Abstract
Androgen-disruptors are environmental chemicals in that interfere with the biosynthesis, metabolism or action of endogenous androgens resulting in a deflection from normal male developmental programming and reproductive tract growth and function. Since male sexual differentiation is entirely androgen-dependent, it is highly susceptible to androgen-disruptors. Animal models and epidemiological evidence link exposure to androgen disrupting chemicals with reduced sperm counts, increased infertility, testicular dysgenesis syndrome, and testicular and prostate cancers. Further, there appears to be increased sensitivity to these agents during critical developmental windows when male differentiation is at its peak. A variety of in vitro and in silico approaches have been used to identify broad classes of androgen disrupting molecules that include organochlorinated pesticides, industrial chemicals, and plasticizers with capacity to ligand the androgen receptor. The vast majority of these synthetic molecules act as anti-androgens. This review will highlight the evidence for androgen disrupting chemicals that act through interference with the androgen receptor, discussing specific compounds for which there is documented in vivo evidence for male reproductive tract perturbations.
Keywords: EDC, androgen-disruptor, androgen receptor, antiandrogen, vinclozolin, DDT
1. Introduction
Male reproductive health is defined by both the proper development of the reproductive system and maintenance of function throughout adult life, including the capacity to reproduce. While female sexual differentiation, considered the default developmental pathway, is largely independent of estrogens and androgens, male sexual differentiation is driven by androgens produced by the fetal testes and is entirely androgen-dependent [1, 2]. Consequently, it is expected that endocrine-disrupting chemicals (EDCs) that interfere with androgen action will have a greater impact on male developmental programming and reproductive tract maturation.
In contrast to estrogenic modes of action, relatively little is known about how androgenic/antiandrogenic EDCs at environmentally relevant concentrations affect male reproductive tract health. Androgens mediate a wide range of developmental and physiological responses in the male and are crucial for testicular and accessory sex gland development and function, pubertal sexual maturation in multiple organs, maintenance of spermatogenesis and maturation of sperm, male gonadotropin regulation through feedback loops and various male secondary characteristics such as bone mass, musculature, fat distribution and hair patterning [2, 3]. Testosterone and its metabolite 5-α-dihydrotestosterone (DHT), the primary androgenic hormones, mediate their biological effects predominantly through binding of the androgen receptor (AR), which is expressed in many end-organs including the hypothalamus, pituitary, liver, prostate, and testes [3]. There are multiple sites whereby EDCs can interfere with androgen-dependent mechanisms and affect male reproductive tract health and these include androgen synthesis, metabolism and clearance, feedback regulation, AR expression in target organs, and direct AR binding [4–9]. This review will focus on EDCs that ligand the AR and in so doing, behave in vitro as AR antagonists and/or, in a few cases, as AR agonists. Further, we will highlight the in vivo evidence that some of these man-made chemicals interfere with biological processes and in so doing, disrupt male reproductive tract health and well-being.
2. Androgen Receptor
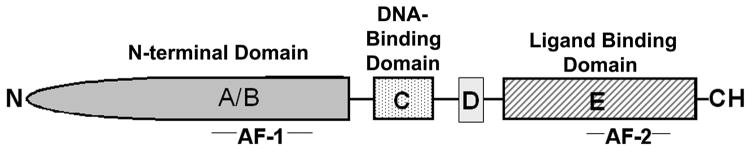
The actions of androgens within target cells are transduced by the low abundance intracellular AR, the number 4 member of the NR3C subgroup of a nuclear receptor superfamily that mediates the action of steroid hormones [10]. The human AR cDNA was first cloned in 1988 [11, 12] and an AR has since been described in a number of species including, mouse [13], rat [14], rabbit [15] monkey [16] and fish [17, 18]. The single-copy androgen receptor gene is localized on the human X chromosome between q11-q13 [19] and contains 8 exons with a total length of 90 kb. As schematized in Figure 1, the large AR gene encodes a 115–120 kD modular protein with five domains that each harbor an autonomous function that is critical to AR action; an N-terminal or A/B domain (NTD) with transactivation function, the DNA-binding or C domain (DBD), a hinge region or D domain and a ligand-binding or E domain (LBD) [20–22].
Figure 1.
Domain structure of the androgen receptor. The androgen receptor is composed of a N-terminal domain (NTD) or A/B domain, with transactivation function mediated through the AF-1 region, a DNA-binding (DBD) or C domain, harboring two zinc finders that recognize AREs in regulated genes, a hinge region or D domain, and a ligand-binding (LBD) or E domain that contains the steroid binding pocket and helices 11 and 12 as well as the activation function-2 region (AF-2).
The first 30 residues of the AR NTD are highly conserved and critical for interactions with the LBD that provide for agonist-induced stabilization of the receptor [23]. This NTD-LBD interaction between 2 AR molecules is a property unique to AR among the steroid receptor family. The NTD also harbors the transcriptional Activation Function-1 (AF-1) domain which specifies the cell and promoter-specific activity and functions as a site for co-receptor protein interaction. Phosphorylation of the NTD via the actions of multiple intracellular kinases is a well characterized post-translational modification that permits ligand-independent AR activation [23, 24]. The AR gene has a unique feature compared to its sex steroid receptor counterparts in that it contains polymorphic repeats of CAG (glutamine) and GGC (glycine) in the NTD, which have been linked to certain chronic diseases [24].
The DBD consists of two zinc-fingers that are encoded by exons 2 and 3, respectively, which recognize and bind to the cis-acting enhancer DNA sequences, or hormone response elements (HRE) located within the regulatory regions of target genes. The first zinc-finger composes the P-box (proximal box) conferring specificity sequences on the receptor protein and also forming a “recognition helix” [25]. P-box residues are identical among the AR, progesterone and glucocorticoid receptors, therefore, these receptors bind a common consensus HRE (or GRE). Amino acids of the second zinc-finger form the “D-box” (distal box) and are more specifically involved in spacer sequence and provide an interface for receptor dimerization [25]. The D domain primarily serves to connect the more highly conserved C and E domains of the receptor. Commonly referred to as the “hinge” region, the D domain also harbors a nuclear localization signal that influences cellular compartmentalization of the receptor.
The AR LBD is a highly structured, multifunctional region that primarily serves to bind androgens and also is the primary site for EDC interactions. The LBD of the AR in humans, rats and mice is identical and provides for high affinity binding of two endogenous androgens, testosterone and 5α-hydroxy-testosterone (DHT), the latter of which binds with much greater affinity [26]. Similar to other steroid receptors, the LBD contains an Activation Function-2 (AF-2) domain located in the C-terminus. While the AF-2 augments ligand-dependent transcriptional activity for most steroid receptors, this function is markedly weaker in the AR where the AF-2 is more involved in interactions with residues in the NTD [23]. Receptor binding to an agonist ligand leads to rearrangement of the LBD such that helix-11 is repositioned and helix-12 swings back to form a “lid” over the binding pocket [27]. This agonist-induced repositioning of helix-12 leads to the formation of a hydrophobic cleft, which serves to recruit co-activators such as p160 to the receptor complex to promote receptor transcriptional activity. In contrast, receptor antagonists are unable to induce a similar repositioning of helix-12, leading to receptor recruitment of co-repressors such as NcoR and SMRT and a structure incompatible with co-activator recruitment, thus making it less likely to activate transcription [28].
