Abstract
Background
Leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) are important immune mediators, often found concomitantly at sites of inflammation. Although, some of the leukotriene-mediated actions are distinctive (e.g. bronchial constriction for cysLTs), many activities such as leukocyte recruitment to tissues and amplification of inflammatory responses are shared by both classes of leukotrienes.
Objective
We used human monocytes to characterize leukotriene specific signaling, gene expression signatures and functions and to identify interactions between LTB4 and cysLTs induced pathways.
Methods
Responsiveness to leukotrienes was assessed using oligonucleotide microarrays, real-time PCR, calcium mobilization, kinase activation and chemotaxis assays.
Results
Human monocytes were found to express mRNA for high- and low-affinity LTB4 receptors, BLT1 and BLT2, but signal predominantly through BLT1 in response to LTB4 stimulation as shown using selective agonists, inhibitors and gene knock-down experiments. LTB4 acting through BLT1 coupled to G protein α inhibitory subunit activated calcium signaling, p44/42 mitogen-activated protein kinase, gene expression and chemotaxis. Twenty-seven genes, including immediate-early genes, transcription factors, cytokines and membrane receptors were significantly upregulated by LTB4. LTB4 and LTD4 had similar effects on signaling, gene expression and chemotaxis indicating redundant cell activation pathways but co-stimulation with both lipid mediators was additive for many monocyte functions.
Conclusion
LTB4 and LTD4 display both redundant and cooperative effects on intracellular signaling, gene expression and chemotaxis in human monocytes. These findings suggest that therapies targeting either leukotriene alone may be less effective than approaches directed at both.
Keywords: asthma, inflammation, monocytes, leukotrienes, receptors
Introduction
Leukotrienes are lipid mediators involved in the pathophysiology of many inflammatory diseases, such as bronchial asthma, allergic rhinitis, atherosclerosis and arthritis (1, 2). Two classes of leukotrienes are synthesized by stimulated leukocytes, LTB4 and cysteinyl leukotrienes (cysLTs)(LTC4, LTD4, LTE4). LTB4 and CysLTs act by binding to distinct sets of specific G-protein coupled receptors (GPCRs) termed, BLT1, BLT2 and CysLT1, CysLT2, respectively (3). CysLTs have a clearly defined role in bronchial asthma and rhinitis, leading to airway constriction, increased vascular permeability and mucus secretion. They are also believed to play an important role in cell trafficking and innate immune responses (2). LTB4 is characterized as a potent chemoattractant for myeloid cells (e.g. neutrophils) related to antibacterial responses and as the mediator associated with development of atherosclerosis and rheumatoid arthritis (4). However, there is evidence suggesting that LTB4 may also play an important role in asthmatic inflammation, as increased levels of LTB4 in sputum, plasma and bronchoalveolar lavage of asthmatic subjects and increased LTB4 synthesis in macrophages and neutrophils from asthmatics have been reported (5–7). The LTB4/BLT1 pathway has also been shown to be essential for neutrophil and effector memory CD8+ cells recruitment into the lungs of allergen-induced airway inflammatory responses in mice (8, 9). In addition, production of leukotrienes is relatively resistant to corticosteroid treatment and in fact, corticosteroids were reported to increase BLT1 expression on inflammatory cells, such as neutrophils and monocytes (10, 11).
Although some of the actions of cysLTs and LTB4 are distinctive (e.g. bronchial contraction for cysLTs), many activities such as leukocyte chemotaxis and amplification of inflammatory responses are shared by both classes of leukotrienes. Many cells (monocytes/macrophages, mast cells, dendritic cells) when stimulated produce both, LTB4 and cysLTs that activate specific receptors simultaneously in the same cell and may lead to parallel, independent receptor specific signaling or may activate similar intracellular signaling pathways. The inhibitory activities of leukotriene receptor antagonists have been studied in models analyzing receptor specific agonists (LTB4/BLT1; LTD4/CysLT1)(12, 13) but it is not known whether some common leukotriene-induced signaling pathways may be redundant when cells are exposed to both classes of leukotrienes in the presence of single receptor antagonists. The question whether co-stimulation of human cells with LTB4 and cysLTs (as may often happen in vivo) may induce additive or synergistic signaling and functional effects in cells expressing LTB4 and cysLT receptors has not been addressed.
We previously showed that CysLT1 is the predominantly expressed cysLT receptor in human elutriated monocytes (14). As human monocytes express receptors for both cysLTs and LTB4, in the current study we aimed to characterize the LTB4 specific signaling, gene signature response and functions in human elutriated monocytes and to look for interactions between LTB4 and LTD4.
METHODS
Materials
LTB4, LTD4, 12 (S)-HETE, 12(S)-HHT, LY255283 (BLT2 antagonist), montelukast and MK571(CysLT1 antagonists) (Cayman Chemical), pertussis toxin (Gαi/o inhibitor), PD98059 (p44/42 MAPK inhibitor)(EMD Chemicals), DMSO (Sigma-Aldrich), anti-phospho-p44/42 and anti-phospho-p38 MAPK, anti- p44/42 and p38 antibodies (Cell Signaling) were obtained from the supplier. CP105696 (BLT1 antagonist) was a generous gift from Pfizer (New York, NY).
