Abstract
Background and Aims
In temperate woody perennials, flower bud development is halted during the winter, when the buds enter dormancy. This dormant period is a prerequisite for adequate flowering, is genetically regulated, and plays a clear role in possibly adapting species and cultivars to climatic areas. However, information on the biological events underpinning dormancy is lacking. Stamen development, with clear differentiated stages, appears as a good framework to put dormancy in a developmental context. Here, stamen developmental changes are characterized in apricot (Prunus armeniaca) and are related to dormancy.
Methods
Stamen development was characterized cytochemically from the end of August to March, over 4 years. Developmental changes were related to dormancy, using the existing empirical information on chilling requirements.
Key Results
Stamen development continued during the autumn, and the flower buds entered dormancy with a fully developed sporogenous tissue. Although no anatomical changes were observed during dormancy, breaking of dormancy occurred following a clear sequence of events. Starch accumulated in particular places, pre-empting further development in those areas. Vascular bundles developed and pollen mother cells underwent meiosis followed by microspore development.
Conclusions
Dormancy appears to mark a boundary between the development of the sporogenous tissue and the occurrence of meiosis for further microspore development. Breaking of dormancy occurs following a clear sequence of events, providing a developmental context in which to study winter dormancy and to evaluate differences in chilling requirements among genotypes.
Keywords: Apricot, cambium activation, carbohydrate partitioning, chilling requirement, dormancy, meiosis, pollen development, Prunus armeniaca, stamen development, vascular differentiation
INTRODUCTION
Stamen and pollen development is a highly conserved process in angiosperms (Hong, 2005; Feng and Dickinson, 2007; Dickinson and Grant-Downton, 2009) with an equally highly conserved genetic control (Borg et al., 2009; Wilson and Zhang, 2009). This consistent developmental process contrasts with the very different conditions in which it occurs and the very different timing of the events involved. Whereas in herbaceous plants the process from stamen initiation to pollen shedding may last for a few weeks (Smyth et al., 1990), in some woody perennials this process may last for several months (Jansson and Douglas, 2007). This is due to the fact that plant expansion to different climates and latitudes has gone through adaptation to cold winters. Two main strategies have been adopted: vernalization, where the plant overwinters in a quiescent stage and cold is required for flower induction (Sung and Amasino, 2005); or dormancy, where flower bud development is initiated in the autumn, enters the winter in a dormant stage (Rohde and Bhalerao, 2007), and resumes growth after dormancy. Whereas biennials and winter annuals usually vernalize, dormancy is common in woody perennial species adapted to temperate climates (Chouard, 1960).
Dormancy is a strategy adopted by phylogenetically very different woody perennial species, such as Populus and Prunus, and plays a clear role in the adaptation of species and cultivars to particular climates and latitudes. The period of time during which the flowers remain dormant is genetically regulated as each genotype or cultivar has particular cold requirements and these requirements are constant over the years and locations (Jansson and Douglas, 2007). In fruit trees, in which cultivars are clones of the same genotype, these differences among cultivars are a clear criterion for cultivar selection to a particular location, and have clear agronomic and economic relevance. Moreover, chilling requirement is the main restriction to the extension of temperate fruit crops to warmer climates as the cold period is required for proper flower bud development and flowering (Perry, 1971).
Winter dormancy in trees is referred to as endodormancy, in which chilling requirements are accumulated, and the flower bud is incapable of responding to higher temperatures and dormancy break. The term ecodormancy is used, once the chilling requirements are fulfilled, when the flower buds respond to higher temperatures with bud burst, but they remain in an apparent quiescent stage due to continued low temperatures (Lang et al., 1987; Horvath et al., 2003). Thus, it is difficult to establish when flower buds have broken endodormancy and resume growth, as no external signs of development can be detected until budburst, several weeks later. In spite of the importance of this parameter, no clear indicator for the breaking of dormancy is yet available, and cold requirements in temperate fruit trees are empirically calculated from temperature data or by observing signs of bud growth. This has resulted in large differences in the estimation of chilling requirements of particular cultivars depending on the method of calculation.
Although chilling requirements vary greatly among genotypes, little is known about the physiological changes underlying these differences (Faust et al., 1997; Rohde and Bhalerao, 2007) and their influence in bud dormancy (Weinbaum et al., 1989; Bartolini et al., 2006). The search for metabolic indicators during cold acclimation (Guy, 1990; Welling and Palva, 2006), dormancy (Smith and Kenfford, 1964; Arora et al., 2003) and de-acclimation (Kalberer et al., 2006) has been intermittent, encouraged by the importance of the subject, but discouraged by the failure to gain a clear picture of the process. More recently, a rapid advance has been taking place in our understanding of how plants perceive temperature. Chromatin remodelling has been shown to be involved in vernalization in Arabidopsis (Henderson and Dean, 2004) and also in other temperature response processes (Kumar and Wigge, 2010), and it has been hypothesized that it could also be involved in dormancy (Horvath et al., 2003; Rohde and Bhalerao, 2007). However, the lack of a well-studied developmental framework hampers the evaluation of this hypothesis.
