Abstract
Background
Common genetic variants in the IRS1 gene have been recently associated with insulin resistance and hyperinsulinemia. We examined whether the best-associated variant modifies the long-term changes in insulin resistance and body weight in response to weight-loss diets in the Pounds Lost Trial.
Methods and Results
We genotyped IRS1 rs2943641 in 738 overweight adults (61% were women) who were randomly assigned to one of four diets varying in macronutrient contents for 2 years. We assessed the progress in fasting insulin, insulin resistance (HOMA-IR) and weight loss by genotypes. At 6 months, participants with the risk-conferring CC genotype had greater decreases in insulin (P=0.009), HOMA-IR (P=0.015) and weight loss (P=0.058) than those without this genotype in the highest carbohydrate diet group, while an opposite genotype effect on changes in insulin and HOMA-IR (P≤0.05) was observed in participants assigned to the lowest carbohydrate diet group. No significant differences were observed across genotypes in other 2 diet groups. The tests for genotype by intervention interactions were all significant (P<0.05). At 2 years, the genotype effect on changes in insulin and HOMA-IR remained significant in the highest carbohydrate diet group (P<0.05). The highest carbohydrate diet led to a greater improvement of insulin and HOMA-IR (P for genotype-time interaction≤0.009) in participants with the CC genotype than those without this genotype across 2-year intervention.
Conclusions
Individuals with the IRS1 rs2943641 CC genotype might obtain more benefits in weight loss and improvement of insulin resistance than those without this genotype by choosing a high-carbohydrate and low-fat diet.
Keywords: diet, genetic variation, insulin, gene-diet interaction, weight-loss trial
Insulin resistance, the most common metabolic disorder associated with obesity, is a major risk factor for type 2 diabetes and cardiovascular disease.1 Besides the important contribution of environmental factors, such as diet and physical activity, genetic variants that influence insulin signaling also play a key role in insulin resistance.2 Identifying the gene-lifestyle interactions on insulin resistance may clarify the mechanisms underlying the development of type 2 diabetes and cardiovascular disease, and provide effective strategies for disease prevention.
A large body of evidence has shown that dietary interventions are beneficial in promoting weight loss and improving insulin action, though there are still extensive debate about the effectiveness of different diets varying in macronutrient components especially fat and carbohydrates.3-5 Emerging data also indicate that genetic background may modify insulin action in response to dietary interventions.6 The insulin receptor substrate 1 (IRS1) gene encodes IRS1 which is a major mediator between the insulin receptor and phosphatidylinositol 3-kinase (PI3K) in the insulin signaling pathway.7, 8 A recent genome-wide association study identified a common genetic variant rs2943641 associated with insulin resistance, hyperinsulinemia, and type 2 diabetes. 9 A very recent intervention trial reported that IRS1 gene variation might modulate the effect of a low-fat and high-carbohydrate diet on insulin sensitivity.10 However, the sample size of that study was small (59 healthy Caucasians).
In the 2-year Pounds Lost trial,11 the longest and largest comparator trial on weight-loss diets so far, four reduced-calorie diets with different compositions of macronutrients resulted in similar weight loss in 811 overweight participants. The diets also improved fasting insulin levels and insulin resistance. In the present study, we aimed to test whether the newly identified IRS1 variant rs2943641 modifies the effects of weight-loss diets on the long-term changes in body weight, fasting insulin and insulin resistance over the intervention.
Methods
The Pounds Lost Trial
The Pounds Lost trial is a 2-year randomized clinical trial to compare the effects of energy-reduced diets with different compositions of fat, protein and carbohydrate on body weight. The study design, methods, and main results have been described in detail elsewhere.11 Briefly, a total of 811 overweight and obese subjects (25≤ body mass index [BMI] ≤40 kg/m2) aged 30 to 70 years were randomly assigned to one of four diets; the target percentages of energy derived from fat, protein, and carbohydrate in the four diets were 20, 15 and 65%; 20, 25 and 55%; 40, 15, and 45%; 40, 25 and 35%. Major criteria for exclusion were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation. Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed. The diets consisted of similar foods and met guidelines for cardiovascular health, and carbohydrate-rich foods were used having a lower glycemic index. After 2 years, 80% of the participant (n=645) completed the trial. The participants flow diagram has been published previously (Supplemental Figure 1).11 The study was approved by the human subjects committee at the Harvard School of Public Health and Brigham and Women's Hospital, Boston; and the Pennington Biomedical Research Center of the Louisiana State University System, Baton Rouge, Louisiana; and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants gave written informed consent.
Participants
Seven hundred and thirty eight participants with DNA sample available were included in the current study (91% of the participants in the Pounds Lost trial). Among them, 61% were women, 80% were white, 15% were African American, 3% were Hispanic, and 2% were Asian or other ethnic groups by self report. Consistent with the entire Pounds Lost trial, the mean (±SD) age was 51±9 years, and the mean BMI was 32.7±3.9 kg/m2. There was no significant difference in the basic characteristics between the participants with and without DNA samples.
Measurements
Body weight was measured in the morning before breakfast on 2 days at baseline, 6 months and 2 years. Dietary intake was assessed in a random sample of 50% of the participants, by a review of the 5-day diet record at baseline and by 24-hour recall during a telephone interview on 3 nonconsecutive days at 6 months and at 2 years. Fasting blood samples, 24-hour urine samples and measurement of resting metabolic rate were obtained on 1 day, and blood pressure was measured on 2 days, at baseline, 6 months, and 2 years. Levels of serum lipids, glucose and insulin were measured at the clinical laboratory at the Pennington Biomedical Research Center. Blood pressure was measured with the use of an automated device (HEM-907XL, Omron). Insulin resistance was estimated by homeostasis model assessment of insulin resistance (HOMA-IR) calculated by the following equation: (fasting insulin [μU/ml] × [fasting glucose [mg/dl]/18.01])/22.5.12 BMI was calculated as weight (kg)/height2 (cm2).