In the “classic” model of steroid receptor action, the AR resides in the nucleus or cytoplasm but is sequestered in a multi-protein inhibitory complex in the absence of hormone. Upon hormone binding, a conformational change occurs in the receptor, transforming it to an “activated” state that is now able to homodimerize, show increased phosphorylation, and bind to HREs within target gene promoters. The ligand/HRE-bound receptor complex interacts with the general transcription apparatus either directly or indirectly via co-regulatory proteins to promote transcription of the target gene [25]. This classic steroid receptor mechanism is dependent on the functions of both AF-1 and AF-2 domains of the receptor, which synergize via the recruitment of co-activator proteins to the DBD, most notably the p160 family members. It is generally believed that the DNA-bound receptor/co-activator complex facilitates disruption of the chromatin and formation of a stable transcription pre-initiation complex. Unique to AR among the sex steroid receptors is that the agonist-liganded AR NTD interacts with co-repressors NCoR and SMRT which function as negative regulators of androgen-stimulated transcriptional activity [28]. Depending on the cell and promoter context, the DNA-bound AR complex may positively or negatively affect expression of the downstream target genes.
3. Evidence Linking AR Disruptors with Disorders of Male Health
The link between environmental chemicals and male infertility has been widely appreciated since 1962 with the publication of Rachel Carson s book, Silent Spring, which highlighted the effects of dichlorodiphenyltrichloroethanes (DDTs) on infertility in birds and other wildlife. While human studies examining altered male reproduction in relation to environmental chemicals were initially limited, evidence has emerged over the years that suggests a link between hormonally active toxicants and developmental reproductive abnormalities [29]. Studies have raised the possibility that EDCs may be contributing to a decline in the human sperm count that has been observed over the last 50–60 years [30–32]. A review of 61 international studies involving 14,947 men between 1938 and 1992 showed that the average sperm count had dropped from 113 million/ml in 1940 to 66 million/ml in 1990, in addition to alterations in sperm morphology and motility [33]. Many epidemiological studies suggest a link between non-persistent (or contemporary-use ) pesticide exposure and altered semen quality [34] suggesting that EDCs may be the proximate cause. Two recent studies based on occupational reports involving simultaneous exposure to several pesticides found associations between pesticide exposures representative of that seen in the general population and reduced semen quality [35, 36].
In addition to altered semen quality, other male reproductive tract anomalies potentially attributable to EDCs have emerged with increased frequency over the past few decades that together have been described as testicular dysgenesis syndrome [37]. These disorders in the human population, which include increased incidences in cryptorchidism, hypospadias, oligospermia, and testicular germ cell cancer, have been linked in some studies to prenatal endocrine-disruptor exposure [37–43]. It is interesting to note that the so-called testicular dysgenesis syndrome has geographical specificity which emphasizes the likelihood that environmental factors contribute to these reproductive tract abnormalities [44]. Although not all cases of these disorders are a result of EDC exposures and have separate etiologies, a unifying hypothesis that links these disorders provides an intriguing and compelling argument that requires further investigation. Other male reproductive abnormalities that have been associated with EDCs that have AR disruptor activity in both human epidemiology studies as well as in animal models included delayed puberty [45] and reduced anogenital distance in newborn boys [46].
Another area of male reproductive health potentially linked to EDC exposures is cancers of the reproductive tract, specifically testicular and prostate cancers. Testicular cancer rates have increased worldwide over the past 35 years with the greatest increase observed in certain European populations. Although direct evidence for increased testicular cancer due to EDC exposures is very limited, there are several reports [47, 48] that suggest a link. In utero diethylstilbestrol exposure has been associated with an increased risk of testicular cancers [49] while maternal levels of chlorinated chemicals suggests a link for these compounds with mixed estrogenic and antiandrogenic activity to testicular cancer rates in sons [50]. Further, a rabbit model for testicular cancer identified exposure to di-n-butylphthalates with antiandrogenic action to testicular carcinoma in situ [51]. There is compelling data for increased prostate cancer risk and exposure of farmers to pesticides, some which are inhibitors of p450 enzymes involved in steroid metabolism [42, 50, 52]. Epidemiologic studies of occupational exposure to PCBs revealed a strong exposure-response relationship for prostate cancer risk [Ritchie, 2003 #3204; Charles, 2003 #3205] and prostate cancer mortality [54]. While estrogenic activity of these compounds is a suspected mode of action, there is also evidence that some PCBs may behave as antiandrogens.
While there are many sites of action for chemicals to interfere with androgen signaling, available evidence primarily classifies these compounds into two broad categories; (i) interference with androgen biosynthesis or metabolism to indirectly modulate androgen function (nonreceptor-mediated disruptors) and (ii) interaction with the androgen receptor to interfere with the ligand-dependent transcriptional function (receptor-mediated disruptors). Furthermore, it has been shown that some pesticides can act by reducing androgen receptor expression [4–6]. For the purposes of this review, we here look at the exposure to endocrine-disrupting pollutants with identified antiandrogenic toxicity, mostly through binding of the androgen receptor to alter proper folding of its ligand-binding domain (LBD), blocking recruitment of co-activators and preventing transcriptional initiation. Androgen-disruptors acting via this mechanism include vinclozolin, DDT, procymidone, linuron, lindane, dieldrin/aldrin, methoxychlor, nonylphenol, and bisphenol-A [7–9]; Table 1). These chemicals will be discussed individually in the following section based upon their chemical classifications.
Table 1.
Endocrine-disrupting pollutants with antiandrogenic toxicity through binding of the AR.
| Androgen-disruptor | Effects and associated mechanism | References |
|---|---|---|
| Diphenylmethanes | ||
| DDT | Decreased fertility, cryptorchidism; inhibition of DHT binding to AR, perturbed Ca++ mobilization, phosphorylation of c-ERB2/c-met; inhibition of p450scc | [9, 74, 130–134] |
| DDE | Cryptorchidism; inhibition of DHT binding to AR, MAPK pathway and PI3K activation, interacts with GPR30 | [9, 74, 133–136] |
| Methoxychlor | Inhibition of somatic growth, reduced accessory sex glandweight, elevation of serum prolactin; suppression ofLeydig cell function; inhibition of spermatogenesis, decreased seminal vesicles and epididymal weight, pubertal delay; induction of CYP28, CYP23A and CAR, alterations in germline DNA methylation, ER activation, antiandrogen and transgenerational effects | [82–85, 87] |
