Abstract
We previously showed that RPE65 does not specifically produce 11-cis retinol only but also 13-cis retinol, supporting a carbocation or radical cation mechanism of isomerization. The intrinsic properties of conjugated polyene chains result in facile formation of radical cations in oxidative conditions. We hypothesized that such radical intermediates, if involved in the mechanism of RPE65, could be stabilized by spin traps. We tested a variety of hydrophilic and lipophilic spin traps for their ability to inhibit RPE65 isomerohydrolase activity. We found that the aromatic lipophilic spin traps such as N-tert-butyl-alpha-phenylnitrone (PBN), 2,2-dimethyl-4-phenyl-2H-imidazole-1-oxide (DMPIO) and nitrosobenzene (NB) strongly inhibit RPE65 isomerohydrolase activity in vitro.
Keywords: RPE65 isomerase, spin traps, N-tert-butyl-alpha-phenylnitrone (PBN), nitrosobenzene, cation-radical
The retinyl ester isomerohydrolase central to the visual cycle was recently confirmed biochemically to be RPE65 (1–3) a protein highly preferentially expressed in the RPE (4). Prior molecular genetics findings, in a targeted mouse model (5) and in human RPE65 gene mutations (6–9), suggested a key role for RPE65 in isomerization. RPE65 is a member of a family of carotenoid oxygenases that specialize in oxidative cleavage of double bonds in various carotenoids. However, RPE65 itself does not perform oxidative cleavage. Rather, by concerted O-alkyl cleavage of all-trans retinyl ester and isomerization of the retinyl moiety it produces 11-cis retinol and a fatty acid (10). We recently presented evidence in support of the carbocation or radical cation mechanism by showing that RPE65 is capable of enzymatic isomerization to 13-cis retinol (11). Indeed, we found that site-directed mutagenesis of a single residue, Phe103, converted the mutant RPE65 into a preferential 13-cis retinol isomerohydrolase and our data were supported by identification of specific 13-cis retinol RPE65 isomerohydrolase in zebrafish (12). In light of this, we concluded that retinoid metabolism in the visual cycle occurs by a carbocation mechanism (SN1 hydrolysis of retinyl ester) (13), or by a cation radical mechanism (one electron oxidation of retinyl ester) (11) as the data could be explained either way. Carotenoid radical cations have a tendency to lose a proton to form a neutral carotenoid radical (14). If the latter mechanism were true, we conjectured that RPE65 could be inhibited by spin trap compounds.
To test this hypothesis we chose a variety of cell permeable spin traps and tested these in a robust minimal visual cycle (canine RPE65/bovine LRAT) reconstructed in HEK293F cells (3). HPLC separation of retinol isomers was conducted as previously described (3). RPE65 isomerohydrolase activity was calculated as ratio of 11-cis retinol or 13-cis retinol to a total amount of all retinol isomers (11-cis, 13-cis and all-trans retinol). We discovered that several lipophilic aromatic spin traps are effective inhibitors of RPE65 isomerase activity. We confirmed our findings using a bovine RPE microsomal fraction preparation.
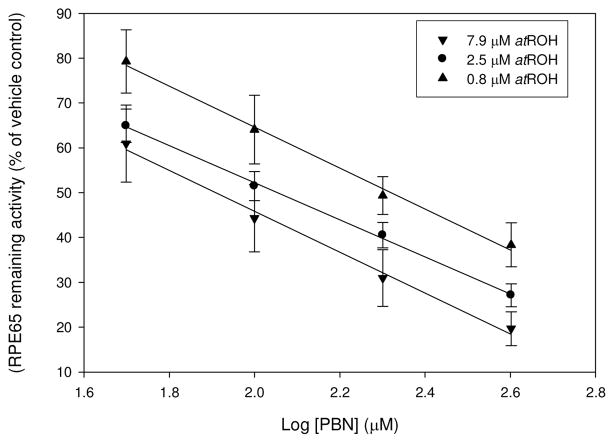
Various spin traps compounds including PBN, 2-methyl-2-nitrosopropane (MNP) and nitrosobenzene have been successfully employed to stabilize and detect carotenoid and retinoid radicals by EPR spectroscopy (14, 15). In our cell-based assay both PBN and nitrosobenzene demonstrated inhibitory effect at 100 μM concentrations (52±3 % and 32±5 % of vehicle control RPE65 activity respectively, Table S1). 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) does not form stable adducts with carotenoid radicals in ESR experiments (14) and predictably did not have an inhibitory effect on RPE65 activity (Figure 1). Nitrosobenzene has a limited usefulness because of its well documented toxicity in cells and animals (16, 17). On the other hand, PBN is a widely used agent that is well tolerated in animals, while a 1,3-disulfonate derivative of PBN, disufenton sodium (NXY059), has been tested in humans as a neuroprotective agent for stroke (18, 19). We found that PBN is an effective uncompetitive inhibitor of RPE65 with Ki = 61 μM which is in the same range as its effects on oxygen radicals (Figure 1, Figure 2). RPE65 protein expression is not reduced at these concentrations, nor is total retinyl ester accumulation, indicating a direct inhibition of RPE65 (data not shown). PBN