Abstract
Background and Scope
Self-incompatibility (SI) in flowering plants ensures the maintenance of genetic diversity by ensuring outbreeding. Different genetic and mechanistic systems of SI among flowering plants suggest either multiple origins of SI or considerable evolutionary diversification. In the grasses, SI is based on two loci, S and Z, which are both polyallelic: an incompatible reaction occurs only if both S and Z alleles are matched in individual pollen with alleles of the pistil on which they alight. Such incompatibility is referred to as gametophytic SI (GSI). The mechanics of grass GSI is poorly understood relative to the well-characterized S-RNase-based single-locus GSI systems (Solanaceae, Rosaceae, Plantaginaceae), or the Papaver recognition system that triggers a calcium-dependent signalling network culminating in programmed cell death. There is every reason to suggest that the grass SI system represents yet another mechanism of SI. S and Z loci have been mapped using isozymes to linkage groups C1 and C2 of the Triticeae consensus maps in Secale, Phalaris and Lolium. Recently, in Lolium perenne, in order to finely map and identify S and Z, more closely spaced markers have been developed based on cDNA and repeat DNA sequences, in part from genomic regions syntenic between the grasses. Several genes tightly linked to the S and Z loci were identified, but so far no convincing candidate has emerged.
Research and Progress
From subtracted Lolium immature stigma cDNA libraries derived from S and Z genotyped individuals enriched for SI potential component genes, kinase enzyme domains, a calmodulin-dependent kinase and a peptide with several calcium (Ca2+) binding domains were identified. Preliminary findings suggest that Ca2+ signalling and phosphorylation may be involved in Lolium GSI. This is supported by the inhibition of Lolium SI by Ca2+ channel blockers lanthanum (La3+) and verapamil, and by findings of increased phosphorylation activity during an SI response.
Keywords: Lolium perenne, perennial ryegrass, grasses, Poaceae, self-incompatibility, calcium inhibitors, lanthanum chloride, verapamil
INTRODUCTION
Self-incompatibility (SI) in flowering plants was first described by Darwin, and has since been recognized for its pivotal role in evolution and diversification as a means to promote outbreeding (Allen and Hiscock, 2008). Various systems have been described in which self-recognition depends on up to several independent multi-allelic loci. Recognition can depend solely on the genetic constitution of the haploid pollen (gametophytic SI, GSI), or involve the parental genomes (sporophytic SI, SSI), and self-fertilization can be prevented at any stage between first pollen/stigma contact and fertilization of the ovule, indicating considerable differentiation and divergence of SI mechanisms within plants (Takayama and Isogai, 2005; see Hiscock and Allen, 2008, for a review on diversity in pollen/stigma interactions). A four-locus SI system found in basal eudicot and monocot species is considered as ancestral, while seemingly simplified one- or two-locus systems may have been derived (Østerbye, 1975; Heslop-Harrison, 1978; discussed in Yang et al., 2008).
SI in grasses
The Poaceae have a two-locus (S and Z) GSI system, in which the presence of identical S and Z alleles in pollen and pistil prevents fertilization. Both S and Z are represented by a polyallelic series: Seventeen S and 17 Z alleles were discriminated in a naturally occurring perennial ryegrass pasture population (Fearon et al., 1994). SI occurs in at least 16 genera. Although first described for Secale cereale (Lundqvist, 1954) and Phalaris coerulescens (Hayman, 1956), the S–Z system has been found in many other species (reviewed in Li et al., 1997), and findings from studied model species will probably also inform investigations in other grass species, facilitating approaches such as map-based cloning of SI constituent genes.
The SI reaction requires at least one more independent locus for full functionality [T locus in Lolium (Thorogood and Hayward, 1991); equivalent to the S5 locus in Secale cereale L. (Voylokov et al., 1998)]. The only variant of this locus is a loss-of-function mutation that acts gametophytically and results in self-compatibility. Self-fertile mutants of the S and Z and T loci have been reported in Phalaris coerulescens (Hayman and Richter, 1992). Thorogood and Hayward (1992) also concluded that self-fertility, transferred from an accession of the self-compatible species Lolium temulentum to L. perenne and L. multiflorum, was determined by a self-fertility variant of the Z locus. It was later suggested that self-fertility was erroneously ascribed to the Z locus but was more likely to be due to a self-fertility mutation of the S locus (Thorogood et al., 2002). Any one or more of these three loss-of-function mutants could explain the emergence of self- compatible lineages in the grasses, such as the tribes Oryzeae and Triticeae. Interestingly, the presence of a self-fertile variant at any one locus does not preclude the existence of fully functional but, due to the complementarity nature of SI loci, unexpressed alleles at other loci. Although the fully sequenced grass model species rice and Brachypodium are self-compatible they could still be of use for the investigation of SI, as one or more of the SI genes are likely to be still present either as fully functional or modified variants.
The notion of a two-locus SI recognition system in grasses has not gone unchallenged and in-vitro pollination experiments in Briza (Murray, 1974) and Lolium (McCraw and Spoor, 1983a, b) conclude that, although gametophytically controlled, SI involves more than two loci. Intriguingly, Thomas and Murray (1975) reported late-acting stylar inhibition of pollen-tube growth in the grass species Cynodon dactylon more in common with S-RNase-type gametophytic systems, which might explain the results of McCraw and Spoor (1983a) who observed nominally self-compatible plants that set little or no seed in Lolium.
