Abstract
The TEL (translocation–Ets–leukemia or ETV6) locus, which encodes an Ets family transcription factor, is frequently rearranged in human leukemias of myeloid or lymphoid origins. By gene targeting in mice, we previously showed that TEL−/− mice are embryonic lethal because of a yolk sac angiogenic defect. TEL also appears essential for the survival of selected neural and mesenchymal populations within the embryo proper. Here, we have generated mouse chimeras with TEL−/− ES cells to examine a possible requirement in adult hematopoiesis. Although not required for the intrinsic proliferation and/or differentiation of adult-type hematopoietic lineages in the yolk sac and fetal liver, TEL function is essential for the establishment of hematopoiesis of all lineages in the bone marrow. This defect is manifest within the first week of postnatal life. Our data pinpoint a critical role for TEL in the normal transition of hematopoietic activity from fetal liver to bone marrow. This might reflect an inability of TEL−/− hematopoietic stem cells or progenitors to migrate or home to the bone marrow or, more likely, the failure of these cells to respond appropriately and/or survive within the bone marrow microenvironment. These data establish TEL as the first transcription factor required specifically for hematopoiesis within the bone marrow, as opposed to other sites of hematopoietic activity during development.
Keywords: TEL gene, hematopoiesis, leukemia
Hematopoiesis is the process by which blood cells of distinct lineages (erythrocytes, B and T lymphocytes, neutrophils, monocyte/macrophages, mast cells, and megakaryocytes) are produced from pluripotent hematopoietic stem cells (HSCs) (Orkin 1996). HSCs lie at the top of the hierarchy and give rise to progenitor cells that exhibit varying developmental potentials. Options available to progenitors become restricted progressively until differentiation along a single pathway takes place. Blood cell formation is initiated early during vertebrate embryogenesis. The first cells produced, embryonic (or primitive) erythrocytes, arise within the blood islands of the extraembryonic yolk sac at embryonic day 7.5 (E7.5). By E11.5, hematopoiesis shifts to the fetal liver, where adult (or definitive) red cells, as well as cells of other lineages, first appear. The site of origin of HSCs has been less certain. Whereas it was previously accepted that HSCs and progenitors migrate from the yolk sac to the fetal liver during development, more recent studies relying on cell transplantation to reconstitute hematopoiesis in adult recipients assign an intraembryonic source for definitive (adult) hematopoiesis within the intraembryonic para-aortic splanchnopleura and aortic–gonadal–mesonephros (AGM) regions (Godin et al. 1993; Medvinsky et al. 1993; Medvinsky and Dzierzak 1996). HSCs arising in these areas are believed to migrate to and colonize the fetal liver and spleen. The presence of multipotential progenitors in the blood of E10 embryos suggests that migration and colonization are mediated via the circulation (Delassus and Cumano 1996). A unique origin of HSCs is challenged by recent evidence demonstrating long-term repopulation by yolk sac progenitors as assayed by reconstitution of fetal recipient animals (Yoder et al. 1997). Thus, the development of a stable, functioning hematopoietic system reflects complex processes involving cellular differentiation, as well as temporal and spatial control of migration, homing, proliferation, and survival of HSCs.
Regulation takes place at multiple levels to ensure proper blood cell development. Cytokines and their cognate receptors mediate signals that participate directly or indirectly in the proliferation, differentiation, or survival of HSC and progenitor cells (see Veiby et al. 1997). Ultimately, these processes are mediated by transcription factors that serve to establish cellular patterns of gene expression (Orkin 1996). In vivo requirements for transcription factors exhibiting hematopoietic or lineage-restricted pattterns of expression have been established by gene targeting studies (see Shivdasani and Orkin 1996).
Among transcriptional proteins essential for aspects of hematopoiesis, several were discovered by virtue of chromosomal translocations associated with human leukemias. These include the SCL/tal-1, Rbtn2/Lmo2, MLL-1, and AML-1 genes (Yu et al. 1995; Okuda et al. 1996; Porcher et al. 1996; Robb et al. 1996; Wang et al. 1996). Another presumed transcription factor involved in leukemia is encoded by the (TEL) (translocation–Ets–leukemia or ETV6) locus (Golub et al. 1994). The involvement of the TEL gene in leukemia is particularly interesting in that different translocations lead to the production of various chimeric proteins, which are associated specifically with distinct forms of the disease (see Golub et al. 1997). Fusion of the oligomerization (or pointed) domain of TEL with the platelet-derived growth factor receptor-β (PDGFβR) chain or with c-Abl leads to constitutive activation of their tyrosine kinases in the pathogenesis of chronic myelomonocytic leukemia (Golub et al. 1994; Papadopoulos et al. 1995; Golub et al. 1996). Fusion of this region with the catalytic domain of the Janus family kinase, JAK2, is associated with various leukemias depending on the precise chimera generated (Lacronique et al. 1997; Peeters et al. 1997). Finally, the fusion of the oligomerization domain to the DNA-binding and transactivation regions of the runt-related AML-1/CBFα2 protein is commonly seen in childhood acute pre-B-cell lymphoblastic leukemia (Golub et al. 1995; Romana et al. 1995), and confers a favorable prognosis (McLean et al. 1996; Shurtleff et al. 1995). TEL-AML1-associated leukemia is unique in that the normal TEL allele is consistently absent (Sato et al. 1995; Stegmaier et al. 1995; Kim et al. 1996; McLean et al. 1996; Raynaud et al. 1996). Loss of heterozygosity suggests that functions of the normal TEL protein may retard or block the development (or progression) of leukemia. This observation predicts a role for TEL itself in some aspect(s) of blood cell formation.
As shown by gene targeting, TEL function is required for viability of the developing mouse. TEL−/− embryos die at E11.5 because of a failure in maintenance of the developing vascular network in the yolk sac (Wang et al. 1997). In addition, apoptosis occurs in selected regions of the embryo. We showed previously that TEL is not required for production of embryonic red cells in the yolk sac, or for in vitro colony formation by definitive hematopoietic progenitors. Although excluding TEL as an essential regulator of the intrinsic differentiation programs of hematopoietic cells, these observations did not address functions in adult hematopoiesis in vivo.
To investigate the role of TEL in fetal liver or bone marrow hematopoiesis within the context of an intact animal, we examined the development of all blood lineages in chimeric mice generated in both wild-type and recombinase-activating-2 gene (RAG-2)−/− backgrounds (Chen et al. 1993). Here, we demonstrate that, although dispensable for fetal liver hematopoiesis, TEL is specifically required for bone marrow hematopoiesis, as early as the immediate postnatal period. Our findings raise several possible mechanisms by which TEL functions in hematopoiesis, and have implications for the consequences of TEL loss in childhood pre-B-cell leukemia.