| Bisphenol A (BPA) | Aberrant development of prostate and urethra, increased anogenital distance, altered periductal stroma, increases susceptibility to prostate hormonal carcinogenesis, inhibition of DHT binding to AR, ligands mutated ARs | [93, 95, 134, 137–139] |
| Flutamides | ||
| Vinclozolin | Hypospadias, undescended testes, delayed puberty, prostate disease among subsequent generations; inhibition of DHT binding to AR, alters germline DNA methylation patterns | [100, 134, 140–143] |
| Linuron | Disruption of reproductive tract development; reduction of epididymal and accessory sex gland weight; increased serum estradiol and luteinizing hormone | [113] |
| Organochlorines | ||
| Lindan (γ-HCH) | Alterations in testes histology; inhibition of DHT binding to AR | [74, 117] |
| Procymidone | Fetal rat: shortened anogenital distance, permanent nipples, hypospadias, ectopic undescended testes, reduced weight and altered histology of prostate (and several other androgen-dependent tissues) | [8] |
| Dieldrin/aldrin | Reproductive performance affected at doses causing maternal intoxication; inhibition of DHT binding to AR | [74] |
| Phthalates | ||
| Butylbenzylphthalate | Reduced anogenital distance and weights of testes, epididymis, ventral prostate, and glans penis, female-like areolas/nipples, hypospadias, cryptorchidism, oligospermia, infertility; inhibition of DHT binding to AR | [80, 134, 144, 145] |
| Others | ||
| Nonyilphenol | MAPK pathway activation, PKC/cAMP modulation, reduced CYP1A1 expression; Reduction of 5α-reductase and 3α-hydroxysteroid-dehydrogenase activities; inhibition of DHT binding to AR | [7, 8] |
4. Classification of AR Disrupting Chemicals
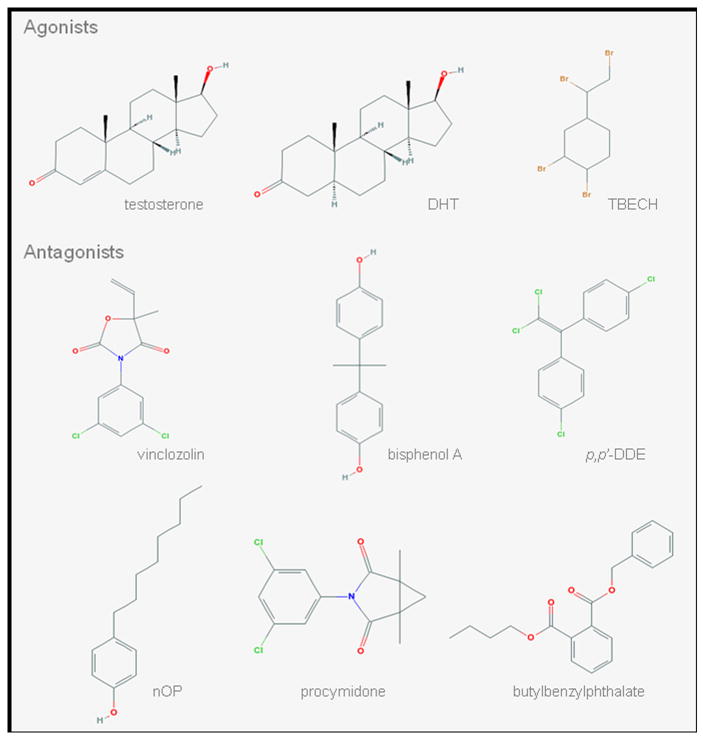
Androgen receptor-mediated disruptors can be classified into agonists and antagonists. An agonist binds to androgen receptor and triggers a response mimicking the action of a naturally occurring androgen. In contrast, an antagonist acts opposite to an agonist and blocks androgen receptor transactivation. Thus far, there are limited studies on screening of androgen receptor binding activity for a large number of chemicals [55–62], which include studies based solely on their chemical structure [63, 64]. Among the systematic investigation of compounds, a pilot study by Araki and colleagues [55] is the first report of industrial or environmental chemicals with AR agonist activity. However, not until a few years ago was 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (TBECH; Figure 2) identified as the first potent environmental activator of the human AR [65] and the only AR agonist for which in vivo study has been performed [66].
Figure 2.
Two-dimentional molecular structures of selected androgen disruptors from each class. DHT, dihydrotestosterone; TBECH, tetrabromoethylcyclohexane; p,p’-DDE, p,p’-1,1-Dichloro-2,2-bis(p-chloroethyl)ethylene; nOP, 4-n-octylphenol
4.1. Agonists
4.1.1. Tetrabromoethylcyclohexanes (TBECHs)
TBECH, a brominated flame retardant used in a variety of products as insulation, stuffing in furnishings, is presence in both sediments and organisms along with its potent activation of AR at nanomolar concentrations caused TBECH to be ranked as one of the 10% most hazardous compounds to ecosystems [67]. TBECH can exist in four diastereoisomeric forms: α and β, found in the commercial flame retardant marketed as Saytex BCL 462 by Albemarle Corp.; and γ and δ, converted from α and β at temperatures > 120°C [68].
In silico analysis of interaction energies and in vitro binding assays showed that TBECH diastereomers γ and δ are more potent activators of the human AR than α or β [69]. TBECH-γδ (50:50) binds to the AR with 22% of DHT s binding affinity and all diastereomers induced expression of the downstream target prostate-specific antigen (PSA) in vitro [69]. Nyholm and colleagues [66] showed that TBECH can be maternally transferred in zebrafish. Future studies are needed to determine the androgenic effects of TBECH in vivo.
4.2. Antagonists
Environmental and industrial chemicals with antiandrogenic action cover wild range of chemical structures including flutamide derivatives, diphenylmethanes, phthalates, organochlorines, and alkylphenols (Figure 2).
4.2.1. Diphenylmethanes
Dichlorodiphenyltrichloroethanes (DDTs) and Congeners
Commercial DDT contains several isomers of which p,p’-1,1,1-trichloro-2,2-bis[p-chlorophenyl]ethane (p,p’-DDT) and its persistent metabolic derivative p,p’-1,1-Dichloro-2,2-bis(p-chloroethyl)ethylene (p,p’-DDE) are the major components. DDT was widely used as a pesticide in the United States until its ban in 1972 after it was found to have adverse effects on male reproductive tract development in wildlife [9, 70]. The Second National Health and Nutrition Examination Survey conducted between 1976 and 1980 revealed a prevalent human exposure in 99.5% of the U.S. population with measurable serum p,p’-DDE levels [71]. Although human exposure has declined significantly since that time, some populations still bear significant levels of p,p’-DDE [72, 73] due to its considerably high environmental half-life, bioaccumulation and the continued use of DDT against malaria in some developing countries.
Regarding its properties as an AR disruptor, DDT isomers p,p’-DDT, o,p’-1,1,1-trichloro-2,2-bis[p-chlorophenyl]ethane (o,p’-DDT) [74], and metabolite p,p’-DDE [57] were shown to reduce binding of DHT to AR in vivo while p,p’-DDE inhibited DHT-induced transcriptional activation in vitro [9]. In addition to AR antagonistic effects of DDT, p,p'-DDE at high concentrations has been shown to function as an inhibitor of 5α-reductase, responsible for converting testosterone to DHT [75] thus it is likely that these compounds interfere with androgen signaling at multiple sites of action.
Although human serum concentrations of DDT and p,p'-DDE were only weakly associated to cryptorchidism or hypospadias in offspring [76–79], fetal and neonatal exposure in male produced demasculinizing effects with a high incidence of epididymal and testicular lesions [9, 80], and reduced prostate growth and inflammation [81].
Methoxychlor
The insecticide methoxychlor is structurally related to DDT. Compared to DDT, however, it has low environmental persistence and therefore was used to replace DDT as a pesticide after the later was banned in the United States. Beyond its considered estrogenic activity, methoxychlor also shows affinity to the AR at comparable or even higher levels than DDTs [57]. While methoxychlor exposure of neonatal rats did not affect male puberty, reproductive organ weights or functions in adulthood [82, 83], exposure throughout gestation, weaning, and lactation resulted in multiple effects including inhibition of somatic growth and accessory sex gland weight, elevated pituitary and serum prolactin levels, delayed puberty, suppression of Leydig cell function, reduced sperm counts [84, 85] and decreased DNA content of the accessory sex glands in the male offspring [86]. Rats fed 2000 ppm methoxychlor for 90 days exhibited decreased prostate size and cell content [87]. Together, these biological endpoints indicate robust antiandrogenic activity of this environmental contaminant with regards to male reproductive health.
