SUMMARY
Purpose
In planning epilepsy surgery, it is important to be able to assess the likelihood of success of surgery for each patient so that the possible risk and benefit can be properly considered. In this study, functional connectivity was investigated as a means for predicting surgical outcome from the preoperative functional magnetic resonance (fMRI) imaging of epilepsy patients.
Methods
Resting-state simultaneous EEG-fMRI data was collected from eighteen intractable epilepsy patients before surgery and from fourteen healthy subjects. For each patient, EEG-spike correlated fMRI analysis were performed and an activation cluster that overlapped the most with the planned resection area for each patient was chosen as the seed for the functional connectivity analysis. After the functional connectivity maps were computed, laterality indices of functional connectivity were contrasted between patients who had seizures after surgeries (seizure-recurrence group) and those who did not have them for at least a year (seizure-free group).
Key Findings
Patients in the seizure-recurrence group had less lateralized functional connectivity than patients in the seizure-free group (t16=2.3, after control subtracted and Fisher transformed, p < 0.05 two-tailed).
Significance
This study suggests the potential for using preoperative fMRI connectivity analysis as a predictive outcome measure. If confirmed by further research, a high laterality will be an important addition to the other predictors of better surgical outcome such as febrile seizures, mesial temporal sclerosis, tumors, abnormal MRI, and EEG/MRI concordance.
Keywords: EEG–fMRI, Focal epilepsy, Functional Connectivity, Functional Neuroimaging
Introduction
Patients with intractable epilepsy are often treated with surgery. Surgical treatments have a high chance of improving epilepsy symptoms. For instance, it has been reported that 60–80% of medial temporal epilepsy patients have cessation of seizures as a result of a variety of surgical approaches applied to different portions of the temporal lobe structures (Primrose 1992, Engel 1993, Spencer 1996). However, epilepsy surgeries are not without risks including post-surgical complications, deficits in cognitive abilities and psychosis (Spencer 1996). Thus, it is important to assess the probability of successful surgery for each patient so that the possible benefit and risk can be properly considered.
One of the factors that effect the surgical outcome may be involvement of multiple brain regions in the epileptogenic process. A good example of this are patients with multifocal seizures, where the seizures appear to originate at different locations in the brain with different episodes or have a broad regional onset to their seizures. As an example the involvement of a large network of brain areas in epilepsy, Gotman et al. (2005) found that spike-and-wave epileptic discharges in generalized epilepsy patients were correlated with activation in the thalamo-cortical network and suppression in the default-mode network. Another example is that poor surgical outcome is observed in patients with bilateral brain abnormalities due to diffuse brain pathology or developmental anomalies. Some have suggested that a network of brain areas, not just a single onset zone, may be responsible for the onset and maintenance of seizures (Spencer 2002). If multiple regions are involved, surgery in one brain region may not lead to a cure of a subject’s epilepsy.
In the early 1990’s, a method of analyzing functional magnetic resonance imaging (fMRI) data to identify networks of brain activities called functional connectivity analysis has been developed. Functional connectivity analysis is based on the finding that regions in the brain that are functionally connected appear to have significant low frequency correlations in the temporal response of their blood oxygenation level dependent (BOLD) fMRI signals (Bandettini et al. 1993, Biswal et al. 1995, Cordes et al. 2000). Functional connectivity is usually computed by selecting one area of the brain as a “seed” and computing the correlation between the fMRI timecourse of the seed and that of other areas or voxels of the brain. Such analyses have revealed that there are consistent intrinsic networks that are stable even under anesthesia (Vincent et al. 2007; Martuzzi et al. 2010) and that the strength of specific circuits can reflect behavioral capabilities (Hampson 2004, Hampson et al. 2006). Functional connectivity in epilepsy patients has been investigated using fMRI data acquired during rest (Pereila et al. 2010, Bettus et al. 2010, Morgan et al. 2010, Zhang et al. 2010, Liao et al. 2010) and during language tasks (Waltes et al. 2006, Voets et al. 2009).
OBJECTIVE AND RATIONALE
To our knowledge there have been no studies published that have investigated the relationship between functional connectivity obtained from resting-state fMRI data and the outcome of epilepsy surgery. In this work, we test the hypothesis that the more bilateral the extent of the network that encompasses the region to be targeted for surgical resection, the worse the surgical outcome. This hypothesis is based on the assumption that a network with strong bilateral functional connectivity that includes the seizure generating region may be less affected by surgical intervention than a unilateral, more localized network.
METHODS
In the following work the fMRI data was obtained prior to either intracranial EEG studies or surgical resective procedures. Two kinds of seeds were used for computing the functional connectivity: (1) seeds selected from significant clusters of spike-correlated fMRI activation (Lemieux et al. 2001, Baudewig et al. 2001, Bénar et al. 2002), where the selection among multiple clusters was based on the written description of the planned resection, and (2) seeds derived from the difference between the post- and pre-surgical anatomical MRI images. Thus the first part of this study that uses the spike-MRI seeded functional connectivity assesses the predictive value of the pre-operative imaging data, whereas the second part of this study that uses the resection area seeded functional connectivity combines pre-operative with post-operative imaging data (the exact area of resection) to assess the predictive power of the pre-operative imaging data in determining outcome.
