Abstract
Background
Several felids are endangered and threatened by the illegal wildlife trade. Establishing geographic origin of tissues of endangered species is thus crucial for wildlife crime investigations and effective conservation strategies. As shown in other species, stable isotope analysis of hydrogen and oxygen in hair (δDh, δ18Oh) can be used as a tool for provenance determination. However, reliably predicting the spatial distribution of δDh and δ18Oh requires confirmation from animal tissues of known origin and a detailed understanding of the isotopic routing of dietary nutrients into felid hair.
Methodology/Findings
We used coupled δDh and δ18Oh measurements from the North American bobcat (Lynx rufus) and puma (Puma concolor) with precipitation-based assignment isoscapes to test the feasibility of isotopic geo-location of felidae. Hairs of felid and rabbit museum specimens from 75 sites across the United States and Canada were analyzed. Bobcat and puma lacked a significant correlation between H/O isotopes in hair and local waters, and also exhibited an isotopic decoupling of δ18Oh and δDh. Conversely, strong δD and δ18O coupling was found for key prey, eastern cottontail rabbit (Sylvilagus floridanus; hair) and white-tailed deer (Odocoileus virginianus; collagen, bone phosphate).
Conclusions/Significance
Puma and bobcat hairs do not adhere to expected pattern of H and O isotopic variation predicted by precipitation isoscapes for North America. Thus, using bulk hair, felids cannot be placed on δ18O and δD isoscapes for use in forensic investigations. The effective application of isotopes to trace the provenance of feline carnivores is likely compromised by major controls of their diet, physiology and metabolism on hair δ18O and δD related to body water budgets. Controlled feeding experiments, combined with single amino acid isotope analysis of diets and hair, are needed to reveal mechanisms and physiological traits explaining why felid hair does not follow isotopic patterns demonstrated in many other taxa.
Introduction
Many carnivore species are currently threatened and are the focus of intense conservation concern [1]. Feline carnivores are often subject to illegal wildlife trade, thus the ability to estimate the geographic provenance of illegal tissue samples would constitute important information in wildlife crime investigations [2]. Probabilistic provenance determination based on O and H isotopes has strong potential to be applied to animal tissues as an investigative tool in wildlife forensic science [3]–[6]. Validation of isotopic methods has relevance and practical application in various fields like wildlife forensics and conservation biology.
Measurements of the stable isotopes of hydrogen (δD) and oxygen (δ18O) of animal keratinous tissues have been used to track the geographic origin and migratory patterns in a wide variety of animals (e.g. [3], [4], [7]–[9]). To date, this approach is based on strong empirical correlations between δD values in animal tissues (δDt) with the isotopic composition of the amount-weighted mean annual or mean-growing season precipitation (δDp). The latter correlates inversely with latitude and elevation across the continents, especially in North America [10]–[12]. Few studies have coupled δD and δ18O measurements of the organic or inorganic fractions of animal tissues despite the strong covariance between these isotopes in environmental waters (hairs and nails: human [8], [13]–[16]; CO2, body water, hair and enamel: woodrat [17]; chitin: brine shrimp [18]; chitin: chironomids [19]; plasma, blood and feathers: birds [20], [21]; fat, blood, muscle, hair and collagen: pig [22]; carbonate and phosphate tooth enamel, bone collagen, subcutaneous fat and hair: laboratory rat [23]). Strong correlations between δDp and δDt have been found for many species [4]. The hydrogen and oxygen isotopic composition of animal tissues (hair, feathers, teeth) is related to the isotopic composition of body water (e.g. [24]–[27]) and ultimately to that of ingested water. Influences on isotopic composition of body water (δDbw, δ18Obw) of animals include abiotic (climate, drinking water) and biotic (diet and physiology) factors [28]–[35]. The incorporation of H and O isotopes from the hydrosphere via diet and drinking water into animal tissues is a complex process and our understanding of how these mechanisms affect the nature and variability of the empirically observed relationships is still poor (e.g. [13]). However, to reliably track the geographic origin of an animal requires a detailed understanding of the metabolic routing of dietary nutrients and mechanisms of H and O isotopic incorporation into animal tissues [36].
Hydrogen and oxygen in animal tissues can be derived from two potential sources: dietary nutrients and body water, whereas oxygen is also derived from inhaled air. The body-water pool, in turn, is derived from ingested drinking-, food-, and metabolic-water produced during the catabolism of food macromolecules [28], [30], [32], [35], [37]–[39]. The relative contributions of all these sources to protein synthesis (i.e. keratin and collagen) are likely to vary among animals [40]–[42]. Controlled experiments are key to understand and model the incorporation of H and O isotopes into proteinaceous tissues like keratins (hair and feathers), collagen, and chitin, and have so far been developed for only a small number of species like woodrat (Neotoma cinerea and Neotoma stephensi; [17]), rat (Rattus norvegicus; [23]), Japanese quail (Coturnix japonica; [24]), house sparrow (Passer domesticus; [21]), humans (Homo sapiens; [8], [13]–[16], [27]), pig (Sus scrofa domesticus; [22]), brine shrimp (Artemia franciscana; [18]) and chironomids (Chironomus dilutus; [19]). These studies revealed that keratin δD and δ18O reflect both biological (diet, physiology) and environmental signals (water, geographic movement, climate; [13]). Deviations from a strong coupling between δDt and δDp, and δ18Ot and δ18Op have been shown (e.g. [13], [43]) and may be linked to: 1) climatic factors like relative humidity [37], [44]; 2) isotopic disequilibrium of food and water contributions to δDt [27]; 3) possible trophic-level effects on δDt [45]; 4) impacts of metabolic rate and drinking water flux on δDbw and δ18Obw [26], [28], [30], [32] (δ18O of phosphate in urinary stone [46], bone [25] and tooth [47]); and 5) dietary and physiological controls on δ18Oh and δDh of hair [13].
Previous studies that successfully applied combined δDt and δ18Ot analysis to track the geographic origin and migration of animals focused on herbivores and omnivores (e.g. [3], [9], [17], [21], [22], [24]). The fact that this method performs particularly well in omnivorous modern humans [8], [13]–[16], [48] is not surprising, because humans are well-hydrated and typically consume a constant local water source (e.g. tap water: [49]–[51]) and consistent homogenous diet across regions (e.g. fast food: [52]). But even for humans, hydrogen isotopic incorporation during keratin synthesis likely varies between different keratinous tissues like nail and hair [53]. Free-ranging carnivores, however, differ significantly in their nutritional, physiological and metabolic characteristics from herbivores and omnivores [54], [55]. The house cat, Felis catus, is the most thoroughly studied mammalian carnivore [54]. Felids are strict carnivores and thus obtain much of their body water from the consumption of prey [54]. Owing to the lack of empirical H/O isotope studies on strict carnivores (other than raptors) it is unclear whether carnivore hairs track the spatially predictable meteoric water signal (despite their integrative high trophic position). However, Kohn [30] hypothesized, that “carnivore bone phosphate should track the meteoric water signal more closely than do herbivores”. For this reason, the concept of geographic source determination based on H/O isotopes using carnivore hairs as an investigative tool in wildlife forensic science needs to be tested.
Here, we provided the first large-scale δD and δ18O analysis of hair samples from wild individuals of two North American feline carnivores, bobcat (Lynx rufus) and puma (Puma concolor). Both species were ideally suited to test the strength of the isotope approach in assigning geographic origins of felidae. The availability of skins from museum collections, high-resolution precipitation δ18O and δD isoscapes for North America and ecological differences between these study animals (e.g. body size, home-range size, habitat use, distribution and prey preferences) allowed us to assess the application and efficacy of H/O isotope fingerprinting for forensic spatial assignment in feline carnivores.
Our study was designed to determine whether puma and bobcat hairs varied predictably in their isotopic composition among isotopically distinct geographic locations and reflected the spatial pattern of isotopic variation in precipitation. Furthermore, we examined if species- or sex-specific effects existed, and whether these could be explained by differences in diet, body size and foraging ecology. Our results demonstrated that the application of water isotopes for provenance determination of feline carnivores was compromised by major controls of their diet, physiology and metabolism on δ18Oh and δDh. The controlling factors and possibilities to quantify these will be discussed.
Materials and Methods
Ethics statement
All CITES permits (MA 125284-0) for the export and use of museum materials from puma and bobcat were issued by the U.S. Fish and Wildlife Service.