Bisphenol A (BPA)
BPA is a synthetic polymer used in the production of polycarbonate plastics and epoxy resins and significant levels have been found in the urine of 93% of the US population in a recent screen by the CDC [88]. While its mode of action is believed to be primarily as an estrogen receptor agonist, studies have also shown AR binding [57] and antagonistic activity for BPA [89]. Thus negative effects of BPA on male reproductive health need to be evaluated in the context of its antiandrogenic capacity in addition to its estrogenic actions. BPA exposures have been linked to reduced sperm counts in a rodent model [90] and a human epidemiology study [91]. Recent assessment of occupational exposure to BPA has linked erectile dysfunction in men with high urinary BPA levels [63].
The effects of BPA with regards to carcinogenic potential, including the prostate gland, have recently been reviewed by an expert panel [92]. In short, there is evidence from rodent models and human prostate cell lines that BPA can influence carcinogenesis, modulate prostate cancer cell proliferation, and for some tumors, stimulate progression [93, 94, 146]. Of particular interest with regards to AR action are the studies by Knudsen and colleagues who examined the influence of BPA on human prostate cancer cells that contained an AR point mutation (AR-T877A) frequently found in advanced prostate cancers of patients who relapse after androgen deprivation therapy [95]. They first observed that 1 nM BPA activates AR-T877A in transcriptional assays and leads to unscheduled cell cycle progression and cellular proliferation in vitro in the absence of androgen. Since BPA had no impact on wild-type AR, these data indicate that this gain-of-function AR mutant attained the ability to utilize BPA as agonist. Subsequent in vivo analyses of the impact of BPA on human prostate tumor growth and recurrence were performed using a mouse xenograft of human cells containing the AR-T877A mutation. At environmental relevant doses of BPA, i.e. at levels seen in human populations, prostate tumor size increased in response to BPA administration [94]. Further, xenografts of mice treated with BPA demonstrated an earlier rise in PSA which is indicative of biochemical failure. These outcomes underscore the need for further study of the effects of BPA on prostate tumor progression and therapeutic efficacy.
4.2.2. Flutamides
Vinclozolin
Vinclozolin [3-(3,5-dichlorophenyl)-5-methyl-5-vinil-oxazolidine-2,4-dione] is a systemic dicarboximide fungicide extensively used to control diseases caused by Botrytis cinerea, Sclerotinia sclerotiorum, and Moniliniam spp. on fruits, vegetables, ornamental plants and vines. It is a well known contaminant of the human diet with endocrine disrupting potential as an AR antagonist through its primary metabolites 2-[[(3,5-dichlorophenyl)-carbamoyl]oxy]-2-methyl-3-butenoic acid and 3′,5′-dichloro-2-hydroxy-2-methylbut-3-enanilide, [96]. Vinclozolin has been shown to inhibit AR transactivation and androgen-dependent gene expression [96–98]. Defects in prostate development and ectopic location of the testes are equivalent to those observed in rats treated with the antiandrogen flutamide, suggesting a similar mechanism of action.
In vivo administration of vinclozolin at different doses, routes, and periods (gestation, lactation, puberty, adulthood) dictates the effects on the male reproductive tract. Chronic prostatitis induced by transient in utero exposure during late gestation (days 14–19) in rats was not evident until puberty or thereafter and are reported to be reversible by pubertal androgen treatments [99]. Peripubertal oral administration of vinclozolin delayed puberty and altered sex accessory gland and epididymal growth in male rats [100]. In contrast, exposure of rats to vinclozolin during midgestation, time of sex determination, promoted multiple adult-onset phenotypes including penile malformation, decreased sperm production and motility, increased spermatogenic cell apoptosis, altered sperm maturation proteins, hypospadias, cleft phallus, suprainguinal ectopic testes, vaginal pouch, epididymal and testicular granulomas, atrophic accessory sex glands, and kidney disease with tumor development [80, 99, 101–106]. In studies performed by Skinner and colleagues, the developmental vinclozolin exposure effects were found to be heritable through multiple generations with continued defects observed in the F3 generation and beyond [107]. An epigenetic basis for the transgenerational disease phenotype of vinclozolin has been established that involves perturbations in DNA methylation patterns on the male germ cell [108]. It is important to note that timing of the exposure is critical for the epigenetic and transgenerational effects on vinclozolin and is related to the establishment of DNA methylation patterns during sex determination in developing male germ cells on fetal days 8–14.
Linuron
Linuron is currently marketed as a selective phenyl urea herbicide for pre- and/or post-emergence control of weeds in crops. The structurally related diuron is used to keep weeds from track systems and sporting grounds [109]. Both compounds are degraded in the environment to 3,4-dichloroanilide and further metabolized into 3,4-dichloroacetanilide [109].
Linuron displays weak affinity to AR [57, 110–112]. In a 2-year feeding trial, linuron increased the incidence of testicular tumors in rats [111]. In short-term in vivo dosing, linuron treatment reduced testosterone- and DHT-dependent tissue weights [80] and altered the expression of androgen-regulated rat ventral prostate genes [113]. In utero exposure to linuron (day 14–18) induced developmental alterations of the testes and epididymides in the male rat offspring [113].
4.2.3. Organochlorines
Hexachlorocyclohexanes
Commercial lindane is a hexachlorocyclohexane (HCH) and consists of several isomers (α, β, γ, δ). The γ-HCH form, which is used as an insecticide, tends to accumulate in body fat of mammalian species and is the most acutely toxic [114, 115]. It has been shown that lindane binds to AR in rat prostate [57, 116], inhibits DHT binding [74], and causes biochemical and histological changes in the rat testis [117]. The β-HCH isomer is a byproduct in the manufacture of lindane and accounts for 90% of the total HCH found in human milk [115]. Male rats fed with β-HCH throughout lactation and weaning developed reproductive toxicity characterized by reduced size of seminiferous tubules and decreased number of interstitial cells along with spermatogenic arrest {Van Velsen, 1986 #3495;[118].
Procymidone
Procymidone is a dicarboximide fungicide structurally related to the well-characterized fungicide vinclozolin. It has low potential for bioaccumulation in the soil and moderate mobility. In vitro, procymidone binds AR [57, 112, 119], inhibits DHT binding in transfected COS (monkey kidney) cells, and inhibits DHT-induced transcriptional activation in CV-1 cells [8].
Procymidone causes reproductive malformations in the fetal male at dosage levels that have little effects on the reproductive tract of the adult male rat [8, 80, 119]. Maternal procymidone exposure during gestation and early lactation caused shortened anogenital distance, permanent nipples, reduced weight of several androgen-dependent tissues (levator ani and bulbocavernosus muscles, prostate, seminal vesicles, Cowper’s gland and glans penis), and reproductive tract malformations (hypospadias, cleft phallus, vaginal pouch, hydronephrosis, occasional hydroureter, epididymal granulomas, and ectopic, undescended testes). In addition, perinatal procymidone treatment had a marked effect on the histology of the lateral and ventral prostatic and seminal vesicular tissues displaying increased incidence of inflammation, similar to that produced by perinatal exposure to vinclozolin [8, 80].
Aldrin and Dieldrin
Aldrin and its major metabolite, dieldrin, were used as insecticides until the early 1970s, when they were widely restricted or banned. Aldrin is rarely found in food but dieldrin accumulates in the mammalian organism causing background levels in the environment [115]. In vitro, aldrin binds AR [57] and dieldrin reduces binding of DHT to AR [74]. In most of the reproduction studies (over one to six generations) carried out with aldrin or dieldrin on mice and rats, the major effect was an increased mortality rate in pups not yet weaned. Reproductive performance was only affected at doses causing maternal intoxication. Single doses of aldrin and dieldrin, equal to about half the LD50, caused severe fetotoxicity and an increased incidence of teratogenic abnormalities in the mouse and hamster [120]. Mating studies of dieldrin-exposed rats suggest male-dependent disturbances in fertility [121].