In crosses of Lolium temulentum with L. multiflorum and L. perenne, distortion had been observed previously for an isozyme locus, GOT/3 (Thorogood and Hayward, 1991), now known to be located on chromosome 3. Thorogood et al. (2002) reported linkage disequilibrium (LD) between markers on chromosomes 1 and 3, which also led to distorted marker segregation ratios in the latter. Mapping of the S locus revealed that maximum LD occurred in the region of the S locus on chromosome 1 and in the region of a restriction fragment length polymorphism wheat probe, WG889, used as a marker, on chromosome 3. The result was that a particular S/WG889 allele combination was very rarely transmitted through the male parent. The WG889 marker has high sequence similarity to a rice SRK gene that is represented by several clustered copies on chromosome 1. This chromosome is broadly synthenic with Lolium chromosome 3 suggesting a causal link. Of further note, it was possible to genotype plants based on a two-locus (SZ) system when classifying incompatibility based on pollen-tube inhibition at the stigmatic surface in the mapping family in which this LD was observed. If this extra locus determines late-acting stylar inhibition this could explain (1) Thomas and Murray's (1975) observations in C. dactylon and (2) McCraw and Spoor's observations (1983) of self-compatibility (as defined by stigmatic inhibition) in Lolium plants that ultimately set no seed.
Lolium perenne as a model to unravel SI in grasses
The outcrossing grass Lolium perenne (perennial ryegrass) is one of the most economically and environmentally important grass species and accounts for 70 % of all agricultural land use in the UK. The economic value of forage grasses in the UK measured by its end products, meat and milk, is £6 billion per annum (King et al., 2008). In addition to conventional agricultural use, grassland is of fundamental importance for tourism, leisure, recreation and the environment, including water quality. Several assets make L. perenne an excellent model for studying SI in allogamous grasses: (a) the species is diploid in its natural state; (b) well-characterized germplasm accessions and breeding populations exist; (c) comprehensive databases and bacterial artificial chromosome (BAC) libraries are available; (c) a large and rising number of molecular markers have been developed; (d) well-defined genetic maps have been constructed; (e) a Lolium physical mapping project is underway; and (f) map positions of SI-related loci have already been determined.
Furthermore, a Lolium reference genome is feasible with the advent of next-generation sequencing technologies. Among the economically important grasses, L. perenne is unusual in still retaining effective SI. As such, L. perenne is a valuable resource for developing experimental models that will clarify both the underlying genetic control and the associated physiological and biochemical responses involved in determining SI.
MAPPING THE S AND Z LOCI
Genetic mapping has been used to pinpoint the locations of the loci involved in the SI response. The isozyme phosphoglycoisomerase (PGI-2) was found to be linked to the S locus in L. perenne (Cornish et al., 1980). PGI-2 and the leaf peroxidase Prx-7 genes co-segregated with S in Secale on chromosome (C) 1R (Gertz and Wricke, 1989). The Z locus co-segregated with the beta-glucosidase and esterase 4/11 genes on C2R and the additional T locus with the esterase 5–7 complex on C5R in rye (Secale cereale) (Fuong et al., 1993). Recent mapping analysis in Secale (Voylokov et al., 1998; Hackauf and Wehling, 2005), Phalaris (Bian et al., 2004) and Lolium (Thorogood et al., 2002) has confirmed syntenic chromosomal locations of S and Z on C1 and C2, respectively, in accordance with the Triticeae consensus map (Armstead et al., 2002; Jones et al., 2002; Sim et al., 2005) (Fig. 1A, B). Evidence of linkage was also found between either the S or the Z locus and the isozyme glutamate oxalo-acetate transaminase, GOT/3, located on C3 (Thorogood and Hayward, 1991). Segregation distortion was observed on C3, where GOT/3 is located (Jones et al., 2002). However, this association to C3 is probably caused by interaction between a C3 gene with either S or a locus closely linked to S (discussed in Thorogood et al., 2002). Additionally, a self-fertility locus has been mapped on C5 of L. perenne, in a position that is likely to be orthologous to the Secale S5 and Phalaris T loci (Thorogood et al., 2005) (summarized by Yang et al., 2008).
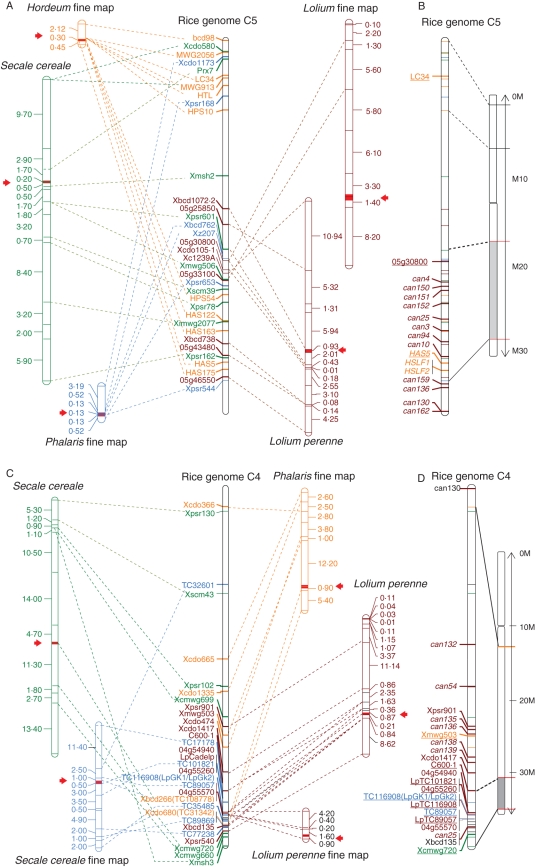
Fig. 1.
Syntenic relationships for the S locus to rice chromosome 5 and chromosome 1 and the Z locus to rice chromosome 4 and chromosome 2 of different grass species. MapChart (Voorrips, 2002) was used to create the maps. (A, B) Syntenic relationships for the S locus of rice chromosome 5 and chromosome 1 of different grass species: markers and candidate genes are indicated in different colours for the different species: Hordeum bulbosum (Kakeda et al., 2009) in orange; Secale cereale (Korzun et al., 2001) in green; Phalaris coerulescens (Bian et al., 2004) in blue and Lolium perenne (Yang et al., 2009) in dark red. (A) Relationship between the different grass markers on chromosome 1 and rice chromosome 5. The red arrows as well as the red part of each chromosome represent the S region. (B) Representation of the physical position of the closest markers (underlined) as well as candidate genes (Kakeda et al., 2009; Yang et al., 2009). The other markers linked to the rice chromosome 5 in (A) are represented by the coloured bars on the chromosome with the same position. (C, D) Syntenic relationships for the Z locus of rice chromosome 4 and chromosome 2 of different grass species: markers and candidate genes were indicated in different colours for the different species: Secale cereale (Korzun et al., 2001; Hackauf and Welhing, 2005) in green and blue, respectively; Phalaris coerulescens (Bian et al., 2004) in orange and Lolium perenne (Yang et al., 2009; Shinozuka et al., 2010) in dark red. (C) Relationship between the different grass markers on chromosome 2 and rice chromosome 4. The red arrows as well as the red part of each chromosome represent the S region. (D) Representation of the physical position of the closest markers (underlined) as well as candidate genes (Yang et al., 2009; Shinozuka et al., 2010). The other markers linked to the rice chromosome 4 in (C) are represented by the coloured bars on the chromosome with the same position.
