Abstract
Renewal of epidermis is achieved by an ordered replication of stem cells and transit amplifying cells followed by terminal differentiation. In mouse epidermis, renewal is organized around highly ordered structures termed epidermal proliferative units, each generated by a single stem cell. It has been difficult to apply these concepts to human epidermis where the basal layer is undulating and the strata have variable thickness. For example it is unclear if stem cells in human epidermis are located at the base of rete ridges or overlying the tip of dermal papilla. Data is available to support both views. To gain a better understanding of epidermal proliferative unit organization in human skin, we have genetically marked xenografts of human foreskin with a lentivirus encoding a fluorescent marker protein and have mapped labeled columns of cells over a 28 week period. By following these columns to their origin in the epidermis we have been able to determine that stem cells are dispersed along the basal compartment. The widths of these columns do vary considerably with the narrowest originating from cells located in the base of the rete ridge. These findings provide new insights into the dynamics of epidermal renewal in human glabrous skin.
Keywords: stem cell, lineage, epidermis
Introduction
Epidermal renewal is achieved by proliferation of stem cells in the basal compartment followed by transit amplification and terminal differentiation of stem cell progeny (Watt, 1998). Early histological studies and subsequent lineage marking experiments with mouse dorsal epidermis demonstrated that descendants of a single stem cell formed a column of cells stretching from the basal layer to the surface. These findings gave rise to the concept of an epidermal proliferation unit or EPU(Potten, 1974; Mackenzie, 1975; Mackenzie, 1997; Kamimura et al., 1997; Ghazizadeh and Taichman, 2001) wherein a single stem cell and its progeny amplifying cells and differentiated cells formed a distinct spatial unit. However, difficulty arose in applying these findings and concepts to human epidermis, as the morphology of human epidermis is considerably different from that of mouse epidermis. Dorsal mouse epidermis has only two living cell layers and the basal compartment is relatively flat, unlike human epidermis which has several to many cell layers and an undulating basal compartment. These undulations result in different numbers of strata and lack of clear alignment between particular basal cells and columns of squames (for a review see Potten and Booth, 2002). Therefore, it is not clear if the proliferative units in human epidermis are arranged in the same way as described for mouse dorsal epidermis. It is also not clear where stem cells are located in the undulating human basal compartment, in rete ridges or over dermal papilla or both. Some reports favor a protective location at the base of rete ridges (Lavker and Sun, 1982; Lavker and Sun, 2000) and other data suggest a location over the tips of dermal papillae (Jones et al., 1995; Jensen et al., 1999). The location of stem cells within the undulating compartment is an important aspect of cutaneous biology as it speaks to the notion that stem cell behavior is related to a particular microenvironment or niche. These issues could be readily resolved if histological markers were available to distinguish stem cells from transit amplifying cells. However, markers that are associated with epidermal stem cells, namely high levels of β1 integrin (Jones et al., 1995), high levels of α6 integrin and low levels of transferrin receptor (Li et al., 1998), p63 (Pellegrini et al., 2001), and more recently AC133-2, an isoform CD133 (Yu et al., 2002), do not provide sufficient resolving power to define stem cell location in vivo (for a review see Gambardella and Barrandon, 2003). Data collected from lineage analysis of mouse dorsal skin or reconstituted human epidermis have also been non-instructive as the basal compartment in both is relatively flat, not undulating (Ghazizadeh and Taichman, 2001;Kolodka et al., 1998).
In this study we have applied lentivirus-mediated genetic marking to evaluate the spatial organization of EPUs in human epidermis. Lentiviral vectors (LVV) integrate into the genome of a transduced cell and thus are stably inherited by all progeny cells. By encoding a fluorescent marker protein in the LVV, all cells with the vector genome will be detectable. With repeated cycles of cell replication, LVV-transduced transit amplifying cells and differentiated cells will be lost through desquamation, whereas a transduced stem cell and its progeny will be retained and will appear as a labeled EPU column. At the base of the labeled column will be a putative stem cell. In this way we were able to learn that epidermal stem cells reside in both rete ridges and over dermal papilla and do not appear to be clustered at any specific location in the basal compartment.
