Abstract
Atherosclerotic carotid stenosis is an important risk factor for stroke. Carotid plaques (CPs) causing stroke may present a distinct type of molecular pathology compared with transient ischemic attack (TIA)-associated or asymptomatic plaques. We compared the gene expression profiles of CPs from stroke patients (n = 12) and asymptomatic patients (n = 9), both with similar risk factors and severity of carotid stenosis (>70%). Sixty probes showed over 1.5-fold expression difference at 5% false discovery rate. Functional clustering showed enrichment of genes in 51 GO categories and seven pathways, the most significant of which relate to extracellular-matrix interaction, PPAR gamma signaling, scavanger receptor activity, and lysosomal activity. Differential expression of ten genes was confirmed in an extended replication group (n = 43), where the most significant expression differences were found in CD36 (2.1-fold change, p = 0.005), CD163 (1.7-fold change, p = 0.007) and FABP4 (2.2-fold change, p = 0.015). These include four genes not previously linked to plaque destabilization: GLUL (2.2-fold change, p = 0.016), FUCA1 (2.2-fold change, p = 0.025), IL1RN (1.6-fold change, p = 0.034), and S100A8 (2.5-fold change, p = 0.047). Strong correlations were found to plaque ulceration, plaque hemorrhage, and markers of apoptosis and proliferation (activated caspase 3, TUNEL, and Ki67). Protein expression of these genes was confirmed by immunohistochemistry and was found in the atheromatous areas of CPs critical for plaque destabilization. This study presents a comprehensive transcriptional analysis of stroke-associated CPs and demonstrates a significant transcriptome difference between stroke-associated and asymptomatic CPs. Follow-up studies on the identified genes are needed to define whether they could be used as biomarkers of symptomatic CPs or have a role in plaque destabilization.
Electronic supplementary material
The online version of this article (doi:10.1007/s00109-011-0773-z) contains supplementary material, which is available to authorized users.
Keywords: Stroke, Atherosclerosis, Microarray, Cerebrovascular, Real-time PCR
Introduction
Atherosclerotic disease of the internal carotid artery is a major risk factor of ischemic stroke, and 20% of patients with anterior and middle cerebral artery infarcts have significant carotid stenoses. Curiously, carotid stenoses behave very differently; some progress to occlusion without causing any symptoms, while others manifest themselves with a sudden massive atherothromboembolic cerebral infarction. Previous ipsilateral symptoms and stenosis of 70% or more are established predictive factors of future ipsilateral stroke [1, 2]. Several histological features are also more frequent in symptomatic carotid plaques (CPs), especially fibrous cap thinning, greater inflammatory cell infiltrations, intraplaque hemorrhage, and plaque rupture [3–6]. Both radiologically and histologically detected ulcerations have been shown to associate with symptoms [3, 5, 7]. The detailed molecular mechanisms underlying these plaque features and CP destabilization are incompletely understood.
Several studies have employed large-scale gene expression analysis as a tool to find novel pathophysiologically important genes or signaling pathways that could trigger CP destabilization. Vemuganti and Dempsey conducted a whole genome microarray analysis of CPs from six symptomatic and four asymptomatic patients and reported 289 differentially expressed genes [8], the significance of which, however, is difficult to evaluate because of the lack of adequate statistical tests or replication. In a slightly different approach, Papaspyridonos and colleagues compared unstable and stable plaque regions from three patients and identified a large number of differentially expressed genes in both intra- and inter-individual comparisons [9]. Twenty-seven of these were replicated in an independent set of 46 unstable and stable plaque regions, and matrix metalloproteinase-9 (MMP-9), cathepsin B, and legumain further at the protein level. Dahl and colleagues compared expression patterns in four symptomatic and four asymptomatic CPs and found 136 genes with more than twofold expression change, none of which were statistically significant [10]. In subsequent studies in a replication group of 21 CPs, they showed one of these genes, visfatin, to be significantly over-expressed both at the RNA and protein level in symptomatic CPs as well as strong protein expression at the site of plaque rupture in acute coronary syndrome patients. Recently, Agardh et al. compared the expression profiles of CPs from patients with recent symptom (≤1 month; n = 14), patients with longer delay (>1 month; n = 17), and asymptomatic patients (n = 9) and showed the over-expression of fatty acid-binding protein 4 (FABP4) in CPs from patients with recent symptoms compared to asymptomatic patients [11]. In addition to FABP4, they report two other genes, IQ motif containing GTPase-activating protein 1 and CD84 antigen, to be upregulated in symptomatic CPs. Also proteomics approaches utilizing a protein array of 512 antibodies [12] and two-dimensional gel electrophoresis in connection with mass spectrometry [13] have been used to compare protein expression in stable and unstable carotid plaques. Despite of a number of studies done, some limiting issues impede drawing firm conclusions on the gene expression changes typical of stroke-associated CPs: the very small number of samples in some studies, diverse clinical diagnoses of patients (amaurosis fugax, TIA, or stroke) and the small number of findings reaching statistical significance.
Previously, we have carried out a microarray study on rare cases of bilateral carotid stenosis when searching for intra-individual local factors influencing plaque stability and identified genes involved in the clearance of free tissue hemoglobin, CD163 molecule (CD163), and heme oxygenase-1, to be important [14]. Now we report a larger microarray study of two extreme patient groups with similar conventional risk factors and severity of carotid stenoses but dissimilar clinical plaque behavior to identify inter-individual differences. Due to the inherent inaccuracy in the diagnosis of temporary symptoms such as in TIA and recent findings suggesting that TIA or stroke may represent distinct plaque pathologies [5, 6], we restricted our analysis to stroke-associated carotid plaques (SCPs) from patients with imaging-confirmed recent ipsilateral ischemic brain infarct and asymptomatic carotid plaques (ACPs) from patients without cerebrovascular symptoms and normal brain imaging.
