Abstract
Increased interest in the potential societal benefit of incorporating health economics as a part of clinical translational science, particularly nutrition interventions, led the Office of Dietary Supplements at the National Institutes of Health to sponsor a conference to address key questions about economic analysis of nutrition interventions to enhance communication among health economic methodologists, researchers, reimbursement policy makers, and regulators. Issues discussed included the state of the science, such as what health economic methods are currently used to judge the burden of illness, interventions, or health care policies, and what new research methodologies are available or needed to address knowledge and methodological gaps or barriers. Research applications included existing evidence-based health economic research activities in nutrition that are ongoing or planned at federal agencies. International and U.S. regulatory, policy and clinical practice perspectives included a discussion of how research results can help regulators and policy makers within government make nutrition policy decisions, and how economics affects clinical guideline development.
Keywords: cost-benefit analysis, primary prevention, nutrition policy, medical economics, nutrition therapy
Introduction
The Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH) recently published its strategic plan for the next 5 years. In this plan, the Office committed itself to expanding its efforts to further research on the role of dietary supplements in health promotion and chronic disease prevention. As part of ODS's continued implementation of its strategic plan, it is assessing ways to enhance methodologies for evaluating the role of dietary supplements and other nutritional interventions in disease settings.
In U.S. healthcare delivery health economic issues have gained increased prominence with President Obama's expressed desire to “raise health care's quality and lower its costs.” Despite the rapid escalation of healthcare costs, research into healthcare economic solutions has not taken center stage in helping to solve these problems. Nutrition is one of the cornerstones of preventive and curative medicine, and it is postulated that better health outcomes can be achieved for dollars spent by ensuring that the population is appropriately nourished. In light of increased interest in the potential societal benefit that might result from incorporating health economics into clinical translational science decision making, ODS hosted the Economic Analysis of Nutrition Interventions for Chronic Disease Prevention: Methods, Research, and Policy workshop on February 23–24, 2010 in Bethesda, Maryland. This event brought together U.S. and international academicians, researchers, policy makers, and regulators to address the following key and questions involving nutrition interventions:
State of the science. What health economic methods are currently used to judge the burden of illness, interventions, or health care policies? What new research methodologies are available or needed to address critical knowledge or methodological gaps or barriers?
Research applications. What evidence-based health economic research activities in nutrition are going on now or planned at the NIH, Centers for Disease Control and Prevention (CDC), Agency for Healthcare Research and Quality (AHRQ), and Economic Research Service in the Department of Agriculture?
Regulatory, policy maker, and clinical practice perspectives. Once these research goals have been met, how can the results help regulators and policy makers at the Government Accountability Office (GAO), Food and Drug Administration (FDA), Office of the Assistant Secretary for Planning and Evaluation in the Department of Health and Human Services (DHHS), and Centers for Medicare and Medicaid Services (CMS) make nutrition policy decisions? What health economic and policy activities are taking place in other countries? How does economics affect the development of clinical guidelines?
The workshop's overriding goal was to enhance communication among health economic methodologists, researchers, reimbursement policy makers, and regulators about needs, capabilities, and future directions. Workshop objectives were to inform policy decision making by:
Improving the methodologies used for health economic research in nutrition.
Identifying areas of congruence between health economic research aims and health policy and regulatory needs.
Establishing a health economic research agenda to foster the use of health economics in clinical and translational health science.
Methods in Economic Analysis of Nutrition Interventions
Methodological Overview of Medical Cost-Effectiveness Analysis
Since 1960, annual health care spending has grown 2.5 % more rapidly than the rest of the economy.1 Much of this growth is due to growth in quantity; in essence, Americans are spending more on health care because they are undergoing more procedures, receiving more care, or both. Concerns about the sustainability of this growth and opportunity costs have fostered growing demand for cost-effectiveness analysis (CEA). CEA may be able to show how best to decrease these costs and identify ways to increase the value obtained with current expenditures. Other countries, such as the United Kingdom and Canada, have used CEA in their national health systems for years, but in the United States, its use is more informal. For example, some private payers use CEA to encourage greater use of cost-effective treatments, and pharmaceutical companies regularly publish CEA studies.
There are three types of CEA:2 1) Cost Minimization. This approach identifies the least expensive method for accomplishing a fixed objective, such as preventing cancer. However, it assumes that the objective will be met and does not measure the health benefit of the intervention. Instead, the goal should be to maximize benefits with the available resources, which is the fundamental aim underlying all CEA approaches; 2) Cost Benefit. In this approach, costs and benefits are measured in dollar terms, and all treatments for which the net benefit is greater than 0 are selected. This approach requires placing a dollar value on health outcomes; 3) Cost-Effectiveness. This approach examines the ratio of changes in cost relative to changes in effectiveness and selects treatments with the lowest cost-effectiveness ratio (the lowest amount of incremental cost relative to the level of incremental effect) to determine the most efficient use of resources.
The theory underlying CEA has a strong basis in “constrained maximization,” the standard approach to maximize utility or benefit, subject to the constraint of the total amount of resources available. The goal to maximize utility therefore depends on how much medical care is consumed subject to a budget constraint (income) minus the expenditures for medical care consumption. The cost and effectiveness comparisons in CEA result in 4 possible outcomes: 1) Cost increases and effectiveness decreases, indicating that the intervention should not be done; 2) Cost increases and effectiveness increases, indicating a need for CEA; 3) Cost decreases and effectiveness decreases, indicating a need for CEA; 4) Cost decreases and effectiveness increases, indicating that the intervention should always be done.
CEA allows researchers to describe the cost-effectiveness of medical interventions in terms of their additional cost per additional unit of benefit (e.g., life-years). For example, screening neonates for phenylketonuria saves money and lives, so this intervention would always be advantageous no matter what value is placed on benefits. Conversely, a screening ultrasound every 5 years for abdominal aortic aneurism costs $907,000 per life-year saved and is not cost effective based on studies suggest that people would give up about $50,000– $200,000 to gain a year of life, providing a baseline willingness to pay for determining which interventions are cost-effective. To aid policymakers, providers and patients, CEAs should be based on multiple perspectives, including private, public, and societal viewpoints, thereby explicitly accounting for the impact of these different perspectives on CEA results.
Quality-adjusted life years (QALYs) are a composite measure reflecting changes in length and quality of life that accounts for morbidity. Based on decision theory, cost-utility analyses require eliciting health-related quality of life (HRQOL) utility weights, or preferences, for health states. These measures are scored on a scale of 0 (dead) to 1 (perfect health). Cost-utility analysis (CUA) use QALYs as the effectiveness measure in CEA and is clearly the dominant methodology. The primary approaches to measuring include linear analog scale, standard gamble, and time trade-off.
Methodological Overview of Quality of Life
A variety of methodologies can be used to measure the effectiveness of interventions to improve health. Concerns in population health include morbidity (illness, or how people feel and how health problems personally affect them) and longevity (length of life).3 To compare widely different interventions for different diseases, the measure of health must encompass differences not only in length but also in quality of life, symptoms, and ability to function. Such a measure would include mortality and life expectancy rates and morbidity-based measures that include health related quality of life (HRQOL) indexes.
The purpose of quality-adjusted survival analysis is to summarize life expectancy with adjustments for quality of life. A QALY is a health outcome measure that assigns to each time period a weight ranging from 0 to 1 that corresponds to the quality of life during that period. The QALY combines morbidity and mortality into a single index, represents life expectancy with adjustments for QOL, and represents a year of life free of all disabilities and symptoms. Ideally, prospective studies measure HRQOL for a disease with and without treatment over time, thereby ultimately estimating the impact of treatment on QALYs. Similarly, cost with and without treatment can be evaluated. To facilitate data availability, in alternative ways of estimating QALYs using existing datasets have been developed. Cross-sectional methodologies allow researchers to estimate HRQOL at a point in time and combine this measurement with life tables and other methods to estimate the longitudinal impact. Currently, a small set of potential HRQOL indices are available. Each of these tools has an associated questionnaire ranging from 5 to nearly 60 questions. The developers of these indexes have collaborated in recent years to try to develop an aggregate index.
Today, utility-based measures to estimate the impact of nutrition at the population level and nutritional interventions at the individual level are available. These generic methods allow comparisons of investments in nutrition with investments in other aspects of health care. Very few applications however currently exist, and we look forward to the development of these methods for comparative effectiveness studies.
