Abstract
Purpose
To determine whether a new model of cryptogenic infantile spasms consisting of prenatal priming with betamethasone and postnatal trigger of spasms by N-methyl-D-aspartic acid responds to chronic ACTH treatment, and has similar EEG signature, efficacy of treatments, and behavioral impairments as human infantile spasms.
Methods
Rats prenatally primed with betamethasone on gestational day 15 were used. Spasms were triggered with N-methyl-D-aspartic acid between postnatal days (P) 10-15 in a single session or in multiple sessions in one subject. The expression of spasms was compared to prenatally saline-injected controls. Effects of relevant treatments (ACTH, vigabatrin, methylprednisolone, rapamycin) were determined in betamethasone-primed rats. In the rats after spasms, behavioral evaluation was performed in the open field and and elevated plus maze on P20-22.
Key Findings
NMDA at P10-15 (the rat “infant” period) triggers the spasms significantly earlier and in greater numbers in the prenatal betamethasone-exposed brain compared to controls. Similar to human condition, the spasms occur in clusters. Repeated trigger of spasms is associated with ictal EEG electrodecrements and interictal large-amplitude waves, a possible rat variant of hypsarrhythmia. Chronic ACTH treatment in a randomized experiment, and chronic pretreatment with methylprednisolone significantly suppress number of spasms similar to human condition. Pretreatment with vigabatrin, but not rapamycin, suppressed the spasms. Significant behavioral changes occurred following multiple bouts of spasms.
Significance
The model of infantile spasms has remarkable similarities with the human condition in semiology, EEG, pharmacological response, and long-term outcome. Thus, the model can be used for search of novel and more effective treatments for infantile spasms.
Keywords: spasms, clustering, ACTH, methylprednisolone, vigabatrin, EEG, behavior
Introduction
Infantile spasms (West syndrome; epileptic spasms) (Berg, et al. 2010, Dulac 1994), a devastating epileptic syndrome of childhood, occur most frequently between 3 to 12 months of age (peaking at 6 months of age) and consist of the triad of age-specific spastic seizures, interictal diffuse EEG abnormalities (hypsarrhythmia), and mental retardation (Dulac 1994). Further characterization involves interictal EEG electrodecrement and a certain therapeutic response to adrenocorticotropin (ACTH) or vigabatrin (Lux, et al. 2004, Lux, et al. 2005, Mackay, et al. 2004). Despite the treatment many patients continue with epilepsy and suffer from mental retardation or severe side effects of the treatment (Dulac, et al. 2002). There are two etiology-based subgroups: (1) Symptomatic infantile spasms are associated with diagnosed brain pathology and relatively poor prognosis, while (2) the cryptogenic/idiopathic group involves suspected but yet unknown brain impairment and better prognosis if therapy is early and sufficient (Berg, et al. 2010, Riikonen 2001).
To aid the progress of new therapies of infantile spasms, we have recently developed a new animal model of cryptogenic infantile spasms consisting of prenatal priming with synthetic corticosteroid betamethasone and postnatal trigger of spasms during infant period with N-methyl-D-aspartic acid, NMDA (Velíšek, et al. 2010, Velíšek, et al. 2007). The model has been tested and corresponds to some previous requirements (Stafstrom 2009, Stafstrom, et al. 2006). Specifically, the spasms are restricted to early postnatal developmental period and a response to a relevant treatment (ACTH) has been confirmed (Kábová, et al. 1999, Stafstrom & Sasaki-Adams 2003, Velíšek, et al. 2007). However, a comprehensive validation is required for more precise definition of this new tool and its use in drug discovery (Stafstrom 2009, Stafstrom & Holmes 2002, Stafstrom, et al. 2006).
Here, we determined the occurrence of spasms through the rat “infant period” from postnatal day 10 (P10) through P15 including repeated EEG/videomonitoring. We hypothesized that the spasms in the model could be progressively triggered throughout this developmental stage and that the EEG would bear strong similarities to human EEG, namely ictal electrodecrement and interictal large-amplitude waves. We also hypothesized that the spasms would cluster similarly to infantile spasms. We also tested the efficacy of two FDA-approved drugs for treatment of infantile spasms, ACTH and vigabatrin (Mackay, et al. 2004, Zupanc 2003), In addition we investigated methylprednisolone treatment, as well as the effects of rapamycin.
Methods
Animals and priming with prenatal betamethasone
Experiments have been approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine and conform to the Revised Guide for the Care and Use of Laboratory Animals [NIH GUIDE, 25(28), 1996]. All efforts have been made to minimize pain and number of animals while keeping sufficient statistical power. Timed pregnant Sprague-Dawley rats were purchased from Taconic Farms (Germantown, NY). Pregnant females were injected on gestational day 15 (G15) with two doses of betamethasone (0.4 mg/kg i.p. at 08:00 and 18:00) or vehicle (normal saline) (Velíšek 2006, Velíšek, et al. 2010, Velíšek, et al. 2007). Animals were kept in the AAALAC-approved facility on 12-hour dark/light cycle with the lights on at 07:00. Offspring were used in the experiments described below (see also Table 1). On average, 4-5 different litters were used in any given experimental subgroup. It should be also emphasized that no more than two pups from a single litter were used within the same experimental subgroup.
Table 1. Distribution of experimental groups, prenatal exposures, and postnatal pretreatments.
For P10 rats 5 mg/kg of NMDA was used, 7.5 mg/kg of NMDA for P12 rats, 12 mg/kg of NMDA for P13 rats, and 15 mg/kg of NMDA for P15 rats were used. NMDA was administered intraperitoneally always in the volume of 10 ml/kg of saline.
