Abstract
The occurrence of spontaneous seizures in mesial temporal lobe epilepsy (MTLE) is preceded by a latent phase that provides a time window for identifying and treating patients at risk. However, a reliable biomarker of epileptogenesis has not been established and the underlying processes remain unclear. Growing evidence suggests that astrocytes contribute to an imbalance between excitation and inhibition in epilepsy. Here, astrocytic and neuronal neurotransmitter metabolism was analyzed in the latent phase of the kainate model of MTLE in an attempt to identify epileptogenic processes and potential biomarkers. Fourteen days after status epilepticus, [1-13C]glucose and [1,2-13C]acetate were injected and the hippocampal formation, entorhinal/piriform cortex, and neocortex were analyzed by 1H and 13C magnetic resonance spectroscopy. The 13C enrichment in glutamate, glutamine, and γ-aminobutyric acid (GABA) from [1-13C]glucose was decreased in all areas. Decreased GABA content was specific for the hippocampal formation, together with a pronounced decrease in astrocyte-derived [1,2-13C]GABA and a decreased transfer of glutamine for the synthesis of GABA. Accumulation of branched-chain amino acids combined with decreased [4,5-13C]glutamate in hippocampal formation could signify decreased transamination via branched-chain aminotransferase in astrocytes. The results point to astrocytes as major players in the epileptogenic process, and 13C enrichment of glutamate and GABA as potential biomarkers.
Keywords: 13C MRS, astrocytes, epileptogenesis, kainate, metabolism, neurons
Introduction
Mesial temporal lobe epilepsy (MTLE) is the most common cause of pharmacoresistant seizures in adults, because 60% to 80% of patients experience seizures despite antiepileptic drug therapy (Semah et al, 1998; Stephen et al, 2001). The patient history often involves an initial precipitating injury early in life, followed by a latent phase of several years before spontaneous seizures occur (Mathern et al, 1995). The latent phase could hold a potential for identifying and treating patients at risk, but a reliable biomarker of epileptogenesis, that is, the development of MTLE, has yet to be established and the underlying pathophysiological processes are still unclear. However, perturbed energy and neurotransmitter metabolism seem to be involved, and a growing amount of data suggest that astrocytes are of special importance. In humans, it has been shown that the concentration of the excitatory neurotransmitter glutamate increases extracellularly in epileptic foci before and during seizures (During and Spencer, 1993). Furthermore, the astrocytic enzyme glutamine synthetase (GS), responsible for converting glutamate to glutamine, is downregulated in sclerotic hippocampi (Eid et al, 2004) and the cycling of glutamate to glutamine, and back, is impaired (Petroff et al, 2002). These changes in glutamate handling are considered to lead to hyperexcitability in epileptogenic areas. However, the underlying mechanisms of altered neurotransmitter metabolism in MTLE are unclear, and its significance in epileptogenesis is difficult to assess in humans. A study investigating the chronic phase of the kainate (KA) model of MTLE in rats has shown decreased turnover of glutamate and perturbed mitochondrial metabolism in both neurons and astrocytes in epileptogenic hippocampal formations (Alvestad et al, 2008). An earlier study using animals in the latent phase of the same model demonstrated increased glutamate turnover, but here whole-brain samples were analyzed, making it impossible to discern metabolic changes in epileptogenic hippocampus from non-epileptogenic regions (Muller et al, 2000). Detailed analysis of astrocytic and neuronal metabolism of energy substrates and neurotransmitters in different brain regions of experimental animals during the latent phase may yield a better understanding of the epileptogenic process and thus point to potential biomarkers and targets for antiepileptogenic treatment.
In the present study, the glutamate agonist KA was used to model MTLE in rats. Injection of KA induces status epilepticus (SE), followed by a latent phase with no behavioral seizures. After a few weeks, spontaneous recurrent seizures occur, thus modeling the clinical course of epileptogenesis and also many pathological features of human MTLE (Ben-Ari, 1985). Brain metabolism during the latent phase was studied using 1H and 13C magnetic resonance spectroscopy (MRS) and high-performance liquid chromatography. Combined with injection of 13C-labeled energy substrates, 13C MRS allows tracking of multiple metabolic pathways in and between neurons and astrocytes, because of differentiated distribution of transporters, enzymes, and metabolites into different compartments (Sonnewald and Kondziella, 2003). Subsequent to injections with [1-13C]glucose and [1,2-13C]acetate, samples were obtained from rats 14 days after KA-induced SE, and from control rats. The brain regions that were sampled were the hippocampal formation (the typical epileptogenic region), entorhinal and piriform cortices (areas of propagation), and neocortex (non-epileptogenic region). We also compared our findings with published results from the chronic phase to address their role in epileptogenesis.
Materials and methods
Animal Experiments
All animals were treated in agreement with the European Convention (ETS 123 of 1986) and all experimental procedures were approved by the Norwegian Animal Welfare Committee. Animals were maintained under standard laboratory conditions with food and water ad libitum, at a room temperature of 22°C, air humidity of 66%, and 12/12 hour-light/dark cycle. The animals were given 1 week to acclimatize to the above conditions before the experiment started.
Kainate (KA; Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline and pH was adjusted to 7.0 before use. Male Sprague–Dawley rats (Taconic M&B, Copenhagen, Denmark) weighing 250±10 g received intraperitoneal injections with 10-mg/kg KA (26 rats) or 9% saline (11 rats). All solutions were administered in a concentration of 1 mL/kg.
The rats were placed in separate cages immediately after KA or saline administration and were monitored with regard to seizures for at least 4 hours. Seizures were scored according to the Racine score (Racine, 1972): stage 1, facial clonus; stage 2, nodding; stage 3, forelimb clonus; stage 4, forelimb clonus with rearing; and stage 5, rearing and falling. Status epilepticus was defined as continuous seizures scored as class 4 or 5 seizures and lasting for more than 90 minutes. Only animals that developed SE, comprising 11 of the 26 rats injected with KA, were included in the study. Thirteen rats did not develop SE, whereas two died. The rats were not monitored by EEG, and therefore we cannot rule out the possibility that some of the rats had electrographic epileptiform discharges, or even seizures, during the 2 weeks following SE. Spontaneous seizures were not observed by the animal caretakers or by the researchers at any point.