Cell culture, calcium mobilization assay, chemotaxis assay and immunoblotting
Human elutriated monocytes from healthy donors were obtained through an institutional review board-approved protocol from the NIH Blood Bank (Bethesda, MD), cultured and calcium mobilisation, chemotaxis and immunoblotting were performed as previously described (14).
BLT1 and BLT2 knockdown
For siRNA knockdown experiments, Silencer Select pre-designed siRNA were used: 5’GGAAAAGGCUGUCUACAUUtt for BLT1 and GCACAAUUGAAAACUUCAAtt for BLT2. Silencer Select Negative Control siRNA (NC siRNA) (Ambion) were used as a negative control. Elutriated monocytes (5×106) were nucleofected with 4 µg of negative control and with either BLT1 or BLT2 specific siRNA using a Human Monocyte Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer’s protocol. After 24 hours, the media was replaced and the cells were used for calcium flux assay.
Microarray analysis
Total RNA was extracted from elutriated monocytes, processed and hybridized to Affymetrix HG U133 plus 2.0 arrays (Affymetrix) as previously reported (14). Data were normalized into S10 values using the MSCL Analyst’s toolbox (P. J. Munson, GeneLogic Workshop of Low Level Analysis of Affymetrix GeneChip Data, 2001, software available at http://abs.cit.nih.gov/geneexpression.html. Genes with FDR values less than 10%, greater than 2 fold-change and greater than 50% present calls in one of the two treatment groups were termed significantly differentially expressed. Two-sided p-values for this test were reported as 1E-12 if their computed values was that or smaller. Raw data and CEL files were submitted to the National Center for Biotechnology Information Gene Expression Omnibus database (GSE24869).
Real-time PCR
mRNA expressions for selected genes were measured using real-time PCR performed on an ABI Prism 7900 sequence detection system (Applied Biosystems) using an RT kit and TaqMan Universal PCR master mix and commercially available probe and primer sets (Applied Biosystems): LTB4R- Hs00272624_s1, LTB4R2-Hs 00252658_s1, CYSLTR1- Hs00272624_s1, CYSLTR2-Hs00252658_s1, GPR17- Hs00171137_m1, FOSB- Hs00171851_m1, and EGR2- Hs00166165_m1. Relative gene expression was normalized to GAPDH transcripts and calculated as fold change compared with control transcripts.
RESULTS
LTB4 induces gene expression in human monocytes
To determine whether LTB4 can affect gene expression in human monocytes and globally identify LTB4 target genes, elutriated monocytes from 5 healthy donors were stimulated with LTB4 (50 nmol/L) or vehicle control (ethanol) for 30 minutes and analyzed with microarrays. LTB4 stimulation significantly up-regulated 27 genes by 2 fold or more (Table 1).
TABLE I.
Elutriated monocytes from 5 separate donors were stimulated with or without LTB4 (50 nmol/L) for 30 minutes and analyzed by microarray as described in the Methods section. Values are mean +/− standard deviation (SD).
| Gene name | Fold Change (±SD) |
P value for 2-sided consistency test |
|
|---|---|---|---|
