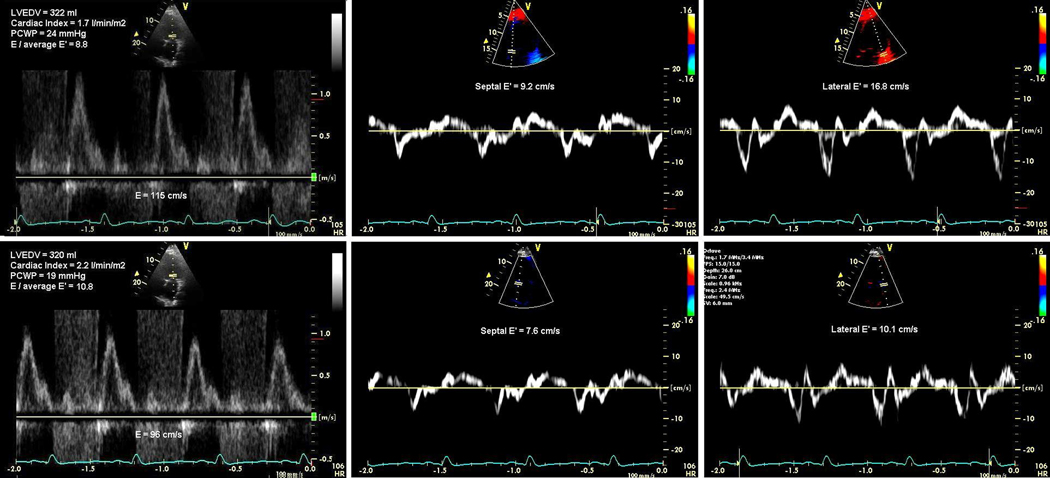
We did not confirm the previously reported finding that changes in the mitral E/Ea track changes in PCWP in ADHF (14). Patients with a reduction in mitral E/Ea could still have significantly elevated PCWP and vice versa at follow-up. Therefore, a potential reduction in mitral E/Ea during serial echocardiographic assessment should not be considered a surrogate for a drop in PCWP in advanced heart failure patients. Importantly, based on the findings of our study, mitral E/Ea should not be used as the only initial or continuing assessment of LV filling pressure to titrate diuretic therapy in the setting of decompensation or advanced systolic heart failure. Figure 6 illustrates a case with dilated cardiomyopathy treated with loop diuretics and vasodilators where a fall in PCWP and increase in CI elicited a contradictory increase in mitral E/Ea. One issue here is the patient’s tachycardia and low cardiac output, causing a hyperdynamic state with increased movement and subsequent velocities of the lateral mitral annulus (high lateral E’). Furthermore, a patient’s treatment may vary significantly over time, particularly in relation to the use of inotropic medications, which may have independent effects on annular motion. Clearly, many confounders may be at play to influence the predictive value of mitral E/Ea.
Figure 6.
Example of changes in pulmonary capillary wedge pressure (PCWP) and mitral E/Ea in one patient from baseline (Upper panel) to follow-up (Lower panel).
Note the discordant PCWP and mitral E/Ea at baseline and at follow-up, and the reduction in PCWP (−5 mmHg) which is associated with an increase in mitral E/Ea (+ 2).
We further highlight the complex relationships between cardiac structure and performance as we observed that the discordance of mitral E/Ea and PCWP was linked to larger LV dimensions, and more impaired cardiac output in patients with ADHF. The presence of more severe LV remodeling seems to indicate the presence of a ‘disconnect’ between LV diastolic function and actual LV filling pressure in patients with ADHF, which limits the clinical utility of mitral E/Ea in estimating filling pressures in patients admitted with ADHF. Conversely, the lack of a significant correlation between mitral E/Ea and PCWP in patients with ADHF can also be explained by the presence of a more pronounced, irreversible, diastolic and systolic dysfunction. Patients with advanced heart failure often have severe LV fibrosis and impaired cardiac output which could restrict systolic and subsequent early diastolic mitral annular motion such that the relationship between left atrial driving pressure (E) and LV relaxation kinetics (Ea) within the LV could become defective, resulting in discordance between echocardiographically measured mitral E/Ea and invasively measured PCWP. In addition, both mitral E and Ea are occurring in early diastole and reflect a host of factors relating to recoil, suction, intraventricular pressure gradients and the previous systolic contraction whereas PCWP is a mean value of diastolic pressure. Taken together, it is therefore not surprising that mitral E/Ea is only a very rough measure of LV end-diastolic pressure. In other words, mitral E/Ea has lower accuracy in assessing PCWP, especially at the more severe end of the heart failure syndrome spectrum because E and Ea are probably altered by volume shifts to a different degree than in cases with less severe heart disease.
