Abstract
Purpose
To evaluate phenotypic expressions of speech sound disorder (SSD) in multigenerational families with evidence of familial forms of SSD.
Method
Members of five multigenerational families (N = 36) produced rapid sequences of monosyllables and disyllables and tapped computer keys with repetitive and alternating movements.
Results
Measures of repetitive and alternating motor speed were correlated within and between the two motor systems. Repetitive and alternating motor speeds increased in children and decreased in adults as a function of age. In two families with children who had severe speech deficits consistent with disrupted praxis, slowed alternating, but not repetitive, oral movements characterized most of the affected children and adults with a history of SSD, and slowed alternating hand movements were seen in some of the biologically related participants as well.
Conclusion
Results are consistent with a familial motor-based SSD subtype with incomplete penetrance, motivating new clinical questions about motor-based intervention not only in the oral but also the limb system.
Keywords: common disease/common variant, common disease/rare variant, disorder subtype, familial phenotype, motor sequencing, motor speed, speech sound disorder
INTRODUCTION
Speech sound disorder (SSD) definition
Speech sound disorder (SSD) is a childhood communication disorder that interferes with the development of speech sound production in the absence of known causes (Pennington & Bishop, 2009). The disorder can be characterized by deficits in articulation, phonological processing, and/or cognitive representation of language (Lewis et al., 2006). SSD is common, although published prevalence rates vary; for instance, 1.1% in Australian school age children (McKinnon, McLeod, & Reilly, 2007), 4% in US 6-year-olds (Shriberg, Tomblin, & McSweeny, 1999), and 15.6% in US preschoolers (Campbell et al., 2003). Differences in prevalence estimates arise, in part, from differences in the age of the children under study, criteria for participation, and assessment methodology. SSD co-occurs with language impairment and dyslexia at rates greater than expected by chance (Pennington & Bishop, 2009; Peterson, McGrath, Smith, & Pennington, 2007).
A universally accepted SSD subtype classification is not yet available. Several classification schemes coexist in the literature. Examples include taxonomies based on error types, a phonetic-phonemic continuum, and suspected etiologies, as recently reviewed (Peter, 2010; Peter & Stoel-Gammon, 2008). Subtypes based on familial or genetic findings have not yet been described.
Childhood apraxia of speech as a motor-based SSD subtype
One proposed SSD subtype is childhood apraxia of speech (CAS), which is thought to interfere with motor planning and/or motor programming processes related to speech production. In its position statement on CAS, the American Speech-Language-Hearing Association (http://www.asha.org/docs/html/PS2007-00277.html) indicated a view of CAS as a “distinct diagnostic subtype of childhood (pediatric) speech sound disorder” and defined it as follows:
[CAS] is a neurological childhood (pediatric) speech sound disorder in which the precision and consistency of movements underlying speech are impaired in the absence of neuromuscular deficits (e.g., abnormal reflexes, abnormal tone). CAS may occur as a result of known neurological impairment, in association with complex neurobehavioral disorders of known or unknown origin, or as an idiopathic neurogenic speech sound disorder. The core impairment in planning and/or programming spatiotemporal parameters of movement sequences results in errors in speech sound production and prosody.
Although this definition views all forms of CAS as a subtype of SSD, some researchers (Potter, Lazarus, Johnson, Steiner, & Shriberg, 2008; Shriberg, Potter, & Strand, 2010; Terband, Maassen, van Lieshout, & Nijland, 2011) hold a more narrow view in which primary, non-acquired forms of CAS form one SSD subtype and secondary, acquired forms are outside the SSD definition. A universally accepted catalogue of diagnostic criteria is not available.
As mentioned in the ASHA position statement, planning and programming of motor sequences in the oral motor system appears to be disrupted by CAS. An example is the finding that repetition rates of the trisyllabic sequence /pataka/ as well as trisyllable/monosyllable rate ratios (Thoonen, Maassen, Gabreels, & Schreuder, 1999; Thoonen, Maassen, Wit, Gabreels, & Schreuder, 1996) effectively distinguished children with CAS from typical controls, whereas there was no group difference in repetition rates for monosyllables. In a study of 11 children with SSD, timing accuracy of oral movements was correlated with timing accuracy in hand movements and the lowest timing accuracy in both motor systems was observed in children with the highest number of CAS characteristics (Peter & Stoel-Gammon, 2008). It is possible that CAS has a genetic etiology. In a study of 11 children with CAS, six had a family history of speech and language difficulties (Thoonen, Maassen, Gabreels, Schreuder, & de Swart, 1997).
In view of the fact that there is no universal consensus regarding how to define SSD subtypes, a genetic etiology would provide a new SSD subtype classification based on a biological model. Consequently, a central question motivating this study was whether distinct SSD subtypes aggregate in families, which would be consistent with a subtype-specific genetic etiology.
Towards SSD subtypes based on genetic etiology
It is thought that SSD in general has a genetic component but family-specific traits or causal genes have not yet been identified. Higher SSD concordance rates in monozygotic versus dizygotic twins (Lewis, 1992), high SSD heritability estimates (Bishop, 2002), and higher susceptibility in biological versus adopted children with affected parents (Felsenfeld & Plomin, 1997) provided early evidence based on behavioral observations that SSD has genetic influences.
More recently, studies have addressed the molecular genetics of speech problems. In rare cases, speech production difficulties of genetic origin are part of a syndrome. FOXP2 mutations cause severe difficulties with speech, receptive and expressive language, reading, writing, cognition, and oral praxis (Fisher, Vargha-Khadem, Watkins, Monaco, & Pembrey, 1998; Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001; MacDermot, et al., 2005; Vargha-Khadem, Watkins, Alcock, Fletcher, & Passingham, 1995; Watkins, Dronkers, & Vargha-Khadem, 2002). Changes in this gene, however, do not explain nonsyndromic SSD. In one study of 49 individuals with disordered speech with apraxic characteristics, only three related individuals had a protein-altering FOXP2 variant (MacDermot, et al., 2005).
As recent reviews have indicated (Lewis, et al., 2006; Pennington & Bishop, 2009; Peter, 2010), the genetic mechanisms causing common, nonsyndromic forms of SSD are not yet well understood. Several studies conducted to investigate gene locations involved in primary SSD have focused on candidate regions implicated in other disorders with comorbid speech deficits (Miscimarra, et al., 2007; Smith, Pennington, Boada, & Shriberg, 2005; Stein, et al., 2006; Stein, et al., 2004). Study samples have consisted of affected children and their siblings (sibs). Genetic linkage analyses targeted candidate regions on chromosomes (chrs) 1, 3, 6, and 15. These were selected because of suspected linkage to other disorders, such as dyslexia and Angelman syndrome, that share phenotypic traits with SSD. Evidence was obtained for linkage of deficits in speech articulation, which are pathognomonic for SSD, to some of these candidate regions, but causal genes have not been identified to date. The authors’ collective findings led to the hypothesis that SSD and dyslexia share a common genetic basis but are also influenced by variants unique to each disorder.
The traditional view of SSD as a common and complex disorder (Stein et al., 2004), in accordance with the common disease/common variant model, may be an accurate assumption for only a subset of cases of familial SSD. The common disease/rare variant model assumes that multiple common variants interact to confer disease susceptibility in a single individual. In the studies mentioned here, the participants were children with SSD and their sibs. Many different families were represented in each sample. The disorder criteria were general, requiring low scores on standardized articulation and phonology tests. It is possible that the samples contained several familial subtypes with varying expressions of SSD. One of the studies reports differential linkage patterns among subgroups of sib pairs where the probands were preschool age (Stein et al., 2004). Of the 77 sib pairs, 34 contributed to linkage results for both multisyllabic word repetition (MWR) and nonsense word repetition (NWR), skills that are characteristically impaired in SSD; 9 of these sib pairs were concordantly affected (i.e., both twins in each pair had low MWR scores) and 2 were concordantly unaffected (i.e., both twins in each pair had typical MWR scores). Given an estimated SSD prevalence of 16% at preschool age, the concordantly affected group may represent an SSD subtype with a phonological memory component, with an estimated prevalence rate of 1.2%, and in the concordantly unaffected group with SSD without such a phonological memory component, that rate would be 0.4% (unadjusted for ascertainment bias). There are, hence, compelling reasons to consider the common disease/rare variant model that posits that rare variants in individual families, in aggregate, explain many cases of SSD. It is possible that in a subset of familial SSD cases, different genetic mechanisms may be at work in different families, which would be consistent with SSD as a heterogeneous disorder rather than a complex disorder.
The common disease/rare variant hypothesis of SSD is in line with a general paradigm shift in thinking about genetic variation in complex diseases (McClellan & King, 2010). Inherited hearing loss is one example of this type of genotype/phenotype association (Dror & Avraham, 2009). Any one of dozens of genes, when disrupted, can cause hearing loss in a given family. Depending on the gene, the pattern of inheritance can be dominant or recessive, and the gene locus can be autosomal, X linked, or mitochondrial. Despite the divergent genetic etiology and the different types of biochemical disruptions in the inner ear across various families, the effect on the auditory system is the same, in that hearing loss results. Similarly, many cases of inherited autism can be explained individually by rare causal variants in a given family (Bucan, et al., 2009). Regarding SSD, the common disease/rare variant model is consistent with the discrepancy between the strong evidence of a genetic etiology based on twin (Lewis, 1992), adoption (Felsenfeld & Plomin, 1997), and heritability studies (Bishop, 2002) on one hand and the lack of unambiguous linkage peaks across samples on the other.
In the past, methodological challenges to studies of SSD genetics in samples representing multiple families have included the likelihood of heterogeneity, unknown penetrance levels, unknown mode of inheritance, rapidly changing speech error profiles as a result of therapy, and the inclusion of adult participants, given that they typically have compensated for their disability. This latter aspect may explain why studies have investigated SSD in affected children and their sibs, not multigenerational families, even though such families typically offer substantially greater power to detect shared inherited gene regions in affected relatives, greater accuracy in gene localization, and greater accuracy of parameter estimation compared to sib pairs and nuclear families (Wijsman & Amos, 1997). Fortunately, new assessment tools now make it possible to address the question of how to incorporate adults with a history of SSD into investigations of SSD genetics. In a recent study, 36 adults with a history of SSD obtained significantly lower scores in taxing speech tasks such as imitating multisyllabic words, nonwords, and tongue twisters than 144 adults without such a history (Lewis et al., 2007). It is unknown what other, if any, characteristics are retained by adults with resolved childhood SSD.