| Experiment | Group identification | Numbers of rats |
Prenatal exposure; gestational day |
Postnatal (AED) pretreatment, postnatal day |
Postnatal trigger of spasms, postnatal day |
|
|---|---|---|---|---|---|---|
| Clustering of spasms (Figure 1) |
P10 | saline | n=7 | saline G15 | none | NMDA P10 |
| betamethasone | n=9 | betamethasone G15 | none | NMDA P10 | ||
|
| ||||||
| P15 | saline | n=6 | saline G15 | none | NMDA P15 | |
| betamethasone | n=5 | betamethasone G15 | none | NMDA P15 | ||
|
| ||||||
| Repeated trigger of spasms (Figure 2) |
saline | n=7 | saline G15 | none | NMDA P12, P13, P15 | |
| betamethasone | n=9 | betamethasone G15 | none | NMDA P12, P13, P15 | ||
|
| ||||||
| Randomized ACTH trial (Figure 3) |
saline | n=11 | betamethasone G15 | saline 2× P12, 3× P13, 3× P14 | NMDA P12, P13, P15 | |
| ACTH | n=11 | betamethasone G15 | ACTH 0.3 mg/kg, 2× P12, 3x P13, 3× P14 |
NMDA P12, P13, P15 | ||
|
| ||||||
| Pharmacology of the model (Figure 4) |
saline | n=4 | betamethasone G15 | saline P14 | NMDA P15 | |
| GVG 100 | n=7 | betamethasone G15 | vigabatrin 100 mg/kg P14 | NMDA P15 | ||
| GVG 250 | n=8 | betamethasone G15 | vigabatrin 250 mg/kg P14 | NMDA P15 | ||
|
| ||||||
| saline | n=16 | betamethasone G15 | saline P15 | NMDA P15 | ||
| MPD 5 | n=10 | betamethasone G15 | methylprednisolone 5 mg/kg P15 |
NMDA P15 | ||
| MPD 20 | n=8 | betamethasone G15 | methylprednisolone 20 mg/kg P15 |
NMDA P15 | ||
| MPD 60 | n=7 | betamethasone G15 | methylprednisolone 60 mg/kg P15 |
NMDA P15 | ||
|
| ||||||
| saline P13-P15 | n=6 | betamethasone G15 | saline P13, P14, P15 | NMDA P15 | ||
| MPD P13-P15 | n=9 | betamethasone G15 | methylprednisolone 60 mg/kg P13, P14, P15 |
NMDA P15 | ||
|
| ||||||
| vehicle 24 hours | n=9 | betamethasone G15 | 1% alcohol P14 | NMDA P15 | ||
| rapamycin 24 hours | n=10 | betamethasone G15 | rapamycin 3 mg/kg P14 | NMDA P15 | ||
|
| ||||||
| vehicle P7-P14 | n=4 | betamethasone G15 | 1% alcohol daily P7-P14 | NMDA P15 | ||
| rapamycin P7-P14 | n=9 | betamethasone G15 | rapamycin 3 mg/kg daily P7 -P14 |
NMDA P15 | ||
|
| ||||||
| Behavioral outcome (Figure 5) |
saline – no spasms | n=11 | betamethasone G15 | none | saline P15 | |
| P15 spasms | n=11 | betamethasone G15 | none | NMDA P15 | ||
| serial spasms | n=6 | betamethasone G15 | none | NMDA P12, P13, P15 | ||
|
| ||||||
| saline-3×3 | n=9 | betamethasone G15 | saline 3× P12, 3× P13, 3× P14 | NMDA P15 | ||
| ACTH-3×3 | n=10 | betamethasone G15 | ACTH 0.3 mg/kg, 3× P12, 3× P13, 3× P14 |
NMDA P15 | ||
|
| ||||||
| saline ×3 | n=4 | betamethasone G15 | saline P13, P14, P15 | NMDA P15 | ||
| MPD 60 ×3 | n=3 | betamethasone G15 | methylprednisolone 60 mg/kg P13, P14, P15 |
NMDA P15 | ||
Triggering of flexion spasms
On P10 or on P15, the offspring prenatally primed with betamethasone or exposed to saline were injected with a single i.p. NMDA injection. For some experiments, NMDA was administered repeatedly on P12, P13, and P15. The NMDA dose was 5.0 mg/kg in P10 rats, 7.5 mg/kg in P12 rats, 12 mg/kg in P13 rats, and 15 mg/kg for P15 rats, based on pilot experiments and our previous publications (Velíšek, et al. 2010, Velíšek, et al. 2007). Immediately after the NMDA injection, the rats were observed for 90 minutes because our pilot experiments demonstrated that the spasms would completely disappear within this time frame. Latency to onset of spasms was recorded. The number of complete spasms was counted to indicate severity of spasms. In some experiments in P10 and P15 rats, we recorded a time for each spasm for illustration of the temporal clustering of spasms.
EEG recordings
Two days prior to onset of EEG recording, we performed surgical implantation of the electrodes under combined ketamine/xylazine anesthesia (50/7 mg/kg i.p.). One screw serving as a reference electrode was placed in the nasal bone, another screw was positioned above the cerebellum to provide electrical ground. Four silver ball electrodes were inserted epidurally on the frontal and occipital cortex bilaterally. Electrodes and screws were covered with dental acrylic. After baseline EEG recordings, rats were challenged with NMDA and EEG continuously recorded until the spasms disappeared. Simultaneous and synchronized video was also captured to allow for evaluation of EEG and behavioral correlates using Pinnacle Solutions, Inc. (Kansas City, MO) 3-channel EEG/video system. Some rats were subjected to repeated EEG recordings.
Drugs tested against spasms
All rats in pharmacology experiments were prenatally exposed to betamethasone and challenged appropriate to the age i.p. doses of NMDA (see above). Drug effects were compared versus respective vehicle. The following drugs were studied:
(1) Randomized chronic ACTH trial
ACTH (full molecule of rat synthetic 39 amino acid-containing hormone, GenScript, Piscataway, NJ) was used in a prospective randomized model: In the morning of P12, the rats received injection of NMDA as described above and the latency to onset and number of spasms were recorded. After the bout of spasms diminished, the rats were randomly distributed into two groups: One started receiving ACTH (0.3 mg/kg of ACTH s.c. at 14:00 and 21:00 hours on P12 and at 7:00, 14:00 and 21:00 hours on P13 and P14). This regimen was derived from human studies, in which (a) the treatment starts after the spasms occur, and (b) median time to ACTH effects is two days (Baram, et al. 1996). With an approximate equivalent of 150-200 IU per 1 mg of ACTH, the rats were receiving 135-180 IU per kg of body weight per day. The second group was injected with vehicle at the same times. On the morning of P15, all rats were challenged with NMDA 15 mg/kg i.p.
(2) Pretreatment
Methylprednisolone hemisuccinate sodium (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline and injected 60 minutes prior to the induction of spasms on P15 in doses of 5, 20, or 60 mg/kg i.p. in the acute administration paradigm. Additionally, we used a long-term paradigm consisting of a single daily dose of 60 mg/kg administered i.p. at 10:00 on P13, P14 and P15, with the last dose one hour prior to triggering of spasms.
Vigabatrin (100 or 250 mg/kg i.p.) was administered in a single dose 24 hours (on P14) prior to the NMDA challenge (on P15) based on the results of our previous study (Velíšek, et al. 1995) as well as a randomized study showing dose-dependent effects of vigabatrin on spasm reduction (Elterman, et al. 2010, Kubová & Mareš 2010).
Rapamycin (LC Laboratories, Woburn, MA), a novel drug in the infantile spasm armamentarium, with anecdotal efficacy against infantile spasms associated with tuberous sclerosis (Muncy, et al. 2009) was used at 3 mg/kg either 24 hours prior induction of spasms (P14) or in a single daily dose of 3 mg/kg from P7-P14. These paradigms were derived from rapamycin blockade of mTORC1 (24 hour pretreatment) as well as mTORC2 (P7-P14 treatment) (Sarbassov, et al. 2006). Rapamycin was dissolved in 1% alcohol. Controls received vehicle (1% alcohol) at the same times. It should be emphasized that both 24 hour and P7-P14 rapamycine pretreatments were biologically active because rapamycin-treated rats in both groups showed significant decreases in weight gains compared to controls (not shown), consistent with the role of rapamycin in the control of food intake (Mori, et al. 2009).