Study species and sampling
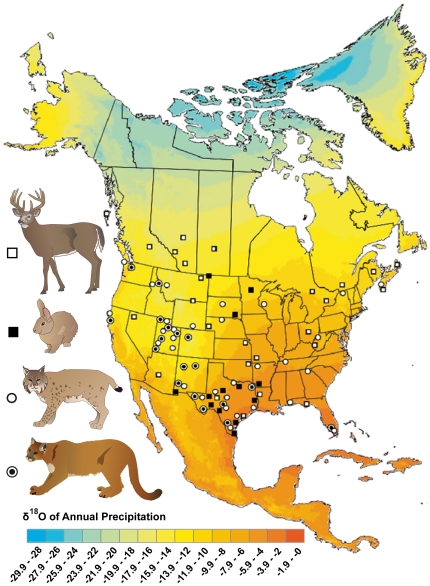
Eighty-eight hair samples from two North American felid species bobcat (Lynx rufus, n = 45) and puma (Puma concolor, n = 30), as well as the eastern cottontail rabbit (Sylvilagus floridanus, n = 13), the latter representing the preferred prey species of the bobcat, were obtained from the Smithsonian National Museum of Natural History in Washington D.C. and the Utah Museum of Natural History, Utah. Published isotope data of bone-phosphate (δ18Obp) and bone collagen (δ18Obc) from white tailed deer (Odocoileus virginianus), constituting the major prey of the puma, were included for comparative analysis [56]. For each specimen, geographic location, sex and elevation was recorded (Table S1). All specimens studied originated from 75 different sites across the United States and Canada (Figure 1). Sample locations ranged in latitude from 25.8 to 48.2°N and longitude from 124.4 to 65.8°W, covering strong altitudinal (2 to 3400 m) and isotopic gradients (δ18Oriv = −17.5‰ to −0.1‰; δDriv = −132.7‰ to 0.6‰).
Figure 1. Map of sampling sites.
Sample locations for both felines bobcat (n = 45) and puma (n = 30) as well as their preferred prey species eastern cottontail rabbit (n = 13) and white-tailed deer* (n = 31), respectively, plotted on the δ18O precipitation map of North America** (*data from [56]; **from http://www.waterisotopes.org).
Stable isotope analysis
Sample preparation and H/O isotope analysis were conducted at Environment Canada. All keratin samples were physically cleaned of adhering debris and washed twice in a 2∶1 mixture of chloroform and methanol to remove lipids from the keratin surface. After cleaning, all samples were air-dried for 24 h. Hair samples were then cut into 0.5 cm increments (H: 350±20 µg; O: 700±50 µg) and weighed into pre-combusted silver foil capsules for H and O isotope ratio analysis. For δD, in order to account for exchangeable hydrogen in hair proteins, we used comparative equilibration with in-house keratin working standards, BWB (−108‰), CFS (−147.7‰), CHS (−187‰), for which the δD value of non-exchangeable H had been previously established [57]. For δ18O, we used the IAEA benzoic acid standards IAEA 601 and 602, with assigned δ18O values of +23.1‰ and +71.4‰, respectively. For H/O isotopic analyses, samples and reference materials were separately pyrolyzed on a Hekatech HTO elemental analyser at 1350°C to H2 and CO for isotopic analysis on an Isoprime™ dual-inlet isotope-ratio mass spectrometer. The reference standards were used to normalize unknown samples to the Vienna Standard Mean Ocean Water-Standard Light Antarctic Precipitation (VSMOW-SLAP) standard scale [57].
Estimates of drinking water isotope compositions (δD, δ18O) for bobcat and puma
The H and O isotopic composition of water ingested by both felid species indirectly from their prey were inferred from modeled isoscape values [58] as well as measured river water values across North America [59], [60]. It was assumed that the place of death of each puma and bobcat reflected their lifetime habitat. For each locality the average δD and δ18O values for precipitation were determined using the Online Isotopes in Precipitation Calculator (OIPC) version 2.2 (http://www.waterisotopes.org). The OIPC provided a model estimation of long-term annually or monthly averaged precipitation isotope ratios at specified locations through spatial modelling of a large database of precipitation isotopic data covering the time period 1960–2004 [10], [58]. The δD and δ18O data of the OIPC model were compared to those measured for local river waters [59], [60]. In general, there was a good correlation between δDriv and δ18Oriv and δDp and δ18Op for relatively small- to medium-sized drainage catchments (<130,000 km2) [9]. As puma and bobcats have smaller home-range sizes (female bobcat: 21.7 km2, [61], [62]; female puma 175.8 km2, [61]) local river water should reflect the average δD and δ18O values of ingested prey-derived drinking water. Therefore we compared the hair δDh and δ18Oh data with the river water data.
Bobcat and puma hair isotope values were plotted against amount-weighted long-term annual, spring (three months mean of March, April, May) and summer (three months mean of June, July and August) precipitation δDp and δ18Op values, because the formation and isotopic incorporation of cat hair is limited to a rather short time period. For instance hair growth in domestic cats is not continuous [63], but rather includes an anagen phase of active growth and a telogen phase of rest [64]. The hair-growth phase takes 6–8 weeks and 70% percent of the hair follicles are in the anagen phase during the summer [65]. Isotopic signals from drinking water and prey consumed during the anagen phase of growth are most likely integrated into the growing hairs. For this reason we related the isotope values of hair δDh and δ18Oh not only to annual average δDp and δ18Op values but also to seasonal spring and summer precipitation to test if a better relation with water isotope values of the likely main hair growing season was obtained (Table S2).
Statistical analysis
First, we analysed the H and O isotopic variation of puma and bobcat hairs among locations and their correlation with the large-scale patterns of isotopic variation in precipitation. We tested whether the correlations significantly changed when using the annual and summer modeled precipitation or local river water data (Table S2). We compared hair H and O isotope data of predators and respective prey species and tried to establish a calibration equation between river water and hair for a feline carnivore. Relationships between mean annual δ18Oriv, δDriv and δ18Oh, δDh of puma, bobcat and rabbit hairs were investigated using linear regressions (Figure 2 and 3). We also examined the relationship between v18Oh and δDh (Figure 4). The effects of species, age, sex, seasonal precipitation and relative humidity on hair isotope values were examined using a General Linear Model (GLM) (Table S2). Statistical tests were conducted using XLSTAT (V 7.5.2).
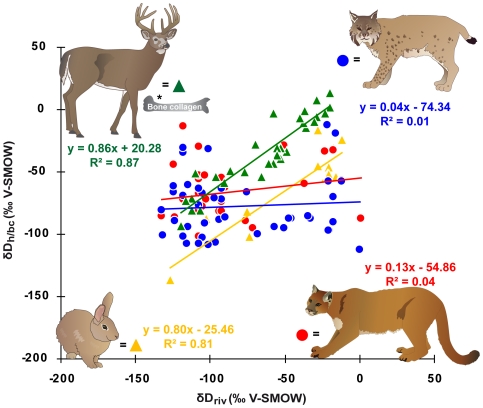
Figure 2. Hydrogen isotope values of keratin relative to river water.
Plot of δD of hair (δDh) from bobcat, puma and eastern cottontail rabbit as well as bone collagen (δDbc) from white-tailed deer* vs. mean annual δD of river water (δDriv) (*data from [56]).
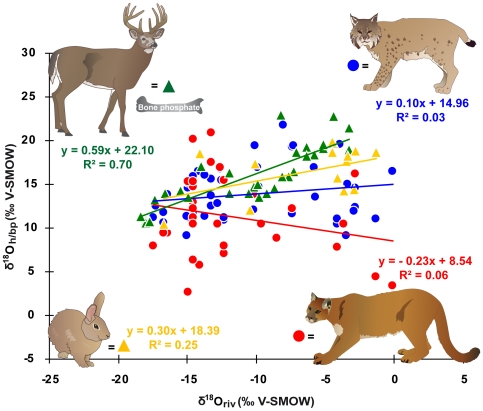
Figure 3. Oxygen isotope values of keratin relative to river water.
Plot of δ18O of hair (δ18Oh) from bobcat, puma and eastern cottontail rabbit and bone phosphate (δ18Obp) from white-tailed deer* vs. mean annual δ18O of river water (δ18Oriv) (*data from [56]).
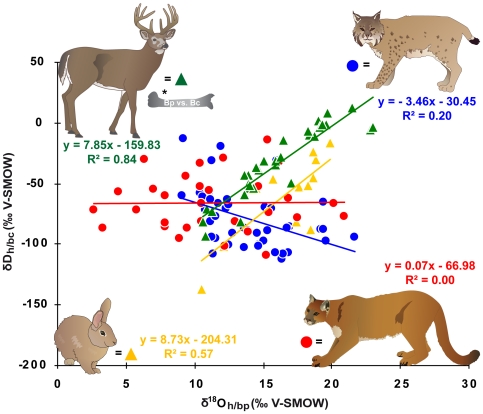
Figure 4. Hydrogen and oxygen isotope ratios of keratin.