4.2.4. Phthalates
Butylbenzylphthalate (BBP)
The diesters of 1,2-benzenedicarboxylic acid (phthalic acid), commonly known as phthalates, are a group of man-made chemicals widely used in industrial applications. They are primarily used as plasticizers in the manufacture of flexible vinyl plastic which, in turn, is used in consumer products, flooring, and wall coverings, food contact applications, and medical devices [122]. They are also used in personal-care products (e.g., perfumes, lotions, cosmetics), as solvents and plasticizers for cellulose acetate, and in making lacquers, varnishes and coatings including those used to provide timed release of some pharmaceuticals [122].
BBP was shown to binds AR in vitro [57]. In utero or perinatal BBP exposure of rats produced a diverse profile of reproductive malformations in the male offspring mainly characterized by reduced weight of the testis, epididymis, ventral prostate and glans penis, reduced anogenital distance, female-like areolas/nipples, and decreased daily sperm production [80]. A large study on male partners of subfertile couples from an infertility clinic in Massachusetts [123, 124]found a dose-response relationship between monobenzyl phthalate (MBzP, the primary hydrolytic metabolite of BBP) and sperm concentrations that fell below the WHO reference value. In contrast to the U.S. study, a Swedish study found no relationship between MBzP levels and any semen parameter [125]. The Swedish study population consisted of young men (median age, 18 years-old; range, 18–21) from the general population, whereas in the U.S. study the median age of the men from an infertility clinic was 35.5 years-old (ranged from 22 to 54). It is unclear whether middle-aged men, compared with young men, are more susceptible to reproductive toxicants.
4.2.5. Alkylphenols
Alkylphenol ethoxylate (APE) are surfactants widely used as industrial (such as textile and paper industry) and laboratory detergents, antioxidant, plastic stabilizers, as well as carriers in agricultural pesticides. In sewage treatment plant effluents, APEs are degraded to more stable, persistent, and hydrophobic alkylphenols such as 4-n-nonylphenol (nNP) and 4-n-octylphenol (nOP). Although alkyl phenols are mainly known for their estrogenic properties, nNP and nOP have been found to be weakly antiandrogenic in recombinant yeast reporter gene assays [8, 59, 89, 126]. Neonatal exposure to nOP negatively affected pubertal spermatogenesis by significant advanced lumen formation and decreased apoptotic rate of germ cells [127], and reduced plasma testosterone [128] in male rat offspring. Male rats exposed during gestation or during the first 21 days of postnatal life caused reduction in testicular size, ventral prostate weight, and daily sperm production [129].
5. Summary
This review has synthesized the current evidence for EDCs acting as disruptors of androgen signaling in the male reproductive tract. Although the list is not comprehensive, it is clear that sufficient data has accrued to indicate that environmental contaminants are capable of deleterious effects on male reproductive tract health through their abilities to act as AR antagonists, or in a few cases, as AR agonists. While mechanistic data mostly arises from in vitro assays and in vivo animal models, there are an increasing number of human studies and epidemiology reports that document clear negative impacts on the human male. While further research at all levels is required to provide detailed understandings of mechanisms and impacts, it is important to take caution at this stage with the continued use of environmental compounds that disrupt male reproductive health. Importantly, continued studies are required to monitor the use of the known compounds as well as to screen all chemicals for potential AR disrupting activity so that caution may be applied with their continued use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swerdloff R. Hypothalamic-Pituitary-Testicular Axis, feedback loops. In: Robaire BCP, editor. Handbook of Andrology. The American Society of Andrology; 2010. pp. 2.1–2.4. [Google Scholar]
- 2.Zirkin BR. Integration of the hypothalamus, pituitary and testis. In: Robaire BCP, editor. Handbook of Andrology. The American Society of Andrology; 2010. pp. 4.1–4.4. [Google Scholar]
- 3.Matsumoto T, Shiina H, Kawano H, Sato T, Kato S. Androgen receptor functions in male and female physiology. Journal of Steroid Biochemistry and Molecular Biology. 2008;109(3–5):236–241. doi: 10.1016/j.jsbmb.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 4.McKinnell C, Atanassova N, Williams K, Fisher JS, Walker M, Turner KJ, Saunders TK, Sharpe RM. Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Andrology. 2001;22(2):323. [PubMed] [Google Scholar]
- 5.Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol. 2002;179(3):185–194. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- 6.List HJ, Smith CL, Martinez E, Harris VK, Danielsen M, Riegel AT. Effects of antiandrogens on chromatin remodeling and transcription of the integrated mouse mammary tumor virus promoter. Exp Cell Res. 2000;260(1):160–165. doi: 10.1006/excr.2000.5018. [DOI] [PubMed] [Google Scholar]
- 7.Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol Ind Health. 1999;15(1–2):80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- 9.Kelce W, Stone C, Laws S, Gray L, Kemppainen J, Wilson E. Persistent DDT metabolit p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 10.N.R.N. Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, Kokontis J, Liao S. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 12.Lubahn D, Joseph D, Sullivan P, Willard H, French F, Wilson E. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 13.He WW, Fischer LM, Sun S, Bilhartz DL, Zhu X, Young CYF, Kelley DB, Tindall DJ. Molecular cloning of androgen receptors from divergent species with a polymerase chain reaction technique: complete cDNA sequence of the mouse androgen receptor and isolation of androgen recepter cDNA probes from dog, guinea pig adn clawed frog. Biochem Biophys Res Comm. 1990;171(2):697–704. doi: 10.1016/0006-291x(90)91202-4. [DOI] [PubMed] [Google Scholar]
- 14.Tan J, Joseph DR, Quarmby VE, Lubahn DB, Sar M, French FS, Wilson EM. The rat androgen receptor: primary structure, autoregulation of its messenger ribonucleic acid, and immunocytochemical localization of the receptor protein. Molecular Endocrinology. 1988;2(12):1276–1285. doi: 10.1210/mend-2-12-1276. [DOI] [PubMed] [Google Scholar]
- 15.Krongrad A, Wilson JD, McPhaul MJ. Cloning and partial sequence of the rabbit androgen receptor: expression in fetal urogenital tissues. J Andrology. 1995;(16):209–212. [PubMed] [Google Scholar]
- 16.Choong CS, Kemppainen JA, Wilson EM. Evolution of the primate androgen receptor: a structural basis for disease. J Mol Evol. 1998;47:334–342. doi: 10.1007/pl00006391. [DOI] [PubMed] [Google Scholar]
- 17.Takeo J, Yamashita S. Two distinct isoforms of cDNA encoding rainbow trout androgen receptors. J Biol Chem. 1999;274:5674–5680. doi: 10.1074/jbc.274.9.5674. [DOI] [PubMed] [Google Scholar]
- 18.Touhata K, Kinoshita M, Tokuda Y, Toyohara H, Sakaguchi M, Yokoyama Y, Yamashita S. Sequence and expression of a cDNA encoding the red seabream androgen receptor. Biochim Biophys Acta. 1999;1450:481–485. doi: 10.1016/s0167-4889(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 19.Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS, Willard HF. Androgen receptor locus on the human X chromosome: regional localization of Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989;44:264–269. [PMC free article] [PubMed] [Google Scholar]
- 20.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 21.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 83:835–839. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. International Journal of Biochemistry & Cell Biology. 2010;42(6):813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.McEwan IJ. Molecular mechanisms of androgen receptor-mediated gene regulation: structure-function analysis of the AF-1 domain. Endocr Relat Cancer. 2004;11:281–293. doi: 10.1677/erc.0.0110281. [DOI] [PubMed] [Google Scholar]