Fourteen days after saline or KA injection, 11 control animals and 11 KA-treated animals were injected intraperitoneally with a saline solution containing [1-13C]glucose (543 mg/kg, 0.3 mol/L) and sodium [1,2-13C]acetate (504 mg/kg, 0.6 mol/L; both 99% 13C enriched, Cambridge Isotopes Laboratories, Woburn, MA, USA). Fifteen minutes after injection of the 13C-labeled compounds, the animals were decapitated. The heads were snap frozen in liquid nitrogen and further stored at −80°C until dissection. During the period from decapitation to freezing, anaerobic glucose metabolism will produce lactate, causing a ‘false' glucose reduction and lactate increase. The two groups were treated identically, thus there is no reason to believe that the metabolites have been affected differently by postmortem effects. Three brain regions were removed as complete as possible while kept cold on dry ice: (1) the hippocampal formation (subiculum, CA1–CA3, and dentate gyrus), (2) the entorhinal cortex (including a small part of the piriform cortex), and (3) the neocortex. Brain samples were extracted with 7% perchloric acid, as previously described (Alvestad et al, 2008). A volume of 200 μL was used for high-performance liquid chromatography analysis, whereas the remaining samples were lyophilized and stored at −20°C until MRS analysis.
High-Performance Liquid Chromatography
Branched-chain amino acids (BCAAs) in brain extracts were quantified by high-performance liquid chromatography after derivatization with o-phthaldialdehyde, using a Hewlett Packard 1100 apparatus (Agilent, Palo Alto, CA, USA) with fluorescence detection.
13C and 1H Magnetic Resonance Spectroscopy
Lyophilized samples were dissolved in a 200-μL solution containing 99.9% D2O (Cambridge Isotopes Laboratories, Woburn, MA, USA) and 0.1% ethylene glycol (Merck, Darmstad, Germany). The samples were neutralized (pH 6.5–7.0) before acquisition.
Magnetic resonance spectra of the neocortex samples were acquired on a Bruker DRX 500-MHz instrument, those of hippocampal formation on a Bruker DRX 600-MHz instrument, and those of entorhinal cortex on a Bruker 600-MHz instrument with a Bruker BioSpin CryoProbe (Bruker Analytik GmbH, Rheinstetten, Germany). Proton-decoupled 13C MR spectra were acquired with a 30° pulse angle, 1.3 second acquisition time, and 0.5 second relaxation delay. The number of scans was typically 20,000 (neocortex), 40,000 (hippocampal formation), and 50,000 (entorhinal cortex). Correction factors for nuclear Overhauser and relaxation effects were applied to the integrals of the individual peaks. The 1H MR spectra were obtained with a 90° pulse angle, 1.36 second acquisition time, and 10-hour relaxation delay; a total of 512 scans were accumulated for each sample. Water suppression was achieved by applying a low-power presaturation pulse at the water frequency.
Data Analysis and Interpretation
The amount of 13C in different metabolites is quantified from integrals of relevant peaks in the spectra using ethylene glycol as an internal standard with known amount of 13C. The interpretation of the MRS data is further based on knowledge of the metabolic fate of [1-13C]glucose and [1,2-13C]acetate in neurons and astrocytes. Glucose is taken up into both neurons and astrocytes, but is metabolized to a greater extent in neurons (Qu et al, 2000). Acetate is, however, only taken up into astrocytes (Waniewski and Martin, 1998). Thus, administering both [1-13C]glucose and [1,2-13C]acetate to the animal allows for a more detailed metabolic analysis of the different cellular compartments.
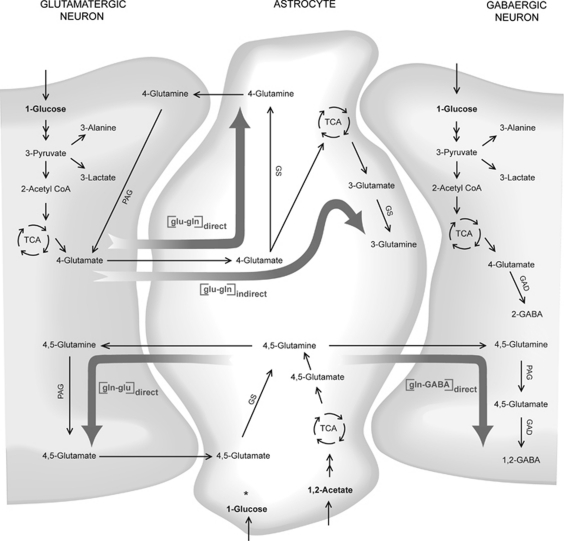
As shown in Figure 1, [1-13C]glucose is converted through glycolysis into one molecule of [3-13C]pyruvate as well as one molecule of unlabeled pyruvate (not shown). The [3-13C]pyruvate is then converted to either [3-13C]lactate or [3-13C]alanine in the cytosol, or to [2-13C]acetyl CoA by pyruvate dehydrogenase in neuronal and astrocytic mitochondria. Further, [2-13C]acetyl CoA can condense with oxaloacetate and enter the tricarboxylic acid cycle (TCA) cycle. The 13C label is then incorporated into the TCA cycle intermediates, and can exit the cycle, for example, at the [4-13C]α-ketoglutarate step to form [4-13C]glutamate. In GABAergic neurons, [4-13C]glutamate is transformed into [2-13C] γ-aminobutyric acid (GABA) by glutamate decarboxylase (GAD). On release into the synaptic cleft, [4-13C]glutamate is taken up into the astrocytes at which it can be converted to [4-13C]glutamine directly by the enzyme GS. However, if the carbon skeleton of glutamate enters the TCA cycle in astrocytes before conversion, [3 or 2-13C]glutamine will eventually be formed. Glutamine is transported to the neurons where it is converted back to glutamate by phosphate-activated glutaminase to maintain the transmitter pool. However, if the 13C-labeled carbons from [1-13C]glucose remain in the neuronal or astrocytic TCA cycle for a second turn, equal amounts of 13C will be incorporated into the second ([2-13C]) and third ([3-13C]) carbon position of glutamate and glutamine, and into the third ([3-13C]) and fourth ([4-13C]) position of GABA. The amount of the metabolite derived from the second TCA turn relative to the first turn, that is, the TCA cycling ratios, can be calculated as follows: 2 × [3-13C]/[4-13C] for glutamate and glutamine, and 2 × [3-13C]/[2-13C] for GABA. The reason for using 2 × [3-13C] is that labeling in [2-13C]glutamate, [2-13C]glutamine, and [4-13C]GABA can also be derived from pyruvate carboxylation in astrocytes, a reaction whereby the TCA cycle can be refilled with intermediates via the astrocytic enzyme pyruvate carboxylase, thus enabling de novo synthesis of glutamate (Shank et al, 1985). The ratio between the pyruvate carboxylase pathway and the pyruvate dehydrogenase pathway can be expressed as ([2-13C]−[3-13C])/[4-13C] for glutamate and glutamine, and ([4-13C]−[3-13C])/[2-13C] for GABA. The 1.1% natural abundant 13C is subtracted from the amount of 13C measured.