Methodological Overview of Costs
Aggregate estimates of current incidence, survival, cost burden and future trends can assist policy and program planning. These estimates can be used to evaluate specific services, components of care or the care trajectory. For nutritional interventions, aggregate costs could be used in broad policy analyses. Longitudinal costs represent longitudinal per-person estimates that are useful for CEA and at the disease category level for tracking costs for individuals over long periods. These estimates typically reflect current patterns of care, not idealized care.
In the “phase of care” approach, segmenting costs into phases that correspond to periods of differing average intensities of care avoids biases from analyzing a population at different disease stages and allows use of the maximal amount of data. Specifically, within the observation window, patients may be in one of three phases: 1) initial high-cost care, 2) late stage also high-cost care, and 3) intermediate stage lower cost care. Data on all available patients can then be used to estimate costs for these different phases of care. Combining these phase-specific estimates with the population-based incidence data on individuals in those stages enables the construction of a pseudo-longitudinal estimation of cost, and combining these phase-specific estimates with prevalence-specific estimates allows the calculation of prevalence cost. Without this type of approach, researchers risk developing misleading comparisons of cost.
This method can be applied to other aspects of cost, such as patient time costs. Engaging in health care activities (such as physician visits) uses time that could be used for other activities, such as generating income. The vast majority of health economics studies, however, do not include time costs. Even when time-related costs are substantial, these studies considered only small convenience samples, focused on specific aspects of care without any comparison of the intervention to “regular” or “routine” care. A National Cancer Institute (NCI), AHRQ, and the Veterans Administration (VA) workshop on cost estimation (http://healthservices.cancer.gov/publications/workshops/hcc) discusses these issues in more detail.4
Methodological Overview of Sensitivity Analysis and Uncertainty
The types of uncertainty associated with economic evaluation5 include: 1) outcomes (e.g., what will be the outcomes for the actual population of interest?); 2) parameters (e.g., what are the probabilities, utilities, and costs that govern the actual outcomes?); 3) methodology (e.g., what costs and consequences should be included and how should they be valued?); and 4) model structures (e.g., what are the structural and causal relationships among the variables of interest?). Parameter uncertainty can include probabilities of disease prevalence and incidence, rates of disease progression, and treatment efficacy; health state utilities; and costs. Sources of uncertainty in parameter estimates include sampling variation and internal and external study validity.
Probabilistic sensitivity analysis for CEA involves representing the uncertainty about the parameters in a given analysis as probability distributions. Adding this type of analysis to the model used to develop cost-effectiveness ratios produces a distribution of cost-effectiveness results. CEA results consist of an incremental effectiveness change (a QALY gain or loss) and an incremental cost or cost saving. Although doing so is statistically complex, a joint distribution of the incremental effectiveness and incremental costs of an intervention can be generated and compared with those of another intervention.
The most common method for calculating distributions of inputs that lead to distributions of CEA outputs is Monte Carlo decision modeling, which requires the use of subjective distributions. Another approach commonly used with clinical trial data is non-parametric bootstrapping with replacement. Methods to generate distributions around outputs include joint probability distribution of incremental cost and incremental effectiveness, distribution of net monetary benefit, and cost-effectiveness acceptability curves. The joint distribution of incremental cost and incremental effectiveness can be used to determine the probability that the result is cost effective relative to some willingness to pay WTP threshold.
Common superficial statements that often appear in the discussion sections of papers, such as “more research is needed” or “we have insufficient evidence from which to draw definite conclusions,” have little value. How do the authors know whether more research is needed? How consequential is this additional research? Mounting a large, expensive study might not be necessary to resolve such a question for which little is at stake. Furthermore, the Centers for Medicare and Medicaid Services (CMS) might not have time to wait for such a study to be conducted before making a decision on coverage. The most helpful type of uncertainty analysis for decision makers is the value-of-information analysis that shows, for example, the importance of finding out a parameter's true value or reducing a parameter's uncertainty.6
ECONOMIC ANALYSIS FOR HEALTH POLICY
Institute of Medicine
The Institute of Medicine (IOM) Committee to Evaluate Measures of Health Benefits for Environmental, Health, and Safety Regulation published recommendations in 2006 for applying CEA in the economic assessment of federal regulations.7 The Office of Management and Budget (OMB) has required some form of economic analysis and centralized review of some federal regulations since the early 1970s. Historically, cost-benefit analysis (CBA) has been the predominant approach used to assess the economic impacts of major U.S. health and safety regulations. In 2003, OMB issued Circular A-4: Regulatory Analysis, requiring that agencies also conduct CEA whenever “a valid effectiveness measure can be developed.” The next year, OMB and several federal agencies asked the IOM to convene a consensus committee to consider technical and ethical issues related to the selection of integrated effectiveness measures, such as quality-adjusted life years (QALYs, see below).
The IOM committee included several members of the U.S. Panel on Cost-Effectiveness in Health and Medicine, convened by the U.S. Public Health Service in 1993 to assess the state of the science and to define best practices for conducting CEAs in health care. The recommendations for conducting and reporting on CEA in the panel's report, Cost-Effectiveness in Health and Medicine, have been adopted by many journals and practitioners as the standard approach.8
The IOM committee deemed QALYs to be the most appropriate metric for regulatory analysis because of their simplicity, wide use, and extensive evaluation. The committee also followed the U.S. panel's endorsement of eliciting direct preferences for health states of interest in the regulatory framework. However, the committee acknowledged that direct elicitations of preferences for health states probably would not be practical in regulatory studies. The committee therefore recommended basing QALY estimates on generic indexes. Because preference-based EuroQol 5D (now known as EQ-5D, www.euroqol.org/) index values had been estimated for the general U.S. population recently, the EQ-5D index was deemed the “leading candidate” for adoption in the U.S. regulatory context. The dimensions of EQ-5D include mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
Implementation of the IOM committee's recommendations has been inhibited by time and resource constraints and by competing priorities. OMB has not issued guidance on implementing the recommendations. For some agencies, most controversial or problematic is the recommendation not to use monetized QALYs in CBA, even though WTP estimates—the theoretically appropriate source of values for CBA—often are not available.
The following four activities would encourage implementation of the IOM committee's recommendations: 1) assess the informational needs of decision makers; 2) develop criteria for matching particular measures to the circumstances in which those measures are most useful instead of routinely reporting numerous results; 3) promote cross-agency collaboration; 4) separate funding for improved data and methods from specific rule making.
An Historic Perspective
“American Exceptionalism,” may explain why the United States appears to be more resistant than other countries to CEA of the incremental costs and health effects associated with different health interventions. American culture emphasizes liberty and freedom in a way that other cultures do not, so the U.S. political system rarely produces big government solutions. Discussions of comparative effectiveness research in the United States have highlighted the danger of government's intervening between patients and doctors.
Medicare program officials have tried, at times, to incorporate CEA into their decision-making processes. In the late 1980s, the Medicare program's proposal to use CEA in making coverage decisions for new medical technology met fierce opposition from the pharmaceutical and device industries, the American Association of Retired Persons, and many medical professional societies. The proposal was eventually withdrawn.
Medicare officials state that they do not use CEA when they make coverage decisions about new technologies, but some signs indicate that this is changing.9 Medicare covers several technologies that have incremental cost-effectiveness ratios of above $100,000 per quality-adjusted life year (QALY) and other inefficient technologies, yet resistance to CEA remains strong.
Private health plans do everything possible to manage utilization but tend not to use CEA in an open, explicit way. Some exceptions exist, however, and the industry appears to be changing slowly. Despite resistance to CEA, the landscape may be changing, in large part due to the unsustainable growth in medical costs. Peter Orszag, when Director of the OMB, has stated that “better information about the costs and benefits of different treatment options…could eventually lower health care spending…” At the highest levels of the federal government, CEA and the notion that it can help inform decision makers is gaining recognition.
A database of cost-effectiveness studies is available at www.cearegistry.org. Analysis of the registry shows that the number of publications on cost-utility analysis (CUA)—CEA that measures health benefits in terms of QALYs— is increasing. About half of these studies originate in the United States, with some funded by NIH. Further analysis also shows that the methodological underpinnings of CEA and CUA are improving. Increasingly, studies are characterizing uncertainty and trying to understand the robustness of conclusions under different assumptions. More economic analyses are being carried out alongside clinical trials, and the focus on good methodological practices is increasing.
Medicare makes 10–15 national coverage decisions each year and program officials have begun citing CEA in a number of these decisions. Regression equations show that the value of technologies, measured as cost-effectiveness, is an independent predictor of what Medicare covers. The Medicare Improvement Patient and Provider Act of 2008 allowed Medicare to cover prevention for the first time without explicit Congressional authority, as long as evidence for the intervention has a high rating from the U.S. Preventive Services Task Force. The legislation also allows the DHHS Secretary to assess the relation between predicted outcomes and the expenditures for prevention services. Medicare now has the authority to use CEA for prevention services and used this authority recently when it decided to cover HIV screening.