Hydrogen and oxygen isotope compositions are shown for hair samples (δDh, δ18Oh) from puma, bobcat and eastern cottontail rabbit as well as collagen (δDbc) and bone phosphate (δ18Obp) data from white-tailed deer* (*data from [56]).
Results
All hair δDh and δ18Oh values were plotted against mean annual δDriv and δ18Oriv values because using either amount-weighted mean annual, summer (June, July and August) or spring (March, April and May) OIPC modeled precipitation values did not significantly change the results (Table S2). The δ18Oh - δ18Op correlation of bobcats was slightly improved by including relative humidity in the regression (R2 = 0.21, p = 0.01, n = 44). Relative humidity did show a significant modest effect on δ18Oh of bobcats (R2 = 0.21, p = 0.002, n = 44) but no effect on δ18Oh of puma (R2 = 0.00, p = 0.818, n = 30). Relative humidity, however, did not affect δDh of bobcats (R2 = 0.05, p = 0.146, n = 44) and puma (R2 = 0.068, p = 0.164, n = 30) (Table S2). The isotope composition of the analyzed hair samples spanned a range of 99.3 ‰ for δDh and 12.6 ‰ for δ18Oh in bobcat, and 95.4 ‰ for δDh, and 18.2 ‰ for δ18Oh in puma (Figures 2 and 3). No significant relationship was found between δDh and δDriv for both species (bobcat: R2 = 0.005, p = 0.65, n = 44; puma: R2 = 0.040, p = 0.291, n = 30) (Figure 2). Likewise δ18Oh and δ18Oriv were not significantly correlated (bobcat: R2 = 0.030, p = 0.261, n = 44; puma: R2 = 0.055, p = 0.211, n = 30) (Figure 3). No effect of sex on the isotopic relationship between hair and water was observed for both species (Table S2). There was a weak correlation between δDh and δ18Oh values of the same hair samples in bobcat (R2 = 0.195, p = 0.003, n = 43) but not in puma (R2 = 0.0002, p = 0.939, n = 30) (Figure 4). Results for the hair isotope compositions of cottontail rabbits exhibited a strong δDh–δDriv (δDh: R2 = 0.81, p<0.0001, n = 13) and a moderate δ18Oh–δ18Oriv (δ18Oh: R2 = 0.25, p = 0.083, n = 13) positive relationship (Figures 2 and 3). The eastern cottontail rabbits also displayed a significant positive correlation between δDh and δ18Oh values of the same hair samples (R2 = 0.571, p = 0.003, n = 13) (Figure 4).
Discussion
Both puma and bobcat lacked the expected correlation between water isotopes in local water and hair, and also exhibited a complete decoupling between δ18Oh and δDh. This finding contrasted strongly with results from numerous previously published studies on keratin tissues of omnivores and herbivores. Hence, tracing the provenance of feline carnivores such as puma and bobcat based on δ18Oh and δDh isoscapes does not appear to be possible, as individuals could not be reliably placed on δ18Op and δDp maps. Potential explanations for this lack of correlation between hair and ambient water isotope compositions are discussed below.
Can relative humidity affect carnivore δ18Oh and δDh?
In our study, relative humidity showed a significant modest effect on δ18Oh of bobcats (R2 = 0.21, p = 0.002) but not on puma (R2 = 0.00, p = 0.818) (Table S2). Previous studies on mammalian bone phosphate showed that relative humidity controls the δ18Obp values of herbivore species with low drinking water requirements (e.g. [30]). For example, δ18Obp values of Australian macropods [37], rabbits and hares [44] have been shown to correlate strongly with changes in relative humidity independent of δ18Op, whereas the δ18Obp of North American deer [38] were influenced by both relative humidity and δ18Op. Low humidity increases the rate of evaporation of surface water and evapotranspiration of leaf- and grass-water and thus leads to oxygen isotopic enrichment effects in plants [66], [67]. Drought-tolerant animals who obtain most of their water from plants thus reflect levels of environmental humidity, in particular their δ18Obp increases with decreasing relative humidity. However, Kohn [30] hypothesized that the importance of relative humidity diminishes with increasing trophic level. Our data support Kohn's hypothesis that predators are less controlled by relative humidity than herbivores. Bobcat δ18Oh compositions were weakly affected by relative humidity (R2 = 0.21, p = 0.002), most likely because they prey upon rabbits, whose δ18Obp compositions are humidity dependent (R2 = 0.86; [44]). In contrast, puma δ18Oh compositions were not influenced by relative humidity (R2 = 0.00, p = 0.818), probably because they feed on white-tailed deer, whose δ18Obp is affected by both relative humidity and δ18Op [38]. Unlike oxygen isotopes, δDh values of both feline carnivores were not influenced by relative humidity (bobcat: R2 = 0.05, p = 0.15; puma: R2 = 0.07, p = 0.16). Similar observations were made for δDbc (bone collagen) of white-tailed deer by Cormie et al. [68]. We conclude that relative humidity particularly affects δ18Ot of predators (e.g. bobcats) that feed on drought -tolerant herbivore species like rabbits. However, relative humidity did not explain the lack of a correlation between δDh-δ18Oh observed in both felids we studied.
Does an isotopic disequilibrium between food and water affect δDh?
It was documented previously [13], [27], that δDh is not well correlated with δDp, if (i) ingested food or water sources (e.g. exotic foods, marine-based diet, high altitude food or snow melt drinking water) are not isotopically related to local meteoric water and/or (ii) migration between isotopically distinct habitats takes place. We tested whether the ingested food sources (i.e. key prey species) of bobcat and puma were in disequilibrium with δDp, and so caused the lack of a correlation between H/O isotopes in precipitation and those in felid hair. In North America, the preferred prey species of puma is the white-tailed deer (Odocoileus virginianus) [69], whose δ18O of bone phosphate (δ18Obp) [38] and δD bone collagen values (δDbc) [56] strongly correlate with δ18Op and δDp, respectively (Figure 2 and 3). In contrast, bobcats mainly prey on lagomorphs [70], whose δ18Oh and δDh values we also found to show a direct relationship with δ18Op and δDp (Figure 2 and 3). Thus the oxygen and hydrogen isotopic composition of prey are not reflected in the hair of their respective predators. Cats are not obligate drinkers [71] and hence isotopic content of drinking water does not explain the lack of a correlation between δDp and δDh in felines.
Migration between isotopically distinct biomes during biosynthesis of hair might also affect the correlation of δDh with δDp. We would have expected this effect based on potential species- or sex-specific behavioral differences characterizing our study species. Puma and bobcat, for instance, have significantly different home range sizes [2], [72], which are also known to vary between seasons and sex. Although carnivores exhibit typical mammalian dispersal behaviour, where males disperse and females are philopatric [73]; we did however not observe an effect of sex on the hair/water isotope correlation for both carnivore species (Table S2). We therefore concluded that the isotopic disequilibrium of food and water does not explain the lack of a relationship between δDh and δDp observed in puma and bobcats.
Does a carnivorous diet affect δDh?
Some studies have suggested a dietary trophic-level effect on H isotope systematics of animal tissues [13], [42], [45], [74], [75]. Possibly, high levels of animal protein consumption leads to a decoupling of δD in keratins from δDp and a deviation from the mean relationship between keratin δD and δ18O [45], [76]. Diet may thus represent a confounding factor in the use of H and O isotopes for geographic tracking [13].