Behavioral testing
Behavioral tests were performed between P20-22. Each day after the behavioral testing, pups were returned to their mother. Behavioral tests were performed blinded to experimental conditions. In the behavioral tests, we compared performance after postnatal NMDA-induced spasms of prenatally betamethasone-exposed rats to saline-injected controls.
Elevated plus maze (anxiety test) (Pellow, et al. 1985, Pellow & File 1986)
Standard elevated plus maze from Columbus Instruments was used as described previously (Velíšek 2006). Latency to enter the last third of the closed arm was measured [referred to as transfer latency; TL; (Velíšek 2006)]. For TL, we chose to use the entry into the last third of a closed arm to skew the measurement towards the indication of anxiety in contrast to simple exploratory activity. Additionally, we recorded the time spent in the closed arm and number of entries into closed arms within the 5 min test session.
Elevated plus maze (memory retention test)
Repeated testing serves as a measure of memory retention (Velíšek 2006). Transfer latency was determined repeatedly on three consecutive days (P20-22). Thus, a memory component should play a role in the repeated tests. Changes in transfer latency over this three-day period were expressed in the Retention Index (RI (Kábová, et al. 1999)). In this study, the RI was calculated as RI= log(TLP20)-log(TLP22). The RI value indicated whether the performance improved over time (RI>0), whether the performance decreased (RI<0) or whether there was no change in performance (RI=0).
Open field activity
Several parameters were determined in an automated open field (Med-Associates, St. Albans, VT, USA) within each 5 min session time (Velíšek 2006). Distance and time traveled (ambulation), time spent in stereotypies, resting, and rearing were calculated for the central part of the open field as well as the total values. Additionally, we determined total number of entries into the central zone (zone crossings) and the relative time spent in the central zone.
Statistics
For every statistical comparison, we first tested (using a two-way ANOVA with factors of prenatal exposure and sex) for sex differences. Because we never found the effect of sex (p always >0.18), male and female pups were combined and the data were re-analyzed. Student’s t-test was used for two-group comparisons. Multiple groups were evaluated with ANOVA or repeated measures (RM)-ANOVA, if the same subjects were investigated repeatedly. If the treatments were completely suppressing spasms in some subjects, we used nonparametric Kaplan-Meier survival statistics with a censored variable and Mantel-Cox logrank test. It should be emphasized that complete absence of all NMDA-induced symptoms may have indicated failure in injecting NMDA: Those cases were excluded from the survival analysis. Only those rats that displayed initial NMDA symptoms at appropriate time points, but did not develop spasms, were included in the survival analysis. Finally, for incidence comparisons, we used Chi-square (multiple groups) or Fisher’s Exact (two groups) tests. Level of significance was preset to p<0.05. However, if post-hoc tests were used, p was adjusted for multiple comparisons. Figures show means ± S.EM.s.
Results
Spasms triggered by NMDA during infant rat period in prenatally compromised brain occur in clusters, earlier and more frequently than in the non-compromised brain
Purpose of this experiment was to determine whether the spasms in the model can be triggered throughout the rat “infancy” (Avishai-Eliner, et al. 2002, Gottlieb, et al. 1977, Velíšek & Moshé 2002) and whether they cluster similar to humans. Although infantile spasms in humans occur most frequently between 6-12 months of age, in some cases they may occur as early as at 2 months of age (Dulac, et al. 2002) more closely corresponding to P10 in rats. Nevertheless, because majority of infantile spasms in humans develops between 6-12 month of age (Dulac, et al. 2002) our additional pharmacology studies were performed in P15 rats, which is developmentally closer to human condition (Avishai-Eliner, et al. 2002).
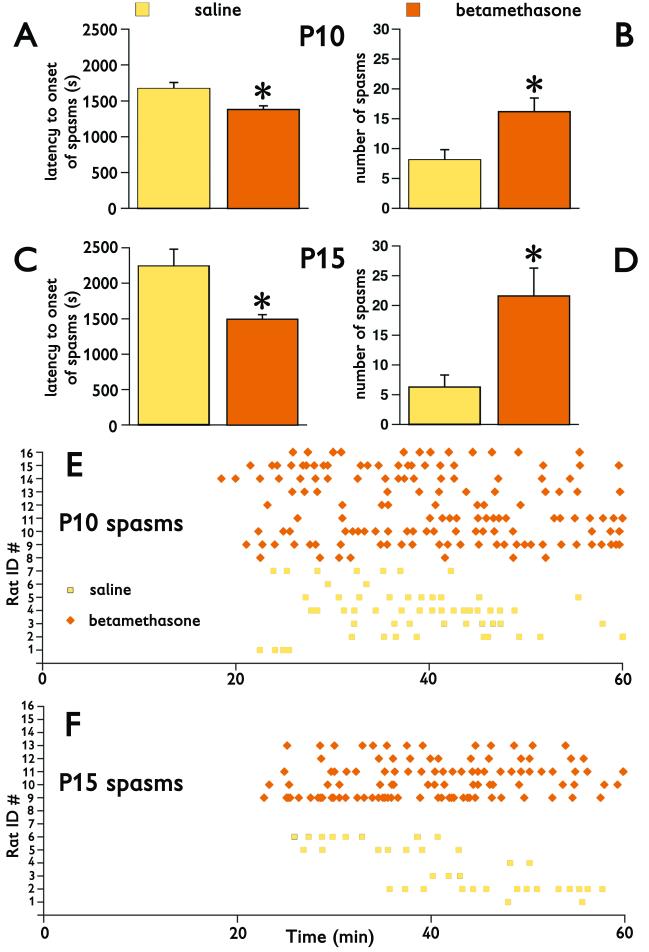
In P10 rats after prenatal betamethasone exposure, the spasms developed earlier (F(1,12)=10.583; p=0.007; Figure 1A) and occurred more frequently (F(1,12)=7.218; p=0.020; Figure 1B) compared to pups prenatally exposed to saline. In P15 rats, we newly found that the spasms also occurred more frequently in betamethasone-exposed rats than in controls (F(1,7)=9.743; p=0.017; Figure 1D). We also reproduced our previous findings (Velíšek, et al. 2007) that after prenatal betamethasone priming the spasms in P15 rats develop earlier (F(1,7)=26.503; p=0.0013; Figure 1C) compared to controls. Graphic representation of the timing of the spasms illustrates that at both P10 and P15, the spasms cluster and prenatal betamethasone exposure remarkably enhances this clustering (Figures 1E, 1F). Clustering of spasms in the model is very similar to clustering of infantile spasms in children.
Figure 1. Prenatal betamethasone exposure accelerates the occurrence of NMDA-triggered spasms, makes the spasms more frequent and more clustered.