Materials and Methods
Viral vectors and in vivo transduction
Second generation self-inactivating LVVs were kindly provided by Dr. Didier Trono (CMU, Switzerland). Recombinant LVV encoding GFP or RFP were constructed as described previously (Ghazizadeh et al., 2004) and virus stocks were generated by transient co-transfection of three plasmids including the packaging construct pCMVΔ8.9, an envelope coding plasmid (pCMV-VSV-G), and the transgene construct pHR’CMV-GFP(or RFP)-WPRE-SIN18 into 293T cells (Zufferey et al., 1998). The virus-containing supernatant was collected 36–72 hours after transfection, filtered through 0.45μm filter (Whatman, Clifton, NJ) and was concentrated 1000 fold by ultra-centrifugation as described elsewhere (Burns et al., 1993). To generate concentrated mixed stocks of GFP- and RFP-encoding viruses, supernatants were mixed prior to ultracentrifugation. Viral titers were determined on 293T cells by flow cytometry on a Becton Dickinson FACScan (BD Biosciences, San Jose, CA) and were between 0.5–1 × 109 transducing units/ml for all viral stocks used for in vivo transduction.
Human skin grafting and in vivo transduction
Human neonatal foreskins were obtained from routine circumcisions, the hypodermal fat was removed and skin was cut to 11 mm circles using a biopsy punch. Circular disks of skin were grafted onto full thickness, circular excisional wounds created on the dorsum of 7-week-old NIH male Swiss nu/nu mice (Taconic labs, NY). Grafts were covered with Vaseline gauze and a polyvinyl bandage to prevent scratching and facilitate tissue engraftment. The dressing was removed after 10–14 days. At 6 weeks post-grafting, when grafts were stabilized, 20μl (containing 1–2 × 107 transducing units) of concentrated virus encoding GFP or a mixture of viruses encoding GFP and RFP were injected intradermally into the grafted skin with the aid of a 30 gauge needle. Animal studies were performed in accordance with institutional guidelines set forth by the State University of New York.
Detection of transgene products and immunofluorescent staining
Skin-directed GFP expression in live animals was assessed at various times following transduction using a fluorescent stereoscope model Bio2-M (Zeiss, Germany) equipped with a mercury 100 W lamp power supply and a wide-band filter set for GFP detection. Mice were anesthetized and placed directly under the stereoscope, images were captured by a Nikon Coolpix 990 camera and processed using Adobe Photoshop.
For histological analysis, tissue samples were harvested at 28 weeks post transduction, fixed in cold 4% paraformaldehyde (Fisher Scientific, Fairlane, NJ) in PBS for 30 minutes, rinsed in PBS and snap-frozen in embedding media. Five μm-thick serial sections were prepared, rehydrated in PBS, and mounted in media containing 4’,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Barlingame, CA). Sections were examined and photographed with an epifluorescent Eclipse 800 microscope (Nikon, Japan) equipped with SPOT camera and image analyzing software.
For immunofluorescent staining, skin samples transduced with GFP encoding virus were cryosectioned, rinsed in PBS, blocked with 5% non-fat milk and incubated with 1:500 dilutions of mouse monoclonal antibody to human CD29 (clone MAR4, BD Pharmingen, San Diego, CA). After extensive washes, sections were incubated with Alexa 594 -conjugated goat anti mouse antibody (Molecular Probes, Eugene, OR). Slides were mounted and examined as described above.
