Abstract
The HM loci in Saccharomyces cerevisiae constitute region-specific but gene-nonspecific repression domains, as a number of heterologous genes transcribed by RNA polymerase II or III are silenced when placed at these loci. The promoters of the Ashbya gossypii TEF gene and the S. cerevisiae TEF1 and TEF2 genes, however, are resistant to transcriptional silencing by the HM silencers in yeast. Moreover, when interposed between the HML α genes and the E silencer, certain segments of these promoters block the repression effect of the silencer on the α genes. All of these fragments contain UASrpg (upstream activation sequence of ribosome protein genes) composed of multiple binding sites for Rap1. In fact, a 149-bp segment consisting essentially of only three tandem Rap1-binding sites from the UASrpg of yeast TEF2 exhibits silencer-blocking activity. This element also exhibits insulating activity and orientation dependence characteristic of known chromatin boundary elements. Finally, the element blocks the physical spread of heterochromatin initiated at a silencer. This segment provides the first example of chromatin domain boundary or insulator elements in yeast.
Keywords: Chromatin boundary elements, promoters, silencers, DNA topology, Rap1, transcriptional silencing
The expression state of a eukaryotic gene depends on its location in the chromosome. This position effect results from the organization of the eukaryotic genome into discrete functional domains, defined in part by local differences in chromatin structure. The extent of each domain appears to be defined and maintained by boundary, or insulator, elements. Examples of boundary elements in chromosomes of metazoans include the insulator of the gypsy retrotransposon, the scs and scs′ elements flanking the 87A1 hsp70 locus in Drosophila, and the chicken β-globin boundary elements (Kellum and Schedl 1991, 1992; Geyer and Corces 1992; Chung et al. 1993). Boundary elements have been defined functionally by their ability both to block trancriptional activation of a promoter by a nearby enhancer and to protect transgenes from positive (activating) or negative (repressing) position effects.
Mating type determination and telomere position effect provide examples of position dependent gene expression in the yeast Saccharomyces cerevisiae. The mating type of a haploid S. cerevisiae strain is determined by the allele (a or α) present at the MAT locus near the centromere of chromosome III. Copies of the mating type genes, including intact structural genes and their promoters, also reside at the homothalic mating loci HML and HMR, respectively. Whereas genes at MAT locus are freely expressed, genes at the HM loci are transcriptionally repressed and only serve as donors of information during a mating type switching event.
Similar to position effect in higher eukaryotes, transcriptional silencing at the HM loci and at telomeres in yeast derives from a heterochromatin-like structure (for review, see Braunstein et al. 1997). First, DNA in transcriptionally silent chromatin in yeast is relatively inaccessible to various modifying agents (Terleth et al. 1989; Gottschling 1992; Singh and Klar 1992; Loo and Rine 1994). Second, similar to DNA in heterochromatin in higher cells, transcriptionally silenced regions in yeast replicate late in S phase (Reynolds et al. 1989). Third, nucleosomes from the silent HM loci and telomeres have reduced acetylation compared to nucleosomes from active regions of the genome (Braunstein et al. 1993) and the pattern of acetylation of histone H4 at HM loci is identical to that in centric heterochromatin in Drosophila (Braunstein et al. 1996). Fourth, DNA within transcriptionally silenced HM loci is more negatively supercoiled than that in active HM loci (Bi and Broach 1997; Cheng et al. 1998), indicating that silent chromatin is more compact. Finally, high-resolution chromatin mapping of HMLα and MATα revealed that the silent HMLα locus has a uniquely organized chromatin structure (Weiss and Simpson 1998).
The combined actions of cis-acting DNA elements and trans-acting factors establish and maintain transcriptional silencing at the HM loci (for review, see Laurenson and Rine 1992). The cis-acting sites, known as the E and I silencers, are small negative regulatory sequences flanking each of the HM loci (Abraham et al. 1984; Feldman et al. 1984) and are both necessary and sufficient for silencing (Brand et al. 1985; Mahoney and Broach 1989; Shei and Broach 1995). They are composed of various combinations of binding sites for proteins Rap1, Abf1, and the origin recognition complex (ORC). The trans-acting factors required for silencing include histones, silencer binding proteins, and the four SIR proteins—Sir1 through Sir4.
The proteins involved in silencing form extensive homotypic and heterotypic interactions. Both Sir3 and Sir4 can homodimerize and heterodimerize and both can bind to Sir2, Rap1 (Moretti et al. 1994; Strahl-Bolsinger et al. 1997), and histones H3 and H4 in vitro (Johnson et al. 1990; Hecht et al. 1995). Sir3 also can bind to histones H2A and H2B (Hecht et al. 1996). Both Sir4 and ORC interact with Sir1 (Triolo and Sternglanz 1996). These interactions prompted the current model for silencing, in which silencers recruit Sir1 and Sir3/Sir4 through their direct interactions with ORC and Rap1, respectively. Sir1 and Sir3/Sir4 in turn recruit Sir2 to the silencer. This complex at the silencer then seeds an array of complexes comprised of Sir2, Sir3, and Sir4 that spreads outward into the adjacent chromatin. In this fashion, Sir1 functions only in initiating silencing (Triolo and Sternglanz 1996), whereas Sir2, Sir3, and Sir4 form an extended complex as an integral part of the silent chromatin (Hecht et al. 1996).
Transcriptional silencing at the HM loci has been considered region-specific but gene-nonspecific, as translocation of the mating type genes resident at silent loci to different sites de-represses them and insertion of heterologous genes into the HM loci results in their repression. LEU2, URA3, ADE2, and TRP1, transcribed by RNA polymerase II (Pol II), and SUP3 and SUP4-o, transcribed by RNA polymerase III, are all repressible by HML or HMR silencers (Brand et al. 1985; Schnell and Rine 1986; Mahoney and Broach 1989; Sussel and Shore 1991; Sussel et al. 1993). The fact that Ty5 retrotransposons inserted within HML can be transcriptionally activated by the pheromone response pathway, and that the heat shock promoter inserted next to HMR can be induced by heat shock, questions the generality of silencer-mediated repression (Lee and Gross 1993; Ke et al. 1997). In both of these cases, though, the silencing apparatus represses basal transcription.
In this report, we show that the promoters of S. cerevisiae TEF2 and TEF1 genes as well as that of the TEF gene of filamentous fungus Ashbya gossypii are resistant to silencing when inserted at HM loci. Moreover, these promoters have silencer-blocking activity that does not require transcription per se. In these promoters, the UASrpg (upstream activation site for ribosomal protein genes) sequence consisting of binding sites for Rap1 is necessary and sufficient for the silencer-blocking activity. UASrpg-containing elements from these promoters also block the spread of the unique chromatin structure correlated with silencing. These elements present the first examples of chromatin boundary/insulator elements in yeast.
Results
Promoters of the A. gossypii and S. cerevisiae TEF genes are resistant to transcriptional silencing
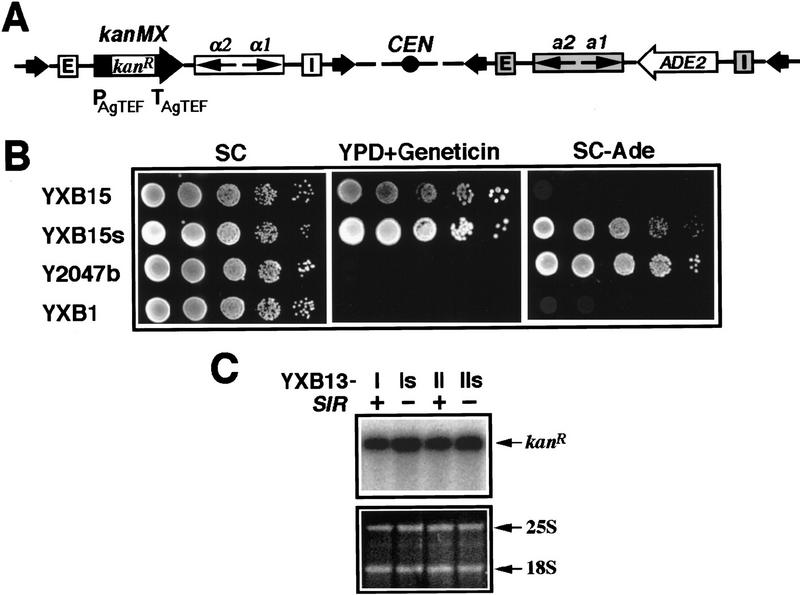
In an attempt to compare features of transcriptional silencing at HMLα and HMRa, we inserted the heterologous kanMX module at HML and the ADE2 gene at HMR (Fig. 1A) in the same strain. The kanMX module consists of the kanR open reading frame (ORF) of Escherichia coli transposon Tn903 fused to the transcriptional control sequences of the TEF gene from filamentous fungus A. gossypii (Fig. 1A). This hybrid kanMX module confers geneticin (G418) resistance to yeast (Wach et al. 1994). Whereas the ADE2 gene at HMR in this SIR+ strain was repressed (cells did not grow on SC − Ade medium; Fig. 1B), the kanMX module was actively transcribed (YXB15 cells grew on YPD + Geneticin medium) even though it was flanked by the E and I silencers of HML (Fig. 1B). Therefore, the kanMX gene was resistant to silencing whereas the ADE2 gene was sensitive to silencing. One explanation for the apparent resistance of the kanMX module to Sir-mediated silencing is that silencing of the gene does in fact occur but that the repressed level of expression still yields sufficient product to confer a geneticin resistance phenotype. To test this possibility, we used Northern analysis to examine directly the transcript levels of the kanMX module inserted in the HML locus in a Sir+ versus Sir− strain. The results of this analysis, in Figure 1C, show that the level of transcription of the kanMX module differs by <20% in the SIR+ versus the sir− background. Therefore, the kanMX module is resistant to Sir-mediated transcriptional silencing.