Genotyping
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood Kit (Qiagen, Chatsworth, CA). Single nucleotide polymorphism (SNP) rs2943641, the best-associated variant with type 2 diabetes and insulin resistance in IRS1 locus,9 was genotyped using the OpenArray™ SNP Genotyping System (BioTrove, Woburn, MA). The genotype success rate was 99%. Replicate quality control samples (10%) were included and genotyped with > 99% concordance. The allele frequency in all participants or in white was in Hardy-Weinberg equilibrium (P>0.05).
Statistical analysis
The primary endpoints for this study were changes in fasting insulin, insulin resistance (estimated by HOMA-IR) and body weight over the intervention. Insulin and HOMA-IR were log-transformed before analysis. General linear models (PROC GLM) for continuous variables and χ2 test (PROC FREQ) for categorical variables were applied for the comparison according to genotype groups at baseline. We compared the changes in the primary endpoints, biomarkers of adherence and nutrient intakes across genotype groups at 6 months and 2 years using generalized linear models. To test for interactions, we examined genotype, intervention (overall 4 diet groups), and genotype-by-intervention interactions as independent predictors of changes in the primary endpoints, adjusted for age, sex, ethnicity and the baseline value for the respective outcome trait, in the generalized linear models. As the majority of the participants were white (80%), we also examined the genotype effects and interactions in white participants in sensitivity analyses. Linear mixed models (PROC MIXED), using variance components structure, were used to test the genotype effect on the trajectory of changes in weight, fasting insulin and HOMA-IR in the participants who provide measurements at baseline, 6 months and 2 years in each of 4 diet groups over the 2-year intervention by including genotype-by-time interaction terms. Secondary analyses were performed to compare the 2 extreme carbohydrate diets (the highest-carbohydrate diet versus the lowest-carbohydrate diet) by the genotype. Consistent with the previous reported association between IRS1 rs2943641 and insulin resistance,9 both additive and dominant inheritance models were tested. All reported P-values are nominal and two-side and a P-value of 0.05 was considered statistical significant. Bonferroni's adjustment was used to adjust P-values for multiple tests for each trait at each time point. We used Quanto 1.2.4 (University of Southern California, Los Angeles, California; http://hydra.usc.edu.gxe) to estimate the detectable effect sizes of genotype-by-diet (2 extreme diets) interactions. The study had 80% power to detect gene-diet interaction effect sizes of 2.6 and 3.4 kg for weight loss, 0.21 and 0.22 log-transformed unit for changes in fasting insulin, and 0.24 and 0.24 log-transformed unit for changes in HOMA-IR at 6 months and 2 years, respectively, under an additive model. Statistical analyses were performed with SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of participants according to the IRS1 rs2943641 genotype. The genotype frequencies were similar between sexes and across the four diet groups, while they differed among ethnic groups (P=0.01). After adjustment for age, sex and ethnicity, the C allele of rs2943641 was significantly associated with increased fasting insulin and HOMA-IR (both β=0.09±0.03, P=0.005), consistent with previous reports.9 We also found associations of this SNP with systolic and diastolic blood pressures (P=0.08 and 0.005, respectively), which was attenuated by further adjustment for fasting insulin (P=0.15 and 0.02, respectively). No other differences in baseline characteristics across the genotype were observed.
Table 1. Baseline Characteristics of the Study Participants*.
| IRS1 rs2943641 Genotype | ||||
|---|---|---|---|---|
| CC (n=331) | CT (n=312) | TT (n=95) | P | |
| Age, yr | 51 ± 9 | 51 ± 9 | 50 ± 9 | 0.55 |
| Sex, n (%) | 0.19 | |||
| Female | 213 (64.4) | 179 (57.4) | 59 (62.1) | |