Results
TEL is expressed in hematopoietic tissues and cell lines
TEL RNA transcripts are expressed widely in the early embryo and adult mouse (Wang et al. 1997). We used RNA in situ hybridization to assess TEL expression at midgestation. As compared with other tissues except the lung, TEL transcripts are expressed highly in the fetal liver and thymus at E14.5 (Fig. 1A,B). TEL mRNAs are also detectable by Northern blotting in cell lines representative of various blood lineages and developmental stages (Fig. 1C). Thus, TEL appears to be relatively abundant in hematopoietic cells.
Figure 1.
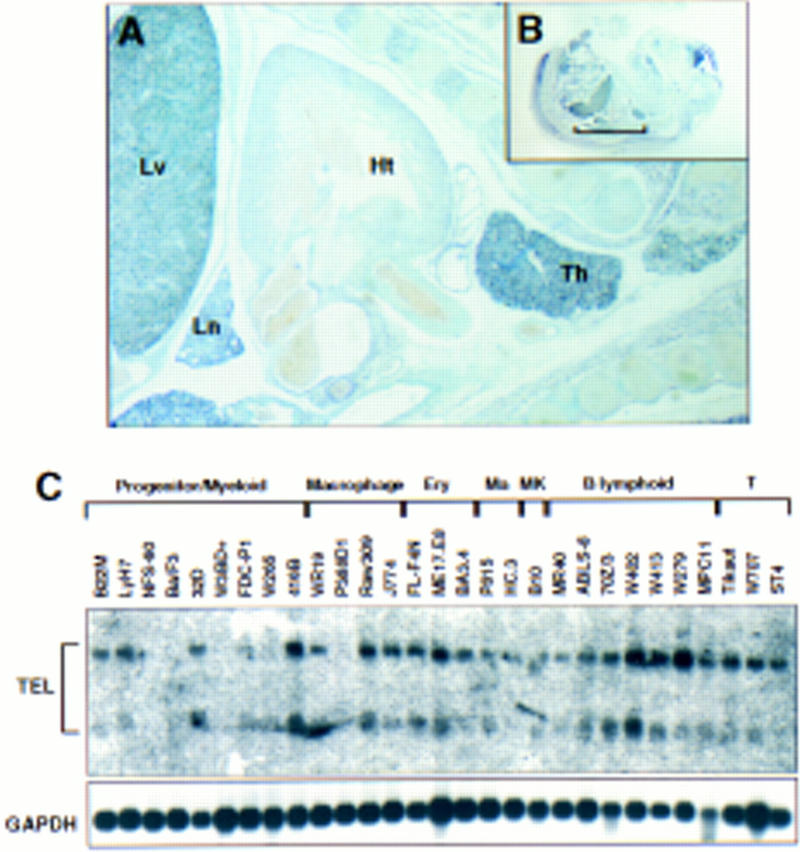
TEL mRNAs are expressed in hematopoietic tissues and cell lines. (A,B) In situ hybridization of TEL transcripts was performed on E14.5 paraffin-embedded embryos as described previously (Wang et al. 1997). (Th) Thymus; (Ht) heart; (Lv) liver; (Ln) lung. (C) Northern blot analysis of poly(A)+ RNA isolated from the indicated murine cell lines. Hybridization with TEL cDNA probes is shown above. Hybridization with GAPDH cDNA as a control is shown below. (Ma) Mast cells; (MK) megakaryocytes; (T) T lymphoid.
Selection for G418-resistant hematopoietic colonies demonstrates that TEL+/− and TEL−/− embryonic stem cells contribute to definitive lineages of chimeric mice at the yolk sac stage
Prior analysis of TEL−/− embryos revealed normal erythropoiesis in the yolk sac and the presence of both erythroid and myeloid colonies in colony assays of yolk sac progenitors (Wang et al. 1997). Such in vitro colonies obtained from the mid-yolk sac arise from definitive progenitors (Kennedy et al. 1997). To circumvent the early lethality of TEL−/− embryos and examine a potential role for TEL in definitive hematopoiesis, we analyzed chimeras made by injection of TEL+/− and TEL−/− embryonic stem cells into wild-type C57BL/6 blastocysts. To distinguish host and ES-derived hematopoietic progenitors, we took advantage of the presence of an active neomycin-resistance gene in the targeted TEL+/− or TEL−/− cells (Fig. 2A). Only colonies derived from injected ES cells would be expected to survive in the presence of G418.
Figure 2.

Assay of ES-cell-derived G418-resistant hematopoietic colonies in TEL/wild-type chimeras. (A) G418 selection strategy to distinguish hematopoietic colonies derived from wild-type (WT) or ES origins in the TEL/wild-type chimeras (see Results). Following injection of TEL+/− and TEL−/− ES cells into wild-type C57BL/6 blastocysts, single-cell suspensions are prepared from hematopoietic organs at different stages [yolk sac (E10.5), fetal liver (E14.5–E16.5), perinatal, adult]. Cells are plated in methylcellulose cultures supplemented with various growth factors, either in the presense or in the absence of G418. The numbers of colonies representing each blood-cell lineage are numerated and colonies are prepared for histological examination. (neo) Neomycin; (hyg) hygromycin; (G418S) G418 sensitive; (G418r) G418 resistant. (B) Macrophage/granulocytic colonies grown in the absence (−) and in the presense (+) of G418 (left panel, 1 mg/ml; right panel, 1.5 mg/ml G418, respectively) were collected from two representative (** in Table 1) TEL/wild-type yolk-sac progenitor assays, DNAs were extracted and subjected to Southern blot analysis. (wt) Wild-type allele; (mt) mutant allele. Note that only targeted alleles (ES-cell-derived) were detected after selection in the presence of G418.
This strategy was validated by experiments summarized in Table 1. Macrophage/granulocyte colonies of yolk sac progenitors were obtained in the presence of G418 from embryos shown to be chimeric by Southern blot analysis. Drug-resistant colonies were not obtained from cells of nonchimeric embryos. Moreover, Southern blotting of G418-resistant colonies grown from TEL−/−/wild-type chimeras revealed only the targeted TEL allele (Fig. 2B). Thus, culture in the presence of G418 selects for ES-derived cells and does not permit survival of wild-type cells. Finally, the fraction of colonies surviving G418 selection closely parallels the degree of chimerism estimated by Southern blotting of embryonic material (Table 1). Therefore, the approach outlined in Figure 2A is a valid means of evaluating the origin of hematopoietic lineages in mouse chimeras at different stages of development.
Table 1.