| Cytokines | |||
| IL1B | interlukin 1 β | 2.6 (0.18) | 8.14E-10 |
| CCL3 | chemokine (C-C motif) ligand 3 | 2.2 (0.08) | 2.43E-10 |
| TNF | tumor necrosis factor | 2.0 (0.05) | 3.36E-11 |
| Membrane | |||
| GPR109B | G protein-coupled receptor 109B | 2.7 (0.09) | 1.01E-12 |
| CD83 | CD83 molecule | 2.1 (0.12) | 8.20E-09 |
| THBD | thrombomodulin | 2.1 (0.08) | 1.13E-09 |
| CD44 | CD44 molecule | 2.0 (0.11) | 1.57E-06 |
| FCAR | Fc fragment of IgA receptor f | 2.0 (0.03) | 1.29E-11 |
| Signal Transduction | |||
| S100A10 | S100 calcium binding protein A | 2.4 (0.08) | 1.68E-12 |
| NEDD9 | neural precursor cell express 9 | 2.2 (0.09) | 8.43E-09 |
| PSCDBP | pleckstrin homology Sec7 | 2.0 (0.05) | 5.73E-10 |
| Transcriptional Activation | |||
| FOSB | FBJ murine osteosarcoma viral | 12.2 (0.19) | 1.00E-12 |
| EGR3 | early growth response 3 | 8.6 (0.11) | 1.00E-12 |
| EGR2 | early growth response 2 | 5.4 (0.15) | 1.00E-12 |
| NR4A1 | nuclear receptor subfamily 4 | 4.2 (0.22) | 1.00E-12 |
| NR4A2 | nuclear receptor subfamily 4 | 3.7 (0.20) | 1.00E-12 |
| ATF3 | activating transcription factor 3 | 2.8 (0.05) | 1.00E-12 |
| KLF10 | Kruppel-like factor 10 | 2.6 (0.07) | 1.00E-12 |
| EGR1 | early growth response 1 | 2.2 (0.24) | 0.00055873 |
| KLF4 | Kruppel-like factor 4 | 2.2 (0.08) | 3.23E-12 |
| Metabolism | |||
| AHR | aryl hydrocarbon receptor | 2.0 (0.06) | 2.76E-09 |
| Cell Cycle and Apoptosis | |||
| PHLDA1 | pleckstrin homology-like domain A1 | 4.1 (0.10) | 1.00E-12 |
| RGC32 | response gene to complement 32 | 2.3 (0.05) | 1.24E-12 |
| MCL1 | myeloid cell leukemia sequence | 2.9 (0.09) | 1.00E-12 |
| Other | |||
| transcribed locus | 4.2 (0.16) | 1.00E-12 | |
| LOC284454 | hypothetical protein LOC284454 | 2.6 (0.14) | 9.55E-10 |
| TRIB1 | tribbles homolog 1 (Drosophila) and LNRF1 | 2.3 (0.08) | 4.13E-12 |
| LONRF1 | LON peptidase N-terminal domain | 2.2 (0.04) | 1.53E-12 |
LTB4 signals through the BLT1 in monocytes
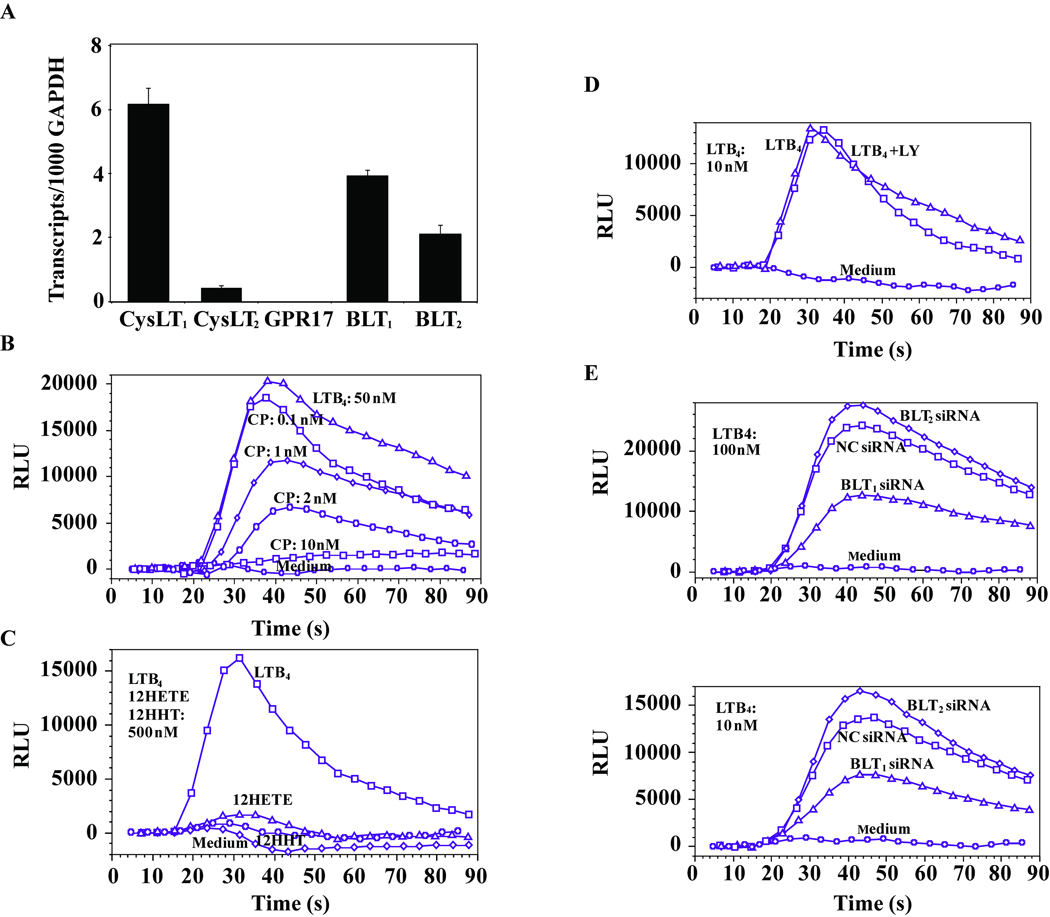
CysLT1, BLT1, and BLT2 were the three receptors predominantly expressed at the mRNA level in monocytes, while low levels of CysLT2 mRNA and no mRNA for GPR17 (a putative cysLT receptor (15)) were found (Figure 1A). LTB4-induced calcium flux was inhibited by the BLT1 selective antagonist, CP105696 (Figure 1B). BLT2-induced signaling by its specific, other than LTB4, agonists: 12-HHT and 12-HETE (16, 17) was not detected (Figure 1C). Similarly, the BLT2 antagonist, LY255283, did not inhibit LTB4-induced calcium flux (Figure 1D). Further, siRNA was used to knock down either BLT1 or BLT2 expression. BLT1 and BLT2 siRNAs decreased their respective transcripts by 88±3.7% and 59±4.2% comparred to cells treated with NC siRNA (TaqMan; n=3, p < 0.05). BLT1 down-regulation caused more than 50 % decrease in LTB4 induced calcium flux whereas the BLT2 knock-down cells showed no inhibition of response to LTB4 (Figure 1E). These data suggest that the BLT1 is the major functional LTB4 receptor expressed in human monocytes.
Figure 1.