Data acquisition
Patients who were consecutively referred and underwent simultaneous EEG-fMRI recording from October 2004 to May 2008 that later underwent epilepsy surgery were selected for the analysis. Only patients with intractable epilepsy who had frequent interictal spikes (more than 10 spikes per hour) were referred for the simultaneous EEG-fMRI. The patient sample consisted of 18 epilepsy patients, 9 males, 7–55 years of age (mean 30.2 years). Clinical information of the patients is summarized in Table 1 and demonstrates that this group includes patients with a wide range of seizure type and suspected epileptogenic location. Simultaneous EEG-fMRI data were also collected from 14 healthy subjects (ages 22–34 years, mean 26.1 years, 7 males). The control population was not specifically selected for the current study, and thus although they were gender matched (50%) they were not age matched precisely (the average ages were 4 years different). All subjects gave written informed consent and the study protocol was approved by the Human Investigation Committee of the Yale School of Medicine (New Haven, CT).
Table 1.
Clinical and laterality-related information
| Pa- tient |
Clinical information | Laterality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of epilepsy | Intericta l spikes |
Pathology | Resection | Seizure -free |
Spike MR localization |
Selected Spike MR seed |
Seed size (voxel)* |
Lateral -ity** |
|
| 1 | AU,SP,CP,GTC | R T | R T glioma | R AMTL, LatT, Ins | No | R AMTL | R AMTL | 41 | 0.20 |
| 2 | AU,SP,CP,GTC | R F,T | R H sclerosis | R AMTL | No | R Ins, L F P | R Ins | 54 | −0.02 |
| 3 | SP | R T | R T cavenous hematoma | R FT | No | R Ins | Med F | 22 | −0.20 |
| 4 | AU,SP,CP,GTC | L F,T | L subdural membrane | L ST, MidT, IF, IP | No | R Ins | L Ins | 22 | −0.40 |
| 5 | AU,CP,GTC | L T | L H sclerosis | L AMTL | No | L F, O | L F | 13 | 0.23 |
| 6 | AU,CP | R F,T,P,O | R F hetero-topia | R F | No | R F, L F | R F | 24 | 0.22 |
| 7 | CP, GTC | L F, R F | R F inflamation | R F | No | R ST | R ST | 31 | −0.16 |
| 8 | CP, GTC | L F, P | Unclear | L F,P | No | L O, IP | L IP | 36 | −0.08 |
| 9 | CP, GTC | L F, P | Unclear | L PMTL, O | No | L IF | L IF | 21 | −0.21 |
| 10 | AU,CP | L T | Unclear | L T,P | No | L F, ST | L ST | 31 | 0.30 |
| 11 | CP | L F,T | L H sclerosis | L AMTL | Yes | L PH, Ins | L PH | 85 | 0.70 |
| 12 | AU,CP,GTC | L F,T | L H sclerosis | L AMTL | Yes | L PH, R Th | L PH | 12 | 0.24 |
| 13 | AB, CP | L T,P | Unclear | L LatT | Yes | L MidT | L MidT | 76 | 0.81 |
| 14 | AU,CP | L F,T | Unclear | L IF | Yes | L MidT, F | L MidT | 68 | 0.55 |
| 15 | AU,SP | L O | L cortical dysplasia | L MedO | Yes | L Cing, O | L O | 12 | 0.17 |
| 16 | AU,SP | R T | R Amy dysplasia | R AMTL | Yes | R F, TO | R F | 28 | 0.01 |
| 17 | SP, CP | L F, R F | LF cortical dysplasia | L F | Yes | R Ins, F, L F | L F | 24 | 0.26 |
| 18 | SP, CP | R T | R H sclerosis | R AMTL | Yes | R LatT | R LatT | 25 | 0.68 |
AU: Aura, SP: Simple Partial Seizure, CP: Complex Partial Seizure, GTC: Generalized Tonic Clonic Seizure, AB: Absence (during childhood) L: Left, R: Right, AMTL: Anteromedial Temporal Lobe, PMTL: Posterior Medial Temporal Lobe, F: Frontal, T: Temporal, P: Parietal, O: Occipital, Lat: Lateral, Med: Medial, S: Superior, Mid: Middle, I: Inferior, H: Hippocampus, Amy: Amygdala, Ins: Insula, h: Thalamus, Cing: Cingulate,
voxel size is 3.4 × 3.4 × 5 [mm]
Control subtracted
Each patient had four to eight six-minute resting-state EEG-fMRI runs during which blood oxygenation level dependent (BOLD) based images were collected using a 3T scanner (Siemens Trio, Siemens Medical, Erlangen, Germany). The fMRI data was acquired with an echo-planar sequence with the following parameters: Repetition Time (TR)=1.55 sec, Echo Time (TE)=30 msec, Flip Angle (FA)= 80 degrees, 25 slices 6mm thick with no gap, Field of View (FOV)=220mm, 64×64 acquisition matrix. In addition to the fMRI images, an anatomical image that had the same dimensions as the fMRI images was acquired using an axial T1 weighted flash sequence (TR=300 msec, TE=2.47 msec, Flip Angle=60 degrees, 256×256 matrix), and a high resolution anatomical image was also acquired using a sagittal MP-RAGE sequence (TR=1.5 sec., TE=2.83 msec., Inversion Time=800 msec., 160 slices, 256×256 matrix) from each subject for image registration purposes.