Figure 1.
The kanMX module is refractory to SIR-mediated transcriptional silencing. (A) Modified HM loci in strain YXB15. The kanMX module (PAgTEF–kanR–TAgTEF) and ADE2 gene were inserted at HML and HMR, respectively. (Open bars) HML silencers. (Shaded bars) HMR silencers. (Arrows in open bars) HMLα genes. (Arrows in shaded bar) HMRa genes. (Solid arrows) FRT sites. See Materials and Methods for details. (B) Growth phenotypes of YXB15 (HMLα:: KanMX HMRa::ADE2 ade2-1 SIR+), YXB15s (HMLα::KanMX HMRa::ADE2 ade2-1 sir3), Y2047b (ΔE–HMLα–SUP4–o–ΔI-HMRa ade2-1 SIR+) and YXB1 (HMLα HMRa ade2-1 SIR+). Cells were grown to late log phase and serial dilutions (10-fold) were spotted on test plates and allowed to grow for 3 days. Geneticin (G418) was used at 250 μg/ml. (C) Northern blot analysis of kanMX expression. Cells of each strain were grown to late log phase before being harvested and their total RNA extracted as described (Kaiser et al. 1994). Ten micrograms of total RNA was loaded in each lane. The gel was stained with ethidium bromide to reveal the 25S and 18S rRNAs as loading controls. The kanR mRNA was detected by Northern blotting and hybridization with a radioactive probe made from the ORF sequence of kanR. Note that strain YXB13-I was the parental strain of YXB15 (Materials and Methods; Fig. 2).
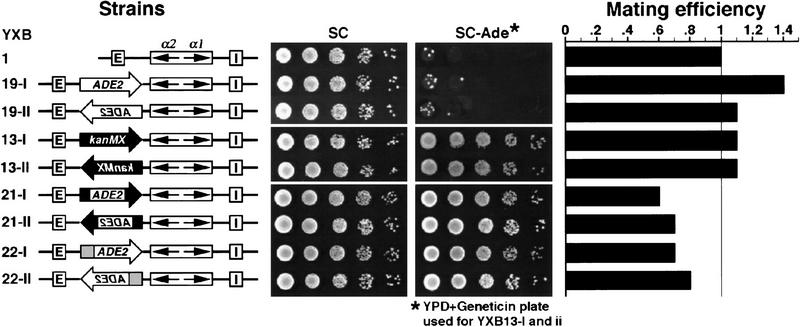
To determine whether the sensitivity of the two different marker genes to silencing was a function of the different HM loci, we examined strains containing the ADE2 gene inserted into HML at the same site as that used for the kanMX module. As evident from the results in Figure 2, the ADE2 gene resident at HML was subject to Sir-dependent repression. The SIR+ version of the strain was auxotrophic for adenine (Fig. 2, strains YXB19-I and YXB19-II) whereas the isogenic sir− strain was prototrophic (data not shown). Because a hybrid gene consisting of the A. gossypii TEF promoter (PAgTEF) fused to the ADE2-coding region inserted into the HML locus was resistant to silencing (Fig. 2, strains YXB21-I and YXB21-II), the difference in sensitivity of ADE2 versus kanMX to Sir-mediated repression can be ascribed to their respective promoters. Therefore, the ability of the kanMX module to overcome silencing is the property of PAgTEF.
Figure 2.
The A. gossypii and yeast TEF promoters confer resistance to transcriptional silencing. (Left) Modified HML loci in the strains tested. YXB19 contains unmodified ADE2 gene (open block arrows) at HML. YXB13 has the kanMX module (solid block arrows) at HML. YXB21 and YXB22 have chimeric genes PAgTEF–ADE2–TAgTEF (solid regions of the block arrows represent AgTEF sequences and open regions represent the ADE2 coding region) and PTEF2–ADE2 (shaded region of the block arrows represents the TEF2 promoter and open regions represent the ADE2-coding region) at HML, respectively. (Middle) Growth phenotypes examined as described in legend to Fig. 1. (Right) Mating efficiency as measured by quantitative mating (Materials and Methods). The mating efficiency of YXB1 was taken as one.
The TEF gene in A. gossypii is the only gene in this organism coding for translation elongation factor-1α (EF-1α) (Steiner and Philippsen 1994). S. cerevisiae, on the other hand, has two redundant genes, TEF1 and TEF2, encoding EF-1α (Nagata et al. 1984; Schirmaier and Philippsen 1984; Cottrelle et al. 1985). To test if the S. cerevisiae TEF promoters are also resistant to transcriptional silencing, we fused the promoter of the TEF2 gene (PTEF2) with the coding region and 3′ flanking region of ADE2 and inserted this chimeric module at the HML locus (Fig. 2, strain YXB22). This PTEF2–ADE2 construct was fully expressed. Strain YXB22 grew on SC–Ade medium and strain YXB22 colonies were white on YPD and indistinguishable from strain YXB22s (sir−) colonies (Fig. 2; data not shown). A similar result was obtained with the yeast TEF1 promoter (data not shown). Therefore, like their A. gossypii counterpart, the S. cerevisiae TEF promoters are resistant to transcriptional silencing.
The HML E and I silencers and the HMR E and I silencers are not equivalent in their strength in transcriptional silencing. By several criteria, the relative order of repression activity is HMR E > HML E > HML I > HMR I (Shei and Broach 1995; Z. Zhang and A.R. Buchman, pers. comm.). Therefore, a gene resistant to HML silencing may not necessarily be resistant to HMR silencing. To address whether the TEF promoters were also resistant to HMR silencing, we inserted the chimeric constructs PTEF1–ADE2 and PTEF2–ADE2 into HMR and showed that they were fully expressed in a SIR+ background (data not shown). Therefore the TEF promoters are resistant to silencing by even the strongest silencer in yeast.
Active and silenced genes can coexist at HML
We have demonstrated that genes driven by the TEF promoters are transcriptionally active when inserted at HML. This could be attributable either to TEF-induced abrogation of silencing across the entire locus or to resistance of the TEF promoters to the transcriptional repression imposed by the silencing apparatus. To distinguish between these possibilities, we investigated whether the presence of these activated genes at HML affected silencing of the α1 and α2 genes resident at the same locus. This was accomplished by testing the mating ability of the strains of interest, as the mating efficiency of a MATa strain is inversely proportional to the expression state of the HMLα genes (Herskowitz 1988). As shown in Figure 2, neither the repressed ADE2 gene (in strain YXB19) nor the active kanMX module at HML (strain YXB13) had any effect on mating and the PAgTEF–ADE2 (in strain YXB21) and PTEF2–ADE2 (in strain YXB22) genes caused only a slight decrease in mating efficiency. These results suggest that although the TEF promoters were active at HML, their presence had little, if any, effect on the silencing of the adjacent α1 and α2 genes.
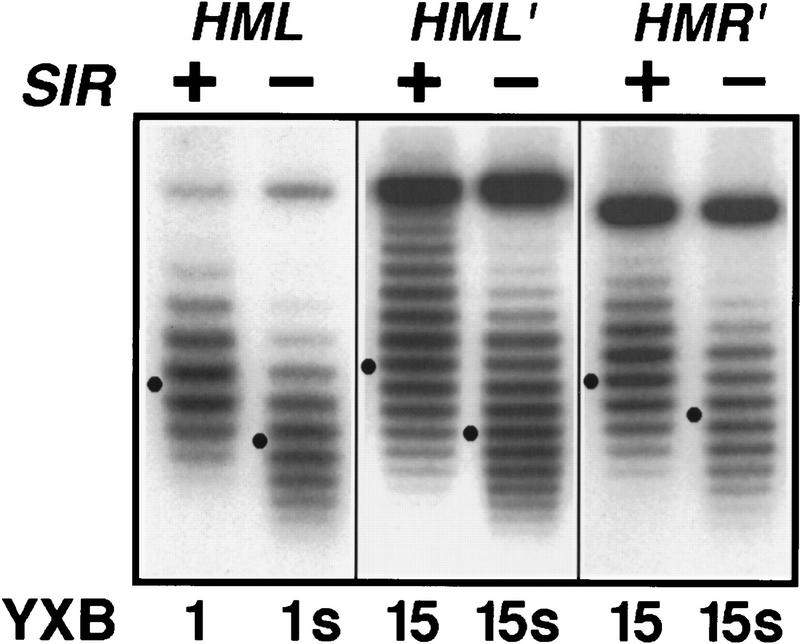
To confirm that the silencing apparatus is intact at HML containing the PTEF-driven genes, we examined the effect of the SIR genes on the topology of the locus. The heterochromatin induced by the silencing apparatus imposes a different topology on the DNA across the HML locus than does the chromatin associated with the active form of the locus. This difference can be detected as an increase in the negative superhelical density of a circular DNA molecule obtained by in vivo excision of the HM locus from the chromosome of a SIR+ versus a sir− strain (Bi and Broach 1997; Cheng et al. 1998). Therefore, a difference in the superhelical density of such a molecule excised from a SIR+ versus a sir− strain would indicate that the locus was packaged in heterochromatin in the SIR+ background.