Figure 1.
Diagram of glial–neuronal metabolism of [1-13C]glucose and [1,2-13C]acetate. The [1-13C]glucose is converted through glycolysis into one molecule of [3-13C]pyruvate, as well as one molecule of unlabeled pyruvate (not shown), in astrocytes and neurons. The [3-13C]pyruvate is then converted to either [3-13C]lactate in the cytosol, [3-13C]alanine in cytosol and mitochondria, oxaloacetate by pyruvate carboxylase in astrocyte mitochondria (for simplicity this pathway is not included), or [2-13C]acetyl CoA in neuronal and astrocytic mitochondria. The [2-13C]acetyl CoA condenses with oxaloacetate and enters the tricarboxylic acid cycle (TCA) cycle. The 13C label is then incorporated into TCA cycle intermediates, and can exit the cycle at the [4-13C]α-ketoglutarate step to form [4-13C]glutamate. In GABAergic neurons, [4-13C]glutamate is transformed into [2-13C]γ-aminobutyric acid (GABA) by glutamate decarboxylase (GAD). On release into the synaptic cleft, [4-13C]glutamate is taken up into astrocytes at which it can be converted to [4-13C]glutamine directly by the enzyme glutamine synthetase (GS), or enter the TCA cycle before conversion to [3 or 2-13C]glutamine. Ratios for the direct and indirect conversion can be calculated (see Materials and methods) and is shown in the figure as [glu–gln]direct and [glu–gln]indirect, respectively. Glutamine is transported back to the neurons where it is converted to glutamate by phosphate-activated glutaminase (PAG). The [1,2-13C]acetate is taken up into astrocytes and converted to [1,2-13C]acetyl CoA, which can enter the TCA cycle and result in the formation of [4,5-13C]glutamate that is rapidly converted to [4,5-13C]glutamine by the astrocytic GS. The ratio calculated for direct conversion of acetate-derived glutamine to glutamate is illustrated by [gln–glu]direct. Glutamine can be transported to neurons and regenerate [4,5-13C]glutamate via PAG, and subsequently give rise to [1,2-13C]GABA via GAD in GABAergic neurons. The ratio for this conversion is illustrated by [gln–GABA]direct in the figure. *Metabolism of [1-13C]glucose in astrocytes leads to the same labeling pattern as shown in neurons in the figure.
After uptake into astrocytes, the metabolism of acetate starts at the acetyl-CoA level. As shown in Figure 1, [1,2-13C]acetate is converted to [1,2-13C]acetyl CoA, which can enter the TCA cycle. Transamination of α-ketoglutarate results in formation of [4,5-13C]glutamate that is rapidly converted to [4,5-13C]glutamine by the astrocytic GS. Glutamine can then be transported to neurons and regenerate [4,5-13C]glutamate via phosphate-activated glutaminase, and subsequently give rise to [1,2-13C]GABA via GAD in GABAergic neurons.
The amount of 13C (corrected for natural abundance) incorporated into metabolites can be expressed as percentage of total metabolite content (which includes both labeled and unlabeled metabolite), termed percentage 13C enrichment. Ratios for glial–neuronal interactions were calculated from percentage 13C enrichment (abbreviated %) of different isotopomers, as explained in the following and illustrated in Figure 1. As an indicator of glutamate–glutamine cycling, the ratio for direct conversion of glutamate to glutamine ([glu–gln]direct in Figure 1) was calculated as follows: %[4-13C]glutamine/%[4-13C]glutamate. The ratio for direct conversion of acetate-derived glutamine to glutamate ([gln–glu]direct in Figure 1) is expressed as follows: %[4,5-13C]glutamate/%[4,5-13C] glutamine. The ratio for indirect conversion of [1-13C]glucose-derived glutamate to glutamine ([glu–gln]indirect in Figure 1) is expressed as follows: TCA cycling ratio for glutamine/TCA cycling ratio for glutamate (cycling ratio, see above). This ratio indicates the proportion of glutamate carbon skeleton that is channeled through the astrocytic TCA cycle before conversion to glutamine. The transfer of 13C label from astrocytes to GABAergic neurons was assessed by calculating a ratio for the conversion of astrocytic acetate-derived glutamine to GABA (through glutamate) in neurons: %[1,2-13C]GABA/%[4,5-13C]glutamine ([gln–GABA]direct in Figure 1).
Results
Total Amounts of Metabolites
Table 1 shows the tissue concentration of N-acetyl aspartate (NAA) as measured by 1H MRS in different brain regions of control rats and in rats at 14 days after KA injection. N-acetyl aspartate was significantly decreased in hippocampal formation and entorhinal cortex of KA-treated animals, whereas it was unchanged in the neocortex. As seen in Table 2, alanine content was increased in the neocortex of KA-treated rats compared with controls. Lactate concentrations were at control levels in all three brain regions. Table 3 shows concentrations of BCAAs in all three brain regions. In the hippocampal formation of KA-treated animals, the amounts of leucine and isoleucine were significantly increased relative to controls, whereas the amount of valine was at control level. No changes were detected in BCAA concentrations in the entorhinal and piriform cortices and in the neocortex. The tissue concentration of succinate was increased in the hippocampal formation from KA-treated animals (1.43±0.88 μmol/g) relative to controls (0.47±0.08 μmol/g, P=0.004), and unchanged in the other regions.
Table 1. Concentration of N-acetyl aspartate.
| Control | KA | |
|---|---|---|
| Hippocampal formation | 4.5±0.5 | 3.1±1.0* |
| Entorhinal/piriform cortex | 5.2±0.7 | 2.5±0.8** |
| Neocortex | 6.0±1.1 | 6.4±0.9 |
KA, kainate; MRS, magnetic resonance spectroscopy.
Concentration of NAA (μmol/g brain tissue) was measured by 1H MRS in different brain regions of control and KA-treated rats 2 weeks after saline (control) or KA treatment. For controls and KA, respectively, sample sizes were n=9 and 11 (hippocampal formation), n=3 and 11 (entorhinal/piriform cortex), and n=10 and 6 (neocortex). Data represent means±s.d. P-value using Student's t-test, only given when significantly different from corresponding control group, P<0.05.
*P=0.001 and **P=0.0001.