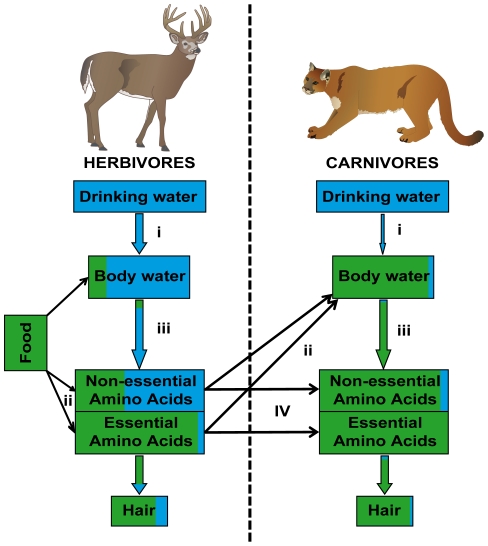
We developed a simple model of hydrogen isotope incorporation in carnivores to illustrate possible trophic-level enrichment and isotopic decoupling of δDh in carnivores. Various fractionation factors and source pools contributing to non-exchangeable hydrogen in hair were considered (Figure 5). Controlled experiments on domestic cats have shown that, on average, only 1% of their total water input originates from drinking water [71]. So, drinking water likely has minor control on deuterium enrichment in felids, leaving the isotopic input of prey as a major determinant of the isotopic signature of carnivore body water. In this aspect, strict carnivores differ significantly from herbivores and omnivores, whose body water is to a large extent (64–80%, see Table 1) obtained from drinking water (Figure 5(i)). Isotope fractionation from drinking water to body water occurs [35], [42], [77] and may play an important role in δDh enrichment of carnivore proteins. Feline carnivores consume prey species whose δDbw and δ18Obw are expected to be higher than δDp and δ18Op due to evaporative enrichment from insensible water loss through skin and breath vapor loss [34], [78]. Consequently, carnivores mainly consuming deuterium-enriched prey should have higher δDbw values over those of their prey. A similar process has been documented in humans for the consumption of cow milk and the resulting enrichment in deuterium of consumer tissue [42], [79]. Otherwise the consumption of D-depleted prey might decrease the carnivore δDbw values particularly during winter when prey species have built up their body fat reserves. Fat reserves are known to have significantly more negative δD values than proteinaceous tissues [24], [76], [80], [81]. The temporary alternation of D-depleted and -enriched carnivore diets relative to δDp, based on differential seasonal consumption of lipids and proteins, respectively, might change the δDbw [35] and is finally recorded in δDh during carnivore hair growth [82].
Figure 5. Hydrogen isotope model of herbivores and carnivores.
Model of hydrogen isotope physiology and the contribution of food and water to non-exchangeable hydrogen in the hair of herbivores and carnivores. Letters represent processes where isotope fractionation occurs (see text for detailed discussion). Blue coloring represents water inputs and green food inputs.
Table 1. Food and drinking water inputs of hydrogen in the body water of different organisms under laboratory conditions.
Hydrogen isotope fractionation can also occur during the oxidation of food to form body water (see Figure 5 (ii)). Carnivores have the ability to digest and utilize high levels of dietary fat and protein and so produce relatively higher levels of metabolic water [54], [83], [84]. Catabolism of macronutrients and production of metabolic water could cause hydrogen isotope fractionation processes leading to deuterium enrichment [35], [41]. In addition, isotopic fractionation most likely happens during the incorporation of body water into tissue amino acids (see Figure 5 (iii)). Water from food, drinking water and metabolism are the three source pools which can be fixed into newly synthesized non-essential amino acids [13]. However, the fraction of hydrogen fixed into amino acids may scale with the extent of non-essential amino acid synthesis in the body. This, in turn, is related to the level and amino acid composition of dietary protein intake [85]. Carnivores exhibit low levels of non-essential amino acid synthesis because their natural meat-rich diet contains all required amino acids [86]. Consequently, low levels of hydrogen fixed into amino acids in vivo could maximize the transfer of hydrogen from diet to hair thereby enhancing the contribution of isotopically heavy, prey-derived hydrogen in carnivore hair [13]. Finally, it is also possible that isotope fractionation occurs during the transfer of food amino acids to tissue amino acids (Figure 5 (iv)). δDh enrichment of carnivore proteins could also occur through selective catabolism of isotopically lighter amino acids [45]. We conclude that there are several possible isotopic fractionation steps during the metabolic incorporation of hydrogen into carnivore hair that could induce enrichment in deuterium and leading to higher δDh and a loss of correlation with δDp.
Effects of carnivore physiology and metabolism on δDh and δ18Oh
If diet rather than drinking water solely controls carnivore δD, we would have expected a variation of the hair/water regression in slope and intercept compared to herbivores and omnivores. Because there was no significant correlation between oxygen and hydrogen isotope compositions of hair and precipitation and δDh and δ18Oh, we therefore suspected the dietary trophic-level effect was potentially obscured by physiological and metabolic adaptations in carnivores [87]. Animals which display deviations from the normal covariance between δD and δ18O values in keratin are carnivorous fish, birds and mammals [45] and ancient human populations with a meat-rich diet [13], [42], [74], which all consume high levels of animal protein and fat. From a purely nutritional perspective, they are all strict carnivores. Through evolution, their adherence to a specialized meat-rich diet induced changes in their metabolic pathways and nutritional requirements [54]. These physiological and metabolic adaptations in strict carnivores could considerably affect the H and O isotope systematics of their keratins.
The H and O isotope compositions of human hair strongly covary, and are closely related to meteoric (drinking) water at the place of residence [8] with the exception of mid 20th century Inuit people [13]. Bowen et al. [13] did not find strong support for ubiquitous effects on the H/O isotope systematics of human hair related to physiological adaptations. However, in pre-globalization times, the typical diet of the Inuit contained high levels of dietary protein and fat from high trophic-level marine animals [88]. Mid 20th century Inuit people thus fed at the highest trophic level of all humans. Since marine food webs have typically longer chain lengths than terrestrial food webs [89], the consumption of marine predators may confer a trophic-level enrichment of Inuit δDh [13]. Historic Inuit are also classified as obligate carnivores among omnivorous humans because they require nutrients that are present only in animal tissue of their diet [90] and so differ from other ancient humans who used a marine-dominated but omnivorous diet like the Ainu from Japan and Thai from Thailand [13].
Measurements of δD in feathers have been successfully applied in many bird species to estimate the origins of migrating and wintering individuals [36]. However, in strictly carnivorous raptors like Amur Falcons (Falco amurensis; [91]) and Cooper's Hawks (Accipiter cooperii; [92]) the linkage between feather δD and δDp was weaker [9], [93]. However, this may be complicated due to the fact that several raptors grow feathers during periods of high work associated with breeding and so may produce more deuterium enriched feathers due to evaporative water loss.
The natural diet of wild felids contains a high proportion of the energy as protein, a variable percentage as fat and a very low percentage as carbohydrate [55]. Metabolic adaptations mainly concern the loss of anabolic pathways required for the synthesis of nutrients universally present in their natural meat-based diet [94]. One of the most striking aspects here is that strict carnivores have lost the ability to produce metabolic compounds that are commonly synthesized by virtually all herbivores and omnivores. For example, cats lack the enzymatic machinery to synthesize some amino and fatty acids, thereby significantly increasing their basal requirement for proteins and essential amino acids. When ingesting prey, wild cats avoid consuming plant materials contained in the intestines [87] and hence the digestion of dietary starches and sugars has adapted to low carbohydrate intake [95].
Currently we lack a testable explanation for our observed and confounding isotopic patterns, but considering the unique felid physiology, we hypothesized that the food metabolism of strict carnivores may exert a vital effect particularly on δDh. This may also affect the relative contributions of all sources to protein synthesis and hair formation. Recent findings from Pecquerie et al. [41] support our hypothesis. They propose two mechanisms involved in stable isotope fractionation during metabolic reactions: First, the selection of molecules for the anabolic or the catabolic pathway routes depends on their isotopic composition. Second, the concept of atom recombination recognizes that molecules are not completely disassembled into elements during chemical reactions [96]. A non-random allocation of atoms of a particular substrate (e.g. food amino acids) to a particular product (e.g. keratin amino acids) impacts isotopic composition of a given product (e.g. hair). While isotope fractionation takes place in metabolic reactions [41], these were particularly modified during the evolutionary history of carnivores. Knowing that approximately two thirds of the hydrogen in human hair are derived from food [27], we suspect that carnivores might be affected by alternate modes of isotopic routing of macronutrients into hair (Table 2).
Table 2. Food and drinking water inputs of hydrogen in hair and feathers of different organisms.
The water metabolism in feline carnivores also differs from herbivores and omnivores. Cats drink to a limited extent [55], [83] and excrete concentrated urine [97]–[99]. In addition they produce relatively high levels of metabolic water, which contributes on average 10% to their total water intake [54], [83]. Drinking water volume, however, exerts a significant physiological control on the isotopic composition of hydrogen and oxygen in human body water [26] (Table 1). Besides various water conservation adaptations, strict carnivores have higher basal metabolic rates than other mammals [100], [101]. A high metabolic rate associated with a low rate of drinking, results in a weak correlation of δ18Obp with δ18Op [25]. We infer that this applies to strict carnivores and assumed that relatively smaller contributions of oxygen in carnivore hair originate from drinking water. In addition, cats lose water primarily through panting [102] vs. from sweat glands of foot pads [103]. Differences in the isotope compositions of liquid water during sweating vs. vapor during panting should affect their body isotopic compositions. Panting animals should thus have higher δ18Obw and δ18Oh values than animals that sweat because water vapour lost in panting is more depleted in 18O [30], [104]. The same should apply to δDbw and δDh.
