Abstract
Signal transduction by the platelet-derived growth-factor receptor β (PDGFR-β) tyrosine kinase is required for proper formation of vascular smooth muscle cells (VSMC). However, the importance of individual PDGFR-β signal transduction pathways in vivo is not known. To investigate the role of two of the pathways believed to be critical for PDGF signal transduction, we have generated mice that bear a PDGFR-β that can no longer activate PI3kinase or PLCγ. Although these mutant mice have normal vasculature, we provide multiple lines of evidence in vivo and from cells derived from the mutant mice that suggest that the mutant PDGFR-β operates at suboptimal levels. Our observations indicate that although loss of these pathways can lead to attenuated PDGF-dependent cellular function, certain PDGFR-β-induced signal cascades are not essential for survival in mice.
Keywords: PDGF, PI3kinase, phospholipase C, chimera, mesangial cells
The platelet-derived growth-factor (PDGF) family induces a variety of cellular responses including cell proliferation, migration, differentiation, and matrix deposition. The PDGF ligands are dimeric molecules encoded by three different genes, PDGF-A, PDGF-B, and the recently described PDGF-C (Li et al. 2000). Two tyrosine kinase receptors, PDGFR-α and PDGFR-β, become activated upon ligand binding and dimerization. PDGFR-α binds all three ligands, but PDGFR-β can only bind the PDGF B-chain with appreciable affinity. Studies in mice have shown that the PDGFR-β and its ligand, PDGF-BB, are required for the formation of the vasculature. Both the PDGFR-β and PDGF-B null mice exhibit defects in pericytes, which are specialized VSMC (Levéen et al. 1994; Soriano 1994; Lindahl et al. 1997a,b). These cells are contractile, singular cells that can be found associated with vascular capillaries and postcapillary venules. In both the PDGFR-β−/− and the PDGF-B−/− mice, the loss of pericytes results in perinatal lethality caused by microaneurisms, especially in the brain and the kidney. The exact mechanism of pericyte failure is unclear, but it is likely owing to the inability of the precursor cells to migrate and/or proliferate in a PDGF-dependent manner.
Like many receptor tyrosine kinases (RTK), the activated β receptor transmits signals by recruiting a variety of SH2-domain-containing proteins to multiple, phosphorylated tyrosine residues. Phospholipase Cγ (PLCγ), the phosphatidylinositol 3′-kinase [P13k] p85 subunit, Ras GTPase-activating protein (RasGAP), the SHP2 phosphatase, and the Src class kinases are all cytosolic molecules that transmit signals downstream from PDGFR-β (for review, see Kazlauskas 1994; van der Geer et al. 1994). A long-standing question regarding these RTK signals is whether each pathway transmits a unique signal or the summation of signals leads to unique biological outcomes. In vitro assays suggest that PI3K and PLCγ binding to the PDGFR-β have qualitative effects on cellular functions such as migration, differentiation, and proliferation (Kundra et al. 1994; Wennstrom et al. 1994; Higaki et al. 1996; Alimandi et al. 1997). PI3K is a lipid kinase that plays a role in actin reorganization, migration, proliferation, and inhibition of apoptosis; PLCγ is a phosphodiesterase whose products lead to intracellular Ca+2 flux and protein kinase C (PKC) activation. Further investigations of mutant β receptors that signal primarily through PI3K or PLCγ have shown that activation of either pathway reconstitutes migration and proliferation in response to PDGF (Valius and Kazlauskas 1993). These results underscore the apparent importance of these two molecules in PDGFR-β signaling as well as their potential overlapping functions in vitro.
One way to analyze the effects of signaling pathways in multiple cell types is to create mice bearing mutant receptors that lack the binding sites for specific downstream molecules. Previously, we have generated mice bearing a PDGFR-β that could no longer bind the PI3-kinase p85 regulatory subunit (F2 mice) (Heuchel et al. 1999). The mice exhibited no developmental defects in any tissue including VSMC. Upon closer examination, we found that the F2 mice were unable to resolve edema in a PDGF-BB-dependent manner. Importantly, mouse embryo fibroblasts (MEFs) derived from these animals exhibited reduced migration and collagen contraction, as expected from results of previous tissue culture experiments (Kundra et al. 1994; Wennstrom et al. 1994; Zent et al. 1998) but showed no reduction in proliferation or adhesion. Because these defects were minor in comparison to the lethal defects of PDGFR-β−/− mice, our results suggested that defects observable in tissue culture experiments may be less manifest in vivo.