The EEG was recorded using two systems. The EEG data from patients 1 to 16 were recorded using an old EEG system that consisted of a modified commercial EEG cap (40 channel QuickCap, CompuMedics, Charlotte, NC, USA) with a linked-ear reference, an in-house made 125 Hz low-pass Butterworth filter for reducing the scanner noise (Negishi et al. 2004), and a commercial EEG recorder (NuAmps, Compumedics) which recorded the signal at 500 Hz sampling rate. The EEG data from patients 17 and 18 were recorded using a new EEG system that consisted of an in-house made carbon wire electrode cap with a bipolar montage, a in-house made pre-amplifier with anti-polarization circuit (Negishi et al. 2008), and a commercial EEG recorder (SynAmps2, Compumedics) which recorded the signal at 1000 Hz sampling rate.
Data processing
Step1: Preprocessing
The scanner noise and cardiac artifacts in the EEG signals was removed offline using in-house software (Negishi et al. 2004, Negishi et al. 2008) and the EEG signal was band-pass filtered at 1 to 50 Hz. The fMRI data was slice-timing corrected and motion-corrected using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/).
Step 2: Spike correlated fMRI analysis
Interictal spikes were read from the EEG either by a neurologist (board certified in clinical neurophysiology) or by a biomedical engineer experienced in reading interictal spikes from the EEG data recorded simultaneously with the fMRI. The spike correlated fMRI analysis was performed using a general linear modeling of the fMRI data, with the interictal spike timings as the event regressors. The general linear modeling was performed using SPM2.
Step 3: Functional connectivity analysis
For the spike correlated fMRI seeded functional connectivity analysis, clusters with more than 10 significantly activated voxels were selected for each patient as the candidate seed regions for the functional connectivity analysis. These clusters were compared with the planned resection area for the patient using a computer program and the cluster that maximally overlapped the planned resection area was selected as the seed for computing functional connectivity. The planned resection area used for this analysis for each patient was defined verbally (e.g. left medial temporal lobe) in the surgery database, which was converted into a map in the MNI space using the Yale Brodmann atlas that was built into the Bioimage Suite (http://www.bioimagesuite.org/). The default voxel threshold was determined as follows so that the seed size (see Table 1) was of the same order of magnitude across patients. The threshold was initially set at the t value corresponding to p=0.01 (False Discovery Rate adjusted), and was doubled if the cluster size was more than 100 voxels, and was halved if the cluster size was less than 10 voxels. This adjustment was repeated until the voxel size fell between 10 and 100. Functional connectivity was computed using BioImage Suite. No efforts were made to remove the effect of respiration and heart beat except for a 0.1 Hz low-pass filtering, a slice mean signal removal, and a third order polynomial drift removal of the fMRI data. See the discussion section for possible effects of the cardiac and respiratory artifacts.
The resection area seeded connectivity analysis was performed using the actual resected areas as the seeds. For this analysis, a difference image between preoperative and postoperative anatomical MRI’s were obtained in BioImage suite and used as the seed for further functional connectivity analysis. Since postoperative MRI’s were available for only nine out of eighteen patients (patients 1, 2, 3, 4, 6, 11, 12, 15, and 18 in Table 1), only these patients’ data were subject to the resection area seeded functional connectivity analysis.
The seeds chosen for each patient (one from spike correlated fMRI analysis and one from the resection area when available) were co-registered to an MNI standard brain and used to compute the functional connectivity in the control subjects. The functional connectivity maps for the control subjects that were computed from the patient-specific seed were then averaged across control subjects after converting the correlation values to z-scores, and the resultant map was used to compute the laterality of the functional connectivity in the controls for the given seed. The functional connectivity maps were converted to statistical maps by taking the Fisher transform (Fisher 1915) and fitting the histogram to a normal distribution (Lowe et al. 1998). A Fisher transform was used because correlation values are bound between −1 and 1, and thus intrinsically have non-Gaussian distributions.
Step 4. Laterality analysis
A laterality index (analogous to that used in fMRI language lateralization, Arora et al. 2009) was defined as the normalized difference between the functional connectivity value in the ipsilateral hemisphere and that of the contralateral hemisphere.
| (1) |
In this formula, L is the laterality index, HI is the number of voxels that had significant functional connectivity (p<0.05) on the same side as the resection, and HC is the number of voxels that had significant functional connectivity (p<0.05) in the side contralateral to the resection.