TEL+/− and TEL−/−ES cells contribute to definitive lineages at the yolk sac stage
| Genotype
|
Chimera
|
Percent ES cell contribution
|
Macrophage/granulocyte colonies
|
|
|---|---|---|---|---|
| −G418
|
+G418
|
|||
| 1 | 0 | >100 | *0 | |
| 2 | 0 | >100 | *0 | |
| −/− | 3 | 50 | 84 ± 20 | 26 ± 2 |
| 4 | 60 | 168 ± 20 | **68 ± 2 | |
| 5 | 80 | 113 ± 20 | **69 ± 6 | |
| +/− | 1 | 20 | 37 ± 3 | 7 ± 0 |
| 2 | 20 | 50 ± 5 | 14 ± 2 | |
Hematopoietic progenitor assays were performed using E10.5 yolk sac cells of TEL/wild-type chimeras in the presence of macrophage/granulocyte colonies (IL-1/IL-3/G-CSF/GM-CSF). Percent ES contribution was estimated by Southern blotting. Note that no colonies were detected in the presence of G418 in chimeras lacking ES cells contribution (*). Hematopoietic colonies (**) were collected and subjected to Southern blot analysis, as shown in Fig. 2B.
TEL−/− ES cells contribute to fetal liver hematopoiesis
We applied this assay to progenitors present in fetal livers of TEL+/− and TEL−/− chimeras. In each instance Southern blotting was used to document chimerism of varying extents in other embryonic tissues (data not shown). As summarized in Table 2, ES-derived (i.e., G418-resistant) erythroid colonies of CFU-e and BFU-e types, as well as non-red (i.e., myeloid) colonies, were observed. Colony morphologies were normal. Thus, expression of the TEL gene is not essential for fetal liver hematopoiesis. This also implies that TEL is not required for the intrinsic commitment and maturation of progenitors of these lineages.
Table 2.
TEL−/− ES cells contribute to fetal liver hematopoiesis
| Chimera
|
Genotype
|
Red colonies
|
|||||
|---|---|---|---|---|---|---|---|
| (CFU-e)
|
(BFU-e)
|
Non-red Colonies
|
|||||
| −G418
|
+G418
|
−G418
|
+G418
|
−G418
|
+G418
|
||
|
|
+/−
|
510 ± 90
|
190 ± 45
|
70 ± 20
|
40 ± 5
|
910 ± 140
|
190 ± 30
|
| −/− | 655 ± 35 | 395 ± 50 | 110 ± 40 | 40 ± 20 | 750 ± 70 | 85 ± 10 | |
| Clone 1 | −/− | 610 ± 130 | 20 ± 10 | 115 ± 35 | 15 ± 5 | 925 ± 45 | 35 ± 10 |
| −/− | 770 ± 90 | 55 ± 10 | 170 ± 10 | 15 ± 5 | 1055 ± 165 | 35 ± 5 | |
| Clone 2 | −/− | 875 ± 75 | 360 ± 45 | 220 ± 20 | 90 ± 5 | 1200 ± 10 | 125 ± 35 |
| Clone 3 | −/− | --------------- N.D. ---------------- | 635 ± 35 | 85 ± 15 | |||
| −/− | 625 ± 45 | 50 ± 0 | |||||
One TEL+/− ES clone and three independently-derived TEL−/− ES cell clones were injected into wild-type blastocysts. Progenitor assays were performed on the resultant TEL/wild-type chimeric fetal livers (E14.5–E16.5). Erythroid (red) colonies, including CFU-e and BFU-e, and myeloid (non-red) colonies were cultured with or without G418 as in Fig. 2A. Numbers represent progenitors/106 fetal liver cells. (N.D.) Not determined.
TEL−/− ES cells do not contribute to bone marrow myelopoiesis and erythropoiesis
The site of hematopoiesis shifts from fetal liver to the bone marrow during the transition from intrauterine to extrauterine life. Given its dispensability for yolk sac and fetal liver hematopoiesis, a deficit in bone marrow hematopoiesis of TEL−/− cells was not expected. However, initial studies using adult marrow cells of >8-week-old mice revealed that TEL−/−, but not TEL+/−, cells failed to generate G418-resistant myeloid colonies in progenitor assays (Table 3A). Chimerism was indicated by ES-cell-derived agouti coat color contribution, and subsequently verified by Southern blotting in the majority of animals (see below; data not shown). Further analysis revealed that the observed deficit of TEL−/− ES-cell-derived progenitors was manifest in the bone marrow by 1 week of age (Table 3A). Additional lineages were assayed to examine the extent of the deficit. TEL−/− bone-marrow-derived mast cells (Table 3A) and megakaryocyte colonies (Table 3B) were also not observed upon G418 selection. Red blood cell contribution was assessed by hemoglobin analysis, relying on genotype differences between host and ES-derived cells. Adult red cells were exclusively of host origin in liveborn chimeras (Fig. 3). These findings indicate that TEL function is required for the production of bone marrow-derived erythroid, myeloid, mast, and megakaryocytic cells.
Table 3.
TEL function is required for development of myeloid, mast cells, and megakaryocytes
| A. TEL−/− ES cells do not contribute to bone marrow myelopoiesis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chimera
|
Percent agouti
|
Macrophage/granulocyte colonies
|
Mast cells
|
||||||
| genotype
|
age (week)
|
−G418
|
+G418
|
−G418
|
+G418
|
||||
| >70 | 2240 ± 240 | 650 ± 5 | + | + | |||||
| 1 | ∼50 | 860 ± 20 | 370 ± 5 | + | + | ||||
| <50 | 660 ± 80 | 205 ± 5 | + | + | |||||
| <50 | 3120 ± 340 | 80 ± 0 | + | + | |||||
| +/− | 3 | <50 | 880 ± 105 | 280 ± 5 | + | + | |||
| >70 | 1285 ± 125 | 405 ± 65 | + | + | |||||
| >8 | >70 | 1400 ± 100 | 350 ± 5 | + | + | ||||
| <70 | 260 ± 30 | 35 ± 5 | + | + | |||||
| <70 | 665 ± 75 | 35 ± 10 | + | + | |||||
| >70 | 875 ± 145 | 5 ± 5 | + | − | |||||
| <50 | 2210 ± 390 | 0 | + | − | |||||
| −/− | 1 | <50 | 2270 ± 10 | 0 | + | − | |||
| <50 | 2100 ± 60 | 0 | + | − | |||||
| <50 | 860 ± 10 | 0 | + | − | |||||
| 3 | >50 | 1315 ± 95 | 0 | + | − | ||||
| <50 | 1095 ± 45 | 0 | + | − | |||||
| >70 | 1165 ± 165 | 0 | + | − | |||||
| >8 | >70 | 460 ± 40 | 0 | + | − | ||||
| <70 | 500 ± 80 | 0 | + | − | |||||
| <70 | 690 ± 60 | 0 | + | − | |||||
| B. Lack of megakaryocytic progenitors in the bone marrows of TEL−/− → wild-type chimeras | |||||||||
| Megakaryocyte colony no./1 × 106 bone marrow cells | |||||||||
| −G418 | +G418 | ||||||||
| 1 | 50 | 155 ± 45 | 60 ± 10 | ||||||
| +/− | 40 | 115 ± 25 | 35 ± 15 | ||||||
| 6 | 80 | 165 ± 35 | 25 ± 3 | ||||||
| 30 | 200 ± 30 | 10 ± 0 | |||||||
| −/− | 1 | 30 | 105 ± 5 | 0 | |||||
| 6 | 60 | 195 ± 15 | 0 | ||||||
| 40 | 185 ± 10 | 0 | |||||||
(A) Bone marrow progenitor assays were performed using TEL/wild-type chimeras with various degrees of ES cell contribution. Bone marrow cells were cultured in methylcellulose supplemented with IL-1/IL-3/GM-CSF/G-CSF to obtain myeloid colonies indicated as no. of myeloid progenitors per 1 × 106 bone marrow cells. To obtain homogeneous populations of mature mast cells, 2 × 105 bone marrow cells/ml were cultured in DMEM supplemented with 10% FCS and IL-3 for 4 weeks. In the presence of G418 no viable cells were obtained from TEL−/−/wild-type chimeras. Similarly, no viable cells were obtained from control wild-type C57BL/6 bone marrow cells upon G418 selection. (+) Mast cell growth; (−) no growth.
