Abstract
Based on the recent literature and collective experience, an international consortium developed revised guidelines for the diagnosis of behavioural variant frontotemporal dementia. The validation process retrospectively reviewed clinical records and compared the sensitivity of proposed and earlier criteria in a multi-site sample of patients with pathologically verified frontotemporal lobar degeneration. According to the revised criteria, ‘possible’ behavioural variant frontotemporal dementia requires three of six clinically discriminating features (disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/compulsive behaviours, hyperorality and dysexecutive neuropsychological profile). ‘Probable’ behavioural variant frontotemporal dementia adds functional disability and characteristic neuroimaging, while behavioural variant frontotemporal dementia ‘with definite frontotemporal lobar degeneration’ requires histopathological confirmation or a pathogenic mutation. Sixteen brain banks contributed cases meeting histopathological criteria for frontotemporal lobar degeneration and a clinical diagnosis of behavioural variant frontotemporal dementia, Alzheimer’s disease, dementia with Lewy bodies or vascular dementia at presentation. Cases with predominant primary progressive aphasia or extra-pyramidal syndromes were excluded. In these autopsy-confirmed cases, an experienced neurologist or psychiatrist ascertained clinical features necessary for making a diagnosis according to previous and proposed criteria at presentation. Of 137 cases where features were available for both proposed and previously established criteria, 118 (86%) met ‘possible’ criteria, and 104 (76%) met criteria for ‘probable’ behavioural variant frontotemporal dementia. In contrast, 72 cases (53%) met previously established criteria for the syndrome (P < 0.001 for comparison with ‘possible’ and ‘probable’ criteria). Patients who failed to meet revised criteria were significantly older and most had atypical presentations with marked memory impairment. In conclusion, the revised criteria for behavioural variant frontotemporal dementia improve diagnostic accuracy compared with previously established criteria in a sample with known frontotemporal lobar degeneration. Greater sensitivity of the proposed criteria may reflect the optimized diagnostic features, less restrictive exclusion features and a flexible structure that accommodates different initial clinical presentations. Future studies will be needed to establish the reliability and specificity of these revised diagnostic guidelines.
Keywords: behavioural variant frontotemporal dementia, diagnostic criteria, frontotemporal lobar degeneration, FTD, pathology
Introduction
The behavioural variant of frontotemporal dementia (bvFTD) is a clinical syndrome characterized by a progressive deterioration of personality, social comportment and cognition. These changes result from frontotemporal lobar degeneration (FTLD) associated with a range of heterogeneous pathologies (Mackenzie et al., 2009, 2010). Despite recent advances in the characterization of bvFTD, the diagnosis of the syndrome remains challenging; while some patients are dismissed as ‘normal’ others may be misdiagnosed as suffering from psychiatric disorders or Alzheimer’s disease (Mendez et al., 1993, 2007; Varma et al., 1999). Early and accurate differential diagnosis of bvFTD is critical, as it has implications for heritability (Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998; Watts et al., 2004; Skibinski et al., 2005; Baker et al., 2006; Cruts et al., 2006; Kumar-Singh and Van Broeckhoven, 2007), prognosis (Rascovsky et al., 2005; Roberson et al., 2005; Chow et al., 2006), therapeutics (Swartz et al., 1997; Moretti et al., 2003; Pasquier et al., 2003; Lebert et al., 2004; Huey et al., 2006; Boxer and Boeve, 2007; Mendez, 2009) and environmental management of patients (Perry and Miller, 2001; Robinson, 2001; Talerico and Evans, 2001; Merrilees and Miller, 2003; Merrilees, 2007; Boutoleau-Bretonniere et al., 2008).
In the absence of definitive biomarkers, the diagnosis of bvFTD is dependent on clinical diagnostic criteria; in other words, the identification of the syndrome’s core or necessary symptoms. The publication of consensus criteria by Neary and colleagues (1998) was a major development in the field. These criteria are widely used in research and practice, but some limitations have become apparent. Among these are the ambiguity of behavioural descriptors and inflexibility in the application of criteria (i.e. the requirement that all five core features be manifest). Most importantly, a number of studies have established the relative insensitivity of these criteria in the early stages of bvFTD when disease-modifying treatments are likely to be most effective (Mendez and Perryman, 2002; Mendez et al., 2007; Rascovsky et al., 2007a; Piguet et al., 2009).
Based on the empirical knowledge accumulated in the past 12 years (Mendez and Perryman, 2002; Mendez et al., 2007; Rascovsky et al., 2007a; Piguet et al., 2009), the International Behavioural Variant FTD Criteria Consortium (FTDC) developed revised guidelines for the diagnosis of bvFTD. The FTDC is comprised of 46 members with extensive experience in bvFTD. After reviewing the world literature, the FTDC developed an initial draft of the bvFTD criteria. This document was further refined over 3 years through correspondence, a web-based interactive forum and in-person meetings. In this report, we propose a revision of diagnostic and research criteria for bvFTD and provide results of an autopsy-confirmed analysis of the sensitivity of these revised diagnostic guidelines.
Materials and methods
Participants
Sixteen brain banks with special interest in clinical assessments of patients with FTLD pathology were identified (see Supplementary Table 1 for participating sites). Sites were asked to select cases who met modified Mackenzie criteria for FTLD (Mackenzie et al., 2009, 2010) (Table 1), and were clinically diagnosed with bvFTD, Alzheimer’s disease, vascular dementia, dementia with Lewy bodies or other neurological or psychiatric conditions at presentation. Cases were excluded if they had concomitant pathology such as Alzheimer’s disease (defined as Braak > 2; CERAD plaque score > sparse), Lewy body disease or significant vascular pathology (defined as large vessel infarct or more than one lacune). Cases were also excluded if they presented with predominant primary progressive aphasia or extra-pyramidal syndromes (e.g. corticobasal syndrome or progressive supranuclear palsy). Patients with multiple syndromes (e.g. bvFTD and corticobasal syndrome) were only included in the study if they were considered bvFTD at initial presentation.
Table 1.
FTLD pathology glossary
| FTLD tau: frontotemporal lobar degeneration with tau immunoreactive inclusions. Subtypes include Pick’s disease, corticobasal degeneration, progressive supranuclear palsy, FTDP-17, sporadic multi-system tauopathy and argyrophilic grain disease |
| FTLD-TDP: frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions |
| FTLD-FUS: frontotemporal lobar degeneration with FUS immunoreactive inclusions (including cases formerly identified as aFTLD-U, FTLD-IF or basophilic inclusion body disease in which FUS immunohistochemistry is positive) |
| FTLD-UPS NOS: frontotemporal lobar degeneration with ubiquitin or P62 only immunoreactive inclusions (negative for all other proteins, as well as cases that have not yet been analysed with FUS or TDP-43) |
| FTLD-IF NOS: frontotemporal lobar degeneration with intermediate filament immunoreactive inclusions (FUS negative, as well as cases that have not yet been analysed with FUS) |
| BIBD NOS: basophilic inclusion body disease (FUS negative, as well as cases that have not yet been analysed with FUS) |
| FTLD-ni NOS: frontotemporal lobar degeneration without immunoreactive inclusions (negative for all proteins, as well as cases that have not yet been analysed with FUS) |
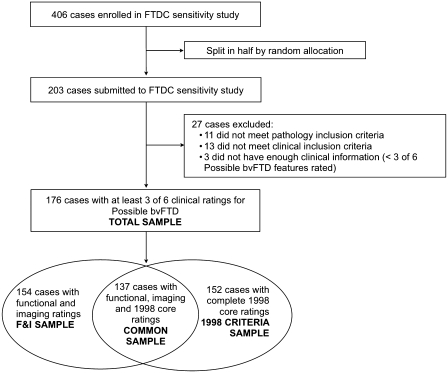
A description of participant selection can be seen in Fig. 1. A total of 406 cases were enrolled in the FTDC registry. Cases were split in half by random allocation to create an original and a replication set. The original set was designated as a sensitivity sample (n = 203), and the replication set was chosen for future specificity studies. The 203 cases in the original sample were reviewed in the context of the proposed FTDC criteria (Table 3). As a result, 11 had pathology exclusion criteria (e.g. concomitant pathology), 13 had clinical exclusion criteria (e.g. primary/predominant aphasic or extra pyramidal presentations) and three cases did not have enough clinical information for inclusion in the study (defined as <3 of the clinical ratings needed to be evaluated for ‘possible’ bvFTD). All cases in the total sample (n = 176) had enough clinical ratings to potentially meet criteria for possible bvFTD. Of the total sample, 154 cases had functional disability and neuroimaging ratings, and 152 cases had complete core ratings for established 1998 criteria. The common sample (n = 137) had sufficient information to potentially evaluate all bvFTD criteria (FTDC possible bvFTD, FTDC probable bvFTD and 1998 criteria for bvFTD). Clinical and demographical characteristics did not differ depending on the sample used (Table 2).
Figure 1.
Case selection and description of samples. bvFTD = behavioural variant FTD; F&I = functional disability and neuroimaging.
Table 3.
International consensus criteria for behavioural variant FTD (FTDC)
| I. Neurodegenerative disease |
| The following symptom must be present to meet criteria for bvFTD |
| A. Shows progressive deterioration of behaviour and/or cognition by observation or history (as provided by a knowledgeable informant). |
| II. Possible bvFTD |
| Three of the following behavioural/cognitive symptoms (A–F) must be present to meet criteria. Ascertainment requires that symptoms be persistent or recurrent, rather than single or rare events. |
| A. Early* behavioural disinhibition [one of the following symptoms (A.1–A.3) must be present]: |
| A.1. Socially inappropriate behaviour |
| A.2. Loss of manners or decorum |
| A.3. Impulsive, rash or careless actions |
| B. Early apathy or inertia [one of the following symptoms (B.1–B.2) must be present]: |
| B.1. Apathy |
| B.2. Inertia |
| C. Early loss of sympathy or empathy [one of the following symptoms (C.1–C.2) must be present]: |
| C.1. Diminished response to other people’s needs and feelings |
| C.2. Diminished social interest, interrelatedness or personal warmth |
| D. Early perseverative, stereotyped or compulsive/ritualistic behaviour [one of the following symptoms (D.1–D.3) must be present]: |
| D.1. Simple repetitive movements |
| D.2. Complex, compulsive or ritualistic behaviours |
| D.3. Stereotypy of speech |
| E. Hyperorality and dietary changes [one of the following symptoms (E.1–E.3) must be present]: |
| E.1. Altered food preferences |
| E.2. Binge eating, increased consumption of alcohol or cigarettes |
| E.3. Oral exploration or consumption of inedible objects |
| F. Neuropsychological profile: executive/generation deficits with relative sparing of memory and visuospatial functions [all of the following symptoms (F.1–F.3) must be present]: |
| F.1. Deficits in executive tasks |
| F.2. Relative sparing of episodic memory |
| F.3. Relative sparing of visuospatial skills |
| III. Probable bvFTD |
| All of the following symptoms (A–C) must be present to meet criteria. |
| A. Meets criteria for possible bvFTD |
| B. Exhibits significant functional decline (by caregiver report or as evidenced by Clinical Dementia Rating Scale or Functional Activities Questionnaire scores) |
| C. Imaging results consistent with bvFTD [one of the following (C.1–C.2) must be present]: |
| C.1. Frontal and/or anterior temporal atrophy on MRI or CT |
| C.2. Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT |
| IV. Behavioural variant FTD with definite FTLD Pathology |
| Criterion A and either criterion B or C must be present to meet criteria. |
| A. Meets criteria for possible or probable bvFTD |
| B. Histopathological evidence of FTLD on biopsy or at post-mortem |
| C. Presence of a known pathogenic mutation |
| V. Exclusionary criteria for bvFTD |
| Criteria A and B must be answered negatively for any bvFTD diagnosis. Criterion C can be positive for possible bvFTD but must be negative for probable bvFTD. |
| A. Pattern of deficits is better accounted for by other non-degenerative nervous system or medical disorders |
| B. Behavioural disturbance is better accounted for by a psychiatric diagnosis |
| C. Biomarkers strongly indicative of Alzheimer’s disease or other neurodegenerative process |
*As a general guideline ‘early’ refers to symptom presentation within the first 3 years (for further discussion see Supplementary material, Appendix 1).
bvFTD = behavioural variant FTD.