Denoting the laterality index for a patient as Lpatient and the laterality index for the control subjects that was computed from the patient’s seed as Lcontrol, the control-subtracted laterality index Lc, was computed as follows.
| (2) |
Because the control-subtracted laterality values only range from −1 to 1, they were Fisher transformed before being subjected to statistical tests in the same way the functional connectivity values were transformed. The hypothesis tested was that patients with unsuccessful outcome (seizure-recurrence group) have more bilateral functional connectivity and thus exhibit significantly lower laterality indices compared to patients with successful outcome (seizure-free group). Successful outcome was defined as seizure free with or without auras at the latest follow-up that took place at least one year after the surgery, which satisfies the ILAE Outcome Criteria 1 or 2 (Wieser et al. 2001) and Engel Category 1A or 1B (Engel et al. 1993). Statistical tests on laterality indices were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA.
RESULTS
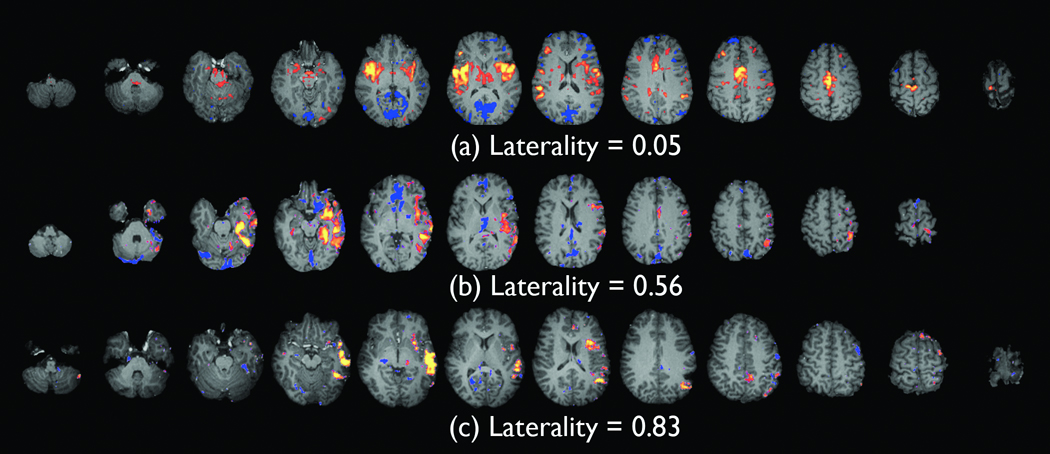
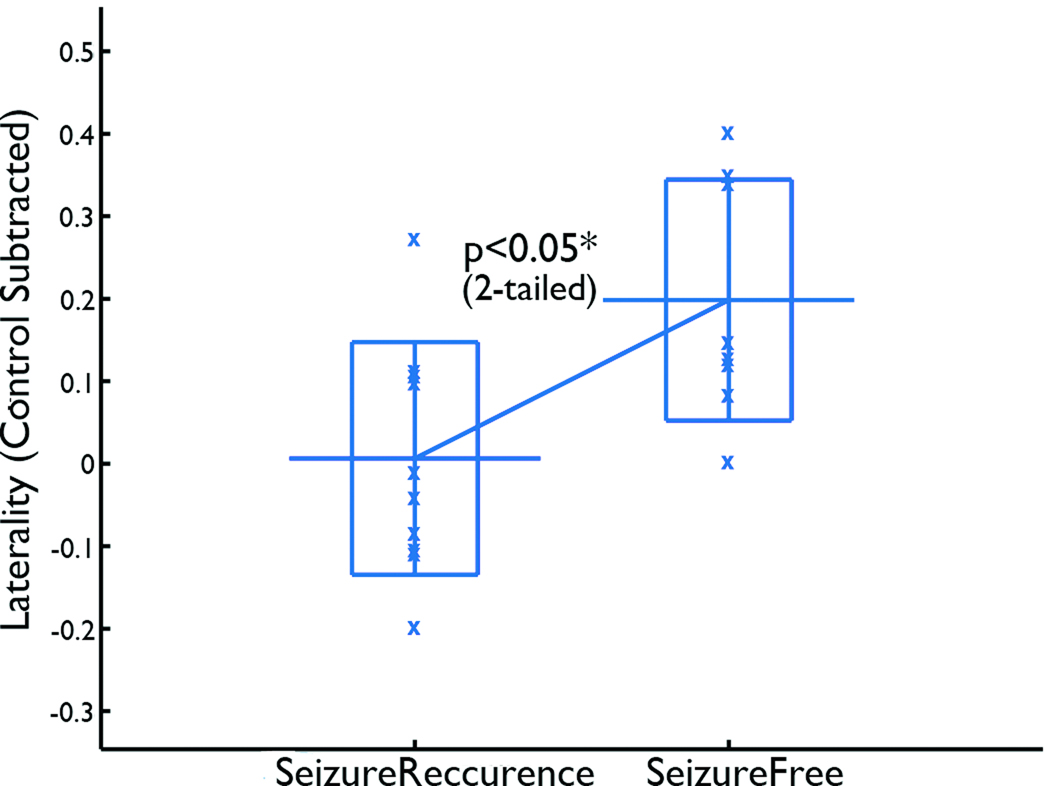
Fig. 1 shows examples of spike correlated fMRI seeded functional connectivity maps with low, medium, and high laterality indices before laterality of the control subjects was subtracted. Note that the functional connectivity map in Fig. 1 (b) includes some negative connectivity medially, and since computation of the laterality index does not distinguish negative and positive functional connectivity, the map (b) has a lower laterality index than the map (c). Fig. 2 shows the laterality indices (control subtracted) of the seizure-recurrence and seizure-free groups. After the Fisher transform, the laterality indices of the seizure-recurrence group were significantly lower than those of the seizure-free group (t16=2.3, p < 0.05, two-tailed. Two-tailed tests were employed for t-tests throughout this paper following the guidance given in Bland and Altman 1994).