Our analysis of the topology of the HM loci containing various inserted genes is shown in Figure 3. Consistent with previous results (Bi and Broach 1997; Cheng et al. 1998), circular molecules spanning the unmodified HML locus excised from the chromosome of a SIR+ versus a sir− strain exhibited a difference in superhelical density of ∼2, with the SIR+ species being more negatively supercoiled (Fig. 3, cf. lanes YXB1 and YXB1s). Similarly, we found that circles spanning the HMR locus carrying the ADE2 gene and circles spanning the HML locus carrying the kanMX module were also more negatively supercoiled in the SIR+ versus a sir− strain (Fig. 3, strains YXB15 and YXB15s). Similar results were obtained with the HML locus containing either PTEF2–ADE2 or PAgTEF–ADE2 (data not shown). Because the extent of Sir-dependent superhelical density change is a function both of length and composition of the DNA, we cannot determine from the precise linking number change whether the entire locus containing the kanMX module, or only a part of the locus, is packaged in heterochromatin. Nonetheless, these results clearly demonstrate that the presence of the kanMX module does not eliminate Sir-dependent silencing at HML.
Figure 3.
Insertion of a silencing-resistant gene at HML does not abolish the SIR-dependent silent chromatin structure. Cells of the indicated strains were grown in YPR medium to log phase before galactose was added and the cultures were incubated for 2.5 hr. DNA isolated from cells was fractionated by agarose gel electrophoresis in the presence of 30 μg/ml chloroquine. Under this condition, the more negatively supercoiled circles migrate more slowly in the gel. HML and HMR circles were revealed by Southern blotting. The Gaussian center of each distribution of topoisomers is indicated (●). (HML′ and HMR′) The modified HML and HMR loci in strain YXB15, respectively (Fig. 1A).
A silencing-resistant gene has silencer-blocking activity
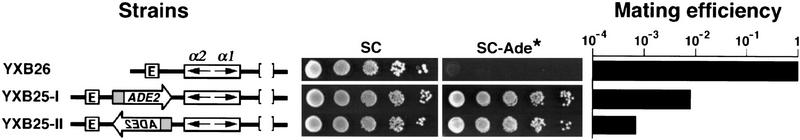
We showed above that the silencing-resistant promoter inserted at HML did not eliminate repression of the adjacent α1 and α2 genes. This could indicate that the presence of a silencing-resistant gene between E and α-mating genes does not affect the ability of E to silence the α-mating genes. Alternatively, the silencing-resistant gene may block E from exerting its silencing effect on α-mating genes, with silencing of the α mating genes in such a situation caused solely by the action of the I silencer. This later hypothesis is not unreasonable, as the HML E and HML I silencers are each capable alone of establishing and maintaining silencing of the α-mating genes at HML (Mahoney and Broach 1989). To distinguish between these two possibilities, we examined expression of the α1/α2 genes at HML in strains in which the PTEF2–ADE2 module was inserted between E and the α genes in a locus that lacked the HML I silencer (Fig. 4, strains YXB25-I and YXB25-II). Because this assay determines the ability of a test sequence to block the activity of the silencer on its normal target when the test gene sits between them, such an assay provides one indication of the boundary activity of the inserted sequence.
Figure 4.
Silencing-resistant genes exhibit silencer-blocking activity. (Left) Modified HML loci in the strains tested. The HML I silencer was deleted from each strain. The PTEF2–ADE2 module was inserted between the E silencer and the HMLα genes in YXB25. (Middle) Growth phenotypes examined as described in legend to Fig. 1. (Right) Mating efficiency as measured by quantitative mating. The mating efficiency of YXB26 was taken as one.
Consistent with earlier observations (Mahoney and Broach 1989), the mating efficiency of a strain in which HML I is deleted (strain YXB26) was comparable with the strain with an intact HML locus (strain YXB1), confirming that the E silencer alone is sufficient for silencing the α-mating genes at HML. Insertion of the PTEF2–ADE2 gene between E and the α-mating genes reduced mating efficiency by 100- to 1000-fold (Fig. 4, strains YXB25-I and YXB25-II) below that of strain YXB26, indicating essentially full derepression of the α-mating genes in these strains. Insertion of the PAgTEF–ADE2 gene had a similar effect (data not shown). These results demonstrate that the silencing-resistant genes exhibit silencer-blocking activity.
UASrpg from the TEF genes can function as heterochromatin boundary elements
To identify the region of PTEF2–ADE2 responsible for silencer-blocking activity and to examine whether active transcription of the gene is required for activity, we interposed various fragments of PTEF2–ADE2 between the E silencer and the α-mating genes in HMLΔI and then tested their effects on silencing of the α-mating genes. As shown in Figure 5A, all of the UASrpg-containing fragments of yeast PTEF2 reduced mating efficiency of cells to some extent (cf. strains YXB29, YXB48, YXB59, and YXB60 with strain YXB26). In particular, a 104-bp fragment containing the TEF2 UASrpg (strain YXB59) was sufficient to reduce the mating efficiency by 90% and the 54-bp UASrpg per se reduced mating efficiency by 70% in one orientation (strain YXB60-II). In contrast, fragments lacking UASrpg had little, if any, effect on mating efficiency (cf. strains YXB49, YXB28, and YXB27 with strain YXB26). Consistent with the above results for yeast PTEF2, a TATA-less 284-bp fragment of the A. gossypii TEF promoter containing UASrpg-like sequences was sufficient to reduce the mating efficiency of cells by 10-fold (Fig. 5B, compare strain YXB31 with strain YXB26). A 119-bp fragment of the 284-bp fragment excluding the UASrpg had little effect on silencing (Fig. 5B, strain YXB47). These data suggest that even in the absence of active transcription, UASrpg-containing elements from TEF promoters have silencer-blocking activity.
Figure 5.
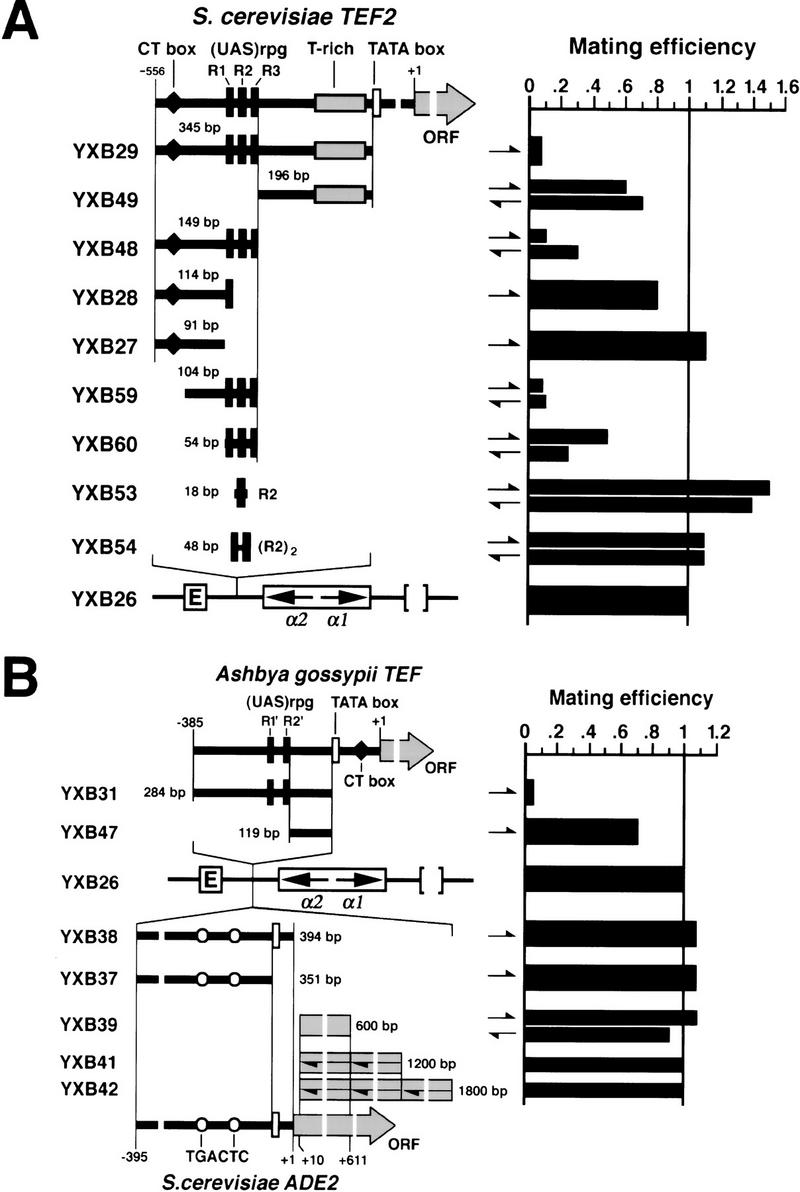
UASrpg exhibits silencer-blocking activity. (A) Various fragments from the yeast TEF2 promoter were inserted at the SpeI site between HML E silencer and the α genes at HMLΔI (left); their effect on α gene expression was analyzed by quantitative mating (right). The direction of an insertion is indicated by a half arrow. In the diagram of the TEF2 promoter the following are indicated: (solid bars) Rap1-binding sites; (open bar) TATA box; (♦) CT box (Gcr1-binding site); (shaded bar) T-rich region. (B) Similar analyses of A. gossypii TEF promoter and the yeast ADE2 gene. (○) TGACTC sequence. Other symbols are as in A.