Study Limitations
There were no direct hemodynamic measurements of LV end-diastolic or left atrial pressure performed, although PCWP is accepted as a well-validated surrogate considering the clinical condition of the patients and the need for serial monitoring (1,31,32). Wedge position was verified by changes in pressure waveforms without fluoroscopic guidance or measured venous blood oxygen content with balloon inflation. To analyze cardiac output, a standard resting metabolic rate was assumed, but overall cardiac outputs assessed by the Fick equation were comparable with those assessed by the thermodilution technique. Regional wall motion abnormalities in severely dilated and/or ischemic ventricles might have altered Ea. However, instead of analyzing only the septal or lateral Ea, we also considered the average of both walls (16). The exact mechanism through which CRT-D influences mitral E and Ea is not known, but pacing the heart leads to altered inter- and intra-ventricular activation sequences with subsequent alterations in wall segmental loading and contraction which probably influences Doppler parameters. Finally, the main aim of the study was to evaluate mitral E/Ea as a surrogate for PCWP, and we did not evaluate the reliability of combinations of different Doppler variables (including pulmonary vein signals) in estimating LV filling pressures. Our data do not imply in any way that Doppler evaluation is not useful in ADHF but merely support the notion of a stepwise approach incorporating all available echocardiographic data (11,16). We also did not evaluate the role of these measurements on the assessment of LV diastolic function of the failing myocardium. Although this is the largest reported cohort of patients with ADHF (especially with LVEF ≤30% and with serial measurements), and the sample size was substantially larger compared with prior reports involving invasive hemodynamic validations, the sample size is still relatively small, notably for sub-group analyses.
CONCLUSION
In decompensated patients with advanced systolic heart failure, tissue Doppler derived mitral E/Ea alone may not be reliable in predicting intracardiac filling pressures, particularly in those with larger LV volumes, more impaired cardiac indices, and the presence of cardiac resynchronization therapy. Our observations underscore a need for a refinement in the broad clinical use of mitral E/Ea to estimate filling pressures, and caution the direct inference of such relationships to patients in the decompensated state with significant LV systolic dysfunction, cardiac remodeling, or biventricular pacing.
CLINICAL PERSPECTIVE.
Early transmitral velocity / tissue Doppler mitral annular early diastolic velocity (E/Ea) has been correlated with pulmonary capillary wedge pressure (PCWP) in a wide variety of cardiac conditions. However, the reliability of mitral E/Ea for predicting PCWP in patients admitted for advanced decompensated heart failure (ADHF) is unknown. A total of 106 prospective consecutive patients with ADHF (ejection fraction [EF] ≤30%, NYHA class III-IV symptoms) underwent simultaneous echocardiographic and hemodynamic evaluation on admission and after 48 hours of intensive medical therapy. We found the predictive value of baseline mitral E/Ea in estimating PCWP to be less robust than previously reported, which appears to be related to larger left ventricular dimensions, more impaired cardiac output, and the presence of cardiac resynchronization therapy. In addition, no reliable direct correlation between baseline or changes in mitral E/Ea and PCWP was found. Taken together, our observations provide an important refinement in the clinical interpretation of the mitral E/Ea ratio as it applies to patient populations where important confounders such as alterations in myocardial structure, severity of systolic dysfunction, or the presence of synchronized pacing may pose challenges in accurately predicting left ventricular filling pressures.
Acknowledgments
Funding Sources
This research was supported in part by the American Society of Echocardiography Sonographers’ Grant and the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, Ohio.
Footnotes
Disclosure Statement
There is no relationship to disclose for all authors.