Flower differentiation in temperate fruit trees starts at the end of the previous summer and goes on through the autumn, with the differentiation of all floral whorls; bud development is arrested during the winter. However, information is surprisingly scarce on the biological events behind dormancy, and, indeed, no biological indicators of this parameter exist, except a posteriori when bud growth can be recorded through an increase in bud weight and later on by bud burst (Brown and Kotob, 1957).
Stamen and pollen development appears as an attractive candidate to provide a framework to study dormancy in detail, as it has clear landmark developmental stages that can be precisely framed within a timescale. This process has been characterized in model species such as Arabidopsis (Sanders et al., 1999), rice (Raghavan, 1988), tobacco (Koltunow et al., 1990) and tomato (Brukhin et al., 2003), but also in some woody perennials, such as poplars (Boes and Strauss, 1994). Comparative studies show that both the developmental process and its genetic control are highly conserved (Scott et al., 2004; Wilson and Zhang, 2009; Pacini, 2010). However, no attention has been given to this process in relation to dormancy.
Fruit trees have an added bonus for the study of dormancy, as the number of cold hours required to break dormancy has been established for a number of cultivars, opening the way to follow stamen and pollen development in relation to dormancy. We were particularly interested in Prunus, because a good number of species, such as peach (P. persica), cherries (P. avium and P. cerasus), plums (P. domestica and P. salicina) and apricot (P. armeniaca) have edible fruits. Moreover, growth of these species in warmer latitudes is halted by the particular cold requirements of each cultivar, as failure to cover these cold requirements results in failures at flowering (Chandler and Tufts, 1933). Although events during pollen development have been described in several Prunus species (Pacini et al., 1986; Reinoso et al., 2002), these have not been considered in relation to dormancy. To fill this gap, here we follow anther and pollen developments in apricot (Prunus armeniaca) paying attention to how stamens enter into dormancy, whether any changes occur during dormancy and what the first events that take place at the breaking of dormancy are.
MATERIALS AND METHODS
Plant material
Two trees of apricot Prunus armeniaca ‘Moniqui’ were selected from an experimental orchard in the CITA in Montañana, Zaragoza (Spain) at 41°44′30″N, 0°47′00″W and 220 m altitude. The trees were grafted on ‘Montizo’ plum rootstock and planted in an orchard at 6 × 6 m. Flower buds were sampled weekly from fruitful short-shoots 10–35 cm in length around the canopy between the time of flower bud differentiation, in August–September, and the time of flowering, in February–March, over 4 years.
Apricot ‘Moniqui’ has been reported as having requirements of 1050–1150 chill units (CU) (Tabuenca, 1979) and 779–956 h below 7 °C (Tabuenca, 1968). The time when these chilling requirements were covered was estimated for the 4 years of experiments according to the number of hours below 7 °C (Weinberger, 1950) and to the Utah model (Richardson et al., 1974) adapted for apricot cultivars (Tabuenca, 1979) for CU. The date of chilling fulfilment in each year was related to developmental events in the flower buds.
Microscope preparations
A first survey of anther development was carried out in paraffin sections. For this purpose, ten flower buds were fixed weekly in FAA [70 % ethanol/acetic acid/formaldehyde (18 : 1 : 1, v/v/v)] (Johansen, 1940), after the removal of bud scales by forceps. Flower buds were dehydrated in a tertiary butyl alcohol series (70, 85, 95 and 100 %, v/v), embedded in Leica Histowax paraffin (Leica Microsystems, Wetzlar, Germany) and sectioned at 10 µm in a Reichert-Jung 1130/Biocut rotatory microtome (Reichert-Jung, Heidelberg, Germany). Prior to staining, sections were rehydrated [three washes in Histoclear (CellPath, Hemel, UK), one in Histoclear/ethanol (1 : 1, v/v) and an ethanol series (100, 70 and 40 %, v/v)] and stained with 0·07 % calcofluor in water for cellulose (Hughes and McCully, 1975).
Five additional flower buds per sample date were fixed in 3 : 1 (v/v) ethanol/acetic acid for 24 h and transferred to 75 % ethanol at 4 °C for storage (Williams et al., 1999). Five additional flower buds per sample date were fixed in glutaraldehyde at 2·5 % in 0·03 m phosphate buffer (Sabatini et al., 1963). Flower buds were dehydrated in an ethanol series and embedded in JB4 plastic resin (Polyscience Inc., Warrington, Philadelphia, PA, USA), sectioned at 2 µm in a Leica 2045 multicut microtome (Leica Microsystems) and mounted on slides covered with 1 % gelatin.