Going forward, the number of published CEAs in the United States will likely continue to increase; resistance to CEAs will likely continue. New comparative effectiveness research will likely not include CEAs by legislative mandate, but CEAs will play a more important role in clinical guidelines and in coverage and reimbursement decisions.
Limitations
The control of American health care costs must be costly to some group if it is to save money. For example, national medical expenditures must equal the income of health care industry entities. As a result, cost control must be controversial;10 the income of some component of the health care system will be less than it otherwise would have been and those whose income is threatened will try to protect their interests in the political process. Furthermore, if cost control must be costly to someone, it follows that no “costless” or benign form of cost control exists.
Three benign approaches to reduce costs have been extensively promulgated: 1) increasing the prevention of illness, morbidity, and death; 2) using health information technology and electronic medical records to reduce errors and adverse drug interactions; 3) conducting comparative effectiveness research on the effects and costs of two or more interventions. Although each of these may be beneficial, the presumption that they would each reduce costs has yet to be demonstrated.
Without a doubt, research on health politics, policy, and economics suggests that efforts to make a population healthier do not make medical care less expensive. This is widely accepted in other countries, such as Canada, but is not a central presumption of the health care reform discussion in the United States. In 1970, the United States and Canada spent approximately the same proportion, about 7%, of their gross national product (GNP) on health care. By 2000, the United States was spending 14% of its GNP on health care compared with 10% in Canada. The best explanation for this difference is that Canadians spend less for the same medical care than Americans. For example, even though Canadians use more bed-days per 1,000 people and have more office visits per capita than Americans, overall medical costs are lower in Canada because the cost per bed-day and office visit is lower.
Economic analyses of nutritional interventions must not ignore the political climate. Nutritional reform should not be oversold in the same way as prevention, health information technology and electronic medical records, and comparative effectiveness research have been in the past.
Research in Economic Analysis of Nutrition Interventions
National Institutes of Health
In fiscal year (FY) 2009, NIH awarded an estimated $194 million for primary economics studies (focused on economics) and about 10% of the $74 million in Tier 2 award funding to economics research, so overall FY 2009 NIH economics research funding was approximately $200 million. Table 1 shows that 67% of the NIH's FY 2009 primary economics research funding was for behavioral and social science research and 42% for health services research. Primary economics awards in nutrition totaled approximately $7 million. NIH economics and nutrition projects include Understanding Disparities in Obesity and its Comorbidities in the United States (funded by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK), Health Benefits and Cost of Human Milk Feeding for Very Low Birthweight Infants (National Institute of Nursing Research), and Economic Contextual Influences on Population Diet and Obesity National Heart lung and Blood Institute (NHLBI). In addition, NIDDK funded 26 of 27 Tier 2 economics-nutrition projects totaling $13.6 million (NHLBI funded the other).
Table 1.
Percent of $194 million in Primary NIH Economics Research by Research, Condition, and Disease Categorization Category, FY 2009
| Research, Condition, and Disease Categorization Category | Total Funding | Percentage of Total NIH Funding for Primary Economics Research |
|---|---|---|
| Behavioral and Social Science | $130 million | 67 |
| Burden of Illness | $4 million | 2 |
| Clinical Trials | $21 million | 11 |
| Comparative Effectiveness | $31 million | 16 |
| Cost Effectiveness | $12 million | 6 |
| Health Services | $82 million | 42 |
| Nutrition | $8 million | 4 |
Office of Dietary Supplements
Approximately half of Americans take at least one dietary supplement on a regular basis and about one-third buy approximately $25 billion worth of dietary supplements including multivitamins/minerals (MVMs) each year.11 In addition, about 65% of the U.S. population uses fortified foods or beverages costing $36 billion per year.12
In 2001, Congress mandated that ODS review the scientific evidence and safety of dietary supplements and identify research needs. ODS and AHRQ's Evidence-Based Practice Center (EPC) program have completed 20 evidence-based reviews since 2003. Topics have included B vitamins and berries, ephedra, MVM supplementation, omega-3 fatty acids, soy, and vitamin D (details on these and other reviews are available at http://ods.od.nih.gov/).
ODS and the Office of Medical Applications of Research (part of the Office of Disease Prevention at NIH) sponsored a 2006 state-of-the-science conference to examine MVM use and chronic disease prevention in adults.6 Notwithstanding several limitations in existing studies, the expert panel made 6 conclusions. First, antioxidants and zinc reduce progression of age-related macular degeneration in adults at the intermediate stage. Selenium, vitamin E, or both might be beneficial for cancer prevention, particularly among men. In 2009, however, two large trials found that vitamin E and selenium had no cancer-related beneficial effects.13,14. Third, few trials have shown that individual or paired vitamins and minerals can prevent chronic disease. No evidence is available to recommend beta-carotene supplementation, and strong evidence suggests that smokers need to avoid beta-carotene. Calcium and vitamin D increase bone mineral density and reduce fracture risk in postmenopausal women. Lastly, niacin; folate; and vitamin B2, B6, and B12 supplements do not reduce chronic disease occurrence in the general population. The panel concluded with a call for improving methods to obtain accurate and current data on total intake of nutrients in foods and dietary supplements.12
A Medline evidence scan using vitamins, minerals, dietary supplements, vitamin D, and omega-3 fatty acids as search terms identified 220,000 studies, but only a small proportion of these publications examined prevention in conjunction with economics. Furthermore, of the approximately 500,000 publications in the Cost-Effectiveness Study Registry created by Neumann and colleagues, only 12 involved vitamins or minerals,15 specifically: vitamin D (4), folate (4), anti-oxidants (3), MVM (1), fortification (3), and primary prevention (4).
The Mineral and Vitamin Intervention Study (MAVIS)-the only cost-utility study of vitamin supplementation identified-found a statistically significant increased incidence ratio of 1.07 for primary care contacts among older Scottish adults who had received MVMs compared with those who had received placebo.16,17 The incremental cost of the intervention was approximately £15 per person over the year, or roughly the cost of the supplements. Participants taking the supplements had a non-statistically significant reduction in quality of life. So MVM increased office visits, increased costs (the vitamins) and reduced quality of life albeit non-statistically significantly.
Some studies have found that folic acid fortification reduced myocardial infarction (MI) rates by 8–13%.18 Researchers have used modeling to predict that folic acid and cyanocobalamin supplementation among those with existing coronary heart disease would result in a reduction of 310,000 deaths over 10 years.18 Specifically, supplementation in men 45 and older and women 55 and older would prevent 300,000 deaths over 10 years, saving approximately $2 billion. A recent update of this study found that folic acid fortification would reduce the incidence of MI, colon cancer, and neural tube defects and that 700 μg folic acid fortification would result in an increase of 266,649 QALYs and a cost decrease of $3.6 billion.19 However, fortification would increase the risk of precipitating or masking vitamin B12 deficiency.19
Americans spend about $61 billion on MVMs and fortification each year. However, evidence for or against MVM use for chronic disease prevention remain insufficient for drawing firm conclusions.
National Center for Complementary and Alternative Medicine (NCCAM)
A comprehensive systematic review of complementary and alternative medicine (CAM) and economics studies in six databases identified 37 CAM economic evaluations of dietary supplements, including herbs, vitamins, and minerals. Of the eight studies meeting minimum quality standards, only two were conducted in the United States; one focused on chromium and biotin for uncontrolled type 2 diabetes and the other on omega-3 supplementation for men with a previous MI.20, 21
In the chromium and biotin study, funded by a dietary supplement company, the investigators found that even the most conservative published annual medical cost savings estimates surpassed the cost of the supplement (roughly $120 per year). The authors therefore declared that the supplement was “cost saving” from a third-party payer perspective.21 According to the product's manufacturer, the study has not had any impact on U.S. health care policy or practice, despite wide dissemination of the results.
Funded by the Council for Responsible Nutrition, the U.S. study of fish oil supplements in men with an MI history used effectiveness data from four trials, the Medicare cost of one hospital visit per death, and the American Hospital Association's estimate of productivity losses.20 The study found that fish oil supplements in this population yielded cost savings to society and cost-effectiveness to the payer ($9,221 per MI-associated death avoided). The funder reported that the study has not affected U.S. health care policy or practice.