In contrast to the weak correlation between feline carnivore hairs δDh and δ18Oh and meteoric water δ18Op and δDp (Figures 2 and 3), a good correlation between claw δDc and δDp was observed in a recently published study of migrating pumas in the USA [6]. The reason why the two keratineous tissues do not reflect meteoric water values in the same way remains unclear. However, a similar paradox is known for human fingernails and hair, with nails displaying a more variable H/O isotope composition and a comparatively weaker correlation between δDc and δDwater (R2 = 0.6) compared to hair (R2 = 0.9) from the same individuals [14], [53]. The reverse trend in feline carnivores may result from different formation rates of hairs [63] and nails [105], alternate modes of isotopic routing of macronutrients into hair and nail as well as different amino acid compositions of hair and nail [106].
Amino acid composition of cat hair
The isotopic values of keratins are generally defined by the isotopic composition of their constituent amino acids [106]. For example, cysteine, serine and glutamate, all non-essential, metabolically active amino acids are present at very high proportions in hair [107]. Their isotopic composition reflects both food and drinking water, with a slight bias towards food. Due to the high relative abundance of non-essential amino acids, their isotope composition can often dominate the bulk H and O isotope hair signature and mask the isotope composition from essential amino acids. The latter are present at lower proportions and routed directly from dietary sources [108]. The constancy of amino acid composition and hence isotopic values between tissues, even for related proteins like nail and hair, cannot be implied [106]. Large isotopic differences between amino acids of different components have been observed [109]–[111], reflecting their formation via different metabolic, synthetic and catabolic processes. However, the amino acid composition of cat hair protein is comparable with that of dog, horse, sheep and human hair [107]. Apparently only the proline content of cat hair protein appears to be lower and glycine appears to be higher than in the other species [107]. Variations in amino acid composition of cat hair might thus be responsible for some of the differences in isotopic patterns we have observed.
Does tanning of museum skins have an effect on the H/O isotopic composition of hairs?
To our knowledge this is the first H/O isotope study on mammal hair which benefits from large museum collections as a valuable source of sample material. However, it has not been assessed whether the tanning process used for preserving hides affects the H/O isotopic composition of taxidermy skins. Tanning chemicals are intended to stop deterioration processes of the skin. At a molecular level tanning chemicals act as solid spacers, which replace the H bonds linking the polypeptide chains of the collagen fiber and thus stabilize the collagen structure of museum skins [112]. Collagen and hair are both proteinaceous tissues and interpeptide H-bonding is abundant and important for maintaining the alpha-helical structure of collagen and hair [113]. Thus, tanning chemicals could potentially alter the non-exchangeable H isotope composition of hairs. However, we hypothesize that tanning chemicals did not affect the H/O isotopic composition of the analyzed felid hairs. First, the rabbit hairs which have most likely undergone the same tanning process as felid hides, showed good isotopic (δDh and δ18Oh) correlation between hair and meteoric waters (Figure 2 and 3). Second, initial results from a small “before and after tanning experiment” using a common mineral tanning technique (aluminium salts [114]) on hairs from different mammal species indicated that there was no significant effect of the tanning process on the H isotopic values of these hair samples (data not shown).
Conclusions
Stable isotope (H, O) data from bobcat and puma hairs from a range of locations across North America revealed that feline carnivores cannot be placed on δ18O and δD isoscapes for forensic investigation purposes. The effective application of water isoscapes for geographic source determination of feline carnivores is most likely compromised by major controls of their diet, physiology and metabolism on δ18Oh and δDh. However, we noted that the integration of H and O isotopes into animal proteins in general remains poorly understood. Isotope fractionation and routing during metabolic and tissue formation processes is complex and presumably varies between herbivores, omnivores and carnivores. Significant research thus remains to be performed to characterize the precise origin and sensitivities of the observed isotope signals. Controlled feeding experiments on strict carnivores like domestic cats are now needed to track isotope routing of macronutrients and their incorporation into different tissue types (e.g. [17], [24]). With the objective to enhance the resolution of H and O isotope analysis of proteins, we suggest compound-specific single amino acid isotope analysis may give improved insights into isotope fractionation processes during protein, and by a comparative isotope analysis of essential versus non-essential amino acids. To date most studies have used bulk tissue protein isotopic values of hydrogen and oxygen [8], [13], [20] but little research has been conducted at the level of single amino acids in hair that was limited to C, N and S isotopes [115]–[117]. Unfortunately, there are no reported applications of hair δ18O and δD compound-specific isotope analysis of amino acids. This represents an important area of future research and will contribute to a better understanding of the observed variations in bulk protein H and O isotope ratios.
Supporting Information
Sample list.
(XLS)
Statistical analysis.
(DOC)
Acknowledgments
We thank Robert Fischer and Suzanne C. Peurach from the mammal collection at the Smithsonian Natural History Museum in Washington D.C., Eric A. Rickart from the Utah Museum of Natural History, and Bryan T. Hamilton from the Great Basin National Park in Nevada for their assistance with the sample collection. We thank Aurélien Bernard and Jürgen Hummel for their constructive and helpful comments. We also thank David Soto for his assistance with the stable isotope assays. The animal symbols used for figures are courtesy of the Integration and Application Network (www.ian.umces.edu/symbols), University of Maryland Center for Environmental Science.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the Deutsche Forschungsgemeinschaft grant TU 148/2-1 Emmy Noether-Group Bone Geochemistry to TT and by a Deutscher Akademischer Austausch Dienst PROMOS Foreign Exchange Grant to SP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baillie JEM, Hilton-Taylor C, Stuart SN. A Global Species Assessment. Gland, Switzerland and Cambridge, UK: IUCN; 2004. IUCN Red List of Threatened Species. [Google Scholar]
- 2.Nowell K, Jackson P. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources; 1996. Wild cats: status survey and conservation action plan.383 [Google Scholar]
- 3.Bowen GJ, Wassenaar LI, Hobson KA. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia. 2005;143:337–348. doi: 10.1007/s00442-004-1813-y. [DOI] [PubMed] [Google Scholar]
- 4.Hobson KA, Wassenaar LI. Tracking animal migration with stable isotopes; In: Hobson KA, Wassenaar LI, editors. London, UK: Academic Press; 2008. 143 [Google Scholar]
- 5.West JB, Bowen GJ, Dawson TE, Tu KP. New York: Springer Verlag; 2009. Isoscapes: Understanding Movement, Pattern, and Process on Earth Through Isotope Mapping.487 [Google Scholar]
- 6.Hénaux V, Powell LA, Hobson KA, Nielsen CK, LaRue MA. Tracking large carnivore dispersal using isotopic clues in claws: an application to cougars across the Great Plains. Methods in Ecology and Evolution 2. 2011. Available: http://dx.doi.org/10.1111/j.2041-210X.2011.00107.x.
- 7.Cryan PM, Bogan MA, Rye RO, Landis GP, Kester CL. Stable hydrogen isotope analysis of bat hair as evidence for seasonal molt and long-distance migration. Journal of Mammalogy. 2004;85:995–1001. [Google Scholar]
- 8.Ehleringer JR, Bowen GJ, Chesson LA, West AG, Podlesak DW, et al. Hydrogen and oxygen isotope ratios in human hair are related to geography. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2788–2793. doi: 10.1073/pnas.0712228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobson KA. Stable isotopes and the determination of avian migratory connectivity and seasonal interactions. The Auk. 2005;122:1037–1048. [Google Scholar]
- 10.Bowen GJ, Revenaugh J. Interpolating the isotopic composition of modern meteoric precipitation. Water Resources Research 39. 2003. Available: http://wateriso.eas.purdue.edu/waterisotopes/media/PDFs/2003WR002086.pdf. Accessed 2011 March 8.