Resin sections were stained with periodic acid-Schiff's reagent (PAS) for insoluble carbohydrates (Feder and O'Brien, 1968), with PAS and Toluidine Blue for general histological observations (Feder and O'Brien, 1968), with 0·07 % calcofluor in water for cellulose (Hughes and McCully, 1975) and with 0·01 % auramine in water (w/v) for cutin (Heslop-Harrison, 1977). To observe nuclei during pollen development, sections of material fixed in 3 : 1 (v/v) ethanol/acetic acid were stained with a solution of 1 mg ml−1 4′,6-diamidino-2-phenylindole (DAPI) in 0·05 m TRIS buffer (pH 7·2) (Williams et al., 1999). To detect mitotic activity and xylem connections, a double staining with DAPI and 0·01 % acridine orange in water was used. Optimum staining was achieved with DAPI 1 : 100 and acridine orange 10 : 25 in phosphate-buffered saline (PBS), applying drops directly on sections for 5 min. Sections were de-stained in three washes of PBS (5 min each), rinsed and mounted in water (Dudley et al., 1987).
Preparations were observed under a UV epifluorescence Leica DM2500 microscope (Leica Microsystems) with a 340–380 bandpass and 425 longpass filter for DAPI and acridine orange, a 450–490 bandpass and 515 longpass filter for auramine, and 355–425 bandpass and 470 longpass filter for calcofluor. Sections were also observed by phase contrast microscopy and with differential interference contrast (DIC). Micrographs were taken with a Leica DC-150 digital camera (Leica Microsystems) for fluorescence microscopy and Leica DC-320 for light microscopy.
RESULTS
Anther development and chilling requirements
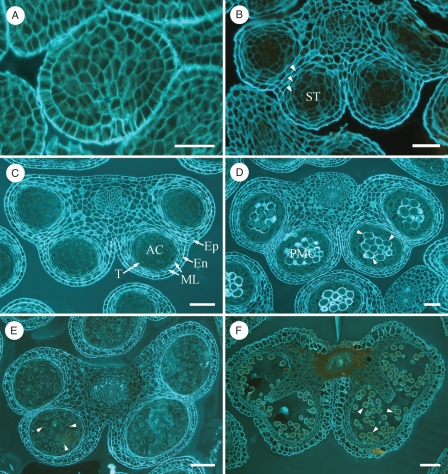
Under our climatic conditions, flower buds started differentiation in August. During the autumn the flower whorls differentiated, and in the winter the flower bud entered dormancy with its development apparently halted. After the dormancy period, the flower bud resumed development, which accelerated towards bud burst. Stamens initiated their differentiation at the end of August, 10 d after the first signs of flower differentiation were apparent, following differentiation of petals and sepals, and prior to the emergence of the pistil primordium. Anthers showed a homogeneous appearance (Fig. 1A) before dormancy. Anther layers differentiated during the autumn (Fig. 1B). During winter, the archesporal cells and all the anther layers (epidermis, endothecium, three to four middle layers and a single layered tapetum) were clearly distinguishable (Fig. 1C). After dormancy, the first apparent change was the laying down of a thick cellulose and callose wall around the pollen mother cells (Fig. 1D), as they entered meiosis. This was followed by microspore development accompanied by conspicuous changes in the stamen (Fig. 1E) up to anthesis, when the anthers and pollen grains were fully mature (Fig. 1F).
Fig. 1.
Anther development in Prunus armeniaca. (A) Anther differentiation before dormancy, showing a homogenous appearance in the summer. (B) A well-defined stamen structure, with early cell layers (arrowheads) and sporogenous tissue (ST) in the autumn. (C) During winter dormancy, all anther layers were differentiating: epidermis (Ep), endothecium (En), middle layers (ML) and a single-layered tapetum (T), and archesporal cells (AC) were distinguishable. (D) After dormancy, the pollen mother cells (PMC) were layered with a thick cellulosic wall (arrowheads). (E) Following meiosis, young microspores (arrowheads) appeared. (F) Mature anther at anthesis with pollen grains (arrowheads). Transverse 2-μm sections of anthers embedded in JB4 and stained with calcofluor. Scale bars: (A–C) = 20 µm; (D) = 40 µm; (E, F) = 100 µm.
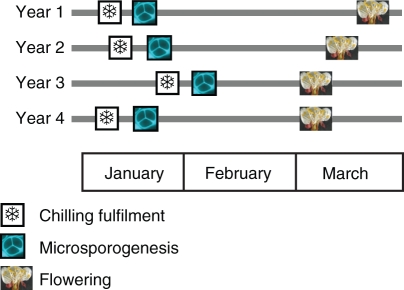
To frame these events within a dormancy context, the dates of chilling fulfilment were empirically calculated with temperature data according to the chilling requirements available for this cultivar in our conditions (Julian et al., 2007, and references therein). Chilling requirements were fulfilled in mid-January in the 4 years of experiments. This occurred with apparently resting closed flower buds, some 5 weeks before bud break, and 8 weeks before anthesis. The dates of chilling fulfilment for the 4 years were related to the observed developmental steps in the anther in each year (Fig. 2). In all 4 years, meiosis closely followed the empirical calculation of chilling requirements.