In contrast to the TEF gene promoters, the promoter from ADE2 did not exhibit silencer blocking activity (Fig. 5B, cf. strains YXB37 and YXB38 with strain YXB26). A 600-bp fragment of the ADE2 ORF (+10 to +611), even when present in two or three tandem copies, also failed to exhibit silencer blocking activity (strains YXB39, YXB41, and YXB42). Despite the fact that the promoter region of the α-mating genes lies 3.8 kb distal to the E silencer in strain YXB42, silencing is maintained, confirming that silencing initiated at E can spread over a long distance. This indicates that the silencer-blocking activity observed for the small (<350 bp) TEF promoter elements is not a distance effect. Finally, the magnitude of the effect obtained with the UAS elements compared with those obtained with the intact genes suggest that other factors in addition to the UAS elements may contribute to the magnitude of the disruption of E-mediated silencing. The UASrpg element, however, is the only sequence tested from PTEF2–ADE2 or PAgTEF that alone elicits significant silencer blocking activity. Furthermore, because the magnitude of the effect of UASrpg is comparable with that of other boundary elements previously described, we conclude that UASrpg is a viable candidate as a yeast boundary element.
The UASrpg sequence of TEF2 consists of three variants (designated R1, R2, and R3 for convenience) of the consensus sequence for Rap1 binding. The R1-containing 114-bp fragment of PTEF2 (Fig. 5A, strain YXB28) did not block silencing significantly. The R2 sequence alone, which binds tightly to Rap1 in vitro (Buchman et al. 1988), also had no silencer-blocking activity (Fig. 5A, strain YXB53). Tandem copies of two or five R2 sequences also failed to exhibit silencer-blocking boundary activity (Fig. 5A, strain YXB54; data not shown). Therefore, the particular combination of the R1, R2, and R3 binding sites for Rap1 in the TEF2 promoter or the particular orientation or spacing of Rap1 sites appears critical for silencer blocking activity.
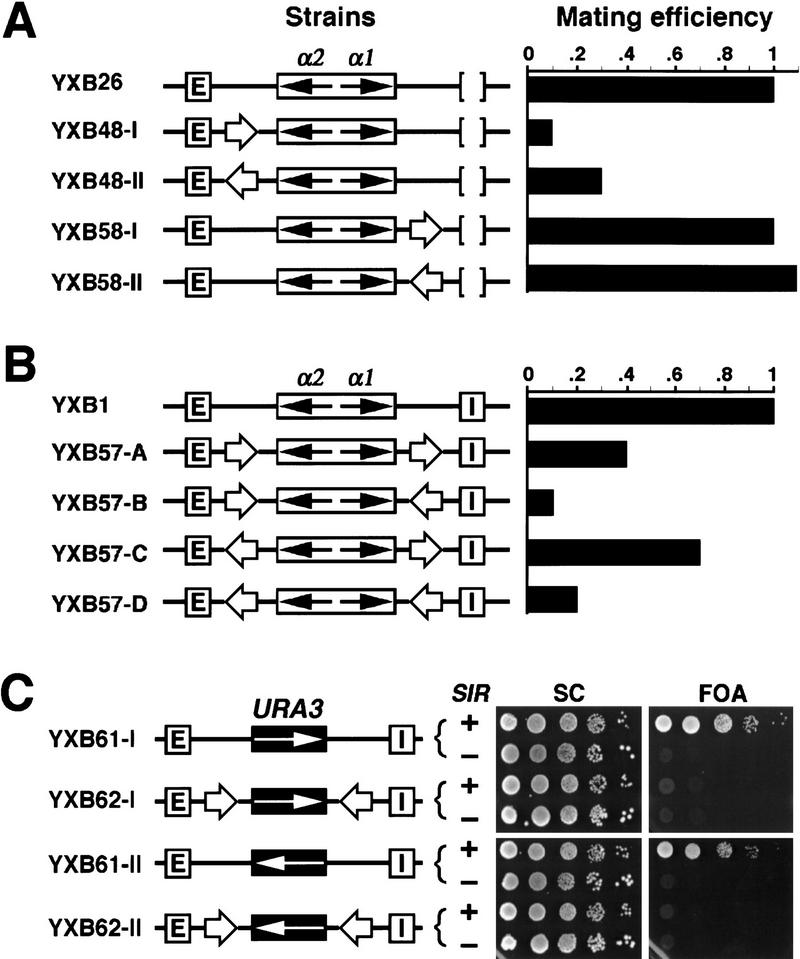
A second criterion for a chromatin boundary element is that its activity be orientation-dependent in the following sense. A boundary element can render a reporter gene impervious to an enhancer or silencer only when it lies between the silencer or enhancer and the reporter gene. We found that the putative boundary elements identified in this study fulfilled this criterion. As shown in Figure 6A, insertion of the 149-bp fragment spanning the UASrpg from TEF2 between E and the HMLα genes at HMLΔI resulted in activation of the α genes (strains YXB48-I and YXB48-II), whereas insertion of the same sequence on the E-distal side of the HMLα genes failed to elicit their activation (strains YXB58-1 and YXB58-II). We concluded from these results that the TEF2 UAS element did not function simply as a general activator of the α-mating genes when inserted in HMLΔI but rather blocked the ability of the E silencer to repress the genes. Therefore, the TEF2 UAS exhibits the orientation properties of a boundary element.
Figure 6.
UASrpg shows position-dependent silencer-blocking activity and insulator activity. (A) UASrpg shows position-dependent silencer-blocking activity. The 149-bp PTEF2 fragment (open arrow; Fig. 5A) was inserted E-proximal (YXB48-I and YXB48-II) or E-distal (YXB58-I and YXB58-II) to the α genes at HMLΔI, respectively. The effect of the insertion on α gene expression was examined by quantitative mating. The mating efficiency of YXB26 was taken as one. (B) UASrpg shows insulator activity. In strains YXB57-A to YXB57-D, HML α genes were bracketed by a pair of the 149-bp element of TEF2 (open arrow), which were in turn flanked by the E and I silencers. The mating efficiency of YXB1 was taken as one. (C) UASrpg can insulate the URA3 gene from Sir-mediated repression. In strains YXB61-I and YXB61-II, the HMLα genes were replaced by the URA3 gene as detailed in Materials and Methods. A pair of the 149-bp fragment of TEF2 were inserted at sites bracketing URA3 in strains YXB61-I and YXB61-II, resulting in strains YXB62-I and YXB62-II. The growth phenotypes of the YXB61 and YXB62 strains and their sir− derivatives on medium containing 5-FOA are shown at right.
Finally, boundary elements have also been defined functionally by their insulator activity. That is, two copies of a bona fide boundary element bracketing a reporter gene can insulate expression of that reporter gene from effects imposed by the surrounding chromosomal environment. Therefore, we tested whether two copies of the TEF2 UAS bracketing the α-mating genes would protect them from silencing by both silencers of HML. Copies of the 149-bp element of PTEF2 were inserted on either side of the α-mating genes at HML, in all four combinations of relative directions of the elements (Fig. 6B, strains YXB57-A to YXB57-D). The 149-bp element caused a significant derepression of the α-mating genes in strains YXB57-B and YXB57-D, but only mild derepression in strains YXB57-A and YXB57-C. Although we do not understand the dependence of insulating activity on the direction of the element, these results confirm that the 149-bp element of PTEF2 possesses all the properties of a boundary element.
To confirm the insulator activity of the PTEF2 UAS, we asked whether this element could insulate a gene other than the α-mating genes from silencing imposed by HML. To do so, we examined the expression of a URA3 gene inserted into the HML locus in place of the α-mating genes in an otherwise ura3− strain. As observed previously and as shown in Figure 6C, expression of the URA3 gene inserted in HML is repressed, as witnessed by the resistance of strains YXB61-I and YXB61-II to 5-fluoro-orotic acid (FOA). We then examined the expression of a URA3 gene inserted at the same site but bracketed by copies of the PTEF2 UAS. As evident from the results in Figure 6C, strains containing such a construct are completely sensitive to FOA (strains YXB62-I and YXB62-II). Therefore, the PTEF2 UAS is capable of insulating not only the α-mating genes but also the URA3 gene from the repression by the HML silencers.