Table 2.
Mean (±SD) demographic characteristics by sample
| Total sample (n = 176) | Functional disability and neuroimaging sample (n = 154) | 1998 Criteria sample (n = 152) | Common sample (n = 137) | |
|---|---|---|---|---|
| Gender (F/M) | 72/104 | 65/89 | 64/88 | 60/77 |
| Age at onset | 58.1 (10.9) | 58.4 (11.1) | 57.8 (10.9) | 58.1 (11.1) |
| Age at initial evaluation | 61.5 (10.9) | 61.7 (11.0) | 61.3 (10.9) | 61.5 (11.0) |
| Age at death | 66.1 (11.6) | 66.4 (11.7) | 65.8 (11.6) | 65.8 (11.6) |
| Education | 14.2 (3.5) | 14.3 (3.4) | 14.2 (3.5) | 14.2 (3.5) |
| MMSE | 22.2 (7.0) | 22.5 (6.9) | 22.2 (7.1) | 22.3 (7.1) |
| Duration: onset–initial evaluation | 3.2 (2.7) | 3.2 (2.6) | 3.2 (2.6) | 3.3 (2.6) |
| Duration: onset–death | 7.8 (3.9) | 7.6 (3.9) | 7.7 (3.9) | 7.6 (3.9) |
| Duration: initial evaluation–death | 4.6 (3.3) | 4.4 (3.1) | 4.5 (3.3) | 4.3 (3.1) |
The classification was modified from existing FTLD criteria (Mackenzie et al., 2009, 2010) to accommodate cases with incomplete immunohistochemistry.
MMSE = Mini-Mental State Examination.
FTDC diagnostic and research criteria for behavioural variant frontotemporal dementia
The FTDC simplified the existing diagnostic criteria and attempted to focus on features that best distinguish bvFTD from psychiatric disorders, Alzheimer’s disease and other dementing conditions. Some of the major advances reflected in the new criteria include: (i) reduced number of diagnostic features; (ii) no arbitrary distinctions between core and supportive features; (iii) greater flexibility in how patients can meet diagnostic criteria; (iv) clearer operational definitions; (v) incorporation of genetic and neuroimaging findings; and (vi) diagnostic hierarchy (distinction between possible, probable or definite bvFTD with FTLD pathology depending on level of diagnostic certainty).
Table 3 presents the proposed FTDC criteria for behavioural variant FTD. Criteria for possible, probable and bvFTD with definite FTLD pathology are described in detail in Appendix 1.
Procedure
For the purpose of pilot validation, a structured criteria rating form was created. This form included general demographics, FTDC criteria (Table 3) and previously established 1998 criteria (referred to below as the 1998 criteria) (Neary et al., 1998). A summary of the 1998 criteria is provided in Supplementary Table 2. A complete version of the rating form is available from the authors.
Un-blinded neurologists or psychiatrists with expertise in bvFTD retrospectively reviewed patient charts from their respective sites. Any clinical information contained in the charts was considered, including history and clinical impressions, caregiver information, standard cognitive and behavioural measures, as well as laboratory and imaging findings. Raters used a web-based version of the criteria rating form to ascertain items of established (Neary et al., 1998) and proposed FTDC criteria at presentation. Individual features were rated as present if the feature was clearly present, absent if clearly absent and ‘don’t know’ if the information contained in the chart was insufficient to make a clear determination. Raters were also asked to estimate the time of symptom onset for FTDC behavioural features. No interrater reliability data were available, as criteria were ascertained by single raters.
In order to avoid exclusion of cases with ‘don’t know’ responses, we employed rules set a priori for fulfilment of criteria. Patients were considered to meet established 1998 criteria if all five core features were rated as present and no exclusion features were rated as present. Patients were considered to meet possible bvFTD if they met criteria for a neurodegenerative disease (i.e. progressive deterioration of behaviour and/or cognition) and presented with three of six possible bvFTD features with no exclusion features rated as present (i.e. medical or psychiatric conditions that could explain the pattern of behavioural or cognitive deficits). Patients met probable bvFTD if they met criteria for possible bvFTD, had functional disability and neuroimaging findings consistent with bvFTD, and had no biomarkers strongly indicative of Alzheimer’s disease or other degenerative process. Given that the entire sample met pathological criteria for FTLD, all cases that met FTDC criteria for possible bvFTD also met criteria for bvFTD with definite FTLD pathology.
The ethics committee at each participating centre approved the research programme.
Data analysis
SPSS 18 and STATA® were used for all statistical analyses. Demographic characteristics are reported as means and standard deviations or proportions when appropriate. Frequency of symptoms is reported as proportions. We compared the sensitivity of the FTDC and 1998 criteria in the common sample using statistical methods for matched binary data (McNemar’s test with each case as a matched pair). Sensitivity of criteria by demographical features was analysed using chi-square tests.
Results
Sample characteristics
All cases in the study (n = 176) met modified Mackenzie criteria for FTLD (see Table 1 for pathology glossary). Pathology classifications were as follows: 70 cases were classified as FTLD tau, 48 FTLD-TDP, 32 FTLD-UPS NOS, 17 FTLD-ni NOS, 6 FTLD-FUS, 1 FTLD-IF NOS and 2 cases with incomplete immunohistochemistry were classified as ‘other’ (FTLD-NOS). Within the tau-positive sample, some cases were specifically identified in the notes as corticobasal degeneration (n = 7), progressive supranuclear palsy (n = 4), argyrophilic grain disease (n = 1), one case with tangle-predominant pathology and argyrophilic grain disease, and one case with an unclassified tauopathy. One of the cases classified as FTLD-TDP had secondary pathological features of argyrophilic grain disease. Within the total sample, 46 cases (26.3%) had a positive family history of a similar primary dementia in a first-degree relative. Of 104 cases with genetic screening, 23 (22.1%) had a pathogenic mutation (16 cases with MAPT and seven cases with PGRN mutations).
The demographic characteristics of each sample can be seen in Table 2. The total sample was primarily Caucasian (96%) with a slight male predominance (59%). Patients were highly educated (14.2 years) and had mild dementia at initial evaluation (average Mini-Mental State Examination = 22.2). Of 172 cases with age of onset reported, 71% had onset before the age of 65 years (average age of onset = 58 years). The average survival from first evaluation was 3.2 years and from symptom onset was 7.8 years. Within the total sample, 26 cases (14.8%) developed features of motor neuron disease while 22 cases (12.5%) exhibited motor features similar to corticobasal syndrome or progressive supranuclear palsy. Some behavioural variant patients with FTD in the total sample demonstrated additional language features such as impaired word or object knowledge (20.4%), motor speech deficits (15.3%) and grammatical deficits in language production or comprehension (7.9%).
At initial presentation, 122/176 (69.3%) of cases received a clinical diagnosis of bvFTD, but this number decreased to 112/176 (63.6%) at the last evaluation. The second most common clinical diagnosis was Alzheimer’s disease in 26/176 (14.8%). First and last clinical diagnoses can be seen in Supplementary Table 3.
Sensitivity of FTDC criteria for behavioural variant frontotemporal dementia
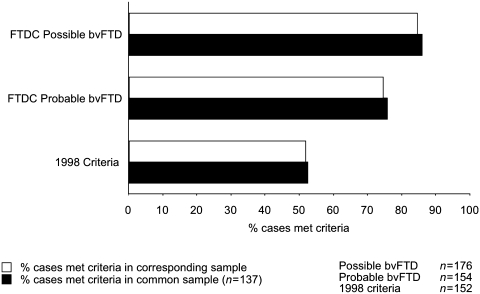
Sensitivity of FTDC and 1998 criteria can be seen in Fig. 2. Of 176 pathology-confirmed FTLD cases, 149 met FTDC criteria for possible bvFTD [sensitivity = 0.85, 95% confidence interval (95% CI) (0.79–0.90)]. Of 154 cases with functional disability and neuroimaging ratings, 115 met criteria for probable bvFTD [sensitivity = 0.75, 95% CI (0.68–0.82)]. In contrast, of 152 cases with complete ratings for 1998 criteria, 79 met criteria for bvFTD [sensitivity = 0.52, 95% CI (0.44–0.60)].
Figure 2.
Sensitivity of FTDC and 1998 criteria as per cent of cases that met criteria in the corresponding sample (white bars) or the common sample (black bars). bvFTD = behavioural variant FTD.
We compared the sensitivity of 1998 and FTDC criteria in the common sample using the McNemar’s test for matched binary data (with each case as a matched pair). This smaller sample includes patients with ratings for both FTDC and 1998 criteria, and is highly representative of the larger samples where patients were evaluated with FTDC criteria (Table 2). Of 137 cases in the common sample, 118 (86%) met criteria for possible bvFTD, 104 (76%) met criteria for probable bvFTD and only 72 (53%) cases met 1998 criteria for bvFTD. Of note, while 65 patients in the common sample failed to meet 1998 criteria, 34 of these cases were nevertheless diagnosed clinically with bvFTD, while six cases were diagnosed with FTD/motor neuron disease at initial presentation. Sensitivity differences between FTDC and 1998 criteria in the common sample were statistically significant (possible bvFTD versus 1998 criteria: McNemar’s Χ2 = 44.08, P < 0.0001; probable bvFTD versus 1998 criteria: McNemar’s Χ2 = 18.75, P < 0.0001). We focus below on the common sample, as both FTDC and 1998 criteria were ascertainable in this group, and these findings closely reflected observations in the larger samples.
Sensitivity of behavioural variant frontotemporal dementia criteria by demographic characteristics
Within the common sample, sensitivity rates for FTDC and 1998 criteria did not differ according to the presence or absence of tau pathology, pathogenic mutations or family history of a similar primary dementia. Both FTDC and 1998 criteria were more sensitive in cases with early onset (onset < 65 years) compared with cases with late onset of the disease (Supplementary Fig. 1). This difference was significant for possible bvFTD (0.92 versus 0.73, Χ2 = 8.4, P < 0.01), probable bvFTD (0.85 versus 0.54, Χ2 = 14.2, P < 0.001) and 1998 criteria (0.61 versus 0.32, Χ2 = 8.6, P < 0.01). Patients with early onset bvFTD had significantly higher rates of disinhibition, loss of sympathy and empathy, perseverative behaviours and imaging findings consistent with bvFTD.