Fig. 2.
Timing of chilling fulfilment, microsporogenesis and flowering in Prunus armeniaca in 4 years of experiments.
Breaking dormancy: the first changes
Once the chilling requirements were fulfilled, the stamens were examined. During dormancy, the whole stamen was devoid of starch. This lack of starch was also clear in the pre-vascular area (Fig. 3A, B), but breaking endodormancy was accompanied by starch accumulation in this area (Fig. 3C, D). However, starch grains soon vanished in the pre-vascular area (Fig. 3E, F), as mitotic activity was clearly observed in the procambium (Fig. 3G, H). This preceded vascular differentiation, and young xylem vessels were soon seen in this area (Fig. 3I, J), which rapidly developed (Fig. 3K) and were well developed some 2 weeks later (Fig. 3L).
Fig. 3.
Mitotic activity and vascular differentiation in the stamen at breaking of dormancy in Prunus armeniaca. (A, B) Pre-vascular area (PA) void of starch during endodormancy. (C, D) Once chilling requirements were fulfilled, starch grains (arrowheads) began to accumulate around the pre-vascular area. (E, F) Starch progressively vanished from the pre-vascular area. (G, H) Concomitantly, mitotic activity (arrowheads) was apparent in the pre-vascular area (white arrow). (I, J) Establishment of young xylem vessels (arrowheads) following mitotic activity. Young (K) and (L) well-developed vascular connections (arrowheads) following breaking of dormancy. Transverse sections of stamens embedded in JB4 and stained with PAS and Toluidine blue (A, C, E); with PAS (B, D, F); with DAPI and acridine orange (G–J), and with calcofluor (K, L). Scale bars: (A, C, E, G, I, K, L) = 40 µm; (B, D, F) = 20 µm; (H, J) = 10 µm.
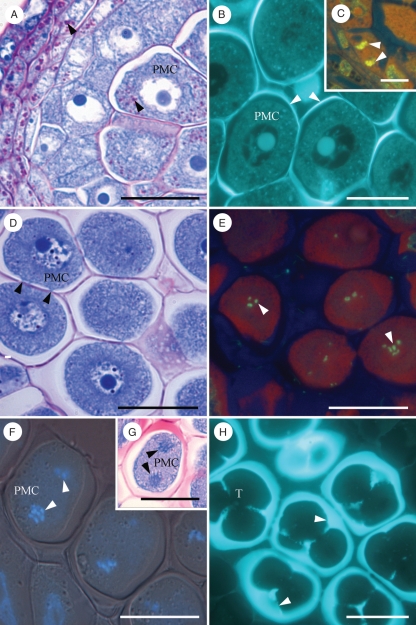
Breaking of dormancy was accompanied by starch accumulation in the stamen. This occurred in a radial way starting in the pre-vascular area and extending to the anther layers: epidermis, middle layer and endothecium; and also in the pollen mother cells (Fig. 4A), but was absent in the tapetum. A thick wall that stained with calcofluor for cellulose and with aniline blue for callose surrounded the pollen mother cells (PMCs) (Fig. 4B). At the same time, the nuclei of the tapetum cells divided (Fig. 4C) and these cells became bi- or multinucleate. Starch vanished from PMCs, which were initially connected by cytomictic channels (Fig. 4D), and meiosis was apparent (Fig. 4E). Concomitantly, the tapetum started fragmentation. Cytomictic channels were no longer present at the end of the first meiotic division (Fig. 4F, G). Meiosis proceeded rapidly and was completed within 1 week. Following telophase II, callose layered around each haploid cell forming the tetrad (Fig. 4H). Meiosis was synchronized within each locule and anther. But after meiosis, asynchrony in the development of the different microspores was observed within a locule of the same anther. In addition, differences in anthers within the same flower bud were detected at all stages.
Fig. 4.
Microsporogenesis in Prunus armeniaca. (A) Starch (arrowheads) accumulated progressively in the anther layers and in the pollen mother cells (PMC). (B) Callose deposition (arrowheads) surrounding the PMC and (C) nuclei division (arrowheads) of tapetum cells indicate the starting of microsporogenesis. (D) Starch vanished from the PMC, which were initially connected by cytomictic channels (arrowheads) and (E) chromosomes (arrowheads) became evident at the end of prophase I. (F) At telophase I (arrowheads), (G) cytomictic channels disappeared isolating each PMC. (H) Following telophase II, a second callose deposition (arrowheads) isolated each microspore forming the tetrad (T). Transverse 2-μm sections of anthers embedded in JB4 and stained with PAS and Toluidine blue (A, D, G); with calcofluor (B, H); with DAPI and acridine orange in (C, E); with DAPI superimposed with phase contrast (F). Scale bars = 20 µm.