The broad purpose of the current research project was to investigate familial forms of SSD in multigenerational families using evidence from behavioral observations as well as from DNA analyses. In this first study, we describe phenotypic expressions of SSD within single families, focusing on aspects of motor processing in the oral and hand motor systems. Future reports will focus on additional behavioral traits and molecular genetic findings.
Specifically, this study addresses the role of repetitive and sequential motor speeds in families where one or more children have been diagnosed with SSD and where other biological relatives also have a history of SSD. We hypothesized that, in a subset of the families who participated in this study, limited motor speeds in repetitive and/or alternating movements might be directly observable in currently affected children as well as in adults with a childhood history of speech difficulties whose speech has since normalized. Hand tasks were included in this study because recent work shows close associations between rapid oral and hand motor performance. For instance, temporal aspects in a nonword imitation task and a hand clap task were significantly correlated in a sample of young children with SSD and typical controls (Peter & Stoel-Gammon, 2008). Performance on a sequential oral motor task of producing rapid repetitions of the trisyllable /pataka/ and a rapid sequential hand motor task was associated with a latent dimension characterized by central processing speed in a large family sample ascertained through proband children with dyslexia (Peter, Matsushita, & Raskind, 2010). It is possible that motor deficits in the oral and hand systems characterize some SSD subtypes in children with currently expressed SSD as well as related adults with a history of SSD but normalized speech.
In repetitive motor speech tasks, such as producing sequences of monosyllables, rate is limited by various factors, including speed and distance of articulator excursion. Because of the nature of the task, the same articulators repetitively produce the airflow constriction underlying the acoustic signal. Similarly, striking a computer key repetitively with the same finger is rate limited by speed and excursion factors and the fact that the same finger is used to strike the key. The key itself contributes yet another rate limit with its depression depth, resistance, and rebound speed. In alternating movements, by contrast, multiple agents are coordinated, resulting in more degrees of freedom and potentially shorter intervals. For instance, the disyllable /pata/ involves bilabial lip closure alternating with tongue/alveolar ridge contact, resulting in two separate repetitive movement oscillations interleaved in time. A meta-analysis of the norms for monosyllables and disyllables in children age 6 years through 13 years (Fletcher, 1972) shows that syllable durations decrease in general as a function of age and that durations of the disyllables are as long or longer compared to those in monosyllables in the younger children, but by age 11 the disyllabic syllables are substantially shorter, indicating a speed advantage in alternating oral movements. The same advantage for trisyllables is approached, but not reached, by age 13 years. Similarly, striking two keys by alternating between two fingers involves two repetition cycles, one for each finger. With intact motor sequencing skill, individual syllables and tap intervals in an alternating task can be expected to be just as short or shorter compared to the intervals in a repetitive task. Parallel to the norms for monosyllable repetition rates in children, norms for repetitive key tapping intervals in children show that interval durations decrease as a function of age (Gualtieri & Johnson, 2006; Prigatano, Gray, & Legacy, 2008), then increase again in late adulthood (Bartzokis, et al., 2008); norms for alternating key strikes are not available. In individuals with motor sequencing difficulty such as individuals with CAS, interval durations in an alternating task may be disproportionally longer than those in a repetitive task, not only in the oral system as previously shown (Thoonen, et al., 1999; Thoonen, et al., 1996) but also in the hand motor system.
To summarize the motivation for this study, SSD is a common disorder affecting the speech production process, but a universally accepted subtype scheme is not yet available. If familial SSD characteristics could be described in affected children and related adults with a history of SSD, this would contribute to a novel, biologically based SSD subtype scheme. Deficits in oral motor sequencing have been described in children with speech errors consistent with CAS, which is a proposed SSD subtype. If motor sequencing deficits are systemic, that is, not just confined to the oral motor system but also observable in other motor systems, and if they persist as such in adults with a history of a motor-based form of SSD, this would constitute a subtype of SSD that is familial and likely of genetic origin. This study, hence, addresses the following research questions:
Are motor speeds in repetitive and alternating tasks associated across motor systems? If so, this would confirm previous reports of cross-system correlations and strengthen our hypothesis that motor speeds across systems are controlled by a shared biological mechanism.
Do repetitive and/or alternating motor speeds distinguish between adults with, and without, a history of SSD in general? If so, motor deficits would represent one of the long-term sequelae of SSD in adults.
Within individual families, do adults with a history of speech difficulties exhibit similar motor rate limits as the related affected children in the same family? If so, this would be consistent with a familial motor-based SSD subtype.
To facilitate a meaningful comparison of motor measures in children and adults, an additional goal of this study was to develop motor measures that were not readily available. Thus, this paper not only addresses questions regarding familial SSD subtypes but also contributes methodological considerations of studying motor measures across the lifespan.
METHOD
Participants
Data for this study were collected at the University of Washington Department of Speech and Hearing Sciences and with the approval of the University of Washington’s Human Subjects Institutional Review Board. Families were ascertained through a proband child with a positive SSD history. Criteria for participation specified proband child age 5 to 9 years, a positive family history of SSD as defined by at least two additional biological relatives with SSD, and the absence of neurologic impairments such as cerebral palsy, diagnosed cognitive-communicative impairments such as autism, impairments in oral function such as dysarthria, and impairments of oral structures such as cleft palate. In each family, both biological parents of the proband and additional relatives, such as siblings, grandparents, great-grandparents, aunts, uncles, and cousins, participated.
Study sessions took place in a quiet laboratory room. In cases where participants were willing to participate in the full study protocol but were not able to travel to the University of Washington, data were collected in quiet study rooms located in clinic or library facilities near their homes. All sessions were video and audio recorded.
To date, five families (N = 57) participated. Thirty-nine participants underwent behavioral testing and provided a family history and, with one exception, DNA. In all cases, the possibility that dialectal variation could explain observed speech production differences was considered and excluded. In what follows, the families are described in terms of SSD history. Four-digit participant codes represent family code, generation number, and a two-digit individual number. Codes are listed only for family members who participated in the behavioral testing and/or provided DNA. Where available, standard scores (SS) from the Goldman-Fristoe Test of Articulation -2 (GFTA-2) (Goldman, 2000) and the Khan-Lewis Phonological Analysis – 2 (KLPA-2) (Khan & Lewis, 2002) are reported, except in cases where no speech sound errors were observed.
In family 001, the proband child was a boy, code 1303, age 5;11 (years;months) with a speech delay for which he had received speech therapy as a preschooler. At the time of testing, his speech was characterized by /r/ missing from his phonetic inventory and a frontal lisp. His GFTA-2 SS was 81 and his KLPA-2 SS was 91. His younger brother, code 1304, age 3;4, also had a speech delay and had received speech therapy. His speech was characterized by /r/ missing from his inventory, inconsistent cluster reduction, velar fronting in initial position, [f]/θ substitution, and [d]/ð substitution. In addition, he produced many vowel errors, e.g., [nef]/knife, [fada]/feather, and [pansə;lz]/pencils. His GFTA-2 standard score (SS) was 92 and his KLPA-2 SS was 89. The boys’ mother, code 1205, reported a childhood history of difficulties learning to read and a habitual [f]/θ substitution even into adulthood; no other speech difficulties were noted. The boys’ father, code 1204, reported a childhood history of speech delay and speech therapy. His brother, code 1201, did not report a childhood history of speech difficulties but his two sons, ages 9;3 (code 1301) and 6;4 (code 1302), had been diagnosed with severe SSD, whereas the boys’ mother, code 1202, did not have a history of speech difficulties. The proband’s father had a second brother, code 1203, who reported that he was told as a child that he sounded like he spoke with a foreign accent but he never received speech therapy. The proband child’s paternal grandmother, code 1102, did not report a history of speech difficulties. The paternal grandfather was deceased and it was unknown whether he had a history of speech difficulties.
In family 002, the proband child was a boy age 8;4, code 2503, with a history of severe SSD. At the time of testing, his speech had greatly improved following intensive therapy and was characterized only by distortions of /r, l/. He had not undergone formal assessment for CAS. His GFTA-2 SS was 86 and his KLPA-2 SS was 71. His two younger brothers, ages 6;5 and 5;5, codes 2504 and 2505, also had been diagnosed with severe SSD. At the time of this study, the 6-year-old brother’s speech was characterized by absence of /r/ from the inventory and slight /l/ distortions. His GFTA-2 SS was 101 and his KLPA-2 SS was 85. When he was 2;11, a speech-language pathologist had observed difficulties with initiation of speech, inconsistent vowel and consonant errors, limited phonetic inventory, and restricted expressive vocabulary and concluded that these characteristics were consistent with CAS. The 5-year-old brother also was unable to produce /r/ and, in addition, his speech was characterized by palatal fronting, deaffrication, and [f]/θ substitution. His GFTA-2 SS was 79 and his KLPA-2 SS was 84. The same speech-language pathologist who had assessed the 6-year-old brother had assessed this brother at age 2;0 and informally observed difficulty with oral motor movements, concluding that the severe speech delay was consistent with CAS as well. Their 3-year-old sister, code 2506, had also been diagnosed with SSD and had received therapy. Neither the mother, code 2405, nor the father, code 2504, reported childhood difficulties with speech development. The maternal grandmother, code 2303, reported severe speech difficulties in childhood but therapy had not been available to her at that time. Her mother, code 2201, the proband’s maternal great-grandmother, did not report a childhood history of speech difficulties. Her husband, code 2202, the proband’s deceased maternal great-grandfather, had an unknown history of childhood speech difficulties, but his sister, code 2203 reported a history of difficulties with speech. As a child, she had been told she was “tongue-tied.” Her speech at the time of the study was characterized by a frontal lisp. Her GFTA-2 SS was 59 and her KLPA-2 SS was 99. The maternal grandmother’s brother had a history of childhood speech difficulties. His adult son, code 2401, reported a childhood history of speech and language delays. His son, age 7;0, code 2501, did not have a diagnosed speech delay, and his speech was characterized by inconsistent [f]/θ substitution. His GFTA-2 SS was 97 and his KLPA-2 SS was 101. His daughter, age 3;7, code 2502, exhibited speech sound errors such as cluster reductions, inconsistent and unusual speech sound substitutions, for instance [v]/w, [v]/r, and [p]/kr, and a number of vowel errors, but she was able to produce /r/ and /l/ accurately. Her GFTA-2 SS was 81 and her KLPA-2 SS was 94.