(A) Latency to onset of spasms in P10 rats after prenatal betamethasone exposure (n=9) is significantly decreased compared to saline exposed controls (n=7; *p<0.05).
(B) Similarly, the number of spasms in P10 rats significantly increased after prenatal betamethasone exposure compared to saline controls (*p<0.05).
(C) In P15 rats, prenatal betamethasone exposure (n=5) also significantly accelerated onset of NMDA-triggered spasms compared to saline controls (n=6; *p<0.05).
(D) The number of spasms in prenatally betamethasone-exposed P15 rats also significantly increased compared to saline controls (*p<0.05).
(E) Time distribution of spasms in individual P10 rats illustrates that after prenatal betamethasone, the spasms occur earlier, they are more frequent, and cluster more than in saline controls. Raw data for (A) and (B) are shown.
(F) Time distribution of spasms in P15 rats convincingly shows earlier onset, larger numbers, and more clustering after prenatal betamethasone exposure compare to saline controls. Raw data for (C) and (D) are shown.
Prenatal betamethasone exposure eliminates attenuation of spasms with repeated NMDA exposure observed in controls
Purpose of this experiment was to determine the outcome of repeatedly triggered spasms in P12-P15 rats. Recurrent EEG/videomonitoring was used to capture possible occurrence of spontaneous seizures.
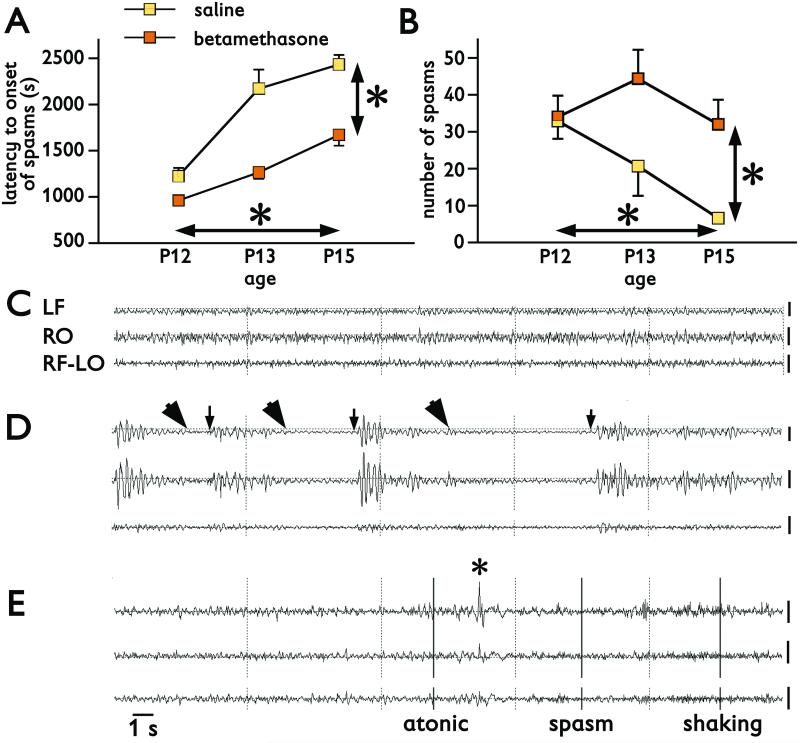
Repeated measures ANOVA was used to compare differences in latency to onset and number of spasms in the same subjects on P12, P13, and P15. Prenatal betamethasone exposure (n=9) significantly accelerated onset of spasms at all three ages compared to prenatally saline-exposed controls (n=7, RM-ANOVA F(1,14)=42.256; *p<0.0001; Figure 2A vertical difference). There were also effects of repeated trigger of spasms with age on the latency to onset of spasms (RM-ANOVA F(2,28)=41.731, *p<0.0001; Figure 2A horizontal difference). Similarly, prenatal exposure to betamethasone (n=9) significantly increased number of spasms during repeated sessions on P12, P13, and P15 compared to controls (n=7; RM-ANOVA F(1,14)=5.659; *p=0.032; Figure 2B). Repeated triggering of spasms with age also affected their number (RM-ANOVA F(2,28)=5.075; *p=0.013; Figure 2B).
Figure 2. Spasms triggered by repeatedly injected NMDA on P12, P13, and P15 and their EEG correlates.
(A) Latency to onset of spasms triggered by repeated NMDA in P12 (7.5 mg/kg), P13 (12 mg/kg), and P15 (15 mg/kg) rats is significantly shorter in prenatally betamethasone-exposed rats compared to saline controls (vertical *p<0.05). Latency to onset of spasms significantly increases with age (vertical *p<0.05).
(B) Similarly, the number of spasms is significantly increased in prenatally betamethasone-exposed rats compared to saline controls (vertical *p<0.05). There was also a significant decrease in number of spasms with age (horizontal *p<0.05).
(C) EEG recordings in a P13 rat prior to NMDA administration (baseline). LF, RO – left frontal and right occipital channel in monopolar montage versus common reference in the nasal bone. RF-LO – a bipolar montage combined from the right frontal and left occipital channel. Background consists of low-amplitude fast activity.
(D) EEG recording in the same P13 rat after the spasms were triggered with 12 mg/kg of NMDA i.p.. Arrowheads mark onsets of spasms, arrows indicate the end of the spasms. Each of the three spasms was associated with a significant decrease in the EEG amplitude, an electrodecrement. Between the spasms, large-amplitude irregular waves occurred, a possible rat correlate to hypsarrhythmia.
(E) EEG recordings in another P13 rat prior to the second NMDA trigger. This rat exhibited and atonic/astatic episode, with the beginning marked in the EEG with the first bar and ending at the asterisk. Then a spasm occurred associated with a decrement in EEG amplitude followed by long-lasting head shaking (vertical bars at the onset and the legend).
Calibration 200 μV at the end of each trace. Time mark 1 s for all recordings.
In comparison to baseline EEG recordings prior to the NMDA trigger (Figure 2C), EEG recordings during spasms were characterized by significant amplitude suppression, i.e., electrodecrement (Figure 2D; arrowheads). Slow irregular waves with large amplitude (a possible rat variant of “hypsarrhythmia”; Figure 2D) appeared in the periods between the spasms. In one rat on P13 (prior to the second NMDA administration) we recorded a clear atonic/astatic episode followed by a brief flexion spasm and head tremor with minimal cortical EEG correlates (Figure 2E). Another rat on P14 (a day without NMDA trigger) displayed a clear motor clonic seizure terminated with a wet dog shake, again without any clear EEG correlate (not illustrated). However, we cannot exclude that these seizures were evoked by persisting circulating NMDA from the previous day trigger.