Microspores were released from the tetrads and young microspores developed rapidly as the anther degenerated. As the microspore developed, starch vanished from the anther layers, following the same order in which it accumulated: from the vascular bundles and the connective tissue to the endothecium. Starch was no longer visible in these layers at bud burst.
DISCUSSION
Stamen development and dormancy
Stamen development of apricot followed a conserved pattern, common to other species. However, results herein show that winter dormancy sets a boundary between the development of the sporogenous tissue and further microspore development. The stamens developed during the autumn until they reached a stage at which all anther layers and sporogenous tissue were well differentiated. The stamens remained in this anatomically quiescent stage during the winter for some 3 months, to resume growth and microspore development after dormancy. The sequence of developmental events observed in apricot stamens fits with previous reports in several Prunus species (Brown, 1952; Pacini et al., 1986; Reinoso et al., 2002), other woody perennials (Boes and Strauss, 1994) and even most gymnosperms (Gifford and Foster, 1987). Indeed, anther differentiation (Feng and Dickinson, 2007, 2010a, b) and pollen development (Knox, 1984; Scott et al., 2004; Hong, 2005; Dickinson and Grant-Downton, 2009; Pacini, 2010) appear to comprise a well-conserved process in angiosperms.
Interestingly, whenever this process has been timed, the phase prior to meiosis, in which stamens enter dormancy, constitutes a relatively long phase. For instance, in Arabidopsis this phase lasts for 2–3 d in relation to a total floral developmental process of 13 d (Smyth et al., 1990). Although pollen development in relation to winter dormancy in temperate trees has been overlooked, it might be worth investigating whether flowers in other species enter dormancy in this same developmental stage, and if winter dormancy also sets a boundary between the sporogenous tissue and further microspore development. If this occurs, it would provide a good framework to look at the early changes accompanying the breaking of dormancy. It would be interesting to ascertain whether the anthers arrest before or after the premeiotic S phase, and also the possible changes in euchromatin remodelling during this apparently long-lasting stage. Although a number of developmental and genetic changes accompany the onset of meiosis, the real switch remains elusive (Dickinson and Grant-Downton, 2009).
Breaking dormancy
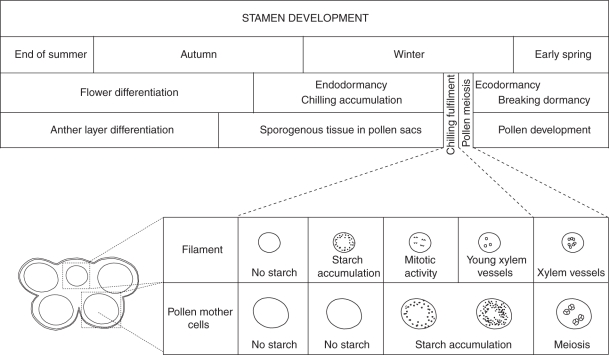
Once the chilling requirements were fulfilled, and prior to any apparent external changes, breaking dormancy was accompanied by starch accumulation in the pre-vascular area of the stamen filament, as a prelude to further vascular differentiation in this area. This starch vanished as the procambium was activated and vascular connections were established. An increase in activity was then observed in the long dormant sporogenous tissue of the anther, announcing the beginning of meiosis (Fig. 5).
Fig. 5.
Diagram representing the sequence of developmental events in the stamen, specifying those occurring at the breaking of dormancy.
The establishment of vascular connections has not been previously associated with breaking of dormancy in flower buds. However, indirect evidence supports this view as cold de-acclimation (Ashworth, 1984; Ashworth et al., 1989) and frost sensitivity (Julian et al., 2007), which are known to occur after rest break, have been related to the establishment of vascular connections in the flower bud. Indeed, this does not appear to be a special feature of flower buds, as cambial reactivation of vegetative buds appears to be the first sign of breaking the quiescent dormant stage in temperate trees (Fonti et al., 2007; Begum et al., 2007, 2008) and is accompanied by a cascade of active gene expression (Schrader et al., 2004; Druart et al., 2007). Interestingly, starch also accumulates close to the cambium in vegetative buds, and is degraded concomitantly with cambium reactivation in both deciduous (Begum et al., 2007; Deslauriers et al., 2009) and evergreen trees (Oribe et al., 2001, 2003).
The establishment of vascular connections is related to the beginning of pollen development. Meiosis is a highly conserved process in eukaryotes (Dickinson and Grant-Downton, 2009), but the causes behind the switch to meiosis in reproductive organs in plants remains elusive (Bennett, 1971; Bhatt et al., 2001; Wilson and Yang, 2004; Harrison et al., 2010). Temperature also appears to play a part, and higher temperatures have been related to the onset of meiosis in a number of species (Citadin et al., 2002). Results herein show that meiosis closely follows the establishment of vascular connections, but the implications of this observation have yet to be explored.