Studies on immediate early gene (IEG) induction have shown that accumulation of mutations on the β receptor resulted in a quantitative, but not a qualitative loss of IEG induction in NIH3T3 cells (Fambrough et al. 1999). Therefore, gene transcription may be determined by the sum of signaling pathways coming from a receptor, and several signaling pathways may be redundant. With regard to proliferation, transformation, and migration, several reports have indicated that PLCγ activation may be redundant or complementary to PI3K-induced cellular responses downstream from PDGFR-β (Valius and Kazlauskas 1993; DeMali et al. 1997). To determine if PLCγ activation could be masking loss of PI3K activities in vivo, we generated mice bearing a PDGFR-β that can activate neither PLCγ nor PI3K. Mice homozygous for the mutant PDGFR-β (PDGFR-βF3, or F3) have no distinguishable phenotype, but when PDGFR-βF3/F3 mutant cells are cultured, they exhibit reduced proliferation and migration in response to PDGF. In addition, deficiencies in VSMC and mesangial cells of PDGFR-βF3/F3 mutant mice were observed in a model of experimental glomerulonephritis and upon generation of chimeric animals. Our data suggest that loss of PI3K and PLCγ activation does not incapacitate the β receptor but does depress the ability of the receptor to carry out normal functions. These results provide additional support to the idea that signals from PDGFR-β may be redundant or complementary, but loss of multiple signaling pathways results in observable defects.
Results
Generation of PDGFR-β signaling mutant allele
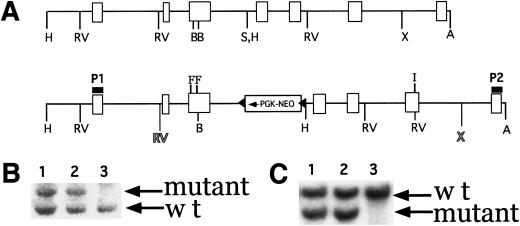
To create a site-specific allele of PDGFR-β, we introduced point mutations into the PDGFR-β locus by gene targeting. Alterations in the coding sequence created point mutations that converted the PI3K binding sites at amino acid residues 739 and 750 from tyrosine to phenylalanine and the PLCγ binding site at residue 1020 from tyrosine to isoleucine (F3 allele). These desired mutations and a neomycin resistance cassette flanked by loxP sites were included in the targeting construct. Figure 1 illustrates the wild type and targeted genomic locus (see Materials and Methods for details on generation of the mice). Mice heterozygous for the F3 allele were intercrossed to generate homozygous mutant offspring that were recovered in normal Mendelian proportions on 129Sv and 129Sv/C57 hybrid backgrounds. Because PDGFR-β null mice are deficient in both pericytes and mesangial cells, we examined the vasculature of the F3 mice to determine if there were any changes in these smooth muscle cell populations. Arterioles, capillaries, and glomeruli of both the developing and adult kidney and brain appeared normal with no apparent hemorrhages, and the aorta and renal arteries were surrounded by a uniform layer of smooth muscle cells as determined by α-smooth muscle actin staining (data not shown). In addition, we observed no upregulation of expression of the PDGFR-β levels in the kidney, when compared to litter mate controls (Fig. 2A and data not shown). Likewise, no increased expression of PDGFR-α was observed in the kidney or the lung (data not shown).
Figure 1.
Generation of mice bearing a mutant PDGFR-β. (A) Wild type genomic and targeted locus. Region of targeting vector is flanked by shaded EcoRV (RV) and XhoI (X) sites. F indicates the exon where the Y ➞ F mutations (PI3kinase binding site) were made, and I indicates the exon where the Y ➞ I (PLCγ binding site) was made. (B) HindIII digest of targeted (lanes 1 and 2) and wild type (lane 3) ES cell clones. Probe P1 was used. Mutant band is at 6.9 kb, and wild type is at 5.2 kb. (C) EcoRV and Asp718 digest of same clones listed in B. Probe P2 was used. Mutant band is at 2.9 kb and wild type is at 4.8 kb. H, HindIII; RV, EcoRV; B, BsaAI; S, SpeI; X, XhoI; A, Asp718. Triangles indicate loxP sites.