Three of the other higher quality studies identified through the systematic review focused on omega-3 fatty acids and secondary MI prevention.22–24 All three showed an increase in costs and a decrease in deaths from supplement use. Other studies found that vitamin K1 for osteoporosis increases quality of life and improves QALYs,25 vitamins C and E and beta-carotene for cataract prevention saves costs,26 and grass pollen extract for allergic rhinitis in asthma patients increases costs and improves QALYs.27 Based on these analyses, demand for clinical economic evaluations of dietary supplements among U.S. decision makers appears to be low, even though such studies can inform decision making.
John E. Fogarty International Center
The many reasons to invest in economics research in the global health context include the potential to increase understanding of the relationships between health, productivity, and development; guide priority setting and resource allocation; inform implementation science; help strengthen health systems; and provide information that could be applicable to U.S. populations.
For example, a study in Guatemala found that exposure to a high-protein supplement in boys younger than age 3 was associated with significantly higher hourly wages in adulthood (46% over the average wage in the study sample).28 Other studies showed that the cash component of a conditional cash transfer program in Mexico was associated with higher body-mass index (BMI) and blood pressure levels in adults.29
The Fogarty Center has participated in the World Health Organization's Disease Control Priorities Project (DCPP) since 2001. DCPP has developed an evidence base to inform decision making by estimating the cost-effectiveness and impact of single interventions and combinations, defining global disease burdens, and summarizing implementation experience in different regions. DCPP has examined more than 250 interventions and developed a “top 10” list of the “best buys” in global health, including the use of vitamin A, iron, and iodine for children and pregnant women to address micronutrient deficiencies. DCPP has also formed the Disease Control Priorities Network to collect valid and comparable information on the costs and consequences of policy alternatives for population health and data on the efficiencies and effectiveness of bundling interventions and their integration into service delivery platforms.
In addition, the Fogarty Center is spearheading the development of the Trans-NIH Center for Global Health Studies for short-term, project-based scholarship in global health science and policy featuring multidisciplinary teams with diverse expertise and experience. The center will identify methods to promote the research-to-policy interface and decision-making tools to guide public health strategies and investments.
National Institute on Aging
NIA spends approximately $80 million per year on health economics research. Major research topics include burden of illness, variations in health care intensity, Medicare and Medicaid interactions pertaining to chronic diseases, behavioral economic approaches to health interventions, national health accounts, and cross-national comparisons of health systems.
Media publicity on resource intensity research has provided an impetus to seek Medicare cost savings that would not compromise patient outcomes. The Dartmouth Atlas group, which is partially funded through one of NIA's program projects, has performed much of this research into variation in care intensity.
NIA also sponsors research by multidisciplinary teams using comparable data from several countries. For example, a 2004 study on people aged 50–74 in the United States and 10 European countries found that Americans had higher heart disease, hypertension, diabetes, cancer, and lung disease rates than their European counterparts.30 A 2006 study of disease prevalence in British and American adults aged 55–64 also found that older Americans were sicker than their counterparts in other rich countries.31 Demographic studies on the age profiles of public spending suggest that the U.S. government spends much more on health care for those older than 75 than other countries.32
National Institute for Child Health and Human Development (NICHD)
NICHD is a non-disease specific institution which examines the health and well being of the population from a very young age. The types of questions that NICHD addresses that pertain to diet and nutrition focus on malnutrition, obesity, policy interventions, health care, child development, and workplace and community interventions. With a long history of fielding longitudinal studies, especially in developing countries, the Institute has such studies in Malaysia, Indonesia, the Philippines, Russia, China, and Mexico that focus on what happens to populations, how families make decisions that affect how children grow up, and how child development is conditioned by the economic environment.
NICHD policy intervention activities include the Mexican government's Programa de Educación, Salud y Alimentación (Education, Health, and Nutrition Program), now called Oportunidades, which aims to develop human capital in poor households. NICHD has also been involved in housing policy and welfare reform activities.
NICHD's health care research activities that include an economic component have addressed HIV/AIDS, prescription drugs, rehabilitation, and obesity. Recently, NICHD has shifted its focus on malnutrition to concerns about obesity in many areas of the world. NICHD now has a major childhood obesity initiative (www.nichd.nih.gov/about/org/od/orsc/), whose mission is to promote a global multilevel, integrative approach to childhood obesity and associated chronic diseases. NICHD's child development research focuses on determining whether investing in health, education, and development results in cost benefits to society.
Centers for Disease Control and Prevention
As a major operating component of the Department of Health and Human Services, the mission of the CDC is “collaborating to create the expertise, information, and tools that people and communities need to protect their health – through health promotion, prevention of disease, injury and disability, and preparedness for new health threats.”
CDC has approximately 60 Ph.D.-level economists or health economics researchers. Most (42) are alumni of the Prevention Effectiveness Post-Doctoral Fellowship Program. These economists are dispersed within CDC, with clusters of at least three health economics researchers within organizational components such as the National Center for Health Statistics (a Federal statistical agency), the National Center for Chronic Disease Prevention and Health Promotion, National Center on Birth Defects and Developmental Disabilities, etc. Health economics research at CDC falls into the following areas: health services research, cost-of-illness studies, economic evaluation of interventions, cross-cutting evidence synthesis, health policy modeling, and economic and econometric analysis.
Cost-of-illness studies at CDC's National Center on Birth Defects and Developmental Disabilities using health insurance claims data include a 2005 economic evaluation of folic acid fortification and birth defects in the United States. This study found that the reduction in neural tube defects (NTDs) associated with folic acid fortification (at 140 μg per 100 g of grain product) was about 30%, much higher than previously projected.33 Although fortification costs $3 million per year, the direct costs averted were estimated to be $146 million per year in 2003 dollars (averted combined direct and indirect costs per year are $425 million). These estimates are conservative as they do not include costs to the family of caring for a child who has spina bifida (estimated at $150,000 in lost earnings to the family)34 or the full medical costs of adults with spina bifida. It is now estimated that the economic benefits are twice that of what was reported in the 2005 study.
Similar results have been reported by others. A folic acid fortification study conducted in Chile found a 50% reduction in NTDs; it was estimated that fortification of bread in Chile, which was implemented in 2000, cost $0.2 million per year and that fortification averted $2 million in costs per year.35 The CE ratio was calculated at $89 per DALY (about the same as has been published for iron and vitamin A supplementation programs). Another study involving a targeted folic acid supplementation program in South Carolina included women who had a pregnancy affected by an NTD.36 These women were offered counseling, supplementation, and were monitored over time. Among the women who accepted the program and were tracked, there were no recurrences (the expected recurrence risk associated with NTDs is 3%). The investigators calculated the number of NTDs averted and determined an incremental cost-effectiveness of $42,587 per QALY gained in the base-case analysis ($15,798 per QALY if healthy births in place of terminations following prenatal diagnosis are included).
CDC economic evaluation programs involve multiple divisions. CDC's Division of Diabetes Translation, which has a vision program that has done work on evaluating the economics of dietary supplementation for the prevention of macular degeneration.37 This division has also done a significant amount of work on preference measures and WTP estimates, as well as economic analysis of randomized clinical trials.CDC's Division of Blood Disorders is examining the cost effectiveness of screening for iron overload and hereditary hemochromatosis.CDC has a series of cross-cutting economic evaluation initiatives, one of which is the Guide to Community Preventive Services (see http://www.thecommunityguide.org/index.html), which addresses population or community level interventions. CDC's National Center for Vital and Health Statistics collects data used in health economics research and conducts analytical work on data widely used in health insurance and health policy research. Lastly,CDC's Division of Cancer Prevention and Control is working on the cost of care and the cost of screening and other interventions, the cost effectiveness of early detection, and the effects on health disparities.
Agency for Healthcare Research and Quality
AHRQ's mission is to improve the quality, efficiency, and effectiveness of health care for all Americans. Clinical economics fits within AHRQ's mission under efficiency, or trying to maximize outcomes given available resources. John Eisenberg, defined “clinical economics” as “The tools of economics …applied to the analysis of medical practice to improve physicians' choices of ways to use social and individual resources for clinical interventions in the hope of improved health.”38 In short, clinical economic research involves examining the costs and outcomes of interventions to inform resource allocation decisions. Although AHRQ's main clinical economics focus has been on methods and resources for cost-effectiveness and related analyses, AHRQ has funded cost-effectiveness analyses examining fecal DNA screening,39 computed tomography screening for colorectal cancer,40 and induction of labor.