Towards the identification of the S and Z genes
Numerous efforts have been made to identify the genes defining the S and Z loci, but to date none of the genes for S or Z on either the pollen or the pistil side is known. Candidates are probably expressed in mature pollen or pistil, and should consist of conserved functional domains, and more variable regions to account for the necessary allelic diversity. Li et al. (1994) reported a putative pollen S-gene clone, Bm2, identified from P. coerulescens. This gene, encoding a functional thioredoxin protein possibly involved in post-translational protein modification (Li et al., 1995), was originally found to co-segregate with the S genotype and was expressed only in mature pollen (Li et al., 1994). However, Baumann et al. (2000) found a recombination distance of 1 cM between Bm2 and S using a larger mapping population, indicating that Bm2 is not located at the S locus.
More recently, combined fine mapping and transcript identification efforts were undertaken in Hordeum bulbosum (Kakeda et al., 2008; Kakeda, 2009) and Lolium perenne (Van Daele et al., 2008; Yang et al., 2009; Shinozuka et al., 2010). Kakeda et al. (2008) employed amplified fragment-length polymorphism (AFLP) markers generated from cDNA (AMFs) of pistil and anther transcripts for fine mapping of the S locus in Hordeum bulbosum. They analysed DNA fragments with an S-haplotype-specific expression, and identified 11 representative S-linked clones from the AMF experiment. Ten of these were converted to PCR markers for detailed linkage analysis. Two anther-derived markers (HAS31 and HAS175) showed complete linkage to the S locus, seven were at a distance of 0·30 cM and another at 0·45 cM. The marker HPS10 hybridized specifically with the DNA from S3 haplotype individuals, and co-segregated completely with S3 in a separate restriction fragment-length polymorphism linkage analysis, and thus was mapped at the S locus. The completely linked genes would be candidates for the S gene, although only HPS10 has potentially sufficient variability, as indicated by its haplotype-specific hybridization signal. More recently, Kakeda (2009) isolated two F-box genes in rapid amplification of cDNA ends (RACE) reactions based on the partial clone HAS175. Intriguingly, this finding could indicate a connection between the grass S–Z system and the S-RNase-based GSI system in which F-box genes have been shown to be involved in S-RNase ubiquitination and degradation (discussed in Kakeda, 2009). Kakeda et al. (2009) reported suppressed recombination around the S locus in H. bulbosum due to its near-centromeric location. This could be a hurdle for a map-based cloning approach for the S locus in grasses generally, or it could simply be a specific characteristic of H. bulbosum. Suppressed recombination around S has also been observed in other SI plant species (e.g. Kamau and Charlesworth, 2005; Rahman et al., 2007) and could hamper map-based cloning.
In a similar approach to Kakeda et al. (2008), Van Daele et al. (2008) generated SI-related transcript-derived fragment (TDF) markers via cDNA-AFLP from pistil mRNA in Lolium. A total of 169 TDFs were expressed in an allele-specific way for both the S and the Z haplotypes. Sequence analysis identified gene functions of general cell metabolism, but also gene functions known to be involved in SI in other systems, such as ubiquitinization and receptor kinases. However, no linkage to any of the SI loci has been demonstrated so far.
Fine-scale mapping in Lolium
In an effort to isolate the S and Z loci, Shinozuka et al. (2010) used comparative genomic information to screen a representative BAC library from L. perenne. For the generation of a fine-scale map around the S and Z loci, Shinozuka et al. (2010) used comparative genomic information to develop cDNA and single sequence repeat-derived markers based on public sequence information of genes and sequences that were known to map close to the S and Z loci in other grasses. The alignment of orthologous sequences facilitated the identification of conserved sequences suitable for the design of PCR primers that were able to amplify intervening corresponding Lolium genomic targets. The new markers were employed for genotyping a Lolium mapping population from a UK variety crossed with a Moroccan ecotype. Although marker order was largely conserved between the grasses, several markers were reversed against the model species rye and rice. In an effort to isolate the Z locus physically, Shinozuka et al. (2010) used three of the markers close to the Z locus to screen a representative BAC library from L. perenne DNA. Three BAC clones containing Z-related DNA were identified and sequenced, and putative genes were analysed by BLAST database searches. Nine gene-like sequences were identified. Four were expressed in anther and pistil; two of these have some degree of nucleotide diversity expected of a Z gene, but the true identity of Z gene(s) will remain elusive until further functional studies are carried out, and mutations directly correlated to particular Z phenotypes are identified.
THE PHYSIOLOGICAL MECHANISMS INVOLVED IN GRASS SI
Considering the economic importance of grasses, surprisingly little is known about the physiological and biochemical events taking place following an incompatible interaction between pollen and stigma. It is known that pollen recognition following an incompatible pollination is very rapid as pollen tube growth is inhibited extremely quickly in grasses, within minutes of pollen contacting the stigma papilla cells. An extreme example is that observed in Gaudinia fragilis in which the incompatible response is observed within 30 s of pollen–stigma contact (Heslop-Harrison, 1982). The deposition of callose at the tube tip follows upon the arrest of growth, and is evidently a consequence, and not a cause, of the inhibition (Heslop-Harrison, 1979). Arrest of growth is generally seen to occur when the emerging and actively growing tube touches the stigma papilla (Heslop-Harrison, 1982), suggesting that it is the contact between the tube tip and the papilla that triggers the SI response leading to pollen tube growth arrest. The timescale of the SI response in grasses suggests that rapid signalling is already effective while gene expression is gearing up. Protein modification and Ca2+ flux signalling may well be involved in these rapid signalling events because their role in SI has been well demonstrated in other systems (Giranton et al., 2000; Franklin-Tong et al., 2002; Miljuš-Đukić et al., 2003).