Frequency of individual features
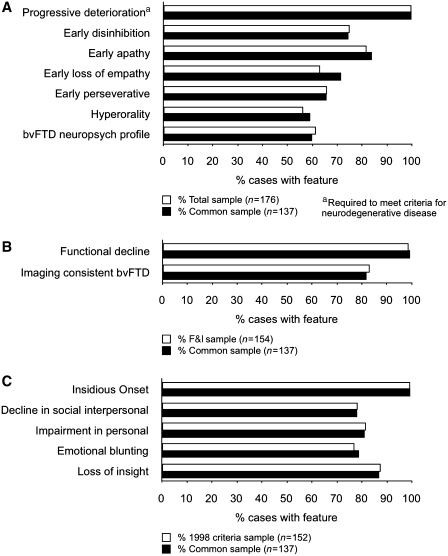
The frequency of individual diagnostic features for 1998 and FTDC criteria can be seen in Fig. 3. All cases in the common sample (137/137) had progressive deterioration of behaviour or cognition consistent with a neurodegenerative disease. Within the common sample, the frequency of possible bvFTD features ranged from 59% (hyperorality) to 84% (early apathy). We were able to ascertain whether these behavioural symptoms were present at onset in a subset of patients; this information can be seen in the Supplementary material, Appendix 1. Closer inspection of ratings for the neuropsychological criterion showed that 120/137 cases in the common sample had unambiguous ratings for a neuropsychological profile consistent with bvFTD (i.e. ‘yes’ or ‘no’ responses, indicating sufficient information to rate this criterion). Of these 120 cases, 114 had ‘deficits in executive tasks’, 89 had ‘relative sparing of episodic memory’ (compared with executive dysfunction) and 99 had ‘relative sparing of visuospatial skills’ (compared with executive deficits). In total, 82/120 cases (68%) had a complete neuropsychological profile most consistent with bvFTD (i.e. executive/generation deficits with relative sparing of memory or visuospatial functions). For probable bvFTD features, 82% of the cases had imaging findings consistent with bvFTD, while 99% exhibited functional decline by history or caregiver report. Within the common sample, individual core features for the 1998 criteria ranged from 78% (decline in social interpersonal conduct) to 99% (insidious onset and gradual progression). By comparison, Supplementary Fig. 2 shows the frequency of supportive features for the 1998 bvFTD criteria. Within the common sample, features such as decline in hygiene, mental rigidity and distractibility were present in 50–57% of cases. Fifty-nine per cent of the cases exhibited altered speech output, with specific language alterations ranging from mutism (13%) to perseveration of speech (35%). Physical signs were relatively uncommon and ranged from low and labile blood pressure (0.7%) to presence of primitive reflexes (26%).
Figure 3.
Frequency of individual features for (A) possible bvFTD, (B) probable bvFTD and (C) 1998 core criteria. Frequency is shown as percentage of cases in the corresponding sample (white bars) or the common sample (black bars). bvFTD = behavioural variant FTD; F&I = functional disability and neuroimaging.
Frequency of behavioural variant frontotemporal dementia exclusionary features
The presence of exclusionary features for FTDC and 1998 criteria can be seen in Fig. 4. In the common sample, 2/137 (1.5%) cases exhibited one or more exclusion features for possible bvFTD criteria. In these two cases, the pattern of cognitive and behavioural deficits was better accounted for by other non-degenerative or medical disorders. Of the 137 cases in the common sample, 26 (19%) presented one or more exclusion features for 1998 criteria. Of note, 15 cases (11%) presented with early, severe amnesia while nine cases (7%) exhibited spatial disorientation. Cases rated as having early, severe amnesia had an older age at onset compared with cases that did not exhibit this exclusion feature [age at onset: 64 versus 57 years, t(133), P < 0.05].
Figure 4.
Frequency of exclusionary features for (A) FTDC criteria and (B) 1998 criteria. Frequency is shown as percentage of cases in the corresponding sample (clear bars) or the common sample (black bars). Two cases were homozygous for the ApoE e4 allele, but ApoE status was not considered a strong biomarker for Alzheimer’s disease (AD).
Sensitivity of behavioural variant frontotemporal dementia by number of features
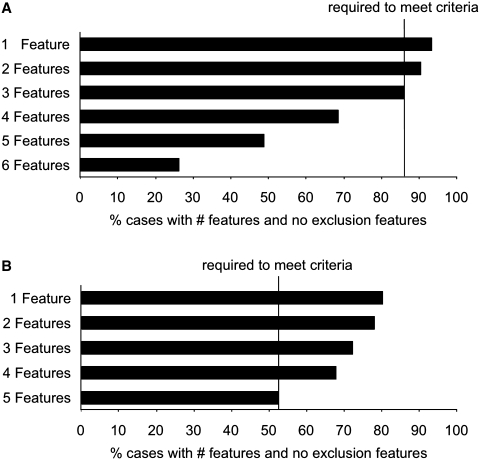
Diagnostic accuracy reflects a combination of diagnostic and exclusion features. This is summarized in Fig. 5, which shows the sensitivity of FTDC and 1998 criteria by number of features present. When taking exclusion features into account, 69% of the patients in the common sample met four of six features for possible bvFTD, 86% met three of six features for possible bvFTD (required to meet criteria) and 90% of cases met two of six features for possible bvFTD. In contrast, only 53% of cases met the five core features required to meet 1998 criteria when taking exclusion features into account.
Figure 5.
Sensitivity of FTDC and 1998 criteria by number of features for (A) FTDC possible behavioural variant FTD and (B) 1998 criteria (common sample, n = 137). Black bars show percent of cases with specified number of diagnostic features and no exclusion features.
Cases that failed to meet FTDC criteria
Within the common sample, 19 cases failed to meet FTDC criteria for possible bvFTD. A summary of observations related to criteria failure for possible bvFTD can be seen in Table 4. Patients who failed to meet criteria were significantly older than patients who met criteria (age at onset: 64 versus 57 years, P < 0.05; age at initial evaluation: 66 versus 61 years, P < 0.05). Six cases presented with early, severe amnesia and were diagnosed with probable Alzheimer’s disease at presentation (mean age at initial evaluation = 72). An additional three cases with rare pathologies (argyrophilic grain disease, argyrophilic grain disease and tangle-predominant pathology, argyrophilic grain disease and TAR DNA binding protein pathology) were significantly older (mean age at initial evaluation = 82 years), and two of three had significant episodic memory deficits. Although the requirement that patients exhibit three of six diagnostic features at presentation was based on prior experience of the Consortium, only six cases were falsely diagnosed because they had only two of six diagnostic features at presentation. Of these six cases, four were initially diagnosed as either ‘non-amnestic mild cognitive impairment’ or bvFTD, and two were diagnosed as bvFTD at last evaluation. Of the remaining cases, two presented with prominent delusions, one presented with prominent spatial disorientation (diagnosed with dementia NOS at presentation, bvFTD at last evaluation) and one case was a PGRN mutation carrier with a known family history. In total, 10/19 patients had marked memory problems at presentation. Failure to meet criteria for possible bvFTD was not due to insufficient information or ‘don’t know’ responses.
Table 4.
Observations related to criteria failure for possible behavioural variant FTD (n = 19/137)
| Observations related to criteria failure for possible bvFTD | Number of cases |
|---|---|
| Early severe amnesia/episodic memory impairments, diagnosed as Alzheimer’s disease at presentation | 6 |
| Cases with 2/6 diagnostic features at presentation, diagnosed as bvFTD or ‘non-amnestic MCI’ either at initial (n = 4) or last evaluation (n = 2) (two cases had ‘don’t know’ responses in 3/6 diagnostic features). | 6 |
| Unusual pathology: AGD (n = 1), AGD and TPSD (n = 1), AGD and TDP (n = 1) | 3 |
| Prominent delusions, diagnosed as depression/delusional disorder (n = 1), bvFTD (n = 1) at presentation | 2 |
| Presented with spatial disorientation, diagnosed as dementia NOS at presentation (bvFTD at last visit) | 1 |
| PGRN mutation with known family history, diagnosed as bvFTD at presentation (only one feature rated as ‘yes’) | 1 |
AGD = argyrophilic grain disease; MCI = mild cognitive impairment; PGRN = progranulin; TPSD = tangle predominant senile dementia.
bvFTD = behavioural variant FTD.
Of the 118 cases in the common sample that met criteria for possible bvFTD, 14 cases failed to meet criteria for probable bvFTD. All 14 cases had imaging findings inconsistent with bvFTD at presentation (e.g. no apparent lobar atrophy or significant posterior atrophy). Compared with cases who met the criteria for probable bvFTD, cases that failed to meet criteria were significantly older (age at onset: 63 versus 56 years, P < 0.05; age at initial evaluation: 67 versus 60 years, P < 0.05), and male predominant (12/14 males = 0.86 versus 54/104 males = 0.52, Χ2 = 5.7, P < 0.05).
Discussion
The proposed FTDC criteria are the result of a 3-year multinational effort to develop empirically derived diagnostic criteria for bvFTD. The present study found that FTDC criteria provide greater sensitivity than previously established criteria in a multi-site sample with known FTLD pathology. Of 137 cases where sufficient clinical data were available to evaluate both FTDC and previously established 1998 criteria, known as the common sample, 118 (86%) met criteria for possible bvFTD, and 104 (76%) met criteria for probable bvFTD. In contrast, the proportion of cases meeting 1998 criteria (53%) was significantly lower. These sensitivity rates were comparable with those found in the larger sample, in which either the FTDC or 1998 criteria could be evaluated. Thus, of 176 pathology-confirmed FTLD cases, 149 (85%) met FTDC criteria for possible bvFTD, while 113/154 (75%) with functional disability and neuroimaging ratings met criteria for probable bvFTD. The increased sensitivity of FTDC criteria is thought to reflect the optimized diagnostic features, less restrictive exclusion features and crucially, a flexible structure that accommodates variation in the symptom profile at presentation. Use of FTDC criteria for bvFTD diagnosis will improve identification of the syndrome, particularly in the earliest stages when disease-modifying therapies are most likely to be effective.
FTDC criteria
Three sets of bvFTD diagnostic criteria have been published over the past two decades that reflect our evolving knowledge about the presentation and progression of the disease (Brun et al., 1994; Neary et al., 1998; McKhann et al., 2001). Among these, most dementia centres adopted the 1998 consensus criteria as the norm for bvFTD diagnosis (Neary et al., 1998). Based on the accumulated experience with the 1998 criteria (Mendez and Perryman, 2002; Mendez et al., 2007; Rascovsky et al., 2007a; Piguet et al., 2009), the International Behavioural Variant FTD Criteria Consortium developed revised guidelines for the diagnosis of bvFTD. Recognizing that the optimum level of diagnostic certainty depends on the clinical and research requirements, the revised FTDC criteria are now structured as a diagnostic hierarchy. Diagnosis of possible bvFTD is based solely on the clinical syndrome and aims to identify patients at the mildest stages of disease. This classification relies on the flexible combination of three of six clinically discriminating features: disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/compulsive behaviours, hyperorality and a dysexecutive neuropsychological profile. Compared with earlier 1998 criteria, possible bvFTD eliminates the distinction between core and supportive features and significantly reduces the number of exclusionary features. Diagnosis of probable bvFTD is based on the clinical syndrome, plus demonstrable functional decline and the frontotemporal imaging findings that reflect the principal anatomical location of neurodegeneration in bvFTD. Furthermore, a diagnosis of probable bvFTD may be withheld if other biomarkers are strongly indicative of Alzheimer’s disease or other degenerative processes. This classification aims to capture patients with a high probability of underlying FTLD pathology and will be useful in studies where high diagnostic certainty is sought (such as clinical trials). The conclusive classification of bvFTD with definite FTLD pathology is limited to patients who exhibit the bvFTD clinical syndrome and who also have a pathogenic mutation or histopathological evidence of FTLD. Patients with the ‘definite’ diagnosis would usually be included in retrospective studies where pathology-proven FTLD cases are needed.