Other clinical economic activities at AHRQ include conferences, surveys and utility assessment. A 2009 conference cosponsored with NCI and the VA and an associated journal supplement on the data and analytic methods, challenges, and future research needs pertaining to obtaining cost data.41 The Medical Expenditure Panel Survey (MEPS), a nationally representative survey of health care utilization and expenditures. For common medical conditions, MEPS is useful for examining health care expenditures and calculating health utilities. The U.S. valuation of the EuroQoL EQ-5D utility survey, which provided nationally representative utility weights for the EQ-5D survey and served as the foundation for several catalogs of utilities.
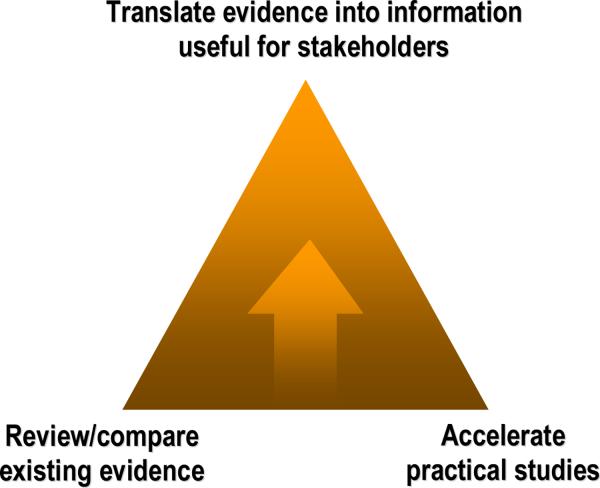
An overall view of the goals of AHRQ's comparative effectiveness program (Effective Health Care Program) is illustrated in Figure 1. The goals of the program are to review and compare existing evidence, accelerate practical studies, and translate evidence into information useful for stakeholders. More information is available at www.effectivehealthcare.ahrq.gov.
Figure 1.
AHRQ's Effective Health Care Program
AHRQ's Evidence-based Practice Center (EPC) program conducts systematic reviews of the medical literature. The EPCs have generated 20 reports on dietary supplements since 2003 (available at: http://www.ahrq.gov/clinic/epcindex.htm#dietsup). Upcoming EPC methods projects include developing a framework for economic evaluation in systematic reviews, decision and simulation modeling in systematic reviews, and measuring the value of information for research.
U.S. Department of Agriculture's Economic Research Service
The Economic Research Service (ERS) at the U.S. Department of Agriculture conducts research to inform public and private decision making on economic and policy issues involving food, farming, natural resources, and rural development. Approximately 50 ERS researchers are studying a broad range of food economics topics, such as food safety, food prices, and diet and health. Although ERS does not conduct CEA, ERS research provides basic building blocks for CEA by other agencies. All ERS publications are available at www.ers.usda.gov.
ERS maintains important data series on food choices and markets. Since 2004, ERS has made major investments in developing new sources of information for a better understanding of food choices, primarily through add-on modules to existing surveys of time use, diet knowledge, and retail purchases. ERS is currently linking National Health and Nutrition Examination Survey data to administrative data to more effectively relate Supplemental Nutrition Assistance Program (SNAP) participation with intake and health outcomes. A new survey will compare what people in low-income households buy with their SNAP benefits to other spending choices they make.
ERS research has found that a 10% decrease in the prices of fruits and vegetables leads to a 2–5% increase in fruit and vegetable consumption by low-income households.42 A 10% discount through coupons leads to more frequent fruit and vegetable purchases and a 2–10% increase in consumption.43 Furthermore, taxes could cause consumers to substitute non-taxed beverages such as bottled water, juice, and milk for sweetened beverages and a 20% tax could reduce caloric sweetened soft drink, juice drink, and sports drink consumption by 24% and childhood overweight prevalence from 16.6% to 13.7%.44
Participation in the Special Supplemental Nutrition Program for Women, Infants, and Children program is not associated with a rise in obesity in early childhood, but low-income children are at higher risk of obesity.45 SNAP is not associated with an increase in BMI or in the likelihood of overweight for most participants.46 Only 20% of schools meet the Department's guidelines for fat content of lunches. ERS is currently examining whether healthier lunches are more costly.47
ERS research has found that 11.5 million individuals live in low-income neighborhoods that are more than one mile from a supermarket,48 and more than 2.3 million individuals who live more than one mile from a supermarket do not have a vehicle. ERS has created the Food Environment Atlas of county-level statistics on food choices, health and well-being, and community characteristics in cooperation with CDC and NIH (http://www.ers.usda.gov/FoodAtlas/).
ERS is examining how behavioral economics can help decision makers understand consumer choices and improve program and policy effectiveness. In a study on food purchasing behaviors, study participants who received a debit card to purchase healthy foods ate fewer calories and a larger percentage of those calories were from healthy options. Participants who had to use cash to purchase food ate more food and a larger proportion of the calories they consumed came from unhealthy foods.49 Other behavioral cues being studied by ERS include the impact of providing information in restaurants, financial incentives for weight loss, and the impact of stress and self control on food choices and body mass index (BMI) outcomes. USDA has done research on steps along the food supply chain and where it is best to intervene, particularly with regard to price interventions. The Healthy Incentives Pilot Program (mandated for SNAP), is focused specifically on providing incentives to SNAP recipients and doing pilots to see what might work. The evaluations of this price-based intervention are being conducted by USDA's Food and Nutrition Service. ERS does work examining the value of research to enhance productivity, looking at innovation in the food system, and tracking the introduction of new products, but more could be done to understand what the incentives are for introducing healthier products.
Policy in Economic Analysis of Nutrition Interventions
Government Accountability Office
The role of GAO, which is part of the legislative branch of government, is to make government more efficient, effective, ethical, equitable, and responsive. GAO examines federal nutrition programs to support congressional decisions on nutrition policy. Examples of GAO evaluations of nutrition interventions include a report on ways to use electronic benefit transfers in SNAP to increase fruit and vegetable consumption. GAO also examined nutrition education delivery in schools when Congress considered reauthorizing the school nutrition program under the Farm Bill. GAO has reported on how the FDA has implemented food labeling requirements and how the increased information in food labels affects food choices. Unfortunately, better nutritional information did not bring about desired behavior changes, and research needs to identify the reasons for this failure. GAO also has reported on successful efforts to reduce childhood obesity.
Economic analyses of nutrition interventions involve understanding food choices in all of the environments in which people choose what to eat. Few cost-effectiveness studies have taken place in these community environments. Better data on food consumption and food choice behavior are needed to design effective policies. A more sophisticated model of food choice architecture is needed. Although peoples' incomes influence their food choices, prices and income alone do not completely explain food choices. Insights from behavioral economics might help researchers better understand food choices, and this enhanced understanding could lead to better nutrition policies and outcomes.
Planning, Evaluation, and Policy Research at the Department of Health and Human Services
The DHHS Office of the Assistant Secretary for Planning and Evaluation (ASPE) advises the DHHS Secretary on policy development in health, disability, aging, human services, and science. ASPE functions include policy analysis and development; policy research, evaluation, and data collection; policy and program planning; and policy implementation. The office has 124 experts in economics and other disciplines. Detailed information on ASPE projects is available at http://www.hhs.gov/aspe.
In 2007, ASPE and several other organizations sponsored a workshop, Nutritional Risk Assessment, to explore issues and challenges faced by nutritionists. Issues addressed include the strengths and challenges of using various risk-assessment approaches to inform dietary and nutritional recommendations, using risk-assessment approaches to evaluate standards for nutrient intake and the relationship of diet and nutrition to chronic disease risk, and identifying next steps to make progress in these areas.
ASPE also identified barriers to the adoption of previous versions of the U.S. Department of Agriculture's Dietary Guidelines for Americans in support of developing the 2010 guidelines. The most commonly cited barrier among at-risk subpopulations was the high cost or perceived high cost of food. The most successful interventions to promote guideline adoption targeted narrow dietary problems and addressed only one or two barriers for each subpopulation.
Food and Drug Administration Nutrition Interventions
Economic analysis brings social science and human behavior into decision making. It provides quantification of exposures, behavior changes, and health effects, and provides an estimate of the opportunities and consequences involved in applying interventions on the large scale. U.S. federal regulatory agencies have been required to carry out economic analyses of various regulations since the 1970s, under a series of laws and executive orders.
Basic requirements for economic analyses of federal regulations include identifying the need for regulation, identifying regulatory options, and estimating the costs and benefits of options. The elements of effective regulatory analysis include: (1) addressing a public health problem, (2) explaining why regulation is the best way to address the problem, (3) providing regulatory options for addressing the problem, (4) identifying specific changes in the behavior of all affected, (5) determining cost changes in behavior, (6) identifying the effectiveness of changes in behavior, (7) determining the value of the reduction in the public health problem, and (8) identifying variability and uncertainty in estimates. Regulatory analysis has a very narrow purpose. It is informing (not deciding or advertising) regulatory (not clinical practice) policy (sufficient for law and decision making). A regulatory analysis needs to be an honest evaluation of a regulation to inform decision making. It is only one input in the decision making process. Examples of FDA economic analyses of major nutrition regulations include nutrition labeling (1993), folic acid fortification (1996), and trans fat labeling (2003).