| Male | 118 (35.6) | 133 (42.6) | 36 (37.9) | |
| Race or ethnic group, n (%) | 0.01 | |||
| White | 258 (43.7) | 259 (43.8) | 74 (12.5) | |
| Black | 61 (54.9) | 34 (30.6) | 16 (14.4) | |
| Hispanic | 5 (20.0) | 17 (68.0) | 3 (12.0) | |
| Asian or other | 7 (63.6) | 2 (18.2) | 2 (18.2) | |
| Diet groups (fat/protein/carbohydrate), n (%)† | 0.63 | |||
| Group 1 (20/15/65%) | 83 (44.4) | 82 (43.8) | 22 (11.8) | |
| Group 2 (20/25/55%) | 91 (50.0) | 69 (37.9) | 22 (12.1) | |
| Group 3 (40/15/45%) | 74 (39.8) | 86 (46.2) | 26 (14.0) | |
| Group 4 (40/25/35%) | 83 (45.4) | 75 (41.0) | 25 (13.7) | |
| Height, m | 1.68 ± 0.09 | 1.69 ± 0.09 | 1.69 ± 0.08 | 0.80 |
| Weight, kg | 94 ± 15 | 93 ± 16 | 94 ± 16 | 0.50 |
| BMI, kg/m2 | 33 ± 4 | 32 ± 4 | 33 ± 4 | 0.47 |
| Waist circumference, cm | 104 ± 12 | 103 ± 13 | 105 ± 14 | 0.91 |
| Blood pressure, mmHg | ||||
| Systolic | 121 ± 13 | 119 ± 13 | 119 ± 13 | 0.08 |
| Diastolic | 77 ± 9 | 75 ± 9 | 74 ± 10 | 0.005 |
| Glucose, mg/dl | 92 ± 13 | 92 ± 11 | 91 ± 12 | 0.30 |
| Insulin, μU/ml‡ | 10.8 (7.1-16.9) | 10.4 (6.9-14.9) | 9.3 (6.4-13.4) | 0.005 |
| HOMA-IR‡ | 2.4 (1.5-4.1) | 2.3 (1.5-3.5) | 2.0 (1.4-2.9) | 0.005 |
| Cholesterol, | ||||
| Total, mg/dl | 204 ± 36 | 200 ± 36 | 202 ± 41 | 0.39 |
| LDL, mg/dl | 127 ± 32 | 124 ± 31 | 127 ± 36 | 0.81 |
| HDL, mg/dl | 49 ± 14 | 49 ± 15 | 48 ± 13 | 0.64 |
| Triglycerides, mg/dl | 147 ± 81 | 142 ± 92 | 137 ± 82 | 0.12 |
| Dietary intake per day | ||||
| Energy, kcal | 1943 ± 546 | 1995 ± 593 | 1949 ± 531 | 0.91 |
| Carbohydrate, % | 44 ± 8 | 45 ± 8 | 46 ± 7 | 0.17 |
| Fat, % | 37 ± 6 | 37 ± 6 | 36 ± 6 | 0.18 |
| Protein, % | 18 ± 4 | 18 ± 3 | 18 ± 3 | 0.56 |
| Urinary nitrogen, g | 12.3 ± 4.6 | 12.1 ± 4.3 | 12.1 ± 4.4 | 0.26 |
| Respiratory quotient | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.84 ± 0.04 | 0.66 |
Data are n (%), means ± SD or median (IQR). P-values were calculated by χ2 test for categorical variables, and F tests after adjusted for age, sex and ethnicity for continuous variables.
The targeted percentages of energy derived from fat, protein and carbohydrate from group 1 to group 4 were 20, 15 and 65%; 20, 25 and 55%; 40, 15, and 45%; 40, 25 and 35%.
These variables were log-transformed before analysis.
Nutrient intake and biomarkers of adherence by the IRS1 genotype
Consistent with the entire Pounds Lost trial, the reported dietary intakes (total energy, fat, protein and carbohydrate) and changes in biomarker of adherence (urinary nitrogen and respiratory quotient) confirmed that participants modified their intake of macronutrients in the direction of the intervention, although the targets were not fully achieved. The mean values of nutrient intakes and biomarkers of adherence at 6 months and at 2 years were similar across IRS1 rs2943641 genotype in each of the four diet groups (all P>0.05) (Table 2). We did not find any significant difference in changes of nutrient intake and biomarkers of adherence at 6 months or at 2 years among IRS1 rs2943641 genotype groups (all P>0.05).
Table 2. Nutrient intake and biomarkers of adherence according to IRS1 rs2943641 genotype and diets at 6 month and 2 years*.
| At 6 months | At 2 years | |||||
|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |
| Diet Group 1 (20/15/65%) | ||||||
| Dietary intake per day† | ||||||
| Energy, kcal | 1639 ± 556 | 1632 ± 467 | 1666 ± 400 | 1525 ± 526 | 1574 ± 494 | 1597 ± 214 |
| Carbohydrate, % | 59 ± 12 | 55 ± 10 | 60 ± 12 | 56 ± 12 | 49 ± 10 | 57 ± 9 |
| Fat, % | 26 ± 8 | 27 ± 7 | 26 ± 10 | 24 ± 8 | 30 ± 8 | 24 ± 5 |
| Protein, % | 17 ± 4 | 18 ± 3 | 17 ± 4 | 19 ± 4 | 20 ± 4 | 21 ± 3 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen, g‡ | 11.5 ± 4.2 | 10.6 ± 3.6 | 11.9 ± 5.7 | 11.6 ± 4.4 | 12.1 ± 5.3 | 12.7 ± 2.6 |
| Respiratory quotient§ | 0.84 ± 0.04 | 0.86 ± 0.05 | 0.85 ± 0.04 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.83 ± 0.04 |
| Diet Group 2 (20/25/55%) | ||||||
| Dietary intake per day† | ||||||
| Energy, kcal | 1644 ± 687 | 1506 ± 470 | 1492 ± 380 | 1528 ± 424 | 1713 ± 599 | 1564 ± 261 |