RESULTS
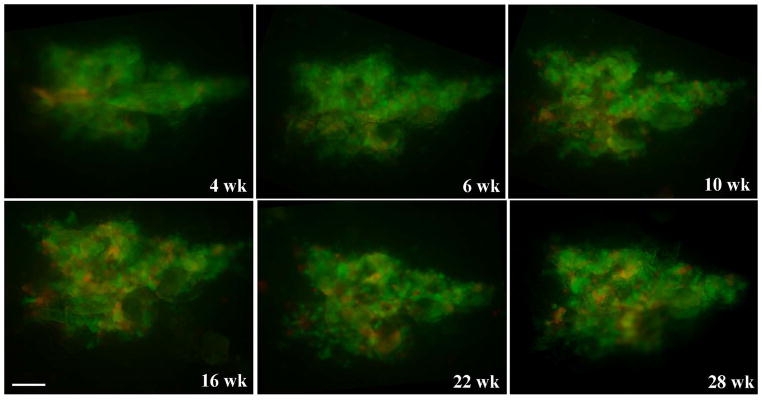
To locate keratinocyte stem cells in human skin, LVV encoding enhanced green fluorescent protein (GFP) or DSRed2 fluorescent protein (RFP) were used to genetically label epidermal keratinocytes in xenografts of human foreskin. Human foreskins were grafted onto nude mice and six weeks later when tissue was normalized, 20 μl of concentrated virus stocks containing 1×107 infectious particles were injected intradermally into the grafted skin. No prior wounding or dermabrasion was needed with LVV transduction. In some experiments, a mixture of GFP and RFP-encoding LVV were used to monitor multiple, independent transduction events. The overall size and gross appearance of grafted skin did not change during the 28-week post-transduction observation period. To verify marker stability, surface or near surface GFP/RFP expression was monitored multiple times over this 28 week period in a non-invasive fashion using fluorescent stereoscopy. Figure 1 shows a cluster of GFP- and RFP-labeled cells of about 1.6 mm2 in size at multiple time points. It is clear that the size and shape of the cluster did not change over time. Transduction with a lentiviral vector is expected to target all epidermal cells including stem cells, transit amplifying cells and post mitotic cells. However, the latter two populations are lost with repeated cycles of replication and desquamation of epidermis (Hall and Watt, 1989; Kolodka et al., 1998). As turnover rate in human epidermis is estimated at about 4 weeks (Potten, 1981), persistent transgene expression over a 28 week period is taken to indicate stem cell transduction. The labeled cluster in Fig.1 represents several adjacent EPUs and the reproducible labeling pattern demonstrates the stability of genetically marked EPUs.
Figure 1.
Persistent and reproducible GFP/RFP surface expression in transduced human epidermis. Human skin xenografts were injected intradermally with a mixture of two viruses encoding GFP and RFP. Surface GFP/RFP expression was assessed by fluorescent stereoscopy at various times indicated using GFP wide band filter set. Scale bar = 0.25 mm.
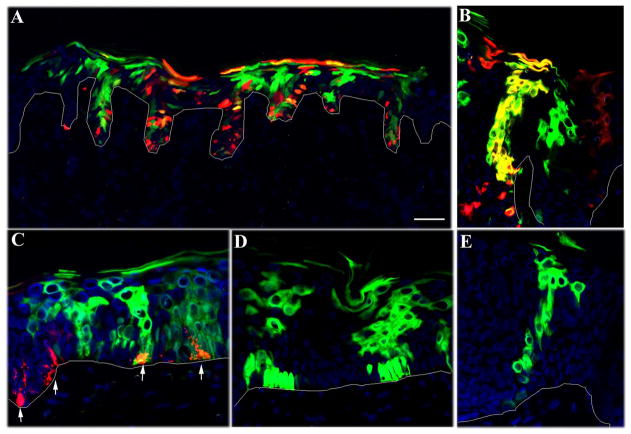
To determine the location of stem cells in the basal compartment of labeled EPUs, serial sections of grafted tissue taken at 28 weeks post-transduction were examined by fluorescent microscopy and the dimensions and position of individual EPU’s were noted. An EPU was defined operationally as a contiguous cluster of fluorescently labeled cells extending from the basal layer to the cornified layer. EPU dimensions were most readily discerned in areas some distance from the injection site, where transduction frequency was reduced and labeled columns more clearly demarcated. When using a mixture of GFP- and RFP-encoding lentiviral vectors, we noted an EPU by a column of green or red labeled cells if the stem cell had been singly transduced or yellow if the stem cell had been transduced with both vectors (Fig. 2A–B). Melanocytes were also transduced, however their unique morphology and pattern of distribution made them easily distinct from keratinocytes (Fig. 2C).