Table 2. Concentrations and 13C enrichments of metabolites related to glycolysis.
|
Hippocampal formation |
Entorhinal/pirifrom cortex |
Neocortex |
||||
|---|---|---|---|---|---|---|
| Control | KA | Control | KA | Control | KA | |
| [1-13C]Glucose | 74±55 | 254±131* | 120±15 | 450±212† | 140±111 | 220±206 |
| Alanine | 0.3±0.1 | 0.4±0.1 | 0.3±0.1 | 0.4±0.1 | 0.3±0.1 | 0.6±0.2‡ |
| [3-13C]Alanine | 41±14 | 29±9§ | 42±9 | 32±9 | 37.7±7.7 | 33.5±13.1 |
| Lactate | 5.5±0.8 | 4.8±1.9 | 5.8±1.3 | 5.1±1.4 | 5.4±0.9 | 6.0±1.5 |
| [3-13C]Lactate | 664±114 | 430±203¶ | 684±109 | 446±127** | 685±130 | 534±192 |
KA, kainate; MRS, magnetic resonance spectroscopy.
Concentrations (μmol/g brain tissue) and amount of 13C (nmol/g brain tissue) in glycolysis-related metabolites, as measured by 1H and 13C MRS in extracts from different brain regions of rats 2 weeks after KA or saline (control) injections. All animals were injected with [1-13C]glucose and [1,2-13C]acetate 15 minutes before decapitation. Sample sizes for 13C and 1H MRS, respectively: controls n=8 and 9, KA n=9 and 11 (hippocampal formation); controls n=3, KA n=8 and 11 (entorhinal/piriform cortex); and controls n=8 and 10, KA n=6 (neocortex). Data represent means±s.d. P-value only given when significantly different from corresponding control group, P<0.05 using Student's t-test.
*P=0.004; †P=0.028; ‡P=0.0004; §P=0.044; ¶P=0.008; and **P=0.019.
Table 3. Concentrations of BCAAs.
|
Hippocampal formation |
Entorhinal/piriform cortex |
Neocortex |
||||
|---|---|---|---|---|---|---|
| Control | KA | Control | KA | Control | KA | |
| Leucine | 0.54±0.25 | 0.77±0.11* | 0.50±0.06 | 0.68±0.19 | 0.60±0.04 | 0.51±0.08 |
| Isoleucine | 0.35±0.1 | 0.50±0.08** | 0.28±0.11 | 0.43±0.12 | 0.33±0.20 | 0.30±0.07 |
| Valine | 0.77±0.38 | 0.93±0.14 | 0.99±0.13 | 0.89±0.16 | 0.93±0.33 | 0.80±0.18 |
BCAA, branched-chain amino acid; HPLC, high-performance liquid chromatography; KA, kainate.
Concentrations of BCAAs (μmol/g brain tissue), as measured by HPLC, in extracts from different brain regions of rats, 2 weeks after KA or saline (control) injections. For controls and KA, respectively, sample sizes were n=9 and 11 (hippocampal formation), n=3 and 11 (entorhinal/piriform cortex), and n=10 and 6 (neocortex). Data represent means±s.d. P-value only given when significantly different from corresponding control group, P<0.05 using Student's t-test.
*P=0.03 and **P=0.02.
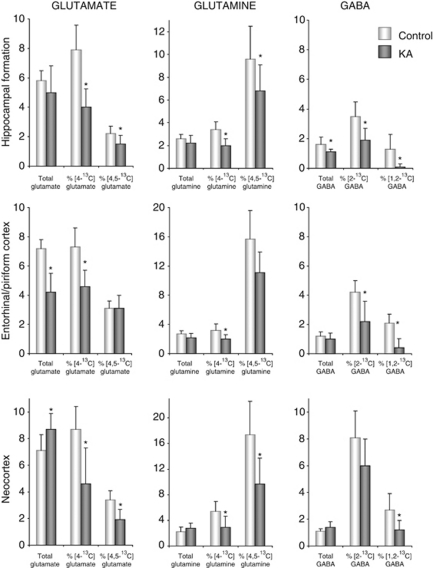
The bars to the left in each bar diagram in Figure 2 show concentrations of glutamate, glutamine, and GABA in different brain regions. In the hippocampal formation of KA-treated animals, no significant changes were found in the amounts of the amino acids glutamate and glutamine as compared with controls, whereas GABA content was significantly decreased. In entorhinal/piriform cortices of KA-injected animals, glutamate content was significantly reduced to ∼60% of that found in controls, whereas the amount of glutamine and GABA was unchanged. In the neocortex, the only difference from control was an increased glutamate concentration in KA-injected animals.
Figure 2.
Total concentrations and percentage of 13C enrichment of glutamate, glutamine, and γ-aminobutyric acid (GABA), as measured by 1H and 13C magnetic resonance spectroscopy (MRS). Total concentrations (μmol/g tissue) of glutamate, glutamine, and GABA, and their percentage of 13C enrichment, as measured by 1H and 13C MRS. The MRS analyses were performed on brain extracts of the hippocampal formation, entorhinal/piriform cortex, and neocortex from animals injected with [1-13C]glucose and [1,2-13C]acetate 15 minutes before decapitation. The experimental groups comprised animals injected with either saline (controls; white bars) or kainate (KA; gray bars) 2 weeks earlier. Sample sizes for 13C and 1H MRS, respectively: controls n=8 and 9, KA n=9 and 11 (hippocampal formation); controls n=3, KA n=8 and 11 (entorhinal/piriform cortex); and controls n=8 and 10, KA n=6 (neocortex). The labeling in [4-13C]glutamate, [4-13C]glutamine, and [2-13C]GABA mostly derives from neuronal metabolism of [1-13C]glucose, the labeling in [4,5-13C]glutamate, [4,5-13C]glutamine, and [1,2-13C]GABA exclusively derives from the metabolism of [1,2-13C]acetate in astrocytes. %, Enrichment is expressed as percentage of 13C-label measured by 13C MRS (nmol/g tissue) relative to the total concentration measured by 1H MRS, signifying turnover of the metabolite. Values are given as mean±standard deviation. *Significantly different from control group using Student's t-test, P<0.05.