BLT1 is the major receptor mediating LTB4-induced calcium mobilization in human monocytes. (A) The relative mRNA expression of CysLT1, CysLT2, GPR17, BLT1 and BLT2 measured by TaqMan is expressed per thousand copies of the control GAPDH gene and presented as mean values ± SD of 3 healthy donors. Monocytes were stimulated with LTB4 (50 nmol/L) in the presence of increasing concentrations of CP105696 (B), or LTB4, 12-HETE, 12-HHT (500 nmol/L) (C) or LTB4 in presence of LY255283 (200 nmol/L) (D) or vehicle control (10 min pretreatment). (E) Monocytes were nucleofected with BLT1 or BLT2 siRNA or negative control oligonucleotides (NC siRNA) as described in the Methods section, cultured for 24 hours and calcium flux was measured in response to LTB4 (100 or 10 nmol/L). Results of calcium flux experiments are shown as relative light units (RLU) from the FLEX 3 station. For the calcium flux experiments, the data are from one of three separate experiments each with similar results.
LTD4 augments the LTB4-induced calcium flux
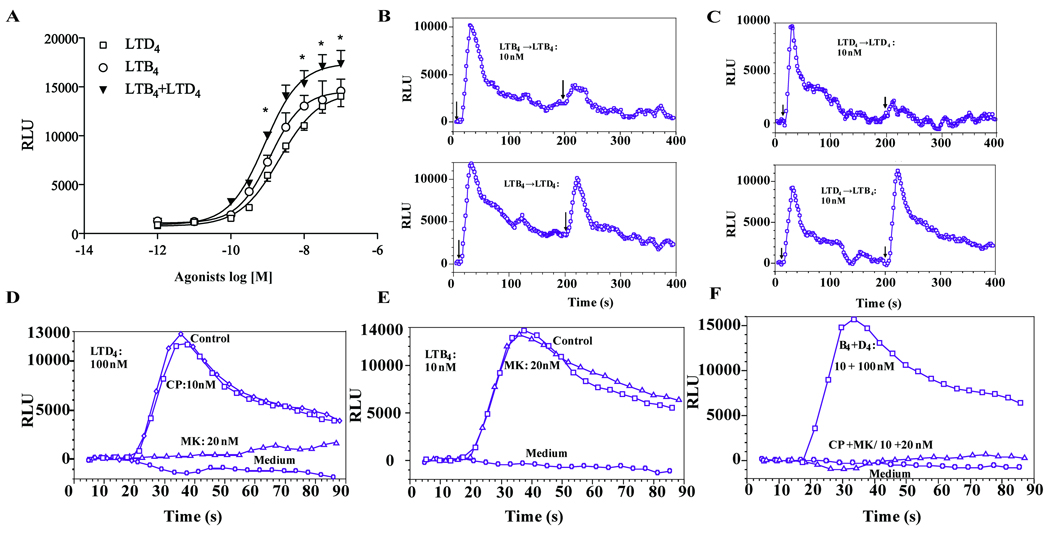
LTB4 induced calcium mobilization with a half maximal effective concentration (EC50) value of 1.17 nmol/L and the response reached plateau at 30 nM (Figure 2A). LTD4 induced calcium flux with an EC50 value of 2.12 nmol/L and reached maximum effect at 100 nM. A significantly augmented calcium flux was observed in response to LTB4/LTD4 costimulation in comparison with LTB4 and LTD4 alone, with a lower EC50 value of 0.84 nmol/L.
Figure 2.
LTB4 and LTD4 act via specific receptors to induce calcium in an additive manner. Monocytes were stimulated with different concentrations of LTB4, LTD4 or LTB4+LTD4 and calcium release was measured, as indicated in Methods (Figure 2A). Data presented as means ± SEM from 3–6 separate experiments each performed in triplicate. *p<0.05, analyzed by ANOVA. Representative traces of calcium flux induced by repeated exposure to LTD4 (10 nmol/L) and LTB4 (10 nmol/L) in monocytes (B and C). Monocytes were pretreated with MK (20 nmol/L) or CP105696 (10 nmol/L) for 20 min and stimulated with LTD4 (100 nmol/L) (D), or LTB4 (10 nmol/L) (E). The effect of treatment with both LTB4 and LTD4 was completely inhibited by pretreatment with both inhibitors (F). Data from one of three separate experiments, each with similar results, are shown.
As shown in Figure 2D, the LTD4-induced calcium flux was completely inhibited by pretreatment with montelukast, but was not inhibited by CP105696. Similarly, the LTB4-induced calcium flux was not inhibited by montelukast, but abolished by CP105696 (Figure 2E). Both inhibitors together completely inhibited the effect of both mediators together (Figure 2F). Stimulation with LTB4 (Figure 2B) potently desensitized monocytes to subsequent stimulation with the same concentration of LTB4 (as expected for homologous desensitization), but it did not have any effect on subsequent LTD4 exposure. Similarly LTD4 desensitized to subsequent stimulation with LTD4 but not to LTB4 (Figure 2C), confirming that LTB4 and LTD4 signal through separate receptors and independent pathways (BLT1 and CysLT1).