Possible and probable FTDC criteria are applied most usefully in the early stages of bvFTD, when there is less overlap with other FTLD phenotypes or neurodegenerative conditions. It may be useful in this context to distinguish between a primary behavioural syndrome using these criteria, a predominant aphasic syndrome using recently published criteria for primary progressive aphasia (Gorno-Tempini et al., 2011), or a predominant amnestic presentation using NIA-Alzheimer’s Association criteria for Alzheimer’s disease (McKhann et al., 2011).
Sensitivity of possible behavioural variant frontotemporal dementia
As a first step in the validation of bvFTD criteria, we compared the sensitivity of proposed FTDC and previously established 1998 criteria in a multi-site sample of patients with bvFTD with known FTLD pathology. This is the largest pathology-confirmed sample of patients with bvFTD reported to date, and sheds some light on the typical presentation of the syndrome. The clinical and demographic characteristics are similar to those of other large bvFTD samples (Johnson et al., 2005; Le Ber et al., 2006).
At initial evaluation, individual features for possible bvFTD were frequent, but not necessarily always present: in the total sample of 176 cases, apathy (84%) and disinhibition (76%) were the most common features, while hyperorality was the least common (59%). This is not surprising, as patients with bvFTD differ in their presentation, particularly at early stages of the disease. For example, while most patients with bvFTD exhibit both disinhibition and apathy well into their disease course, patients may initially present as primarily disinhibited or primarily apathetic (Le Ber et al., 2006). This variable presentation may reflect differences in the earliest localization of disease and/or underlying neuropathological features (Hu et al., 2007; Massimo et al., 2009). The flexible structure of possible bvFTD criteria attempts to account for this variability at initial presentation. Sensitivity of possible bvFTD was further enhanced by the use of less restrictive exclusionary features. Within the common sample, only two cases (1.5%) exhibited a pattern of deficits that was better explained by other medical conditions, and no cases were better explained by a psychiatric diagnosis. The specification of three core features for a possible diagnosis was based on the consortium experience but appears to be optimal. In the common sample, 86% of the patients had three of the six required clinical features. Increasing the number to four features decreased sensitivity to 69%, while relaxing the requirement to two features increased sensitivity only by 4%, with potential detriment to specificity. The question of the optimal criterion will need to be addressed in prospective studies that evaluate the combined sensitivity and specificity of the proposed FTDC criteria.
Within the common sample, we found a significant difference in sensitivity according to age of disease onset. The criteria for possible bvFTD were significantly more sensitive in cases with early onset (onset < 65 years of age) compared with those with late onset of the disease (0.92 versus 0.73, respectively). Compared with patients with early onset, patients with late onset bvFTD had significantly lower rates of disinhibition, loss of sympathy/empathy and perseverative, compulsive behaviours. The lower sensitivity of possible bvFTD in older patients may be due to the presence of unusual FTLD-spectrum pathologies or primarily amnestic presentations. Although included in the FTLD spectrum, cases with argyrophilic grain disease pathology (argyrophilic grain disease, argyrophilic grain disease and tangle-predominant pathology, argyrophilic grain disease and TAR DNA binding protein pathology) may not present with a typical behavioural syndrome. Further studies are required to examine the cognitive and behavioural characteristics of patients with rare FTLD pathologies such as these. On the other hand, the occurrence of amnestic presentations in this sample is not surprising, as marked anterograde amnesia has been documented as either the sole or dominant symptom in up to 10% of pathology-confirmed bvFTD cases (Hodges et al., 2004; Graham et al., 2005; Knopman et al., 2005; Piguet et al., 2009). The preponderance of primarily amnestic (versus behavioural) presentations in elderly subjects with bvFTD may be related to hippocampal sclerosis, for example, which was recently reported in 43% of FTLD cases with late onset of disease ( > 65 years of age) (Baborie et al., 2010). On a cautionary note, the preponderance of memory deficits in the elderly may bias overall clinical impressions by increasing the salience of prominent amnesia while downplaying the patient’s behavioural symptoms. Future prospective studies will need to confirm these preliminary observations regarding age differences in amnestic versus behavioural presentations.
Only six cases failed to be diagnosed as possible bvFTD because they exhibited only two of six diagnostic features. Prospective studies are needed to determine whether cases with two diagnostic features for possible behavioural variant FTD warrant increased vigilance for the eventual emergence of the typical bvFTD syndrome. Since a reduced number of diagnostic criteria may adversely affect specificity, prospective studies of the specificity of the FTDC criteria are required to examine the threshold number of features for diagnosis.
Comparison of FTDC and 1998 criteria
The consensus criteria published by Neary and colleagues (1998) greatly advanced the field of frontotemporal degenerations, and have been widely used in research and clinical practice. In an effort to further refine criteria by incorporation of recent empirical knowledge, the FTDC developed revised guidelines for the diagnosis of bvFTD. Both the 1998 and revised FTDC criteria rely on the presence of distinct clinical features for the diagnosis of bvFTD. A major difference, however, is that the 1998 criteria require the presence of all five core diagnostic features: insidious onset and gradual progression, early decline in personal and social interpersonal conduct, emotional blunting and loss of insight. Although individual core features are common at presentation, they are not ubiquitous. In the present sample, for example, the frequency of 1998 core features at initial presentation ranged from 78% for emotional blunting, to 99% for insidious onset and gradual progression. The ambiguity in behavioural descriptions (e.g. ‘emotional blunting’, ‘regulation of personal conduct’), and the need for inferences about a patient’s cognitive or emotional state (e.g. ‘loss of insight’), also have the potential to lower interrater reliability and the ultimate validity of these items for diagnosis (Rascovsky et al., 2007b). The 1998 criteria are further restricted by a large number of exclusion features (11 exclusion features and three relative exclusion features). Of 137 cases in the common sample, 26 (19%) presented one or more exclusion features for these diagnostic guidelines. Our observations show that the presence of early severe amnesia or spatial disorientation should not be exclusionary, and elimination of these exclusions improved the sensitivity of the FTDC criteria compared with the 1998 criteria. Exclusion based solely on impaired neuropsychological memory performance can lead to underdiagnosis of bvFTD (Hornberger et al., 2010), while spatial disorientation (when applied without reference to timing) may result in erroneous rejection of the diagnosis in patients who are in the late stages of their illness.
The strict, five-feature core requirement, coupled with the number and nature of exclusion features, may be responsible for the low sensitivity of 1998 criteria observed in the present study. When taking exclusion features into account, only 53% of patients met all five core features at initial presentation. Even when the number of core features was relaxed, only 72% of the patients met three of the five core features required for diagnosis. Furthermore, the three most common 1998 core features were insidious onset and gradual progression, loss of insight and impairment in regulation of personal conduct. These three features are very common in neurodegenerative diseases and may yield suboptimal discrimination when attempting to differentiate bvFTD from other forms of dementia (Ishii et al., 2009; Orfei et al., 2010; Starkstein et al., 2010).
The low sensitivity of 1998 criteria found in the present study mirrors the findings in recent retrospective studies. For instance, Mendez and Perryman (2002) examined the sensitivity of 1998 criteria in a sample of 53 patients with a clinical diagnosis of bvFTD and frontal hypoperfusion on SPECT. Only a third of these patients met 1998 criteria at presentation, but this number increased to 83% at a 2-year follow-up. This low initial sensitivity was replicated by the same authors in a sample of 134 patients with a suspected diagnosis of bvFTD (Mendez et al., 2007). Another retrospective study (Piguet et al., 2009) evaluated the sensitivity of 1998 criteria in a well-characterized cohort of 45 patients with bvFTD with a 3-year follow-up (18 with confirmed FTLD pathology). Only 58% of the patients in this sample met 1998 criteria for bvFTD at presentation. In contrast to sensitivity rates reported in retrospective studies, a prospective study (Pijnenburg et al., 2008) found a much higher sensitivity of 1998 criteria for bvFTD (79%), but there was no pathological confirmation in the majority of cases. Of note, diagnostic features in the above study were ascertained by a caregiver questionnaire about the 1998 clinical features and patients were diagnosed based on 1998 criteria (with 1-year clinical follow-up as gold standard). As expected, the restrictions that lower the sensitivity of 1998 criteria also lead to increased levels of specificity. This is particularly true when attempting to discriminate bvFTD from Alzheimer’s disease or other dementing conditions. In studies with dementia comparison groups, the specificity of 1998 criteria ranged from 90 to 100% (Mendez et al., 2007; Pijnenburg et al., 2008).
Sensitivity of probable behavioural variant frontotemporal dementia
The designation of probable bvFTD by the FTDC criteria restricts diagnosis to patients with demonstrable functional decline and typical bvFTD anatomical findings. These criteria are particularly suited for studies where high diagnostic certainty is required (such as clinical trials). Although 86% of patients in the common sample met criteria for possible bvFTD, only 76% of cases met criteria for probable bvFTD. Patients who failed to meet criteria for probable bvFTD were significantly older (age at onset: 63 versus 56; age at initial evaluation: 67 versus 60), and all 14 cases had imaging findings inconsistent with bvFTD at presentation (e.g. no apparent lobar atrophy or significant posterior atrophy). Although disproportionate atrophy in medial frontal, orbital–insular and anterior temporal regions may help distinguish bvFTD from other conditions (Frisoni et al., 1996; Rosen et al., 2002a; Varma et al., 2002; Grossman et al., 2004; Boccardi et al., 2005; Short et al., 2005; Whitwell and Jack, 2005; Bocti et al., 2006; Perry et al., 2006; Du et al., 2007; Mendez et al., 2007; Richards et al., 2008; Schroeter et al., 2008; Seeley et al., 2008; Davies et al., 2009; Kipps et al., 2009a; Lindberg et al., 2009; Whitwell et al., 2009), this imaging pattern is not necessarily present in all cases or at very early stages of disease (Perry et al., 2006). In fact, structural imaging in the form of frontal or anterior temporal lobe atrophy has been reported in 50–64% of cases with bvFTD (Knopman et al., 2005; Mendez et al., 2007; Pijnenburg et al., 2008). Low sensitivity of structural imaging may be particularly related to age at disease onset. A recent study of pathology-confirmed FTLD cases showed that, while a majority of presenile cases (onset < 65) showed moderate to severe frontotemporal atrophy and ventricular dilation at autopsy, only 12/30 (40%) of elderly patients showed severe frontotemporal atrophy (Baborie et al., 2010). Compared with structural imaging, functional imaging changes (e.g. predominant frontotemporal hypometabolism or hypoperfusion in SPECT or PET, or perfusion changes observed with arterial spin labelling MRI) may provide additional sensitivity (Mendez et al., 2007; Hu et al., 2010), suggesting that behavioural and functional abnormalities may precede structural imaging changes in bvFTD. Of interest, there was a striking male predominance in cases that failed the imaging requirements for probable bvFTD compared with those who did (12/14 males = 0.86 versus 54/104 males). The reasons behind this gender difference are unclear, but may relate to ascertainment bias or greater reliance on imaging features to diagnose females with ambiguous behavioural profiles.