Materials and methods
Patients
The patients were selected from the Helsinki Carotid Endarterectomy Study (HeCES) cohort, which included 92 patients who underwent carotid endarterectomy due to high-grade carotid stenoses (>70% [1]) during 1997–2000 in the Helsinki University Central Hospital (methods in detail [15–17]). Recruitment was in-group consecutive, i.e., the recruitment of asymptomatic patients continued 2 months longer to get a more balanced cohort symptom-wise. All patients underwent thorough clinical examination and interview by a stroke neurologist, DSA angiography, transcranial Doppler ultrasound, brain imaging (CT or MRI), routine blood analysis, and analysis of serum lipids and fibrinolytic system. The clinical data was gathered in a HeCES databank together with plaque-level histological and gene expression data. The division into symptomatic or asymptomatic CPs was based on the presence or absence of prior ipsilateral ischemic cerebral or retinal symptoms within 120 days prior to surgery. All human studies were performed in accordance with the Declaration of Helsinki and informed consent was obtained from all patients. The study protocol was approved by the University of Helsinki ethics committee of ophtalmology, otorhinolaryngology, neurology, and neurosurgery (#339/E9/04).
This microarray study includes CPs only from symptomatic patients with clinical stroke and radiologically confirmed ischemic brain infarction in the ipsilateral carotid territory (n = 12) and asymptomatic patients with no history of ischemic cerebral or retinal symptoms and normal brain imaging (n = 9). In addition, we performed quantitative real-time RT-PCR in an extended group of patients with a clinically defined ischemic stroke or retinal infarct in the ipsilateral carotid territory (n = 25) and clinically asymptomatic patients (n = 18). This group of patients (n = 43) is referred to as the “extended replication group” in the text. There is a significant gender difference between the stroke and asymptomatic patients in both the microarray (p = 0.023) and the extended replication (p = 0.055) groups. Patient characteristics are given in Table 1.
Table 1.
Patient characteristics and macroscopic plaque features of the analyzed patient groups
| Microarray group | Extended replication group | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Symptomatic | Asymptomatic | p valuea | All | Symptomatic | Asymptomatic | p valuea | |
| Number | 22 | 13 | 9 | 43 | 25 | 18 | ||
| Gender, M/F | 16/6 | 12/1 | 4/5 | 0.023 | 27/16 | 19/6 | 8/10 | 0.055 |
| Age, yearsb | 64 ± 8 | 62 ± 6 | 66 ± 9 | 65 ± 8 | 65 ± 9 | 65 ± 7 | ||
| Degree of ICA stenosesb, c | 79 ± 10 | 82 ± 12 | 74 ± 5 | 78 ± 9 | 79 ± 10 | 76 ± 7 | ||
| Cerebrovascular symptom | ||||||||
| Stroke, % | 59 | 100 | 0 | <0.001 | 58 | 100 | 0 | <0.001 |
| Time from symptom to CEA, daysb | 44 ± 32 | 52 ± 39 | ||||||
| Comorbidities, % | ||||||||
| Diabetes mellitus, type I or II | 23 | 31 | 11 | 28 | 32 | 22 | ||
| Dyslipidemia | 68 | 69 | 67 | 58 | 64 | 50 | ||
| Peripheral arterial disease | 27 | 23 | 33 | 21 | 16 | 28 | ||
| Coronary heart disease | 41 | 31 | 56 | 37 | 32 | 44 | ||
| Current smoking | 27 | 31 | 22 | 20 | 17 | 24 | ||
| Arterial hypertension | 68 | 85 | 44 | 67 | 72 | 61 | ||
| Medications, % | ||||||||
| ASA | 55 | 39 | 78 | 62 | 44 | 88 | 0.004 | |
| ACE inhibitor | 5 | 8 | 0 | 17 | 16 | 18 | ||
| Statin | 46 | 46 | 44 | 40 | 40 | 39 | ||
| Anticoagulant | 36 | 54 | 11 | 33 | 52 | 6 | 0.035 | |
| Laboratory measuresb | ||||||||
| Hematocrit | 41 ± 3 | 42 ± 3 | 40 ± 3 | 41 ± 4 | 42 ± 3 | 39 ± 3 | 0.005 | |
| High-sensitivity CRP | 6.5 ± 6.8 | 5.3 ± 3.4 | 8.2 ± 9.9 | 8.7 ± 15.0 | 6.2 ± 11.6 | 12.1 ± 18.5 | ||
| LDL | 3.3 ± 1.2 | 3.8 ± 1.7 | 3.1 ± 1.0 | 3.4 ± 1.0 | 3.6 ± 1.0 | 3.1 ± 0.8 | ||
| HDL | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.2 | 0.002 | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.5 ± 0.5 | 0.048 |
| Fibrinogen | 4.0 ± 1.1 | 4.1 ± 1.1 | 3.8 ± 1.2 | 4.7 ± 4.5 | 5.1 ± 5.8 | 4.0 ± 1.3 | ||
| tPA antigen | 8.7 ± 3.1 | 9.5 ± 2.3 | 7.8 ± 3.7 | 8.0 ± 3.0 | 8.6 ± 2.4 | 7.3 ± 3.5 | ||
| Macroscopic plaque features, % | ||||||||
| Ulceration | 30 | 55 | 0 | 0.014 | 46 | 60 | 28 | 0.035 |
| Intraplaque hemorrhage | 53 | 67 | 33 | 55 | 63 | 44 | ||
| Intramural thrombus | 19 | 33 | 0 | 18 | 29 | 6 | ||
| Loose atheroma | 19 | 25 | 11 | 24 | 33 | 11 | ||
| Calcification | 62 | 50 | 78 | 69 | 63 | 72 | ||
CEA carotid endarterectomy
a p values shown for significant group differences
bAge, degree of ICA stenoses, time between symptom and CEA and laboratory measurements are given as mean and standard deviation
cDegree of internal carotid artery stenoses according to the NASCET criteria
Sample preparation
The CPs were removed en bloc in endarterectomy. The vascular surgeon (ES) inspected CPs after gentle wash of saline, and made macroscopic observations about surface ulceration, intraplaque hemorrhage, intramural thrombus, loose atheroma, and calcification. The plaques were cut into five longitudinal slices, all containing a portion of atheroma core, each of which were used for a specific purpose: histological examination, RNA and protein extraction, and biochemical analyses. Total RNA was extracted with Trizol®-reagent (Invitrogen Life Technologies) and purified with an RNeasy Total RNA Isolation Kit (Qiagen) according to the manufacturers' recommendations. RNA quality was assessed using the RNA 6000 Nano LabChip Kit (Agilent Bioanalyser 2100; Agilent Technologies). Details on RNA quality assessment can be found in Supplemental Material.