Figure 2.
Distribution of fluorescently-labeled EPU in skin sections. Human skin was transduced in situ with a mixture vectors encoding GFP (green) and RFP (red) in A–C or a single vector encoding GFP in D–E. Tissue sections were analyzed at 28 weeks by fluorescent microscopy. (A) The overall distribution of labeled EPUs is shown in a low power section of skin. (B) EPUs labeled by a single virus are evident as either green or red columns while the EPU labeled with both vectors appears as orange. (C–E) EPUs originating from relatively flat areas of the basal compartment contain 2–6 cells at the base. The arrows in C denote transduced melanocytes (red) in the basal layers of epidermis. Scale bar = 100 μm in A and 40 μm in B–E.
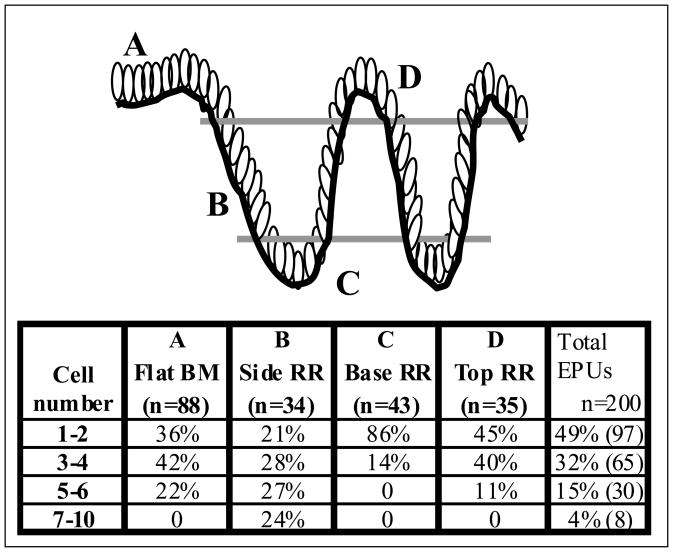
A total of 200 labeled EPUs derived from three transduced tissues (two transduced with a mixture of viruses encoding GFP and RFP and one with GFP alone) were analyzed and scored based on their location and width in the basal compartment (Fig. 3). These data revealed that first, there was no preferential site of origin of EPUs in the basal layer as they originated from the top, sides and base of rete ridges as well as from relatively flat regions of the basement membrane. Second, the number of basal cells in EPUs is highly variable especially for those located in the rete ridges.
Figure 3.
Tabulation of the origin and size of EPUs in human epidermis. Serial sections of transduced human skin were analyzed by fluorescent microscopy at 28 weeks post transduction. A total of 200 EPUs were examined. The basement membrane origin of labeled EPUs is noted as either flat (column A) or side, base or top of the rete ridge (columns B, C and D, respectively). The number of cells at the base of the labeled EPU is noted as well (rows). The percentage of labeled EPUs in each category is reported. Labeled EPUs were seen as originating from all regions of the basal compartment with no preference for any single region.
Labeled EPUs originating from relatively flat regions of basement membrane had the most uniform morphology (Fig. 2C–E). The number of basal cells in an EPU cross-section was variable, however as shown in Figure 3 (column A) more than 78% of EPUs (n=88) in this region were between 1–4 cells in diameter suggesting that at least 10% of basal cells in these areas are stem cells.