Metabolism of [1-13C]Glucose
Table 2 depicts concentrations of metabolites related to glycolysis, as well as amount of 13C in these (corrected for natural abundance). Compared with controls, KA-injected rats had significantly increased levels of unmetabolized [1-13C]glucose in mesial temporal lobe structures, indicating decreased glucose metabolism in these regions. The amount of [3-13C]lactate and [3-13C]alanine was decreased in the hippocampal formation, whereas only [3-13C]lactate was significantly decreased in the entorhinal/piriform cortex. In the neocortex, [3-13C]alanine was increased, whereas [3-13C]lactate was unchanged. The plasma level of glucose and the 13C enrichment of glucose in brain tissue from KA-treated animals were not significantly different from controls (results not shown).
The 13C labeling arising from the first turn of the TCA cycle is shown in the bars in the middle of each diagram in Figure 2. The incorporation from [1-13C]glucose into [4-13C]glutamate and [4-13C]glutamine was decreased in all three brain regions of KA-treated animals compared with controls. The incorporation into [2-13C]GABA was reduced in mesial temporal lobe structures, and not in the neocortex. More specifically, in hippocampal formations of KA animals, the percentage 13C enrichment was roughly halved in [4-13C]glutamate and [2-13C]GABA, and reduced by ∼40% of that in controls in [4-13C]glutamine.
In the hippocampal formation of KA-treated rats, relatively more of the 13C label from [1-13C]glucose found in glutamine was derived from the second turn in the TCA cycle relative to the first turn. This is shown by increased TCA cycling ratio in this metabolite (1.29±0.14 in controls versus 1.65±0.37 in KA-treated rats, P=0.02; Supplementary Table 1). The percentage of 13C enrichment in succinate was decreased in all three brain regions of KA-treated rats compared with controls (hippocampal formation: 0.76±0.63 versus 2.99±0.67, P=0.0001; entorhinal/piriform cortex: 0.75±0.86 versus 2.36±1.17, P=0.04; and neocortex: 2.29±1.72 versus 8.04±3.82, P=0.0096).
Metabolism of [1,2-13C]Acetate
The bars to the right of each diagram in Figure 2 depict the percentage of 13C incorporation from [1,2-13C]acetate into glutamate, glutamine, and GABA. The percentage of [4,5-13C]glutamate and [4,5-13C]glutamine were both significantly decreased to ∼70% of control levels in hippocampal formation of KA animals, and to 56% of control levels in the neocortex. In the entorhinal/piriform cortex, the percentage of [4,5-13C]glutamate and [4,5-13C]glutamine was not significantly altered. The percentage [1,2-13C]GABA was reduced to 44% of control levels in the neocortex of KA animals and to 21% of control levels in the entorhinal/piriform cortex, whereas it was reduced to 6% of control levels in the hippocampal formation.
Astrocytic versus Neuronal Metabolism
The proportion of 13C label derived from acetate, relative to that from glucose, was increased in glutamate in the hippocampal formation of KA-treated rats (0.29±0.06 in the control group versus 0.38±0.11 in the KA group, P<0.05). The acetate/glucose utilization ratio for glutamine formation was unaltered in all the three brain regions studied (ratios not shown). For GABA, a reduced use of acetate relative to glucose as a precursor was observed in hippocampi of KA-treated rats (0.34±0.17 in the control group versus 0.04±0.11 in the KA group, P<0.001), whereas this was unaltered in the other brain regions. The contribution of astrocytic de novo synthesis of glutamate, glutamine, and GABA via pyruvate carboxylase relative to metabolism via pyruvate dehydrogenase was unchanged in KA-treated animals (pyruvate carboxylase/pyruvate dehydrogenase ratios not shown). The ratios for direct conversion of glutamate to glutamine ([glu–gln]direct) were unchanged in all brain areas. The ratio for direct conversion of glutamine to glutamate ([gln–glu]direct; Figure 1) was increased in the entorhinal/piriform cortex (controls 0.20±0.02 and KA 0.28±0.04, P=0.018). The ratio for indirect conversion of glutamate to glutamine ([glu–gln]indirect; Figure 1) was increased in KA-injected rats compared with controls in the hippocampal formation (3.33±1.22 versus 2.30±0.24, P=0.03) and the neocortex (4.62±2.70 versus 2.45±0.26, P=0.04), and remained unchanged in the entorhinal/piriform cortex. The ratio of glutamine–GABA transfer ([gln–GABA]direct; Figure 1) was decreased in hippocampal formation (0.14±0.08 in the control group versus 0.01±0.04 in the KA group, P=0.001), as well as in the entorhinal cortex (0.13±0.02 versus 0.03±0.05, P=0.009).
Discussion
The present study shows that energy and neurotransmitter hypometabolism precedes the occurrence of spontaneous seizures in the KA model of TLE. The latent phase was characterized by decreased turnover of glutamate and glutamine in all the brain areas that were studied, whereas decreased GABA turnover was most pronounced in the mesial temporal lobe. The altered metabolic profile in the epileptogenic region can be explained by complex and interconnected processes, such as impaired glycolysis, neuronal cell loss, mitochondrial dysfunction in both neurons and astrocytes, as well as impaired glutamate–glutamine–GABA cycling.
Reduced Glycolysis in Mesial Temporal Lobe
Metabolism of glucose was reduced in the hippocampal formation and entorhinal cortex of KA-injected animals, as evidenced by an accumulation of unmetabolized [1-13C]glucose in these structures. Previous studies using 14C-2-deoxyglucose autoradiography have shown an initial increase in glucose utilization in limbic structures hours after systemic KA injections, followed by a decrease in glucose utilization 1 to 4 days later, with the exception of the amygdala (Ben-Ari et al, 1981; Lothman and Collins, 1981). The 13C MRS enables a complete analysis of glucose metabolism in the cytosol and the TCA cycle, and not only glucose uptake and phosphorylation as obtained with 2-deoxyglucose autoradiography and [18F]fluorodeoxyglucose positron emission tomography. The present study shows that both cytosolic and mitochondrial glucose metabolism is specifically impaired in the hippocampal formation in the latent phase. The amounts of [3-13C]lactate and [3-13C]alanine were decreased in the hippocampal formation of KA-treated animals, indicating decreased production of their precursor pyruvate. Accordingly, the reduced glucose consumption in hippocampal formation in the latent phase reflects, at least in part, reduced glycolysis. A similar impairment of glucose metabolism is not seen in the neocortex, and is thus specific for the mesial temporal lobe. In the normal brain, glycolysis is directly coupled to neural function and glutamatergic neurotransmission (Sibson et al, 1998). It is not clear whether the decrease in glycolysis during the latent phase represents a detrimental or a protective process in response to KA injection. However, recent studies may support the latter, as they have shown an anticonvulsant, and to some extent antiepileptogenic, effect of decreasing glycolysis by means of treatment with fructose-1,6-bisphosphate or 2-deoxyglucose in animal models of epilepsy (Garriga-Canut et al, 2006; Lian et al, 2007). Interestingly, glycolytic metabolism appears normal in the chronic phase, as no significant changes were seen in [1-13C]glucose, [3-13C]lactate, or [3-13C]alanine in the epileptogenic mesial temporal lobe 3 months after KA treatment (Alvestad et al, 2008). This might imply a failure of the potential protective glycolytic decrease when moving from latent to chronic phase. In chronic epilepsy in humans, glucose utilization is decreased in epileptogenic zones interictally and increased ictally, as detected by fluorodeoxyglucose positron emission tomography (Semah et al, 1995; Meltzer et al, 2000). Interictal glucose hypometabolism seems to become more pronounced with increasing duration of epilepsy (Akman et al, 2010), and one cannot exclude the possibility that this also could be the case for KA-treated rats with longer epilepsy duration than in our previous study (Alvestad et al, 2008).