| Carbohydrate, % | 53 ± 8 | 54 ± 10 | 50 ± 8 | 50 ± 7 | 53 ± 12 | 50 ± 6 |
| Fat, % | 26 ± 7 | 25 ± 7 | 27 ± 7 | 27 ± 7 | 29 ± 10 | 30 ± 7 |
| Protein, % | 21 ± 4 | 22 ± 4 | 23 ± 2 | 21 ± 4 | 20 ± 5 | 21 ± 4 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen, g‡ | 12.0 ± 5.1 | 12.1 ± 3.3 | 10.8 ± 4.2 | 11.8 ± 4.2 | 12.3 ± 3.7 | 11.6 ± 3.1 |
| Respiratory quotient§ | 0.84 ± 0.04 | 0.85 ± 0.04 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.84 ± 0.05 | 0.83 ± 0.03 |
| Diet Group 3 (40/15/45%) | ||||||
| Dietary intake per day† | ||||||
| Energy, kcal | 1546 ± 536 | 1678 ± 501 | 1778 ± 414 | 1465 ± 469 | 1730 ± 664 | 1163 ± 241 |
| Carbohydrate, % | 50 ± 8 | 48 ± 9 | 50 ± 10 | 49 ± 8 | 49 ± 14 | 46 ± 13 |
| Fat, % | 34 ± 7 | 34 ± 6 | 32 ± 7 | 33 ± 7 | 33 ± 11 | 30 ± 4 |
| Protein, % | 18 ± 4 | 17 ± 4 | 20 ± 6 | 20 ± 5 | 18 ± 4 | 25 ± 8 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen, g‡ | 9.6 ± 3.6 | 11.0 ± 5.3 | 11.7 ± 4.3 | 11.2 ± 3.9 | 10.9 ± 4.3 | 12.1 ± 2.6 |
| Respiratory quotient§ | 0.83 ± 0.05 | 0.84 ± 0.04 | 0.83 ± 0.05 | 0.82 ± 0.05 | 0.83 ± 0.04 | 0.84 ± 0.06 |
| Diet Group 4 (40/25/35%) | ||||||
| Dietary intake per day† | ||||||
| Energy, kcal | 1696 ± 525 | 1603 ± 510 | 1553 ± 434 | 1366 ± 442 | 1445 ± 468 | 1429 ± 377 |
| Carbohydrate, % | 42 ± 5 | 44 ± 8 | 42 ± 5 | 42 ± 8 | 44 ± 9 | 43 ± 9 |
| Fat, % | 35 ± 5 | 33 ± 9 | 36 ± 6 | 36 ± 8 | 34 ± 8 | 35 ± 5 |
| Protein, % | 23 ± 4 | 23 ± 5 | 22 ± 4 | 21 ± 5 | 21 ± 6 | 21 ± 5 |
| Biomarkers of adherence | ||||||
| Urinary nitrogen, g‡ | 13.0 ± 5.4 | 12.5 ± 4.3 | 11.3 ± 4.1 | 13.2 ± 6.5 | 12.2 ± 4.0 | 12.5 ± 5.6 |
| Respiratory quotient§ | 0.84 ± 0.05 | 0.84 ± 0.04 | 0.83 ± 0.03 | 0.82 ± 0.04 | 0.83 ± 0.04 | 0.82 ± 0.03 |
Data are means ± SD.
Data were included for 79 to 85 participants per diet group at 6 months and 38 to 45 at 2 years.
Data were included for 127 to 141 participants per diet group at 6 months and 85 to 103 at 2 years.
Data were included for 145 to 155 participants per diet group at 6 months and 103 to 124 at 2 years.
Interactions between the IRS1 genotype and dietary intervention on changes in weight, fasting insulin and HOMA-IR at 6 months and 2 years
At 6 months, we observed significant interactions between IRS1 rs2943641 genotype and dietary intervention (4 groups) on changes in weight (Padd=0.037, Pdom=0.058), fasting insulin (Padd=0.024, Pdom =0.011) and HOMA-IR (Padd=0.025, Pdom =0.013), adjusted for age, sex, ethnicity and the baseline value for the respective outcome trait (Table 3). Participants with the CC genotype had greater weight loss than those without this genotype in the highest carbohydrate diet group (Padd=0.015, Pdom=0.058), while no significant genotype effects were observed in other 3 diet groups (all P>0.14). Participants with the CC genotype had greater decreases in insulin levels than those without this genotype in the highest carbohydrate diet group (Padd=0.006, Pdom=0.009), while an opposite genotype effect was observed in participants assigned to the lowest carbohydrate diet group (Padd=0.098, Pdom=0.05). No significant differences were observed across the IRS1 genotypes in other 2 diet groups (all P>0.14). Moreover, among participants in the highest carbohydrate diet group, after further adjustment for weight loss, the genotype effect on changes in insulin (P=0.04) remained significant; while the genotype effect on weight loss became non-significant after further adjustment for changes in insulin or HOMA-IR (P>0.36). The results for changes in HOMA-IR were similar to those for fasting insulin (Table 3, P=0.05 after further adjustment for weight loss). After adjustment for multiple tests for each trait (4 within-diet tests, significant P-value was 0.0125 [0.05/4]), the genotype effect on changes in fasting insulin and HOMA-IR in the highest carbohydrate diet group remained significance.
Table 3. Changes in weight, fasting insulin and HOMA-IR according to IRS1 rs2943641 genotype and diets at 6 month and 2 years*.