- 11.Dansgaard W. Stable Isotopes in Precipitation. Tellus. 1964;16:436–468. [Google Scholar]
- 12.Rozanski K, Araguás-Araguás L, Gonfiantini R. Isotopic patterns in modern global precipitation. Climate change in continental isotopic records. 1993;78:1–36. [Google Scholar]
- 13.Bowen GJ, Ehleringer JR, Chesson LA, Thompson AH, Podlesak DW, et al. Dietary and Physiological Controls on the Hydrogen and Oxygen Isotope Ratios of Hair from Mid-20th Century Indigenous Populations. American Journal of Physical Anthropology. 2009;139:494–504. doi: 10.1002/ajpa.21008. [DOI] [PubMed] [Google Scholar]
- 14.Fraser I, Meier-Augenstein W, Kalin R. The role of stable isotopes in human identification: a longitudinal study into the variability of isotopic signals in human hair and nails. Rapid Communications in Mass Spectrometry. 2006;20:1109–1116. doi: 10.1002/rcm.2424. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien DM, Wooller MJ. Tracking human travel using stable oxygen and hydrogen isotope analyses of hair and urine. Rapid Communications in Mass Spectrometry. 2007;21:2422–2430. doi: 10.1002/rcm.3108. [DOI] [PubMed] [Google Scholar]
- 16.Thompson AH, Chesson LA, Podlesak DW, Bowen GJ, Cerling TE, et al. Stable Isotope Analysis of Modern Human Hair Collected From Asia (China, India, Mongolia, and Pakistan). American Journal of Physical Anthropology. 2010;141:440–451. doi: 10.1002/ajpa.21162. [DOI] [PubMed] [Google Scholar]
- 17.Podlesak DW, Torregrossa AM, Ehleringer JR, Dearing MD, Passey BH, et al. Turnover of oxygen and hydrogen isotopes in the body water, CO2, hair, and enamel of a small mammal. Geochimica et Cosmochimica Acta. 2008;72:19–35. [Google Scholar]
- 18.Nielson KE, Bowen GJ. Hydrogen and oxygen in brine shrimp chitin reflect environmental water and dietary isotopic composition. Geochimica et Cosmochimica Acta. 2010;74:1812–1822. [Google Scholar]
- 19.Wang YV, O'Brien DM, Jenson J, Francis D, Wooller MJ. The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia. 2009;160:225–233. doi: 10.1007/s00442-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 20.Hobson KA, Bowen GJ, Wassenaar LI, Ferrand Y, Lormee H. Using stable hydrogen and oxygen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia. 2004;141:477–488. doi: 10.1007/s00442-004-1671-7. [DOI] [PubMed] [Google Scholar]
- 21.Wolf N, Bowen GJ, del Rio CM. The influence of drinking water on the δD and δ18O values of house sparrow plasma, blood and feathers. The Journal of Experimental Biology. 2011;214:98–103. doi: 10.1242/jeb.050211. [DOI] [PubMed] [Google Scholar]
- 22.Tuross N, Warinner C, Kirsanow K, Kester C. Organic oxygen and hydrogen isotopes in a porcine controlled dietary study. Rapid Communications in Mass Spectrometry. 2008;22:1741–1745. doi: 10.1002/rcm.3556. [DOI] [PubMed] [Google Scholar]
- 23.Kirsanow K, Tuross N. Palaeogeography, Palaeoclimatology, Palaeoecology: in press; 2011. Oxygen and hydrogen isotopes in rodent tissues: Impact of diet, water and ontogeny. [Google Scholar]
- 24.Hobson KA, Atwell L, Wassenaar LI. Influence of drinking water and diet on the stable-hydrogen isotope ratios of animal tissues. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8003–8006. doi: 10.1073/pnas.96.14.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luz B, Kolodny Y. Oxygen isotope variation in bone phosphate. Applied Geochemistry. 1989;4:317–323. [Google Scholar]
- 26.O'Grady SP, Wende AR, Remien CH, Valenzuela LO, Enright LE, et al. Aberrant Water Homeostasis Detected by Stable Isotope Analysis. PloS ONE 5. 2010 doi: 10.1371/journal.pone.0011699. Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0011699. Accessed 2011 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp ZD, Atudorei V, Panarello HO, Fernandez J, Douthitt C. Hydrogen isotope systematics of hair: archeological and forensic applications. Journal of Archaeological Science. 2003;30:1709–1716. [Google Scholar]
- 28.Bryant JD, Froelich PN. A model of oxygen isotope fractionation in body water of large mammals. Geochimica et Cosmochimica Acta. 1995;59:4523–4537. [Google Scholar]
- 29.Bryant JD, Koch PL, Froelich PN, Showers WJ, Genna BJ. Oxygen isotope partitioning between phosphate and carbonate in mammalian apatite. Geochimica et Cosmochimica Acta. 1996;60:5145–5148. [Google Scholar]
- 30.Kohn MJ. Predicting animal δ18O: Accounting for diet and physiological adaptation. Geochimica et Cosmochimica Acta. 1996;60:4811–4829. [Google Scholar]
- 31.Longinelli A. Oxygen isotopes in mammal bone phosphate: a new tool for paleohydrological and paleoclimatological research? Geochimica et Cosmochimica Acta. 1984;48:385–390. [Google Scholar]
- 32.Luz B, Kolodny Y. Oxygen isotope variations in phosphate of biogenic apatites, IV. Mammal teeth and bones. Earth and Planetary Science Letters. 1985;75:29–36. [Google Scholar]
- 33.McKechnie AE, Wolf BO, Martínez del Rio C. Deuterium stable isotope ratios as tracers of water resource use: an experimental test with rock doves. Oecologia. 2004;140:191–200. doi: 10.1007/s00442-004-1564-9. [DOI] [PubMed] [Google Scholar]
- 34.Schoeller DA, Leitch CA, Brown C. Doubly labeled water method: in vivo oxygen and hydrogen isotope fractionation. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology. 1986;251:1137–1143. doi: 10.1152/ajpregu.1986.251.6.R1137. [DOI] [PubMed] [Google Scholar]
- 35.Tatner P. A model of the natural abundance of oxygen-18 and deuterium in the body water of animals. Journal of Theoretical Biology. 1988;133:267–280. [Google Scholar]
- 36.Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- 37.Ayliffe LK, Chivas AR. Oxygen isotope composition of the bone phosphate of Australian kangaroos: potential as a palaeoenvironmental recorder. Geochimica et Cosmochimica Acta. 1990;54:2603–2609. [Google Scholar]
- 38.Luz B, Cormie AB, Schwarcz HP. Oxygen isotope variations in phosphate of deer bones. Geochimica et Cosmochimica Acta. 1990;54:1723–1728. [Google Scholar]
- 39.Luz B, Kolodny Y, Horowitz M. Fractionation of oxygen isotopes between mammalian bone-phosphate and environmental drinking water. Geochimica et Cosmochimica Acta. 1984;48:1689–1693. [Google Scholar]
- 40.Karasov WH, del Rio CM. Princeton: University Press; 2007. Physiological ecology: how animals process energy, nutrients, and toxins.242 [Google Scholar]
- 41.Pecquerie L, Nisbet RM, Fablet R, Lorrain A, Kooijman SALM. The impact of metabolism on stable isotope dynamics: a theoretical framework. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:3455–3468. doi: 10.1098/rstb.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynard LM, Hedges REM. Stable hydrogen isotopes of bone collagen in palaeodietary and palaeoenvironmental reconstruction. Journal of Archaeological Science. 2008;35:1934–1942. [Google Scholar]
- 43.Lott CA, Meehan TD, Heath JA. Estimating the latitudinal origins of migratory birds using hydrogen and sulfur stable isotopes in feathers: influence of marine prey base. Oecologia. 2003;134:505–510. doi: 10.1007/s00442-002-1153-8. [DOI] [PubMed] [Google Scholar]
- 44.Delgado Huertas A, Iacumin P, Stenni B, Sánchez Chillón B, Longinelli A. Oxygen isotope variations of phosphate in mammalian bone and tooth enamel. Geochimica et Cosmochimica Acta. 1995;59:4299–4305. [Google Scholar]
- 45.Birchall J, O'Connell TC, Heaton THE, Hedges REM. Hydrogen isotope ratios in animal body protein reflect trophic level. Journal of Animal Ecology. 2005;74:877–881. [Google Scholar]
- 46.Levinson AA, Luz B, Kolodny Y. Variations in oxygen isotopic compositions of human teeth and urinary stones. Applied Geochemistry. 1987;2:367–371. [Google Scholar]
- 47.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. A stable isotope aridity index for terrestrial environments. Proceedings of the National Academy of Sciences. 2006;103:11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daux V, Lécuyer C, Heran MA, Amiot R, Simon L, et al. Oxygen isotope fractionation between human phosphate and water revisited. Journal of Human Evolution. 2008;55:1138–1147. doi: 10.1016/j.jhevol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Bowen GJ, Ehleringer JR, Chesson LA, Stange E, Cerling TE. Stable isotope ratios of tap water in the contiguous United States. Water Resources Research 43. 2007. Available: http://wateriso.eas.purdue.edu/waterisotopes/media/PDFs/USATapMap.pdf.