Decreased Neuronal Mitochondrial Metabolism
In a parallel set of animals studied in the same laboratory, neuronal loss was apparent in mesial temporal lobe structures, especially in hippocampal formation, 2 weeks after KA injection (Hammer et al, 2008). Loss of glutamatergic neurons is most prominent, as seen by extensive loss of CA1 and CA3 pyramidal cells in the hippocampal formation and loss of layer III neurons in the entorhinal cortex (Hammer et al, 2008). N-acetyl aspartate, an amino acid primarily synthesized in neurons (Baslow, 2003), is used as a marker of cell loss and/or mitochondrial viability. A ∼30% decrease in NAA was found in mesial temporal lobe structures of KA-injected animals.
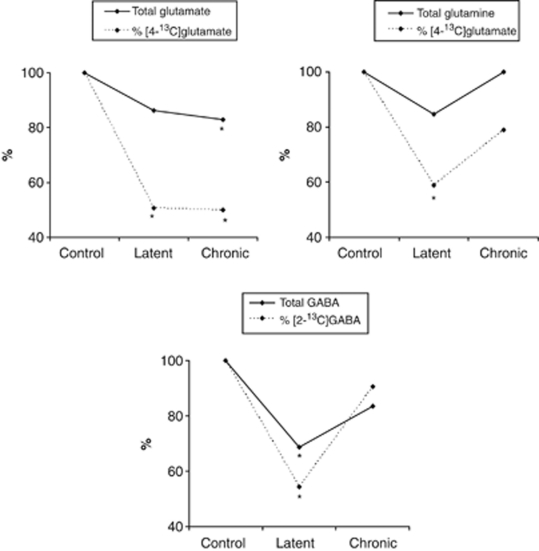
There were pronounced changes in glutamate metabolism of KA-injected animals. Glutamate levels were not directly related to extent of neuronal cell death, because total glutamate content was unchanged in the damaged hippocampal formation and increased in the neocortex. The percentage enrichment with [4-13C]glutamate was decreased by ∼50% in the hippocampal formation and neocortex, and by ∼40% in the entorhinal/piriform cortex. The enrichment of glutamate with [4-13C]glutamate primarily reflects glutamate turnover in neurons, because glutamatergic neurons contain the major pool of glutamate in the brain (Storm-Mathisen et al, 1983; Ottersen and Storm-Mathisen, 1985), and ∼70% of acetyl CoA derived from [1-13C]glucose is metabolized in neuronal mitochondria (Qu et al, 2000). When moving from the latent to the chronic phase of KA-induced epilepsy, turnover of glutamate was still decreased in the hippocampal formation, and at control levels in the entorhinal/piriform cortex and neocortex (Figure 3 and Alvestad et al, 2008). The decrease in glutamate turnover is, thus, transient in the areas of propagation and the non-epileptogenic tissue, whereas in the epileptogenic hippocampal formation the decrease continues into the chronic phase, potentially contributing to both the development and maintenance of spontaneous seizures.
Figure 3.
Glutamate, glutamine, and γ-aminobutyric acid (GABA) in the hippocampal formations of animals in the latent and chronic phase of the kainate (KA) model of mesial temporal lobe epilepsy. Total concentrations (μmol/g tissue) of glutamate, glutamine, and GABA, and their percentage 13C enrichment, as measured in the hippocampal formation by 1H and 13C magnetic resonance spectroscopy (MRS). Values are given for KA-treated animals as percentage relative to controls (100%). The experimental groups comprised animals in the latent phase (2 weeks after KA injection) and animals in the chronic phase (13 weeks after KA injection, calculated from Alvestad et al, 2008), with their respective age-matched saline-treated controls. The [1-13C]glucose was injected 15 minutes before decapitation. For 1H MRS: latent controls n=9, latent KA n=11, chronic controls n=6, and chronic KA n=8. For 13C MRS: latent controls n=8, latent KA n=9, chronic controls n=6, and chronic KA n=8. %, Enrichment signifies turnover of the metabolite, and is expressed as percentage of 13C-label in the fourth position of glutamate and glutamine and in the second position of GABA, as measured by 13C MRS, relative to the total concentration measured by 1H MRS. *Significantly different from control group using Student's t-test, P<0.05.
The turnover of a metabolite, expressed as percent enrichment, represents the net balance of synthesis and degradation. In hippocampal formation, reduced glutamate synthesis could be caused by the decrease in glycolytic activity, resulting in reduced ATP production and decreased precursor input to the TCA cycle. Indeed, it has been shown that glycolytically derived ATP is necessary for glutamatergic neurotransmission, independent of global cellular ATP levels (Ikemoto et al, 2003).However, reduced glutamate synthesis could also be a consequence of mitochondrial malfunction, as indicated by the reduced NAA, as well as the increased content of 13C and decreased 13C incorporation into succinate. The TCA cycle intermediate succinate is converted to fumarate by the enzyme succinate dehydrogenase, which constitutes complex II of the mitochondrial electron transport chain. An accumulation of succinate might, thus, indicate that downstream oxidative phosphorylation is impaired.