Microarray analysis and quantitative real-time RT-PCR
The microarray experiments were performed according to the manufacturer's recommendations and the MIAME guidelines [18] using Affymetrix GeneChip® U133A arrays containing 22,283 probe sets. The flowchart of the microarray data analysis is shown in Fig. 1. Detailed description on analysis steps can be found in Supplemental Material.
Fig. 1.

Microarray data analysis flowchart. The number of probe sets remaining after each analysis step is shown. Dashed line arrows to the right indicate probe sets that were used for different clustering analysis. RMA Robust Multi-array Average. Asterisk (*) listed in Supplemental Table 3
Quantitative real-time RT-PCR (qPCR) was performed using TaqMan® Gene Expression Assays and the ABI PRISM®7000 Sequence Detection System (Applied Biosystems) according to the manufacturer's recommendations. Beta-actin was used as the endogenous control gene and relative quantitation was performed using either the comparative delta–delta–Ct or the relative standard curve method. For details see the Supplemental Material and Supplemental Table 1.
Immunohistochemistry and microscopy
Microscopic analyses were performed in adjacent segments of the plaques used for RNA extraction. CPs were assigned to AHA-classes based on microscopic histology [19]. The amounts of macrophages, activated T cells, mast cells, and smooth muscle cells were determined by immunohistochemistry [16]. In addition, the protein products of the following genes were immunostained in the microarray group either in fresh frozen or paraffin-embedded sections using methods previously described [14, 15, 20]: CD36 molecule (CD36), perilipin 2 (PLIN2), FABP4, glutamate-ammonia ligase (GLUL), chemokine (C–C motif) ligand 18 (CCL18), interleukin 1 receptor antagonist (IL1RN), heme oxygenase 1 (HMOX1), and S100 calcium binding protein A8 (S100A8; Supplemental Table 2). Light microscopy was performed using Axioplan 2, AxioCam color, and MRGrab 1.0.0.4 respectively (all from Carl Zeiss) by one investigator (KN), who was blinded to the clinical data. For details see Supplemental Material.
Statistics
The statistical analyses, apart from the microarray analyses, were performed using SPSS10.0.7 for Windows (SPSS Inc.). Pearson's chi square or Fisher's exact test was used to evaluate the differences in non-continuous variables, and the independent-samples t test was employed for continuous variables. The results from qPCR were evaluated by the Mann–Whitney U test. Correlations between real-time qPCR results and clinical data and plaque characteristics were calculated by nonparametric Spearman's Rank Order Correlation Coefficient with Benjamini–Hoechberg multiple testing correction.
Results
Macroscopic and histological characterization of CPs
We first explored macroscopic and histological differences between SCPs and ACPs. There were no differences in macroscopic intraplaque hemorrhages, intramural thrombus, loose atheroma, or calcification. The SCPs had more macroscopic ulcerations (Table 1). On microscopic examination, all CPs represented complicated AHA class VI lesions containing microscopic surface ulceration (VIa), hematoma/hemorrhage (VIb), and/or thrombotic deposit (VIc, see Supplemental Figure 3) [19]. No significant quantitative differences were found in the immunostained area for macrophages, activated T cells, mast cell numbers, or smooth muscle cell staining grades (Supplemental Figures 4 and 5).
Microarray analysis
Figure 1 shows the flowchart of microarray analysis. Firstly, we employed hierarchical clustering of all the CPs and reliably expressed genes (n = 3,433, see Fig. 1) to examine the relationships between CPs. The resulting Condition tree (Fig. 2) shows two main branches, one including ten of the 12 SCPs and the second, six of the nine ACPs. Thus, the global gene expression profiles can classify CPs according to the symptom to asymptomatic and stroke-associated CPs. The classification accuracy remains the same, if clustering is performed for all genes (n = 22,283, see Fig. 1).
Fig. 2.
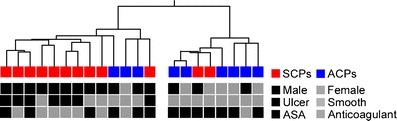
Hierarchical clustering of CPs (Condition tree). The samples were classified into a tree based on their similarities. The length of the branch between two samples indicates how correlated their expression profiles are. The boxes below represent samples. The characteristics that were unevenly distributed between symptomatic and asymptomatic groups are shown
Ninety-two reliably expressed genes (103 probe sets) showed ≥1.5-fold expression difference between stroke-associated and asymptomatic CPs (Supplemental Table 3). SAM analysis [21] of the 103 probe sets showed that 60 probes sets were significantly differentially expressed at 5% false discovery rate (Table 2). Since microarray analysis was shown to be sensitive to the choice of preprocessing algorithm [22], we analyzed the hybridization data also by two other commonly used algorithms, GC-RMA [23], and MAS5 (http://www.affymetrix.com), followed by comparable filtering steps (see Supplemental Material for details). Based on SAM analysis, 77 and 75 probe sets were significant at 5% FDR in GC-RMA and MAS5 normalized data, respectively. Thirty-seven were shared by all three normalization methods (Supplemental Figure 6).
Table 2.