Carbohydrate partitioning in the stamen
Stamen development was accompanied by conspicuous changes in starch accumulation and degradation. This occurred in a precisely orchestrated way, in particular areas at particular times and was a prelude to activity in these areas. Following breaking of dormancy, starch accumulated first in the connective tissue and then in the anther layers in an orderly way, from the outer to the inner layers. Although the timing of starch accumulation in relation to breaking of dormancy has been overlooked, the accumulation of starch in the anther appears to be a conserved phenomenon in a number of unrelated species (Pacini et al., 1986; Clement et al., 1994; Lora et al., 2009). Moreover, sugar partitioning during pollen development is a key factor for viable pollen (Zhang et al., 2010).
Starch also accumulated in the pollen mother cell prior to meiosis and, after meiosis, microspore development was accompanied by starch vanishing from the stamen. This also occurred in an orderly way, following an inverse order to starch accumulation, from the inner to the outer layers of the anther wall, which is similar to observations in other angiosperms (Clement and Pacini, 2001). Starch disappearance was followed by the degradation of the anther layers, as reported in other species (Goldberg et al., 1993), starting with the tapetum, which follows the programmed cell death pattern (Wu and Cheung, 2000).
The results described here set a framework for stamen and pollen development in relation to winter dormancy. Dormancy in apricot established a boundary between the differentiation of the sporogenous tissue and further microspore development. The first observed change at breaking of dormancy was the establishment of vascular connections in the stamen followed by meiosis. This sequence of events sets the stage for the study of gene expression during pollen development in temperate woody perennials and also for studies of chilling requirements and adaptation in these species. Moreover, the similarity of events observed here in flower buds to those recently described for vegetative buds – in terms of breaking of dormancy and the establishment of vascular connections – sets a common ground for a rapid advance in this field.
ACKNOWLEDGEMENTS
We are grateful to an anonymous referee who made excellent suggestions that helped to substantially improve the manuscript and Professor J. I. Hormaza for helpful discussion and advice. This work was supported by Ministerio de Ciencia e Innovación – European Regional Development Fund, European Union (Grant numbers: AGL2006-13529-CO2-00, AGL2009-12621-C02-00); Gobierno de Aragón (Grupo de Excelencia de Aragón A-43); and Instituto Nacional de Investigación y Tecnología Agraria y Agroalimentaria (Doctoral fellowship INIA2003-8 to C.J.).
LITERATURE CITED
- Arora R, Rowland LJ, Tanino K. Induction and release of bud dormancy in woody perennials: a science comes of age. Hortscience. 2003;38:911–921. [Google Scholar]
- Ashworth EN. Xylem development in Prunus flower buds and the relationship to deep supercooling. Plant Physiology. 1984;74:862–865. doi: 10.1104/pp.74.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth EN, Davis GA, Wisniewski ME. The formation and distribution of ice within dormant and deacclimated peach flower buds. Plant Cell and Environment. 1989;12:521–528. [Google Scholar]
- Bartolini S, Viti R, Guerriero R. Xylem differentiation and microsporogenesis during dormancy of apricot flower buds (Prunus armeniaca L.) European Journal of Horticultural Science. 2006;71:84–90. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata) Annals of Botany. 2007;100:439–447. doi: 10.1093/aob/mcm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R. Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiology. 2008;28:1813–1819. doi: 10.1093/treephys/28.12.1813. [DOI] [PubMed] [Google Scholar]
- Bennett MD. Duration of meiosis. Proceedings of the Royal Society of London Series B-Biological Sciences. 1971;178:277–299. [Google Scholar]
- Bhatt AM, Canales C, Dickinson HG. Plant meiosis: the means to 1N. Trends in Plant Science. 2001;6:114–121. doi: 10.1016/s1360-1385(00)01861-6. [DOI] [PubMed] [Google Scholar]
- Boes TK, Strauss SH. Floral phenology and morphology of black cottonwood, Populus trichocarpa (Salicaceae) American Journal of Botany. 1994;81:562–567. [Google Scholar]
- Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. Journal of Experimental Botany. 2009;60:1465–1478. doi: 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- Brown DS. Relation of irrigation practice to the differentiation and development of apricot flower buds. Botanical Gazette. 1952;114:95–102. [Google Scholar]
- Brown DS, Kotob FA. Growth of flower buds of apricot, peach, and pear during the rest period. Proceedings of the American Society for Horticultural Science. 1957;69:158–164. [Google Scholar]
- Brukhin V, Hernould M, Gonzalez N, Chevalier C, Mouras A. Flower development schedule in tomato Lycopersicon esculentum cv. sweet cherry. Sexual Plant Reproduction. 2003;15:311–320. [Google Scholar]