The FDA estimates costs based on an engineering cost model for product changes, the cost of negative health consequences, and the cost for consumers and producers of behavioral changes. Benefit estimation at FDA is a product of the number of illnesses prevented, number of QALYs saved per illness prevented, and monetary value of a statistical life year. Several approaches are available for estimating willingness to pay to reduce risk and these different approaches result in a wide range of estimates.
Many FDA projects involve estimates of the effects of food labeling. The consumer studies experts in FDA's Center for Food Safety and Applied Nutrition estimate how much labeling affects consumers' food choices. Labeling regulations can influence product formulation, as demonstrated by the experience with trans fat labeling. When products are reformulated to improve a set of products' overall nutrition profile, the nutrition intake even of consumers who do not use nutrition labeling improves. FDA has conducted multiple economic analyses of nutrition interventions. For example, the standardized “Nutrition Facts” labeling on food packaging and established standards for and authorized nutrient content claims and health claims. FDA estimated that these standards prevented 39,000 cases of coronary heart disease and cancer and saved 13,000 lives as a result over a 20-year period, resulting in 81,000 life-years saved. Monetized benefits totaled $3.6 billion. FDA required the reporting of trans fat amounts on food labels and authorized a trans-fat-free claim. The rule will prevent an estimated 600 to 1,200 heart attacks and 250 to 500 deaths annually, resulting in 2,000–4,000 life-years saved annually. The annual monetized benefits associated with this rule total $1–2 billion. Many more products have been reformulated to remove trans fats than anticipated in these estimates. FDA regulations required the fortification of enriched grain products with folic acid to prevent NTDs. When the rule was published in 1996, economists estimated that it would prevent 25–125 NTDs and 5–30 deaths per year, with annual monetized benefits of $220–$700 million. Subsequent studies have shown a much larger effect.
Centers for Medicare and Medicaid Services
Medicare is a national program that health insurance companies administer in 15 U.S. regions. The Medicare Modernization Act of 2003 stipulates that after the Medicare program issues a draft decision, it must publish its final version within 9–12 months. During this period, the Medicare program offers two 30-day public comment periods; one is required by law for any proposed decision and one occurs whenever Medicare opens a decision. Medicare must make its final decision public no later than 60 days following the close of the mandated public comment period. This is challenging because, in some cases, CMS receives more than 6,000 public comments and all public comments must be catalogued and responded to.
Medicare rarely makes decisions regarding nutrition-related matters because Congress has not identified most nutritional interventions as insurance benefits under Medicare. Rather, Medicare beneficiaries typically self-administer nutritional interventions or obtain over-the counter nutrition supplements on their own. The challenges that are common in the comparative analysis of nutritional and other health interventions include using secondary health outcomes from clinical trials done primarily to address other questions, weighing public input and other important but methodologically weaker factors in decisions, the sensitivity of recommendations to changes in inputs, and addressing uncertainties about the consequences of adopting a particular strategy.
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Approach for Incorporating Resource Use into Clinical Guidelines
Over the past two decades, guideline panels have begun to rate the quality of medical evidence and the strength of health-related recommendations, including nutritional recommendations, to provide informative summaries for consumers. Virtually every clinical organization in the United States has not only produced its own guidelines, but has also developed its own system for grading its recommendations, as have many national and international organizations. These myriad systems create much confusion.
Ten years ago, an international group of methodologists and guideline developers began to create a common international system, Grades of Recommendation, Assessment, Development and Evaluation (GRADE), to grade evidence quality (Table 2) and strength of recommendation (Table 3). The GRADE rating system is described in detail in a 2008 issue of the British Medical Journal.50
Table 2.
Quality of the Evidence
| Study Design | Quality of Evidence | Lower if | Higher if |
|---|---|---|---|
| Randomised trial → | High | Risk of bias - 1 Serious - 2 Very serious Inconsistency - 1 Serious - 2 Very serious Indirectness - 1 Serious - 2 Very serious Imprecision - 1 Serious - 2 Very serious Publication bias - 1 Likely - 2 Very likely |
Large effect + 1 Large + 2 Very large Dose response + 1 Evidence of a gradient All plausible confounding + 1 Would reduce a demonstrated effect or + 1 Would suggest a spurious effect when results show no effect |
| Moderate | |||
| Observational study → | Low | ||
| Very low |
Table 3.
Strength of Recommendations
| Determinants of strength of recommendation | |
|---|---|
| Factor | Comment |
| Balance between desirable and undesirable effects | The larger the difference between the desirable and undesirable effects, the higher the likelihood that a strong recommendation is warranted. The narrower the gradient, the higher the likelihood that a weak recommendation is warranted |
| Quality of Evidence | The higher the quality of evidence, the higher the likelihood that a strong recommendation is warranted |
| Values and preferences | The more values and preferences vary, or the greater the uncertainty in values and preferences, the higher the likelihood that a weak recommendation is warranted |
| Costs (resource allocation) | The higher the costs of an intervention- that is, the greater the resources consumed- the lower the likelihood that a strong recommendation is warranted |
In the past few years, more than 50 organizations have adopted the GRADE approach. The U.S. Preventive Services Task Force, an independent panel that systematically reviews effectiveness evidence and develops recommendations for clinical preventive services, uses many elements of the GRADE approach but continues to use its own system.
The two topics evaluated by GRADE are: (1) the quality of a body of evidence (i.e., the extent to which there is confidence that the estimates are adequate to support a decision), which is rated as high, moderate, low, or very low; and (2) the strength of a recommendation, which is graded as strong or weak. Randomized trials start as high-quality evidence, but limitations, including risk of bias, inconsistency, indirectness, imprecision, and publication bias, may lower their quality ratings. Observational studies start as low-quality evidence, but factors such as very large effects or a dose-response relationship may increase their quality ratings. The output of the GRADE evaluation is an evidence profile, which permits different ratings of quality of evidence for different outcomes, and presents the best estimates of relative and absolute effects.
Resource use is considered an outcome in GRADE. Because of the complexity of its assessment, some clinical guideline panels choose not to consider resource use. When a panel does decide to consider resource use, GRADE's approach is to identify the viewpoint (that is, costs to whom, since different payers bear the costs across and within societies), label the important resource use items, find relevant evidence, evaluate the quality of the evidence, and value resources in terms of cost. As with other outcomes, a systematic review is needed that includes the quality of the evidence and a summary of findings. Also, both resource use and costs are documented. Quality issues may arise, just as with other outcomes, and directness often is a major issue. Costs vary more than other outcomes, and even when resource use is the same, the implications and opportunity costs differ in different jurisdictions.
GRADE defines the strength of recommendations as the degree of confidence that the desirable effects of adhering to a recommendation outweigh the undesirable effects. In the case of “strong” recommendations, the benefits clearly outweigh the downsides, or vice versa for weak recommendations. The strength of recommendations may be downgraded if the quality of evidence is low or the desirable and undesirable consequences are closely balanced. Values and preferences are important when making tradeoffs, especially with regard to costs. It is important that guideline panels make their values and preferences explicit.
International Perspectives (United Kingdom, Germany and Canada)
The United Kingdom's National Institute for Health and Clinical Excellence (NICE) is part of the U.K. National Health Service (NHS) and was created in 1999 to provide an evidence-based approach to evaluating the clinical effectiveness and cost-effectiveness of new medical technologies and procedures.51 NICE issues guidance to the NHS on the use of health care interventions, assesses new treatment methods and procedures, and evaluates clinical guidelines and public health interventions.
NICE assesses interventions systematically by conducting scoping exercises, reviewing submissions from the technology's key sponsors, and independently reviewing the published evidence. Based on these activities, NICE issues guidance to the NHS and then monitors and reviews the implementation of this guidance.
The clinical data and the economic modeling that NICE systematically reviews tend to be of higher quality than the actuarial analyses, which are only performed at the end of the review process and are not always done well. Many NICE evaluations do not consider indirect costs because of technical problems, although doing so would be desirable. These problems include uncertainty about how to measure productivity losses due to illness. Failure to consider indirect costs is rarely a major impediment to implementing NICE recommendations.