Strengths and caveats
The present study is the result of a multinational effort to devise empirically derived criteria for bvFTD, and represents the largest pathology-confirmed bvFTD sample reported to date. Although the study design makes our findings representative and generalizable, some caveats of the study should be kept in mind. The greatest limitation of the present study was the absence of appropriate neurological or psychiatric comparison groups to assess specificity of the FTDC criteria. In our stepwise approach to criteria development, we are pursuing the strategy of evaluating the specificity of FTDC criteria once sensitivity has been established. Validation of any diagnostic criteria is an iterative process, and we acknowledge that the FTDC criteria may require revisions in light of future specificity findings. Unfortunately, appropriate specificity studies may require a prospective design with considerable time requirements. Constructing a suitable comparison group retrospectively to estimate specificity is challenging and prone to bias for several reasons. First, the sample of pathologically proven non-FTLD cases would have to be very large. Second, it is likely that information relevant to the FTDC criteria would never have been collected in cases where FTLD was not suspected clinically. For example, information about the core behavioural symptoms characteristic of bvFTD is generally not recorded in patients with typical amnesic Alzheimer’s disease or other forms of dementia. Patients with the phenocopy syndrome also present problems. These patients are behaviourally indistinguishable from patients with true bvFTD when the 1998 criteria are applied (Hornberger et al., 2009; Kipps et al., 2009a). Phenocopy cases should be distinguishable in that they do not have functional decline or imaging changes. Given these factors, it is possible that specificity could be erroneously under or over-estimated. In order to assess specificity properly, a prospective study of a large number of unselected patients with dementia should be carried out in which the elements of the FTDC criteria are sought at the time of initial diagnosis. Ideally, such a study should have independent biomarker confirmation of the pathological diagnosis, a very considerable logistic undertaking.
The use of autopsy-confirmed cases ensures that our patients had FTLD pathology, but we acknowledge that retrospective autopsy-based samples can be prone to selection bias. It should be noted, however, that most participating brain banks were associated with Alzheimer’s centres or memory clinics treating a range of degenerative conditions where autopsy is generally pursued for all types of dementia. While individuals erroneously diagnosed with a psychiatric illness may have been less likely to undergo autopsy, in our experience, it is unlikely for patients with dementia to retain a primary psychiatric diagnosis late in their disease course. Another caveat of the present study is the ascertainment of diagnostic features based on retrospective and unblinded review of records. Although raters were instructed to rate features as positive only when clearly present, a priori knowledge of the underlying pathology may have sensitized raters to features consistent with bvFTD. Conversely, some FTDC features were not known at the time of patient evaluation, so the retrospective nature of the study may have underestimated the true frequency of such diagnostic criteria (e.g. loss of sympathy or empathy). The variability of information across centres may also contribute to low frequency rates, particularly when evaluating features such as imaging and neuropsychological profiles. Prospective studies that include standardized tests, questionnaires and imaging parameters may help elucidate the utility of the revised FTDC criteria. Finally, while we relied on easily observable features with clear operational definitions, prospective studies with multiple, blinded raters with different levels of expertise will be needed to determine the reliability of the FTDC criteria.
Summary
In summary, early and accurate diagnosis of bvFTD is crucial for the appropriate care of patients afflicted with this devastating disorder. In the absence of definitive biomarkers, diagnosis of bvFTD should be made on the basis of sensitive clinical criteria coupled with diagnostic methods that are practical and easily available. Even as sensitive and specific biomarkers for FTLD become a reality, definition of the bvFTD clinical syndrome is important for routine screening, as well as optimal management of patients and their families. The proposed FTDC criteria provide a sensitive standard for bvFTD diagnosis, allowing for early recognition of the syndrome when disease-modifying therapies are expected to be most effective. Future reliability and specificity studies will ultimately clarify the relative strength of these revised diagnostic guidelines.
Funding
National Institutes of Health (P01-AG17586, R01-NS44266, P01-AG32953, R01-AG15116, P50-AG016574, P01-AG019724, P50-AG023501, R01-AG034499-02); Department of Health Services grant from the state of California (CA DHS 07-65807).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- bvFTD
behavioural variant frontotemporal dementia
- FTDC
International Behavioural Variant FTD Criteria Consortium
- FTLD
frontotemporal lobar degeneration
- SPECT
single-photon emission computed tomography
Appendix 1: FTDC diagnostic and research criteria for behavioural variant frontotemporal dementia
I. Neurodegenerative disease
In order to meet criteria for any bvFTD diagnosis, the patient must show a progressive deterioration of behaviour and/or cognition by observation or history (as provided by a knowledgeable informant). This core symptom aims to distinguish bvFTD from acute medical events or stable conditions such as long-standing psychiatric disease.
II. Possible bvFTD
The diagnosis of possible bvFTD is based on personality, social comportment and cognitive features that discriminate bvFTD from other conditions. While it is important to interpret diagnostic features of a case in the clinical context, ratings of behavioural features can be difficult and potentially open to observer bias. As such, we encourage ratings that are based on overt behaviours, as opposed to inferences about a patient’s cognitive or emotional state. For quantification of these behaviours, scales such as the Neuropsychiatric Inventory (Cummings et al., 1994), the Cambridge Behavioural Inventory (Bozeat et al., 2000; Wedderburn et al., 2008) or the Frontal Behavioural Inventory (Kertesz et al., 2000) are available to guide behavioural ratings. For some patients, standard psychiatric evaluation will be required. Determination of a cognitive profile should be based on formal neuropsychological testing. Tests of social cognition, assessing emotion, theory of mind and decision-making can provide further objective markers of cognitive dysfunction (Gregory et al., 2002; Snowden et al., 2003; Rosen et al., 2004b; Lough et al., 2006; Eslinger et al., 2007, 2011; Torralva et al., 2009a; Kipps et al., 2009b). However, these are not yet widespread in clinical practice and have not therefore been incorporated into the neuropsychological criterion for bvFTD.
In order to meet criteria for possible bvFTD, three of the following behavioural or cognitive symptoms (A–F) must be present. We selected this threshold to accommodate individual differences in clinical presentation. Ascertainment requires that symptoms be persistent or recurrent, rather than single or rare events. As a general guideline ‘early’ refers to symptom presentation within the first 3 years (for further discussion see Supplementary material, Appendix 1).
A. Early behavioural disinhibition
Early behavioural disinhibition is a hallmark feature of the bvFTD clinical syndrome. Many comparative studies show that disinhibition discriminates bvFTD from Alzheimer’s disease, dementia with Lewy bodies and vascular dementia (Brun et al., 1994; Barber et al., 1995; Levy et al., 1996; Mendez et al., 1998; Hirono et al., 1999; Bozeat et al., 2000; Kertesz et al., 2000; Bathgate et al., 2001; Rosen et al., 2002b; De Deyn et al., 2005; Srikanth et al., 2005; de Vugt et al., 2006; Blair et al., 2007; Heidler-Gary et al., 2007; Liscic et al., 2007; Rankin et al., 2008). Disinhibition may present as one of the following (A.1–A.3):
A.1. Socially inappropriate behaviour
Examples of behaviours that violate social norms include inappropriately approaching, touching or kissing strangers, verbal or physical aggression, public nudity or urination, inappropriate sexual acts and criminal behaviour (such as theft or shoplifting).
A.2. Loss of manners or decorum
This category includes a range of behaviours that violate social graces. Examples include inappropriate laughter, cursing or loudness, offensive jokes or opinions, or crude or sexually explicit remarks. Patients may also display a general lack of etiquette (e.g. failing to wait in line, eating with mouth open), loss of respect for interpersonal space and a lack of response to social cues (e.g. patient will continue talking despite other’s attempts to end a conversation). Some bvpatients with FTD exhibit poor hygiene or grooming (e.g. wearing malodorous, stained, torn or inappropriate clothing) or impolite physical behaviours (e.g. flatulence, scratching or fondling private parts, picking teeth, belching or spitting).
A.3. Impulsive, rash or careless actions
The revised criteria acknowledge that not all behavioural disinhibition leads to obvious breaches in social or interpersonal conduct; in fact, it can manifest as impulsive behaviours that may or may not be performed in a social context. These include reckless driving, new-onset gambling, stealing (usually food or ‘shiny’ objects), buying or selling objects without regard for consequences, or indiscriminate sharing of personal information (e.g. credit card information, social security number).
B. Early apathy or inertia
Apathy/inertia is the most common initial symptom in bvFTD (Diehl-Schmid et al., 2006; Le Ber et al., 2006; Mendez et al., 2008a), and appears to be more severe and pervasive in bvFTD than in other dementias (Levy et al., 1996; Kertesz et al., 2000; Boone et al., 2003; Liu et al., 2004; Engelborghs et al., 2005; Perri et al., 2005; Srikanth et al., 2005; de Vugt et al., 2006; Jenner et al., 2006; Shinagawa et al., 2006; Blair et al., 2007; Chow et al., 2009). In order to meet this criterion, one of the following symptoms (B.1–B.2) must be present:
B.1. Apathy
Apathy is defined as a loss of motivation, drive or interest (Robert et al., 2009). It can manifest as passivity or lack of spontaneity. The patient may lack initiative and cease to engage in important or previously rewarding activities (e.g. job, hobbies).
B.2. Inertia
Inertia refers to decreased initiation of behaviour (i.e. the patient requires prompts or cues to initiate or continue routine activities). For example, it may be reported that a patient requires specific directives to start and finish brushing his teeth, or that a patient no longer starts or sustains conversation.
C. Early loss of sympathy or empathy
Loss of empathy refers to an inability to read the emotional expressions of others or imagine their experiences (Rankin et al., 2006). It is a common feature at initial presentation, and is often coupled with indifference and a general decrease in social engagement (Le Ber et al., 2006). This feature is especially useful in the differentiation of bvFTD from Alzheimer’s disease (Barber et al., 1995; Kertesz et al., 2000; Boone et al., 2003; Rankin et al., 2005; Mendez et al., 2006). In everyday life, loss of sympathy or empathy may present as one of the following (C.1–C.2):
C.1. Diminished responsiveness to other people’s needs and feelings
A positive rating on this feature should be based on specific examples that reflect a lack of understanding or indifference to the feelings of others—e.g. hurtful comments or inexplicable disregard for others pain or distress.
C.2. Diminished social interest, interrelatedness or personal warmth
While the preceding feature referred to overt behaviours that denote a marked loss of empathy, this feature refers to a more general decline in social engagement, with emotional detachment, coldness, lack of eye contact, etc. Relatives and friends might experience the patient as uncharacteristically distant (e.g. no longer touches, hugs or seeks out their company).
D. Early perseverative, stereotyped or compulsive/ritualistic behaviour
Perseverative, stereotyped or compulsive behaviours have been added to the revised criteria, as they are commonly observed in pathology confirmed cases (Ames et al., 1994), and consistently discriminate bvFTD from other primary dementias (Miller et al., 1997; Hirono et al., 1999; Bozeat et al., 2000; Kertesz et al., 2000; Bathgate et al., 2001; Shigenobu et al., 2002; Nyatsanza et al., 2003; Liu et al., 2004; Mendez et al., 2005; Srikanth et al., 2005; de Vugt et al., 2006; Shinagawa et al., 2006; Blair et al., 2007). A positive rating on this feature can occur if the patient exhibits any one of the following (D.1–D.3):
D.1. Simple repetitive movements
These movements include tapping, clapping, rubbing, scratching, picking at skin or clothing, humming, rocking, throat clearing, pursing of lips or lip smacking.
D.2. Complex, compulsive or ritualistic behaviours
Examples include counting and cleaning rituals, collecting or hoarding, checking, repetitive trips to the bathroom (without need), ordering objects and walking fixed routes. Pacing (without a compulsive quality) should not be included, as it can occur in other primary dementias or as a psychotropic medication effect.
D.3. Stereotypy of speech
These are single words, phrases or entire themes or stories that the patient habitually repeats despite their lack of communicative value.