Protein phosphorylation
Protein phosphorylation/dephosphorylation by specific protein kinases/phosphatases is one of the most important mechanisms for controlling many fundamental processes in all living organisms, including plants (Huber, 2007). Phosphorylation events play a crucial role in the signalling cascade of Papaver GSI and Brassica SSI. Phosphorylation of SRK (S-receptor kinase) triggers the activation of proteasomal protein degradation in Brassica SI (see Ivanov et al., 2010, for recent review), while phosphorylation of the p26 pyrophosphatases and p56 mitogen-activated protein kinase is crucial for the SI response in Papaver (Rudd et al., 1996, 2003). Moreover, a Nicotiana alata pollen Ca2+-dependent protein kinase has been shown to specifically phosphorylate the SI S-RNase (Kunz et al., 1996). For the grasses there is only indirect evidence of kinase participation from studies which used kinase inhibitor tests in Secale cereale (Wehling et al., 1994). Wehling et al. (1994) also found a pronounced increase in phosphorylation activity in rye pollen grains incubated with ‘self’ stigma proteins, and loss of SI in self-compatible rye mutants was associated with significantly decreased basic phosphorylation activity in pollen grains. Thus there is some indirect evidence for the involvement of phosphorylation events triggered upon an incompatible pollen–pistil interaction in grasses. However, a detailed analysis of potential phosphorylation events involved in grass SI signalling is lacking.
Involvement of calcium as a second messenger
The importance of cytosolic free calcium Ca2+ for the regulation of pollen tube growth is well known (Franklin-Tong, 1999; Holdaway-Clarke and Hepler, 2003; Ge et al., 2007). The presence of a tip-focused Ca2+ gradient is essential for pollen tube growth as disruption of this gradient invariably results in cessation of growth (Pierson et al., 1994). Franklin-Tong et al. (2002) showed that in Papaver, which has a gametophytic SI system, a rapid increase of cytosolic free Ca2+ in the shank region of the pollen tube is induced by the SI response in incompatible pollen. Concurrently, the high apical Ca2+, which is a key characteristic of growing pollen tubes, is lost. Recently, in Brassica rapa, which has a sporophytic SI system, Ca2+-signalling-related actin reorganization and probably depolymerization has been found in papilla cells after self-pollination, which in turns regulates hydration and germination of pollen (Iwano et al., 2007). In analogy to Papaver and Brassica SI, there is some evidence for the involvement of Ca2+-mediated signalling in grass SI. Application of the Ca2+ channel blockers verapamil and La3+ chloride to isolated stigmas resulted in suppression of the SI response upon self-pollination in Secale cereale (rye) (Wehling et al., 1994). Based on rapid pollen tube growth inhibition, the SI response in grasses could be hypothesized to involve signalling events initiated by a receptor–ligand interaction similar to that found in Papaver and Brassica SI. It is tempting to hypothesize that the stigma S and Z determinants act as signal molecules, by interacting with the ‘self’ pollen S and Z partners at the tube plasma membrane, inducing a Ca2+-mediated signal cascade that results in pollen tube inhibition. Such a mechanism, using Ca2+ as a second messenger, would allow for the rapid transduction of the ‘self’ signal into the germinating pollen grain, resulting in the rapid inhibition of tube elongation. However, the involvement of a Ca2+-mediated signalling cascade has still not unequivocally been established in grass SI.
Proteolysis or ubiquitination?
Two well-characterized pathways are mainly responsible for intracellular proteolysis: the ubiquitin–proteasome pathway and the autophagy–lysozome/vacuole pathway (for a review see Zhang et al., 2009). It has been shown that proteolytic events play important roles in self-pollen rejection during SI (Zhang et al., 2009; Meng et al., 2011, Solanaceae SI). Ubiquitin-mediated proteolysis is involved in SI of the Brassicaceae and the ubiquitin–proteasome pathway and the vacuole pathway appear to be involved in SI of the Solanaceae. Programmed cell death, an event related to proteolysis, is involved in SI of the Papaveraceae and may also play a role in the S-RNase-based SI system. Recent results also show that SI triggers changes to the vacuole network in Brassica stigmatic papillae (Iwano et al., 2007), suggesting a role for the vacuole pathway in the SI response. The fact that major proteolytic pathways are involved in single-locus SI systems suggests that similar events might occur in grass SI. The potential involvement of proteolytic pathways in grass SI has only been reported in rye: a putative ubiquitin-specific protease (UBP) gene that co-segregates with the Z locus is specifically expressed in the pistil (Hackauf and Wehling, 2005). However, it remains to be determined whether the UBP gene represents a candidate for the Z gene in any grass species.