In family 003, the proband was a boy, age 5;11, code 3301, whose parents had concerns regarding his speech development, especially given a family history of SSD. He had not yet undergone formal assessment. His speech was characterized by absence of /r/ from the inventory, [f]/θ substitution in medial and final position, and [d]/ð substitution. His GFTA-2 SS was 89 and his KLPA-2 SS was 87. His father, code 3201, did not report a history of speech difficulties but his mother, code 3202, had a childhood history of speech difficulties. One of the mother’s adult brothers, code 3204, and one of her adult sisters, code 3203, reported childhood histories of speech difficulties. No written records of their childhood speech therapy were available, but according to the parents, the brother, as a young child, omitted final consonants. Her father, code 3101, the proband’s maternal grandfather, reported a childhood history of late developing speech. Her mother, code 3102, the proband’s maternal grandmother, did not report any childhood speech difficulties. The proband had several cousins on his mother’s side of the family, reportedly with speech delays, who could not participate in the study because they were not yet old enough or lived out of the country.
In family 004, the proband was a girl, age 9;0, code 4303, who had speech therapy as a preschooler but whose speech had since normalized. Her younger sister, age 4;5, code 4304, was unable to produce /r/ and she had a frontal lisp. Her GFTA-2 SS was 95 and her KLPA-2 SS was 98. Her older brother, age 10;8, code 4302, had no history of speech difficulties. The children’s father, code 4203, and mother, code 4202, did not report childhood speech difficulties but the paternal grandmother, code 4102, reported receiving speech therapy as a child for what was explained to her as tongue thrust, with tongue placement consistent with a frontal lisp. Her adult speech was still characterized by a frontal lisp. She reported that one of her nieces had also been treated for tongue thrusting, and several of this niece’s children wore orthodontic appliances to correct speech sound distortions due to a forward tongue placement. Detailed data from these relatives, however, were not available.
Family 005 was unusual in that both parents of the proband child, a girl, age 5;2, code 502, had histories of childhood speech difficulties and there were other relatives on both sides of the family with histories of speech difficulties. The proband child had previously been diagnosed with a speech disorder consistent with severe CAS and had been receiving speech therapy since age 3 years. The CAS diagnosis was based in part on the presence of 32 of 49 characteristics from the Apraxia Profile (Hickman, 1997) and a severity rating of severe impairment in the areas of simple phonemic level, complex phonemic level, and spontaneous length in the Kaufman Speech Praxis Test for Children (Kaufman, 1995), conducted when she was 4;11. At the time of the study, the proband’s consonant inventory was substantially reduced. She substituted [d] for most obstruents and consonant clusters (e.g., (e.g., [dØbɔl]/shovel; [daun]/clown; [dipɔ]/zipper.) Phonological process analysis revealed the presence of the following processes: deletion of final consonants, stopping, cluster simplification, liquid simplification, palatal and velar fronting, cluster reduction, and initial voicing. In some cases, several processes converged on a single target. For instance, [dØbɔl]/shovel shows the effects of three phonological processes, palatal fronting, stopping, and initial devoicing, all acting on the target /ʃ/. Her GFTA-2 SS was 42 and her KLPA-2 SS was 41. Of note, this girl had difficulty with saliva control. Her brother, age 7;9, also had a history of speech difficulties but they were less severe and largely resolved by the time of testing following therapy, with stimulable [f]/θ substitution being the only residual error. His GFTA-2 SS was 90 and his KLPA-2 SS was 89.
The proband’s father, code 5403, and his sister, code 5404, both had childhood histories of speech difficulties, and so did the proband’s paternal grandmother, code 5308. The proband’s paternal aunt had two daughters, ages 8;8 and 6;3, codes 5503 and 5504. The 8-year-old girl had a history of severe SSD. According to a speech evaluation at age 3;10, using the Clinical Assessment of Articulation and Phonology (Secord & Donohue, 2002), her speech error patterns included consistent cluster reduction, velar and palatal fronting, and frequent syllable reduction, vocalization, gliding, and stopping. The six-year-old girl had undergone a speech evaluation at age 3;5. Her speech sound substitutions ([t]/_, [d]/_,[f]/θ, [b]/v, [w]/r), omissions [final /r/), and cluster reductions (all /l/ and /r/ clusters) were ruled to be at the low end of the normal range for her age.
The proband had two male fourth cousins who were brothers. One of them, age 14, code 5505, had a diagnosis of Asperger syndrome and the other, age 10, code 5506, had been diagnosed with severe speech difficulties consistent with disrupted praxis in addition to hemiplegia, possibly related to a suspected stroke suffered at birth. These two brothers participated in the full protocol but their oral motor scores were excluded from the present study due to concerns about confounding effects from their primary diagnoses. The 10-year-old boy did not participate in the rapid naming tasks.
The proband’s mother, code 5402, reported childhood speech difficulties that had resolved with therapy. Her mother, code 5304, the proband’s maternal grandmother reported no speech difficulties and neither did her father, code 5303, the proband’s maternal grandfather, although his brother had received speech therapy as a child for a brief period. The proband’s mother’s half-sister, age 14, code 5401, had a history of mild speech delay and had received therapy for help with /r/.
Protocol
Articulation was assessed with the GFTA-2. The GFTA-2 quantifies consonant errors on the single word level by phoneme and word position. Phonological processes were quantified with the KLPA-2, using the word productions from the GFTA-2. Two questionnaires about hand preference (adapted from the Edinburgh Handedness Inventory) and educational and developmental backgrounds (generated specifically for this study) were filled out. Parents provided the requested information for their children. The data collection further included DNA and a variety of additional behavioral tasks, not further detailed here, covering aspects of speech production, language ability, reading, spelling, phonological memory, and verbal and nonverbal processing.
For the present study, motor data were available from 11 children (9 ever affected) and 25 adults (12 ever affected), 36 participants together. The term “ever affected” refers to individuals who had a history of SSD, whether or not they showed evidence of SSD at the time of testing. This term is used in contrast with “never affected,” which refers to individuals without a history of SSD.
To investigate motor processing in the oral and hand systems, repetitive and alternating movement tasks were administered. Following the methods in Fletcher (Fletcher, 1972), participants were instructed to produce series of monosyllables (/pa, /ta/, /ka/), disyllables (/pata/, /taka/), and trisyllables (/pataka/) as fast and as accurately as they could. Each trial was preceded by a brief model and a practice run. At least 20 productions of the monosyllables, 15 of the disyllables, and 10 of the trisyllables were collected. In the case of inaccurate production in the disyllable task, for instance saying /papata …/ instead of /patapata …/, the inaccurately produced syllables were included in the calculation of average syllable duration.
To measure maximum key tapping rates during a repetitive task, participants were instructed to tap the spacebar of a laptop computer using their index finger as many times as possible during a 10-second interval, following published protocols of this activity (Gualtieri & Johnson, 2006). Tap intervals were recorded using a program designed with LabView (National Instruments). Ten trials were administered, five for the right hand and five for the left, starting with the right hand regardless of a participant’s handedness status, and switching hands after each trial to minimize fatigue. Each trial began with clicking on a button to start the trial, followed by a wait period that varied randomly between 2 and 4 seconds and a visual start signal. In the alternating version of this activity, participants were instructed to tap two different computer keys with the index and middle fingers of the same hand, going back and forth between the two keys as fast as possible. The left and right arrow keys, which are separated by the down arrow key, were programmed to receive the inputs from this activity. For both types of the key tapping task, the instructions included a brief model and the keyboard was positioned such that participants could reach the target keys comfortably for each trial. In all study sessions, the finger tapping task was administered before the oral motor task.
Data Reduction
Data were reduced and analyzed by a team consisting of the first author and qualified undergraduate and graduate students. Syllable durations were measured using the freely available acoustic software Praat (Boersma, 2001), version 5.1.25. Short durations were interpreted as consistent with rapid motor speed, although it is acknowledged that short durations can also be achieved with relatively small articulator excursions, not necessarily representing rapid movement. As in previous studies of nonword repetition and maximum syllable repetition rates (Peter & Stoel-Gammon, 2008; Thoonen et al., 1999; Thoonen et al., 1996), the first token in a series was excluded from analysis to minimize nonlinear initiation effects, and the last token prior to an inhalation was excluded to minimize final lengthening effects and also because, in open syllables, the vowel endpoints can not always be established reliably due to variations in the acoustic environment. Inhalations were excluded. Most adults completed the target set of syllables within one breath, but many children were not able to do so. Whether or not the tokens were produced in one breath had minimal, if any, effects on the syllable durations because inhalations and first and last syllables in a breath group were excluded from the analysis.
Norms were only available for some of the measures of interest. For instance, norms for the mono- and disyllabic repetitions are available for ages 2;6 (years;months) through 6 years (Robbins & Klee, 1987) and 6 through 13 years (Fletcher, 1972) and they show decreasing mean syllable duration rates as a function of age. One goal of this study, hence, was to develop measures that can be used in children as well as adults. In addition to raw syllable durations, averaged per participant, duration ratios for monosyllables and multisyllables were calculated to factor out the raw speeds. A ratio > 1 indicated that the multisyllables had shorter durations than the monosyllables, implying shorter intervals and, by inference, more rapid movement in the multisyllables, compared to the monosyllables, which is consistent with intact sequencing skills as typically seen in older children. For the purposes of the present study, the ratio of the monosyllable /pa/ and the disyllable /pata/ was selected because young children with SSD frequently have difficulty producing /k/. Norms for ages 6 through 13 (Fletcher, 1972) show that sequencing ability in children increases as a function of age, as /papa, pata/ ratios range from .99 for age 6 years to 1.2 for age 13 years. In an attempt to factor out age, z-scores were calculated for each participant using these norms (Fletcher, 1972). For the participants younger than age 6 years, norms were imputed using norms for children age 2;6 (years;months) through 6 years (Robbins & Klee, 1987). For children age 6 years, these two scales differ by 53 ms, likely because of methodological differences. The norms for the older children were based on time-by-count measures, whereas the norms for the younger children were based on count-by-time measures of 3-second intervals. Because norms for older children and adults throughout the lifespan are not available, the norms from the 13-year-olds were used for all individuals 13 years and older, even though it is possible that these norms slightly underestimate age-adjusted oral motor speeds in the older adults (Peter et al., 2010). To observe relative deficits in sequencing places of articulation in the /pata/ task, the z-scores from the disyllable durations were subtracted from the z-scores from the monosyllable durations. A large discrepancy was interpreted as faster monosyllable rates compared to disyllable rates and, hence, a relative deficit in motor sequencing.