(B) Progenitor assays were performed in the presence of kit-ligand (KL) and thrombopoietin. Pure and mixed (with erythroid) megakaryocytic colonies were enumerated on day 7. The identity of megakaryocytes was confirmed by May-Grunwald-Giemsa stain and histochemical staining for acetylcholinesterase activity.
Figure 3.
TEL−/− ES cell progneitors do not contribute to mature erythroid lineages in chimeras. Red blood cells from young and adult chimeric mice were subject to hemoglobin analysis. [H (Hbbs)] Specific for host (C57BL/6) blastocyst cells; [ES (Hbbd)] specific for ES (129/Sv) cells. (*) Lack of adult β-hemoglobin contribution from TEL−/− ES-cell-derived chimeras.
TEL is also required for efficient lymphopoiesis in the adult
To address the possible involvement of TEL in lymphopoiesis, we generated chimeras by injection of TEL+/− or TEL−/− ES cells into RAG-2−/− blastocysts. Because RAG-2−/− mice do not produce mature B and T lymphocytes (Chen et al. 1993), examination of the number and phenotype of B and T cells in such chimeras allows for a rapid and stringent complementation assay of lymphoid potential.
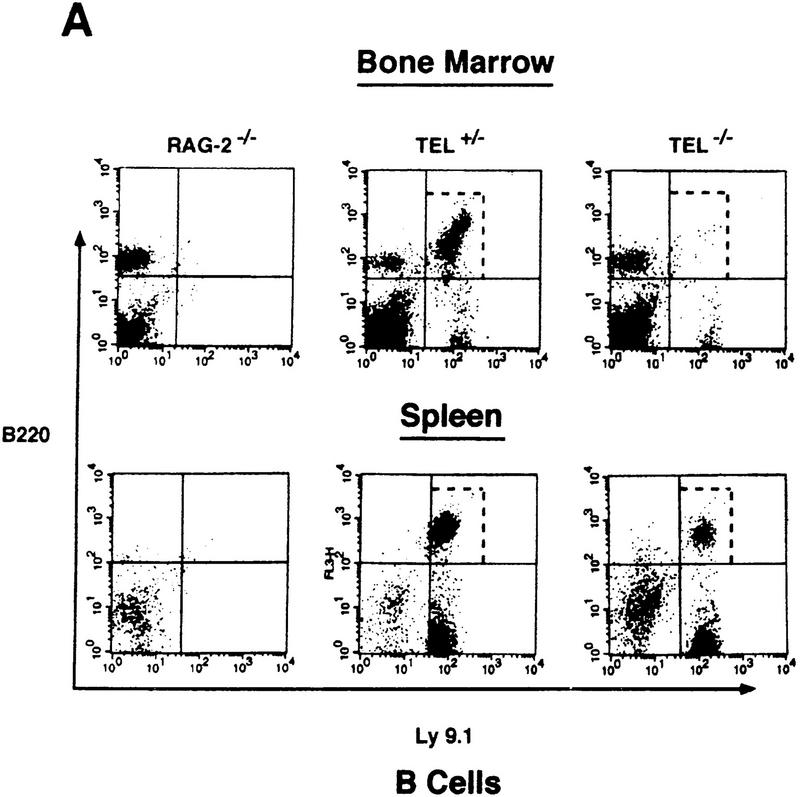
We first examined the B- and T-cell phenotype in adult TEL/RAG-2 chimeras by FACS analysis. As shown in Figure 4A, a dramatic reduction in both the frequency and absolute number of TEL−/− B220+ B cells in the bone marrow was observed. This B-cell defect appears to lie at the progenitor level as revealed by the lack of pre-B-cell progenitors in the bone marrow assayed at 1–6 weeks of age (Table 4). Note, however, that despite the paucity of TEL−/− B220+ B cells in the bone marrow, B220+/IgM+ mature B cells are present in the spleen (Fig. 4A; data not shown).
Figure 4.
TEL−/− ES-derived cells do not reconstitute efficiently lymphoid lineages in RAG-2−/− chimeras. Flow cytometry was performed on hematopoietic compartments of the TEL/RAG-2 chimeras. Anti-B220 antibody was used as B-cell marker for bone marrow and spleen cells. Anti-Ly 9.1 antibody distinguishes ES-derived from RAG-2−/− blastocyst-derived lymphocytes (the latter are Ly9.1−). (A) Anti-CD4 and CD8 antibodies were used as T-cell markers for thymus and lymph-node cells. (B) The total numbers of lymph-node T cells are indicated. The dotted rectangles highlight the differences in the frequency of reconstituting lymphocytes between the TEL+/− and TEL−/− ES-cell-derived chimeras.
Table 4.
Lack of pre-B cell progenitors in the bone marrows of TEL−/− → wild-type chimeras
| Chimera
|
Percent agouti | Pre-B cell colony no./ 1 × 106 bone marrow cells
|
||
|---|---|---|---|---|
| genotype | age (weeks) | −G418 | +G418 | |
| 1 | 80 | 345 ± 50 | 140 ± 5 | |
| 15 | 640 ± 40 | 4 ± 1 | ||
| +/− | 2 | 80 | 895 ± 35 | 203 ± 23 |
| 60 | 775 ± 75 | 71 ± 8 | ||
| 6 | 80 | 307 ± 13 | 69 ± 6 | |
| 30 | 285 ± 25 | 19 ± 1 | ||
| 40 | 480 ± 0 | 0 | ||
| 1 | 40 | 398 ± 38 | 0 | |
| 30 | 210 ± 25 | 0 | ||
| 60 | 935 ± 5 | 0 | ||
| −/− | 2 | 40 | 1060 ± 40 | 0 |
| 40 | 595 ± 65 | 0 | ||
| 6 | 60 | 370 ± 0 | 0 | |
| 40 | 333 ± 47 | 0 | ||
Pre-B cell progrenitor assays were performed in 1- to 6-week-old chimeric mice. Bone marrow cells (2 × 105) were cultured in methylcellulose supplemented with IL-7 (H3630, Stem Cell Technology), and pre-B colonies were enumerated on day 10. Numbers represent pre-B progenitors/1 × 106 bone marrow cells.