| At 6 months | At 2 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | Padd | Pdom | CC | CT | TT | Padd | Pdom | |
| Weight loss, kg† | ||||||||||
| Diet group 1 (20/15/65%) | -6.5 ± 1.1 | -4.7 ± 1.2 | -3.7 ± 1.5 | 0.015 | 0.018 | -2.9 ± 1.6 | -1.3 ± 1.7 | -3.2 ± 2.1 | 0.70 | 0.37 |
| Diet group 2 (20/25/55%) | -4.8 ± 1.1 | -5.6 ± 1.2 | -6.6 ± 1.6 | 0.14 | 0.19 | -3.2 ± 1.5 | -2.9 ± 1.6 | -2.6 ± 2.1 | 0.74 | 0.77 |
| Diet group 3 (40/15/45%) | -3.4 ± 1.7 | -4.3 ± 1.8 | -4.7 ± 2.1 | 0.30 | 0.29 | -2.4 ± 1.5 | -2.9 ± 1.3 | -2.9 ± 1.9 | 0.75 | 0.72 |
| Diet group 4 (40/25/35%) | -4.9 ± 1.2 | -5.3 ± 1.2 | -6.3 ± 1.6 | 0.30 | 0.44 | -3.0 ± 2.0 | -4.5 ± 2.1 | -3.8 ± 2.4 | 0.38 | 0.22 |
| P for interaction | 0.037 | 0.058 | 0.84 | 0.59 | ||||||
| Change in log-insulin, μU/ml‡ | ||||||||||
| Diet group 1 (20/15/65%) | -0.26 ± 0.09 | -0.10 ± 0.09 | 0.01 ± 0.12 | 0.006 | 0.009 | -0.21 ± 0.10 | 0.02 ± 0.10 | -0.19 ± 0.14 | 0.16 | 0.017 |
| Diet group 2 (20/25/55%) | -0.24 ± 0.09 | -0.35 ± 0.09 | -0.27 ± 0.12 | 0.34 | 0.15 | -0.21 ± 0.10 | -0.23 ± 0.10 | -0.23 ± 0.14 | 0.80 | 0.79 |
| Diet group 3 (40/15/45%) | -0.23 ± 0.08 | -0.19 ± 0.07 | -0.17 ± 0.10 | 0.52 | 0.56 | -0.06 ± 0.10 | -0.10 ± 0.09 | 0.003 ± 0.12 | 0.77 | 0.86 |
| Diet group 4 (40/25/35%) | -0.01 ± 0.12 | -0.15 ± 0.12 | -0.13 ± 0.15 | 0.098 | 0.05 | -0.10 ± 0.13 | -0.21 ± 0.14 | -0.26 ± 0.17 | 0.11 | 0.11 |
| P for interaction | 0.024 | 0.011 | 0.27 | 0.068 | ||||||
| Change in log-HOMA-IR‡ | ||||||||||
| Diet group 1 (20/15/65%) | -0.27 ± 0.10 | -0.11 ± 0.10 | 0.01 ± 0.13 | 0.009 | 0.015 | -0.17 ± 0.11 | 0.06 ± 0.11 | -0.15 ± 0.15 | 0.18 | 0.023 |
| Diet group 2 (20/25/55%) | -0.25 ± 0.10 | -0.36 ± 0.11 | -0.29 ± 0.13 | 0.34 | 0.18 | -0.19 ± 0.10 | -0.20 ± 0.11 | -0.21 ± 0.15 | 0.83 | 0.83 |
| Diet group 3 (40/15/45%) | -0.21± 0.10 | -0.19 ± 0.08 | -0.17 ± 0.12 | 0.67 | 0.72 | -0.05 ± 0.12 | -0.07 ± 0.10 | 0.02 ± 0.14 | 0.77 | 0.97 |
| Diet group 4 (40/25/35%) | 0.00 ± 0.13 | -0.16 ± 0.13 | -0.16 ± 0.16 | 0.057 | 0.029 | -0.05 ± 0.15 | -0.18 ± 0.15 | -0.24 ± 0.19 | 0.093 | 0.093 |
| P for interaction | 0.025 | 0.013 | 0.27 | 0.075 | ||||||
Data are means ± SE after adjustment for age, sex, ethnicity and the baseline value for the respective outcome trait.
Data were included for 69 to 84 (CC), 65 to 73 (CT) and 18 to 23 (TT) participants per diet group at 6 months (total n=661), and 56 to 73 (CC), 55 to 68 (CT) and 17 to 22 (TT) participants per diet group at 2 years (total n=600).
Data were included for 61 to 75 (CC), 62 to 68 (CT) and 17 to 22 (TT) participants per diet group at 6 months (total n=606) and 48 to 61 (CC), 50 to 64 (CT) and 14 to 18 (TT) participants per diet group at 2 years (total n=523); These 2 variables were log-transformed before analysis.
Because the IRS1 rs2943641 genotype was associated with baseline fasting insulin and HOMA-IR, we further examined the interactions between baseline levels of insulin or HOMA-IR (tertiles) and 4 diet groups on changes in fasting insulin or HOMA-IR. There was no significant results (all P for interaction >0.37). Our data suggest that the observed interactions were less likely to be driven by baseline levels of insulin or HOMA-IR.
At 2 years, most participants regained body weight, and there was no significant interaction or genotype effect on weight loss. For changes in fasting insulin and HOMA-IR, the genotype-diet interactions were attenuated, with a marginal significance under the dominant genetic model (P=0.068 and 0.075, respectively). The genotype effect also became weaker but remained significant in the highest carbohydrate diet group (P=0.017 and 0.023, respectively). However, the results did not remain statistical significance after adjustment for multiple tests for each trait (n tests = 4).