UASrpg blocks the spread of the Sir-dependent chromatin structure initiated at a silencer
We have shown that fragments spanning UASrpg block the ability of the E silencer to repress the α genes at HML when inserted between the silencer and the α-gene promoters. We wanted to determine whether it did so by blocking the spread of Sir-dependent heterochromatin initiated at the silencer. To explore this possibility, we constructed strains in which we could simultaneously examine the topology of different segments of the HML locus. To do so, we constructed strains carrying the HML loci diagrammed in Figure 7A. Each of these loci contain three copies of the FLP recombination target site (FRT) in direct orientation, two bracketing the entire locus and one lying between the E silencer and the α1/α2 genes, at the site we use for inserting the boundary element (insertion of the FRT between E and the α1/α2 genes does not affect E-mediated silencing of those genes; Holmes and Broach 1996; data not shown). As a result of this configuration, induction of FLP yields excision of two circles. One circle corresponds to the region encompassing the E silencer up to the point of insertion of the boundary element; the other circle corresponds to the region distal to the site of insertion of the boundary element and encompasses the α1/α2 genes. Because the excised circles are of significantly different sizes, we could independently examine the migration of both of them in the same track on chloroquin agarose gels.
Figure 7.
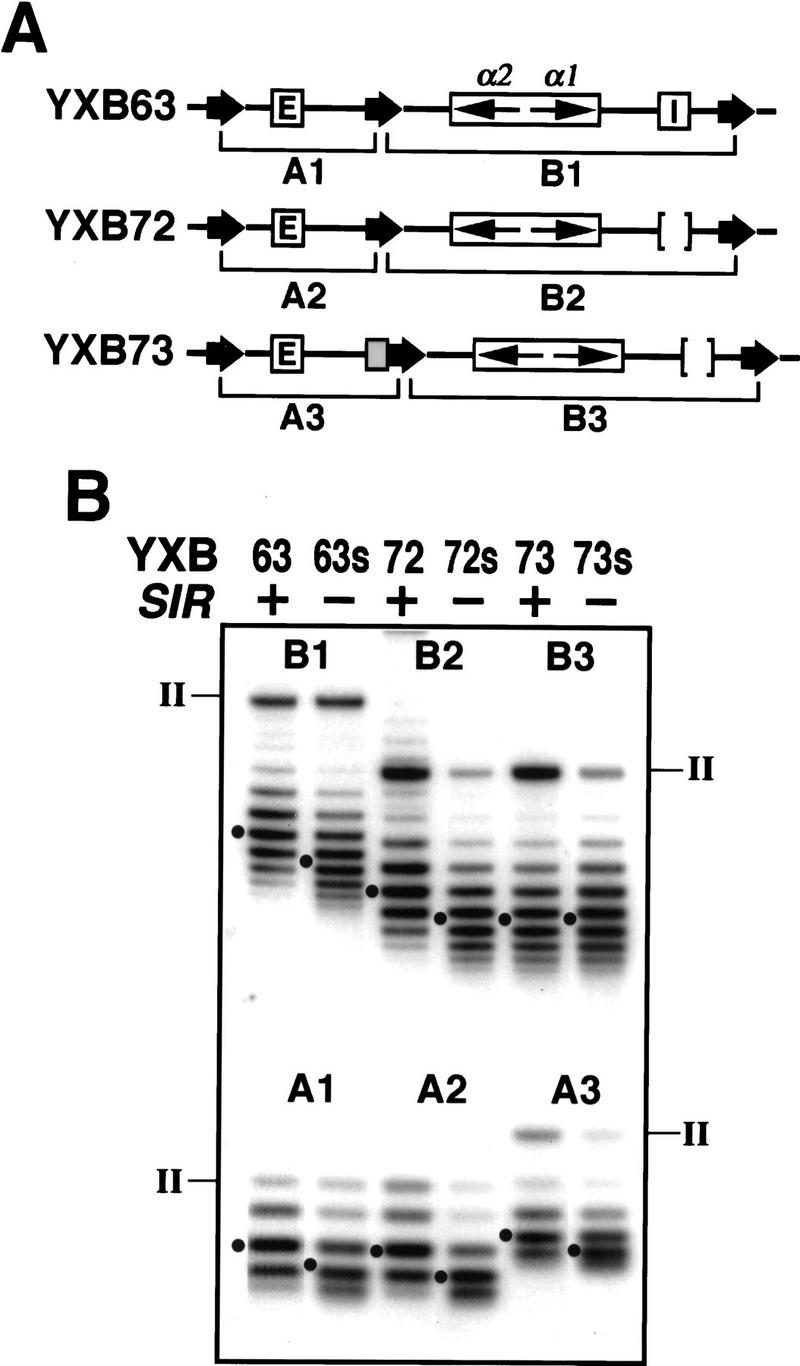
UASrpg blocks the spread of SIR-dependent silent chromatin structure initiated at a silencer. (A) Diagram of the HML locus in strains in which separate segments of the locus can be simultaneously excised as circles for topological examination. (Thick arrows) FRT sites; (shaded box) the 149-bp fragment of PTEF2 containing UASrpg. (B) Topological states of separate parts of HML. DNA circles from the HML locus of each strain diagramed in A were analyzed as described in Materials and Methods. The topoisomers of the two circles in each strain are significantly different in size (∼1 vs. ∼3 kb) so that they can be fractionated without overlap in the same track of the gel. The nicked (from II) circles are indicated. The Gaussian center of each distribution of topoisomers is indicated (●).
The results of this analysis are shown in Figure 7. As expected from our previous observations, both circles (A1 and B1) excised from the two halves of the otherwise wild-type HML locus were more negatively supercoiled in a SIR+ (strain YXB63) than in a sir− background (strain YXB63s). Therefore, in a wild-type locus, the Sir-dependent chromatin structure extends over both the silencer region and the α1/α2 genes. The same Sir-dependent pattern was observed with circles (A2 and B2) excised from strains YXB72 and YXB72s, which contain an HML locus identical to that in strains YXB63 and YXB63s except for deletion of the I silencer. Therefore, consistent with expression studies, the E silencer alone is sufficient to impose a Sir-dependent chromatin structure across the α1/α2 genes. Insertion of the UASrpg boundary element markedly altered this pattern. In a locus containing the boundary element between the E silencer and the α1/α2 genes, the region upstream of the boundary element showed the same Sir-specific change in topology as was found for the locus lacking the boundary (circle A3 in strains YXB73 and YXB73s compared with circle A2 in strains YXB72 and YXB72s). In contrast, the topology of DNA across the α-mating genes, that is, downstream of the boundary element (circle B3), was identical in the SIR+ and sir− strains (YXB73 and YXB73s). Further, the topology of the DNA across the mating type genes downstream of the boundary element in the SIR+ strain was identical to that of the mating type genes in the absence of a boundary but in a sir− strain (cf. B2 with B3 in lanes 72s and 73). Therefore, the chromatin structure downstream of the boundary element corresponds to that of the derepressed locus. From this we conclude that the UASrpg blocks the spread of Sir-dependent heterochromatin initiated at the silencer.
Discussion
Genes resistant to transcriptional silencing in yeast
We have shown in this study that promoters of the TEF genes from budding yeast S. cerevisiae or filamentous fungus A. gossypii are refractory to transcriptional silencing. In S. cerevisiae, both TEF1 and TEF2 code for translation elongation factor 1α (EF-1α), one of the most abundant proteins in eukaryotic cells. The TEF genes belong to a large family of genes (ribosomal protein genes or RPG) encoding components of the translation machinery, which are coordinately regulated (for review, see Planta and Raue 1988; Shore 1994). Most yeast RPGs contain an upstream activation site (UASrpg) consisting of at least one, and usually two or more, Rap1-binding sites located ∼250–450 bp upstream of the ATG start codon. UASrpgs mediate transcriptional activation of RPGs. In addition, a T-rich stretch often lies downstream of UASrpg and can enhance RPG transcription (see Fig. 5A). Like its S. cerevisiae counterparts, AgTEF also has a UASrpg consisting of two Rap1 binding sites in its promoter and is resistant to silencing in yeast. All three promoters contain a binding site for Gcr1, a factor originally identified as a glycolysis regulator required for efficient transcription of glycolytic genes (Santangelo and Tornow 1990). We predict, but have not tested, that all of the ribosome protein genes and other housekeeping genes that have UASrpg in their promoters would be resistant to transcriptional silencing.
How do TEF promoters escape transcriptional silencing? Aparicio and Gottschling (1994) showed that overexpression of the transcriptional activator Ppr1 could reverse silencing of a URA3 gene subject to telomere position effect. They provided evidence consistent with a model in which an activator complex and the silencing apparatus compete for assembly at the promoter site. Whichever complex formed first in a particular cell cycle would determine the expression state of the locus during that cell cycle. The resistance of TEF promoters can be understood in the context of this model, by assuming that an activator complex remains stably bound to the UASrpg throughout the cell cycle or that the avidity of the complex for UASrpg is sufficiently high that it always out competes the silencer apparatus for formation at the TEF promoter. Which elements of the PTEF confer silencer resistance? The T-rich sequence in yeast TEF promoters likely does not contribute to silencing resistance as it is absent from AgTEF. Gcr1 binds to Rap1 and its activity at promoters is UASrpg-dependent, indicating that Gcr1 acts through Rap1. Gcr1 binds to CTTCC (CT-box) sequences present in promoters of many glycolytic genes but DNA binding is not important for Gcr1p function as deletion of the CT-box in the DNA and/or removal of the DNA-binding domain from Gcr1p do not affect its ability to support efficient transcription. Therefore, silencing resistance, like boundary activity as described below, likely resides in the Rap1-binding sites.