LTB4 and LTD4 signal in additive way through Gαi/o-coupled BLT1 and p44/42 MAPK in monocytes
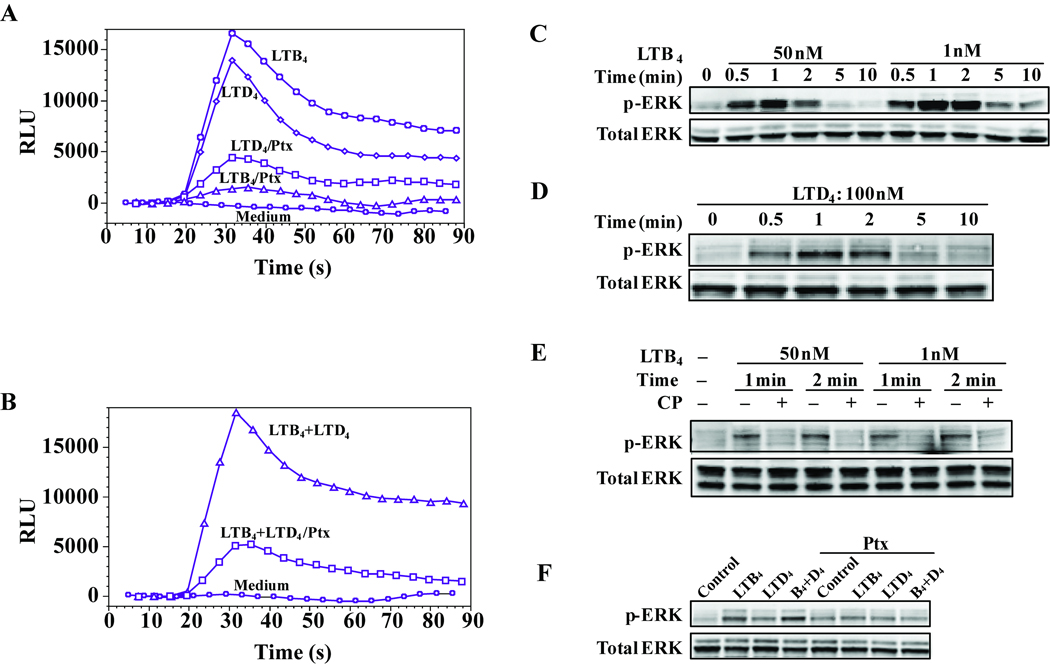
LTB4-induced calcium mobilization was fully inhibited by pertussis toxin (Ptx) pretreatment (Figure 3A) while residual calcium flux (less than 30 %) in response to LTD4 was observed. We further tested the Ptx effect on combined LTB4 and LTD4 induced calcium flux and found more than 75 % inhibition of calcium flux by Ptx (Figure 3B), with the residual calcium mobilization contributed by LTD4 signaling via its CysLT1 receptor (Figure 3A). We then analyzed MAPK kinase activation. LTB4 or LTD4 induction of p44/42 phosphorylation was observed in 30 seconds and returned to baseline in 5 minutes (Figure 3C and 3D). The LTB4 induced phosphorylation of p44/42 was abrogated by pretreatment with CP105696 (Figure 4E). An additive effect on p44/42 MAPK phosphorylation was observed when cells were stimulated with LTB4 and LTD4 together (Figure 4F) that was inhibited by Ptx pretreatment. While LTD4 induction of p38 phosphorylation has been previously reported,(14) we detected no consistent p38 phosphorylation in response to LTB4 stimulation (data not shown).
Figure 3.
LTB4 and LTD4 signal through Gαi/o-coupled BLT1 and p44/42 MAPK in monocytes. Monocytes were pretreated with pertussis toxin (100 ng/mL) overnight and then stimulated with LTB4 (50 nmol/L) or LTD4 (100 nmol/L) (A), or stimulated with LTB4 and LTD4 together (B). Monocytes were stimulated with LTB4 (C) or LTD4 (D) at different time points and phosphorylation of p44/42 was assayed by immunoblotting. (E) Cells were preincubated with CP105696 (10 nmol/L) for 20 min and stimulated with LTB4 for 1 or 2 min. (F) Cells were preincubated with pertussis toxin (100 ng/mL) overnight and stimulated with LTB4 (50 nmol/L), LTD4 (100 nmol/L) or LTB4 and LTD4 for 2 min. Data are representative of three independent experiments.
Figure 4.