In the sensitivity analyses including only white participants, the results were similar. At 6 months, there were significant interactions between genotype and diet on changes in weight, insulin and HOMA-IR under both additive and dominant genetic models (all P<0.05). At 2 years, the results were attenuated but with the same trend
The trajectory of changes in weight, fasting insulin and HOMA-IR
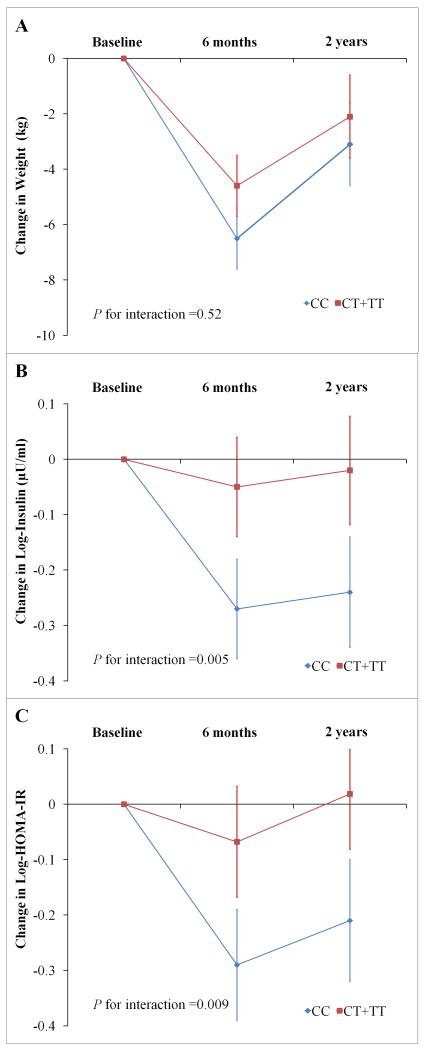
We then used linear mixed models to assess the genotype by time effect over the 2-year intervention, and found significant interactions between genotype and intervention time on changes in fasting insulin (Padd=0.045, Pdom=0.005) and HOMA-IR (Padd=0.05, Pdom=0.009) among participants in the highest carbohydrate diet group; in which participants with the CC genotype had a greater improvement of fasting insulin and insulin resistance than those without this genotype across 2-year intervention (Figure 1). These results did not change after further adjustment for weight loss. However, we did not find the genotype-time interactions on weight loss in this diet group (Padd=0.86, Pdom=0.52). The genotype-time interactions were not observed in other diet groups (Supplemental Figure 2, all P >0.31).
Figure 1. Genotype Effect of IR1 rs2943641 on Trajectory of Changes in Weight, Fasting Insulin and HOMA-IR in Participants Assigned to the Highest-carbohydrate Diet overall 2 Years.
A: changes in weight, data were included for 187, 162 and 158 participants at baseline, 6 months and 2 years, respectively. B: changes in fasting insulin, data were included for 185, 150 and 135 participants at baseline, 6months and 2 years, respectively. C: changes in HOMA-IR, data were included for 185, 150 and 135 participants at baseline, 6months and 2 years, respectively. Data are means ± SE after adjustment for age, sex and ethnicity. P values were tested for the interaction between genotype and intervention time. Insulin and HOMA-IR were log-transformed before analysis.
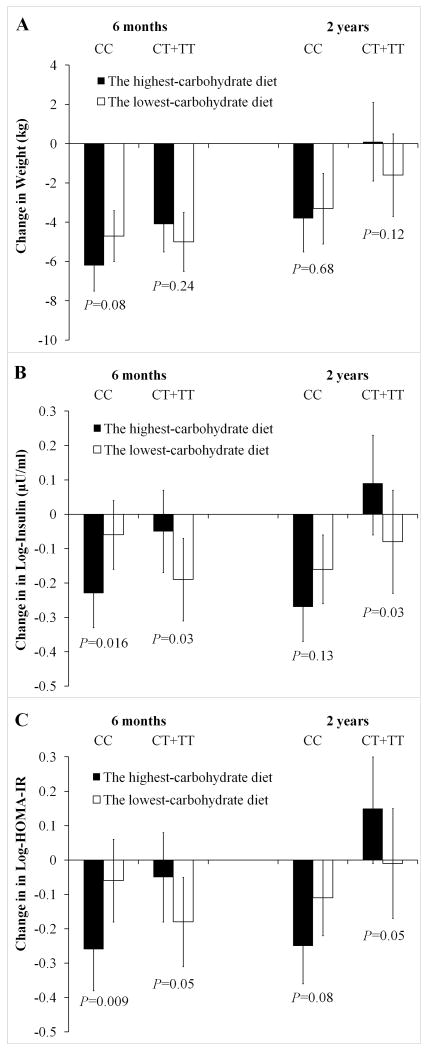
Effects of the highest-carbohydrate and lowest-carbohydrate diets by the IRS1 genotype
Given the opposite effects of the IRS1 rs2943641 genotype in response to the highest-carbohydrate and lowest-carbohydrate diets, we then tested the effects of these 2 extreme carbohydrate diets by the IRS1 rs2943641 genotype as secondary analyses (Figure 2). For participants with the CC genotype, the highest-carbohydrate diet decreased weight, fasting insulin and HOMA-IR more than the lowest-carbohydrate diet (P=0.08, 0.016 and 0.009, respectively). In contrast, the lowest-carbohydrate diet decreased weight, fasting insulin and HOMA-IR more than the highest-carbohydrate diet among participants without CC genotype (P=0.24, 0.03 and 0.05, respectively). At 2 years, the diet effects were attenuated (Figure 2). After adjustment for multiple comparisons (2 within-genotype group [CC and CT+TT genotypes] tests for each trait at each time point, significant P-value was 0.025 [0.05/2]), the diet effect on changes in fasting insulin and HOMA-IR at 6 months among participants with the CC genotype remained statistical significance.