- 24.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Laudet V, Gronemeyer H. The nuclear receptor factsbook. Academic Press; San Diego: 2002. [Google Scholar]
- 26.Hiipakka RA, Liao S. Molecular mechanisms of androgen action. Trends. Endocrinol Metab. 1998;9:317–324. doi: 10.1016/s1043-2760(98)00081-2. [DOI] [PubMed] [Google Scholar]
- 27.Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek SR, Jr, Weinmann R, Einspahr HM. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci USA. 2001;98:4904–4909. doi: 10.1073/pnas.081565498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson MC, Shen HC, Hollenberg AN, Balk SP. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Molecular cancer therapeutics. 2008;7(10):3187–3194. doi: 10.1158/1535-7163.MCT-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert SFaED. Integrating Epigenetics, Medicine, and Evolution. Sinauer Associates, Inc; 2009. The Nature of Endocrine Disruptors, Ecological Developmental Biology; pp. 197–244. [Google Scholar]
- 30.Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105(11):1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swan SH. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Internatl J Androl. 2006;29(1):62–68. doi: 10.1111/j.1365-2605.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 33.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Brit Med J. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, Wang C, Brazil C, Overstreet JW Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Envir Hlth Perspect. 2003;111(12):1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meeker JD, Ryan L, Barr DB, Herrick RF, Bennett DH, Bravo R, Hauser R. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environmental Health Perspectives. 2004;112(17):1665–1670. doi: 10.1289/ehp.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16(5):972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 38.Bay K, Asklund C, Skakkebaek NE, Andersson AM. Testicular dysgenesis syndrome: possible role of endocrine disruptors. Best Practice & Research. Clinical Endocrinology and Metabolism. 2006;20(1):77–90. doi: 10.1016/j.beem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mollecular and Cellular Endocrinology. 2006;254–255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Cooper RL, Kavlock RJ. Endocrine disruptors and reproductive development: a weight-of-evidence overview. J Endocrinology. 1997;152(2):159–166. doi: 10.1677/joe.0.1520159. [DOI] [PubMed] [Google Scholar]
- 41.Morrison H, Savitz D, Semenciw R, Hulka B, Mao Y, Morison D, Wigle D. Farming and prostate cancer mortality. Am J Epidemiol. 1993;137(3):270–280. doi: 10.1093/oxfordjournals.aje.a116674. [DOI] [PubMed] [Google Scholar]
- 42.Alavanja MC, Samanic C, Dosemeci M, Lubin J, Tarone R, Lynch CF, Knott C, Thomas K, Hoppin JA, Barker J, Coble J, Sandler DP, Blair A. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am J Epidemiol. 2003;157(9):800–814. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- 43.Meyer TE, Coker AL, Sanderson M, Symanski E. A case-control study of farming and prostate cancer in African-American and Caucasian men. Occup Environ Med. 2007;64(3):155–160. doi: 10.1136/oem.2006.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, Chellakooty M, Damgaard IN, Mau C, Reunanen M, Skakkebaek NE, Toppari J. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363(9417):1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 45.Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, Sippell W, Abbott DH, Soto A, Tyl RW, Bourguignon JP, Skakkebaek NE, Swan SH, Golub MS, Wabitsch M, Toppari J, Euling SY. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- 46.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Envir Hlth Prospect. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safe S. Environmental estrogens:roles in male reproductive tract problems and in breast cancer. Rev Environ Health. 2002;17:253–262. doi: 10.1515/reveh.2002.17.4.253. [DOI] [PubMed] [Google Scholar]
- 48.Skakkebaek NE, Rajpert-De Meyts E, Jørgensen N, Carlsen E, Petersen PM, Giwercman A, Andersen AG, Jensen TK, Andersson AM, Müller J. Germ cell cancer and disorders of spermatogenesis: an environmental connection? Acta Pathologica, Microbiologica, Immunologica Scandinavica. 1998;106(1):3–11. doi: 10.1111/j.1699-0463.1998.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 49.Martin OV, Shialis T, Lester JN, Scrimshaw MD, Boobis AR, Voulvoulis N. Testicular dysgenesis syndrome and the estrogen hypothesis: a quantitative meta-analysis. Environ Health Perspectives. 2008;116(2):149–157. doi: 10.1289/ehp.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins GS, EL . Environmental contaminants and cancers of the reproductive tract. In: Woodruff TJ, Janseen SJ, Guillette LJ Jr, Giudice LC, editors. Environmental Impacts on Reproductive Health and Fertility. Cambridge University Press; New York, NY: 2010. pp. 194–213. [Google Scholar]
- 51.Veeramachaneni DN. Impact of environmental pollutants on the male: effects on germ cell differentiation. Animal Reprod Sci. 2008;105(1–2):144–157. doi: 10.1016/j.anireprosci.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prins GS. Endocrine disruptors and prostate cancer risk. Endocrine Related Cancer. 2008;16(3):649–653. doi: 10.1677/ERC-08-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charles LE, Loomis D, Shy CM, Newman B, Millikan R, Nylander-French LA, Couper D. Electromagnetic fields, polychlorinated biphenyls, and prostate cancer mortality in electric utility workers. Am J Epidemiology. 2003;157(8):683–691. doi: 10.1093/aje/kwg044. [DOI] [PubMed] [Google Scholar]
- 54.Prince MM, Ruder AM, Hein MJ, Waters MA, Whelan EA, Nilsen N, Ward EM, Schnorr TM, Laber PA, Davis-King KE. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Environ Health Perspect. 2006;114(10):1508–1514. doi: 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araki N, Ohno K, Nakai M, Takeyoshi M, Iida M. Screening for androgen receptor activities in 253 industrial chemicals by in vitro reporter gene assays using AR-EcoScreen cells. Toxicol In Vitro. 2005;19(6):831–842. doi: 10.1016/j.tiv.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Araki N, Ohno K, Takeyoshi M, Iida M. Evaluation of a rapid in vitro androgen receptor transcriptional activation assay using AR-EcoScreen cells. Toxicol In Vitro. 2005;19(3):335–352. doi: 10.1016/j.tiv.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chemical research in toxicology. 2003;16(10):1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- 58.Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect. 2004;112(5):524–531. doi: 10.1289/ehp.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, Huhtaniemi IT. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. Journal of Steroid Biochemistry and Molecular Biology. 2004;88(2):157–166. doi: 10.1016/j.jsbmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Schreurs RH, Sonneveld E, van der Saag PT, van der Burg B, Seinen W. Examination of the in vitro (anti)estrogenic, (anti)androgenic and (anti)dioxin-like activities of tetralin, indane and isochroman derivatives using receptor-specific bioassays. Toxicol Lett. 2005;156(2):261–275. doi: 10.1016/j.toxlet.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Tamura H, Ishimoto Y, Fujikawa T, Aoyama H, Yoshikawa H, Akamatsu M. Structural basis for androgen receptor agonists and antagonists: interaction of SPEED 98-listed chemicals and related compounds with the androgen receptor based on an in vitro reporter gene assay and 3D-QSAR. Bioorganic & medicinal chemistry. 2006;14(21):7160–7174. doi: 10.1016/j.bmc.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 62.Vinggaard AM, Niemela J, Wedebye EB, Jensen GE. Screening of 397 chemicals and development of a quantitative structure--activity relationship model for androgen receptor antagonism. Chemical research in toxicology. 2008;21(4):813–823. doi: 10.1021/tx7002382. [DOI] [PubMed] [Google Scholar]