Figure 1.
Examples of spike correlated fMRI-seeded functional connectivity maps with low (a), medium (b), and high (c) laterality indices. The laterality values displayed are before laterality values of the controls are subtracted. The distinct lateralization of the functional connectivity can be clearly recognized in (b) and (c).
Figure 2.
Control-subtracted laterality index of the spike correlated fMRI seeded functional connectivity. Patients in the non-seizure-free group had significantly lower laterality indices than patients in the seizure-free group. The long horizontal bar shows the average, the rectangle show the range between the average plus and minus the standard deviation, and the “x” shows a data point.
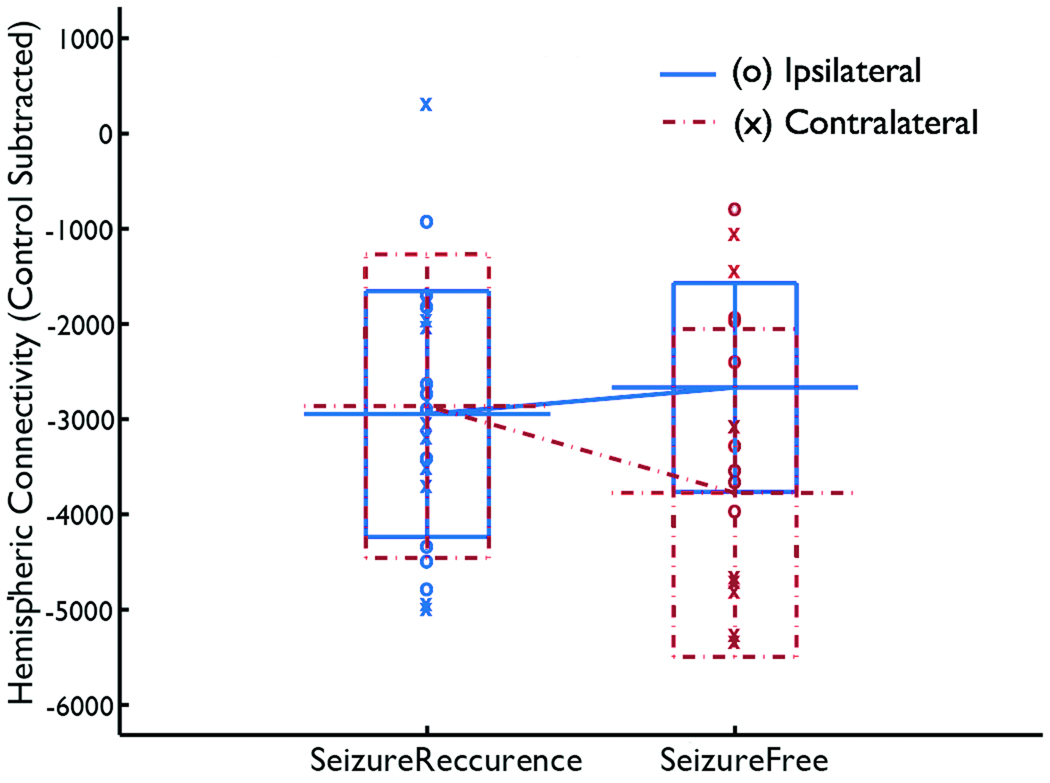
Because a decrease in the laterality index can be caused by decreased functional connectivity in the affected hemisphere (HI) or increased functional connectivity in the contralateral hemisphere (HC) or both, it is natural to ask whether the change in one of these alone can be a predictor of the surgical outcome. This question was addressed by a logistic regression analysis, using control-subtracted ipsilateral connectivity and control-subtracted contralateral connectivity as covariates and testing whether these two variables can predict the surgical outcome. Logistic regression analysis tests whether the output probability is modeled as a logistic function of the weighted sum of covariates, where the significance of the model is evaluated by the chi-squared test and the effect of each variable being included in the model can be evaluated using the Wald statistic. The result (Fig. 3) showed that the two variables together were not significantly predictive of the outcome (, p>0.05), suggesting that the laterality index was more predictive than using ipsilateral and contralateral connectivity linearly combined, possibly because of the normalization used in the computation of the laterality index (denominator in equation (1)). Neither control-subtracted contralateral connectivity (Wald static = 3.17, df=1, 0.05<p<0.1) nor control-subtracted ipsilateral connectivity (Wald static = 2.56, df=1, 0.1<p) was predictive of the outcome, although the former had a higher significance.