Figure 2. Effect of the Highest-carbohydrate and the Lowest-carbohydrate Diets on Changes in Weight (A), Fasting Insulin (B) and HOMA-IR (C) by IRS1 Genotypes at 6 Months and 2 Years.
Data are means ± SE and P-values are for comparisons between the 2 extreme diet groups, after adjustment for age, sex and ethnicity. Insulin and HOMA-IR were log-transformed before analysis.
Discussion
In this study, we examined the genotype effect of the IRS1 variant rs2943641 on weight loss and insulin resistance in response to four weight-loss diets varying in macronutrient components in the Pounds Lost trial. We found potential gene-intervention interactions on improvement of fasting insulin and HOMA-IR at 6 month and 2 years of follow-up. Participants with the CC genotype showed greater improvement of insulin resistance in response to the highest-carbohydrate diet than those without this genotype.
At baseline, we confirmed the reported association between the IRS1 rs2943641 C allele and insulin resistance and hyperinsulinemia.9 We observed parallel gene-diet interactions on weight loss and improvement of insulin resistance at 6 months. The genotype effect on improvement of fasting insulin and HOMA-IR remained after adjustment for weight loss. Our results suggest that this effect on the changes in insulin resistance might be independent of weight loss, though weight loss has been considered as a cornerstone for improving insulin resistance.3 In contrast, the genotype effect on weight loss diminished after adjustment for changes in fasting insulin or HOMA-IR, suggesting that improvement in insulin resistance may influence weight loss. We have carefully adjusted for the covariates correlated with the genotype such as ethnicity and baseline levels of fasting insulin or HOMA-IR in our analysis. The sensitivity analysis in white participants showed similar results. These analyses indicate that our findings are unlikely to be due to confounding. In addition, there was no interaction between baseline levels of fasting insulin/HOMA-IR and diets.
As expected, the gene-diet interactions on weight loss and insulin resistance were largely attenuated at 2 years. This may be due to diminished adherence that occurred between 6 months and 2 years in the Pounds Lost trial,11 similar to other weight-loss trials.13-17 A relatively large number of dropouts (n=179) at 2 years may also reduce the statistical power. Nonetheless, we still observed that participants with the IRS1 rs2943641 CC genotype showed a greater improvement of insulin resistance compared to those without this genotype, assigned to the highest-carbohydrate diet over the 2-year period. This result indicates a stable and long-term effect of the IRS1 rs2943641 CC genotype on improvement of insulin resistance in response to a weight-loss diet with high carbohydrate. However, it is difficult to tease out which macronutrient is responsible for the observed interactions because the percentages of fat, carbohydrates, and protein changed simultaneously. Since there was little energy difference (about 2%, based on the assessment of diet recall data and urinary nitrogen exertion) derived from protein among the diet groups,18, 19 increased carbohydrate as percentage of energy intake mostly reflects decreased fat intake. Thus, either dietary fat or carbohydrates may modulate the effect of the IRS1 rs2943641. In addition, it should be noted that “high-quality” carbohydrate-rich foods (low glycemic index) were used in this intervention study.
It is interesting that participants with the IRS1 rs2943641 CC genotype associated with higher degree of insulin resistance,9 but showed greater improvement of insulin resistance than those with other genotypes in response to the high-carbohydrate/low-fat diet. Our findings are consistent with a recent study, in which healthy subjects with a risk allele of IRS1 rs1801278 (G972R) for insulin resistance also showed more improvement in insulin sensitivity after eating a high-carbohydrate diet compared with the fat-rich diets.10 The potential mechanisms underlying these findings are unknown but might be related to lipid-induced insulin resistance.20 Previous studies have shown that chronic high-fat diets and increased plasma free fatty acids levels impair insulin signaling by alteration in tyrosine/serine phosphorylation of IRS1 leading to decreased activation of IRS1–associated PI3K activity.21-24 Thus, it is possible that the activation of IRS1–associated PI3K activity may be enhanced in the IRS1 rs2943641 CC subjects when they consume a low-fat and high-carbohydrate diet, in comparison with subjects with other genotypes. However, although IRS1 rs2943641 has been suggested to be associated with IRS1-associated PI3K activity,9 it is unclear whether this variant affects the phosphorylation in key residues of IRS1. The precise mechanisms responsible for the interaction between IRS1 gene variation and diet on insulin action remain to be further clarified.
To the best of our knowledge, this is the first study to date to assess the gene-diet interactions on improvement of insulin resistance with weight-loss in a large and long-term randomized trial. Our data may provide novel information to the development of effective strategies for dietary interventions based on genetic background. However, several limitations of this study warrant consideration. Insulin resistance was assessed by HOMA-IR rather than by using euglycemic glucose clamp technique, since it was not feasible to apply such a technique in this large population-based trial. Because the adherence to various diets declined after 6 months, the power to detect a long-term genotype effect in response to the real difference in macronutrients intake among the diet groups was reduced. Although it is the largest weight-loss trial so far, this study may be underpowered in detecting modest interactions and associations. Because the primary endpoints (insulin and HOMA-IR) and the repeated measurement at 6 months and 2 years were correlated, it is too conservative to treat them as independent analyses, and over-adjustment for multiple comparisons may increase the type 2 error and the risk of false negative. We therefore controlled for multiple tests for each trait at each time point. Of note, our primary analysis was based on priori hypothesis driven by previous studies,9, 10 which is biologically plausible. Most of the participants are whites (80%) in our study and it remains to be determined whether our findings could be generalized in other ethnic groups. In addition, the participants of our study are overweight or obese, therefore further studies are warranted to investigate whether our findings are applicable in general populations. Because the C-allele of this SNP was common in the general populations (frequency = 0.63, 0.92 and 0.77 for Caucasians, East Asians and Africans, HapMap CEU, HCB and YRI data, respectively), this variant could be a proxy of genetic background for choosing diets varying in macronutrients in prevention of insulin resistance and cardiovascular disease.