- 50.Chesson LA, Valenzuela LO, O'Grady SP, Cerling TE, Ehleringer JR. Links between Purchase Location and Stable Isotope Ratios of Bottled Water, Soda, and Beer in the United States. Journal of Agricultural and Food Chemistry. 2010;58:7311–7316. doi: 10.1021/jf1003539. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy CD, Bowen GJ, Ehleringer JR. Temporal variation of oxygen isotope ratios δ18O in drinking water: Implications for specifying location of origin with human scalp hair. Forensic Science International. 2011;208:156–166. doi: 10.1016/j.forsciint.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 52.Chesson LA, Podlesak DW, Thompson AH, Cerling TE, Ehleringer JR. Variation of hydrogen, carbon, nitrogen, and oxygen stable isotope ratios in an American diet: Fast food meals. Journal of Agricultural and Food Chemistry. 2008;56:4084–4091. doi: 10.1021/jf0733618. [DOI] [PubMed] [Google Scholar]
- 53.Fraser I, Meier-Augenstein W. Stable 2H isotope analysis of modern day human hair and nails can aid forensic human identification. Rapid Communications in Mass Spectrometry. 2007;21:3279–3285. doi: 10.1002/rcm.3209. [DOI] [PubMed] [Google Scholar]
- 54.MacDonald M, Rogers Q, Morris J. Nutrition of the domestic cat, a mammalian carnivore. Annual Review of Nutrition. 1984;4:521–562. doi: 10.1146/annurev.nu.04.070184.002513. [DOI] [PubMed] [Google Scholar]
- 55.Zoran DL. The carnivore connection to nutrition in cats. Journal of the American Veterinary Medical Association. 2002;221:1559–1567. doi: 10.2460/javma.2002.221.1559. [DOI] [PubMed] [Google Scholar]
- 56.Cormie AB, Luz B, Schwarcz HP. Relationship between the Hydrogen and Oxygen Isotopes of Deer Bone and Their Use in the Estimation of Relative-Humidity. Geochimica et Cosmochimica Acta. 1994;58:3439–3449. [Google Scholar]
- 57.Wassenaar L, Hobson K. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes in Environmental and Health Studies. 2003;39:211–217. doi: 10.1080/1025601031000096781. [DOI] [PubMed] [Google Scholar]
- 58.Bowen GJ, Wilkinson B. Spatial distribution of δ18O in meteoric precipitation. Geology. 2002;30:315–318. [Google Scholar]
- 59.Coplen TB, Kendall C, VA GSR. Stable hydrogen and oxygen isotope ratios for selected sites of the US Geological Survey's NASQAN and benchmark surface-water networks. 2000. Available: http://pubs.usgs.gov/of/2000/ofr00-160/. Accessed 2011 March 8.
- 60.Dutton A, Wilkinson BH, Welker JM, Bowen GJ, Lohmann KC. Spatial distribution and seasonal variation in 18O/16O of modern precipitation and river water across the conterminous USA. Hydrological Processes. 2005;19:4121–4146. [Google Scholar]
- 61.Kitchener A. Ithaca: Comstock Publishing Associates; 1991. The natural history of the wild cats.288 [Google Scholar]
- 62.Sandell M. The mating tactics and spacing patterns of solitary carnivores. Carnivore behavior, ecology, and evolution. 1989;1:164–182. [Google Scholar]
- 63.Baker K. Hair growth and replacement in the cat. The British Veterinary Journal. 1974;130:327. doi: 10.1016/s0007-1935(17)35835-9. [DOI] [PubMed] [Google Scholar]
- 64.Galbraith H. Nutritional and hormonal regulation of hair follicle growth and development. Proceedings of the Nutrition Society. 1998;57:195–205. doi: 10.1079/pns19980032. [DOI] [PubMed] [Google Scholar]
- 65.Ryder ML. Seasonal changes in the coat of the cat. Research in Veterinary Science. 1976;21:280. [PubMed] [Google Scholar]
- 66.Epstein S, Thompson P, Yapp CJ. Oxygen and hydrogen isotopic ratios in plant cellulose. Science. 1977;198:1209–1215. doi: 10.1126/science.198.4323.1209. [DOI] [PubMed] [Google Scholar]
- 67.Farquhar GD, Lloyd J, Taylor JA, Flanagan LB, Syvertsen JP, et al. Vegetation effects on the isotope composition of oxygen in atmospheric CO2. Nature. 1993;363:439–443. [Google Scholar]
- 68.Cormie A, Luz B, Schwarcz H. Relationship between the hydrogen and oxygen isotopes of deer bone and their use in the estimation of relative humidity. Geochimica et Cosmochimica Acta. 1994;58:3439–3449. [Google Scholar]
- 69.Iriarte JA, Franklin WL, Johnson WE, Redford KH. Biogeographic variation of food habits and body size of the America puma. Oecologia. 1990;85:185–190. doi: 10.1007/BF00319400. [DOI] [PubMed] [Google Scholar]
- 70.Lariviere S, Walton LR. Lynx rufus. Mammalian Species. 1997;563:1–8. [Google Scholar]
- 71.Kane E, Rogers Q, Morris J. Feeding behavior of the cat fed laboratory and commercial diets. Nutrition Research. 1981;1:499–507. [Google Scholar]
- 72.Lindzey F. Wild furbearer management and conservation in North America Toronto, Canada: Ontario Ministry of Natural Resources. In:; 1987. Mountain lion. pp. 656–668. [Google Scholar]
- 73.Johnson C. Sex-biased philopatry and dispersal in mammals. Oecologia. 1986;69:626–627. doi: 10.1007/BF00410373. [DOI] [PubMed] [Google Scholar]
- 74.Arnay-De-La-Rosa M, Gonzalez-Reimers E, Yanes Y, Velasco-Vazquez J, Romanek CS, et al. Paleodietary analysis of the prehistoric population of the Canary Islands inferred from stable isotopes (carbon, nitrogen and hydrogen) in bone collagen. Journal of Archaeological Science. 2010;37:1490–1501. [Google Scholar]
- 75.Schimmelmann A, Deniro MJ. Stable Isotopic Studies on Chitin. III. The D/H and 18O/16O Ratios in Arthropod Chitin. Geochimica et Cosmochimica Acta. 1986;50:1485–1496. [Google Scholar]
- 76.DeNiro MJ, Epstein S. Hydrogen Isotope Ratios of Mouse-Tissues Are Influenced by a Variety of Factors Other Than Diet. Science. 1981;214:1374–1375. doi: 10.1126/science.7313700. [DOI] [PubMed] [Google Scholar]
- 77.Gretebeck RJ, Schoeller DA, Socki RA, Davis-Street J, Gibson EK, et al. Adaptation of the doubly labeled water method for subjects consuming isotopically enriched water. Journal of Applied Physiology. 1997;82:563–570. doi: 10.1152/jappl.1997.82.2.563. [DOI] [PubMed] [Google Scholar]
- 78.d'Angela D, Longinelli A. Oxygen isotopes in living mammal's bone phosphate: further results. Chemical Geology: Isotope Geoscience section. 1990;86:75–82. [Google Scholar]
- 79.Masud Z, Vallet C, Martin GJ. Stable isotope characterization of milk components and whey ethanol. Journal of Agricultural and Food Chemistry. 1999;47:4693–4699. doi: 10.1021/jf9900027. [DOI] [PubMed] [Google Scholar]
- 80.Estep MF, Hoering TC. Biogeochemistry of the stable hydrogen isotopes. Geochimica et Cosmochimica Acta. 1980;44:1197–1206. [Google Scholar]
- 81.Smith B, Epstein S. Biogeochemistry of the stable isotopes of carbon and hydrogen in salt marsh biota. Plant Physiology. 1970;46:738–742. doi: 10.1104/pp.46.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warinner C, Tuross N. Brief communication: Tissue isotopic enrichment associated with growth depression in a pig: Implications for archaeology and ecology. American Journal of Physical Anthropology. 2010;141:486–493. doi: 10.1002/ajpa.21222. [DOI] [PubMed] [Google Scholar]
- 83.Hendriks W, Wamberg S, Tarttelin M. A metabolism cage for quantitative urine collection and accurate measurement of water balance in adult cats (Felis catus). Journal of Animal Physiology and Animal Nutrition. 1999;82:94–105. [Google Scholar]
- 84.Mellanby K. Metabolic water and desiccation. Nature. 1942;150:21. [Google Scholar]