Key tap intervals were obtained from the LabView output files and averaged for each participant. Similar to the syllable repetition task, raw durations were converted to z-scores using norms for ages 5 years through 7 years (Gray, Livingston, Marshall, & Haak, 2000) and 8 years to 83 years (Gualtieri & Johnson, 2006). The norms for the younger children were available for the dominant and nondominant hand, and those for older children and adults were available for the right and left hand; therefore, the z-scores averaged for both hands were used for normalizing the present data. The norms for the key tapping task also differed in that the data for young children were obtained using a special key tapping device, whereas those for the older children and adults were obtained using a computer keyboard; therefore, norms for the younger children were imputed by comparing both scales at the age overlap. Similar to the syllable repetition tasks, a ratio of repetitive and alternating tap intervals was calculated. Because norms for intertap intervals from alternating key tapping were not available, a z-score difference could not be calculated. Table 1 summarizes the motor measures with respect to the tasks, measured ability, and standardization.
Table 1.
Measures of repetitive and alternating motor speeds.
| Variable | Measured Ability | Standardization Source | Standardization Age |
|---|---|---|---|
| /pa/ Syllable Duration (ms) | Raw speed of repetitive oral movement | N/A | N/A |
| /pa/ Z Score | Age-adjusted speed of repetitive oral movement | Robbins & Klee (1987), Fletcher (1972) | 2;6 - 6;11, 6;0 - 13;11 |
| /pata/ Syllable Duration (ms) | Raw speed of alternating oral movement | N/A | N/A |
| /pata/ Z Score | Age-adjusted speed of alternating oral movement | Robbins & Klee (1987), Fletcher (1972) | 2;6 - 6;11, 6;0 - 13;11 |
| /pa, pata/ Duration Ratio | Oral sequencing ability based on raw durations | N/A | N/A |
| /pa, pata/ Z Score Difference | Oral sequencing ability based on z scores | Robbins & Klee (1987), Fletcher (1972) | 2;6 - 6;11, 6;0 - 13;11 |
| Rep. Key Tap Intervals (ms) | Raw speed of repetitive finger movement | N/A | N/A |
| Rep. Key Tap Inverval Z Score | Age-adjusted speed of repetitive finger movement | Gray et al. (2000), Gualtieri & Johnson (2006) | 5;1 - 7;11, 8;1 - 83;11 |
| Alt. Key Tap Intervals (ms) | Raw speed of alternating finger Movement | N/A | N/A |
| Rep./Alt. Key Tap Interval Ratio | Finger sequencing ability based on raw durations | N/A | N/A |
Reliability
Approximately 15% of the motor data and 50% of the data from the standardized speech testing were checked for reliability. Standard scores (SS) from the articulation testing in currently affected children differed, on average, by 3.25 SS. Differences > 1 SS were reconciled prior to phonological analysis by reviewing the video records. The mean syllable durations from the mono- and disyllabic production task differed by < 1 ms. Because the key tap intervals were captured with a software program rather than retrieved and analyzed manually, they were considered accurate and reliable. The program’s data capture mechanism was tested prior to the study sessions.
Statistical Analysis
To investigate the associations among the repetitive and alternating motor measures in the oral and hand motor systems (research question 1), correlations among and between the temporal measures in the oral and the hand motor system were calculated. This was done separately in the three types of variables, raw durations in ms, repetitive/alternating duration ratio, and z-score. Statistical significance was Bonferroni adjusted for multiple testing, although it should be noted that the variables are not independent of each other and the Bonferroni correction is more conservative than necessary. Seven tests were carried out, arriving at an adjusted alpha of .0071. To observe potential linear and/or nonlinear effects of age on the variables of interest, each of these measures was checked by visual inspection of scatterplots and with multiple regression models using age in months and a squared term of age in months. Significant correlations with the linear age term was interpreted as an indication that the measured variable increased or decreased as a function of time, whereas significant correlations with the quadratic age term indicated an increasing, then decreasing, trajectory or a decreasing, then increasing, trajectory as a function of age.
To investigate whether motor speeds distinguish between adults with and without a history of SSD regardless of family-specific subtypes (research question 2), one-tailed t tests between these two groups were conducted for each of the variables of interest. To investigate whether limits in motor control of speed and/or sequencing characterize adults with a history of speech difficulties in similar ways as biologically related children (research question 3), variables of interest were compared between ever affected and never affected participants in families with affected children who showed motor speed deficits.
RESULTS
Associations among and between oral and hand task performance scores
Correlations were computed for three types of variables, durations in ms units, ratios of repetitive/alternating unit durations, and z-scores. Of interest were correlations within each of the two motor domains (oral, hand) as well as cross-domain correlations (Table 2). Strongest within- and cross-domain correlations were seen in the raw durations, followed by z-scores and repetitive/alternating durational ratios.
Table 2.
Pairwise correlation coefficients (p values) for variables of interest and t scores (p values) from multiple regression models evaluating the effects of age in months and squared age in months. Asterisks indicate statistical significance.
| Domain | Variable | Oral | Hand | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| /pa/ Dur. (ms) | /pa/ Z Score | /pata/ Dur. (ms) | /pata/ Z Score | /pa, pata/ Ratio | /pa, pata/ Z Score Diff. | KT Rep. Dur. (ms) | KT Rep. Z Score | KT Alt. Dur. (ms) | KT Rep., Alt. Ratio | ||
| Oral | /pa/ Dur. (ms): r | 1.00 | |||||||||
| /pa/ Z Score | 1.00 | ||||||||||
| /pata/ Dur. (ms): r | 0.71* | 1.00 | |||||||||
| p | <0.0001 | ||||||||||
| /pata/ Z Score: r | 0.55* | 1.00 | |||||||||
| p | 0.0008 | ||||||||||
| /pa, pata/ Ratio: r | 1.00 | ||||||||||
| /pa, pata/ Z Score Diff.: r | 1.00 | ||||||||||
| Hand | KT Rep. Dur. (ms): r | 0.81* | 1.00 | ||||||||
| p | <0.0001 | ||||||||||
| KT Rep. Z Score: r | 0.33 | 1.00 | |||||||||
| p | 0.0646 | ||||||||||
| KT Alt. Dur. (ms): r | 0.55* | 0.85* | 1.00 | ||||||||
| p | 0.0008 | <0.0001 | |||||||||
| KT Rep., Alt. Ratio: r | 0.30 | 1.00 | |||||||||
| p | 0.0912 | ||||||||||
| Age | Age (Months): t | -7.43* | .96 | -4.81* | .53 | 1.48 | -0.04 | -8.52* | -0.13 | -5.70* | 6.19* |
| p | <0.0001 | 0.3450 | <0.0001 | 0.5970 | 0.1490 | 0.9660 | <0.0001 | 0.8970 | <0.0001 | <0.0001 | |
| Squared Age (Months): t | -6.10* | -1.27 | 4.34* | -1.35 | -1.72 | 0.75 | 6.81* | -0.07 | -4.85 | -5.77* | |
| p | <0.0001 | 0.2120 | <0.0001 | 0.1880 | 0.0950 | 0.4570 | <0.0001 | 0.9460 | <0.0001 | <0.0001 | |
Within the oral motor system, the syllable durations from the monosyllabic (/pa/) and the disyllabic (/pata/) repetition tasks were significantly correlated (r = .71, p < .0001). Within the hand motor system, the intertap interval durations from the repetitive and alternating task also were correlated significantly (r = .85, p < .0001).
Across motor systems, durations from the monosyllabic /pa/ series and the repetitive key tapping task were correlated significantly (r = .81, p < .0001). Durations in both measures followed a closely overlaid quadratic trajectory across the lifespan (Figure 1), showing a steep decrease in children and a slight increase in adults as a function of age. For both variables, regression models confirmed these trajectories with greater associations for the linear age term, compared to the squared age term (Table 2). Similarly, alternating interval durations from the disyllabic /pata/ series and the key tapping task involving two separate keys were correlated significantly (r = .55, p = .0008). As in the case of the repetitive movement task, alternating durations from the oral and the hand task followed a closely overlaid quadratic trajectory across the lifespan (Figure 2). As in the variables from the repetitive movement tasks, regression models confirmed these trajectories with greater associations for the linear age term, compared to the squared age term (Table 2).
Figure 1.
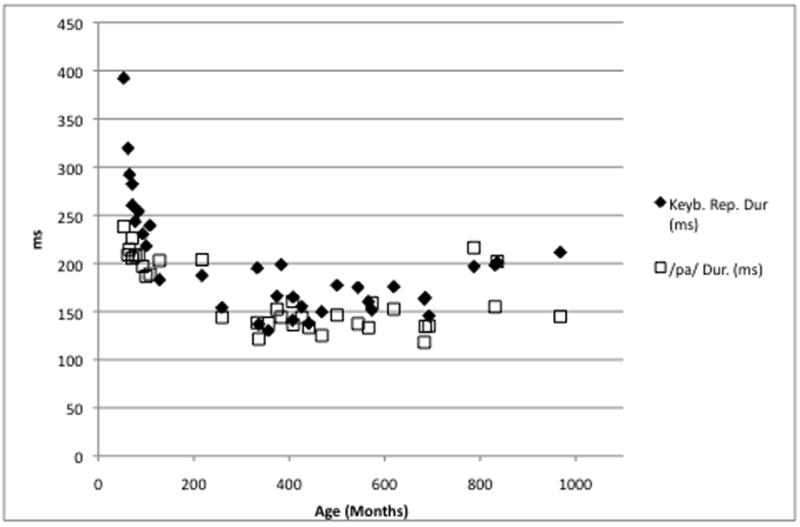
Durations (ms) from the monosyllable repetition task (/pα/) and the repetitive key tapping task as a function of age in months.