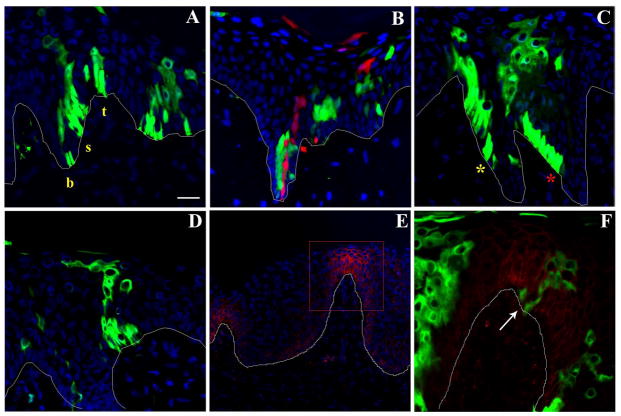
EPU’s originating from undulating areas of the basal layer were less well-organized and often did not follow a straight path through suprabasal layers (Fig 4). Figure 4A shows three GFP-labeled clusters originating from the base, side and top of a rete ridge. The narrowest EPUs originated in the base of rete ridges and most of these contained a single cell at the base, possibly the stem cell itself. Figure 4B demonstrates two independently labeled stem cells and their progeny in the base of a rete ridge. The progeny of the labeled stem cells were positioned directly above, implying an upward migration of the immediate progeny into the differentiated compartment in the suprabasal layers. On the contrary, EPUs originating from the side of the rete ridges occupied a broader position in the basal layer with the majority composed of 5–7 basal cells (Fig. 3). To assume a columnar organization, cells in these EPUs appeared to follow an upward migratory path along the sloping basement membrane (Fig. 4C). These data suggest that stem cell progeny follows a path perpendicular to the surface of epidermis regardless of the stem cell position in the rete ridge. Such organization may be necessary to overcome structural complexity associated with the abrupt change in the orientation of undulating basal layer.
Figure 4.
Lineage analysis of labeled EPUs in human epidermis. Skin samples were treated as described in Fig. 2 and representative sections demonstrating: (A) the distribution of GFP-labeled EPUs in the base (b), side (s) and top (t) of a rete ridge; (B) two adjacent EPUs positioned in the base of a rete ridge, one labeled with GFP (green) and another with RFP (red); (C) two GFP-labeled EPUs denoted with red and yellow asterisks originating from the side of a rete ridge are presented to reveal columnar organization and upward migration of progeny in basal layer; (D) an EPU originating from the tip of dermal papillae is shown; (E–F) immunofluorescent staining of GFP-labeled human skin sections for β-1 integrin (red); (F) high- power micrograph of boxed area in section shown in E demonstrating a GFP-labeled EPU (denoted by an arrow) originating from the tip of dermal papillae. Progeny of stem cells localized to these areas follow an upward migration pattern. Scale bar= 40 μm for A–D; 100 μm for E and 20μm for F.
The findings that keratinocyte stem cells in culture express high levels of β-1 integrin and that, in intact skin, clusters of β-1 integrin bright cells are seen in discrete patches at the tip of dermal papillae, have led to the suggestion that stem cells are located at the dermal papillae and that amplifying cells migrate to the base of rete ridge where they begin their upward, suprabasal migration (Jones et al., 1995; Jensen et al., 1999). In our study, histological analysis of xenografts of human skin immunostained with antibody to β-1 integrin confirmed higher levels of β-1 integrin at the tips of dermal papillae (Fig. 4E); however, genetically labeled EPU’s did not appear to originate from dermal papillae in any preferential way. Labeled EPUs in this region were organized into columnar units and no evidence could be obtained to support lateral migration of labeled cells into the base of the rete ridge (Fig. 4D and F).