Rap1, also known as Grf1, TBA, or TUF, is an essential nuclear protein present at exceedingly high abundance (104 molecules per nucleus) for a sequence-specific DNA-binding protein (for review, see Shore 1994). The 13-bp consensus sequence for Rap1 binding lies not only in the enhancer domains (UASs) of numerous genes but also in the silencers of the HM loci and in telomeric (C1–3A) repeats. Rap1 is a global regulator that affects transcriptional activation, transcriptional repression, telomere length, circular plasmid segregation and meiotic recombination. Rap1 performs these diverse functions by interacting with different factors in proper contexts. For instance, as a transcriptional repressor, Rap1 binds to both HML E and HML I and HMR E silencers and recruits Sir3 and/or Sir4 to silencers through direct interaction. Similarly, Rap1 interacts with Rif1 and various telomere-specific proteins in executing its role in regulating telomere length. Defining how Rap1 elicits resistance to repression, however, poses some difficulty, especially because in other contexts the insertion of a single Rap1-binding site enhanced, rather than abrogated, repression of a reporter gene inserted into an attenuated HML locus (Boscheron et al. 1996). In addition, the UAS element necessary for expression of α1 and α2 contains a Rap1-binding site (Giesman et al. 1991) that rather than render the genes resistant to silencing at HML, makes them exquisitely sensitive to transcriptional silencing. Therefore, whereas Rap1 is critical to the resistance of certain promoters to transcriptional silencing, other as yet undefined factors or contexts determine whether the Rap1 binding site will enhance silencing or render a promoter resistant to silencing.
Rap1-binding sites can constitute silencer-blocking chromatin boundary element in yeast
In this study we have shown that the silencing-resistant TEF genes from yeast or A. gossypii can serve as boundaries to contain the spread of heterochromatin. The UASrpg of PTEF2 is both necessary and sufficient for silencer-blocking activity and the UASrpg-containing elements excluding the TATA-box from the AgTEF promoter can also function as boundary elements. These elements block silencing when present between the silencer and the reporter gene but not when present downstream of the reporter gene, indicating these elements do not simply reverse silencing by acting as transcriptional enhancers. Finally, the UASrpg of PTEF2 can insulate both the mating type genes and the URA3 gene from the repressive effects of a heterochromatin domain. Therefore, we have shown that the UASrpg of PTEF2 exhibits all the properties of chromatin boundary elements identified in metazoans, and moreover, that the UASrpg of PTEF2 functions as a physical barrier to the spread of Sir-dependent heterochromatin.
As in silencer resistance, the Rap1-binding sites are critical for chromatin boundary activity. Boundary activity, however, likely requires the coordinated actions of more than one Rap1-binding sites aligned in a proper organization. In PTEF2, three Rap1p-binding sites are positioned in direct orientation and the distances between them are 6 and 3 bp, respectively; in PTEF1, two Rap1p sites in direct orientations are separated by 4 bp; and in PAgTEF, two Rap1p sites in direct orientation are separated by 17 bp. Whereas the UASrpg (R1-R2-R3) of TEF2 possesses orientation-dependent silencer-blocking activity, R1 or R2 alone has no silencer-blocking activity. Furthermore, two directly repeated copies of R2 separated by 11 bp showed no silencer-blocking activity, even though the two Rap1 sites (separated by 17 bp) in AgTEF had silencer-blocking activity. These data suggest that the organization (orientation and spacing) of Rap1-binding sites is important for their silencer-blocking activity. In this context, it is noteworthy that Rap1 binding can induce a 50–60° bend in DNA 5′ to the recognition sequence (Vignais and Sentenac 1989; Vignais et al. 1990; Gilson et al. 1993; Muller et al. 1994). For multiple juxtaposed Rap1-binding sites, the overall effect of Rap1 binding on DNA structure would depend on their orientations and spacing. Despite this observation, Buck and Shore (1995) showed that a carboxy-terminal domain of Rap1 fused to the GAL4 DNA-binding domain could mediate silencing when targeted to a mutated silencer with the Rap1 site replaced by the GAL4 site. Therefore, binding of Rap1 to DNA per se is not required for silencing. It will be interesting to test the effect of such a construct on boundary activity.
The best-characterized chromatin boundary elements include the specialized chromatin structure (scs and scs′) at the boundaries of the Drosophila 87A hsp70 genes, the insulator element in the of Drosophila gypsy retrotransposon, and the sequences associated with the 5′ constitutive hypersensitive site in the chicken β-globin locus (Corces and Gerasimova 1997). The scs′ element contains the binding site for the BEAF-32 protein, and the gypsy insulator is composed of 12 binding sites for su(Hw) (suppressor of Hairy-wing). Like Rap1 in yeast, BEAF-32 binds to many sites in the Drosophila genome (specifically, to interband regions that separate polytene bands of Drosophila third-instar larval chromosomes) and may have general structural and functional roles throughout the genome. In addition, like Rap1, BEAF-32 binds to at least one promoter, that of the aurora gene (Glover et al. 1995). The su(Hw) proteins also bind to many (∼200) sites on polytene chromosomes from third-instar larvae, which are not sites of gypsy elements, and may also have a role in the higher order organization of the Drosophila genome. A second component of the gypsy insulator is the mod(mdg4) protein (modifier for mdg4), which interacts with su(Hw). In the absence of mod(mdg4), the gypsy insulator becomes a silencer that mediate bidirectional repression of nearby genes via heterochromatin formation. This is caused by the presence of su(Hw) alone at the insulator. Interestingly, su(Hw) can also function as a transcriptional activator (Corces and Geyer 1991). Therefore, similar to Rap1 in yeast, su(Hw) can function in gene repression and activation as well as serve as a component of chromatin boundaries in a context-dependent manner.
A model for boundary activity
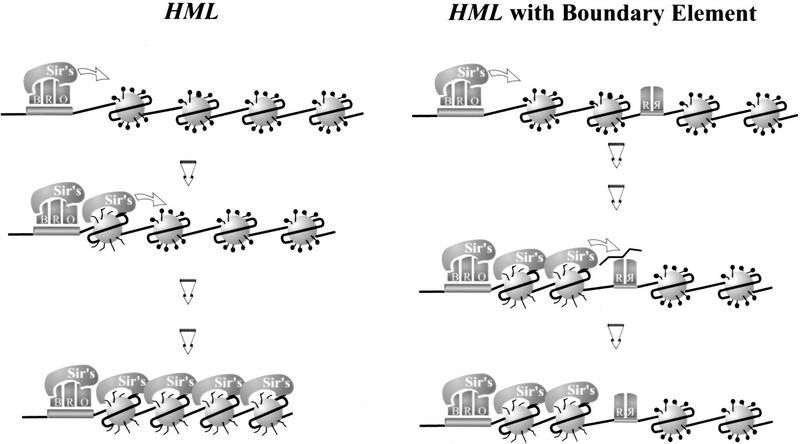
In Figure 8 we present a model that would account for the observations described in this report. Our model postulates that Rap1 bound to sites within the UASrpg excludes formation of several nucleosomes across the region. This would be consistent with our observation that the UASrpg on its own alters local chromatin structure, independent of its effect on silencing (X. Bi and J.R. Broach, unpubl.). In addition, if propagation of the silencing complex along chromatin requires sequential interaction of the Sir complex with contiguous nucleosomes, as has been suggested (Hecht et al. 1996; Braunstein et al. 1997), then the nucleosomal ‘hole’ in the chromatin would block the outward migration of the silencing complex. This nucleosome exclusion activity could account for the function of UASrpg as an enhancer, in that excluding nucleosomes could render the neighboring DNA accessible to other transcription factors and to assembly of the machinery necessary for initiation of transcription. In addition, the silencer blocking activity of UASrpg would render the highly active genes in which it acts refractory to any repressive effects emanating from adjacent genes.
Figure 8.
A model for the heterochromatin boundary activity of UASrpg. Transcriptionally silenced chromatin initiates from a silencer and emanates outward as a result of the spreading of the SIR complex along contiguous nucleosomes, leading to an extended complex of Sir proteins across hypoacetylated nucleosomes (left; see introductory section and Discussion for detailed descriptions). Binding of multiple Rap1 molecules to UASrpg excludes formation of several nucleosomes across the region and this nucleosomal ‘hole’ in the chromatin blocks the migration of the SIR complex (right). See Discussion for a detailed description. (B) Abf1; (R) Rap1; (O) ORC; (Sir’s) complex of Sir2, Sir3, and Sir4.
We have shown that UASrpg possess chromatin boundary activity. This element, however, is not present at the boundary between the HML heterochromatin region and the adjacent euchromatic region. In fact, testing individual DNA segment in the region surrounding HML in the boundary assay described in this report failed to identify any chromatin boundary elements flanking HML (X. Bi, M. Braunstein, G. Shei, and J.R. Broach, unpubl.). Therefore, whereas the yeast genome contains sequences that will block the spread of heterochromatin, the yeast cell can use a different mechanism to restrict the spread of heterochromatin and limit its effects to specific domains.