Figure 2.
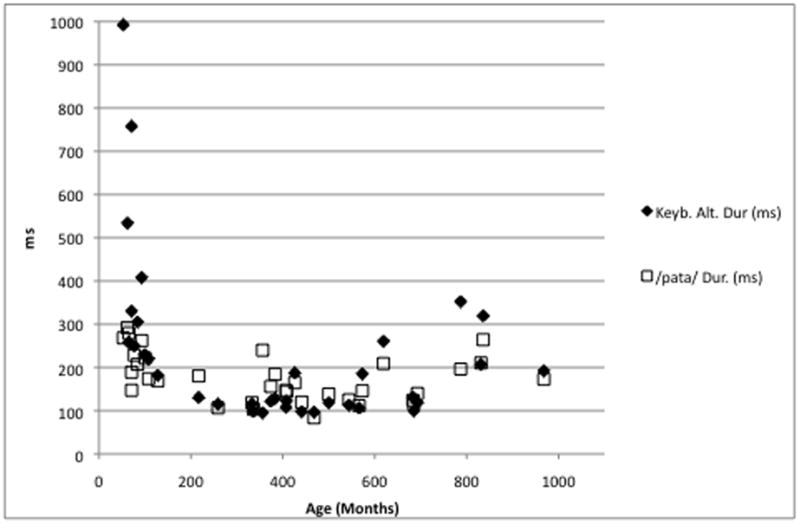
Durations (ms) from the disyllable repetition task (/pαta/) and the alternating key tapping task as a function of age in months.
Regarding the durational ratios of intervals from repetitive and alternating movement, which are independent of raw durations, the ratio of the durations from the monosyllabic and disyllabic task was not correlated significantly with the ratio of the durations from the repetitive and alternating key task (r = .30, p = .0912). The repetitive/alternating duration ratio from the key tapping task followed a quadratic trajectory with some outliers across the lifespan (figure 3) with the highest ratios and, hence, the greatest speed advantage in the alternating task, seen in participants between the ages of 18 and 57 years. The majority of participants 18 years and older exceeded a ratio of 1, indicating a speed advantage for the alternating mode. Five of the six participants who did not show this speed advantage were members of families 002 or 005 (Figure 3) and biologically related to the probands. It is not clear what role the age of participants 2201, 2203, and 5308 played in these low scores. Regression models showed that age in months was correlated with this ratio to a greater extent than the squared term of age in months, consistent with a strong increase of sequencing ability with increasing age and a decrease in older adults.
Figure 3.
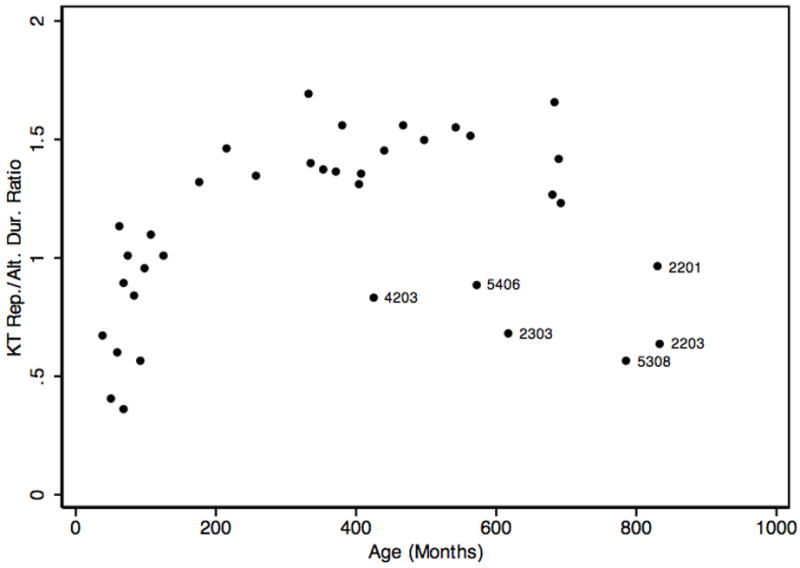
Ratios of repetitive/alternating key tapping (KT) durations as a function of age in months. Labels are participant codes for adults with ratios <1.
The analogous trajectory from the monosyllable/disyllable ratios was not as clearly quadratic (Figure 4; Table 2), although it showed a steep increase in the children and adolescents. Given the norms (Fletcher, 1972) showing that by age 11 children produce disyllables with shorter durations than monosyllables, a monosyllable/disyllable ratio > 1 would be expected for adults. Most adults showed this speed advantage for alternating oral movements. Seven of the ten participants who did not show this advantage were biological relatives of the probands in families 002 or 005. It is not clear what role the ages of participants 2201, 2203, and 5202 played in these low ratios. Four of the adult participants (2201, 2203, 2303, and 4203) failed to reach a ratio >1 in both tasks, consistent with motor sequencing deficits in both the oral and the hand task. Neither the linear nor the quadratic term of age was associated with age to a significant extent (table 2).
Figure 4.
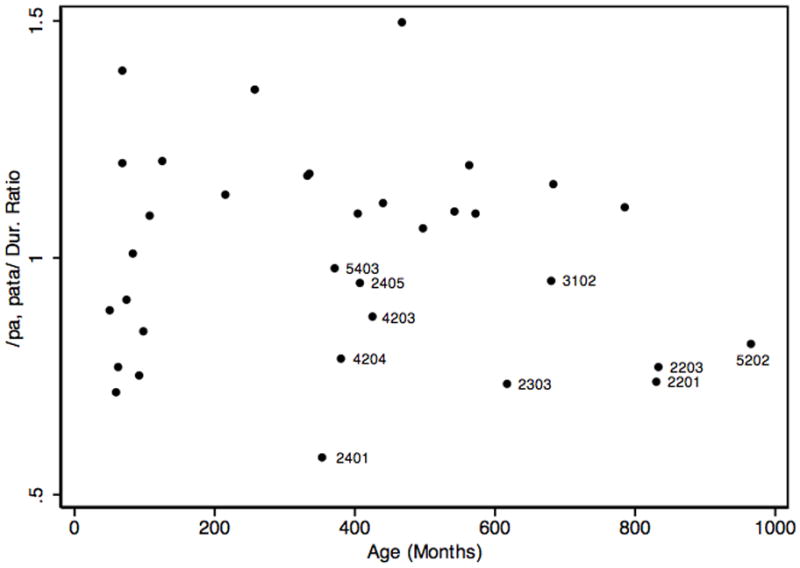
Ratios of monosyllable and disyllable durations as a function of age in months. Labels are participant codes for adults with ratios <1.
Regarding standardized scores within the oral motor system, z-scores from the monosyllabic and disyllabic task were correlated significantly (r = .55, p = .0008). Across motor systems, the correlation of z-scores from the repetitive oral task (/pa/) and the repetitive key tapping task did not reach statistical significance (r = .33, p = .0646). Age-adjusted norms are not available for the alternating key tapping task. The regression models (Table 2) show that age was not associated with any of the z-scores to a significant extent, consistent with what would be expected when using age norms to convert raw durations z-scores. Figure 5 shows the distribution of the z-score difference from the mono- and disyllabic task as a function of age. Of 12 scores that exceeded 1 SD, indicating a substantial deficit in oral motor sequencing, six came from family 002, two from family 004, and four from family 005. Table 2 summarizes the pairwise correlations among the variables of interest and the multiple regression statistics from the two age terms.
Figure 5.
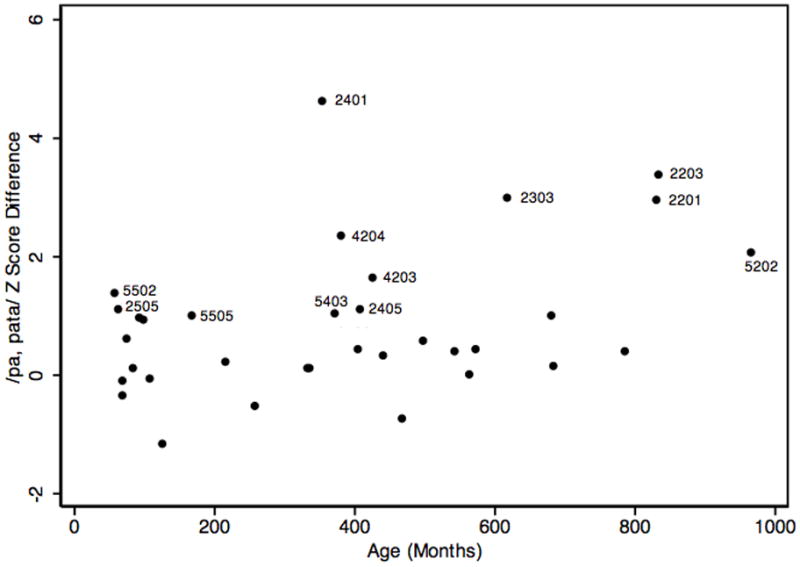
/pα, pαta/ z-score differences as a function of age in months. Labels are participant codes for differences > 1.
Regarding measures suitable for evaluating familial or genetic effects on repetitive and sequential motor speeds across the lifespan, these findings indicate that raw durations are strongly influenced by age, decreasing in childhood and adolescence and increasing again in older adulthood. Therefore, raw durations should not be used to evaluate familial or genetic effects on motor speeds across the lifespan. As measures of sequential motor ability, durational ratios based on repetitive and alternating tasks should be interpreted by taking the lifetime trajectories into account, because it appears that the ratios increase in childhood, then decrease in later adulthood as a function of age, even when raw durations are factored out. The z-scores from the oral and hand tasks appear to control for most of this age effect and, hence, are useful for evaluating familial or genetic effects on repetitive and alternating motor speeds in children and adults. Because z-scores for alternating key tapping are not available, the repetitive/alternating interval ratio can be used to determine divergences from expectations at a given age.