Microarray probe sets showing statistically significant expression difference between stroke-associated and asymptomatic carotid plaques
| Probe ID | Gene symbola | Gene namea | Fold-change | Mann–Whitney U p b | SAM q (%)b |
|---|---|---|---|---|---|
| 202206_at | ARL4C | ADP-ribosylation factor-like 4 C | 1.6 | 0.001 | 1.72 |
| 213418_at | HSPA6 | Heat shock 70 kDa protein 6 | 1.8 | 0.001 | 1.72 |
| 221760_at | MAN1A1 | Mannosidase, alpha, class 1A, member 1 | 1.5 | 0.002 | 1.72 |
| 210512_s_at | VEGFA | Vascular endothelial growth factor A | 2.0 | 0.003 | 1.72 |
| 200921_s_at | BTG1 | B-cell translocation gene 1, anti-proliferative | 1.6 | 0.004 | 1.72 |
| 202207_at | ARL4C | ADP-ribosylation factor-like 4C | 1.6 | 0.004 | 1.72 |
| 212192_at | KCTD12 | Potassium channel tetramerisation domain containing 12 | 1.6 | 0.004 | 1.72 |
| 201670_s_at | MARCKS | Myristoylated alanine-rich protein kinase C substrate | 1.6 | 0.006 | 1.72 |
| 202998_s_at | LOXL2 | Lysyl oxidase-like 2 | 1.7 | 0.006 | 1.72 |
| 218149_s_at | ZNF395 | Zinc finger protein 395 | 1.5 | 0.006 | 1.72 |
| 202499_s_at | SLC2A3 | Solute carrier family 2, member 3 | 1.7 | 0.009 | 1.72 |
| 205099_s_at | CCR1 | Chemokine (C–C motif) receptor 1 | 1.6 | 0.009 | 1.72 |
| 212154_at | SDC2 | Syndecan 2 | 1.8 | 0.009 | 1.72 |
| 217028_at | CXCR4 | Chemokine (C–X–C motif) receptor 4 | 1.8 | 0.009 | 1.72 |
| 202310_s_at | COL1A1 | Collagen, type I, alpha 1 | 1.7 | 0.011 | 1.72 |
| 212820_at | DMXL2 | DMX-like 2 | 1.7 | 0.011 | 1.72 |
| 201438_at | COL6A3 | Collagen, type VI, alpha 3 | 1.7 | 0.013 | 1.72 |
| 221210_s_at | NPL | N-acetylneuraminate pyruvate lyase | 1.8 | 0.013 | 1.72 |
| 201193_at | IDH1 | Isocitrate dehydrogenase 1 (NADP+), soluble | 1.6 | 0.016 | 1.72 |
| 202934_at | HK2 | Hexokinase 2 | 1.7 | 0.016 | 1.72 |
| 213655_at | YWHAE | Tyrosine 3-monooxygenase | 1.6 | 0.016 | 1.72 |
| 215049_x_at | CD163 | CD163 molecule | 1.6 | 0.016 | 1.72 |
| 217983_s_at | RNASET2 | Ribonuclease T2 | 1.7 | 0.016 | 1.72 |
| 32128_at | CCL18 | Chemokine (C–C motif) ligand 18 | 2.2 | 0.016 | 1.72 |
| 203645_s_at | CD163 | CD163 molecule | 1.7 | 0.019 | 1.72 |
| 209924_at | CCL18 | Chemokine (C–C motif) ligand 18 | 2.0 | 0.019 | 1.72 |
| 212671_s_at | HLA-DQA1/HLA-DQA2/LOC650946 | Major histocompatibility complex, class II DQ alpha 1 | 1.8 | 0.019 | 1.72 |
| 201645_at | TNC | Tenascin C | 1.6 | 0.023 | 1.72 |
| 202838_at | FUCA1 | Fucosidase, alpha-L-1 | 1.9 | 0.023 | 1.72 |
| 212582_at | OSBPL8 | Oxysterol binding protein-like 8 | 1.6 | 0.023 | 1.72 |
| 203814_s_at | NQO2 | NAD(P)H dehydrogenase, quinone 2 | 1.5 | 0.028 | 1.72 |
| 217202_s_at | GLUL | Glutamate-ammonia ligase | 1.7 | 0.028 | 1.72 |
| 200648_s_at | GLUL | Glutamate-ammonia ligase | 1.9 | 0.033 | 1.72 |
| 202087_s_at | CTSL | Cathepsin L | 1.5 | 0.033 | 1.72 |
| 202436_s_at | CYP1B1 | Cytochrome P450, family 1, subfamily B, polypeptide 1 | 1.6 | 0.033 | 1.72 |
| 202859_x_at | IL8 | Interleukin 8 | 1.9 | 0.033 | 1.72 |
| 202902_s_at | CTSS | Cathepsin S | 1.5 | 0.033 | 1.72 |
| 204438_at | MRC1/MRC1L1 | Mannose receptor, C type 1 | 1.6 | 0.033 | 1.72 |
| 208146_s_at | CPVL | Carboxypeptidase, vitellogenic like | 1.5 | 0.036 | 1.72 |
| 201147_s_at | TIMP3 | TIMP metallopeptidase inhibitor 3 | 1.9 | 0.039 | 1.72 |
| 202345_s_at | FABP5 | Fatty acid-binding protein 5 | 1.6 | 0.047 | 1.72 |
| 206488_s_at | CD36 | CD36 molecule | 2.2 | 0.047 | 1.72 |
| 219607_s_at | MS4A4A | Membrane-spanning 4-domains, subfamily A, member 4 | 1.8 | 0.047 | 1.72 |
| 202912_at | ADM | Adrenomedullin | 1.5 | 0.055 | 1.72 |
| 203665_at | HMOX1 | Heme oxygenase 1 | 2.2 | 0.055 | 1.72 |
| 203980_at | FABP4 | Fatty acid-binding protein 4 | 2.6 | 0.055 | 1.72 |
| 212657_s_at | IL1RN | Interleukin 1 receptor antagonist | 1.6 | 0.055 | 1.72 |
| 214038_at | CCL8 | Chemokine (C–C motif) ligand 8 | 1.6 | 0.055 | 1.72 |
| 215223_s_at | SOD2 | Superoxide dismutase 2, mitochondrial | 1.5 | 0.055 | 1.72 |
| 204580_at | MMP12 | Matrix metallopeptidase 12 | 2.3 | 0.065 | 1.72 |
| 209122_at | PLIN2 | Perilipin 2 | 1.8 | 0.065 | 1.72 |
| 217294_s_at | ENO1 | Enolase 1 | 1.7 | 0.065 | 1.72 |
| 200832_s_at | SCD | Stearoyl-CoA desaturase | 1.6 | 0.076 | 1.72 |
| 209351_at | KRT14 | Keratin 14 | 1.8 | 0.076 | 1.72 |
| 209555_s_at | CD36 | CD36 molecule | 1.9 | 0.076 | 1.72 |
| 201785_at | RNASE1 | Ribonuclease, RNase A family, 1 | 1.5 | 0.118 | 3.26 |
| 202917_s_at | S100A8 | S100 calcium binding protein A8 | 1.6 | 0.118 | 3.26 |