CHARACTERIZATION/IDENTIFICATION OF CALCIUM-RELATED TRANSCRIPTS POTENTIALLY INVOLVED IN LOLIUM SI
Yang et al. (2009) constructed subtractive hybridization cDNA libraries to identify genes closely linked to the onset of the SI response. Because of the rapid response in Lolium (Heslop-Harrison, 1982), the first reacting SI components are probably already present in the pistil before pollination, and libraries of mature stigma, before and after pollination, were subtracted with immature stigma cDNAs to enrich for genes active within this time window. From a total of 890 clones hybridizing more strongly with post-pollination cDNAs rather than stigma cDNA, 82 genes were selected which mapped to either the S or the Z loci on LG1 and LG2 and which corresponded to rice sequences in regions syntenic around these loci. After further exclusion of pollen-specific genes, and genes without tissue-specific expression, ten sequences were analysed in more detail as potential S or Z gene candidates, or SI response genes. These genes were expressed in pollinated stigmas, where low levels of expression were detectable in mature stigmas only with the aide of real-time PCR (Yang et al., 2009). Nine genes expressed in a similar pattern, with a rapid increase and a maximum expression 2–10 min after pollination, followed by decreased expression over the next 20 min (for details see Yang et al., 2009). Only Can151 had a second increase in expression between 5 and 10 min after pollination. Some sequences were identified as related to proteins involved in growth processes (cell wall, lipid metabolism), and may regulate these processes in pollen tube growth or in pollen–pistil interactions. Three kinase genes and a kinase-partner protein were identified, with sequence similarity to allele-specific TDFs found by Van Daele et al. (2008), indicating a potential role for kinase activity in the Lolium SI response.
Two genes, Can3 and Can94, identified in this suppression subtractive hybridization experiment (Yang et al., 2009) were further characterized as they represented promising stigmatic candidate genes for the S and Z loci in L. perenne. The expression patterns of both Can3 and Can94 were tested in in-vitro pollination experiments (see detailed results in Yang et al., 2009). Mature isolated ovaries placed on agar were dusted with self-pollen, and both sequences were rapidly up-regulated, reaching peak expression within 2 min. Transcript levels remained high until 10 min after pollination and dropped thereafter. Extended cDNAs were generated using 5′ and 3′-RACE (Can3 1808 bp: accession number AM991123; Can94 1115 bp: accession number AM991124). Can3 comprised two partially overlapping open reading frames (ORFs) (bp 802–1587, bp 218 – 868) of 261 and 217 amino acids, and Can94 one ORF (bp 169–915) of 248 amino acids. At present it cannot be predicted if the Can3 message encodes two separate polypeptides, although similar transcripts of comparable length were detected in Triticum (AK332180, 88 % identity) and Oryza sativum (AK071552, 85 % identity; tentatively identified as calcineurin B like (CBL)-interacting serine/threonine protein kinase). Functional domains were predicted using InterProScan (http://www.ebi.ac.uk/Tools/InterProScan/). Both Can3 and Can94 have a calcium calmodulin-dependent protein kinase-related domain. Can3 has a serine/threonine protein kinase domain and a protein kinase catalytic domain whereas Can94 has four calcium-binding EF-hand domains (Table 1). To demonstrate the function of these transcripts it would be desirable to introduce them into Lolium genotypes which are amenable to plant transformation and investigate their effects in vivo.
Table 1.
Function domains identified for the two SI-involved candidate genes Can3 and Can94 by comparing their translated ORFS with the InterPro database* for protein function prediction
| Name | Amino acids | InterPro entry | Function domain |
|---|---|---|---|
| Can3, ORF1 (261 amino acids, nt 802–1587) | 1–71 | IPR000719 | Protein kinase, catalytic domain |
| 112–172 | IPR004041 | NAF domain | |
| 21–242 | IPR020636 | Calcium/calmodulin-dependent protein kinase-like | |
| Can3, ORF2 (216 amino acids, nt 218–868) | 12–216 | IPR000719 | Protein kinase, catalytic domain |
| 60–215 | IPR020636 | Calcium/calmodulin-dependent protein kinase-like | |
| 193–213 | n.a. | Transmembrane-region | |
| Can94 (248 amino acids, nt 169–915) | 1–228 | IPR020636 | Calcium/calmodulin-dependent protein kinase-like |
| 68–96 | IPR002048 | Calcium-binding EF-hand | |
| 104–132 | IPR002048 | Calcium-binding EF-hand | |
| 140–168 | IPR002048 | Calcium-binding EF-hand | |
| 173–201 | IPR002048 | Calcium-binding EF-hand |
* http://www.ebi.ac.uk/Tools/InterProScan/. The Can3 transcript contains two slightly overlapping ORFs of 261 and 216 amino acids in length which were analysed independently.
PROTEOMICS APPROACHES TO IDENTIFY SI PROTEINS
The grass SI reaction has also been preliminarily investigated at the level of changed protein accumulation and enzymatic activity in isolated styles treated with pollen coat eluates in Lolium multiflorum (Kalinowski et al., 2006). The protein profile in two-dimensional PAGE analysis changed considerably between self-compatible and self-incompatible combinations. Esterase activity was reduced in the SI samples, while protease activity increased. The observed effects at the protein level are most likely part of a later response stage of SI, although phosphatase/kinase signalling could well influence isozyme patterns and activities. More detailed protein identification and activity profiles will be necessary before the results can be integrated with efforts described above to identify the first truly SI-related transcripts.
The effect of calcium inhibitors on the SI response in Lolium
Ca2+ ions are one of the most widespread second messengers used in signal transduction, and as mentioned previously, calcium signalling is involved in Papaver and Brassica SI. To determine if a Ca2+-mediated signalling cascade is triggered upon an incompatible pollen–pistil interaction in Lolium, we tested the effect of Ca2+ inhibitors on the SI response in semi in vivo pollinated pistils of L. perenne plants of the International Lolium Genome Initiative population (Jones et al., 2002). Ovaries with attached stigmas were isolated from closed florets of spikelets which were close to anthesis and placed on Petri dishes containing either standard medium or experimental medium. Standard medium containing 2·5 % agar, 25 % sucrose and 25 p.p.m. boric acid was used as a control (Thorogood et al., 2002). The experimental medium contained various calcium inhibitors: 50 µm calcimycin, 10 mm EGTA, 1 mm lanthanum chloride (La3+), 10 µm ruthenium red and 100 µm verapamil. After incubation on the medium for 24 h, stigmas were pollinated with self-pollen, which had been collected in cellophane bags. Each experiment was repeated three times, with five ovaries at each time. Tewnty-four hours after in-vitro pollination, styles and stigmas were separated from the ovaries, stigmas were stained with a drop of aniline blue-lactophenol and pollen tube growth was observed by microscopy.