In conclusion, we found that the IRS1 variant rs2943641 modified insulin resistance response to weight-loss diets in a 2-year randomized trial. Participants with the IRS1 rs2943641 CC genotype might obtain more benefits in weight loss and improvement of insulin resistance than those without this genotype in response to high-carbohydrate/low-fat diet. These novel findings provide supportive evidence for the notion of a personalized nutrition intervention in preventing diseases related to obesity and insulin resistance, such as type 2 diabetes and cardiovascular disease.
Supplementary Material
Clinical Perspective.
Although recent data from gene-environment interaction analyses provide support for the notion of a “personalized” nutrition approach, evidence from clinical trials is scares. Genome-wide association studies have identified common genetic variants in the IRS1 locus associated with insulin resistance and hyperinsulinemia, as well as type 2 diabetes and coronary heart disease. In a two-year randomized weight-loss trial (Pounds Lost), we genotyped the best associated variant (single nucleotide polymorphism rs2943641) in 738 over-weight adults, to examine the modifications of the IRS1 gene variation on the long-term changes in body weight, fasting insulin and insulin resistance in response to weight-loss diets with different compositions of macronutrients. Our results indicated that participants with the IRS1 rs2943641 CC genotype might obtain more benefits in weight loss and improvement of insulin resistance than those without this genotype in response to a high-carbohydrate/low-fat diet. Our data may provide novel information to the development of effective strategies for dietary interventions based on genetic background in preventing diseases related to obesity and insulin resistance, such as type 2 diabetes and cardiovascular disease.
Acknowledgments
We thank the participants in the trial for their dedication and contribution to the research.
Sources of Funding: This study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981), the General Clinical Research Center (RR-02635) and the Boston Obesity Nutrition Research Center (DK46200). Dr. Lu Qi was a recipient of the American Heart Association Scientist Development Award (0730094N).
Footnotes
Disclosures: None.
Clinical Trial Registration Information Preventing Overweight Using Novel Dietary Strategies (Pounds Lost); NCT00072995 (http:www.clinicaltrials.gov/).
References
- 1.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Manco M, Mingrone G. Effects of weight loss and calorie restriction on carbohydrate metabolism. Curr Opin Clin Nutr Metab Care. 2005;8:431–439. doi: 10.1097/01.mco.0000172585.09762.8e. [DOI] [PubMed] [Google Scholar]
- 4.Wood RJ, Fernandez ML. Carbohydrate-restricted versus low-glycemic-index diets for the treatment of insulin resistance and metabolic syndrome. Nutr Rev. 2009;67:179–183. doi: 10.1111/j.1753-4887.2009.00186.x. [DOI] [PubMed] [Google Scholar]
- 5.Kirk E, Reeds DN, Finck BN, Mayurranjan MS, Patterson BW, Klein S. dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Miranda J, Perez-Martinez P, Marin C, Fuentes F, Delgado J, Perez-Jimenez F. Dietary fat, genes and insulin sensitivity. J Mol Med. 2007;85:213–226. doi: 10.1007/s00109-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 7.Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, Iii, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 8.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa Y, Kasuga M, Yazaki Y, Aizawa S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 9.Rung J, Cauchi S, Albrechtsen A, Shen L, Rocheleau G, Cavalcanti-Proenca C, Bacot F, Balkau B, Belisle A, Borch-Johnsen K, Charpentier G, Dina C, Durand E, Elliott P, Hadjadj S, Jarvelin MR, Laitinen J, Lauritzen T, Marre M, Mazur A, Meyre D, Montpetit A, Pisinger C, Posner B, Poulsen P, Pouta A, Prentki M, Ribel-Madsen R, Ruokonen A, Sandbaek A, Serre D, Tichet J, Vaxillaire M, Wojtaszewski JFP, Vaag A, Hansen T, Polychronakos C, Pedersen O, Froguel P, Sladek R. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- 10.Marín C, Pérez-Martínez P, Delgado-Lista J, Gómez P, Rodríguez F, Yubero-Serrano EM, García-Ríos A, Camargo A, Pérez-Jiménez F, López-Miranda J. The insulin sensitivity response is determined by the interaction between the G972R polymorphism of the insulin receptor substrate 1 gene and dietary fat. Mol Nutr Food Res. 2010;55:328–335. doi: 10.1002/mnfr.201000235. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 14.Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 17.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 18.Katan MB. Weight-loss diets for the prevention and treatment of obesity. N Engl J Med. 2009;360:923–925. doi: 10.1056/NEJMe0810291. [DOI] [PubMed] [Google Scholar]
- 19.Purnell JQ. Obesity: Calories or content: what is the best weight-loss diet? Nat Rev Endocrinol. 2009;5:419–420. doi: 10.1038/nrendo.2009.145. [DOI] [PubMed] [Google Scholar]
- 20.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 24.Frangioudakis G, Ye JM, Cooney GJ. Both saturated and n-6 polyunsaturated fat diets reduce phosphorylation of insulin receptor substrate-1 and protein kinase B in muscle during the initial stages of in vivo insulin stimulation. Endocrinology. 2005;146:5596–5603. doi: 10.1210/en.2005-0481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.