| 221730_at | COL5A2 | Collagen, type V, alpha 2 | 1.5 | 0.136 | 3.26 |
| 203381_s_at | APOE | Apolipoprotein E | 1.6 | 0.177 | 4.00 |
| 204259_at | MMP7 | Matrix metallopeptidase 7 | 1.5 | 0.201 | 4.72 |
aFor the probe set detecting transcripts from several homologous genes, symbols are separated by hyphen. Gene name is only given for the first one
b p = pairwise non-adjusted p values from Mann–Whitney U test, q = the minimum false discovery rate at which the gene is called significant from significance analysis of microarrays (SAM)
To identify pathophysiologically important gene groups, we performed gene enrichment analysis on Gene Ontology (GO) categories and Kyoto Encyclopedia of Genes and Genomes (KEGG) and Biocharta pathways for the differentially expressed genes (n = 103, Supplemental Table 3). Fifty-one GO categories showed enrichment with the most significant (p < 0.001) categories: extracellular matrix, extracellular space, lysosome, lytic vacuole, vacuole, fibrillar collagen, collagen, scavenger receptor activity, peptidoglycan metabolism, response to wounding and organ development (categories with ≥3 genes and p < 0.01 are shown in the Supplemental Figure 7 and Supplemental Table 4). Six KEGG and one Biocharta pathway showed more than two differentially expressed genes, and the most significant pathways (p < 0.001, in order of significance) were the extracellular matrix-receptor interaction, PPAR signaling, and antigen processing and presentation pathways (Supplemental Table 5).
Confirmation of differentially expressed genes in the extended replication group
Differential expression of 18 genes discovered in the microarray experiment was tested by qPCR in the same samples and congruent fold change was found for 16 genes (89%, Fig. 3). Next we analyzed these 18 genes in the extended replication group (n = 43), which included CPs from clinically defined stroke patients (n = 25) and asymptomatic patients (n = 18), and qPCR confirmed significant differential expression of ten genes between SCPs and ACPs (Fig. 3). Confirmed expression changes with the most significant association (p < 0.01) with symptoms were CD36 (2.1-fold change, p = 0.005) and CD163 (1.7-fold change, p = 0.007).
Fig. 3.
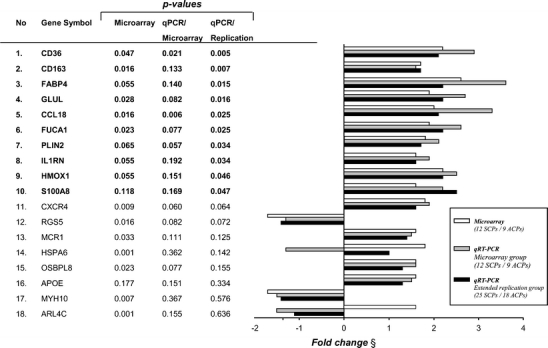
Confirmation of differential gene expression by qPCR. Genes are organized according to statistical significance (significant bolded). Microarray refers to non-adjusted p values from microarray analysis also given in Table 2 and Supplemental Table 3. QPCR/microarray refers to the real-time qPCR analysis results in the microarray group and qPCR/replication in the extended replication group. All p values from Mann–Whitney U test
Correlations of differentially expressed genes with clinical data and plaque features
We investigated whether the ten genes with confirmed expression changes showed any association with clinical patient characteristics or morphological plaque features (Supplemental Table 6). After multiple testing correction, we did not find significant associations to known atherosclerosis risk factors, comorbidities, medications, or plaque cell-type markers. Instead, several genes showed association to plaque ulceration (seven out of ten genes), intraplaque hemorrhage (four out of ten), and markers of cell death and proliferation (activated caspase 3, four out of ten; Ki67 proliferation marker, four out of ten; TUNEL positivity in the intima, two out of ten). Additionally, three genes showed positive correlation to the degree of ICA stenosis.
Immunohistochemical verification of proteins encoded by differentially expressed genes
The expression and histological localization of nine genes (CD36, CD163, FABP4, PLIN2, GLUL, CCL18, IL1RN, HMOX1, and S100A8) with qPCR-confirmed differential expression in SCPs were investigated at the protein level using immunohistochemistry (Fig. 4). CD36, CD163, FABP4, PLIN2, HMOX1, GLUL, and S100A8 showed expression mainly in macrophages. HMOX1, GLUL, S100A8, and IL1RN were also expressed in other cell types as judged by morphology: HMOX1, GLUL, and IL1RN in smooth muscle cells and S100A8 in scarce intimal polymorphonuclear cells. IL1RN was also expressed in endothelial cells, both in the neovessels as well as in the luminal endothelium. CCL18 showed a diffuse intimal staining pattern typical for secreted chemokines. CD36, CD163, FABP4, PLIN2, S100A8, HMOX1, and GLUL showed strong staining around the lipid core and in atheromatous areas.
Fig. 4.