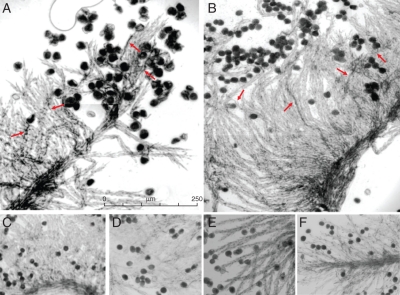
After semi in-vivo self-pollination, self-pollen tubes in isolated stigmas on control medium were arrested at or near the stigma surface (Fig. 2F). Treatment of isolated stigmas with the inorganic Ca2+ channel blocker La3+ resulted in the SI response being partially overcome in L. perenne, such that self-pollen tube growth was observed in 60 % of the stigmas and pollen tubes elongated down the style (Fig. 2A, Table 2). The effect of the organic Ca2+-permeable channel blocker verapamil was similar to that of La3+ with 38 % of self-pollen tubes showing growth down the style (Fig. 2B, Table 2). No pollen tube growth was observed in self-pollinated stigmas treated with the calcium ionophore calcimycin, the Ca2+ chelator EGTA or the organic Ca2+ channel blocker ruthenium red (Fig. 2C–E, Table 2). More detailed studies are necessary to distinguish between the effects of these inhibitors on the SI response and pollen tube growth per se. However, the clear effects of the Ca2+ channel blockers La3+ and verapamil make a strong case for the involvement of Ca2+ signalling in the Lolium SI response. This receives further indirect support from our SI candidate gene studies that identified Ca2+ binding and calmodulin-related protein domains in transcripts Can3 and Can94.
Fig. 2.
Results of treatment with calcium inhibitors. Unpollinated stigmas were placed on agar plates containing: (A) 1 mm LaCl3, (B) 100 µm verapamil hydrochloride, (C) 10 mm EGTA, (D) 10 µm ruthenium red and (E) 50 µm calcimycin; (F) standard agar plate (control). Pollen tube growth (indicated by red arrows) was detected in (A) and (B). No pollen tube growth was observed in (C), (D) or (E), the same as control (F).
Table 2.
Effects of calcium inhibitors on self-incompatible pollen tube growth
| Experiment 1 |
Experiment 2 |
Experiment 3 |
Average pollen tube growth (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of pollen | No. pollen tubes | Pollen growth (%) | No. of pollen | No. of pollen tubes | Pollen growth (%) | No. of pollen | No. of pollen tubes | Pollen growth (%) | ||
| Control | 98 | 0 | 0 | 105 | 0 | 0 | 126 | 0 | 0 | 0 |
| La3+ | 84 | 48 | 57·1 | 84 | 53 | 63·1 | 137 | 81 | 59·1 | 59·8 ± 3** |
| Verapamil | 205 | 77 | 37·6 | 144 | 49 | 34 | 167 | 71 | 42·5 | 38 ± 4* |
| Calcimycin | 151 | 0 | 0 | 107 | 0 | 0 | 100 | 0 | 0 | 0 |
| EGTA | 130 | 0 | 0 | 100 | 0 | 0 | 114 | 0 | 0 | 0 |
| Ruthenium red | 105 | 0 | 0 | 126 | 0 | 0 | 100 | 0 | 0 | 0 |
Percentage pollen tube growth is given as mean ± s.d. of three replicate experiments. **P < 0·001; *P < 0·01 as determined by Student's t-test.
CONCLUSIONS
Despite a number of efforts undertaken over the past six decades, largely based on recombination mapping, the nature and genetics of the gametophytic S–Z SI system in grasses is still not known and the S and Z loci remain to be identified. Our current mapping efforts of both S and Z loci in Lolium perenne have led to the identification of a small number of candidate genes within physical regions of 100 kb or less. Our recent studies of SI in Lolium have given some promising preliminary evidence that calcium signalling and protein phosphorylation are involved in the recognition and/or inhibition of incompatible pollen. Work in progress has identified transcripts predicted to code for proteins which contain calcium-binding domains and that SI in L. perenne is sensitive to chemical reagents with known effects on Ca2+ channelling across membranes. Although we are still far from understanding the precise role of intracellular Ca2+ concentrations in the L. perenne SI response, these initial results suggest that an increase in Ca2+ is associated with the SI response and that the role of Ca2+ in mediating L. perenne SI may be analogous to its role in the incompatibility responses of other plant species.
Studies on the mechanistic aspects of SI in grasses need to be further explored to understand the processes involved in SI and even if the S and/or Z loci were identified their physiological functions and processes need to be studied in greater detail.
ACKNOWLEDGEMENTS
Funding was provided through Teagasc core funding through the National Development Plan of Ireland. B.Y. and C.M. were financed by a Teagasc Walsh Fellow PhD studentship.