- Citadin I, Raseira MCB, Herter FG, et al. Meiosis stage as an indicator for peach endodormancy end. Revista Brasileira de Fruticultura. 2002;24:23–28. [Google Scholar]
- Clement C, Pacini E. Anther plastids in angiosperms. Botanical Review. 2001;67:54–73. [Google Scholar]
- Clement C, Chavant L, Burrus M, Audran JC. Anther starch variations in Lilium during pollen development. Sexual Plant Reproduction. 1994;7:347–356. [Google Scholar]
- Chandler W, Tufts W. Influence of the rest period on opening of buds of fruit trees in spring and on development of flower buds of peach trees. Proceedings of the American Society for Horticultural Science. 1933;30:180–186. [Google Scholar]
- Chouard P. Vernalization and its relations to dormancy. Annual Review of Plant Physiology and Plant Molecular Biology. 1960;11:191–238. [Google Scholar]
- Deslauriers A, Giovannelli A, Rossi S, Castro G, Fragnelli G, Traversi L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiology. 2009;29:1223–1235. doi: 10.1093/treephys/tpp061. [DOI] [PubMed] [Google Scholar]
- Dickinson HG, Grant-Downton R. Bridging the generation gap: flowering plant gametophytes and animal germlines reveal unexpected similarities. Biological Reviews. 2009;84:589–615. doi: 10.1111/j.1469-185X.2009.00088.x. [DOI] [PubMed] [Google Scholar]
- Druart N, Johansson A, Baba K, et al. Environmental and hormonal regulation of the activity–dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant Journal. 2007;50:557–573. doi: 10.1111/j.1365-313X.2007.03077.x. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Jacobs TW, Long SR. Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti. Planta. 1987;171:289–301. doi: 10.1007/BF00398674. [DOI] [PubMed] [Google Scholar]
- Faust M, Erez A, Rowland LJ, Wang SY, Norman HA. Bud dormancy in perennial fruit trees: physiological basis for dormancy induction, maintenance, and release. Hortscience. 1997;32:623–629. [Google Scholar]
- Feder N, O'Brien TP. Plant microtechnique: some principles and new methods. American Journal of Botany. 1968;55:123–142. [Google Scholar]
- Feng XQ, Dickinson HG. Packaging the male germline in plants. Trends in Genetics. 2007;23:503–510. doi: 10.1016/j.tig.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Feng XQ, Dickinson HG. Cell–cell communication in plant reproduction. Biochemical Society Transactions. 2010a;38:571–576. doi: 10.1042/BST0380571. [DOI] [PubMed] [Google Scholar]
- Feng XQ, Dickinson HG. Tapetal cell fate, lineage and proliferation in the Arabidopsis anther. Development. 2010b;137:2409–2416. doi: 10.1242/dev.049320. [DOI] [PubMed] [Google Scholar]
- Fonti P, Solomonoff N, Garcia-Gonzalez I. Earlywood vessels of Castanea sativa record temperature before their formation. New Phytologist. 2007;173:562–570. doi: 10.1111/j.1469-8137.2006.01945.x. [DOI] [PubMed] [Google Scholar]
- Gifford EM, Foster AS. Morphology and evolution of vascular plants. 3rd edn. New York: W. H. Freeman; 1987. [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM. Anther development – basic principles and practical applications. Plant Cell. 1993;5:1217–1229. doi: 10.1105/tpc.5.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CL. Cold-acclimation and freezing stress tolerance – role of protein-metabolism. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:187–223. [Google Scholar]
- Harrison CJ, Alvey E, Henderson IR. Meiosis in flowering plants and other green organisms. Journal of Experimental Botany. 2010;61:2863–2875. doi: 10.1093/jxb/erq191. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Dean C. Control of Arabidopsis flowering: the chill before the bloom. Development. 2004;131:3829–3838. doi: 10.1242/dev.01294. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. The pollen stigma interaction: pollen tube penetration in Crocus. Annals of Botany. 1977;41:913–922. [Google Scholar]
- Hong M. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annual Review of Plant Biology. 2005;56:393–434. doi: 10.1146/annurev.arplant.55.031903.141717. [DOI] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Chao WS, Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends in Plant Science. 2003;8:534–540. doi: 10.1016/j.tplants.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hughes J, McCully ME. The use of an optical brightener in the study of plant structure. Stain Technology. 1975;50:319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Jansson S, Douglas CJ. Populus: a model system for plant biology. Annual Review of Plant Biology. 2007;58:435–458. doi: 10.1146/annurev.arplant.58.032806.103956. [DOI] [PubMed] [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. [Google Scholar]
- Julian C, Herrero M, Rodrigo J. Flower bud drop and pre-blossom frost damage in apricot (Prunus armeniaca L.) Journal of Applied Botany and Food Quality-Angewandte Botanik. 2007;81:21–25. [Google Scholar]
- Kalberer SR, Wisniewski M, Arora R. Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Science. 2006;171:3–16. [Google Scholar]