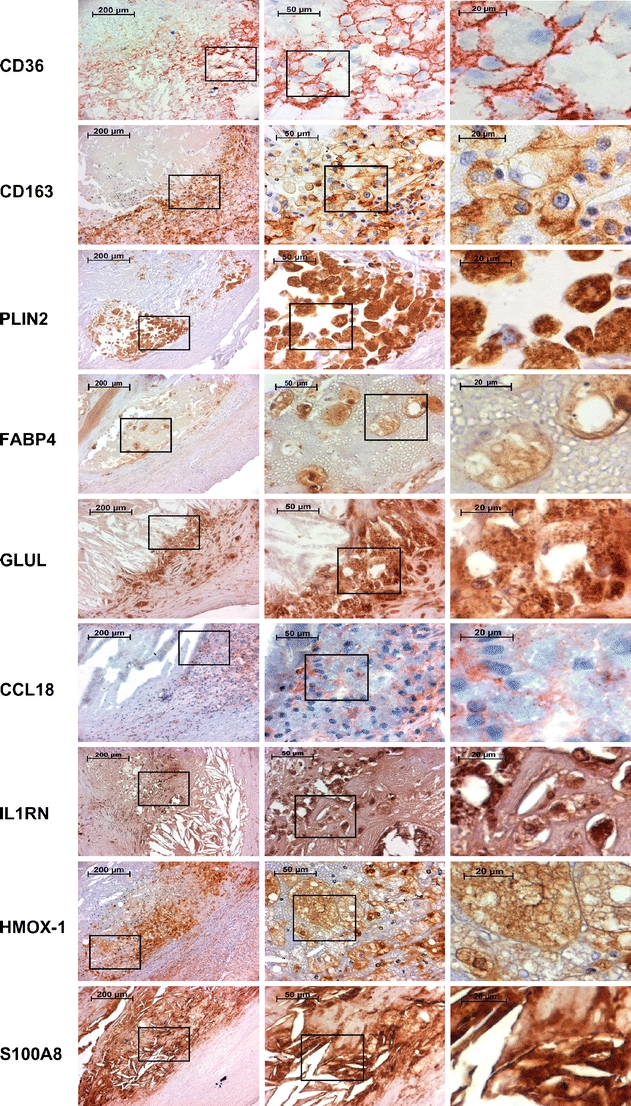
Immunohistochemical stainings of CD36, CD163, PLIN2, FABP4, GLUL, CCL18, IL1RN, HMOX1, and S100A8. The first column displays images obtained from the edge of an atheroma. The second and the third columns are magnifications from foam cell-rich areas indicated by rectangles
Discussion
The evolution of an atherosclerotic CP into an unstable plaque leading to atherothromboembolic strokes, is still incompletely understood. We adopted large-scale microarray analysis to identify genes and cellular pathways that could relate to plaque destabilization. Microarray analysis was focused on two phenotypic extremes: symptomatic CPs from patients with recent ipsilateral ischemic brain infarct and asymptomatic CPs from patients that have never experienced cerebrovascular symptoms and had normal brain imaging. We demonstrate that stroke-associated CPs possess a characteristic gene expression profile, which distinguishes them from asymptomatic CPs. In addition to confirming several previously recognized genes linked to unstable atherosclerosis, our analysis revealed expression changes in genes with no previously known association with symptomatic or unstable atherosclerotic plaques. Functional clustering data imply intrinsic differences between ACPs and SCPs in cellular processes, including inflammatory responses, intracellular lipid and carbohydrate metabolism and transport, lysosomal activity, and reorganization of the extracellular matrix.
The microarray analysis revealed differential expression with more than 1.5-fold change in 103 probe sets representing 92 genes. SAM analysis of these 103 probe sets found 60 probe sets to be statistically significant at 5% FDR (Fig. 1 and Table 2 ). This represents approximately 0.3% of the probe sets in the original microarray. A small number of differentially expressed genes are expected, since we compare samples that all represent advanced atherosclerotic tissue with no clear histological differences. In addition, we used a stringent filtering criteria and accepted 15% of the microarray probe sets for statistical analysis. The decision on this high filtering cutoff was based on a fact that in a heterogenous tissue such as atherosclerotic plaque, any expression signal measured is a sum of signals from numerous cell populations. Typically, this leads to low fold changes, when only a proportion of cells express a given gene or different cell population show reverse expression changes overriding each others' effect. In this kind of situation, detection of low fold “true” changes from background fluctuation is difficult. Therefore, we chose to concentrate on transcripts whose expression level and difference are high enough to allow for their reliable detection with a goal of finding reproducible list of differentially expressed genes, even if we recognize that this probably increases type II errors.
Eighteen genes were selected for replication by qPCR both in the microarray group and in the extended replication group. Several criteria were used in the selection: statistical significance, consistency across preprocessing methods, functional clustering analysis, and novelty. Replication rate was 89% (16 out of 18 genes) in the microarray group. Only ARL4C and HSPA6 failed to show differential expression by qPCR despite being the two most significant genes in the microarray analysis. Both genes have only one exon, which might cause problems in the correct measurement of single-exon genes by the array's probe design. Findings were similar in the extended replication group: all except ARL4C and HSPA6 showed congruent fold changes in magnitude and direction (Fig. 3). The expression change was statistically significant for ten of the 18 genes (56%). We confirmed protein expression of these genes in the CPs by immunohistochemistry and show that they are expressed in the atheromatous areas of CPs critical for plaque destabilization (Fig. 4).
Many of the identified genes show correlation to plaque features that can be considered markers of CP instability, namely ulceration [5, 7], intraplaque hemorrhage [5], and apoptosis [24] (Supplemental Table 6). In line with the finding of the higher frequency of macroscopic ulcerations in our stroke-associated CPs (see Table 1), the expression of 80% of the qPCR-confirmed genes showed correlation to macroscopic ulcerations. Plaque ulceration is an integral phase in the development of sudden thromboembolism and in this way a morphological correlate of plaque instability [7, 25–27]. It is likely that ulceration affects gene expression patterns and may partly explain them, i.e., through the process of wound healing. However, ulceration and thromboembolism are not “one-time events” but rather the unstable plaques have a chronic course of disease with repeated thromboembolic events. For example, 36% of patients in our extended replication group had TIA within 6 months prior to ischemic stroke and NASCET showed that without CEA the risk of new event stays high several months until it drops gradually within 2 to 3 years to the same annual 3% level as found in asymptomatic patients [1]. It is possible that the recurrent cycles of rupture and healing with the concomitant ulceration of endothelial lining and intraplaque hemorrhages are integral to the instability of symptomatic plaques.