Randomized long-term ACTH trial suppresses spasms and decreases mortality after repeatedly triggered spasms
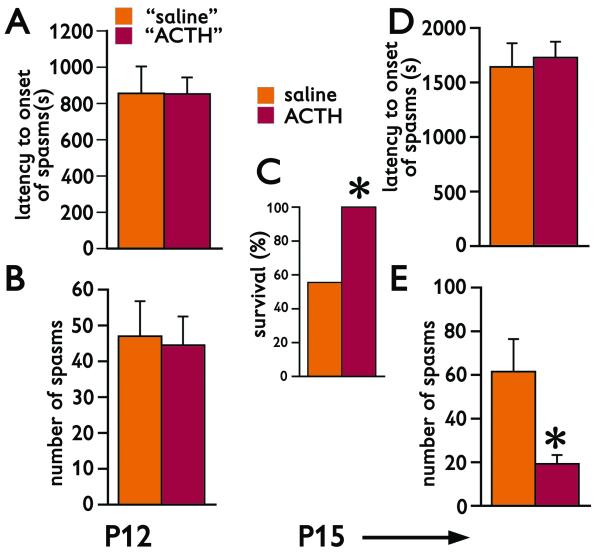
Purpose of this experiment was to investigate the effects of long-term ACTH therapy administered after the first bout of repeatedly triggered spasms. Total of 26 rats prenatally-exposed to betamethasone were used on P12. The rats received NMDA (7.5 mg/kg) and spasms were recorded. Out of those 26 rats 4 died during the spasms or immediately after the bout of spasms. Remaining 22 rats were randomly distributed into two groups [see Figure 3A for latency to onset of spasms (Student’s t-test; p=0.984) and Figure 3B for number of spasms (Student’s t-test; p=0.849) in the randomized groups]. After the first bout of spasms, the group marked “ACTH” started a treatment with ACTH, while the other group was injected with vehicle instead of ACTH. On P13 morning, another bout of spasms was induced between early morning and afternoon ACTH treatments. ACTH treatment continued until P14 evening. On P15 morning, we triggered the spasms for the last time. Thus, the rats received NMDA on P12, P13, and P15 (Table 1). The first interesting observation was the effect of ACTH treatment on the survival following the repeated NMDA-induced spasms. All 11 rats treated with ACTH survived till the end of the experiment, while only 6 out of 11 rats injected with vehicle survived (Figure 3C; Fisher’s Exact test; *p=0.035). While there was no difference in the latency to onset of the spasms after the trigger on P15 (Figure 3D; Student’s t-test; p=0.7422), long-term treatment with ACTH significantly suppressed number of spasms compared to vehicle (Figure 3E; Student’s t=test; *p=0.003), an effect similar to human condition.
Figure 3. Randomized ACTH significantly suppresses spasms and decreases lethal outcome.
(A) Latency to onset of spasms in the first bout of spasms triggered on P12 by 7.5 mg/kg of NMDA i.p. “Saline” and “ACTH” represents the P12 data (mean±S.E.M.) of rats randomized to the saline or ACTH treatments, respectively. Randomized treatment started immediately after the spasms of the first bout disappeared and continued through evening of P14. Second bout of spasms was induced between the treatments on P13. There was no triggering of spasms on P14.
(B) Number of spasms in the first bout of spasms triggered on P12 by 7.5 mg/kg of NMDA i.p. Details as above.
(C) Survival rate of rats through P15 (prior to entering the P15 trigger of spasms). Saline and ACTH indicate the data of rats receiving saline or ACTH, respectively. ACTH-treated rats had 100% survival, a significant increase compared to 56% in the saline group (Fisher’s Exact test, *p=0.035).
(D) Latency to onset of spasms in the bout triggered on P15 was not affected by chronic ACTH treatment.
(E) Chronic ACTH treatment significantly decreased number of spasms in the P15 bout compared to saline controls (Student’s t-test; *p=0.003).
Pretreatments with vigabatrin and chronic, but not acute, methylprednisolone also suppress spasms in the model
Purpose of these experiments was validation of the model using pretreatments with additional drugs used for treatment of infantile spasms. NMDA in these experiments was administered only on P15 (Table 1).
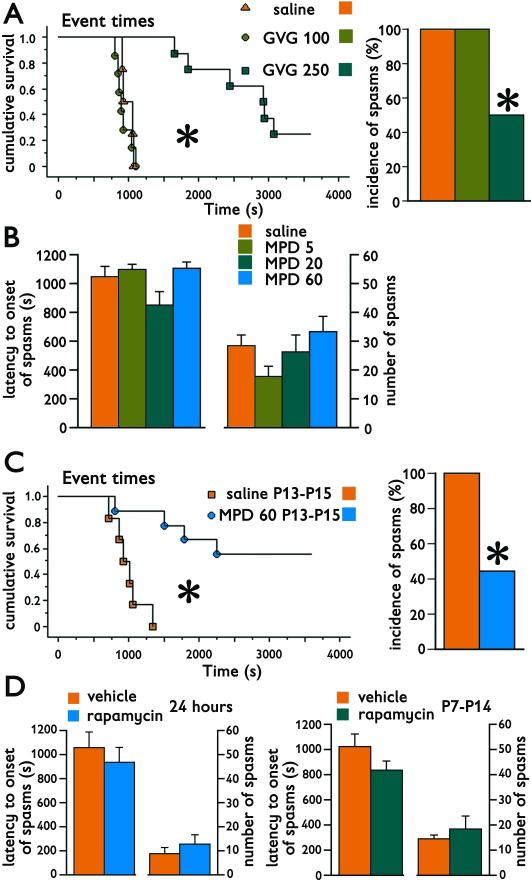
Vigabatrin (GVG) in the dose 100 mg/kg administered on P14, i.e., 24 hours prior to the NMDA trigger, did not induce any sedative effects and was not effective against the spasms. A dose of 250 mg/kg, while inducing partial sedation, significantly delayed the latency to onset of spasms (Kaplan-Meier survival statistics with Mantel-Cox logrank test; Chi square=19.421; *p<0.0001; Figure 4A, left) and significantly decreased the incidence of spasms (Chi-square=6.967; *p=0.031; Figure 4A, right). After GVG (250 mg/kg; n=8) only 50% of subjects developed spasms versus 100% in both the control (n=4) and the 100 mg/kg GVG group (n=7).
Figure 4. Pharmacologic validation of the model of cryptogenic infantile spasms using pretreatments prior to NMDA trigger of spasms.
(A) Vigabatrin (GVG) administration at 24 hours prior to trigger of spasms was evaluated using a non-parametric survival statistics because spasms were suppressed in some subjects completely. Indeed, the 250 mg/kg dose of vigabatrin significantly delayed onset of spasms (left; cumulative survival; Mantel-Cox logrank test, *p<0.0001). This dose of vigabatrin also significantly suppressed incidence of spams (in %; right; Chi-square test; *p=0.031).