Materials and methods
Plasmid and yeast strain constructions
The kanMX module consists of the A. gossypii TEF promoter (PAgTEF, the SpeI–NcoI fragment), the ORF of the E. coli kanR gene of Tn903 (the NcoI–ScaI fragment) and the terminator sequence of A. gossypii TEF (TAgTEF, the ScaI–NotI fragment) (Wach et al. 1994; see Fig. 1A). The NotI–kanMX–NotI fragment of pFA6–kanMX (Wach et al. 1994) was inserted into plasmid pRS416 (Sikorski and Hieter 1991) at the NotI site to make pRS–kanMX. The XbaI–kanMX–SpeI fragment of pRS–kanMX was inserted at the SpeI site of pYXB1 (Bi and Broach 1997) in two orientations, resulting in pYXB13-I and pYXB13-II, respectively (see Fig. 2). Plasmid pYXB1 contains the BamHI fragment of yeast chromosome III containing HMLα (in pUC19) with two FLP1 recombination target sites (FRT; Broach and Volkert 1991) in direct orientation inserted at the Bsu36I and SnaBI sites bracketing HMLα. Plasmid pUC18–HMR was made by inserting the HindIII fragment of yeast chromosome III containing HMRa into pUC18 at the HindIII site. Plasmid pUC18–Δhmr::SUP4–o was derived from pUC18–HMR by replacing its NruI–HMRα–XhoI fragment with the EcoRV–SUP4–o–XhoI fragment from pMB21 (Braunstein 1996). Two FRT sites were inserted into pUC18–HMR at the XhoI and SnaBI sites bracketing HMRa to make pFHMRF.
Each plasmid described below was made by inserting a PCR-amplified fragment of interest (with appropriate restriction sites added to both ends) into a properly digested vector. The insert was then confirmed by DNA sequencing. Plasmid pYXB15 was made by inserting the ADE2 gene (coordinates −550 to +1990, with the ORF being +1 to +1716), with two BamHI sites added to both ends, at the BglII site of pFHMRF. pYXB19 was constructed by inserting ADE2 (coordinates −300 to +1990, ORF being +1 to +1716) at the SpeI site of pYXB1. The ORF of ADE2 (+1 to +1716) was used to replace the NcoI–kanR–ScaI fragment of pYXB13, generating pYXB21. Plasmid pYXB21-H was derived from pYXB21 by replacing its SpeI–PAgTEF–NcoI fragment with PTEF2 (promoter region of S. cerevisiae TEF2 gene, coordinates −556 to −1). Plasmid pYXB22 was constructed from pYXB21-H by substituting the BglII–BlpI fragment (consisting of part of ADE2 ORF and the TAgTEF sequence) with the BglII–BlpI fragment of pYXB19, effectively resulting in the replacement of TAgTEF with TADE2 (+1717 to +1994). Plasmid pYXB26 was derived from pYXB1 by deleting a 462-bp fragment (coordinates 4889–5173 of the BamHI–HML–BamHI fragment) containing the HML I silencer. Plasmid pYXB25 was derived from pYXB22 by deleting the I silencer of HML as just described for the construction of pYXB26. Plasmid pYXB29 was derived from pYXB26 by inserting the 345-bp fragment of PTEF2 shown in Figure 5A at the SpeI site. The following plasmids were similarly derived from pYXB26. Plasmids pYXB49, 48, 28, 27, 59, 60, 53, and 54 were constructed using various fragments of PTEF2, respectively (see Fig. 5A). pYXB31, 47 were made using fragments from PAgTEF, respectively (Fig. 5B). pYXB37, 38, 39, 41, and 42 were made using fragments of ADE2, respectively (Fig. 5B). The 149-bp fragment of PTEF2 (Fig. 5A) was inserted at the Bst1107I site of pYXB26 resulting in plasmid pYXB58 (Fig. 6A). Two copies of the 149-bp fragment were inserted at the SpeI and the Bst1107I sites of pYXB1, respectively, in all four possible combinations of orientations to make plasmids pYXB57-A, pYXB57-B, pYXB57-C, and pYXB57-D (Fig. 6B). Plasmid pUC26 contains the BamHI fragment of yeast chromosome III containing HML in pUC19. A 294-bp sequence (coordinates 3319–3613) of the BamHI fragment, containing the promoters for the HMLα1 and α2 genes, was replaced by a 1.1-kb HindIII–URA3–HindIII fragment of pDM22 (Mahoney and Broach 1989), generating pYXB61. Plasmid pYXB62 was derived from pYXB61 by inserting two copies of the 149-bp fragment of PTEF2 (Fig. 5A) at the SpeI and Bst1107I sites flanking URA3 in opposite orientations (Fig. 6C). Plasmid pYXB63 was derived from pYXB1 by inserting an additional FRT site at the SpeI site of HML (Fig. 7A). Plasmid pYXB72 and 73 (Fig. 7A) were similarly derived from pYXB26 and pYXB48-I, respectively. For all the above plasmids harboring modified HML sequences (except pYXB26 and pYXB57), -I or -II denotes that a fragment is inserted at HML in the same or opposite orientation as that of the α1 gene (the direction of a fragment being the 5′ to 3′ orientation of the coding strand of the gene it resides in). See Figures 1, 2, and 4–7 for illustrations. Plasmid pMB21 (Braunstein 1996) is an integrating plasmid containing SIR3, SUP4–o, and TRP1 genes.
Yeast strains with modifications at HML were derived from Y2047b (MATa HMRa HMLα EΔ79-113::SUP4–o IΔ242 LEU2–GAL10–FLP1 ura3-52 ade2-1 lys1-1 his5-1 can1-100 [cir0]; Holmes and Broach 1996). The SUP4–o allele, which can suppress the can1-100 mutation is present at HML(ΔEΔI) in Y2047b, rendering it sensitive to canavanine-killing. The HML-containing BamHI fragment of pYXB13-I was used to transform Y2047b to canavanine resistant (due to the loss of SUP4–o), resulting in strain YXB13-I (Fig. 2). All the other strains except YXB14, YXB15, YXB61, and YXB62 were made similarly using the BamHI fragments of corresponding plasmids (see Figs. 1, 2, and 4–7). The HindIII–Δhmr::SUP4–o–HindIII fragment of pUC18–Δhmr::SUP4–o was used to transform YXB13-I to lysine prototrophy (attributable to the suppression of lys1-1 mutation by SUP4–o) to make strain YXB14. Strain YXB14 was then transformed to canavanine resistant with the HindIII fragment of pYXB15, resulting in strain YXB15 (Fig. 1). The SIR3 gene in some of the above strains was disrupted by URA3 as described previously (Mahoney and Broach 1989) resulting in the s strains (YXB13-Is, YXB13-IIs, etc.). YXB61s was constructed by transforming DMY2 (MATa ura3-52 ade2-1 lys1-1 leu2-3,112 his5-1 can1-100 sir3::LEU2; Mahoney and Broach 1989) to Ura+ using the EcoRI–hmlΔα1α2::URA3–PvuII fragment of pYXB61. YXB62s was similarly constructed using the EcoRI–PvuII fragment of pYXB62. YXB61s and YXB62s were then transformed to Lys+ using pMB21 digested by XbaI whose site is within the TRP1 gene, resulting in YXB61 and YXB62, respectively, which are SIR+. The relevant genotype of each strain was confirmed by Southern blotting. Growth of yeast strains was done according to standard procedures (Kaiser et al. 1994) unless otherwise specified.
Quantitative mating assay
Quantitative mating was performed as described by Sprague (1991). Strains were grown to a density of 0.5–1.5 × 107 per ml. About 3 × 106 cells were mixed with ∼107 cells of the tester strain DC17α and collected on a nitrocellulose filter. In addition, cells of each haploid strain were collected on separate filters. After incubation on YPD for 5 hr, cells from the mating-mix filter were plated on SD medium to select for diploids. Cells of single strains were also plated on SD medium to check for revertants. Cells of the strain being tested were plated on −Leu plate. Mating efficiency is calculated as the number of colonies (from the mating-mix) on SD medium divided by that of the strain being tested (on −Leu medium).
Analysis of DNA circles excised from the HM loci by FLP1
Yeast strains were grown in YPR (1% yeast extract, 2% Bacto-peptone, and 2% raffinose) to early log phase (OD600 = 0.6). Galactose was then added to the culture to induce the expression of FLP1. After 2.5 hr of incubation, cells were collected by centrifugation. Nucleic acid was then isolated using the glass bead method (Kaiser et al. 1994) and fractionated on agarose gels in 0.5× TPE (45 mm Tris, 45 mm phosphate, 1 mm EDTA at pH 8.0) supplemented with chloroquine at a concentration of 30 μg/ml. DNA circles were detected by Southern blotting.
Acknowledgments
This work was supported by National Institutes of Health grant GM48540 to J.R.B. and by postdoctoral fellowship PF4298 from the American Cancer Society to X.B.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jbroach@molecular.princeton.edu; FAX (609) 258-1975.