The number of TEL−/− CD4+CD8+ immature T cells in the thymus was also reduced dramatically relative to that seen with TEL+/− ES cells (Fig. 4B). As with TEL−/− B cells, the defect appears to lie at the progenitor level, as TEL−/− CD25+ prothymocytes failed to accumulate (data not shown). In addition, despite the low number of immature thymic T cells, mature single CD4+ or CD8+ T cells appeared to migrate to and repopulate the lymph node in the RAG-2−/− background (Fig. 4B). The total number of these mature T cells was ∼sixfold less than that seen in TEL+/− chimeras (Fig. 4B).
We also examined progenitors in chimeras during the transition from fetal liver to bone marrow hematopoiesis. As shown in Figure 5, FACS analysis of thymic cells of E18 TEL/RAG-2 chimeras revealed the presence of TEL−/− CD4+CD8+ immature T cells in numbers approximating that of TEL+/− cells. Thus, TEL−/− progenitors exit the fetal liver, migrate, and home to fetal thymus, despite their paucity in the thymus after birth.
Figure 5.
TEL−/− ES cells contribute to thymic T cells prior to birth. TEL+/− and TEL−/− ES cells were injected into RAG-2−/− blastocysts, and flow cytometry of thymocytes was performed at E18. The majority of the thymocytes at E18 are CD4+CD8+. Representative FACS analysis from 2 TEL+/− and 2 TEL−/− chimeras are shown. The control represents an embryo that lacked detectable ES cell contribution.
These findings indicate that TEL is not required intrinsically for the proliferation and differentiation of committed lymphoid lineages. As TEL−/− splenic B cells and lymph node T cells proliferate in response to stimulation by LPS or anti-CD3 antibody, respectively (data not shown), TEL is also not essential for some aspects of cellular responsiveness. Because the majority of the lymphoid progenitors reside in the bone marrow during postnatal life, our data suggest, however, that TEL is required for maintaining a normal pool of lymphoid progenitors in the bone marrow.
TEL−/− ES cells do not contribute to hematopoietic organs in the adult
Because deficiency affects all lineages, our results are compatible with a critical role for TEL in the development/maintenance of HSCs or multipotential progenitor cells within the bone-marrow compartment. To examine whether such defects are reflected at the level of TEL−/− ES-cell contribution to the hematopoietic organs, we performed Southern blotting of tissues from young and adult mice. As shown in Figure 6A, both TEL+/− and TEL−/− cells contributed readily to nonhematopoietic tissues, including brain, heart, liver, kidney, and muscle. In marked contrast, contribution to the hematopoietic organs was observed in TEL+/− but not TEL−/− chimeras (Fig. 6A; data not shown). As shown by progenitor assays (Tables 1 and 2), however, TEL−/− ES cells readily contribute to hematopoietic tissues of the embryo–yolk sac and fetal liver (Fig. 6B). These data support a restricted role for TEL in the bone marrow (Fig. 7).
Figure 6.
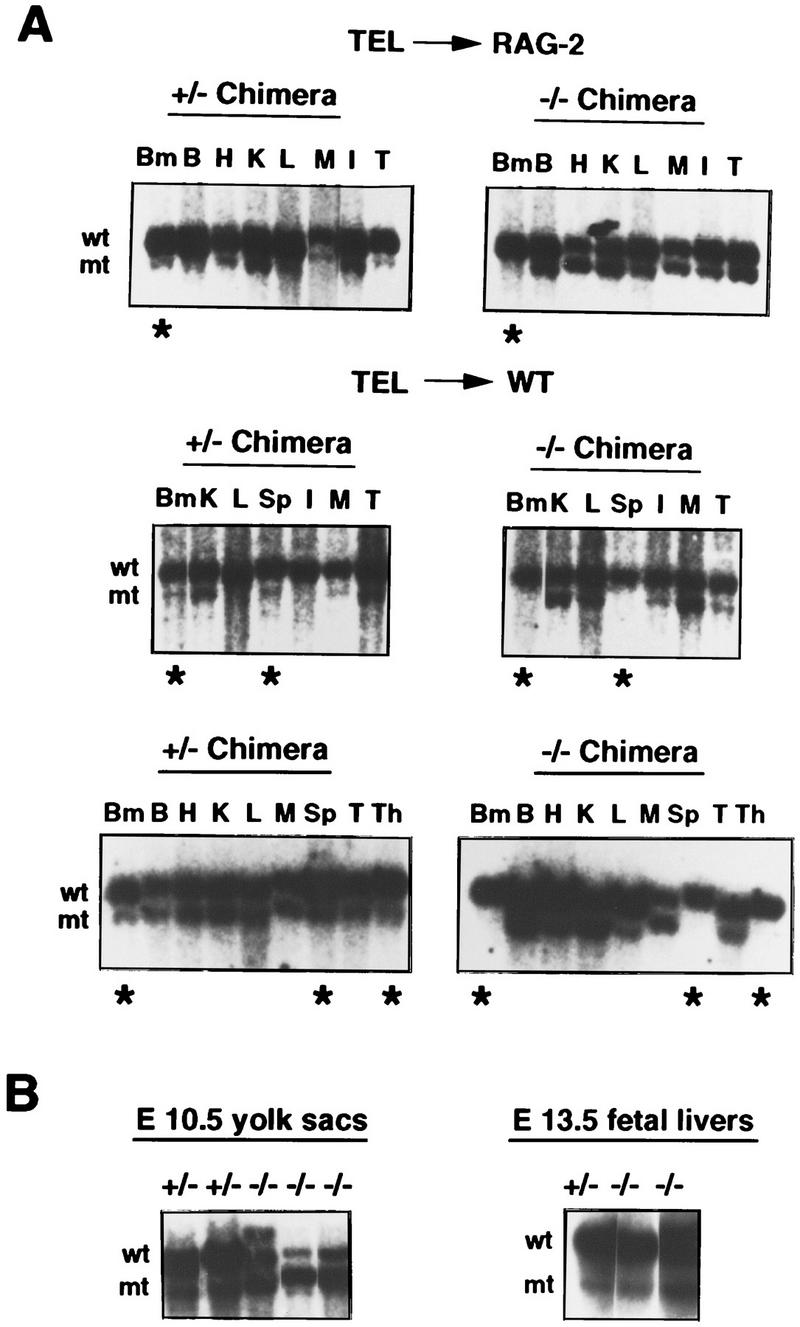
TEL−/− ES cells fail to contribute to adult hematopoietic tissues in chimeras. Southern blots were performed using DNAs of various adult tissues from TEL+/− or TEL−/− ES-cell-derived chimeras in the RAG-2 or wild-type (WT) backgrounds (A) and hematopoietic cells derived from yolk sacs or fetal livers (B). (wt) Wild-type alleles; (mt) mutant alleles; (Bm) bone marrow; (B) brain; (H) heart; (K) kidney; (L) liver; (M) muscle; (I) intestine; (Sp) spleen; (T) tail; (Th) thymus; (*) hematopoietic tissues (BM, Sp, and Th).