When NICE first announces plans to study an innovation, it issues a call to a broad list of stakeholder groups, including professional organizations and patient advocacy groups. In addition, every NICE committee includes a patient representative. However, patient recommendations may be overruled by budgetary considerations in NICE decisions.
NICE has evaluated many nutritional interventions over the past decade. Unfortunately, the evaluations have concluded that no evidence exists to support these interventions' use. However, a NICE review did result in a recommendation (but not a mandate) that health professionals should consider using omega-3 fatty acid ethyl esters in patients within 3 months after a MI who are not consuming 7 g of omega-3 fatty acids per week by dietary means.
NICE makes decisions for public health evaluations by examining the intervention types, how relevant they are to NHS costs, and how strong the evidence is. Recommendations about very broad interventions that the health care sector is not solely responsible for implementing, such as interventions involving exercise, rely primarily on goodwill for implementation because funding is rarely available to implement them. The NHS tends to follow NICE's recommendations regarding medical interventions, such as vaccinations, more rigidly. The NHS enforces guidance regarding nutritional interventions, such as nutrient supplementation for certain populations, if the evidence is very strong. However, the evidence for nutritional intervention is often weak; in these cases, NICE words its guidance less definitively, saying, for example, that “clinicians may consider” a specific course of action.
The negative recommendations of NICE tend to be followed more rigidly in a cost-containment environment than the positive ones. Enforcement of coverage decisions is always problematic but it is easier in specialist than primary care. Primary care physicians who follow NICE guidance closely in their prescribing receive incentives.
Some of the issues raised by NICE's experience that are relevant in the United States are the importance of clear authority, rigorous assessments of medical technologies and procedures, extensive stakeholder involvement in these reviews, and transparency in decision-making about these technologies and procedures.
Similarly, Canada's mechanisms for reviewing health care technologies for funding decisions use economic analysis, and economists now play a prominent role on the committees that are making policy decisions. Economic analysis in decision making has begun to widen beyond its established role in pharmaceuticals, although this is still its major use. In Germany, the two formal health technology assessment agencies are the German Agency for Health Technology Assessment (DAHTA) at the German Institute of Medical Documentation and Information (DIMDI) and the Institute for Quality and Efficiency in Health Care (IQWiG).52 DIMDI's approach to technology assessment is similar to that already described for the NICE. IQWiG's mandate is to examine all types of technologies, although its' focus to date has been on expensive new drugs and special problems. German law requires comparison with existing technologies (without economic assessment). If a new intervention is superior to existing ones, IQWiG undertakes an economic evaluation. IQWiG must follow German Ministry of Health's Statement that: “the approach of excluding drugs with costs above a fixed uniform threshold value from reimbursement is not compatible with legal regulations in Germany.” In Germany and Canada, the focus is on drugs and technology with no assessments or analyses on dietary supplements to date.
Conclusions
With the maturation of methodologies, economic evaluations of nutrition interventions, including dietary supplements, in health promotion and chronic disease prevention have proceeded at both the research and policy levels in the United States. In contrast, economic evaluations of health technologies have been incorporated into the process for developing policy recommendations in the UK, Canada and Germany. Because these issues are difficult to understand, further study of economic issues related to nutrition interventions in chronic disease, however, would be valuable. Because ODS considers nutrition interventions at the population level, such evaluations require a great deal of data for modeling and must account for broad health effects that capture not only benefits but potential risks. Complicating such economic analyses is the variability in patient access, provider practice patterns and health reimbursement for disease management and prevention such as obesity and other chronic diseases. Potential future directions for ODS include 1) focusing on the unique methodological issues related to studying dietary supplements, such as considering the impact of third-party payments for dietary supplements instead of out-of-pocket funding; 2) using value of information analysis to help guide the setting of research priorities; 3) determining quality of life associated with nutritional interventions at the population level, and 4) applying an epidemiological approach as an alternative to standard regression analyses by controlling for time-dependent confounding when the confounders are a cause of the exposure and outcome.
ODS will apply the lessons from this workshop in its mission areas and work with other federal agency partners to consider the implications of these lessons in its planning. ODS will identify economic analyses of ongoing and planned studies, especially those that are directly related to dietary supplements and those that are more broadly relevant to nutrition interventions for chronic disease prevention. ODS also plans to form partnerships with federal agencies to advance research in this area, and it welcomes discussions on future research and policy directions to move the science forward.
Acknowledgements
The workshop was jointly sponsored by the Office of Dietary Supplements, the National Center for Complementary and Alternative Medicine, the National Cancer Institute, and the National Institute for Nursing Research, all of the National Institutes of Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
References
- 1.Hartman M, Martin A, Nuccio O, Catlin A, National Health Expenditure Accounts Team Health spending growth at a historic low in 2008. Health Aff. 2010;29(1):147–155. doi: 10.1377/hlthaff.2009.0839. [DOI] [PubMed] [Google Scholar]
- 2.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford University Press; Oxford: 2005. [Google Scholar]
- 3.Kaplan RM. The significance of quality of life in health care. Qual Life Res. 2003;12(Suppl 1):3–16. doi: 10.1023/a:1023547632545. [DOI] [PubMed] [Google Scholar]
- 4.Lipscomb J, Yabroff KR, Brown ML, Lawrence W, Barnett PG. Health care costing: data, methods, current applications. Med Care. 2009;47(7)(Supp 1):S1–S6. doi: 10.1097/MLR.0b013e3181a7e401. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer D. Addressing uncertainty in medical cost-effectiveness analysis implications of expected utility maximization for methods to perform sensitivity analysis and the use of cost-effectiveness analysis to set priorities for medical research. J Health Econ. 2001;20(1):109–29. doi: 10.1016/s0167-6296(00)00071-0. [DOI] [PubMed] [Google Scholar]
- 6.Groot Koerkamp B, Weinstein MC, Stijnen T, Heijenbrok-Kal MH, Hunink MGM. Uncertainty and patient heterogeneity in medical decision models. Med Decis Making. 2010;30:194–205. doi: 10.1177/0272989X09342277. [DOI] [PubMed] [Google Scholar]
- 7.Committee to Evaluate Measures of Health Benefits for Environmental Health and Safety Regulation, editor. Valuing Health for Regulatory Cost-Effectiveness Analysis. The National Academies Press; Washington DC: 2006. [Google Scholar]
- 8.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. Oxford; New York: 1996. [Google Scholar]
- 9.Chambers JD, Neumann PJ, Buxton MJ. Does Medicare have an implicit cost-effectiveness threshold? Med Decis Making. 2010;30:E14–2. doi: 10.1177/0272989X10371134. [DOI] [PubMed] [Google Scholar]
- 10.Marmor T, Oberlander J, White J. The Obama administration's options for health care cost control: hope versus reality. Ann Intern Med. 2009;150(7):485–489. doi: 10.7326/0003-4819-150-7-200904070-00114. [DOI] [PubMed] [Google Scholar]
- 11.Office of Dietary Supplements . Strategic Plan 2010–2014. Office of Dietary Supplements, National Institutes of Health, U.S. Department of Health & Human Services; Bethesda, MD: 2010. NIH Publication Number 10-7527. [Google Scholar]