instability in vivo has been reported and its neu-roprotective effects attributed to the degradation products t-butylhydroxylamine (TBH) and consequently MNP (20). Neither of these compounds had an effect on RPE65 activity in our cell-based assay at 200 μM. DMPIO, a structural and functional analog of PBN, demonstrated a significant inhibitory potency while a structural analogs that lacks an N-oxide moiety, n-benzylidene-tert-butylamine (NBTB), demonstrated much less inhibitory effect on RPE65 activity. Moreover, spin traps inhibit 13-cis isomer formation to the same extent as 11-cis isomer (Table 1). The uncompetitive nature of PBN inhibition was shown by the lowering of IC50 as substrate concentration was increased (Figure 2). These findings are consistent with the proposed mechanism of trapping of retinyl ester radical intermediate by PBN. We modeled RPE65 on a composite structural template (VP14, ACO and RPE65; see Supporting Information) to ensure an “open” conformation of the protein. Docking of a PBN-retinyl palmitate spin adduct was performed using AutodockVina and compared with docking of the PBN molecule alone (Figure 3), predicting that while the spin adduct could dock favorably (−9.5 kcal/mol), PBN alone could not (−5.6 kcal/mol), remaining distant from the iron center, ruling out the possibility of iron chelation, and so not expected to directly affect the catalytic activity. Modeling attempts with the RPE65 structure alone were unsuccessful due to the “closed” substrate binding cleft of the RPE65 crystal in the absence of substrate (21) and possibly in the presence of reaction products (P. Chander, in preparation).
Figure 1. RPE65 activity in the presence of various spin traps.
A: HEK293F cells transfected as described (see Supporting Information) were incubated in the presence of 0–500 mM of POBN, NXY059, SPBN, DMPO, MNP and PBN at 2.5 mM all-trans retinol. Activity was calculated relative to the vehicle control; n≥3 for each data point. B: structures of spin traps and a structural analog used in this work.
Figure 2. Inhibition of RPE65 activity in the presence of PBN at various substrate concentrations (IC50 determination).
HEK293F cells transfected as described (see Supporting Information) were incubated in the presence of 0–400 mM PBN in DMSO (vehicle) at substrate concentrations of 0.79, 2.5 or 7.9 mM all-trans retinol (half-log concentration steps). Activity was calculated relative to DMSO vehicle control. Data plotted as regression against log10 PBN concentration; n≥4 for each data point.
Table 1.
RPE65 activity (%) in the presence of PBN, possible degradation products, a spin trap analog DMPIO, and structural analog NBTB without spin trap properties at 2.5 μM substrate.
| Inhibitor (200 μM) | RPE65 activity (11-cis isomer % of vehicle) | RPE65 activity (13-cis isomer % of vehicle) |
|---|---|---|
| PBN | 50±6 | 43±6 |
| TBH | 103±6 | 107±10 |
| MNP | 94±5 | 95±7 |
| NBTB | 86±6 | 91±6 |
| DMPIO | 68±6 | 63±1 |
Figure 3. Simulated docking of PBN-retinyl palmitate spin adduct on RPE65 model.

Models of a spin adduct of all-trans-retinyl palmitate and PBN (RP-PBN; purple) and PBN (yellow) were docked independently on a composite RPE65 model as described (see Supporting Information), but depicted here together.
In considering more hydrophilic spin traps we analyzed the effect on RPE65 activity of N-tert-butyl-(2-sulfophenyl)-nitrone (SPBN), α-(4-Pyridyl 1-oxide)-N-tert-butylnitrone (POBN) and NXY059. However, none of these inhibited RPE65 activity in our cell-based assay up to 400 μM (Figure 1). Therefore, we concluded that spin trap hydrophobicity is a key element to access the substrate tunnel and to bind to a reaction radical intermediate.
However, when we treated cells with a number of unrelated hydrophobic spin traps, only the aromatic N-oxides DMPIO and 2-(2-Carboxyethyl)-2-methyl-4-phenyl-2H-imidazole-1-oxide (MCPIO) demonstrated a moderate inhibitory effect at 100 μM concentration (Table S1). There was no substantial change in cell viability (~ 90 %) and RPE65 protein expression in treated cells (Table S2). We confirmed in the isolated HEK293 microsomal fraction that PBN and DMPIO significantly inhibit RPE65 activity at 200 μM inhibitor (46 % and 56 % of vehicle respectively). Also we demonstrated that production of 11-cis retinol from bovine RPE microsomal fraction is inhibited by PBN at 400 μM (53 ± 3 % of wildtype RPE65 activity).