Seven of the qPCR-replicated genes also showed correlation to intraplaque hemorrhages (IPH). In the large meta-analysis of Gao and colleagues [28], incidence of IPH was higher in symptomatic plaques, but the evidence mainly derived from early small-sized studies, whereas recent larger studies yielded insignificant associations. We did not find difference in the incidence of IPH between ACPS and SCPS in our study groups or in the whole HeCES cohort (Table 1) [17]. However, these observations do not exclude the role of IPH in plaque destabilization. In patients with asymptomatic moderate carotid stenosis, MRI-depicted IPH was associated with future ipsilateral cerebrovascular events [27]. IPH or marked intraplaque vessel formation were also shown to be the only plaque features to predict cardiovascular events after CEA [29]. IPH can destabilize plaques both by ensuing oxidative and inflammatory stress as well as exacerbating the local lipid burden by entailing red blood cell membranes abundant in cholesterol [14, 20, 30, 31]. Our own data suggests that IPH lead to a more-pronounced inflammatory gene induction in symptomatic than in asymptomatic CPs, which could be explained by differences in genetic factors or plaque microenvironment [14]. This kind of phenomenon could explain ambiguous results from previous observational studies.
The results obtained from the present study concur well with the results from our earlier intra-patient expression analysis [14] (15/92 (16%) of the differentially expressed genes) as well as with the results from Papaspyridonos and colleagues comparing unstable and stable plaque regions [9] (33/92 (36%) of the differentially expressed genes). Both matrix metallopeptidase 9 and legumain reported by Papaspyridonos and colleagues [9] are significantly over-expressed in the SCPs of our material. Instead, we do not find increased expression of cathepsin B, but cathepsins D, L, and S. We detect the strong over-expression of FABP4 in the stroke-associated CPs, as was recently reported for symptomatic CPs by two groups [11, 32]. Increased expression of visfatin is not found in our material, but 20 of the genes showing differential expression (although not reaching statistical significance) in the microarray study by Dahl and colleagues [10], were also differentially expressed in our stroke-associated CPs. Converging evidence from these studies accumulates for the important role of several genes in the destabilization of CPs, especially cathepsin L, CD36, fatty acid-binding proteins 4 and 5 (FABP4 and FABP5), heme oxygenase 1, interleukin 1 receptor antagonist (IL1RN), interleukin 8, matrix metalloproteinases 7 and 12, N-acetylneuraminate pyruvate lyase (NPL), non-muscle myosin 10 (MYH10) and perilipin 2 (PLIN2, previously known as ADFP).
There is a significant difference between the proportion of women in the symptomatic and asymptomatic groups (Table 1). Skewed gender distribution is an inherent epidemiological phenomenon incorporated in all consecutive series of symptomatic carotid disease [1, 2]. In this study, the gender difference is partly due to the fact that the microarray analysis was restricted to the phenotypic extremes of the disease defined by strict clinical and radiological criteria and hence constituted a subgroup of the larger gender balanced HeCES cohort. To partially compensate for the gender imbalance, genes located in sex chromosomes were excluded from the microarray data analysis. Despite of this imbalance, RNA expression levels of only three of the 18 genes replicated by qPCR showed any correlation to gender (Supplemental Table 6). Also, hierarchical clustering of samples based on global gene expression patterns show that symptom and macroscopic ulcerations are stronger determining factors of gene expression than gender (Fig. 2).
In this study, we report a comprehensive transcriptional analysis of CPs from individuals with clinically relevant carotid disease that links plaque-level gene expression data to thromboembolic strokes. Since the assignment to symptom groups was performed purely from a clinical standpoint, we consider the reported transcriptome to genuinely represent clinical vulnerability of CPs. Perhaps the most striking finding of the present study is that the resulting transcriptome clearly differentiates stroke-associated CPs from asymptomatic CPs at the level of individual patients, corresponding to the clinical course (Fig. 2). Unlike many systemic markers analyzed in our patient material, the plaque-level analysis revealed significant differences related to carotid plaque behavior. This indicates that local expression changes within the plaque might be more crucial in the longstanding development of plaque vulnerability than incidental systemic factors that might have a more transient triggering role in acute plaque destabilization. These results suggest potential new pharmacological targets to be explored for the management, stabilization, or treatment of unstable large artery atherosclerosis.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
(PDF 812 kb)
Acknowledgments
Taru Puhakka, Saija Eirola, and Riitta Kärkkäinen are thanked for their skillful technical assistance. This study was funded by Helsinki University Central Hospital (EVO) research grants, the Academy of Finland and the Sigrid Jusélius, Lundbeck, Aarne Koskelo, Maire Taponen, and Päivikki and Sakari Sohlberg foundations. Wihuri Research Institute is maintained by the Jenny and Antti Wihuri foundation.