LITERATURE CITED
- Allen AM, Hiscock SJ. The evolution and phylogeny of self-incompatibility systems in angiosperms. In: Franklin-Tong V, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. New York: Springer; 2008. pp. 73–101. [Google Scholar]
- Armstead IP, Turner LB, King IP, Cairns AJ, Humphreys MO. Comparison and integration of genetic maps generated from F-2 and BC1-type mapping populations in perennial ryegrass. Plant Breeding. 2002;121:501–507. [Google Scholar]
- Baumann U, Juttner J, Bian XY, Langridge P. Self-incompatibility in the grasses. Annals of Botany. 2000;85:203–209. [Google Scholar]
- Bian X-Y, Friedrich A, Bai J-R, et al. High-resolution mapping of the S and Z loci of Phalaris coerulescens. Genome. 2004;47:918–930. doi: 10.1139/g04-017. [DOI] [PubMed] [Google Scholar]
- Cornish MA, Hayward MD, Lawrence MJ. Self-incompatibility in ryegrass III. The joint segregation of S and PGI-2 in Lolium perenne L. Heredity. 1980;44:55–62. [Google Scholar]
- Fearon CH, Cornish MA, Hayward MD, Lawrence MJ. Self-incompatibility in ryegrass. X. Number and frequency of alleles in a natural population of Lolium perenne L. Heredity. 1994;73:254–261. [Google Scholar]
- Franklin-Tong VE. Signalling in pollination. Current Opinion in Plant Biology. 1999;2:490–495. doi: 10.1016/s1369-5266(99)00017-5. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE, Holdaway-Clarke TL, Straatman KR, Kunkel JG, Hepler PK. Involvement of extracellular calcium influx in the self-incompatibility response of Papaver rhoeas. Plant Journal. 2002;29:333–345. doi: 10.1046/j.1365-313x.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- Fuong FT, Voylokov AV, Smirnov VG. Genetic studies of self-fertility in rye (Secale cereale L.). 2. Theoretical and Applied Genetics. 1993;87:619–623. doi: 10.1007/BF00221888. The search for isozyme marker genes linked to self-incompatibility loci. [DOI] [PubMed] [Google Scholar]
- Ge LL, Tian HQ, Russell SD. Calcium function and distribution during fertilization in angiosperms. American Journal of Botany. 2007;94:1046–1060. doi: 10.3732/ajb.94.6.1046. [DOI] [PubMed] [Google Scholar]
- Gertz A, Wricke G. Linkage between the incompatibility locus Z and a β-glucosidase locus in rye. Plant Breeding. 1989;102:255–259. [Google Scholar]
- Giranton J-L, Dumas C, Cock JM, Gaude T. The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proceedings of the National Academy of Sciences USA. 2000;97:3759–3764. doi: 10.1073/pnas.070025097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackauf B, Wehling P. Approaching the self-incompatibility locus Z in rye (Secale cereale L.) via comparative genetics. Theoretical and Applied Genetics. 2005;110:832–845. doi: 10.1007/s00122-004-1869-4. [DOI] [PubMed] [Google Scholar]
- Hayman DL. The genetical control of incompatibility in Phalaris coeruleus Desf. Australian Journal of Biological Sciences. 1956;9:321–333. [Google Scholar]
- Hayman DL, Richter J. Mutations affecting self-incompatibility in Phalaris-coerulescens Desf (Poaceae) Heredity. 1992;68:495–503. [Google Scholar]
- Heslop-Harrison J. Genetics and physiology of angiosperm incompatibility systems. Proceedings of the Royal Society of London Series B – Biological Sciences. 1978;202:73–92. [Google Scholar]
- Heslop-Harrison J. Pollen–stigma interaction in grasses: a brief review. New Zealand Journal of Botany. 1979;17:537–546. [Google Scholar]
- Heslop-Harrison J. Pollen–stigma interaction and cross-incompatibility in the grasses. Science. 1982;215:1358–1364. doi: 10.1126/science.215.4538.1358. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytologist. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Hepler PK. Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- Huber SC. Exploring the role of protein phosphorylation in plants: from signalling to metabolism. Biochemical Society Transactions. 2007;35:28–32. doi: 10.1042/BST0350028. [DOI] [PubMed] [Google Scholar]
- Ivanov R, Fobis-Loisy I, Gaude T. When no means no: guide to Brassicaceae self-incompatibility. Trends in Plant Sciences. 2010;15:387–394. doi: 10.1016/j.tplants.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Iwano M, Shiba H, Matoba K, et al. Actin dynamics in papilla cells of Brassica rapa during self- and cross-pollination. Plant Physiology. 2007;144:72–81. doi: 10.1104/pp.106.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ES, Mahoney NL, Hayward MD, et al. An enhanced molecular marker based genetic map of perennial ryegrass (Lolium perenne) reveals comparative relationships with other Poaceae genomes. Genome. 2002;45:282–295. doi: 10.1139/g01-144. [DOI] [PubMed] [Google Scholar]
- Kakeda K. S locus –linked F-box gene expressed in anthers of Hordeum bulbosum. Plant Cell Reports. 2009;28:1453–1460. doi: 10.1007/s00299-009-0745-8. [DOI] [PubMed] [Google Scholar]
- Kakeda K, Ibuki T, Suzuki J, et al. Molecular and genetic characterization of the S locus in Hordeum bulbosum L., a wild self-incompatible species related to cultivated barley. Molecular Genetics and Genomics. 2008;280:509–519. doi: 10.1007/s00438-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Kalinowski A, Radlowski M, Bocian A. Effects of interaction between pollen coat eluates and pistil at the molecular level in self-compatible and self-incompatible plants of Lolium multiflorum Lam. Journal of Applied Genetics. 2006;47:319–329. doi: 10.1007/BF03194641. [DOI] [PubMed] [Google Scholar]
- Kamau E, Charlesworth D. Balancing selection and low recombination affect diversity near the self-incompatibility loci of the plant Arabidopsis lyrata. Current Biology. 2005;15:1773–1778. doi: 10.1016/j.cub.2005.08.062. [DOI] [PubMed] [Google Scholar]
- King J, Thorogood D, Edwards KJ, et al. Development of a genomic microsatellite library in perennial ryegrass (Lolium perenne) and its use in trait mapping. Annals of Botany. 2008;101:845–853. doi: 10.1093/aob/mcn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz C, Chang A, Faure JD, Clarke AE, Polya GM, Anderson MA. Phosphorylation of style S-RNases by Ca2+-dependent protein kinases from pollen tubes. Sexual Plant Reproduction. 1996;9:25–34. [Google Scholar]