The reduced turnover of glutamate in the hippocampal formation and neocortex can be explained not only by reduced synthesis but also by reduced degradation of glutamate. A decreased percentage of 13C label in glutamate indicates reduced synthesis but the total amount of glutamate is in fact unchanged in the hippocampal formation, and even increased in the neocortex. The total amount of glutamate comprises both labeled and unlabeled glutamate, and thus, less unlabeled glutamate must have been degraded to maintain glutamate amount at control levels. In line with this, both neuronal turnover (percent 13C enrichment) and total concentration of GABA were decreased in mesial temporal lobe structures of KA animals, reflecting reduced GAD-mediated GABA synthesis from glutamate. Other groups have reported that following KA injection, there is a marked loss of GABAergic interneurons in the hippocampus, and the cells that are most vulnerable for cell death are those normally exerting inhibition onto the dendritic region of glutamatergic cells (Buckmaster and Jongen-Relo, 1999; Magloczky and Freund, 2005). In our study, we cannot distinguish between the contributions of interneuron death and/or decreased GAD activity to the observed decrease in GABA synthesis. Nevertheless, loss of GABAergic activity may have a role in epileptogenesis by decreased control of excitatory input. In the chronic phase of KA-induced epilepsy, both GABA content and turnover were at control levels in the hippocampal formation (Figure 3 and Alvestad et al, 2008). This ‘normalization' of GABA function agrees with findings of normal GAD activity in hippocampi from the chronic phase of KA-induced epilepsy (Baran et al, 2004), and also coincides with increasing levels of GAD mRNA in remaining GABAergic neurons in the chronic phase of pilocarpine-induced epilepsy (Esclapez and Houser, 1999). Other groups have reported that the remaining GABAergic neurons are mostly basket cells, normally exerting inhibition onto the perisomatic region of the pyramidal cells, and considered responsible for synchronized activity within large cell populations (Magloczky and Freund, 2005). Hence, the observed ‘recovery' of GABAergic function in the chronic phase does not necessarily balance the increased excitation during seizures, but could be responsible for synchronization of seizure activity.
Decreased Astrocytic Mitochondrial Metabolism and Perturbed Interaction with Neurons
Astrocytic mitochondrial metabolism was assessed by the use of [1,2-13C]acetate and considerable alterations were observed in KA-injected rats. Enrichment with [4,5-13C]glutamate, [4,5-13C]glutamine, and [1,2-13C]GABA was decreased in the hippocampal formation and neocortex. In all brain regions, there was also a decrease in [4-13C]glutamine enrichment, 60% of which is derived from astrocytic metabolism (Hassel et al, 1997). The slow turnover of metabolites in astrocytes was seen in spite of the massive gliosis occurring in the hippocampal formation after KA injection (Hammer et al, 2008), suggesting that reactive astrocytes have severely altered metabolism compared with normal astrocytes. Several of the metabolic alterations observed could be consequences of decreased activity of branched-chain aminotransferase (BCAT). According to the proposed BCAT nitrogen shuttle, the mitochondrial BCAT in astrocytes catalyzes the reversible transamination of BCAAs (leucine and isoleucine) with α-ketoglutarate to form α-ketoisocaproate and glutamate (Yudkoff et al, 1996; Hutson et al, 1998). Decreased transamination via BCAT in astrocytes could, thus, explain the accumulation of leucine and isoleucine together with decreased 13C enrichment of glutamate in hippocampal formation in the present study. Furthermore, relatively more of the 13C label from [1-13C]glucose found in glutamine was derived from a second turn of the TCA cycle, consistent with less α-ketoglutarate leaving the astrocytic TCA cycle for transamination with BCAAs. According to the proposed BCAT nitrogen shuttle hypothesis, following transamination, glutamate is aminated to glutamine and transported to neurons together with α-ketoisocaproate, where the reversed transamination reaction occurs. Neurons depend on glutamine supply from astrocytes for replenishing the neurotransmitter pool of glutamate and GABA, because they are not capable of de novo synthesis (Patel et al, 2001; Rae et al, 2003). Yudkoff et al (2003) have previously demonstrated that transamination of leucine was diminished in the pentylenetetrazole seizure model, and proposed that pentylenetetrazole treatment may favor the reverse reaction, consuming glutamate. There is no sign of this potentially protective effect in animals after spontaneous seizures have occurred, because there was no change in BCAAs in the chronic phase of KA-induced epilepsy (unpublished data from Alvestad et al, 2008). The potential antiepilept(ogen)ic effect of inhibiting BCAA transamination warrants further research. In fact, inhibition of BCAT and stimulation of glutamine transport is one of the proposed mechanisms of action of the antiepileptic drug and leucine analog, gabapentin (Hutson et al, 1998). However, gabapentin seems to inhibit the cytosolic, and predominantly neuronal, isoform of the enzyme to a larger extent than the mitochondrial isoform that is localized in astrocytes, and the functional relevance of this mechanism to the antiepileptic effect of the drug is unclear.
Most of the glutamate released from neurons is taken up by astrocytes and converted to glutamine directly, or metabolized through the astrocytic TCA cycle as oxidative fuel (Hassel et al, 1995), as illustrated in Figure 1. Ratios calculated from 13C MRS data make it possible to distinguish between these two possibilities (see Materials and Methods). In the entorhinal/piriform cortex of rats 14 days after KA injection, the ratio for direct conversion of glutamine to glutamate was increased. The ratio of indirect conversion of glutamate to glutamine was increased in the hippocampal formation and neocortex, suggesting that relatively more of the glutamate from neurons is channeled through the astrocytic TCA cycle before glutamine synthesis rather than converted directly to glutamine. In the hippocampal formation, this is consistent with the increased level of GS reported in a previous study of the latent phase in the same animal model (Hammer et al, 2008), and could possibly be because of a need for alternative energy fuel in astrocytes in light of impaired mitochondrial metabolism of other TCA substrates, as seen in the decreased labeling from [1,2-13C]acetate. This could also serve as a buffering system for any excess glutamate that may develop. In the neocortex, the total glutamate concentration was increased in this study, and although the overall glutamate level in hippocampal formation was unchanged, extracellular glutamate concentration around synapses could still be increased locally during the development of hyperexcitable tissue. Indeed, it has been shown that with increasing extracellular glutamate concentration, more glutamate goes through the astrocytic TCA cycle before glutamine formation (McKenna et al, 1996). In contrast to findings in the latent phase, both direct and indirect conversions of glutamate to glutamine were unchanged in the chronic phase, as well as the percentage enrichment with [4-13C]glutamine (calculated from Alvestad et al, 2008). This parallels the decline in GS in the hippocampal formation observed when moving from the latent to the chronic phase in the same model (Hammer et al, 2008), and might reflect a reduced capacity to handle sudden increases in extracellular glutamate concentrations as epilepsy develops. Interestingly, GS levels are significantly reduced compared with controls in humans with long-standing epilepsy, as previously mentioned (Eid et al, 2004).