(B) Methyprednisolone (MPD) in doses 5 (n=10); 20 (n=8); or 60 mg/kg (n=7) administered 60 min prior to the trigger of spasms did not have any effect on either latency to onset of spasms (left) or number of spasms in individual subjects (right) in comparison to controls (n=16).
(C) Methylprednisolone administered in three injections in a single daily dose of 60 mg/kg on P13, P14, and P15 (n=9) significantly delayed onset of spasms (left; cumulative survival; Mantel-Cox logrank test; *p=0.0008) compared to vehicle controls (n=6). Indeed, this chronic methylprednisolone treatment also decreased incidence of spasms (in %; right; Fisher’s Exact test; *p=0.044).
(D) Rapamycin administered in a single dose of 3 mg/kg i.p. 24 hours prior to trigger of spasms did not affect either latency to onset or number of spasms (left). Eight days of rapamycin administration (single 3 mg/kg dose i.p. daily P7-P14) also did not affect latency to onset or number of spasms (right). However, in both treatment paradigms rapamycin significantly decreased weight gain (not shown).
Methylprednisolone administered acutely in doses 5-60 mg/kg one hour prior to the trigger of spasms did not affect either latency to onset of spasms (ANOVA, F(3,37)=2.238; p=0.100) or number of spasms (F(3,37)=1.825; p=0.160; Figure 4B). On the other hand, a chronic treatment with 60 mg/kg of methylprednisolone daily on P13-P15 (n=9) significantly delayed the onset of spasms (Kaplan-Meier survival statistics with Mantel-Cox logrank test; Chi-square=11.210; *p=0.0008; Figure 4C, left) compared to vehicle-injected controls (n=6). In addition, some methylprednisolone-treated rats did not develop the spasms at all, i.e., the treatment also significantly decreased incidence of spasms occurring now in only 4 out of 9 subjects after treatment compared to 100% in controls (Fisher’s Exact test *p=0.044; Figure 4C, right).
Finally, we used rapamycin, a macrolid from Streptomyces hygroscopicus, an immunosuppressive drug currently used in transplantation medicine as well as for treatment of neoplasms. Rapamycin has been tested as a novel treatment of infantile spasms, especially those associated with tuberous sclerosis (Muncy, et al. 2009) and may have antiepileptogenic features (Pitkanen & Lukasiuk 2011). Rapamycin administered in a single dose of 3 mg/kg i.p. (n=10) 24 hours prior to trigger of spasms (on P14; Table 1) did not affect latency to onset of spasms (Student’s t-test; p=0.504) or number of spasms (p=0.458) compared to vehicle-injected controls (n=9; Figure 4D, left). Similarly, rapamycin administered in eight doses of 3 mg/kg between P7-P14 (n=9) did not affect either latency to onset (Student’s t-test; p=0.176) or number of spasms (p=0.631) compared to vehicle-injected controls (n=4; Figure 4D, right). The same dose of rapamycin was effective against the spasms in a model of symptomatic infantile spasms (Scantlebury, et al. 2010). Additionally, our treatment was biologically active as rapamycine both after a single dose (Student’s t-test, p=0.0008) and chronic P7-14 administration (p<0.0001) significantly decreased weight gain compared to matched controls injected with vehicle (data not shown).
Behavior
Purpose of this experiment was investigating the effects of single and repeated episodes of spasms on behaviors as well as the effects of long-term ACTH or methylprednisolone treatments on behavior after spasms. Only prenatally betamethasone-exposed rats were used because only in these rats, the ACTH treatment can be effective to control spasms (Table 1).
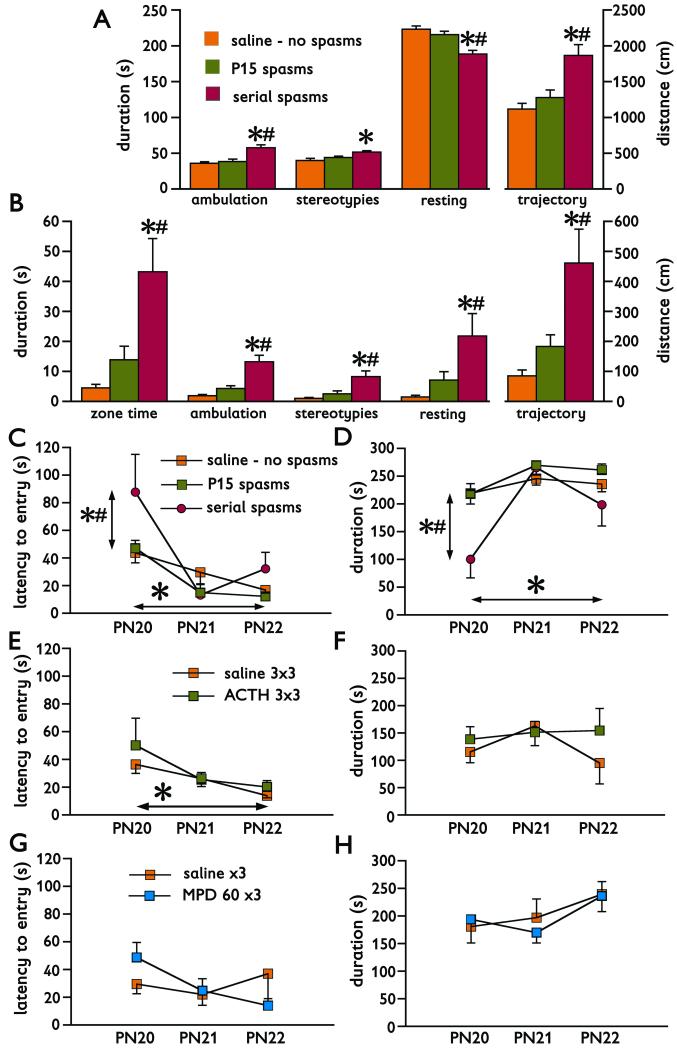
First, we tested P20 behaviors after either after a single experience of spasms on P15 or repeated bouts of spasms on P12, 13, and 15. In the open field, we evaluated behaviors separately for the entire area relevant for exploratory behavior and for the central part (perceived by the rats as less safe). Throughout the behavioral parameters recorded, rats experiencing serial spasms (n=6) always differed from controls (no spasms; n=11) and in most cases from the rats experiencing a single bout of spasms on P15 (n=11; see Figures 5A and 5B and their legend for detailed statistics). Thus, the rats after serial spasms were hyperactive and spent more time and performed more activities in the central area of the open field than controls or rats experiencing one bout of spasms, an effect consistent with impaired anxiety. Despite all these differences, the velocity of movements of all rats was not significantly different (F(2,25)=2.048; p=0.15; not illustrated). In the elevated plus maze, the rats experiencing repeated bouts of spasms also displayed a significant difference in behavior compared to controls and rats experiencing one bout of spasms. Specifically, there was an increased latency to entry of the closed arm of the elevated plus maze (F(2,26)=4.224; *p=0.0258; Figure 5C). There was also a significant effect of repeated testing (RM-ANOVA F(2, 52)=22.480, *p<0.0001; Figure 5C). The rats after serial spasms also spent significantly less time in the closed arms than the other two groups (F(2,26)=5.225; *p=0.012; Figure 5D). Repeated days of testing also significantly affected this parameter (RM-ANOVA F(2, 52)=21.191; *p<0.0001; Figure 5D).