Figure 3.
Ipsilateral (blue solid lines) and contralateral functional connectivity (red dashed lines) used as covariates for predicting the surgical outcome. Neither control-subtracted contralateral connectivity nor control-subtracted ipsilateral connectivity alone was predictive of the outcome.
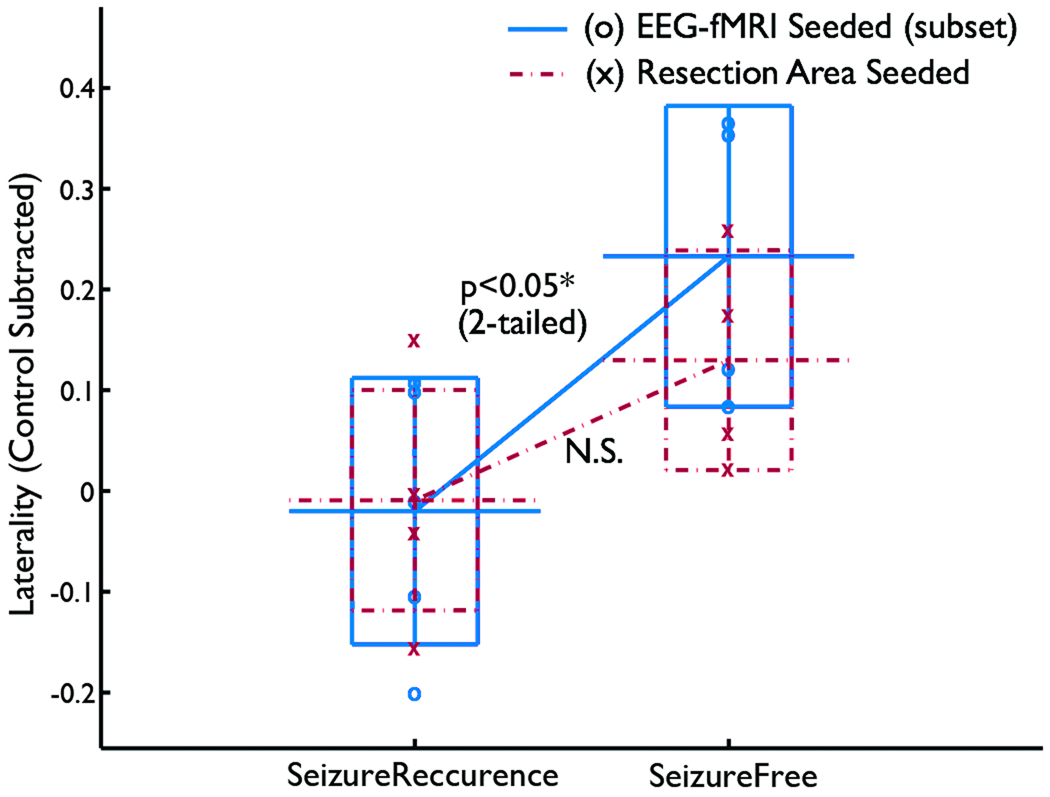
The laterality of resection area seeded functional connectivity was not significantly smaller in the seizure-recurrence group than in the seizure-free group (Fig. 4) (t7=1.90, p>0.05, two-tailed). However, because this was based on smaller number of patients (5 in the seizure-recurrence group and 4 in the seizure-free group), the result cannot be directly compared with the result from the analysis based on spike correlated fMRI above. When the spike correlated fMRI seeded functional connectivity analysis was applied on the same set of patients, there was still a difference in the laterality between the seizure-recurrence and seizure-free groups (t7=2.70, p<0.05 two-tailed). To compare the two seeding methods, a repeated measure of variance test was performed with the outcome as a between-subject factor and the analysis method (resection area seeded vs. spike correlated fMRI seeded) as a within subject factor. The result showed a significant effect of the outcome (F=8.71, df=1, p<0.05) but non-significant interaction (F=0.014, df=1, p>0.05), indicating no significant difference between the two seeding methods. In summary, the control subtracted laterality of the resection area seeded functional connectivity was not significantly different between the seizure-recurrence and seizure-free groups in this particular set of patients, but a larger study is necessary to compare the significance of resection area seeded functional connectivity analysis with that of the spike correlated fMRI seeded functional connectivity analysis.
Figure 4.
Comparison between the resection area-seeded functional connectivity analysis and the spike correlated fMRI seeded functional connectivity analysis based on 9 patients. The resection area-seeded functional connectivity analysis did not show a significant difference of laterality between seizure-recurrence and seizure-free groups, whereas the spike correlated fMRI seeded connectivity did. However, the lack of interaction indicates that there was not a significant difference between two seeding methods.
DISCUSSION
Effects of physiological noise on the estimation of functional connectivity
As mentioned in the methods section, no efforts were made to remove the effect of respiration and heartbeat. It has been known that physiological noise reduces the specificity in functional connectivity (Lowe et al. 1998). In the current case, the physiological noise should impact both the seizure-recurrence and seizure-free groups alike and thus should not bias the comparison between the two groups. Also, the effect of reduced spatial specificity is minimal, since the analysis is based only on laterality of the connectivity. However, existence of physiological noise may increase the noise in the functional connectivity and thus weaken the validity of the statistical inference based on the laterality index.