- 85.Kromhout D. Coronary heart disease epidemiology. New York: Oxford University Press; 2005. Fish consumption, n-3 fatty acids, and coronary heart disease. pp. 264–275. [Google Scholar]
- 86.Morris JG. Nutritional and metabolic responses to arginine deficiency in carnivores. Journal of Nutrition. 1985;115:524–531. doi: 10.1093/jn/115.4.524. [DOI] [PubMed] [Google Scholar]
- 87.Kirk CA, Debraekeleer J, Armstrong PJ. Normal cats. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, editors. Small animal clinical nutrition 4th ed. Marceline, MO: Walsworth Publishing Co; 2000. pp. 291–351. [Google Scholar]
- 88.Buchardt B, Bunch V, Helin P. Fingernails and diet: stable isotope signatures of a marine hunting community from modern Uummannaq, North Greenland. Chemical Geology. 2007;244:316–329. [Google Scholar]
- 89.Cohen JE. Marine and Continental Food Webs - 3 Paradoxes. Philosophical Transactions of the Royal Society of London B. 1994;343:57–69. [Google Scholar]
- 90.Gibson R, Sinclair A. Are Eskimos obligate carnivores? Lancet. 1981;1:1100. doi: 10.1016/s0140-6736(81)92263-7. [DOI] [PubMed] [Google Scholar]
- 91.Symes CT, Woodborne S. Migratory connectivity and conservation of the Amur Falcon Falco amurensis: a stable isotope perspective. Bird Conservation International. 2010;20:134–148. [Google Scholar]
- 92.Meehan TD, Rosenfield RN, Atudore VN, Bielefeldt J, Rosenfield LJ, et al. Variation in hydrogen stable-isotope ratios between adult and nestling Cooper's Hawks. Condor. 2003;105:567–572. [Google Scholar]
- 93.Smith AD, Donohue K, Dufty AM., Jr Intrafeather and intraindividual variation in the stable-hydrogen isotope δD content of raptor feathers. The Condor. 2008;110:500–506. [Google Scholar]
- 94.Legrand-Defretin V. Differences between cats and dogs: a nutritional view. Proceedings of the Nutrition Society. 1994;53:15–24. doi: 10.1079/pns19940004. [DOI] [PubMed] [Google Scholar]
- 95.Kienzle E. Carbohydrate metabolism of the cat 1. Activity of amylase in the gastrointestinal tract of the cat. Journal of Animal Physiology and Animal Nutrition. 1993;69:92–101. [Google Scholar]
- 96.Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews. 2009;84:91–111. doi: 10.1111/j.1469-185X.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 97.Anderson R. Water balance in the dog and cat. Journal of Small Animal Practice. 1982;23:588–598. [Google Scholar]
- 98.Chew RM. Water metabolism of mammals. In: Mayer RV, Van Gelder RG, editors. Physiological mammalogy. New York Academic Press; 1965. pp. 43–178. [Google Scholar]
- 99.Schmidt-Nielsen K, Schmidt-Nielsen B. Water Metabolism of Desert Mammals. Physiological Reviews. 1952;32:135–166. doi: 10.1152/physrev.1952.32.2.135. [DOI] [PubMed] [Google Scholar]
- 100.McNab BK. The influence of food habits on the energetics of eutherian mammals. Ecological Monographs. 1986;56:1–19. [Google Scholar]
- 101.McNab BK. The standard energetics of mammalian carnivores: Felidae and Hyaenidae. Canadian Journal of Zoology. 2000;78:2227–2239. [Google Scholar]
- 102.Doris PA, Baker MA. Effect of Dehydration on Thermoregulation in Cats Exposed to High Ambient-Temperatures. Journal of Applied Physiology. 1981;51:46–54. doi: 10.1152/jappl.1981.51.1.46. [DOI] [PubMed] [Google Scholar]
- 103.Adams T. Characteristics of eccrine sweat gland activity in the footpad of the cat. Journal of Applied Physiology. 1966;21:1004–1012. doi: 10.1152/jappl.1966.21.3.1004. [DOI] [PubMed] [Google Scholar]
- 104.Wong W, Cochran W, Klish W, Smith EO, Lee L, et al. In vivo isotope-fractionation factors and the measurement of deuterium-and oxygen-18-dilution spaces from plasma, urine, saliva, respiratory water vapor, and carbon dioxide. American Journal of Clinical Nutrition. 1988;47:1–6. doi: 10.1093/ajcn/47.1.1. [DOI] [PubMed] [Google Scholar]
- 105.Homberger DG, Ham K, Ogunbakin T, Bonin JA, Hopkins BA, et al. The structure of the cornified claw sheath in the domesticated cat (Felis catus): implications for the claw shedding mechanism and the evolution of cornified digital end organs. Journal of Anatomy. 2009;214:620–643. doi: 10.1111/j.1469-7580.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.O'Connell T, Hedges R, Healey M, Simpson A. Isotopic comparison of hair, nail and bone: modern analyses. Journal of Archaeological Science. 2001;28:1247–1255. [Google Scholar]
- 107.Hendriks W, Tarttelin M, Moughan P. The amino acid composition of cat (Felis catus) hair. Animal Science. 1998;67:165–170. [Google Scholar]
- 108.Commerford S, Carsten A, Cronkite E. The distribution of tritium among the amino acids of proteins obtained from mice exposed to tritiated water. Radiation Research. 1983;94:151–155. [PubMed] [Google Scholar]
- 109.Gaebler O, Vitti TG, Vukmirovich R. Isotope effects in metabolism of 14N and 15N from unlabeled dietary proteins. Biochemistry and Cell Biology. 1966;44:1249–1257. doi: 10.1139/o66-142. [DOI] [PubMed] [Google Scholar]
- 110.Hare PE, Fogel ML, Stafford TW. The isotopic composition of carbon and nitrogen in individual amino acids isolated from modern and fossil proteins. Journal of Archaeological Science. 1991;18:277–292. [Google Scholar]
- 111.Macko SA, Fogel ML, Hare P, Hoering T. Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chemical Geology: Isotope Geoscience section. 1987;65:79–92. [Google Scholar]
- 112.Péquignot A, Tumosa C, Endt DW. The effects of tanning and fixing processes on the properties of taxidermy skins. Collection Forum 21. 2006. Available: http://www.crcc.cnrs.fr/IMG/pdf/Collection_Forum_2006-2.pdf.
- 113.Lees S. Water content in type I collagen tissues calculated from the generalized packing model. International Journal of Biological Macromolecules. 1986;8:66–72. [Google Scholar]
- 114.Covington AD. Modern tanning chemistry. Chemical Society Reviews. 1997;26:111–126. [Google Scholar]
- 115.McCullagh JSO, Tripp JA, Hedges REM. Carbon isotope analysis of bulk keratin and single amino acids from British and North American hair. Rapid Communications in Mass Spectrometry. 2005;19:3227–3231. doi: 10.1002/rcm.2150. [DOI] [PubMed] [Google Scholar]
- 116.Petzke KJ, Fuller BT, Metges CC. Advances in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13:532–540. doi: 10.1097/MCO.0b013e32833c3c84. [DOI] [PubMed] [Google Scholar]
- 117.Raghavan M, McCullagh JSO, Lynnerup N, Hedges REM. Amino acid δ13C analysis of hair proteins and bone collagen using liquid chromatography/isotope ratio mass spectrometry: paleodietary implications from intra-individual comparisons. Rapid Communications in Mass Spectrometry. 2010;24:541–548. doi: 10.1002/rcm.4398. [DOI] [PubMed] [Google Scholar]
- 118.Takeda H, Lu HM, Miyamoto K, Fuma S, Yanagisawa K, et al. Comparative biokinetics of tritium in rats during continuous ingestion of tritiated water and tritium-labeled food. International Journal of Radiation Biology. 2001;77:375–381. doi: 10.1080/09553000010017117. [DOI] [PubMed] [Google Scholar]
- 119.Wallach ADWAD, Inbar MIM, Scantlebury MSM, Speakman JRSJR, Shanas USU. Water requirements as a bottleneck in the reintroduction of European roe deer to the southern edge of its range. Canadian Journal of Zoology. 2007;85:1182–1192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample list.
(XLS)
Statistical analysis.
(DOC)