The metabolic interaction between astrocytes and GABAergic neurons was compromised in the mesial temporal lobe structures in the latent phase, as shown by a decreased glutamine to GABA transfer ratio in KA-injected animals compared with controls. In the hippocampal formation, this is corroborated by the finding of decreased [1,2-13C]GABA and a reduced use of acetate, which is metabolized by astrocytes, relative to the use of glucose as a substrate for GABA synthesis. The underlying mechanisms are not evident, but decreased transamination through BCAT, as proposed above, could contribute by impairing the BCAT nitrogen shuttle and glutamine release from astrocytes. Reduced utilization of astrocytic glutamine for GABA synthesis could affect the overall level of inhibition, as it has been demonstrated that GABAergic neurotransmission is dependent on adequate glutamine transport (Liang et al, 2006). It is not known whether the compromised astrocyte–neuron interaction continues after spontaneous seizures occur, because transfer ratios of astrocytic glutamine to GABA cannot be calculated from data from the chronic phase. Although the importance for epileptogenesis is difficult to assess, the finding adds to the lines of evidence in this study, indicating that astrocytes are, at least partly, responsible for changes in both glutamate and GABA metabolism during epileptogenesis.
Conclusions
The complex and interconnected changes in energy and neurotransmitter metabolism found in this study may be characteristic of the epileptogenic process in response to the initial KA insult. Glutamate turnover is decreased both before and after spontaneous seizures occur, potentially contributing to both the development and maintenance of epileptic seizures. Several transient metabolic changes, observed in latent and not in chronic phase, were identified in mesial temporal lobe structures, such as reduced glycolysis in parallel with decreased neural activity and decreased GABA content and turnover, as well as decreased transamination of BCAAs through BCAT. A failure of these mechanisms might be involved in the shift from latent to chronic phase. Astrocytes were found to be partly responsible for changes in both glutamate and GABA metabolism, possibly because of a compromised BCAT nitrogen shuttle and glutamine supply. Further investigation is needed to explore the potential antiepilept(ogen)ic effect of inhibiting astrocytic BCAT. Decreased turnover of GABA and glutamate, demonstrated by 1H and 13C MRS, may hold potential as clinically useful biomarkers.
Acknowledgments
The authors thank Lars Evje and Bente Urfjell for technical assistance and Carina Knudsen for artwork.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Akman CI, Ichise M, Olsavsky A, Tikofsky RS, Van Heertum RL, Gilliam F. Epilepsy duration impacts on brain glucose metabolism in temporal lobe epilepsy: results of voxel-based mapping. Epilepsy Behav. 2010;17:373–380. doi: 10.1016/j.yebeh.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvestad S, Hammer J, Eyjolfsson E, Qu H, Ottersen OP, Sonnewald U. Limbic structures show altered glial-neuronal metabolism in the chronic phase of kainate induced epilepsy. Neurochem Res. 2008;33:257–266. doi: 10.1007/s11064-007-9435-5. [DOI] [PubMed] [Google Scholar]
- Baran H, Kepplinger B, Draxler M, Skofitsch G. Choline acetyltransferase, glutamic acid decarboxylase and somatostatin in the kainic acid model for chronic temporal lobe epilepsy. Neurosignals. 2004;13:290–297. doi: 10.1159/000081964. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Riche D, Ghilini G, Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6:1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Relo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412:488–505. [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Hammer J, Alvestad S, Osen KK, Skare O, Sonnewald U, Ottersen OP. Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia. 2008;56:856–868. doi: 10.1002/glia.20659. [DOI] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2–13C]acetate and [1–13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–2782. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Berkich D, Drown P, Xu B, Aschner M, LaNoue KF. Role of branched-chain aminotransferase isoenzymes and gabapentin in neurotransmitter metabolism. J Neurochem. 1998;71:863–874. doi: 10.1046/j.1471-4159.1998.71020863.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- Lian XY, Khan FA, Stringer JL. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27:12007–12011. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Adelson D, Brenner RP, Crumrine PK, Van Cott A, Schiff DP, Townsend DW, Scheuer ML. Planned ictal FDG PET imaging for localization of extratemporal epileptic foci. Epilepsia. 2000;41:193–200. doi: 10.1111/j.1528-1157.2000.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Muller B, Qu H, Garseth M, White LR, Aasly J, Sonnewald U. Amino acid neurotransmitter metabolism in neurones and glia following kainate injection in rats. NeurosciLett. 2000;279:169–172. doi: 10.1016/s0304-3940(99)00983-0. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Different neuronal localization of aspartate-like and glutamate-like immunoreactivities in the hippocampus of rat, guinea-pig and Senegalese baboon (Papio papio), with a note on the distribution of gamma-aminobutyrate. Neuroscience. 1985;16:589–606. doi: 10.1016/0306-4522(85)90194-0. [DOI] [PubMed] [Google Scholar]
- Patel AB, Rothman DL, Cline GW, Behar KL. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res. 2001;919:207–220. doi: 10.1016/s0006-8993(01)03015-3. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U. (13)C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci. 2000;22:429–436. doi: 10.1159/000017472. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rae C, Hare N, Bubb WA, McEwan SR, Broer A, McQuillan JA, Balcar VJ, Conigrave AD, Broer S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation. J Neurochem. 2003;85:503–514. doi: 10.1046/j.1471-4159.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- Semah F, Baulac M, Hasboun D, Frouin V, Mangin JF, Papageorgiou S, Leroy-Willig A, Philippon J, Laplane D, Samson Y. Is interictal temporal hypometabolism related to mesial temporal sclerosis? A positron emission tomography/magnetic resonance imaging confrontation. Epilepsia. 1995;36:447–456. doi: 10.1111/j.1528-1157.1995.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence. Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U, Kondziella D. Neuronal glial interaction in different neurological diseases studied by ex vivo 13C NMR spectroscopy. NMR Biomed. 2003;16:424–429. doi: 10.1002/nbm.837. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Kwan P, Brodie MJ. Does the cause of localisation-related epilepsy influence the response to antiepileptic drug treatment. Epilepsia. 2001;42:357–362. doi: 10.1046/j.1528-1157.2001.29000.x. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nelson D, Nissim I, Erecinska M. Neuronal metabolism of branched-chain amino acids: flux through the aminotransferase pathway in synaptosomes. J Neurochem. 1996;66:2136–2145. doi: 10.1046/j.1471-4159.1996.66052136.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A. Metabolism of brain amino acids following pentylenetetrazole treatment. Epilepsy Res. 2003;53:151–162. doi: 10.1016/s0920-1211(02)00260-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.