- 63.Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, Wang J, Weng X, Ferber JR, Herrinton LJ, Zhu Q, Gao E, Checkoway H, Yuan W. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Human reproduction (Oxford England) 2010;25(2):519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Wang Y, Ding J, Wang Y, Chang Y, Zhang S. Insilico prediction of androgenic and nonandrogenic compounds using Random Forest. QSAR & Combinatorial Science. 2009;28(4):396–405. [Google Scholar]
- 65.Larsson A, Eriksson LA, Andersson PL, Ivarson P, Olsson PE. Identification of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane as an androgen agonist. Journal of medicinal chemistry. 2006;49(25):7366–7372. doi: 10.1021/jm060713d. [DOI] [PubMed] [Google Scholar]
- 66.Nyholm JR, Norman A, Norrgren L, Haglund P, Andersson PL. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio) Chemosphere. 2008;73(2):203–208. doi: 10.1016/j.chemosphere.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 67.U.S. EPA; U.S.E.P. Agency. Apendix A: WMPT Summary Spreadsheet. Washington, D.C: 2000. Waste Minimization Prioritization Tool: Background Document for the Tier PBT Chemical List. [Google Scholar]
- 68.Arsenault G, Lough A, Marvin C, McAlees A, McCrindle R, MacInnis G, Pleskach K, Potter D, Riddell N, Sverko E, Tittlemier S, Tomy G. Structure characterization and thermal stabilities of the isomers of the brominated flame retardant 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane. Chemosphere. 2008;72(8):1163–1170. doi: 10.1016/j.chemosphere.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 69.Khalaf H, Larsson A, Berg H, McCrindle R, Arsenault G, Olsson PE. Diastereomers of the brominated flame retardant 1,2-dibromo-4-(1,2 dibromoethyl)cyclohexane induce androgen receptor activation in the hepg2 hepatocellular carcinoma cell line and the lncap prostate cancer cell line. Environ Health Perspect. 2009;117(12):1853–1859. doi: 10.1289/ehp.0901065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 71.Stehr-Green PA. Demographic and seasonal influences on human serum pesticide residue levels. Journal of toxicology and environmental health. 1989;27(4):405–421. doi: 10.1080/15287398909531312. [DOI] [PubMed] [Google Scholar]
- 72.Dewailly E, Dodin S, Verreault R, Ayotte P, Sauve L, Morin J, Brisson J. High organochlorine body burden in women with estrogen receptor-positive breast cancer. Journal of the National Cancer Institute. 1994;86(3):232–234. doi: 10.1093/jnci/86.3.232. [DOI] [PubMed] [Google Scholar]
- 73.Waliszewski SM, Pardio Sedas VT, Infanzon RM, Rivera J. Determination of organochlorine pesticide residues in human adipose tissue: 1992 study in Mexico. Bulletin of environmental contamination and toxicology. 1995;55(1):43–49. doi: 10.1007/BF00212387. [DOI] [PubMed] [Google Scholar]
- 74.Danzo BJ. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ Health Perspect. 1997;105(3):294–301. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo S, King I, Alléra A, Klingmüller D. Effects of various pesticides on human 5alpha-reductase activity in prostate and LNCaP cells. Toxicol In Vitro. 2007;21(3):502–508. doi: 10.1016/j.tiv.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, Wilcox AJ. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol. 2002;155(4):313–322. doi: 10.1093/aje/155.4.313. [DOI] [PubMed] [Google Scholar]
- 77.Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect. 2005;113(2):220–224. doi: 10.1289/ehp.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosie S, Loff S, Witt K, Niessen K, Waag KL. Is there a correlation between organochlorine compounds and undescended testes? Eur J Pediatr Surg. 2000;10(5):304–309. doi: 10.1055/s-2008-1072381. [DOI] [PubMed] [Google Scholar]
- 79.Mol NM, Sorensen N, Weihe P, Andersson AM, Jorgensen N, Skakkebaek NE, Keiding N, Grandjean P. Spermaturia and serum hormone concentrations at the age of puberty in boys prenatally exposed to polychlorinated biphenyls. European journal of endocrinology/European Federation of Endocrine Societies. 2002;146(3):357–363. doi: 10.1530/eje.0.1460357. [DOI] [PubMed] [Google Scholar]
- 80.Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15:94–118. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- 81.You L, Brenneman KA, Heck H. In utero exposure to antiandrogens alters the responsiveness of the prostate to p,p’-DDE in adult rats and may induce prostatic inflammation. Toxicol Appl Pharmacol. 1999;161(3):258–266. doi: 10.1006/taap.1999.8804. [DOI] [PubMed] [Google Scholar]
- 82.Gellert RJ, Heinrichs WL, Swerdloff R. Effects of neonatally-administered DDT homologs on reproductive function in male and female rats. Neuroendocrinology. 1974;16(2):84–94. doi: 10.1159/000122555. [DOI] [PubMed] [Google Scholar]
- 83.Gellert RJ, Wilson C. Reproductive function in rats exposed prenatally to pesticides and polychlorinated biphenyls (PCB) Environ Res. 1979;18(2):437–443. doi: 10.1016/0013-9351(79)90119-1. [DOI] [PubMed] [Google Scholar]
- 84.Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12(1):92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 85.Bal HS. Effect of methoxychlor on reproductive systems of the rat. Proceedings of the Society of Expierimental Biology Medicine. 1984;176(2):187–196. doi: 10.3181/00379727-176-41861. [DOI] [PubMed] [Google Scholar]
- 86.Cooke PS, Eroschenko VP. Inhibitory effects of technical grade methoxychlor on development of neonatal male mouse reproductive organs. Biol Reprod. 1990;42:585–596. doi: 10.1095/biolreprod42.3.585. [DOI] [PubMed] [Google Scholar]
- 87.Shain SA, Shaeffer JC, Boesel RW. The effect of chronic ingestion of selected pesticides upon rat ventral prostate homeostasis. Toxicol Appl Pharmacol. 1977;40(1):115–130. doi: 10.1016/0041-008x(77)90123-5. [DOI] [PubMed] [Google Scholar]
- 88.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Envir Hlth Prospect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216(2–3):197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, Ye X, Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reproductive Toxicology. 2010;30(4):532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keri R, Ho SM, Hunt PA, Knudsen KE, Soto AM, Prins GS. An Evaluation of Evidence for the Carcinogenic Activity of Bisphenol A: Report of NIEHS Expert Panel on BPA. Reproductive Toxicology. 2007;24:240–252. doi: 10.1016/j.reprotox.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho SM, Tang WY, Belmonte J, Prins GS. Developmental exposure estradiol and bisphenol A (BPA) increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodisesterase type 4 variant (PDE4D4) in the rat prostate. Cancer Research. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wetherill YB, Hess-Wilson JK, Comstock CE, Shah SA, Buncher CR, Sallans L, Limbach PA, Schwemberger S, Babcock GF, Knudsen KE. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Thereapy. 2006;5(12):3181–3190. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- 95.Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005;65(1):54–65. [PubMed] [Google Scholar]
- 96.Kelce W, Monosson E, Gamcsik M, Laws S, Gray L. Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxi Appl Pharm. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 97.Kelce WR, Gray LE, Wilson EM. Antiandrogens as environmental endocrine disruptors. Reproductive Fertility Development. 1998;10(1):105–111. doi: 10.1071/r98051. [DOI] [PubMed] [Google Scholar]
- 98.Kelce WR, Wilson EM. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med. 1997;75(3):198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- 99.Cowin PA, Gold E, Aleksova J, O’Bryan MK, Foster PM, Scott HS, Risbridger GP. Vinclozolin exposure in utero induces postpubertal prostatitis and reduces sperm production via a reversible hormone-regulated mechanism. Endoccrinology. 2010;151(2):783–792. doi: 10.1210/en.2009-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE., Jr Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health. 1999;15(1–2):65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- 101.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. Journal of Andrology. 2006;(27):6. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gray LEJ, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicology and Applied Pharmacology. 1994;129(1):46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- 103.Yu D, Jia WW, Gleave ME, Nelson CC, Rennie PS. Prostate-tumor targeting of gene expression by lentiviral vectors containing elements of the probasin promoter. Prostate. 2004;59(4):370–380. doi: 10.1002/pros.20010. [DOI] [PubMed] [Google Scholar]