References
- Abraham J, Nasmyth KA, Strathern JN, Klar AJS, Hicks JB. Regulation of mating type information in yeast: negative control requiring sequences both 5′ and 3′ to the regulated region. J Mol Biol. 1984;176:307–331. doi: 10.1016/0022-2836(84)90492-3. [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Gottschling DE. Overcoming telomeric silencing: A transactivator competes to establish gene expression in a cell cycle-dependent way. Genes & Dev. 1994;8:1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- Bi X, Broach JR. DNA in transcriptionally silenced chromatin assumes a distinct topology that is sensitive to cell cycle progression. Mol Cell Biol. 1997;17:7077–7087. doi: 10.1128/mcb.17.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscheron C, Maillet L, Marcand S, Tsai-Pflugfelder M, Gasser SM, Gilson E. Cooperation at a distance between silencers and proto-silencers at the yeast HML locus. EMBO J. 1996;15:2184–2195. [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Breeden L, Abraham J, Sternglanz R, Nasmyth K. Characterization of a ‘silencer’ in yeast: A DNA sequence with properties opposite to those of transcriptional enhancer. Cell. 1985;41:41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Braunstein M. The relationship between histone acetylation and transcriptional silencing in Saccharomyces cerevisiae. Ph.D. thesis. Princeton, NJ: Princeton University; 1996. [Google Scholar]
- Braunstein M, Rose A, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes & Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Sobel RE, Allis CD, Turner BM, Broach JR. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein M, Holmes SG, Broach JR. Heterochromatin and regulation of gene expression in Saccharomyces cerevisiae. In: v. Driel R, Otte AP, editors. Nuclear organization, chromatin structure, and gene expression. Oxford, UK: Oxford University Press; 1997. pp. 250–275. [Google Scholar]
- Broach JR, Volkert FC. Circular DNA plasmids of yeast. In: Broach JR, Jones EW, Pringle JR, editors. The molecular biology of the yeast Saccharomyces: Genome dynamics, protein synthesis, and energetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1991. pp. 287–331. [Google Scholar]
- Buchman AR, Lue NF, Kornberg RD. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988;8:5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck SW, Shore D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes & Dev. 1995;9:370–384. doi: 10.1101/gad.9.3.370. [DOI] [PubMed] [Google Scholar]
- Cheng TH, Li Y-C, Gartenberg MR. Persistence of an alternate chromatin structure at silenced loci in the absence of silencers. Proc Natl Acad Sci. 1998;95:5521–5526. doi: 10.1073/pnas.95.10.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Whiteley M, Felsenfeld G. A 5′ element of the chick β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- Corces VG, Geyer PK. Interactions of retrotransposons with the host genome: The case of the gypsy element of Drosophila. Trends Genet. 1991;7:69–73. doi: 10.1016/0168-9525(91)90277-W. [DOI] [PubMed] [Google Scholar]
- Corces VG, Gerasimova TI. Chromatin domains and boundary elements. In: v. Driel R, Otte AP, editors. Nuclear organization, chromatin structure and gene expression. Oxford, UK: Oxford University Press; 1997. pp. 83–98. [Google Scholar]
- Cottrelle P, Thiele D, Price VL, Memet S, Micousin J-Y, Marck C, Buhler J-M, Sentenac A, Fromageot P. Cloning, nucleotide sequencing and expression of one of two genes coding for yeast elongation factor EF-1α. J Biol Chem. 1985;260:3090–3096. [PubMed] [Google Scholar]
- Feldman JB, Hicks JB, Broach JR. Identification of sites required for repression of a mating type locus in yeast. J Mol Biol. 1984;178:815–834. doi: 10.1016/0022-2836(84)90313-9. [DOI] [PubMed] [Google Scholar]
- Geyer PK, Corces VG. DNA position-specific repression of by a Drosophila zinc finger protein. Genes & Dev. 1992;6:1865–1873. doi: 10.1101/gad.6.10.1865. [DOI] [PubMed] [Google Scholar]
- Giesman D, Best L, Tatcell K. The role of RAP1 in the regulation of the MATα locus. Mol Cell Biol. 1991;11:1069–1079. doi: 10.1128/mcb.11.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser S. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Gottschling DE. Telomere-proximal DNA is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci. 1992;89:4062–4065. doi: 10.1073/pnas.89.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M. Histones H3 and H4 N-termini interact with SIR3 and SIR4 proteins: A molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repression by SIR3 from telomeric heterochromatin. Nature. 1996;383:92–96. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Life cycle of the budding yeast S. cerevisiae. Microbiol Rev. 1988;52:536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SG, Broach JR. Silencers are required for inheritance of the repressed state in yeast. Genes & Dev. 1996;10:1021–1032. doi: 10.1101/gad.10.8.1021. [DOI] [PubMed] [Google Scholar]
- Johnson LM, Kayne PS, Kahn ES, Grunstein M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc Natl Acad Sci. 1990;87:6286–6290. doi: 10.1073/pnas.87.16.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics: A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Ke N, Irwin PA, Voytas DF. The pheromone response pathway activates transcription of Ty5 retrotransposons located within silent chromatin of Saccharomyces cerevisiae. EMBO J. 1997;16:6272–6280. doi: 10.1093/emboj/16.20.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- ————— A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol. 1992;12:2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenson P, Rine J. Silencers, silencing, and heritable transcriptional states. Microbiol Rev. 1992;56:543–560. doi: 10.1128/mr.56.4.543-560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Gross DS. Conditional silencing: The HMRE mating-type silencer exerts a rapidly reversible position effect on the yeast HSP82 heat shock gene. Mol Cell Biol. 1993;13:727–738. doi: 10.1128/mcb.13.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo S, Rine J. Silencers and domains of generalized repression. Science. 1994;264:1768–1771. doi: 10.1126/science.8209257. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Broach JR. The HML mating-type cassette of Saccharomyces cerevisiae is regulated by two separate but functionally equivalent silencers. Mol Cell Biol. 1989;9:4621–4630. doi: 10.1128/mcb.9.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Freeman K, Goodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes & Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- Muller T, Gilson E, Schmidt R, Giraldo R, Sogo J, Gross H, Gasser SM. Imaging the asymmetrical DNA bend induced by repressor activator protein 1 with scanning tunneling microscopy. J Struct Biol. 1994;113:1–12. doi: 10.1006/jsbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- Nagata S, Nagashima K, Tsunetsugu-Yakota Y, Fujimura K, Miyazaki M, Kaziro Y. Polypeptide chain elongation factor 1α (EF-1α) from yeast: Nucleotide sequence of one of two genes for EF-1α from Saccharomyces cerevisiae. EMBO J. 1984;8:1825–1830. doi: 10.1002/j.1460-2075.1984.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planta RJ, Raue HA. Control of ribosome biogenesis in yeast. Trends Genet. 1988;4:64–68. doi: 10.1016/0168-9525(88)90042-x. [DOI] [PubMed] [Google Scholar]
- Reynolds AE, McCarroll RM, Newlon CS, Fangman WL. Time of replication of ARS elements along yeast chromosome III. Mol Cell Biol. 1989;9:4488–4494. doi: 10.1128/mcb.9.10.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo GM, Tornow J. Efficient transcription of the glycolytic gene ADH1 and three translational component genes requires the GCR1 product, which can act through TUF/GRF/RAP binding sites. Mol Cell Biol. 1990;10:859–862. doi: 10.1128/mcb.10.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmaier F, Philippsen P. Identification of two genes coding for the translation elongation factor EF-1α of S. cerevisiae. EMBO J. 1984;13:3311–3315. doi: 10.1002/j.1460-2075.1984.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell R, Rine J. A position effect on the expression of a tRNA gene mediated by the SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6:494–501. doi: 10.1128/mcb.6.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shei GJ, Broach JR. Yeast silencers can act as orientation-dependent gene inactivation centers that respond to environmental signals. Mol Cell Biol. 1995;15:3496–3506. doi: 10.1128/mcb.15.7.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D. RAP1: A protean regulator in yeast. Trends Genet. 1994;10:408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1991;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Klar AJS. Active genes in budding yeast display enhanced in vivo accessibility to foreign DNA methylases: A novel in vivo probe for chromatin structure in yeast. Genes & Dev. 1992;6:186–196. doi: 10.1101/gad.6.2.186. [DOI] [PubMed] [Google Scholar]
- Sprague GF. Assay of yeast mating reaction. In: Guthrie C, Fink GR, editors. Guide to yeast genetics and molecular biology. San Diego, CA: Academic Press; 1991. pp. 77–93. [Google Scholar]
- Steiner S, Philippsen P. Sequence and promoter analysis of the highly expressed TEF gene of the filamentous fungus Ashbya gossypii. Mol Gen Genet. 1994;242:263–271. doi: 10.1007/BF00280415. [DOI] [PubMed] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes & Dev. 1997;11:83–93. doi: 10.1101/gad.11.1.83. [DOI] [PubMed] [Google Scholar]
- Sussel L, Shore D. Separation of transcriptional activation and silencing functions of the RAP-1-encoded repressor/activator protein 1: Isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci. 1991;88:7749–7753. doi: 10.1073/pnas.88.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Vannier D, Shore D. Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:3919–3928. doi: 10.1128/mcb.13.7.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleth C, van Sluis CA, van der Putte P. Differential repair of UV damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1989;12:4433–4439. doi: 10.1093/nar/17.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- Vignais ML, Sentenac A. Asymmetric DNA bending induced by the yeast multifunctional factor TUF. J Biol Chem. 1989;264:8463–8466. [PubMed] [Google Scholar]
- Vignais ML, Huet J, Buhler JM, Sentenac A. Contacts between the factor TUF and RPG sequences. J Biol Chem. 1990;265:14669–14674. [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruption in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Weiss K, Simpson RT. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HMLα. Mol Cell Biol. 1998;18:5392–5403. doi: 10.1128/mcb.18.9.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]