Motor speeds in adults with, and without a history of SSD
None of the variables of interest distinguished significantly between adults with and without a history of SSD in general. For the monosyllable durations in ms, t (p) = -1.29 (.8904); the disyllable durations in ms, t (p) = -.34 (3690); the ratio from the mono- and disyllable durations, t (p) = -1.01 (.1609); the z-score difference from the mono- and disyllable durations, t (p) = .67 (.2532); the key tapping repetitive tap intervals in ms, t (p) = -.74 (.2326); the key tapping alternating tap intervals in ms, t (p) = -.85 (.2036); the ratio from the repetitive and alternating intervals, t (p) =.00 (.4991); and the z-score from the repetitive key tapping intervals, t (p) = .84 (.2049), respectively. Group differences for the individual z-scores from the oral tasks were not computed because in the adults, they were linear transformations of the raw durations. Analogous group differences in the children were not computed because the majority had a history of SSD.
Familial limits of motor speeds
None of the affected children showed deficits in repetitive motor speeds, whether in the key tapping task as documented with z-scores of repetitive tapping durations, nor in the monosyllable repetitions as documented with the analogous z-scores. Slowed repetitive speeds in both motor tasks were observed in the adult participants 2203 and 3204. Slowed repetitive key tapping but not slowed monosyllable rates were seen in the adult participants 3202 and 4204. Slowed monosyllabe rates but not slowed key tapping rates were seen in the adult participants 4302 and 5308.
Affected children in two families, 002 and 005, showed limits in motor sequencing speeds. In family 002, the three brothers, codes 2503, 2504, and 2505, all with histories of severe SSD and two, with speech histories consistent with CAS, had short monosyllable durations and their disyllable durations were within a quarter of a standard deviation from the population mean. Subtracting the z-scores for the disyllables from those from monosyllables, however, showed a relative deficit in the motor sequencing element inherent in the disyllabic task. A corresponding deficit was not observed in the key tapping task, where repetitive/alternating ratios were between 0.95 and 1.13, although it is acknowledged that age norms for this variable are not available. Neither parent had a history of speech difficulties, but the unaffected mother, code 2405, showed a similar discrepancy between z-scores from the monosyllable and disyllable tasks and so did the unaffected maternal great-grandmother, code 2201. In addition, three affected adults, codes 2303, 2203, and 2401, showed very long disyllable durations and large z-score discrepancies between mono- and disyllable durations ranging from 2.97 to 4.60. Two of these, codes 2303 and 2203, also had very low repetitive/alternating key tapping ratios, indicating that their alternating intervals were much longer than their repetitive intervals, consistent with slower sequencing speeds, whereas the third affected adult, code 2401, showed intact finger sequencing skills. As shown in figures 3, 4, and 5, several members of this family, all biologically related to the proband, had duration ratios that deviated from expectation for age in both types of motor task and large z-score differences in the mono- and disyllable task.
Similarly, the proband child in family 005, code 5502, who had severe speech deficits consistent with CAS, showed slowed sequencing in the syllable repetition task. Her monosyllabic z-score was substantially above expectation for her age and the disyllabic z-score was less than half a standard deviation below the mean. The z-score difference of 1.38 documented a relative deficit in her sequencing ability. Her key tapping also showed slowed alternating durations compared to repetitive durations, although an age-adjusted interpretation cannot be offered. Her brother, code 5501, also affected with SSD but less severely so, showed the same relative strength of repetitive speed and limited sequencing speed in both motor systems, although, again, age-adjusted norms of the alternating key tap intervals are not available. In the paternal branch of the family, both the father, code 5403, and the grandmother, code 5408, produced the disyllables with substantially longer syllable durations compared to the monosyllables. The grandmother also showed the same slowed motor sequencing during the key tapping task but the father did not. A third cousin of the father, code 5406, did not report a history of speech difficulties but showed relatively slower speeds in the alternating version of the key tapping and syllable repetition tasks, compared to the repetitive version. As was the case for family 002, deviations from expectation for age in both types of motor task are evident in figures 3, 4, and 5 for several members of this family, all biologically related to the proband.
In the maternal branch of family 005, none of the related family members, whether ever SSD affected or not, showed repetitive or alternating motor speed deficits in the key tapping task. Z-scores from the repetitive task were - 0.11 or higher, and ratios of repetitive/alternating durations were greater than 1.16, consistent with intact finger sequencing ability. Regarding disyllable durations, the proband’s mother, code 5402, and great-grandmother, code 5202, both had z-scores above expectations. The mother produced the disyllables at much greater speeds than expected, whereas the great-grandmother showed substantially slower speeds, resulting in a large z-score difference.
DISCUSSION
The purpose of this study was to investigate familial subtypes of SSD in five families with multigenerational histories of SSD. The focus was on the role of motor speeds in the oral and hand motor systems.
Suitable measures of repetitive and alternating motor speeds across the lifespan
An evaluation of the effects of age on the variables of interest showed that raw durations of repetitive and alternating movement in the oral and the finger task varied significantly as a function of age. A steep decline in duration across childhood (indicating improving motor facility) and a slight increase in older adulthood were observed as a function of age. Ratios of repetitive/alternating durations, which were constructed as a measure of sequencing ability independent of actual durations, also showed an effect of age, with an increase of sequencing ability across childhood and a slight decrease in older adulthood. This shows that relative motor sequencing ability varies across the lifespan even when raw durations are factored out. The z-scores from the mono- and disyllable task and the repetitive key tapping task showed the least effect of age. The z score difference from the mono- and disyllable task was interpreted as a robust measure of oral motor sequencing across the lifespan that can be used to evaluate familial or genetic effects on motor sequencing ability. An analogous measure is not available for the key tapping task, but divergences of the repetitive/alternating ratio given expectation for age can be used to estimate deficits in finger motor sequencing across the lifespan. It should be kept in mind, however, that this sample contained individuals with deficits in repetitive and alternating motor speeds. To fully investigate the effect of age on motor speeds, norming data should be generated in a typical sample of adults.
Regarding the role of age in repetitive finger motor speeds, previous studies (Bartzokis, et al., 2008; Gualtieri & Johnson, 2006; Prigatano, et al., 2008) have shown that raw key tapping speeds increase in childhood and decrease with advancing age in adults. The results from the present study are consistent with these findings and extend them to the motor speech system, although our sample contained individuals with SSD histories, which may have influenced performance on this task. Normative data for repetitive motor speech speeds, available only for children age 2;6 through 13;11, reveal an increase in speed as a function of age. Our results are consistent with this trend and also show a decrease in speed with advancing age in adults, similar to the findings in the finger motor literature, although it is acknowledged again that in some of the adult participants, it is not certain whether the repetitive motor rates are influenced by age, a familial or genetic effect, or both.
Regarding the role of age in alternating motor speeds, norms for disyllables (Fletcher, 1972) show that by 11 years, children develop a speed advantage for alternating places of articulation, presumably due to progressively greater oral motor sequencing ability. Analogous norms for alternating key strikes are not available. This study shows not only that alternating movement speeds in a finger task and in a syllable repetition task follow a quadratic trajectory (i.e., increase of sequencing ability during childhood and a decline with advancing age in adulthood), but also that a similar quadratic trajectory is likely for the ratio of repetitive and alternating intervals in a hand task, independent of raw durations. Our sample, however, contained several children and adults with histories of apraxic-like speech difficulties, which may have confounded the effect of age on the ratios. A decrease of alternating movement speeds in adults was observed in our study of global processing speeds in a large sample of families with dyslexia (Peter et al., 2010), where the decreases were noted not only for timed measures of finger motor and oral motor sequencing but also rapid naming of stimuli with and without category switches and alphabet writing. Age adjustments for performance on measures of phonological memory and articulation in adults with monotonically increasing raw scores as a function of age have recently been presented (Stein, et al., 2010). Norms for older children and adults throughout the lifespan (in populations that do not have familial histories of speech or language impairments) should be developed for measures whose raw scores follow a linear or quadratic trajectory across the lifespan.
It was noted that the raw interval durations in the finger and oral task were highly similar to each other in most participants. It is unknown whether these similarities are coincidental or whether they reflect systemic rate limits inherent in the central and/or peripheral nervous system. In the youngest children, however, key tapping intervals were substantially longer than their syllable durations, especially in the alternating mode. This discrepancy may, in part, result from the fact that young children have smaller hands than older children and adults, placing them at a physical disadvantage with respect to the dimensions of the key depression depth and distance between two different keys. It is also possible that finger motor sequencing skills develop later than oral motor sequencing skills in children. Environmental factors may play a role as well. Younger children have less experience pressing computer keys than older children and adults, but they have accumulated considerable motor practice in the oral system in the process of speech acquisition, including during the babble phase.
Correlations among Measures of Oral and Hand Motor Speeds
We found that interval durations in the oral and the hand motor system were positively and significantly correlated with each other, even showing highly similar interval durations. This was the case for both the repetitive and alternating movements. Ratios of repetitive/alternating durations and z-scores showed a trend towards these cross-modal associations but not with statistical significance. In the case of the z-scores, this suggests that participant age may, in part, account for the strengths of the cross-modal associations seen in the raw durational measures. A larger participant sample would be necessary to probe cross-modal associations with controls for age and motor deficits as well as for familial relationships.
Cross-modal associations in hand and finger tasks are consistent with our previous findings in a small sample of children with and without SSD, where timing accuracy was correlated in a hand and a nonword imitation task (Peter & Stoel-Gammon, 2008), and a large sample of families with evidence of familial dyslexia, where z-scores from a timed finger sequencing and a timed motor speech task loaded on a general factor characterized by global processing speed (Peter, et al., 2010). Given the associations between the oral and hand motor system during repetitive and sequential tasks, it is reasonable to ask whether children with speech disorders characterized by motor sequencing difficulties might also show these difficulties in other motor systems and whether biologically related adults with a history of such speech disorders also show deficits in oral and/or hand sequencing tasks.
Motor Speeds in Adults with a History of SSD
Overall, the adults with a history of SSD did not show evidence of repetitive or alternating motor deficits, compared to the never affected adults. Therefore, it is unlikely that motor deficits are among the long-term sequelae of all familial forms of SSD in this sample. Uniform motor sequelae across families would be consistent with the common disease/rare variant model where a common disease is influenced by common and shared genetic variants. The absence of motor sequelae shared across all families allows for the possibility of finding these sequelae within some individual families.