E. Hyperorality and dietary changes
Changes in dietary and eating behaviour are common manifestations of bvFTD (Passant et al., 2005; Diehl-Schmid et al., 2006), and can range from altered food preferences to oral exploration of inedible objects. Although this feature is shared with other FTLD syndromes (Snowden et al., 2001; Ikeda et al., 2002; Liscic et al., 2007; Whitwell et al., 2007), dietary changes consistently discriminate bvFTD from Alzheimer’s disease (Miller et al., 1997; Bozeat et al., 2000; Bathgate et al., 2001; Ikeda et al., 2002; Rosen et al., 2002b; Liu et al., 2004; Srikanth et al., 2005; Jenner et al., 2006; Blair et al., 2007; Mendez et al., 2008b). This combined feature can present as one of the following symptoms (E.1–E.3):
E.1. Altered food preferences
In the context of bvFTD, this change in food habits usually presents as carbohydrate cravings (particularly sweets), or food fads (i.e. rigid, stereotyped or idiosyncratic food preferences).
E.2. Binge eating, increased consumption of alcohol or cigarettes
Patients consume excessive amounts of food and continue to eat despite (in some cases) acknowledging satiety (Woolley et al., 2007). Some patients exhibit new, resumed or compulsive smoking or ingestion of alcohol.
E.3. Oral exploration or consumption of inedible objects
In extreme cases, hyperorality may manifest as oral exploration, chewing or ingestion of inedible objects, a feature consistent with the Kluver-Bucy syndrome (Mendez and Foti, 1997).
F. Neuropsychological profile: executive/generation deficits with relative sparing of memory and visuospatial functions
The neuropsychological profile of bvFTD is now treated as a criterion in its entirety. Features such as ‘early and severe amnesia’ and ‘spatial disorientation’ (poor spatial localization and disorientation in highly familiar surroundings) cease to be exclusion criteria, as that would disqualify a significant proportion of patients with bvFTD. Some studies have demonstrated marked anterograde amnesia in pathologically confirmed cases (Graham et al., 2005; Knopman et al., 2005; Piguet et al., 2009), while ‘spatial disorientation’ without reference to time from disease onset may erroneously reject patients in the late stages of their illness. Although deficits in specific cognitive functions alone are unlikely to reliably differentiate bvFTD from other conditions (Hutchinson and Mathias, 2007), the overall pattern of impairments (specifically, relative sparing of memory and visuospatial functions in comparison to executive dysfunction) may aid in differential diagnosis (for review see Grossman, 2002; Wittenberg et al., 2008). Determination of a cognitive profile should be based on formal neuropsychological testing. In order to meet this criterion, patients must present with all three of the following features (F.1–F.3):
F.1. Deficits in executive tasks
Patients with bvFTD often present with deficits in executive function, a term that encompasses complex cognitive abilities such as working memory, planning, generation, abstraction, problem solving and mental flexibility. In order to meet this criterion, the patient must demonstrate cognitive impairment on at least one standardized test of executive ability (defined as performance at or below the fifth percentile compared with age- and education-matched norms). Although patients with bvFTD may perform within normal limits on traditional executive function tests (e.g. Wisconsin Card Sorting Test, Stroop), they consistently fail verbal and non-verbal generation tasks, and may show deficits in planning, mental flexibility, response inhibition and reversal learning (Lindau et al., 1998; Hodges et al., 1999; Perry and Hodges, 2000; Rascovsky et al., 2002, 2008; Slachevsky et al., 2004; Perri et al., 2005; Walker et al., 2005; Heidler-Gary et al., 2007; Hornberger et al., 2008; Huey et al., 2009; Krueger et al., 2009; Libon et al., 2009; Mendez et al., 2009; Torralva et al., 2009a, b). The presence of errors in the performance of various cognitive tests (e.g. perseverations or rule violations) is considered an item of this criterion, as it can aid in the differential diagnosis of bvFTD (Kramer et al., 2003; Thompson et al., 2005; Libon et al., 2007b; Carey et al., 2008).
F.2. Relative sparing of episodic memory
Preservation of episodic memory relative to executive dysfunction, can be valuable in differential diagnosis, particularly when the distinction involves bvFTD and Alzheimer’s disease (Elfgren et al., 1994; Pachana et al., 1996; Lindau et al., 1998; Perry and Hodges, 2000; Rascovsky et al., 2002; Kramer et al., 2003; Rosen et al., 2004a; Walker et al., 2005; Heidler-Gary et al., 2007; Libon et al., 2007a, b; Giovagnoli et al., 2008). This relative preservation can be observed in both verbal and non-verbal domains, and is most evident when memory tests lack a heavy retrieval or executive burden (e.g. long list of words, reproduction of complex figures).
F.3. Relative sparing of visuospatial skills
Most patients with bvFTD retain the ability to navigate their environment, copy simple and complex line drawings, assemble blocks and judge spatial positions until very late in their disease (Elfgren et al., 1994; Mendez et al., 1996, 2009; Miller et al., 1997; Rascovsky et al., 2002, 2008; Perri et al., 2005; Giovagnoli et al., 2008). When evaluating patients with known executive impairments, care should be taken to avoid complex constructional tasks with heavy executive demands.
III. Probable bvFTD
The diagnosis of probable bvFTD is based on functional and imaging findings that discriminate this disorder from other dementias, psychiatric disorders and non-degenerative conditions such as the phenocopy syndrome. Individuals with a phenocopy syndrome may have identical clinical features to those with bvFTD, but the phenocopy syndrome is not progressive: functional abilities are preserved and imaging abnormalities are absent (Davies et al., 2006; Kipps et al., 2007b, 2009a; Mioshi et al., 2009; Piguet et al., 2009). The aetiology of ‘phenocopy’ cases remains unknown (Hornberger et al., 2009; Piguet et al., 2011). Given their good long-term prognosis, it seems less likely that they have a neurodegenerative disorder. Although some authors speculate that phenocopy cases may fit the autism–Asperger’s spectrum or psychiatric disorder, there is currently no published evidence to support this claim (Piguet et al., 2011). In order to meet criteria for probable bvFTD, a patient must first meet criteria for possible bvFTD (A), plus both of the following (B and C):
B. Exhibits significant functional decline
Patients with bvFTD typically present with moderate to severe disability, even at early stages of the disease (Rosen et al., 2004a; Rascovsky et al., 2005; Mioshi et al., 2007). Even though formal neuropsychological testing may reveal little cognitive difficulty, these patients cannot maintain gainful employment or live independently. In order to meet criteria for probable bvFTD, this functional decline must be demonstrated by caregiver report or instruments that measure basic and instrumental activities of daily living [e.g. Clinical Dementia Rating Scale (CDR), Functional Activities Questionnaire (FAQ), Disability Assessment for Dementia (DAD), Assessment of Motor or Process Skills (AMPS), (Pfeffer et al., 1982; Doble et al., 1997; Morris, 1997)].
C. Imaging results consistent with behavioural variant FTD
In order for this criterion to be met, one of the following (C.1–C.2) must be present:
C.1. Frontal and/or anterior temporal atrophy on MRI or CT
The few studies that have explored the utility of structural changes in the diagnosis of individual cases (Varma et al., 2002; Kipps et al., 2007a; Mendez et al., 2007) have been largely consistent with observations from group studies indicating that disproportionate frontal, insular or anterior temporal lobe atrophy (or combination thereof) may help distinguish bvFTD from healthy individuals, non-progressive behavioural syndromes and other dementias (Frisoni et al., 1996; Rosen et al., 2002a; Grossman et al., 2004; Boccardi et al., 2005; Short et al., 2005; Whitwell and Jack, 2005; Bocti et al., 2006; Perry et al., 2006; Du et al., 2007; Richards et al., 2008; Schroeter et al., 2008; Seeley et al., 2008; Davies et al., 2009; Kipps et al., 2009a; Lindberg et al., 2009; Whitwell et al., 2009). While MRI is preferred to CT, it should be noted that structural changes are not necessarily present in all cases or at very early stages of disease (Perry et al., 2006). Follow-up studies are sometimes useful to demonstrate that frontal and anterior temporal atrophy is progressive, particularly in older patients.
C.2. Frontal and/or anterior temporal hypoperfusion or hypometabolism on PET or SPECT
Functional imaging studies such as PET or SPECT have been shown to increase the sensitivity of detection of bvFTD (Mendez et al., 2007; Womack et al., 2011). As in the case of structural imaging, few studies have investigated the utility of PET/SPECT changes at the individual level (Read et al., 1995; McNeill et al., 2007; Mendez et al., 2007; Kipps et al., 2009a). While PET is preferred to SPECT, functional imaging studies using visual ratings or group-averaged findings suggest that predominant frontal or frontotemporal hypometabolism or hypoperfusion may aid in the differential diagnosis of bvFTD (Starkstein et al., 1994; Read et al., 1995; Charpentier et al., 2000; Sjogren et al., 2000; Salmon et al., 2003, 2006; Diehl et al., 2004; Franceschi et al., 2005; Jeong et al., 2005; Le Ber et al., 2006; Nakano et al., 2006; Peters et al., 2006; McNeill et al., 2007; Schroeter et al., 2008).
IV. bvFTD with definite FTLD pathology
This conclusive diagnostic category is based on the presence of a known pathogenic mutation or histopathological evidence of FTLD (on biopsy or autopsy). In order to meet criteria for bvFTD with definite FTLD pathology, a patient must present with possible or probable bvFTD (A), plus one of the one of the following (B–C):
B. Histopathological evidence of FTLD on biopsy or at post-mortem
Although distinguished by the selective degeneration of frontal and anterior temporal lobes, FTLD is histopathologically heterogeneous. Recent consensus criteria (Mackenzie et al., 2009, 2010) classify FTLD on the basis of the presumed molecular defect (i.e. the protein abnormality presumed to be pathogenic or most characteristic). In general terms, FTLD can be assigned to one of three major molecular subgroups: FTLD with tau inclusions (FTLD tau), FTLD with TAR DNA-binding protein inclusions (FTLD-TDP) or cases immunoreactive for fused in sarcoma protein (FTLD-FUS). For the purpose of this study, consensus criteria have been modified to accommodate cases with incomplete immunohistochemistry. Please refer to Table 1 for pathology glossary and descriptions.
C. Presence of a known pathogenic mutation
Under the new framework, an individual presenting with the bvFTD clinical syndrome and a verified pathogenic mutation is now considered to meet criteria for bvFTD with definite FTLD pathology. Autosomal dominant bvFTD may be caused by mutations in several genes, including those encoding the microtubule-associated protein tau (MAPT) (Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998), charged multi-vesicular body protein 2B (CHMP2B) (Skibinski et al., 2005), valosin-containing protein (VCP) (Watts et al., 2004) and progranulin (PGRN) (Baker et al., 2006; Cruts et al., 2006).
V. Exclusionary criteria for bvFTD
In order to diagnose bvFTD, one should exclude medical, neurological and psychiatric conditions that could otherwise account for the behavioural and cognitive changes presented by the patient. A diagnosis of bvFTD may not be given if the patient presents with any one of the following (A–B):
A. Pattern of deficits is better accounted for by other non-degenerative nervous system or medical disorders
These comprise a variety of conditions including delirium, cerebrovascular disease, cerebellar disorder, trauma, infections, systemic disorders (e.g. hypothyroidism) or substance-induced conditions.
B. Behavioural disturbance is better accounted for by a psychiatric diagnosis
The behavioural syndrome should not be better accounted for by psychiatric conditions such as depression, schizophrenia, bipolar disorder, late-onset psychosis or a pre-existing personality disorder.