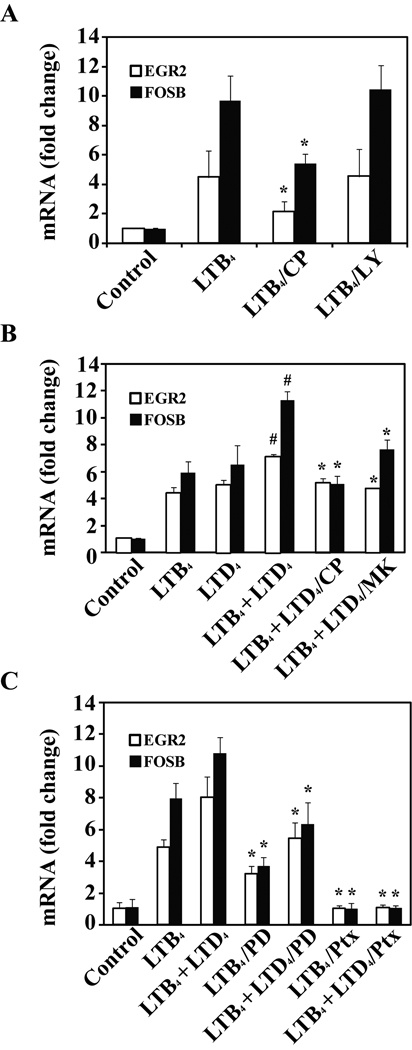
The additive effect of LTB4 and LTD4 co-stimulation on gene expression. (A) Monocytes were preincubated with CP105696 (10 nmol/L), or LY255283 (200 nmol/L) or vehicle for 20 min and stimulated with LTB4 (50 nmol/L) for 30 min. (B) Cells were pretreated with CP105696 (10 nmol/L), or MK571 (100 nmol/L) or vehicle for 20 min and then stimulated with LTB4 (50 nmol/L), LTD4 or both for 30 minutes. (C) Cells were pretreated with PD98059 (10 µmol/L) for 1 hr or pertussis toxin (100 ng/mL) overnight and stimulated with LTB4 (50 nmol/L) or LTB4 and LTD4 (50 nmol/L, 100 nmol/L) for 30 min. After the treatment, total RNA was extracted and subjected to TagMan analysis. Data are shown as a fold change in comparison with vehicle control treated cells. Mean ± SD from 3 experiments done in triplicate. *p < 0.05 for agonist(s) plus inhibitor comparred to agonist(s) alone; #p<0.01 for LTB4+LTD4 comparred to LTB4 or LTD4 alone.
The additive effect of LTB4 and LTD4 co-stimulation on gene expression
We validated the array expression data for 2 immediate early genes (IEG) (FOSB and EGR2) using TaqMan real time PCR. EGR2 and FOSB were induced by LTB4 treatment and this effect was inhibited by CP105696 but not by the BLT2 antagonist, LY255283 (Figure 4A). Figure 4B demonstrates the additive effect of LTB4 (50 nmol/L) and LTD4 (100 nmol/L) on EGR2 and FOSB gene expression. The combined effect of LTD4 and LTB4 stimulation was partially inhibited by pretreatment with the BLT1 and CysLT1 antagonists, CP105696 and MK571, respectively (Figure 4B). The effect of LTD4 and LTB4 was substantially inhibited by PD98059 and completely inhibited by Ptx (Figure 4C), again suggesting similar but separate signaling pathways leading to immediate early gene expression.
LTD4 augments LTB4 induced chemotaxis of monocytes
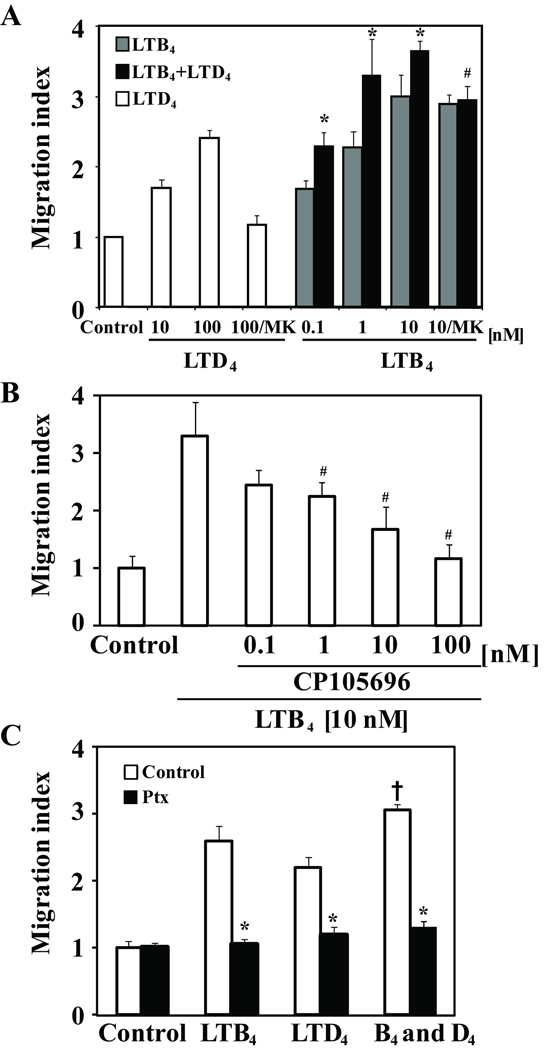
A significant chemotactic activity in response to LTB4, inhibited by the BLT1 inhibitor, CP105696 (Figure 5A–B), was observed. LTD4 also induced chemotactic response that was abrogated by MK571 (Figure 6A). Treatment of cells with LTB4 and LTD4 induced an additive effect (Figure 5A, C) that was only partially inhibited by MK571 and fully inhibited by Ptx pretreatment, showing that chemotaxis of monocytes in response to leukotrienes is mediated through Gai/o coupled receptor activation.
Figure 5.
LTD4 augments LTB4 induced chemotaxis of monocytes. (A) Chemotactic activity of monocytes to different concentrations of or LTB4, LTD4 or LTB4 and LTD4 (100 nmol/L) in the presence or absence of MK571 (MK; 100 nmol/L). *p < 0.05 for LTB4+LTD4 comparred to LTB4 alone. #p<0.05 for LTB4+LTD4 comparred to LTB4+LTD4 plus MK571. (B) Cells were preincubated with different concentrations of CP105695 for 20 min and stimulated with LTB4 (10 nmol/L). #p<0.05 for LTB4 comparred to LTB4 plus CP105696. (C) Cells were preincubated with pertussis toxin (100 ng/mL) overnight and stimulated with LTB4 (10 nmol/L), LTD4 (100 nml/L) or LTB4 and LTD4. *p < 0.05 for agonist(s) plus Ptx comparred to agonist(s) alone. +p<0.05 comparing LTB4 and LTD4 to LTB4 alone. Data from 3 different donors are presented as the migration index in comparison with vehicle control (mean ±SD).