Comparison with other research on the functional connectivity of epilepsy patients
Pereila et al. (2010) found impaired ipsilateral connectivity in their study on medial TLE (tempoal lobe epilepsy) patients with hippocampal sclerosis. Using normalized correlations between pairs of epilepsy related regions of interests (five regions of interests in each side), Bettus et al. (2010) found decreased correlation in most medial temporal lobe epilepsy patients bilaterally, with more decrease in the ipsilateral side than in the contralateral side. Increased correlation was found only on the contralateral lobe in some patients. They found the existence of increased correlation on the opposite side to be a strong indicator of lateralization. These results are consistent with our findings, in that they identified impaired ipsilateral functional connectivity and increased contralateral functional connectivity as correlates of epilepsy.
Morgan et al. (2010) investigated the anterior hippocampal region in left TLE patients and found increased negative functional connectivity in the network including thalamic, brainstem, frontal, and parietal regions. Zhang et al. (2010) investigated the resting state fMRI data from medial TLE patients using Independent Component Analysis (ICA) and found decreased functional connectivity in dorsal mesial prefrontal cortex, mesial temporal lobe, and inferior temporal cortex in both left medial TLE and right medial TLE patients. They also found increased functional connectivity in the posterior cingulate cortex in right medial TLE but not in left medial TLE patients. Liao et al. (2010) have shown changes in functional connectivity in bilateral hipocampal screlosis patients. They found increased functional connectivity within the medial temporal lobes and decreased functional connectivity within the frontal and parietal lobes, as well as between frontal and parietal lobes in patients relative to healthy controls. These results cannot be directly compared with the results presented in the current paper, since our method does not distinguish negative and positive correlations and also does not distinguish connectivity in different regions in the brain beyond hemispheric differences.
Some researchers investigated language related functional connectivity in epilepsy patients. Voets et al. (2009) analyzed fMRI acquired during complex sentence encoding using tensor ICA method, and found decreased connectivity between bilateral medial temporal lobes in left medial TLE patients. Waltes et al. (2006) compared the language network between epilepsy patients and controls, using regions activated by language tasks as the seed. They found disrupted functional connectivity between the seed area and other language areas in the left temporal lobe epilepsy patients. These results can not be compared directly with our results, since our results does not use language related fMRI data, but it is of an interest that they reported decreased connectivity in epilepsy patients. In the future, it would be interesting to see whether connectivity in the contralateral area is increased in language task related fMRI data and in resting fMRI data.
Functional connectivity as an indicator of the surgical outcome
The results of the current study showed that the laterality index computed from the EEG correlated fMRI seeded functional connectivity was lower in the seizure-recurrence group than in the seizure-free group. A review of past literatures (Tonini et al. 2004) found febrile seizures, mesial temporal sclerosis, tumors, abnormal MRI, EEG/MRI concordance, and extensive surgical resection to be the strongest predictor of attaining seizure-free outcomes. If confirmed by further research, the laterality of functional connectivity will be a valuable addition to the predictors of surgical outcome.
It may be argued that EEG correlated fMRI activities, being based on interictal epileptic discharges rather than seizure onset zones, are not adequate as the seeds for functional connectivity analysis. In fact, during the review of intracranial recording from the patients in the current study it was observed that electrodes that continuously show interictal activities are often not the ones that show earliest changes at the seizure onsets (not shown). However, scalp EEG data often correctly localizes the seizure onset hemisphere in temporal lobe epilepsy patients (Blume et al. 2001). We feel that the use of the interictal spike correlated seeds is justifiable since we are trying to identify functional connectivity networks in the resting-state fMRI data, rather than to directly localize a seizure onset zone.
Note that for one patient (patient number 4 in Table 1), the EEG-fMRI activation cluster with the highest significance was located contralateral to the side of the resection and the laterality index was negative. However, the spike correlated fMRI seed was chosen on the ipsilateral side of the surgery because it overlapped with the resection area most. The patient did not become seizure free after surgery. It could be argued that inclusion of this patient unfairly lowers the average laterality index in the seizure-recurrence group in favor of our hypothesis. However, we argue that the fact that the spike correlated fMRI was found on the opposite site of the clinical consensus indicates the bilateral nature of the seizure related brain activities, and thus is consistent with the fact that the patient did not become seizure-free. Moreover, the hypothesis was confirmed even when this patient was excluded (F=2.54, df=15, p<0.05 two-tailed).