In the absence of definitive biomarkers, criterion C can be positive for possible bvFTD but must be negative for probable bvFTD. To clarify, a diagnosis of probable bvFTD does not require biomarker or genetic screening for Alzheimer’s disease or other degenerative conditions. However, when available, the presence of sensitive and specific biomarkers indicative of other degenerative conditions will preclude a diagnosis of probable bvFTD.
C. Biomarkers strongly indicative of Alzheimer’s disease or other neurodegenerative process
These include pathogenic mutations for other conditions (e.g. Presenilin, APP), extensive amyloid related radioligand binding (e.g. PIB) (Rabinovici et al., 2007), or the presence of sensitive and specific CSF markers (Dubois et al., 2000; Rowe et al., 2007; Shaw et al., 2009). Biomarker studies are rapidly evolving, and this criterion will require revisions once sensitive and specific biomarkers are determined for Alzheimer’s disease and other degenerative conditions. Similarly, a positive FTLD biomarker criterion should be added to the diagnosis of probable bvFTD once additional FTLD biomarkers become available.
References
- Ames D, Cummings JL, Wirshing WC, Quinn B, Mahler M. Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci. 1994;6:100–13. doi: 10.1176/jnp.6.2.100. [DOI] [PubMed] [Google Scholar]
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn DJ, Richardson A, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2010;121:365–71. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Barber R, Snowden JS, Craufurd D. Frontotemporal dementia and Alzheimer's disease: retrospective differentiation using information from informants. J Neurol, Neurosurg Psychiatry. 1995;59:61–70. doi: 10.1136/jnnp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Blair M, Kertesz A, Davis-Faroque N, Hsiung GY, Black SE, Bouchard RW, et al. Behavioural measures in frontotemporal lobar dementia and other dementias: The Utility of the Frontal Behavioural Inventory and the Neuropsychiatric Inventory in a National Cohort Study. Dement Geriatr Cogn Disord. 2007;23:306–15. doi: 10.1159/000101908. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Bocti C, Rockel C, Roy P, Gao F, Black SE. Topographical patterns of lobar atrophy in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:364–72. doi: 10.1159/000091838. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Swartz R, Lu P, Lee A. Relationship between positive and negative symptoms and neuropsychological scores in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc. 2003;9:698–709. doi: 10.1017/S135561770395003X. [DOI] [PubMed] [Google Scholar]
- Boutoleau-Bretonniere C, Vercelletto M, Volteau C, Renou P, Lamy E. Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;25:272–7. doi: 10.1159/000117394. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord. 2007;21:S79–87. doi: 10.1097/WAD.0b013e31815c345e. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol, Neurosurgery Psychiatry. 2000;69:178–86. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Englund B, Gustafson L, Passant U, Mann DMA, Neary D, et al. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol, Neurosurg Psychiatry. 1994;57:416–18. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Damon J, Halabi C, Dean D, Delis DC, et al. Discriminant validity and neuroanatomical correlates of rule monitoring in frontotemporal dementia and Alzheimer's disease. Neuropsychologia. 2008;46:1081–7. doi: 10.1016/j.neuropsychologia.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Charpentier P, Lavenu I, Defebvre L, Duhamel A, Lecouffe P, Pasquier F, et al. Alzheimer's disease and frontotemporal dementia are differentiated by discriminant analysis applied to (99 m)Tc HmPAO SPECT data. J Neurol Neurosurg Psychiatry. 2000;69:661–3. doi: 10.1136/jnnp.69.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow TW, Binns MA, Cummings JL, Lam I, Black SE, Miller BL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66:888–93. doi: 10.1001/archneurol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow TW, Hynan LS, Lipton AM. MMSE scores decline at a greater rate in frontotemporal degeneration than in AD. Dement Geriatr Cogn Disord. 2006;22:194–9. doi: 10.1159/000094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol. 2006;63:1627–31. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR. Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology. 2009;51:491–503. doi: 10.1007/s00234-009-0521-z. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Engelborghs S, Saerens J, Goeman J, Marien P, Maertens K, et al. The Middelheim Frontality Score: a behavioural assessment scale that discriminates frontotemporal dementia from Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:70–9. doi: 10.1002/gps.1249. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: a comparison between Alzheimer's disease and frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Forstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging. 2004;25:1051–6. doi: 10.1016/j.neurobiolaging.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Pohl C, Perneczky R, Forstl H, Kurz A. Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;22:352–7. doi: 10.1159/000095625. [DOI] [PubMed] [Google Scholar]
- Doble SE, Fisk JD, MacPherson KM, Fisher AG, Rockwood K. Measuring functional competence in older persons with Alzheimer's disease. Int Psychogeriatr. 1997;9:25–38. doi: 10.1017/s1041610297004171. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, et al. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–66. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Elfgren C, Brun A, Gustafson L, Johanson A, Minthon L, Passant U, et al. Neuropsychological tests as discriminators between dementia of Alzheimer's type and Frontotemporal dementia. Int J Geriatr Psychiatry. 1994;9:635–42. [Google Scholar]
- Engelborghs S, Maertens K, Nagels G, Vloeberghs E, Marien P, Symons A, et al. Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry. 2005;20:1028–37. doi: 10.1002/gps.1395. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2011;23:74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, et al. Oops! Resolving social dilemmas in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2007;78:457–60. doi: 10.1136/jnnp.2006.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57:216–25. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Beltramello A, Geroldi C, Weiss C, Bianchetti A, Trabucchi M. Brain atrophy in frontotemporal dementia. J Neurol, Neurosurg Psychiatry. 1996;61:157–65. doi: 10.1136/jnnp.61.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Erbetta A, Reati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer's disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Davies R, Xuereb J, Halliday G, Kril J, Creasey H, et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain. 2005;128:597–605. doi: 10.1093/brain/awh348. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125:752–64. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Grossman M. Frontotemporal dementia: a review. J Int Neuropsychol Soc. 2002;8:566–83. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Heidler-Gary J, Gottesman R, Newhart M, Chang S, Ken L, Hillis AE. Utility of behavioral versus cognitive measures in differentiating between subtypes of frontotemporal lobar degeneration and Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;23:184–93. doi: 10.1159/000098562. [DOI] [PubMed] [Google Scholar]
- Hirono N, Mori E, Tanimukai S, Kazui H, Hashimoto M, Hanihara T, et al. Distinctive neurobehavioral features among neurodegenerative dementias. J Neuropsychiatry and Clinical Neurosci. 1999;11:498–503. doi: 10.1176/jnp.11.4.498. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Annals Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Ward R, Garrard P, Bak T, Perry R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology. 1999;13:31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–9. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Kipps C, Hodges JR. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008;71:1481–8. doi: 10.1212/01.wnl.0000334299.72023.c8. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Shelley BP, Kipps CM, Piguet O, Hodges JR. Can progressive and non-progressive behavioural variant frontotemporal dementia be distinguished at presentation? J Neurol Neurosurg Psychiatry. 2009;80:591–3. doi: 10.1136/jnnp.2008.163873. [DOI] [PubMed] [Google Scholar]
- Hu WT, Mandrekar JN, Parisi JE, Knopman DS, Boeve BF, Petersen RC, et al. Clinical features of pathologic subtypes of behavioral–variant frontotemporal dementia. Arch Neurol. 2007;64:1611–6. doi: 10.1001/archneur.64.11.1611. [DOI] [PubMed] [Google Scholar]
- Hu WT, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75:881–8. doi: 10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Goveia EN, Paviol S, Pardini M, Krueger F, Zamboni G, et al. Executive dysfunction in frontotemporal dementia and corticobasal syndrome. Neurology. 2009;72:453–9. doi: 10.1212/01.wnl.0000341781.39164.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AD, Mathias JL. Neuropsychological deficits in frontotemporal dementia and Alzheimer’s disease: a meta-analytic review. J Neurol Neurosurg Psychiatry. 2007;78:917–28. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:371–6. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Weintraub N, Mervis JR. Apathy: a common psychiatric syndrome in the elderly. J Am Med Dir Assoc. 2009;10:381–93. doi: 10.1016/j.jamda.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Jenner C, Reali G, Puopolo M, Silveri MC. Can cognitive and behavioural disorders differentiate frontal variant-frontotemporal dementia from Alzheimer's disease at early stages? Behav Neurol. 2006;17:89–95. doi: 10.1155/2006/812760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. 18F-FDG PET Findings in Frontotemporal Dementia: An SPM Analysis of 29 Patients. J Nucl Med. 2005;46:233–9. [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–30. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460–8. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Davies RR, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement Geriatr Cogn Disord. 2007a;23:334–42. doi: 10.1159/000100973. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR, Fryer TD, Nestor PJ. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009a;132:2566–78. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009b;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Fryer TD, Hodges JR. Behavioural variant frontotemporal dementia: not all it seems? Neurocase. 2007b;13:237–47. doi: 10.1080/13554790701594870. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol. 2005;57:480–8. doi: 10.1002/ana.20425. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73:349–55. doi: 10.1212/WNL.0b013e3181b04b24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Van Broeckhoven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–14. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, et al. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–65. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dementia Geriatr Cogn Disord. 2004;17:355–9. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Arch Neurol. 1996;53:687–90. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Massimo L, Moore P, Coslett HB, Chatterjee A, Aguirre GK, et al. Screening for frontotemporal dementias and Alzheimer’s disease with the Philadelphia Brief Assessment of Cognition: a preliminary analysis. Dement Geriatr Cogn Disord. 2007a;24:441–7. doi: 10.1159/000110577. [DOI] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73:535–42. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007b;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Lindau M, Almkvist O, Johansson SE, Wahlund LO. Cognitive and behavioral differentiation of frontal lobe degeneration of the non-Alzheimer's type and Alzheimer's Disease. Dementia Geriatr Cogn Disord. 1998;9:205–13. doi: 10.1159/000017048. [DOI] [PubMed] [Google Scholar]