- 104.Cowin PA, Foster P, Pedersen J, Hedwards S, McPherson SJ, Risbridger GP. Early-onset endocrine disruptor-induced prostatitis in the rat. Environ Health Perspect. 2008;116(7):923–929. doi: 10.1289/ehp.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Auger J, Eustache F, Maceiras P, Broussard C, Chafey P, Lesaffre C, Vaiman D, Camoin L, Auer J. Modified expression of several sperm proteins after chronic exposure to the antiandrogenic compound vinclozolin. Toxicology Science. 117(2):475–484. doi: 10.1093/toxsci/kfq199. [DOI] [PubMed] [Google Scholar]
- 106.Gray LE, Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15(1–2):48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- 107.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disuptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5(9):e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.U.S. EPA; U.S.E.P.A.O.o.P. Programs. Fact Sheet No. 28. 1984. Pesticide Fact Sheet for Linuron. [Google Scholar]
- 110.Bauer ER, Meyer HH, Stahlschmidt-Allner P, Sauerwein H. Application of an androgen receptor assay for the characterisation of the androgenic or antiandrogenic activity of various phenylurea herbicides and their derivatives. The Analyst. 1998;123(12):2485–2487. doi: 10.1039/a804606i. [DOI] [PubMed] [Google Scholar]
- 111.Cook JC, Mullin LS, Frame SR, Biegel LB. Investigation of a mechanism for Leydig cell tumorigenesis by linuron in rats. Toxicol Appl Pharmacol. 1993;119(2):195–204. doi: 10.1006/taap.1993.1060. [DOI] [PubMed] [Google Scholar]
- 112.Waller CL, Juma BW, Gray LE, Jr, Kelce WR. Three-dimensional quantitative structure--activity relationships for androgen receptor ligands. Toxicol Appl Pharmacol. 1996;137(2):219–227. doi: 10.1006/taap.1996.0075. [DOI] [PubMed] [Google Scholar]
- 113.Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC, Gray LE., Jr Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicology Science. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- 114.IPCS. Enviromental Health Criteria 124. Lindane, International Programme on Chemical Safety, World Health Organization; Genova: 1991. [Google Scholar]
- 115.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simic B, Kniewald Z, Davies JE, Kniewald J. Reversibility of the inhibitory effect of atrazine and lindane on cytosol 5 alpha-dihydrotestosterone receptor complex formation in rat prostate. Bulletin of environmental contamination and toxicology. 1991;46(1):92–99. doi: 10.1007/BF01688260. [DOI] [PubMed] [Google Scholar]
- 117.Srinivasan K, Ramesh HP, Radhakrishnamurty R. Changes induced by hexachlorocyclohexane isomers in rat liver and testis. Bulletin of environmental contamination and toxicology. 1988;41(4):531–539. doi: 10.1007/BF02020997. [DOI] [PubMed] [Google Scholar]
- 118.Dalsenter PR, Faqi AS, Webb J, Merker HJ, Chahoud I. Reproductive toxicity and toxicokinetics of lindane in the male offspring of rats exposed during lactation. Hum Exp Toxicol. 1997;16(3):146–153. doi: 10.1177/096032719701600303. [DOI] [PubMed] [Google Scholar]
- 119.Hosokawa S, Murakami M, Ineyama M, Yamada T, Yoshitake A, Yamada H, Miyamoto J. The affinity of procymidone to androgen receptor in rats and mice. The Journal of toxicological sciences. 1993;18(2):83–93. doi: 10.2131/jts.18.83. [DOI] [PubMed] [Google Scholar]
- 120.IPCS. Enviromental Health Criteria 91. Aldrin and Dieldrin International Programme on Chemical Safety, World Health Organization; Geneva: 1989. [Google Scholar]
- 121.ATSDR. Toxicological Profile for Aldrin/Dieldrin. Association for Toxic Substances and Drug Registry; Atlanta: 1987. [Google Scholar]
- 122.ATSDR. Toxicological Profile for di-n-octyl phtalate (DNOP) Association for Toxic Substances and Disease Registry; Atlanta: 2001. [Google Scholar]
- 123.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003;14(3):269–277. [PubMed] [Google Scholar]
- 124.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 125.Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16(4):487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- 126.Paris F, Balaguer P, Terouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193(1–2):43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 127.Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141(10):3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- 128.Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update. 2001;7(3):236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- 129.Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995;103(12):1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ben Rhouma K, Tébourbi O, Krichah R, Sakly M. Reproductive toxicity of DDT in adult male rats. Hum Exp Toxicol. 2001;20(8):393–397. doi: 10.1191/096032701682692946. [DOI] [PubMed] [Google Scholar]
- 131.Martz F, Straw JA. Metabolism and covalent binding of 1-(o-chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane (o,p,’-DDD). Correlation between adrenocorticolytic activity and metabolic activation by adrenocortical mitochondria. Drug Metab Dispos. 1980;8(3):127–130. [PubMed] [Google Scholar]
- 132.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275(24):18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 133.Watson WH, Yager JD. Arsenic: extension of its endocrine disruption potential to interference with estrogen receptor-mediated signaling. Tox Sci. 2007;98(1):1–2. doi: 10.1093/toxsci/kfm111. [DOI] [PubMed] [Google Scholar]
- 134.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. The Journal of endocrinology. 1998;158(3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 135.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. Journal of Steroid Biochemistry and Molecular Biology. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 136.Stoica GE, Franke TF, Moroni M, Mueller S, Morgan E, Iann MC, Winder AD, Reiter R, Wellstein A, Martin MB, Stoica A. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22(39):7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 137.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Experimental Biology and Medicine. 2000;224:61–68. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 138.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proceedings of the National Academy of Science. 2005;102(19):7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz de Toro M, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod. 2001;65:1271–1277. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 140.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 141.Shono T, Suita S, Kai H, Yamaguchi Y. Short-time exposure to vinclozolin in utero induces testicular maldescent associated with a spinal nucleus alteration of the genitofemoral nerve in rats. J Pediatr Surg. 2004;39(2):217–219. doi: 10.1016/j.jpedsurg.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 142.Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A, Hass U. Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl. 2008;31(2):241–248. doi: 10.1111/j.1365-2605.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- 143.Anway M, Skinner M. Transgenerational effects of the endocrine disruptor vinclozolin on the prostate transcriptome and adult onset disease. Prostate. 2008;68(5):515–529. doi: 10.1002/pros.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicology Science. 2000;58(2):350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 145.Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ’testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Human reproduction (Oxford, England) 2003;18(7):1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- 146.Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal Exposure to Oestradiol and Bisphenol A Alters the Prostate Epigenome and Increases Susceptibility to Carcinogenesis. Basic and Clinical Pharmacology & Toxicology. 2008;102:134–138. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]