Discussion
We report here a description of LTB4 mediated signaling, target gene induction and chemotaxis in human monocytes, followed by an analysis of the cooperative and redundant responses induced by the co-administration of LTB4 and LTD4. In our model of human elutriated monocytes, LTB4 stimulation induced calcium mobilization, p44/42 MAPK phosphorylation, gene expression and chemotaxis, showing that LTB4 is a potent stimulus for monocyte activation. Potent inhibition of LTB4 induced calcium flux, MAPK kinase activation, immediate-early gene expression as well as chemotaxis was observed with CP105696 treatment indicating that BLT1 is responsible for all studied LTB4-induced activities, further confirmed by BLT1 and BLT2 selective knock down experiments. This is in agreement with previous studies showing that LTB4 induced MCP-1 production (12) and LTB4 triggered adhesion of monocytes to endothelium (18) were inhibited by the BLT1 antagonist, CP105696.
We provide here evidence in human monocytes that LTB4 stimulates BLT1 coupled to pertussis toxin (Ptx) sensitive Gαi/o causing intracellular calcium mobilization, p44/42 kinase activation, gene expression and chemotaxis. Our data in monocytes confirm early observations of predominant Gαi/o coupling of human BLT1 observed in neutrophils (19) and differ compared to models of heterologously overexpressed BLT1 where Ptx sensitive and insensitive G protein coupling was described, (20) underlining the importance of studying primary human cells.
We show for the first time in monocytes that LTB4 induced the expression of 27 genes, belonging to families of immediate early genes, transcription activators, cytokines and membrane receptors. Several genes induced by LTB4 in monocytes are associated with acute phase immune responses, e.g. IL-1β, TNFα and CCL3, consistent with the role of LTB4 as an important mediator of innate immune responses. The role of identified transcription factors in regulation of gene expression in human monocytes has not been well studied. Egr genes are involved in monocyte activation and differentiation (21). Egr proteins have also been shown to interact with other transcription factors such as NF-κB and thereby modulate the transcription of genes encoding inflammatory cytokines (22). On the other hand, Egr2 may be involved in the negative regulation of T-cell proliferation and inflammation (23). Previous studies suggested that LTB4 might induce MCP-1 (CCL2) and IL-6 in monocytes (12, 24). Our data confirm that CCL2 was also up-regulated (1.9 fold), but it did not cross the 2 fold threshold chosen for gene selection, while IL-6 gene was not significantly changed. However, others studying changes in gene expression used 6–12 hours of LTB4 stimulation. The induction of EGR2 and FOSB mRNA by LTB4 was only partially inhibited by the BLT1 antagonist, CP105696, not affected by the BLT2 antagonist, LY255283, but fully abolished by Ptx pretreatment (Figure 4), suggesting that LTB4 might regulate gene expression in monocytes acting also through a different than BLT1 or BLT2, Ptx-sensitive receptor.
We compared our data with early gene signatures generated for LTD4 in monocytes and for LTD4 and thrombin in human umbilical vein endothelial cells (HUVECs) (14, 25). A similar set of genes was up-regulated in all these studies, including genes such as FOSB, EGR2, EGR3, NR4A2 and ATF3. This is an interesting observation as in each case different GPCRs were involved in cell activation, namely BLT1, CysLT1, CysLT2 and PAR1 for LTB4 and LTD4 activated monocytes and LTD4 and thrombin activated HUVECs, respectively. This suggests that signals from distinct GPCR subfamily members activate similar gene expression programs in response to ligands formed concomitantly in vivo at an inflammatory site. This implies the existence of an additional level of regulation that controls cell responsiveness and perhaps requires multiple different GPCRs to signal simultaneously in order to reach a combined threshold to fully activate the cell. Such a regulatory mechanism would be responsible for keeping cells non- or weakly responsive to single mediator(s) until a set of activating stimuli reach a threshold required for full cell activation. In support of this hypothesis, we observed an additive, enhanced gene expression in response to LTB4 and LTD4 costimulation and a similar enhancing effect on gene expression was reported by Uzonyi et al. in HUVECs stimulated with LTD4 and thrombin (25). As most GPCRs become quickly desensitized to subsequent stimulation with the same agonist (as we showed here for LTB4 and LTD4), it is possible that only simultaneous activation of many different GPCRs by different mediators could lead to a functional activation of a particular cell in vivo. Such an additive effect of LTB4 and LTD4 costimulation was not only observed for gene expression, but was also seen at the level of signaling (calcium flux and p44/42 phosphorylation) and monocyte chemotaxis. The mechanism of this additive effect is not clear. Both agonists signal in parallel through specific receptors, as selective antagonists for BLT1 and CysLT1 affected only the receptor-specific part of the response. If these signaling pathways were completely nonredundant, one might expect a synergistic effect triggered by both agonists. A partial additive effect was observed in our study and no synergism, demonstrating redundancy in intracellular signaling induced by LTB4 and LTD4 in monocytes.