Thus, we describe for the first time a new class of RPE65 inhibitors that are based on trapping of a reaction intermediate with hydrophobic aromatic N-oxides. Inhibitory potency decreases with increase in polarity from NB to PBN to DMPIO and to MCPIO (Table S1). On the other hand, NBTB, a structural analog of PBN without spin trap properties, does not inhibit RPE65 activity significantly and thus we conclude that the N-oxide group is involved in RPE65 inhibition. The inhibition of RPE65 activity is a possible route for slowing the visual cycle and preventing accumulation of A2E byproducts involved in the etiology of Stargardt macular dystrophy. Interestingly, PBN protects rats against light damage at 50 mg/kg systemic administration (22), an effect ascribed to scavenging of oxygen radicals due to rhodopsin photoproducts. However, our data suggests that this protective effect may be upstream, at least in part, due to inhibition of RPE65 activity. Reduced 11-cis retinoid flux via inhibition of RPE65 would reduce rhodopsin photocycling and so reduce generation of free radicals caused by light damage. This is pertinent as Rpe65−/− mice and C57BL/6 mice with the hypomorphic L450M RPE65 variant are both much less susceptible to light damage than wildtype mice (23). We conclude that inhibition by spin traps of RPE65 lends strong support to a radical cation-based mechanism of retinol isomerization by RPE65.
Supplementary Material
Acknowledgments
TP was supported by the Howard Hughes Medical Institute (HHMI), while MA was a HHMI/Montgomery County (MD) Public Schools/NIH student intern when this work was performed.
Footnotes
This research was supported by the Intramural Research Program of the National Eye Institute, NIH
SUPPORTING INFORMATION AVAILABLE: Detailed experimental methods and additional tables are available online at http://pubs.acs.org.
References
- 1.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. The Journal of biological chemistry. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 5.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Nature genetics. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 6.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumara-manickavel G, Denton MJ, Gal A. Nature genetics. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 7.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Nature genetics. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 8.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson DA, Gyurus P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, Sieving PA, Gal A. Investigative ophthalmology & visual science. 2000;41:4293–4299. [PubMed] [Google Scholar]
- 10.Law WC, Rando RR. Biochemistry. 1988;27:4147–4152. doi: 10.1021/bi00411a037. [DOI] [PubMed] [Google Scholar]
- 11.Redmond TM, Poliakov E, Kuo S, Chander P, Gentleman S. The Journal of biological chemistry. 2010;285:1919–1927. doi: 10.1074/jbc.M109.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi Y, Moiseyev G, Chen Y, Farjo K, Nikolaeva O, Ma JX. 2011;278:973–987. doi: 10.1111/j.1742-4658.2011.08019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBee JK, Kuksa V, Alvarez R, de Lera AR, Prezhdo O, Haeseleer F, Sokal I, Palczewski K. Biochemistry. 2000;39:11370–11380. doi: 10.1021/bi001061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konovalova TA, Kispert LD, Polyakov NE, Leshi-na TV. Free radical biology & medicine. 2000;28:1030–1038. doi: 10.1016/s0891-5849(00)00192-1. [DOI] [PubMed] [Google Scholar]
- 15.Iwahashi H, Negoro Y, Ikeda A, Kido R. Journal of chromatography. 1987;391:199–205. doi: 10.1016/s0021-9673(01)94316-x. [DOI] [PubMed] [Google Scholar]
- 16.Fujii H, Zhao B, Koscielniak J, Berliner LJ. Magn Reson Med. 1994;31:77–80. doi: 10.1002/mrm.1910310113. [DOI] [PubMed] [Google Scholar]
- 17.Haseloff RF, Mertsch K, Rohde E, Baeger I, Grigor’ev IA, Blasig IE. FEBS letters. 1997;418:73–75. doi: 10.1016/s0014-5793(97)01349-5. [DOI] [PubMed] [Google Scholar]
- 18.Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hardemark HG, Rodichok L. Stroke; a journal of cerebral circulation. 2008;39:1751–1758. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 19.Lapchak PA, Chapman DF, Zivin JA. Stroke; a journal of cerebral circulation. 2001;32:147–153. doi: 10.1161/01.str.32.1.147. [DOI] [PubMed] [Google Scholar]
- 20.Atamna H, Robinson C, Ingersoll R, Elliott H, Ames BN. Faseb J. 2001;15:2196–2204. doi: 10.1096/fj.01-0134com. [DOI] [PubMed] [Google Scholar]
- 21.Kiser PD, Golczak M, Lodowski DT, Chance MR, Palczewski K. Proc Natl Acad Sci U S A. 2009;106:17325–17330. doi: 10.1073/pnas.0906600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranchon I, Chen S, Alvarez K, Anderson RE. Investigative ophthalmology & visual science. 2001;42:1375–1379. [PubMed] [Google Scholar]
- 23.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.