Family-Specific SSD Subtypes
The families were selected for this study because they showed evidence of familial SSD, but we found that phenotypic expression varied between the families. In family 004, difficulty with /r/ and fronted tongue placement for /s, z/ characterized the speech a young family member, consistent with forward tongue placement as also reported in her grandmother and several other biological relatives. This may represent a subtype of SSD with oral structural components, although it is acknowledged that direct data were not available from some of the members of this family. In families 1 and 3, other traits, not yet probed, may characterize the inherited SSD subtype.
Slowed repetitive movement rates did not appear to aggregate in any of the families but slowed alternating movement rates showed evidence of familial aggregation. In families 002 and 005, speech-language pathologists had previously concluded that the speech characteristics of affected children were consistent with CAS, a proposed SSD subtype with deficits in sequential motor processes. In both families, the general mode of inheritance, based on affectation status, is consistent with autosomal dominance with cases of nonpenetrance such as the proband’s mother in family 002. Slowed oral sequencing rates characterized the affected relatives, and slowed finger sequencing was seen in some but not all of the affected relatives, indicating that oral sequencing speeds are more closely associated with the presence of the SSD subtype in these families than finger sequencing speeds. Slowed oral and/or finger sequencing seen in some unaffected relatives, 2405, 5202, and 5406, may indicate that sequencing deficits are a predisposing but not determining factor for SSD.
Of all participants, the proband in family 005 had the most severe speech difficulty as documented with the lowest GFTA-2 score. Because both of her parents had positive familial SSD histories, it is possible that she inherited a risk allele from each of her parents, together disrupting her speech development more severely compared to her brother, who may have only inherited one of the risk alleles. The paternal branch of the family provided more evidence for a motor-based disorder than the maternal branch did. The exact nature of the speech difficulties in the maternal branch could not be determined with the available data.
Taken together, the results from this study are consistent with the common disease/rare variant model in that we describe family-specific SSD subtypes based on behavioral data. Subtypes include a familial SSD form with a structural component, a familial form of motor-based SSD, and potentially at least one familial form that does not fit either of the other two profiles. In one of these forms, motor sequencing difficulties in the oral motor system, as captured in a z-score difference and ratios of monosyllable and disyllable repetition durations, were indicative of this SSD subtype. Corresponding sequencing difficulties in the key tapping task, as estimated from ratios of repetitive/alternating durations, were seen less frequently in ever affected individuals. These results are consistent with Thoonen et al.’s (1996, 1999) findings that the alternating but not repetitive oral movements distinguish between children with praxis-related speech deficits and typical peers. Furthermore, they expand on the observation by Thoonen et al. (1997) that some children with praxis-related speech deficits have a family history of speech and language difficulties by showing that motor sequencing difficulties characterize not only the affected children but also many of the ever-affected relatives. Given that difficulty with motor sequencing was observed in the proband’s unaffected mother in family 002 and a distantly related unaffected relative, code 5406, in family 005, it is possible that difficulty with motor sequencing is a risk factor contributing to the full expression of SSD in combination with other factors including environmental influences.
FUTURE STUDIES AND CLINICAL IMPLICATIONS
Regarding the participants from the present study, in future studies, we plan to address the role of additional phenotypes, including phonological memory, verbal and nonverbal processing, and reading, and also the results from genotypic investigations. Future studies also should be conducted to attempt to replicate the results from this study in additional family samples with evidence of familial SSD. They should test our results in additional syllable types and with additional measures of sequencing including accuracy of attempted tokens. Sequencing ability in oral tasks and perhaps also in the finger tasks may be an appropriate component phenotype for molecular genetic studies of SSD.
Future studies also should address clinical questions related to motor sequencing ability. If deficits in motor sequencing are found to be a core characteristic of certain SSD subtypes with a genetic etiology, then future research should investigate whether therapy directed at speech sequencing can ameliorate the severity in affected children. It may further be of interest to investigate whether therapy targeting sequencing in other motor systems can influence a central sequencing deficit and thereby support the effectiveness of speech therapy, Utmost care should be taken to follow the mandates of evidence-based practice in designing therapy for children with familial motor-based SSD subtypes. Because the cause-effect relationship between general motor sequencing deficits and motor-based SSD subtypes is not clear, interventions targeting motor sequencing with the goal of ameliorating speech deficits should not be implemented unless evidence for the efficacy of such interventions becomes available.
Acknowledgments
We thank the families whose participation made this study possible. Many thanks to the following undergraduate and graduate students for their assistance with data collection and analysis: Leah Anderson, Lynn Bak, Yayin Chen, Erica Gonzales, Mariya Legesse, Amelie Lehmkühler, Jonathan Mahaffie, David Ramm, and Nancy Yuan. The software for the key tapping task was designed by Elias Peter. We gratefully acknowledge the following funding sources: NIDCD T32DC00033 (B. Peter), American Speech-Language-Hearing Foundation New Century Scholars Research Grant (B. Peter), and R01HD054562 (W.H. Raskind).
Biographies
Beate Peter, Ph.D., CCC-SLP, is Affiliate Instructor in the Department of Speech and Hearing Sciences at the University of Washington in Seattle and also maintains clinical work with children who have communication disorders. She holds a B.S. degree in Speech and Hearing Sciences, a M.S. degree in Speech-Language Pathology, and a Ph.D. degree in Speech and Hearing Sciences, all earned at the University of Washington. Dr. Peter recently completed three years of postdoctoral training in medical genetics under the mentorship of Wendy Raskind, M.D., Ph.D. In addition to the Certificate of Clinical Competence, issued by the American Speech-Language-Hearing Association, she holds the Graduate Certificate in Statistical Genetics, issued by the Department of Biostatistics at the University of Washington.
Wendy Herlihy Raskind, M.D., Ph.D., is Professor of Medicine/Medical Genetics and Psychiatry & Behavioral Sciences and Adjunct Professor of Genome Sciences at the University of Washington, Seattle. She holds a B.A. degree in Mathematics from Brown University. Her MD degree and her Ph.D. degree in Genetics were conferred by the University of Washington. Dr. Raskind has extensive experience identifying genes for mendelian disorders and candidate loci for endophenotypes of complex disorders including dyslexia and autism. She has been involved in research on neurologic and behavioral disorders for more than 19 years.
Continuing Education Questions
Peter, B. & Raskind, W.H. A multigenerational family study of oral and hand motor sequencing ability provides evidence for a familial speech sound disorder subtype
-
According to the literature reviewed in the Introduction, which of the following most accurately describes current thinking regarding genetic influences on speech sound disorder (SSD) prior to the present study?
SSD is a rare disorder that is caused by disruptions of a single gene called FOXP2.
SSD is a common disorder that is caused by many genes acting together.
SSD is a common disorder that is often caused by a disruption on the Y chromosome, explaining higher prevalence rates among males compared to females.
SSD is a rare disorder caused by spontaneous mutations in genes that have not yet been identified, explaining why so many children with SSD do not have a family history of SSD.
- The influence of age on the measures of repetitive and alternating movements can be summarized as follows:
- The fastest repetitive and alternating speeds are seen in children six through 14 years of age.
- The fastest repetitive and alternating speeds are seen in older children and young and middle aged adults.
- The fastest repetitive and alternating speeds are seen in older adults.
- Age does not influence repetitive and alternating speeds appreciably.
- Regarding associations of motor speeds in the oral and hand motor system, the results from this study support the following conclusion:
- The rate limits in the oral motor system are completely independent of the hand motor system, so that short interval durations in one system are not associated with interval durations in the other.
- Repetitive interval durations in one system are not associated with those in the other system, but alternating interval durations are closely associated between motor systems.
- The two systems are tightly coupled, so that similar repetitive and alternating interval durations are generally seen in both systems, except for very young children whose finger tapping intervals were longer than their syllable durations.
- Close associations between syllable durations and finger tapping intervals were seen only in individuals with a history of SSD. In ever unaffected participants, the durations from the two motor systems varied at random.
- The motor-related familial SSD subtype described in this report is best summarized as follows:
- In ever affected children and adults, slowed repetitive, but not alternating, motor speeds were noted predominantly in the oral motor system.
- In ever affected children and adults, slowed repetitive, but not alternating, motor speeds were noted predominantly in the hand motor system.
- In ever affected children and adults, slowed alternating, but not repetitive, motor speeds were noted predominantly in the oral motor system.
- In ever affected children and adults, slowed alternating, but not repetitive, motor speeds were noted predominantly in the hand motor system.
- In their sum, the findings from this report support the following new view of genetic influences on SSD:
- SSD is influenced not so much by genetic factors but rather, by environmental factors such as hearing disordered speech from others.
- SSD follows the common disease/common variant model where the expression of SSD traits does not differ substantially among individual families.
- SSD follows the common disease/rare variant model where distinct disorder subtypes are evident in individual families.
- SSD subtypes cannot be established with a multigenerational design because adults with a history of SSD have compensated their disability.
Solutions
-
According to the literature reviewed in the Introduction, which of the following most accurately describes current thinking regarding genetic influences on speech sound disorder (SSD) prior to the present study?
SSD is a rare disorder that is caused by disruptions of a single gene called FOXP2.
SSD is a common disorder that is caused by many genes acting together.
SSD is a common disorder that is often caused by a disruption on the Y chromosome, explaining higher prevalence rates among males compared to females.
SSD is a rare disorder caused by spontaneous mutations in genes that have not yet been identified, explaining why so many children with SSD do not have a family history of SSD.
- The influence of age on the measures of repetitive and alternating movements can be summarized as follows:
- The fastest repetitive and alternating speeds are seen in children six through 14 years of age.
- The fastest repetitive and alternating speeds are seen in older children and young and middle aged adults.
- The fastest repetitive and alternating speeds are seen in older adults.
- Age does not influence repetitive and alternating speeds appreciably.
- Regarding associations of motor speeds in the oral and hand motor system, the results from this study support the following conclusion:
- The rate limits in the oral motor system are completely independent of the hand motor system, so that short interval durations in one system are not associated with interval durations in the other.