The result based on a subset of patients who had postoperative MRI’s showed that there was no significant difference in the laterality of the resection area seeded functional connectivity between the seizure-recurrence group and the seizure-free group. However, there may be an effect of different seed sizes between the two seeding methods, as the EEG-fMRI based seeds had smaller area sizes (mean 33.0 voxels, std. (standard deviation) 23.7 voxels, voxelsize = 3.4×3.4×5 mm) compared to resection area based seeds (mean 283 voxels, std. 245 voxels). Larger seed areas could result in weaker functional connectivities, since the timecourse of a large seed is an average of timecourses of many voxels that may include voxels outside the affected area, or voxels whose timecourses are not phase-locked to each other. However, since larger seed areas result in lower functional connectivity in both ipsilateral and contralateral hemispheres, the laterality index may not be less sensitive to the area size than the actual functional connectivity measures.
The use of the resected area as a seed may have a disadvantage in that these are structurally determined seeds. The use of spike correlated fMRI localization enables one to define a functionally determined seed that may well be part of a network related to interictal but nevertheless epilepsy related activity. By contrast, if the resection area is used as the seed, the resultant functional connectivity may not capture enough of the variance in the fMRI data that is directly related to epilepsy.
On the other hand, it should be noted that the information on the surgical area affects the resection area seeded analysis directly, which can be an advantage for the resection area seeded analysis. For instance, the size of the planned resection, which is a significant predictor of the outcome (Tonini et al. 2004), will change the size of the seed in the resection area seeded analysis, whereas it does not change the size of the seed of the spike correlated fMRI seeded analysis. Moreover, from a surgical planning point of view, it would be interesting to investigate different resection strategies and estimate the impact of resection on the network connectivity using the planned resection area as the seed. Thus the resection area seeded correlation analysis merits a further, larger scale study.
Acknowledgment
We would like to thank Sunkyung Yu, Xiangyu Cong, and James Dizura for advice on statistics. This research was supported by NIH NS047605, EB009666, and EB000473.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Constable RT. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowitz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Mugn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Bittermann HJ, Paulus W, Frahm J. Simultaneous EEG and Functional MRI of epileptic activity: a case report. Clin Neurophysiol. 2001;112:1196–1200. doi: 10.1016/s1388-2457(01)00562-4. [DOI] [PubMed] [Google Scholar]
- Bénar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J. The BOLD response to interictal epileptiform discharges. Neuroimage. 2002;17:1182–1192. doi: 10.1006/nimg.2002.1164. [DOI] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2010;81(10):1147–1154. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughhton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar imaging. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bland LM, Altman DG. One and two sided tests of significance. BMJ. 1994;309:248. doi: 10.1136/bmj.309.6949.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume WT, Holloway GM, Wiebe S. Temporal epileptogenesis: localizing value of scalp and subdural interictal and ictal EEG data. Epilepsia. 2001;42(4):508–514. doi: 10.1046/j.1528-1157.2001.02700.x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand E. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993. pp. 609–622. [Google Scholar]
- Fisher RA. Frequency distribution of the values of the correlation coefficient in samples of an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Olson IR, Leung H-C, Skudlarski P, Gore JC. Changes in functional connectivity of human MT/v5 with visual motion input. Neuroreport. 2004;15(8):1315–1319. doi: 10.1097/01.wnr.0000129997.95055.15. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Josephs O, Allen P, Toms N, Scott C, Krakow K, Turner R, Fish DR. Event-related fMRI with simultaneous and continuous EEG: description of the method and initial case report. Neuroimage. 2001;14:780–787. doi: 10.1006/nimg.2001.0853. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. PloS One. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal E, Ellingson ML, Spanaki MV, Spencer SS. Long-Term Outcome After Epilepsy Surgery. Epilepsia. 1996;37(9):807–808. doi: 10.1111/j.1528-1157.1996.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting state fluctuation. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49(1):823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;1323:152–160. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Nixon T, Constable RT. Removal of time-varying gradient artifacts from EEG data acquired during continuous fMRI. Clin Neurophysiol. 2004;115:2181–2192. doi: 10.1016/j.clinph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Laufer I, Nixon T, Constable RT. An EEG recording system with carbon wire electrodes for simultaneous EEG-fMRI recording. J Neurosci Methods. 2008;173:99–107. doi: 10.1016/j.jneumeth.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose DC, Ojemann GA. Outcome of resective surgery for temporal lobe epilepsy. In: Lüders H, editor. Epilepsy Surgery. New York: Raven Press; 1991. pp. 601–611. [Google Scholar]
- Spencer SS. Long-Term Outcome After Epilepsy Surgery. Epilepsia. 1996;37(9):807–813. doi: 10.1111/j.1528-1157.1996.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, Tetto A, Vitelli E, Vitezic D, Wiebe S. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62(1):75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adock JE, Stacey R, Hart Y, Carpenter K, Matthews PM, Beckman CF. Functional and structural changes in the memory network associated with left temporal lobe epilepsy. Hum Brain Mapp. 2009;30(12):4070–4081. doi: 10.1002/hbm.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59(2):335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wiezer HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, Sperling MR, Lüders H. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, Wang Z, Li K, Chen H, Liu Y. Altered spontaneous neuronal activity of the default-mode network in mesial temporal lobe epilepsy. Brain Res. 2010;1323:152–160. doi: 10.1016/j.brainres.2010.01.042. [DOI] [PubMed] [Google Scholar]