Disclosures
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Jani Saksi and Petra Ijäs contributed equally to this article
References
- 1.NASCET collaborators North American Symptomatic Carotid Endarteroctomy Trial. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.No author (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351:1379–1387 [PubMed]
- 3.Golledge JM, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.STR.31.3.774. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis. 2002;44:349–356. doi: 10.1053/pcad.2002.122475. [DOI] [PubMed] [Google Scholar]
- 5.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford Plaque Study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 6.Peeters W, Hellings WE, de Kleijn DP, de Vries JP, Moll FL, Vink A, Pasterkamp G. Carotid atherosclerotic plaques stabilize after stroke. Insights into the natural process of atherosclerotic plaque destabilization. Arterioscler Thromb Vasc Biol. 2009;29:128–133. doi: 10.1161/ATVBAHA.108.173658. [DOI] [PubMed] [Google Scholar]
- 7.Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. Stroke. 1994;25:304–308. doi: 10.1161/01.STR.25.2.304. [DOI] [PubMed] [Google Scholar]
- 8.Vemuganti R, Dempsey RJ. Carotid atherosclerotic plaques from symptomatic stroke patients share the molecular fingerprints to develop in a neoplastic fashion: a microarray analysis study. Neuroscience. 2005;131:359–374. doi: 10.1016/j.neuroscience.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee P, Suckling KE, James CH, Greaves DR, Patel L. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:1837–1844. doi: 10.1161/01.ATV.0000229695.68416.76. [DOI] [PubMed] [Google Scholar]
- 10.Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, Damas JK, Tunheim SH, Ueland T, Smith C, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 11.Agardh HE, Folkersen L, Ekstrand J, Marcus D, Swedenborg J, Hedin U, Gabrielsen A, Paulsson-Berne G. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med. 2010;269:200–210. doi: 10.1111/j.1365-2796.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 12.Slevin M, Elasbali AB, Miguel Turu M, Krupinski J, Badimon L, Gaffney J. Identification of differential protein expression associated with development of unstable human carotid plaques. Am J Pathol. 2006;168:1004–1021. doi: 10.2353/ajpath.2006.050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lepedda AJ, Cigliano A, Cherchi GM, Spirito R, Maggioni M, Carta F, Turrini F, Edelstein C, Scanu AM, Formato M. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis. 2009;203:112–118. doi: 10.1016/j.atherosclerosis.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ijäs P, Nuotio K, Saksi J, Lehtonen-Smeds EM, Soinne L, Saimanen E, Karjalainen-Lindsberg M, Salonen O, Sarna S, Tuimala J, et al. Microarray analysis reveals over-expression of CD163 and HO-1 in symptomatic carotid plaques. Arterioscler Thromb Vasc Biol. 2007;27:154–160. doi: 10.1161/01.ATV.0000251991.64617.e7. [DOI] [PubMed] [Google Scholar]
- 15.Nuotio K, Lindsberg PJ, Carpén O, Soinne L, Lehtonen-Smeds EM, Saimanen E, Lassila R, Sairanen T, Sarna S, Salonen O, et al. Adhesion molecule expression in symptomatic and asymptomatic carotid stenosis. Neurology. 2003;60:1890–1899. doi: 10.1212/01.wnl.0000065914.33177.9e. [DOI] [PubMed] [Google Scholar]
- 16.Lehtonen-Smeds EM, Mäyränpää M, Lindsberg PJ, Soinne L, Saimanen E, Jarvinen AA, Salonen O, Carpen O, Lassila R, Sarna S, et al. Carotid plaque mast cells associate with atherogenic serum lipids, high grade carotid stenosis and symptomatic carotid artery disease—results from the Helsinki Carotid Endarterectomy Study. Cerebrovasc Dis. 2005;19:291–301. doi: 10.1159/000084497. [DOI] [PubMed] [Google Scholar]
- 17.Soinne L, Saimanen E, Malmberg-Ceder K, Kovanen P, Lindsberg PJ, Kaste M, Lassila R. Association of the fibrinolytic system and hemorheology with symptoms in patients with carotid occlusive disease. Cerebrovasc Dis. 2005;20:172–179. doi: 10.1159/000087201. [DOI] [PubMed] [Google Scholar]
- 18.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 19.Stary H, Chandler A, Dinsmore R, Fuster V, Glagov S, Insull W, Rosenfeld M, Schwartz C, Wagner W, Wissler R. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 20.Nuotio K, Isoviita PM, Saksi J, Pitkämäki J, Ijäs P, Soinne L, Saimanen E, Salonen O, Kovanen PT, Kaste M, et al. Adipophilin expression is increased in symptomatic carotid atherosclerosis and co-localizes with red blood cells and cholesterol crystals. Stroke. 2007;38:1791–1798. doi: 10.1161/STROKEAHA.106.478867. [DOI] [PubMed] [Google Scholar]
- 21.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irizarry RA, Wu Z, Jaffee HA. Comparison of Affymetrix GeneChip expression measures. Bioinformatics. 2006;22:789–794. doi: 10.1093/bioinformatics/btk046. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Irizarry RA. Preprocessing of oligonucleotide array data. Nat Biotech. 2004;22:656–658. doi: 10.1038/nbt0604-656b. [DOI] [PubMed] [Google Scholar]
- 24.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–1380. doi: 10.1161/01.ATV.0000031341.84618.A4. [DOI] [PubMed] [Google Scholar]
- 25.Sitzer M, Muller W, Siebler M, Hort W, Kniemeyer H, Jancke L, Steinmetz H. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke. 1995;26:1231–1233. doi: 10.1161/01.STR.26.7.1231. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke. 2000;31:615–621. doi: 10.1161/01.STR.31.3.615. [DOI] [PubMed] [Google Scholar]
- 27.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI-initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 28.Gao P, Chen Z, Bao Y, Jiao L, Ling F. Correlation between carotid intraplaque hemorrhage and clinical symptoms—systematic review of observational studies. Stroke. 2007;38:2382–2390. doi: 10.1161/STROKEAHA.107.482760. [DOI] [PubMed] [Google Scholar]
- 29.Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, de Vries JP, Seldenrijk KA, De Bruin PC, Vink A, Velema E, de Kleijn DP, Pasterkamp G. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–50. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 30.Isoviita PM, Nuotio K, Saksi J, Turunen R, Ijäs P, Pitkäniemi J, Soinne L, Kaste M, Kovanen PT, Lindsberg PJ. An imbalance between CD36 and ABCA1 protein expression favors lipid accumulation in stroke-prone ulcerated carotid plaques. Stroke. 2010;2:389–393. doi: 10.1161/STROKEAHA.109.567636. [DOI] [PubMed] [Google Scholar]
- 31.Michel JB, Virmani R, Asrbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peeters W, de Kleijn DP, Vink A, van de Weg S, Schoneveld AH, Sze SK, van der Spek PJ, de Vries JP, Moll FL, Pasterkamp G. Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 812 kb)