- Repetitive interval durations in one system are not associated with those in the other system, but alternating interval durations are closely associated between motor systems.
- The two systems are tightly coupled, so that similar repetitive and alternating interval durations are generally seen in both systems, except for very young children whose finger tapping intervals were longer than their syllable durations.
- Close associations between syllable durations and finger tapping intervals were seen only in individuals with a history of SSD. In ever unaffected participants, the durations from the two motor systems varied at random.
- The motor-related familial SSD subtype described in this report is best summarized as follows:
- In ever affected children and adults, slowed repetitive, but not alternating, motor speeds were noted predominantly in the oral motor system.
- In ever affected children and adults, slowed repetitive, but not alternating, motor speeds were noted predominantly in the hand motor system.
- In ever affected children and adults, slowed alternating, but not repetitive, motor speeds were noted predominantly in the oral motor system.
- In ever affected children and adults, slowed alternating, but not repetitive, motor speeds were noted predominantly in the hand motor system.
- In their sum, the findings from this report support the following new view of genetic influences on SSD:
- SSD is influenced not so much by genetic factors but rather, by environmental factors such as hearing disordered speech from others.
- SSD follows the common disease/common variant model where the expression of SSD traits does not differ substantially among individual families.
- SSD follows the common disease/rare variant model where distinct disorder subtypes are evident in individual families.
- SSD subtypes cannot be established with a multigenerational design because adults with a history of SSD have compensated their disability.
References
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV. Motor immaturity and specific speech and language impairment: evidence for a common genetic basis. Am J Med Genet. 2002;114(1):56–63. doi: 10.1002/ajmg.1630. [DOI] [PubMed] [Google Scholar]
- Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5(9/10):341–345. [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5(6):e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TF, Dollaghan CA, Rockette HE, Paradise JL, Feldman HM, Shriberg LD, et al. Risk factors for speech delay of unknown origin in 3-year-old children. Child Dev. 2003;74(2):346–357. doi: 10.1111/1467-8624.7402002. [DOI] [PubMed] [Google Scholar]
- Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- Felsenfeld S, Plomin R. Epidemiological and offspring analyses of developmental speech disorders using data from the Colorado Adoption Project. J Speech Lang Hear Res. 1997;40(4):778–791. doi: 10.1044/jslhr.4004.778. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Vargha-Khadem F, Watkins KE, Monaco AP, Pembrey ME. Localisation of a gene implicated in a severe speech and language disorder. Nat Genet. 1998;18(2):168–170. doi: 10.1038/ng0298-168. [DOI] [PubMed] [Google Scholar]
- Fletcher SG. Time-by-count measurement of diadochokinetic syllable rate. J Speech Hear Res. 1972;15(4):763–770. doi: 10.1044/jshr.1504.763. [DOI] [PubMed] [Google Scholar]
- Gray RM, Livingston RB, Marshall RM, Haak RA. Reference group data for the Reitan-Indiana Neuropsychological Test Battery for Young Children. Percept Mot Skills. 2000;91(2):675–682. doi: 10.2466/pms.2000.91.2.675. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Hickman LA. Phonological Disability in Children: Studies in Language Disability and Remediation. Second Edition. New York: Elsevier Publishing Company, Inc; 1997. [Google Scholar]
- Kaufman NR. Kaufman Speech Praxis Test for Children. Detroit: Wayne State University Press; 1995. [Google Scholar]
- Khan L, Lewis N. Khan-Lewis Phonological Analysis. Second Edition. Circle Pines: American Guidance Service; 2002. [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413(6855):519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lewis BA. Pedigree analysis of children with phonology disorders. J Learn Disabil. 1992;25(9):586–597. doi: 10.1177/002221949202500908. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Freebairn LA, Hansen AJ, Miscimarra L, Iyengar SK, Taylor HG. Speech and language skills of parents of children with speech sound disorders. Am J Speech Lang Pathol. 2007;16(2):108–118. doi: 10.1044/1058-0360(2007/015). [DOI] [PubMed] [Google Scholar]
- Lewis BA, Shriberg LD, Freebairn LA, Hansen AJ, Stein CM, Taylor HG, et al. The genetic bases of speech sound disorders: evidence from spoken and written language. J Speech Lang Hear Res. 2006;49(6):1294–1312. doi: 10.1044/1092-4388(2006/093). [DOI] [PubMed] [Google Scholar]
- MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, et al. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76(6):1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J, King MC. Genetic heterogeneity in human disease. Cell. 2010;141(2):210–217. doi: 10.1016/j.cell.2010.03.032. [DOI] [PubMed] [Google Scholar]
- McKinnon DH, McLeod S, Reilly S. The prevalence of stuttering, voice, and speech-sound disorders in primary school students in Australia. Lang Speech Hear Serv Sch. 2007;38(1):5–15. doi: 10.1044/0161-1461(2007/002). [DOI] [PubMed] [Google Scholar]
- Miscimarra L, Stein C, Millard C, Kluge A, Cartier K, Freebairn L, et al. Further evidence of pleiotropy influencing speech and language: analysis of the DYX8 region. Hum Hered. 2007;63(1):47–58. doi: 10.1159/000098727. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Bishop DV. Relations among speech, language, and reading disorders. Annu Rev Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Peter B. New frontiers in understanding speech sound disorder: Unraveling the mysteries of genetic causes. In: Harrison AE, editor. Speech disorders: Causes, treatment and social effect. New York: Nova SciencePublishers; 2010. [Google Scholar]
- Peter B, Matsushita M, Raskind WH. Global processing speed in children with low reading ability and in children and adults with typical reading ability: exploratory factor analytic models. Journal of Speech, Language, and Hearing Research. 2010 doi: 10.1044/1092-4388(2010/10-0135). Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B, Stoel-Gammon C. Central timing deficits in subtypes of primary speech disorders. Clin Linguist Phon. 2008;22(3):171–198. doi: 10.1080/02699200701799825. [DOI] [PubMed] [Google Scholar]
- Peterson RL, McGrath LM, Smith SD, Pennington BF. Neuropsychology and genetics of speech, language, and literacy disorders. Pediatr Clin North Am. 2007;54(3):543–561. vii. doi: 10.1016/j.pcl.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Potter NL, Lazarus JA, Johnson JM, Steiner RD, Shriberg LD. Correlates of language impairment in children with galactosaemia. J Inherit Metab Dis. 2008;31(4):524–532. doi: 10.1007/s10545-008-0877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigatano GP, Gray JA, Legacy J. Predictors of quantitative and qualitative Halstead finger-tapping scores in low socioeconomic status school-age children. Child Neuropsychol. 2008;14(3):263–276. doi: 10.1080/09297040701399288. [DOI] [PubMed] [Google Scholar]
- Robbins J, Klee T. Clinical assessment of oropharyngeal motor development in young children. J Speech Hear Disord. 1987;52(3):271–277. doi: 10.1044/jshd.5203.271. [DOI] [PubMed] [Google Scholar]
- Secord W, Donohue J. Clinical Assessment of Articulation and Phonology. Greenville: Super Duper Publications; 2002. [Google Scholar]
- Shriberg LD, Potter NL, Strand EA. Prevalence and Phenotype of Childhood Apraxia of Speech In Youth with Galactosemia. J Speech Lang Hear Res. 2010 doi: 10.1044/1092-4388(2010/10-0068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Tomblin JB, McSweeny JL. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J Speech Lang Hear Res. 1999;42(6):1461–1481. doi: 10.1044/jslhr.4206.1461. [DOI] [PubMed] [Google Scholar]
- Smith SD, Pennington BF, Boada R, Shriberg LD. Linkage of speech sound disorder to reading disability loci. J Child Psychol Psychiatry. 2005;46(10):1057–1066. doi: 10.1111/j.1469-7610.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- Stein CM, Lu Q, Elston RC, Freebairn LA, Hansen AJ, Shriberg LD, et al. Heritability Estimation for Speech-Sound Traits with Developmental Trajectories. Behav Genet. 2010 doi: 10.1007/s10519-010-9378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CM, Millard C, Kluge A, Miscimarra LE, Cartier KC, Freebairn LA, et al. Speech sound disorder influenced by a locus in 15q14 region. Behav Genet. 2006;36(6):858–868. doi: 10.1007/s10519-006-9090-7. [DOI] [PubMed] [Google Scholar]
- Stein CM, Schick JH, Gerry Taylor H, Shriberg LD, Millard C, Kundtz-Kluge A, et al. Pleiotropic effects of a chromosome 3 locus on speech-sound disorder and reading. Am J Hum Genet. 2004;74(2):283–297. doi: 10.1086/381562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terband H, Maassen B, van Lieshout P, Nijland L. Stability and composition of functional synergies for speech movements in children with developmental speech disorders. J Commun Disord. 2011;44(1):59–74. doi: 10.1016/j.jcomdis.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Thoonen G, Maassen B, Gabreels F, Schreuder R. Validity of maximum performance tasks to diagnose motor speech disorders in children. Clinical Linguistics & Phonetics. 1999;13(1):1–23. [Google Scholar]
- Thoonen G, Maassen B, Gabreels F, Schreuder R, de Swart B. Towards a standardised assessment procedure for developmental apraxia of speech. Eur J Disord Commun. 1997;32(1):37–60. doi: 10.3109/13682829709021455. [DOI] [PubMed] [Google Scholar]
- Thoonen G, Maassen B, Wit J, Gabreels F, Schreuder R. The integrated use of maximum performance tasks in differential diagnostic evaluations among children with motor speech disorders. Clinical Linguistics & Phonetics. 1996;10(4):311–336. [Google Scholar]
- Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci U S A. 1995;92(3):930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, Vargha-Khadem F. Behavioural analysis of an inherited speech and language disorder: comparison with acquired aphasia. Brain. 2002;125(Pt 3):452–464. doi: 10.1093/brain/awf058. [DOI] [PubMed] [Google Scholar]
- Wijsman EM, Amos CI. Genetic analysis of simulated oligogenic traits in nuclear and extended pedigrees: summary of GAW10 contributions. Genet Epidemiol. 1997;14(6):719–735. doi: 10.1002/(SICI)1098-2272(1997)14:6<719::AID-GEPI28>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]