Figure 7.
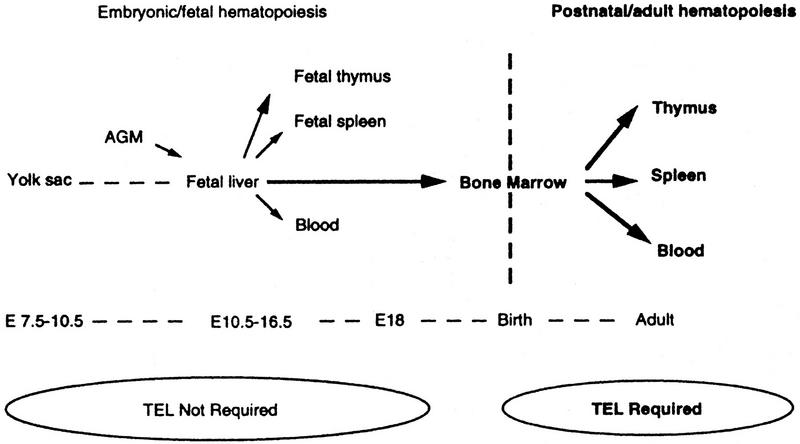
The requirement for TEL in the development of the hematopoietic system. Arrows depict the colonization routes of HSC/multipotent progenitors.
Intraembryonic hematopoietic progenitors initially colonize the fetal liver, and thereafter migrate to other hematopoietic organs (spleen, thymus, and bone marrow). In the adult, the bone marrow is the primary source of multipotential progenitors, which then seed other hematopoietic sites via the circulation. TEL function might be required in one, or multiple, processes, including migration and/or homing from the fetal liver to other sites, or progenitor survival/proliferation within the marrow microenvironment. Although cellular migration cannot be studied conveniently in chimeras, the presence of low numbers of TEL−/− hematopoietic progenitors in the bone marrow, spleen, and liver of newborn animals and in the spleen and liver of E18 embryos (data not shown) suggests that TEL−/− hematopoietic cells initially seed sites of hematopoietic activity, but in the absence of stable colonization of the bone-marrow microenvironment, hematopoiesis is not sustained.
Discussion
The TEL gene is involved in leukemias of both myeloid and lymphoid origins (Golub et al. 1997). During early embryonic development TEL is required for yolk-sac angiogenesis and for survival of several cell types (Wang et al. 1997). Here we have used mouse chimera analysis (Pevny et al. 1991; Porcher et al. 1996) to address additional roles for TEL in later development, specifically within hematopoiesis. Our studies reveal a unique requirement for TEL in establishing stable bone-marrow hematopoiesis in early postnatal life and in the adult (Fig. 7). The specificity of TEL loss of function for hematopoiesis in this site, as contrasted with other sites during development, points to the existence of novel regulatory pathways in bone-marrow HSCs or progenitors. Moreover, our results may relate to the pathogenesis of childhood pre-B leukemia associated with TEL/AML1 translocations and loss of heterozygosity at the TEL locus.
TEL serves a critical, nonredundant function in bone-marrow hematopoiesis
The expression of the TEL locus in hematopoietic cells, taken together with its rearrangement in leukemia, hinted at a potential role for TEL in hematopoiesis. Our prior data relying on in vitro differentiation of TEL−/− ES cells and progenitor assay of TEL−/− yolk-sac progenitor cells, however, demonstrated that TEL function is dispensable for the committment and differentiation of erythroid-myeloid lineages. This conclusion is confirmed by the studies reported here. The presence of a low number of otherwise normal TEL−/− B and T lymphocytes in TEL−/−/RAG-2−/− chimeras further indicates that TEL is not essential for development of lymphoid cells.
Whereas TEL is dispensable for the intrinsic differentiation of blood lineages, however, it is required for in vivo hematopoiesis within the bone marrow. This is an unexpected finding that points to critical differences in the nature of hematopoietic HSCs (or progenitors) that develop within different anatomic sites or differences in the responses of these cells to varying microenvironments (see below).
The bone marrow microenvironment is comprised of diverse cell types, including reticular fibroblasts, adipocytes, macrophages, endothelial cells, and extracellular matrix (Mayani et al. 1992; Papayannopoulou and Craddock 1997). Because our results are derived from the analysis of multiple chimeras of varying contribution of host to the microenvironment, we infer that the bone-marrow defect is cell-autonomous and hematopoietic-cell specific. Our findings, however, do not exclude additional functions of TEL specific to cells of the microenvironment, as these would not be detected in our assays. It might also be argued that the hematopoietic defects reflect unknown, TEL-independent genetic alterations in the TEL−/− ES clones that might impair contribution of ES cells to hematopoietic, but not other, compartments in chimeras. This explanation is highly unlikely for at least two reasons. First, as controls for the experiments, we have used TEL+/− ES clones that were selected in parallel with TEL−/− clones. In numerous experiments, we document that TEL+/− clones contribute efficiently to the hematopoietic system of chimeras (see Table 3A). Second, the temporal appearance of the hematopoietic defect is remarkable in its onset. Contribution of TEL−/− ES cells to fetal liver progenitors is detected readily at E14.5–16, whereas contribution to bone-marrow progenitors is virtually absent just several days later. We conclude that the failure of TEL−/− hematopoietic progenitors to achieve stable colonization of the bone marrow reflects a nonredundant, selective, and cell-autonomous function for TEL.
Possible mechanisms for bone-marrow hematopoietic defects in the absence of TEL
The establishment of bone-marrow hematopoiesis necessitates migration of HSCs (or progenitors) from the fetal liver (or other intraembryonic sites) and subsequent retention, survival, and proliferation of these cells within the marrow microenvironment. The interaction of the microenvironment with hematopoietic cells is critical for blood-cell production. Stroma provides a solid support for hematopoiesis, in part facilitating availability to membrane-localized growth factors. Hematopoietic site-restricted interactions may also be inferred from experiments in sheep indicating that liver-derived fetal progenitors home preferentially to fetal bone marrow (Zanjani et al. 1993).