- Lindberg O, Ostberg P, Zandbelt BB, Oberg J, Zhang Y, Andersen C, et al. Cortical morphometric subclassification of frontotemporal lobar degeneration. AJNR Am J Neuroradiol. 2009;30:1233–9. doi: 10.3174/ajnr.A1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscic RM, Storandt M, Cairns NJ, Morris JC. Clinical and psychometric distinction of frontotemporal and Alzheimer dementias. Arch Neurol. 2007;64:535–40. doi: 10.1001/archneur.64.4.535. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–8. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–8. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–8. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill R, Sare GM, Manoharan M, Testa HJ, Mann DM, Neary D, et al. Accuracy of single-photon emission computed tomography in differentiating frontotemporal dementia from Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2007;78:350–5. doi: 10.1136/jnnp.2006.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF. Frontotemporal dementia: therapeutic interventions. Front Neurol Neurosci. 2009;24:168–78. doi: 10.1159/000197896. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Cherrier M, Perryman KM, Pachana N, Miller BL, Cummings JL. Frontotemporal dementia versus Alzheimer’s disease: differential cognitive features. Neurology. 1996;47:1189–94. doi: 10.1212/wnl.47.5.1189. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Foti DJ. Lethal hyperoral behaviour from the Kluver-Bucy syndrome. J Neurol Neurosurg Psychiatry. 1997;62:293–4. doi: 10.1136/jnnp.62.3.293-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Lauterbach EC, Sampson SM. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci. 2008a;20:130–49. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Licht EA, Shapira JS. Changes in Dietary or Eating Behavior in Frontotemporal Dementia Versus Alzheimer's Disease. Am J Alzheimers Dis Other Demen. 2008b;23:280–5. doi: 10.1177/1533317507313140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, McMurtray AM, Licht EA, Saul RE. Frontal-executive versus posterior-perceptual mental status deficits in early-onset dementias. Am J Alzheimers Dis Other Demen. 2009;24:220–7. doi: 10.1177/1533317509332626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, McMurtray A, Licht E, Shapira JS, Saul RE, Miller BL. The scale for emotional blunting in patients with frontotemporal dementia. Neurocase. 2006;12:242–6. doi: 10.1080/13554790600910375. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Perryman KM. Neuropsychiatric features of frontotemporal dementia: evaluation of consensus criteria and review. J Neuropsychiatry Clin Neurosci. 2002;14:424–9. doi: 10.1176/jnp.14.4.424. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Perryman KM, Miller BL, Cummings JL. Behavioral differences between frontotemporal dementia and Alzheimer's disease: a comparison on the BEHAVE-AD rating scale. Int Psychogeriatr. 1998;10:155–62. doi: 10.1017/s1041610298005262. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Selwood A, Mastri AR, Frey WH. Pick’s disease versus Alzheimer’s disease: a comparison of clinical characteristics. Neurology. 1993;43:289–92. doi: 10.1212/wnl.43.2.289. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, McMurtray A, Licht E, Miller BL. Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol. 2007;64:830–5. doi: 10.1001/archneur.64.6.830. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, Miller BL. Stereotypical movements and frontotemporal dementia. Mov Disord. 2005;20:742–5. doi: 10.1002/mds.20465. [DOI] [PubMed] [Google Scholar]
- Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2007;21:S64–9. doi: 10.1097/WAD.0b013e31815bf774. [DOI] [PubMed] [Google Scholar]
- Merrilees JJ, Miller BL. Long-term care of patients with frontotemporal dementia. J Am Med Dir Assocn. 2003;4:S162–4. doi: 10.1097/01.JAM.0000095366.91533.22. [DOI] [PubMed] [Google Scholar]
- Miller BL, Ikonte C, Ponton M, Levy M, Boone K, Darby A, et al. A study of the Lund-Manchester research criteria for frontotemporal dementia: clinical and single-photon emission CT correlations. Neurology. 1997;48:937–42. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Kipps CM, Dawson K, Mitchell J, Graham A, Hodges JR. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology. 2007;68:2077–84. doi: 10.1212/01.wnl.0000264897.13722.53. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Kipps CM, Hodges JR. Activities of daily living in behavioral variant frontotemporal dementia: differences in caregiver and performance-based assessments. Alzheimer Dis Assoc Disord. 2009;23:70–6. doi: 10.1097/wad.0b013e318182d293. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49:13–9. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9:173–6. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- Nakano S, Asada T, Yamashita F, Kitamura N, Matsuda H, Hirai S, et al. Relationship between antisocial behavior and regional cerebral blood flow in frontotemporal dementia. Neuroimage. 2006;32:301–6. doi: 10.1016/j.neuroimage.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nyatsanza S, Shetty T, Gregory C, Lough S, Dawson K, Hodges JR. A study of stereotypic behaviours in Alzheimer's disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74:1398–402. doi: 10.1136/jnnp.74.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfei MD, Varsi AE, Blundo C, Celia E, Casini AR, Caltagirone C, et al. Anosognosia in mild cognitive impairment and mild Alzheimer's disease: frequency and neuropsychological correlates. Am J Geriatr Psychiatry. 2010;18:1133–40. doi: 10.1097/JGP.0b013e3181dd1c50. [DOI] [PubMed] [Google Scholar]
- Pachana NA, Boone KB, Miller BL, Cummings JL, Berman N. Comparison of neuropsychological functioning in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc. 1996;2:505–10. doi: 10.1017/s1355617700001673. [DOI] [PubMed] [Google Scholar]
- Passant U, Elfgren C, Englund E, Gustafson L. Psychiatric symptoms and their psychosocial consequences in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S15–8. doi: 10.1097/01.wad.0000183084.22562.5a. [DOI] [PubMed] [Google Scholar]
- Pasquier F, Fukui T, Sarazin M, Pijnenburg Y, Diehl J, Grundman M, et al. Laboratory investigations and treatment in frontotemporal dementia. Annals of Neurology. 2003;54(Suppl 5):S32–5. doi: 10.1002/ana.10573. [DOI] [PubMed] [Google Scholar]
- Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, et al. Alzheimer's disease and frontal variant of frontotemporal dementia – a very brief battery for cognitive and behavioural distinction. J Neurol. 2005;252:1238–44. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Graham A, Williams G, Rosen H, Erzinclioglu S, Weiner M, et al. Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord. 2006;22:278–87. doi: 10.1159/000095128. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology. 2000;54:2277–84. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Miller BL. Behavior and treatment in frontotemporal dementia. Neurology. 2001;56:S46–51. doi: 10.1212/wnl.56.suppl_4.s46. [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21:373–9. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Pfeffer RJ, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–29. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–72. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Shelley BP, Kipps CM, Hodges JR. Sensitivity of current criteria for the diagnosis of behavioral variant frontotemporal dementia. Neurology. 2009;72:732–7. doi: 10.1212/01.wnl.0000343004.98599.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg YA, Mulder JL, van Swieten JC, Uitdehaag BM, Stevens M, Scheltens P, et al. Diagnostic accuracy of consensus diagnostic criteria for frontotemporal dementia in a memory clinic population. Dement Geriatr Cogn Disord. 2008;25:157–64. doi: 10.1159/000112852. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–25. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, O'Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Santos-Modesitt W, Kramer JH, Pavlic D, Beckman V, Miller BL. Spontaneous social behaviors discriminate behavioral dementias from psychiatric disorders and other dementias. J Clin Psychiatry. 2008;69:60–73. doi: 10.4088/jcp.v69n0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007a;21:S14–8. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Ross L, Salmon D, Mendez M, Ziegler R, Farias S, et al. Inter-rater reliability of diagnostic criteria for Frontotemporal Dementia (FTD): Findings from the California Non-AD Diagnostic Reliability Consortium. Neurology. 2007b;68(Suppl 1):A354. [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Galasko D. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:373–83. doi: 10.1017/S135561770808051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology. 2002;58:1801–8. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Lipton A, Leverenz JB, DeCarli C, Jagust W, et al. Rate of progression differs in frontotemporal dementia and Alzheimer's disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- Read SL, Miller BL, Mena I, Kim R, Itabashi H, Darby A. SPECT in dementia: clinical and pathological correlation. J Am Geriatr Soc. 1995;43:1243–47. doi: 10.1111/j.1532-5415.1995.tb07400.x. [DOI] [PubMed] [Google Scholar]
- Richards BA, Chertkow H, Singh V, Robillard A, Massoud F, Evans AC, et al. Patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Neurobiol Aging. 2008;30:1626–36. doi: 10.1016/j.neurobiolaging.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65:719–25. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24:98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Robinson KM. Rehabilitation applications in caring for patients with Pick's disease and frontotemporal dementias. Neurology. 2001;56:S56–8. doi: 10.1212/wnl.56.suppl_4.s56. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002a;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Hartikainen KM, Jagust W, Kramer JH, Reed BR, Cummings JL, et al. Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology. 2002b;58:1608–15. doi: 10.1212/wnl.58.11.1608. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Narvaez JM, Hallam B, Kramer JH, Wyss-Coray C, Gearhart R, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alz Dis Assoc Disord. 2004a;18:202–7. [PubMed] [Google Scholar]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, Levenson RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord. 2004b;17:277–81. doi: 10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Salmon E, Garraux G, Delbeuck X, Collette F, Kalbe E, Zuendorf G, et al. Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. Neuroimage. 2003;20:435–40. doi: 10.1016/s1053-8119(03)00346-x. [DOI] [PubMed] [Google Scholar]
- Salmon E, Kerrouche N, Herholz K, Perani D, Holthoff V, Beuthien-Baumann B, et al. Decomposition of metabolic brain clusters in the frontal variant of frontotemporal dementia. Neuroimage. 2006;30:871–8. doi: 10.1016/j.neuroimage.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia–a meta-analysis. Neurobiol Aging. 2008;29:418–26. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenobu K, Ikeda M, Fukuhara R, Maki N, Hokoishi K, Nebu A, et al. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiatry Res. 2002;110:175–87. doi: 10.1016/s0165-1781(02)00094-x. [DOI] [PubMed] [Google Scholar]
- Shinagawa S, Ikeda M, Fukuhara R, Tanabe H. Initial symptoms in frontotemporal dementia and semantic dementia compared with Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:74–80. doi: 10.1159/000090139. [DOI] [PubMed] [Google Scholar]
- Short RA, Broderick DF, Patton A, Arvanitakis Z, Graff-Radford NR. Different patterns of magnetic resonance imaging atrophy for frontotemporal lobar degeneration syndromes. Arch Neurol. 2005;62:1106–10. doi: 10.1001/archneur.62.7.1106. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Gustafson L, Wikkelso C, Wallin A. Frontotemporal dementia can be distinguished from Alzheimer's disease and subcortical white matter dementia by an anterior-to-posterior rCBF-SPET ratio. Dementia Geriatr Cogn Dis. 2000;11:275–85. doi: 10.1159/000017250. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol. 2004;61:1104–7. doi: 10.1001/archneur.61.7.1104. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol, Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, Craufurd D, et al. Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia. 2003;41:688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–41. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer's disease, vascular dementia and frontotemporal dementia. J Neurol Sci. 2005;236:43–8. doi: 10.1016/j.jns.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Brockman S, Bruce D, Petracca G. Anosognosia is a significant predictor of apathy in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2010;22:378–83. doi: 10.1176/jnp.2010.22.4.378. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Migliorelli R, Teson A, Teson A, Sabe L, Vasquez S, et al. Specificity of changes in cerebral blood in patients with frontal lobe dementia. J Neurol, Neurosurg Psychiatry. 1994;57:790–96. doi: 10.1136/jnnp.57.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clinical Psychiatry. 1997;58:212–6. [PubMed] [Google Scholar]
- Talerico KA, Evans LK. Responding to safety issues in frontotemporal dementias. Neurology. 2001;56:S52–5. doi: 10.1212/wnl.56.suppl_4.s52. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Stopford CL, Snowden JS, Neary D. Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:920–7. doi: 10.1136/jnnp.2003.033779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009a;132:1299–309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Lopez P, Manes F. INECO Frontal Screening (IFS): a brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc. 2009b;15:777–86. doi: 10.1017/S1355617709990415. [DOI] [PubMed] [Google Scholar]
- Varma AR, Adams W, Lloyd JJ, Carson KJ, Snowden JS, Testa HJ, et al. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer's disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand. 2002;105:261–9. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol, Neurosurg Psychiatry. 1999;66:184–8. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AJ, Meares S, Sachdev PS, Brodaty H. The differentiation of mild frontotemporal dementia from Alzheimer's disease and healthy aging by neuropsychological tests. Int Psychogeriatr. 2005;17:57–68. doi: 10.1017/s1041610204000778. [DOI] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, et al. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry. 2008;79:500–3. doi: 10.1136/jnnp.2007.122028. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR., Jr Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top Magn Reson Imaging. 2005;16:409–25. doi: 10.1097/01.rmr.0000245457.98029.e1. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage. 2007;35:207–13. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev. 2008;18:91–102. doi: 10.1007/s11065-008-9056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack KB, Diaz-Arrastia R, Aizenstein HJ, Arnold SE, Barbas NR, Boeve BF, et al. Temporoparietal Hypometabolism in Frontotemporal Lobar Degeneration and Associated Imaging Diagnostic Errors. Arch Neurol. 2011;68:329–37. doi: 10.1001/archneurol.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–33. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.