Discussion
Most of our knowledge on cell lineage in human epidermis is based on the studies of keratinocytes in culture or regenerated epidermis where stem cells are removed from their microenvironment. In the present study we have used direct lentivirus-mediated gene transfer to a human foreskin xenograft model to genetically mark stem cells in vivo and to study their lineage in a minimally disturbed, steady state condition. At the time of in vivo transduction, the expression of epithelial markers including K14 and involucrin, and proliferation marker Ki67 in the transplanted skin were normalized (data not shown). Furthermore the overall size and gross appearance of grafted skin did not change during the 28-week post-transduction observation period. We showed that in human epidermis where there is an undulating basal layer and varying number of strata, labeled stem cells and their progeny were organized into narrow EPUs. Analysis of labeled EPUs revealed the following findings: (1) There was no preferred site of origin of EPUs in the basal compartment. Of the 200 EPUs analyzed, 44% were traced to the relatively flat regions of the basement membrane, 17% to the side of rete ridges, 22% to the base of rete ridges and 17% to the tip of dermal papillae. (2) The number of basal cells in an EPU cross-section ranged from 1 to 10 with the highest numbers seen in EPUs originating from the sides of rete ridges while the lowest numbers were seen in EPUs originating from the base of the rete ridge. (3) In all EPUs examined, migration from the basal compartment was perpendicular to the skin surface. Where the EPU originated from the side of the rete ridge, upward migration was along the sloping basement membrane.
We found no evidence for clustering of stem cells in association with a specialized structure or niche either at the top or at the base of rete ridges (Jensen et al., 1999; Lavker and Sun, 1982). Our lineage analysis suggests that epidermal stem cells are located along the basal layer as depicted in Figure 5. The narrow EPUs originating from the base of rete ridges and the extended basal compartment in EPUs that originated along the sides of rete ridges implies that a higher number of stem cells are located at the base of the rete ridge. Previous histological and pulse labeling studies of monkey palm epidermis have indicated the presence of cells with stem cell characteristics in this region (Lavker and Sun, 1982). Based on these findings it was proposed that stem cells are clustered at the bottom of epidermal rete ridges in a physically protective niche (Lavker and Sun, 1983). Although our analysis supports the presence of a higher number of stem cells at these sites, the structural constraints of the undulating basal layer are more likely the cause of this phenomenon rather than presence of a specialized protective niche. The presence of EPUs originating from the top of rete ridges rules out the necessity for a protective niche in the deeper regions of epidermis.
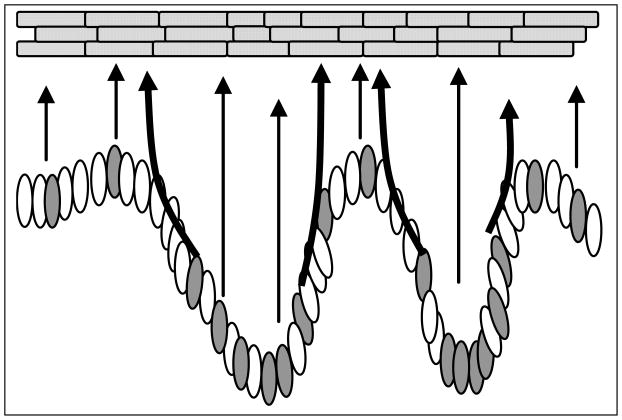
Figure 5.
Schematic diagram showing upward migration patterns in EPUs. Stem cells (gray) are distributed along the undulating basal layer in the base, side and top of the rete ridge structures and give rise to columns of cells directly above. The arrows show the migratory path of stem cell progeny in basal or suprabasal layers perpendicular to the surface of epidermis.
Using high level β-1 integrin expression as a stem cell marker, Watt and collaborators proposed that stem cells are clustered on the tip of dermal papillae, surrounded by integrin-dull transit amplifying cells (Jones et al., 1995). In this model, stem cell progeny migrate over the basement membrane away from stem cell compartment before its upward migration into the suprabasal layers (Jensen et al., 1999). Our studies confirmed the heterogeneous expression of β-1 integrin in basal keratinocytes, however stem cells were not restricted to integrin-bright regions and there was no evidence for lateral migration of stem cell progeny into the integrin-dull regions. These data suggest a re-evaluation of β-1 integrin levels as a marker for epidermal stem cells.