Adhesive interactions of hematopoietic cells with stroma and cells of the microenvironment are important in vivo (Coulombel et al. 1997; Papayannopoulou and Craddock 1997). Some of these are mediated by integrin interactions with their respective ligands and are critical in vivo (Hynes 1996). For example, migration or homing of progenitors to the fetal liver requires β1 integrin (Hirsch et al. 1996). Maintenance of postnatal lymphopoiesis is dependent on expression α4 integrin (Arroyo et al. 1996). Moreover, integrins, notably α4β1, α5β1 and their cognate ligands, V-CAM and fibronectin, have been shown to be necessary for proper cell–cell and/or cell–extracellular matrix adhesion in the development of various tissues, including the hematopoietic system (Hynes 1996; Papayannopoulou and Craddock 1997). Further analysis of the α4 knockout reveals the existence of hematopoietic defects outside the lymphoid compartment (A. Arroyo and R. Hynes, pers. comm.). This might recommend α4 as a candidate target gene for TEL. However, our preliminary analysis demonstrates ostensibly normal expression of α4 in TEL−/− fetal liver cells. Moreover, undifferentiated TEL−/− ES cells bind fibronectin and V-CAM, cognate ligands for α4 integrin (data not shown). Thus, if TEL regulates the expression of integrins, or other adhesive molecules, their identity is as yet unknown.
Beyond mere adhesion of HSCs or progenitors to marrow stroma elements, the responses of cells to these interactions and to other stimuli of the microenvironment are likely to be critical in stable colonization and subsequent blood-cell production. TEL function might be essential in one (or multiple) aspect(s) of these pathways. Regardless of the specific target genes in HSCs or progenitors, it seems likely that TEL serves critical functions in the response of HSCs or progenitors to the bone-marrow microenvironment.
Speculations regarding TEL function and leukemia
In instances of leukemias in which gene fusions are expressed, translocations are undoubtedly inciting genetic events (Rabbitts 1994). Indeed, recent evidence indicates that at least in one variety of pediatric leukemia, translocation between the MLL and AF4 loci occurs antenatally (Gale et al. 1997). Rearrangement of the TEL and AML1 loci may have a similarly early origin in development (Ford et al. 1998). If such somatic events precede onset of evident disease by several years, it seems highly probable that secondary genetic events contribute to its evolution and progression. Such hypotheses conform to the prevailing view that oncogenesis is a multistep process (Nowell 1976).
The TEL locus was discovered through its fusion with the PDGFβR gene in chronic myelomonocytic leukemia (CMML), and has been shown subsequently to be rearranged with the c-abl, JAK 2, and AML-1 loci in CMML, T-ALL, and pre-B-ALL (see Golub et al. 1997). In the TEL fusions with PDGFβR, c-abl, and JAK2, TEL-induced oligomerization results in constitutive activation of downstream-signaling pathways (Carroll et al. 1996; Lacronique et al. 1997). Such is not the case for the TEL/AML1 fusion protein, which is presumed to act by different mechanisms, perhaps including alteration of AML1 function, which is essential on its own for definitive hematopoiesis (Okuda et al. 1996; Wang et al. 1996). A distinctive feature of TEL/AML1-associated leukemia is nearly invariant loss of heterozygosity at chromosome 12p, which includes the site of the TEL locus, as well as that of the cyclin inhibitor p27(Kip1) (Sato et al. 1995; Stegmaier et al. 1995; Kim et al. 1996; Raynaud et al. 1996). Expression of normal TEL mRNA in cells of rare patients in early samples, but not at later times, is consistent with TEL loss serving as a secondary genetic “hit” in disease progression (Kim et al. 1996). Based on the novel consequences of the absence of TEL for hematopoiesis demonstrated here, we speculate regarding their implications for evolution of childhood pre-B-TEL-AML1-associated leukemia.
The inability of TEL−/− HSCs (or progenitors) to stably colonize the bone marrow might reflect defective adhesion or defective adhesion-mediated cellular responses. Studies, largely in the context of Phildelphia+–CML, have suggested that leukemic cells are impaired in both respects (Verfaillie 1997). Leukemic progenitors may circulate prematurely in the blood, and also proliferate excessively because of altered adhesive properties. Thus, if TEL regulates adhesion receptors or pathways responsive to adhesion, its loss would predict altered behavior of leukemic progenitors. Alternatively, TEL may mediate aspects of adhesion-independent cellular responses to the bone-marrow microenvironment. On one hand, our finding that loss of TEL in otherwise normal hematopoietic progenitors cripples their capacity for effective hematopoiesis in the bone marrow appears counterintuitive as a potential contributing factor in the pathogenesis of TEL/AML1 pre-B-cell leukemia. If we postulate, however, that unique properties of the TEL/AML1 fusion protein rescue otherwise doomed TEL−/− HSCs (or a subset of progenitors) in the bone-marrow microenvironment for subsequent survival, proliferation, and competition with normal progenitors, such apparent discrepancies may be reconciled. In principle, these aspects of the pathogenesis of childhood pre-B-cell leukemia might be addressed with suitable genetic manipulations in the mouse. It seems likely that the bone marrow-restricted deficit of TEL−/− hematopoietic cells described here is relevant to the evolution of this form of leukemia.
Materials and methods
Blotting and in situ hybridization
Northern and Southern blotting were described previously (Wang et al. 1997). In situ hybridization was performed with digoxigenin-11-UTP (Boehringer-Mannheim)-labeled riboprobes generated from a 550-bp EcoRI–Eco47III fragment spanning the 5′ TEL-untranslated region (Wang et al. 1997).
Generation of chimeras
TEL+/− and TEL−/− ES clones were injected into C57BL/6 or RAG-2−/− blastocysts (Chen et al. 1993). Bone marrow, thymus, spleen, or lymph-node cells of E18, newborn or 1–16 weeks old mice were collected for hematopoietic progenitor assay or FACS analysis. Internal organs were used for extraction of DNA. Chimerism was assessed by agouti coat-color contribution in the adult mice and/or by Southern blot analysis (Porcher et al. 1996). Two independent clones each of TEL+/− and TEL−/− ES cells were used for injections. Data on the myeloerythroid lineages were derived from the study of 7–14 chimeras. For RAG-2−/− blastocyst experiments, 7 TEL+/−, and 10 TEL−/− chimeras were analyzed.
Hematopoietic progenitor assays from chimeric mice
Yolk sac, fetal liver, spleen, and bone-marrow progenitor assays were performed as described (Porcher et al. 1996; Wang et al. 1997). Cells (1–2 × 105) were plated into methylcellulose medium supplemented with various growth factors (Porcher et al. 1996) either in the presence (1.5 mg/ml) or absence of G418. Pre-B-cell colony assays were performed in the presence of IL-7-containing methylcellulose (Stem Cell Technologies).
Hemoglobin assay
Hemoglobin analysis was performed as described (Porcher et al. 1996).
FACS analysis
Preparation and staining of lymphoid cells were performed as described (Porcher et al. 1996).