- Li XM, Nield J, Hayman D, Langridge P. Cloning a putative self-incompatibility gene from the pollen of the grass Phalaris coerulescens. Plant Cell. 1994;6:1923–1932. doi: 10.1105/tpc.6.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nield J, Hayman D, Langridge P. Thioredoxin activity in the C terminus of Phalaris S protein. The Plant Journal. 1995;8:133–138. doi: 10.1046/j.1365-313x.1995.08010133.x. [DOI] [PubMed] [Google Scholar]
- Li X, Paech N, Nield J, Hayman D, Langridge P. Self-incompatibility in the grasses: evolutionary relationship of the S gene from Phalaris coerulescens to homologous sequences in other grasses. Plant Molecular Biology. 1997;34:223–232. doi: 10.1023/a:1005802327900. [DOI] [PubMed] [Google Scholar]
- Lundqvist A. Studies on self-sterility in rye. Secale cereale L. Hereditas. 1954;40:278–294. [Google Scholar]
- McCraw JM, Spoor W. Self-incompatibility in Lolium species.1. Lolium rigidum Gaud and Lolium multiflorum L. Heredity. 1983a;50:21–27. [Google Scholar]
- McCraw JM, Spoor W. Self-incompatibility in Lolium species. 2. Lolium-perenne L. Heredity. 1983b;50:29–33. [Google Scholar]
- 646.Meng X, Sun P, Kao T-H. S-RNase-based self-incompatibility in Petunia inflata. Annals of Botany. 2011;108:637. doi: 10.1093/aob/mcq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljuš-Đukić J, Ninković S, Nešković M. Effects of protein phosphatase inhibitors and calcium antagonists on self-incompatible reaction in buckwheat. Biologia Plantarum. 2003;46:475–478. [Google Scholar]
- Murray BG. Breeding systems and floral biology in the genus Briza. Heredity. 1974;33:285–292. [Google Scholar]
- Østerbye U. Self-incompatibility in Ranunculus acris L. 1. Genetic interpretation and evolutionary aspects. Hereditas. 1975;80:91–112. [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, et al. Pollen-tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: effect of BAPTA-type buffers and hypertonic media. Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MH, Tsuchiya T, Suwabe K, et al. Physical size of the S locus region defined by genetic recombination and genome sequencing in Ipomoea trifida, Convolvulaceae. Sexual Plant Reproduction. 2007;20:63–72. [Google Scholar]
- Rudd JJ, Franklin FC, Lord JM, Franklin-Tong VE. Increased phosphorylation of a 26-kD pollen protein is induced by the self-incompatibility response in Papaver rhoeas. The Plant Cell. 1996;8:713–724. doi: 10.1105/tpc.8.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd JJ, Osman K, Franklin FC, Franklin-Tong VE. Activation of a putative MAP kinase in pollen is stimulated by the self-incompatibility (SI) response. FEBS Letters. 2003;547:223–227. doi: 10.1016/s0014-5793(03)00710-5. [DOI] [PubMed] [Google Scholar]
- Shinozuka H, Cogan NOI, Smith KF, Spangenberg GC, Forster JW. Fine-scale comparative genetic and physical mapping supports map-based cloning strategies for the self-incompatibility loci of perennial ryegrass (Lolium perenne L.) Plant Molecular Biology. 2010;72:343–355. doi: 10.1007/s11103-009-9574-y. [DOI] [PubMed] [Google Scholar]
- Sim S, Chang T, Curley J, Warnke SE, Barker RE, Jung G. Chromosomal rearrangements differentiating the ryegrass genome from the Triticeae, oat, and rice genomes using common heterologous RFLP probes. Theoretical and Applied Genetics. 2005;110:1011–1019. doi: 10.1007/s00122-004-1916-1. [DOI] [PubMed] [Google Scholar]
- Takayama S, Isogai A. Self-incompatibility in plants. Annual Review of Plant Biology. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Murray BG. A new site for self-incompatibility in the Gramineae. Incompatibility Newsletter. 1975;6:22–23. [Google Scholar]
- Thorogood D, Hayward MD. The genetic control of self-compatibility in an inbred line of Lolium perenne L. Heredity. 1991;67:175–181. [Google Scholar]
- Thorogood D, Hayward MD. Self-compatibility in Lolium temulentum L. – Its genetic control and transfer into Lolium perenne L. and L. multiflorum Lam. Heredity. 1992;68:71–78. [Google Scholar]
- Thorogood D, Kaiser WJ, Jones JG, Armstead I. Self-incompatibility in ryegrass 12. Genotyping and mapping the S and Z loci of Lolium perenne L. Heredity. 2002;88:385–390. doi: 10.1038/sj.hdy.6800071. [DOI] [PubMed] [Google Scholar]
- Thorogood D, Armstead IP, Turner LB, Humphreys MO, Hayward MD. Identification and mode of action of self-compatibility loci in Lolium perenne L. Heredity. 2005;94:356–363. doi: 10.1038/sj.hdy.6800582. [DOI] [PubMed] [Google Scholar]
- Van Daele I, Van Bockstaele E, Martens C, Roldán-Ruiz I. Identification of transcribed derived fragments involved in self-incompatibility in perennial ryegrass (Lolium perenne L.) using cDNA-AFLP. Euphytica. 2008;163:67–80. [Google Scholar]
- Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. Journal of Heredity. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- Voylokov AV, Korzun V, Börner A. Mapping of three self-fertility mutations in rye (Secale cereale L.) using RFLP, isozyme and morphological markers. Theoretical and Applied Genetics. 1998;97:147–153. [Google Scholar]
- Wehling P, Hackauf B, Wricke G. Phosphorylation of pollen proteins in relation to self-incompatibility in rye (Secale cereale L.) Sexual Plant Reproduction. 1994;7:67–75. [Google Scholar]
- Yang B, Thorogood D, Armstead I, Barth S. How far are we from unravelling self-incompatibility in grasses? New Phytologist. 2008;179:740–753. doi: 10.1111/j.1469-8137.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- Yang B, Thorogood D, Armstead I, Franklin FCH, Barth S. Identification of genes expressed during the self-incompatibility (SI) response in perennial ryegrass (Lolium perenne L.) Plant Molecular Biology. 2009;70:709–723. doi: 10.1007/s11103-009-9501-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao Z, Xue Y. Roles of proteolysis in plant self-incompatibility. Annual Review of Plant Biology. 2009;60:21–42. doi: 10.1146/annurev.arplant.043008.092108. [DOI] [PubMed] [Google Scholar]