Several approaches have been used to estimate the number of epidermal stem cells. Kinetic considerations based on label retaining studies and the size of EPUs in mouse epidermis predict that 5–10% of basal cells are stem cells (Potten, 1974; Bickenbach and Chism, 1998). Similar studies has not been possible in human; however, the pattern of X chromosome inactivation in normal human epidermis suggested a size ranging from 35 to more than 300 basal cells in diameter for human EPUs (Asplund et al., 2001; Chaturvedi et al., 2002). This is likely an overestimation, as X chromosome inactivation occurs during embryogenesis during a period of extensive epidermal expansion. While regional differences and the non-uniform arrangement of labeled EPUs in human epidermis prevents a precise calculation of stem cell number, our analysis suggests that at least 10% of basal cells are stem cells.
Epidermis has evolved to provide a protective covering for the body. The relatively invariant architecture of epidermis and the presence of extensive intercellular junctions restrict cell movement in human epidermis (Potten and Booth, 2002). As depicted in Figure 5, our analysis indicate that the progeny of stem cells in an undulating basal layer follow an upward migratory path, perpendicular to the skin surface, whether this path is in basal or suprabasal layers. This may be accomplished by controlling the size of the transit amplifying compartment in the basal layer. This compartment appears to be largest when stem cells are located at the side of rete ridge and smallest when located at the base. The non-uniform distribution of stem cells and the variable size of transit amplifying compartment may be required to achieve the structural integrity in regions where the orientation of basal layer and the thickness of epidermis are rapidly changing.
Acknowledgments
We are grateful to Dr. Didier Trono for providing lentiviral vector plasmids; Ning Lin and Robin Harrington for expert technical assistance. This research was supported by grants from the NIH to SG (K01-AR02100) and to LT (R01-DE04511).
Abbreviations
- LVV
Lentiviral vectors
- EPU
Epidermal proliferative unit
- GFP
enhanced green fluorescent protein
- RFP
DS Red2 fluorescent protein
References
- Asplund A, Guo Z, Hu X, Wassberg C, Ponten F. Mosaic pattern of maternal and paternal keratinocyte clones in normal human epidermis revealed by analysis of X-chromosome inactivation. J Invest Dermatol. 2001;117:128–131. doi: 10.1046/j.0022-202x.2001.01385.x. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular somatitis virus G glycoprotein pseudotyped retroviral vectors:concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Chu MS, Carrol BM, Brenner BSJW, Nickoloff BJ. Estimation of size of clonal unit for keratinocytes in normal human skin. Arch Pathol Lab Med. 2002;126:420–424. doi: 10.5858/2002-126-0420-EOSOCU. [DOI] [PubMed] [Google Scholar]
- Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr Opin Cell Biol. 2003;15:771–777. doi: 10.1016/j.ceb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Katz AB, Harrington R, Taichman LB. Lentivirus-Mediated Gene Transfer to Human epidermis. J Invest Dermatol Symp Proc. 2004;9:269–275. doi: 10.1111/j.1087-0024.2004.09302.x. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–188. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Kamimura J, Lee D, Baden HP, Brissette J, Dotto GP. Primary mouse keratinocytes cultures contain hair follicle progenitor cells with multiple differentation potential. J Invest Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Sun T-T. Heterogeneity in epidermal basal kertinocytes: morphological and functional correlations. Science. 1982;215:1239–1240. doi: 10.1126/science.7058342. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun T-T. Epidermal stem cells. J Invest Dermatol. 1983;81:121s–127s. doi: 10.1111/1523-1747.ep12540880. [DOI] [PubMed] [Google Scholar]
- Li AL, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IC. Ordered structure of the epidermis. J Invest Dermatol. 1975;65:45–51. doi: 10.1111/1523-1747.ep12598037. [DOI] [PubMed] [Google Scholar]
- Mackenzie IC. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J Invest Dermatol. 1997;109:377–383. doi: 10.1111/1523-1747.ep12336255. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 1974;7:77–88. doi: 10.1111/j.1365-2184.1974.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Potten CS. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888–899. doi: 10.1046/j.1523-1747.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Watt FM. Epidermal stem cells: marker, patterning and the control of stem cell fate. Phil Trans R Soc Lond. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]