Acknowledgments
We thank Kerrianne Cunniff for technical assistance. T.R.G. is a recipient of a Burroughs Wellcome Fund Career Award in the Biomedical Sciences. D.G.G., F.W.A., and S.H.O. are Investigators of the Howard Hughes Medical Institute. These studies were supported in part by a Center of Excellence in Molecular Hematology award of the National Institute of Diabetes and Digestion and Kidney Diseases, National Institutes of Health.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Orkin@rascal.med.harvard.edu; FAX (617) 355-7262.
References
- Arroyo A, Yang JT, Rayburn H, Hynes RO. Differentiation requirement for α4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- Carroll M, Thomasson MH, Barker GF, Golub TR, Gilliland DG. The TEL/PDGFRβ fusion in CMML is a transforming protein that self-associates and activates PDGFRβ kinase-dependent signaling pathways. Proc Natl Acad Sci. 1996;93:14845–14850. doi: 10.1073/pnas.93.25.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: An assay of gene function in lymphocyte development. Proc Natl Acad Sci. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombel L, Auffray I, Gaugler MH, Rosenblatt M. Expression and function of integrins on hematopoietic progenitor cells. Acta Haematol. 1997;97:13–21. doi: 10.1159/000203655. [DOI] [PubMed] [Google Scholar]
- Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4:97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- Ford AM, Bennett CA, Price CM, Bruin MCA, Wering ERV, Greaves M. Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. Proc Natl Acad Sci. 1998;95:4584–4588. doi: 10.1073/pnas.95.8.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, Greaves MF. Backtracking leukemia to birth: Identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci. 1997;94:13950–13954. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lievre F, Marcos MAR. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Golub TR, Barker GF, Bohlander SK, Hiebert S, Ward DC, Bray-Ward P, Morgan E, Raimondi SC, Rowley JD, Gilliland DG. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub TR, Goga A, Barker GF, Afar DE, McLaughlin J, Bohlander SK, Rowley JD, Witte ON, Gilliland DG. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol Cell Biol. 1996;16:4107–4116. doi: 10.1128/mcb.16.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub TR, Barker GF, Stegmaier K, Gilliland DG. The TEL gene contributes to the pathogenesis of myeloid and lymphoid leukemias by diverse molecular genetic mechanisms. Curr Top Microbiol Immunol. 1997;220:67–79. doi: 10.1007/978-3-642-60479-9_5. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: What have we learned from them? Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive hematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Moldwin RL, Vignon C, Bohlander SK, Suto Y, Giordano L, Gupta R, Fears S, Nucifora G, Rowley JD, Smith SD. TEL-AML1 translocations with TEL and CDKN2 inactivation in acute lymphoblastic leukemia cell lines. Blood. 1996;88:785–794. [PubMed] [Google Scholar]
- Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard OA. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- Mayani H, Guilbert LJ, Jamowska-Wieczorek A. Biology of the hematopoietic microenvironment. Eur J Haematol. 1992;49:225–233. doi: 10.1111/j.1600-0609.1992.tb00053.x. [DOI] [PubMed] [Google Scholar]
- McLean TW, Ringold S, Neuberg D, Stegmaier K, Tantravahi R, Ritz J, Koeffler HP, Takeuchi S, Janssen JWG, Seriu T, Bartram CR, Sallan SE, Gilliland DG, Golub TR. TEL/AML-1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood. 1996;88:4252–4258. [PubMed] [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–66. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Okuda T, v. Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Orkin SH. Development of the hematopoietic system. Curr Opin Genet Devel. 1996;6:597–602. doi: 10.1016/s0959-437x(96)80089-x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos P, Ridge SA, Boucher CA, Stocking C, Wiedemann LM. The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res. 1995;55:34–38. [PubMed] [Google Scholar]
- Papayannopoulou T, Craddock C. Homing and trafficking of hemopoietic progenitor cells. Acta Haematol. 1997;97:97–104. doi: 10.1159/000203665. [DOI] [PubMed] [Google Scholar]
- Peeters P, Raynaud SD, Cools J, Wlodarska I, Grosgeorge J, Philip P, Monpoux F, Rompaey LV, Baens M, Berghe HV, Marynen P. Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood. 1997;90:2535–2540. [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai S-F, D’Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T-cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/s0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Raynaud S, Cave H, Baens M, Bastard C, Cacheux V, Grosgeorge J, Guidal-Giroux C, Guo C, Vilmer E, Marynen P, Grandchamp B. The 12;21 translocation involving TEL and deletion of the other TEL allele: Two frequently associated alterations found in childhood acute lymphoblastic leukemia. Blood. 1996;87:2891–2899. [PubMed] [Google Scholar]
- Robb L, Elwood NJ, Elefanty AG, Kontgen F, Li R, Barnett LD, Begley CG. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15:4123–4129. [PMC free article] [PubMed] [Google Scholar]
- Romana SP, Mauchauffe M, Le Coniat M, Chumakov I, Le Paslier D, Berger R, Bernard OA. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- Sato Y, Suto Y, Pietenpol J, Golub TR, Gilliland DG, Davis EM, Le Beau MM, Roberts JM, Vogelstein B, Rowley JD, Bohlander SK. TEL and KIP1 define the smallest region of deletions on 12p13 in hematopoietic malignancies. Blood. 1995;86:1525–1533. [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Shurtleff SA, Buijs A, Behm FG, Rubnitz JE, Raimondi SC, Hancock ML, Chan GC, Pui CH, Grosveld G, Downing JR. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia. 1995;9:1985–1989. [PubMed] [Google Scholar]
- Stegmaier K, Pendse S, Barker GF, Bray-Ward P, Ward DC, Montgomery KT, Krauter KS, Reynolds C, Sklar J, Donnelly M, Bohlander SK, Rowley JD, Sallan SE, Gilliland DG, Golub TR. Frequent loss of heterozygosity at the TEL gene locus in acute lymphoblastic leukemia of childhood. Blood. 1995;86:38–44. [PubMed] [Google Scholar]
- Veiby OP, Mikhail AA, Snodgrass HR. Growth factors and hematopoietic stem cells. Hematol Oncol Clin N Am. 1997;11:1173–1184. doi: 10.1016/s0889-8588(05)70487-1. [DOI] [PubMed] [Google Scholar]
- Verfaillie CM. Stem cells in chronic myelogenous leukemia. Hematol Oncol Clin N Am. 1997;11:1079–1114. doi: 10.1016/s0889-8588(05)70484-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Kuo F, Fujiwara Y, Gilliland DG, Golub TR, Orkin SH. Yolk sac angiogenic defect and intra-embryonic apoptosis in mice lacking the Ets-related factor TEL. EMBO J. 1997;16:4374–4383. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GAJ, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Zanjani ED, Ascensao JL, Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to fetal bone marrow. Blood. 1993;81:399–404. [PubMed] [Google Scholar]