- Knox RB. The pollen grain. In: Johri BM, editor. Embryology of angiosperms. Heidelberg: Springer-Verlag; 1984. pp. 197–238. [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB. Different temporal and spatial gene-expression patterns occur during anther development. Plant Cell. 1990;2:1201–1224. doi: 10.1105/tpc.2.12.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. H2A.Z-Containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL. Endodormancy, paradormancy, and ecodormancy – physiological terminology and classification for dormancy research. Hortscience. 1987;22:371–377. [Google Scholar]
- Lora J, Testillano PS, Risueno MC, Hormaza JI, Herrero M. Pollen development in Annona cherimola Mill. (Annonaceae). Implications for the evolution of aggregated pollen. BMC Plant Biology. 2009;9:129. doi: 10.1186/1471-2229-9-129. doi:10.1186/1471-2229-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees-Structure and Function. 2003;17:185–192. [Google Scholar]
- Pacini E. Relationships between tapetum, loculus, and pollen during development. International Journal of Plant Sciences. 2010;171:1–11. [Google Scholar]
- Pacini E, Bellani LM, Lozzi R. Pollen, tapetum and anther development in two cultivars of sweet cherry (Prunus avium) Phytomorphology. 1986;36:197–210. [Google Scholar]
- Perry TO. Dormancy of trees in winter. Science. 1971;171:29–36. doi: 10.1126/science.171.3966.29. [DOI] [PubMed] [Google Scholar]
- Raghavan V. Anther and pollen development in rice (Oryza sativa) American Journal of Botany. 1988;75:183–196. [Google Scholar]
- Reinoso H, Luna V, Pharis RP, Bottini R. Dormancy in peach (Prunus persica) flower buds. V. Anatomy of bud development in relation to phenological stage. Canadian Journal of Botany-Revue Canadienne De Botanique. 2002;80:656–663. [Google Scholar]
- Richardson EA, Seeley SD, Walker DR. A model for estimating the completion of rest for ‘Redhaven’ and ‘Elberta’ peach trees. Hortscience. 1974;9:331–332. [Google Scholar]
- Rohde A, Bhalerao RP. Plant dormancy in the perennial context. Trends in Plant Science. 2007;12:1360–1385. doi: 10.1016/j.tplants.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Sabatini DD, Bench K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. Journal of Cell Biology. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, et al. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sexual Plant Reproduction. 1999;11:297–322. [Google Scholar]
- Scott RJ, Spielman M, Dickinson HG. Stamen structure and function. Plant Cell. 2004;16:S46–S60. doi: 10.1105/tpc.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Moyle R, Bhalerao R, et al. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant Journal. 2004;40:173–187. doi: 10.1111/j.1365-313X.2004.02199.x. [DOI] [PubMed] [Google Scholar]
- Smith H, Kenfford NP. Chemical regulation of dormancy phases of bud development. American Journal of Botany. 1964;51:1002–1012. [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Amasino RM. Remembering winter: toward a molecular understanding of vernalization. Annual Review of Plant Biology. 2005;56:491–508. doi: 10.1146/annurev.arplant.56.032604.144307. [DOI] [PubMed] [Google Scholar]
- Tabuenca MC. Necesidades de frío invernal en variedades de albaricoquero. Anales de la Estación Experimental de Aula Dei. 1968;9:10–24. [Google Scholar]
- Tabuenca MC. Duración del periodo de reposo a distintas temperaturas y evaluación de las necesidades de frío en variedades de albaricoquero y almendro. Anales de la Estación Experimental de Aula Dei. 1979;14:519–531. [Google Scholar]
- Weinbaum SA, Polito VS, Muraoka TT. Assessment of rest completion and its relationship to appearance of tetrads in anthers of Nonpareil almond. Scientia Horticulturae. 1989;38:69–76. [Google Scholar]
- Weinberger JH. Chilling requirements of peach varieties. Proceedings of the American Society for Horticultural Science. 1950;56:122–128. [Google Scholar]
- Welling A, Palva ET. Molecular control of cold acclimation in trees. Physiologia Plantarum. 2006;127:167–181. [Google Scholar]
- Wilson ZA, Yang CY. Plant gametogenesis: conservation and contrasts in development. Reproduction. 2004;128:483–492. doi: 10.1530/rep.1.00306. [DOI] [PubMed] [Google Scholar]
- Wilson ZA, Zhang DB. From Arabidopsis to rice: pathways in pollen development. Journal of Experimental Botany. 2009;60:1479–1492. doi: 10.1093/jxb/erp095. [DOI] [PubMed] [Google Scholar]
- Williams JH, Friedman WE, Arnold ML. Developmental selection within the angiosperm style: using gamete DNA to visualize interspecific pollen competition. Proceedings of the National Academy of Sciences of the USA. 1999;96:9201–9206. doi: 10.1073/pnas.96.16.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Cheung AY. Programmed cell death in plant reproduction. Plant Molecular Biology. 2000;44:267–281. doi: 10.1023/a:1026536324081. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liang WQ, Yang XJ, et al. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell. 2010;22:672–689. doi: 10.1105/tpc.109.073668. [DOI] [PMC free article] [PubMed] [Google Scholar]