This observation may have important clinical implications. First, in vivo when multiple mediators are present at a site of inflammation, activation and functions of cells expressing BLT1 and CysLT1 might not be effectively inhibited by selective receptor antagonists as as these receptors have redundant functions. This finding could be relevant for choosing treatment strategies for diseases where enhanced synthesis of both leukotrienes have been identified, such as asthma, rhinitis and atherosclerosis. Blocking both receptors may be more effective than blocking either alone. Second, the observed additive effect of LTB4/LTD4 costimulation if clinically relevant, may favor leukotriene synthesis inhibitors (5-lipoxygenase or FLAP inhibitors) over antagonists of single leukotriene pathways as a therapeutic strategy for allergy and asthma, but properly designed clinical trials are needed to fully verify such a hypothesis.
Acknowledgments
This research was supported by the Intramural Research Program of the Clinical Center, NIH.
Footnotes
None of the authors reports a conflict of interest related to material in this manuscript.
All authors participated either in the performance of experiments or in the interpretation of data presented in this manuscript.
References
- 1.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173(3):1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 2.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;174(2):589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 3.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55(1):195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69(2–3):123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw AJ, Hay H, Cromwell O, Collins JV, Kay AB. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989;84(1):19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 6.Shindo K, Matsumoto Y, Hirai Y, Sumitomo M, Amano T, Miyakawa K, et al. Measurement of leukotriene B4 in arterial blood of asthmatic patients during wheezing attacks. J Intern Med. 1990;228(2):91–96. doi: 10.1111/j.1365-2796.1990.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco Y, Hosni R, Chabannes B, Gormand F, Moliere P, Grosclaude M, et al. Leukotriene B4 level in stimulated blood neutrophils and alveolar macrophages from healthy and asthmatic subjects. Effect of beta-2 agonist therapy. Eur J Clin Invest. 1992;22(11):732–739. doi: 10.1111/j.1365-2362.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 8.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4(10):982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 9.Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176(5):3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 10.Stankova J, Turcotte S, Harris J, Rola-Pleszczynski M. Modulation of leukotriene B4 receptor-1 expression by dexamethasone: potential mechanism for enhanced neutrophil survival. J Immunol. 2002;168(7):3570–3576. doi: 10.4049/jimmunol.168.7.3570. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson A, Sabirsh A, Bristulf J, Kidd-Ljunggren K, Ljungberg B, Owman C, et al. Pro- and anti-inflammatory substances modulate expression of the leukotriene B4 receptor, BLT1, in human monocytes. J Leukoc Biol. 2005;77(6):1018–1025. doi: 10.1189/jlb.1204740. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Zhao A, Wong F, Ayala JM, Struthers M, Ujjainwalla F, et al. Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol. 2004;24(10):1783–1788. doi: 10.1161/01.ATV.0000140063.06341.09. [DOI] [PubMed] [Google Scholar]
- 13.Woszczek G, Chen LY, Alsaaty S, Nagineni S, Shelhamer JH. Concentration-dependent noncysteinyl leukotriene type 1 receptor-mediated inhibitory activity of leukotriene receptor antagonists. J Immunol. 2010;184(4):2219–2225. doi: 10.4049/jimmunol.0900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woszczek G, Chen LY, Nagineni S, Kern S, Barb J, Munson PJ, et al. Leukotriene D(4) induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J Allergy Clin Immunol. 2008;121(1):215–221. e1. doi: 10.1016/j.jaci.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25(19):4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med. 2008;205(4):759–766. doi: 10.1084/jem.20072329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276(15):12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, et al. Mechanisms of leukotriene B4--triggered monocyte adhesion. Arterioscler Thromb Vasc Biol. 2003;23(10):1761–1767. doi: 10.1161/01.ATV.0000092941.77774.3C. [DOI] [PubMed] [Google Scholar]
- 19.Powell WS, MacLeod RJ, Gravel S, Gravelle F, Bhakar A. Metabolism and biologic effects of 5-oxoeicosanoids on human neutrophils. J Immunol. 1996;156(1):336–342. [PubMed] [Google Scholar]
- 20.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda S, Nakamura T, Stone R, Hass R, Bernstein S, Datta R, et al. Expression of the early growth response 1 and 2 zinc finger genes during induction of monocytic differentiation. J Clin Invest. 1991;88(2):571–577. doi: 10.1172/JCI115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wieland GD, Nehmann N, Muller D, Eibel H, Siebenlist U, Suhnel J, et al. Early growth response proteins EGR-4 and EGR-3 interact with immune inflammatory mediators NF-kappaB p50 and p65. J Cell Sci. 2005;118(Pt 14):3203–3212. doi: 10.1242/jcs.02445. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Symonds AL, Martin JE, Kioussis D, Wraith DC, Li S, et al. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J Exp Med. 2008;205(10):2295–2307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rola-Pleszczynski M, Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80(4):1004–1011. [PubMed] [Google Scholar]
- 25.Uzonyi B, Lotzer K, Jahn S, Kramer C, Hildner M, Bretschneider E, et al. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci U S A. 2006;103(16):6326–6331. doi: 10.1073/pnas.0601223103. [DOI] [PMC free article] [PubMed] [Google Scholar]