- 12.Huang H-Y, Caballero B, Chang S, et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006;145(5):372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 13.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann PJ, Fang C-H, Cohen JT. 30 years of pharmaceutical cost-utility analyses: growth, diversity and methodological improvement. Pharmacoeconomics. 2009;27(10):861–872. doi: 10.2165/11312720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Avenell A, Campbell MK, Cook JA, et al. Effect of multivitamin and multimineral supplements on morbidity from infections in older people (MAVIS trial): pragmatic, randomised, double blind, placebo controlled trial. BMJ. 2005;331(7512):324–329. doi: 10.1136/bmj.331.7512.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilonzo MM, Vale LD, Cook JA, et al. A cost-utility analysis of multivitamin and multimineral supplements in men and women aged 65 years and over. Clin Nutr. 2007;26(3):364–370. doi: 10.1016/j.clnu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Tice JA, Ross E, Coxson PG, et al. Cost-effectiveness of vitamin therapy to lower plasma homocysteine levels for the prevention of coronary heart disease: effect of grain fortification and beyond. JAMA. 2001;286(8):936–943. doi: 10.1001/jama.286.8.936. [DOI] [PubMed] [Google Scholar]
- 19.Bentley TG, Weinstein MC, Willett WC, Kuntz KM. A cost-effectiveness analysis of folic acid fortification policy in the United States. Public Health Nutr. 2009;12(4):455–467. doi: 10.1017/S1368980008002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmier JK, Rachman NJ, Halpern MT. The cost-effectiveness of omega-3 supplements for prevention of secondary coronary events. Manag Care. 2006;15:43–50. [PubMed] [Google Scholar]
- 21.Fuhr JP, Jr, He H, Goldfarb N, Nash DB. Use of chromium picolinate and biotin in the management of type 2 diabetes: an economic analysis. Dis Manag. 2005;8(4):265–275. doi: 10.1089/dis.2005.8.265. [DOI] [PubMed] [Google Scholar]
- 22.Franzosi MG, Brunetti M, Marchioli R, et al. Cost-effectiveness analysis of n-3 polyunsaturated fatty acids (PUFA) after myocardial infarction: results from Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto (GISSI)-Prevenzione Trial. Pharmacoeconomics. 2001;19(4):411–420. doi: 10.2165/00019053-200119040-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lamotte M, Annemans L, Kawalec P, Zoellner Y. A multi-country health economic evaluation of highly concentrated N-3 polyunsaturated fatty acids in secondary prevention after myocardial infarction. Pharmacoeconomics. 2006;24(8):783–795. doi: 10.2165/00019053-200624080-00005. [DOI] [PubMed] [Google Scholar]
- 24.Quilici S, Martin M, McGuire A, Zoellner Y. A cost-effectiveness analysis of n-3 PUFA (Omacor) treatment in post-MI patients. Int J Clin Pract. 2006;60(8):922–932. doi: 10.1111/j.1742-1241.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson M, Lloyd-Jones M, Papaioannou D. Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health Technol Assess. 2009;13(45):iii–xi. 1–134. doi: 10.3310/hta13450. [DOI] [PubMed] [Google Scholar]
- 26.Trevithick JR, Massel D, Robertson JM, et al. Modeling savings from prophylactic REACT antioxidant use among a cohort initially aged 50–55 years: a Canadian perspective. Journal of Orthomolecular Medicine. 2006;21:212–220. [Google Scholar]
- 27.Nasser S, Vestenbaek U, Beriot-Mathiot A, Poulsen PB. Cost-effectiveness of specific immunotherapy with Grazax in allergic rhinitis co-existing with asthma. Allergy. 2008;63(12):1624–1629. doi: 10.1111/j.1398-9995.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371(9610):411–416. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- 29.Fernald LC, Gertler PJ, Hou X. Cash component of conditional cash transfer program is associated with higher body mass index and blood pressure in adults. J Nutr. 2008;138(11):2250–2257. doi: 10.3945/jn.108.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avendano M, Glymour MM, Banks J, Mackenbach JP. Health disadvantage in US adults aged 50 to 74 years: a comparison of the health of rich and poor Americans with that of Europeans. Am J Public Health. 2009;99(3):540–548. doi: 10.2105/AJPH.2008.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 32.Lee R, Lee S-H, Mason A. Charting the economic lifecycle. In: Prskawetz A, Bloom DE, Lutz W, editors. Population Aging, Human Capital Accumulation, and Productivity Growth, a Supplement to Population and Development Review 33. Population Council; New York: 2008. pp. 208–237. [Google Scholar]
- 33.Grosse SD, Waitzman NJ, Romano PS, Mulinare J. Re-evaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am J Public Health. 2005;95:1917–1922. doi: 10.2105/AJPH.2004.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilford JM, Grosse SD, Goodman AC, Li K. Labor market productivity costs for caregivers of children with spina bifida: a population-based analysis. Med Decis Making. 2009;29:23–32. doi: 10.1177/0272989X08322014. [DOI] [PubMed] [Google Scholar]
- 35.Llanos A, Hertrampf E, Cortes C, Pardo A, Grosse SD, Uauy R. Cost-effectiveness of a folic acid fortification program in Chile. Health Policy. 2007;83:295–303. doi: 10.1016/j.healthpol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence prevention program. Am J Prev Med. 2008;35:572–577. doi: 10.1016/j.amepre.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Rein DB, Saaddine JB, Wittenborn JS, et al. Cost-effectiveness of vitamin therapy for age-related macular degeneration. Ophthalmology. 2007;114:1319–1326. doi: 10.1016/j.ophtha.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg JM. Clinical economics: a guide to the economic analysis of clinical practices. JAMA. 1989;262(20):2879–2886. doi: 10.1001/jama.262.20.2879. [DOI] [PubMed] [Google Scholar]
- 39.Agency for Healthcare Research and Quality . Cost-effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. Agency for Healthcare Research and Quality; Rockville, MD: 2007. [Accessed June 15, 2010]. Available at: http://www.cms.gov/determinationprocess/downloads/id52TA.pdf. [PubMed] [Google Scholar]
- 40.Agency for Healthcare Research and Quality . Cost-effectiveness of CT Colonography to Screen for Colorectal Cancer. Agency for Healthcare Research and Quality; Rockville, MD: 2009. [Accessed June 15, 2010]. Available at: http://www1.cms.gov/determinationprocess/downloads/id58TA.pdf. [PubMed] [Google Scholar]
- 41.Yabroff KR, Brown ML, Lawrence WF, Barnett PG, Lipscomb J, editors. Health care costing: data, methods, future directions. Medical Care. 2009;47(7 Suppl 1):S1–S142. doi: 10.1097/MLR.0b013e3181a7e401. [DOI] [PubMed] [Google Scholar]
- 42.Dong D, Lin B-H. Fruit and Vegetable Consumption by Low-Income Americans: Would a Price Reduction Make a Difference? U.S. Department of Agriculture; Washington, DC: 2009. [Accessed June 30, 2010]. ERR-70. Available at: http://www.ers.usda.gov/Publications/ERR70/ERR70.pdf. [Google Scholar]
- 43.Dong D, Leibtag E. Promoting Fruit and Vegetable Consumption: Are Coupons More Effective than Pure Price Discounts? U.S. Department of Agriculture; Washington, DC: 2010. [Accessed June 30, 2010]. ERR-96. Available at: http://www.ers.usda.gov/Publications/ERR96/ERR96.pdf. [Google Scholar]
- 44.Lin B-H, Smith TA, Lee J-Y. The effects of a sugar-sweetened beverage tax: consumption, calorie intake, obesity, and tax burden by income. Agricultural & Applied Economics Association Meeting; Denver, CO. 2010. [Accessed June 15, 2010]. Available at: http://ageconsearch.umn.edu/bitstream/61167/2/AAEA%20_05122010_.pdf. [Google Scholar]
- 45.Ver Ploeg M. WIC and the Battle against Childhood Overweight. [Accessed June 30, 2010];Economic Brief Number 13. 2007 April; Available at: http://www.ers.usda.gov/Publications/EB13/EB13.pdf.
- 46.Ver Ploeg M, Ralston K. Food Stamps and Obesity: What Do We Know? U.S. Department of Agriculture; Washington, DC: 2008. [Accessed June 30, 2010]. EIB-34. Available at: http://www.ers.usda.gov/Publications/EIB34/EIB34.pdf. [Google Scholar]
- 47.Newman C, Guthrie J, Mancino L, Ralston K, Musiker M. Meeting Total Fat Requirements for School Lunches: Influence of School Policies and Characteristics. U.S. Department of Agriculture; Washington, DC: 2009. [Accessed June 30, 2010]. ERR-87. Available at: http://www.ers.usda.gov/Publications/ERR87/ERR87.pdf. [Google Scholar]
- 48.Ver Ploeg M, Breneman V, Farrigan T, et al. Access to Affordable and Nutritious Food—Measuring and Understanding Food Deserts and Their Consequences: Report to Congress. U.S. Department of Agriculture; Washington, DC: 2009. [Accessed June 30, 2010]. AP-036. Available at: http://www.ers.usda.gov/Publications/AP/AP036/AP036.pdf. [Google Scholar]
- 49.Just DR, Mancino L, Wansink B. Could Behavioral Economics Help Improve Diet Quality for Nutrition Assistance Program Participants? U.S. Department of Agriculture; Washington, DC: 2007. [Accessed June 30, 2010]. ERR-43. Available at: http://www.ers.usda.gov/publications/err43/err43.pdf. [Google Scholar]
- 50.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond MF, Schwartz JS, Jönsson B, Luce BR, Neumann PJ, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24:244–58. doi: 10.1017/S0266462308080343. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzer R, Siebert U. Methods, procedures, and contextual characteristics of health technology assessment and health policy decision making: comparison of health technology assessment agencies in Germany, United Kingdom, France, and Sweden. Int J Technol Assess Health Care. 2009;25:305–14. doi: 10.1017/S0266462309990092. [DOI] [PubMed] [Google Scholar]