Figure 5. Behavioral changes after spasms.
(A) Changes in behavior recorded on P20 in the open field in controls without spasms (n=11), after single bout of spasms on P15 (n=11) and after serial spasms on P12, P13, and P15 (n=6). In all measured behaviors, the group experiencing serial spasms was significantly different from controls (*p<0.05) and P15 spasms (#p<0.05), except for the duration of stereotypies, in which case difference was found only between controls and rats after serial spasms (always ANOVA with post-hoc Fisher PLSD test). Increased ambulation time, decreased resting time and longer trajectory traveled indicate an increased behavioral/exploratory activity in the rats after serial spasms.
(B) Behaviors recorded in the central zone of the open field. Because the rats tend to stay close to safer walls (thigmotaxis), increased time spent and activity in the central area indicate decrease in anxiety. In these behaviors, rats experiencing serial spasms always significantly differed from controls without spasms (*p<0.05) and rats with a single bout of spasms on P15 (#p<0.05; ANOVA with post-hoc Fisher PLSD test).
(C) Latency to entry of the closed arm of the elevated plus maze. Three consecutive sessions were performed on P20, P21, and P22. Rats experiencing serial spasms differed from both controls without spasms (*p<0.05) and rats experiencing spasms only on P15 (#p<0.05; ANOVA with post hoc Fisher PLSD test; vertical difference). There was also an effect of repeated elevated plus maze sessions on the performance: with repeated sessions the latency shortened indicating learning in the aversive environment (light sources were pointed to the open arms; *p<0.05; horizontal difference). This effect was seen in controls and rats with P15 spasms.
(D) Similarly, total time spent in the closed arm was shorter in the rats experiencing serial spasms compared to both controls with no spasms (*p<0.05) and the rats with spasms on P15 (#p<0.05). Findings in the rats experiencing serial spasms shown in (C) and (D) indicate decreased anxiety. The time spent inside the open arm is also a function of the repeated session, though inverse to the latency to entry.
(E) Pretreatment of rats with ACTH (3 days, 3 doses of 0.3 mg/kg per day) did not alter behavioral outcome in the elevated plus maze after a single bout of spasms induced on P15 (n=10) if compared to vehicle-injected controls with spasms (n=9). The effect of repeated sessions (learning) has been preserved (*p<0.05; horizontal difference).
(F) There was no effect of long-term ACTH pretreatment for a single bout of spasms induced on P15 on the time spent in the closed arm of the elevated plus maze.
(G) Long-term treatment with methylprednisolone (n=3; 60 mg/kg dose on P13, P14, and P15) did not alter latency to entry of the closed arm of the elevated plus maze after spasms compared to vehicle treated controls with spasms (n=4).
(H) Similarly, there was no effect of long-term methylprensiolone treatment on the time spent in the closed arm of the elevated plus maze.
Lack of difference after treatments with ACTH or methylprednisolone and controls in (E) through (H) can be attributed to a minimal difference in behaviors between controls without spasms and rats experiencing a single bout of spasms on P15 illustrated in (A) through (D). Thus, there was no room for improvement of behaviors after treatment.
Next, we investigated effects of long-term ACTH therapy on behavior after spasms. In the open field, chronic therapy, which suppressed the spasms, did not have any effects on behavior (data not shown) compared to controls receiving saline and experiencing spasms on P15. This outcome can be explained by only minimal differences in open field behaviors between controls without spasms and rats with a single bout of spasms (Figures 5A and 5B), which cannot be further affected (improved) by treatment. In the elevated plus maze, there was also no effect of ACTH treatment probably for similar reasons as in the open field. The effect of repeated daily sessions on the latency to entry of the closed arm was however preserved (RM-ANOVA F(2,34)=3.384; *p=0.0457; Figure 5E). Similarly, there was no effect of long-term ACTH treatment on the time spent in the closed arm (F(1,17)=0.748; p=0.399; Figure 5F).
Finally, we tested the effects of methylprednisolone treatment on the behavior in the elevated plus maze after spasms. There was no effect of treatment with methylprednisolone on the latency to entry the closed arm (F(1,5)=0.001; p=0.975; Figure 5G) or on the time spent in the closed arm (F(1,5)=0.030; p=0.869; Figure 5H). Again, the reason was probably a small influence of a single bout of spasms on the behaviors, which could not be further improved by treatment.
Discussion
This study provides an important validation of the new model of cryptogenic infantile spasms and demonstrates the following: Flexion spasms triggered by postnatal NMDA in the brain prenatally exposed to corticosteroids reliably occur between P10-P15 in the rat, a period corresponding early postnatal development in humans (infant period) (Avishai-Eliner, et al. 2002). Expression of the spasms is significantly accelerated, more frequent, and more clustered in prenatally betamethasone-exposed brain compared to saline-exposed controls. Finding of clustering of the spasms is similar to human infantile spasms (Dulac, et al. 2002). Our model is consistent with a recently published study indicating an increased risk to develop spasms in children, whose mothers experienced increased stress during pregnancy (Shang, et al. 2010).
Repeated expression of spasms is associated with EEG patterns relevant to human infantile spasms: ictal electrodecrement and interictally large-amplitude irregular waves indicative of rat variant of hypsarrhythmia (Dulac, et al. 2002, Hrachovy & Frost 2003, Kábová, et al. 1999). Similarly, repeated expression of spasms is associated with behavioral impairments, providing corroboration for findings in humans that the early treatment of infantile spasms is important for better long-term (behavioral/cognitive) outcome (Riikonen 2001).
The most important validation of the model, however, comes from the pharmacological studies. We demonstrate that ACTH treatment initiated after the first bout of spasms not only significantly suppresses the number of spasms, but also significantly decreases mortality: This effect is consistent with the effects of ACTH in human infantile spasms (Kellaway, et al. 1983). Similarly, we demonstrate that the effects of vigabatrin against the spasms in this model correspond to the effect of vigabatrin in human infantile spasms (Baram 2003, Lux, et al. 2004, Lux, et al. 2005, Tolman & Faulkner 2009) and the data corroborate previous findings of vigabatrin effects against NMDA-triggered spasms in prenatally unchallenged brain (Kubová & Mareš 2010). The differences between the effects of acutely administered methylprednisolone and chronic treatment are also interesting: Acute administration did not reveal an effective dose although the dose-response curve was constructed. However, chronic administration of methylprednisolone had powerful effects against the spasms consistent with effects of prednisone and prednisolone in infantile spasms (Baram, et al. 1996). The need for longer treatment with ACTH and steroid therapy indicates that a feedback within the HPA axis may need to be activated (Baram 2001, Brunson, et al. 2002), and reaching the maximum efficacy requires certain period of time (in humans median of 2 days for ACTH and 3.5 days for prednisone (Baram, et al. 1996)).