REFERENCES
- 1.Haskell RJ, French WI. Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting ventricular enddiastolic pressure. Am J Cardiol. 1988;61:136–141. doi: 10.1016/0002-9149(88)91319-7. [DOI] [PubMed] [Google Scholar]
- 2.Keogh AM, Baron DW, Hikie JB. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy for cardiac transplantation. Am J Cardiol. 1990;65:903–908. doi: 10.1016/0002-9149(90)91434-8. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA. Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol. 1995;26:1417–1423. doi: 10.1016/0735-1097(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 4.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 5.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M. Canadian Critical Care Clinical Trials Group. A randomized controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 6.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 9.Diwan A, McCulloch M, Lawrie GM, Reardon MJ, Nagueh SF. Doppler estimation of left ventricular filling pressures in patients with mitral valve disease. Circulation. 2005;111:3281–3289. doi: 10.1161/CIRCULATIONAHA.104.508812. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, 3rd, Zoghbi WA, Quiñones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 11.Oh JK. Echocardiography as a noninvasive Swan-Ganz catheter. Circulation. 2005;111:3192–3194. doi: 10.1161/CIRCULATIONAHA.105.548644. [DOI] [PubMed] [Google Scholar]
- 12.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 13.Sohn DW, Song JM, Zo JH, Chai IH, Kim HS, Chun HG, Kim HC. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–931. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 14.Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, Nagueh SF. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–2439. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 15.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 16.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2002;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 17.Kidawa M, Coignard L, Drobinski G, Krzeminska-Pakula M, Thomas D, Komajda M, Isnard R. Comparative value of tissue Doppler imaging and m-mode color Doppler mitral flow propagation velocity for the evaluation of left ventricular filling pressure. Chest. 2005;128:2544–2550. doi: 10.1378/chest.128.4.2544. [DOI] [PubMed] [Google Scholar]
- 18.Bruch C, Stypmann J, Gradaus R, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of filling pressures in patients with primary or secondary pure mitral regurgitation. Am J Cardiol. 2004;93:324–328. doi: 10.1016/j.amjcard.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Bruch C, Grude M, Müller J, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of left ventricular filling pressures in patients with systolic and diastolic heart failure. Am J Cardiol. 2005;95:892–895. doi: 10.1016/j.amjcard.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Steimle AE, Stevenson LW, Chelimsky-Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD, Kartashov A, Tillisch JH. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. 1997;19:1165–1172. doi: 10.1161/01.cir.96.4.1165. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by twodimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura R, Tajik A. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 23.Temporelli P, Giannuzzi P, Nicolosi G, Latini R, Franzosi M, Gentile F, Tavazzi L, Maggioni AP. GISSI-3 Echo Substudy Investigators. Doppler derived mitral deceleration time as a strong prognostic marker of left ventricular remodeling and survival after acute myocardial infarction. Results of the GISSI-3 echo substudy. J Am Coll Cardiol. 2004;43:1646–1653. doi: 10.1016/j.jacc.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Rossvoll O, Hatle L. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures. J Am Coll Cardiol. 1993;21:1687–1696. doi: 10.1016/0735-1097(93)90388-h. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura RA, Abel MD, Hatle LK, Tajik AJ. Relation of pulmonary vein to mitral flow velocities by transesophageal Doppler echocardiography: effect of different loading conditions. Circulation. 1990;81:1488–1497. doi: 10.1161/01.cir.81.5.1488. [DOI] [PubMed] [Google Scholar]
- 26.Hurrell DG, Nishimura RA, Ilstrup DM, Appleton CP. Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1997;30:459–467. doi: 10.1016/s0735-1097(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 27.Ha J, Lulic F, Bailey K, Pellikka P, Seward J, Tajik A, Oh J. Effects of treadmill exercise on mitral inflow and annular velocities in healthy adults. Am J Cardiol. 2003;91:114–115. doi: 10.1016/s0002-9149(02)03016-3. [DOI] [PubMed] [Google Scholar]
- 28.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Sohn DW. Mitral annulus velocity in the estimation of left ventricular filling pressure: prospective study in 200 patients. J Am Soc Echocardiogr. 2000;13:980–985. doi: 10.1067/mje.2000.107156. [DOI] [PubMed] [Google Scholar]
- 30.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 31.Werko L, Varnauskas E, Elliasch H, Lagerlof H, Senning A, Thomasson B. Further evidence that the pulmonary capillary venous pressure pulse in man reflects cyclic pressure changes in the left atrium. Circ Res. 1953;1:337–339. doi: 10.1161/01.res.1.4.337. [DOI] [PubMed] [Google Scholar]
- 32.Rahimtoola SH, Loeb HS, Ehsani A, Sinno MZ, Chuquimia R, Lal R, Rosen KM, Gunnar RM. Relationship of pulmonary artery to left ventricular diastolic pressures in acute myocardial infarction. Circulation. 1972;46:291–297. doi: 10.1161/01.cir.46.2.283. [DOI] [PubMed] [Google Scholar]