Mechanisms beyond our model of cryptogenic infantile spasms are still enigmatic. We identified a significant involvement of hypothalamic arcuate and supraoptic nuclei in our model (Velíšek, et al. 2007), thus molecular changes in the hypothalamic nuclei may bring clues for the regulation of the spasms in prenatally betamethasone-exposed brain. Indeed, a recent study (Kuzmiski, et al. 2010) showed that in the hypothalamic paraventricular nucleus, a previous experience of stress has long-term priming effects and involves a depression of postsynaptic NMDA receptors via activation of corticotropin releasing factor (CRF) receptor 1 in conjunction with enhanced presynaptic plasticity and increased glutamate release. This priming has a potential to affect the function of the HPA axis (Kuzmiski, et al. 2010). Interestingly, we observed downregulation of NR1 subunits of NMDA receptors in the hypothalamic nuclei, as well as CRF upregulation following our prenatal betamethasone exposure (unpublished). CRF control of presynaptic glutamate release as well as its postsynaptic modulation of glutamate transmission has been corroborated in the amygdala (for review see (Gallagher, et al. 2008)). Thus, increased susceptibility to develop spasms in our model may be related to increased hypothalamic glutamate release due to prenatal betamethasone priming.
In conclusion, we show here that our model of cryptogenic infantile spasms can be a useful tool for search of novel and more effective treatments of infantile spasms with improved long-term outcome and fewer side effects.
Acknowledgment
Supported by the grants NS-059504, NS-056093, and NS-072966 from the NINDS-NIH, and by the grant #6-FY08-214 from March of Dimes Foundation. Outstanding technical help of Ms. Zunju Hu is greatly appreciated.
Footnotes
Disclosure of Conflicts of Interest None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ. What are the reasons for the strikingly different approaches to the use of ACTH in infants with West syndrome? Brain Dev. 2001;23:647–648. doi: 10.1016/s0387-7604(01)00308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ. Treatment of infantile spasms: the ideal and the mundane. Epilepsia. 2003;44:993–994. doi: 10.1046/j.1528-1157.2003.44086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Baram TZ. ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability. Int Rev Neurobiol. 2002;49:185–197. doi: 10.1016/s0074-7742(02)49013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac O. Infantile Spasms and West Syndrome. W. B. Saunders; London: 1994. [Google Scholar]
- Dulac O, Soufflet C, Chiron C, Kaminska A. What is West syndrome? Int Rev Neurobiol. 2002;49:1–22. doi: 10.1016/s0074-7742(02)49003-4. [DOI] [PubMed] [Google Scholar]
- Elterman RD, Shields WD, Bittman RM, Torri SA, Sagar SM, Collins SD. Vigabatrin for the treatment of infantile spasms: final report of a randomized trial. J Child Neurol. 2010;25:1340–1347. doi: 10.1177/0883073810365103. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol. Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD., Jr Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J Clin Neurophysiol. 2003;20:408–425. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Kábová R, Liptáková S, Šlamberová R, Pometlová M, Velíšek L. Age-specific N-methyl-D-aspartate-induced seizures: perspectives for the West syndrome model. Epilepsia. 1999;40:1357–1369. doi: 10.1111/j.1528-1157.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kellaway P, Frost JD, Hrachovy RA. Infantile spasms. In: Morselli PL, Pippenger CE, Penry JK, editors. Antiepileptic drug therapy in pediatrics. Raven Press; New York: 1983. pp. 115–136. [Google Scholar]
- Kubová H, Mareš P. Vigabatrin but not valproate prevents development of age-specific flexion seizures induced by N-methyl-D-aspartate (NMDA) in immature rats. Epilepsia. 2010;51:469–472. doi: 10.1111/j.1528-1167.2009.02305.x. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Marty V, Baimoukhametova DV, Bains JS. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat Neurosci. 2010;13:1257–1264. doi: 10.1038/nn.2629. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC., 3rd Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG, Jr., Guan KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- Riikonen R. Long-term outcome of patients with West syndrome. Brain Dev. 2001;23:683–687. doi: 10.1016/s0387-7604(01)00307-2. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang NX, Zou LP, Zhao JB, Zhang F, Li H. Association between prenatal stress and infantile spasms: a case-control study in China. Pediatr Neurol. 2010;42:181–186. doi: 10.1016/j.pediatrneurol.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Infantile spasms: a critical review of emerging animal models. Epilepsy Curr. 2009;9:75–81. doi: 10.1111/j.1535-7511.2009.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Holmes GL. Infantile spasms: criteria for an animal model. Int Rev Neurobiol. 2002;49:391–411. doi: 10.1016/s0074-7742(02)49023-x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Moshé SL, Swann JW, Nehlig A, Jacobs MP, Schwartzkroin PA. Models of pediatric epilepsies: strategies and opportunities. Epilepsia. 2006;47:1407–1414. doi: 10.1111/j.1528-1167.2006.00674_1.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Sasaki-Adams DM. NMDA-induced seizures in developing rats cause long-term learning impairment and increased seizure susceptibility. Epilepsy Res. 2003;53:129–137. doi: 10.1016/s0920-1211(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Tolman JA, Faulkner MA. Vigabatrin: a comprehensive review of drug properties including clinical updates following recent FDA approval. Expert Opin Pharmacother. 2009;10:3077–3089. doi: 10.1517/14656560903451690. [DOI] [PubMed] [Google Scholar]
- Velíšek L. Prenatal exposure to betamethasone decreases anxiety in developing rats: Hippocampal neuropeptide Y as a target molecule. Neuropsychopharmacology. 2006;31:2140–2149. doi: 10.1038/sj.npp.1301016. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Chachua T, Yum MS, Poon KL, Velíšková J. Model of cryptogenic infantile spasms after prenatal corticosteroid priming. Epilepsia. 2010;51(Suppl 3):145–149. doi: 10.1111/j.1528-1167.2010.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšek L, Jehle K, Asche S, Velíšková J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Moshé SL. Effects of brief seizures during development. In: Sutula T, Pitkanen A, editors. Do Seizures Damage the Brain. Elsevier; 2002. pp. 355–364. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Velíšková J, Ptachewich Y, Ortíz J, Shinnar S, Moshé SL. Age-dependent effects of gamma-aminobutyric acid agents on flurothyl seizures. Epilepsia. 1995;36:636–643. doi: 10.1111/j.1528-1157.1995.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Zupanc ML. Infantile spasms. Expert Opin Pharmacother. 2003;4:2039–2048. doi: 10.1517/14656566.4.11.2039. [DOI] [PubMed] [Google Scholar]