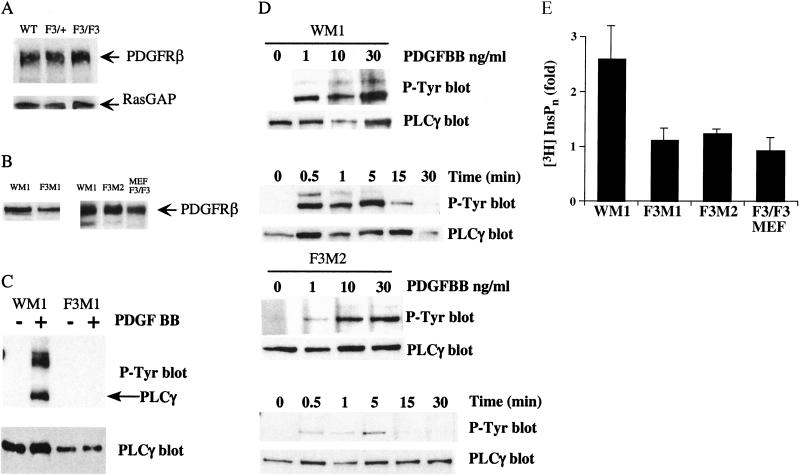
Figure 2.
Expression and signaling of the mutant PDGFR-βF3/F3. Whole kidney lysates from mice (A) and cells (B) were blotted for protein levels. (A) Upper panel, PDGFR-β protein levels. Lower panel, RasGAP levels as a protein loading control. (B) Whole cell lysates from mesangial cells blotted for PDGFR-β protein levels. Lysates loaded in each lane are from 8 × 103 cells. (C–D) PLCγ phosphorylation. PLCγ was immunoprecipitated and blotted for tyrosine phosphorylation. The same filter was stripped and blotted for PLCγ. The lower band in the P-Tyr blot corresponds to PLCγ. (C) Wild type and F3M1 mesangial cells were stimulated with 30 ng/mL PDGF-BB. (D) Upper panels, mesangial cells were stimulated with the indicated concentration of PDGF-BB for 5 min and lysed. Lower panels, cells were stimulated with 30 ng/mL PDGF-BB for the indicated time and lysed. (E) IP3 assay. Cells were labeled o.n. with myo-[3H]inositol and then stimulated with PDGF-BB or 10% FBS. Cells were analyzed for inositol phosphate release. Data represent the mean and standard deviation of two independent experiments. Results are expressed as fold release, where the uninduced sample was used as the baseline and given the value of 1. In one experiment, 10% FBS was used as a positive control, where fold increase was as follows: WM1, 1.6; F3M1, 1.4; F3M2, 3.0; and F3/F3 MEFS, 1.4.
To determine if the F3 mutation led to a hypomorphic allele, we crossed the F3 mice to mice bearing the PDGFR-β null allele and recovered transheterozygotes at the expected frequency with no histological abnormalities. These data indicate that there were no apparent developmental defects in mice bearing only one copy of the mutant PDGFR-β. Taken altogether these results suggest that the mutant F3 PDGF-β receptor can function adequately in vivo to drive VSMC proliferation and/or migration.
Biochemical analysis
To determine if the mutations resulted in loss of the intended signaling pathways, we generated cell lines from the F3/F3 mice and wild type siblings. Because PDGFR-β null mice lack kidney mesangial cells, and others have reported a dependence on PDGF for mesangial cell proliferation (Johnson et al. 1992; Choudhury et al. 1997), we inferred that this particular cell type may be sensitive to alterations in PDGF signaling. Mesangial cells are specialized smooth muscle cells within the kidney glomerulus. We generated one wild type (WM1) and two mutant (F3M1 and F3M2) mesangial cell lines (see Materials and Methods for details on cell line derivation). In addition, we generated several MEF cell lines. Immunoblotting, flow cytometry, and stimulation by PDGF-AA, indicated that no PDGFR-α was present on our three mesangial cell lines (Fig. 3 and data not shown). In contrast, the cells did express PDGFR-β (Fig. 2B).
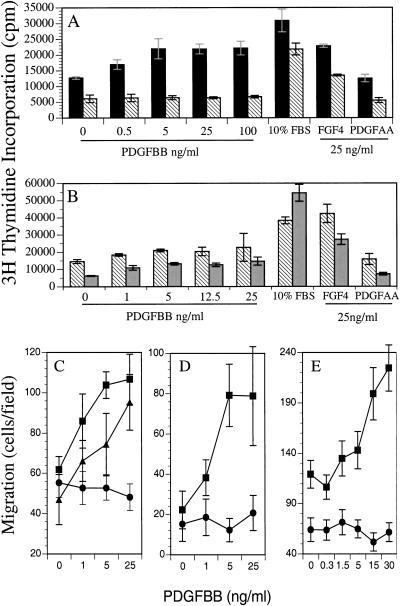
Figure 3.
Lack of mitogenesis and migration in PDGFR-βF3/F3 cells. (A,B). [3H]thymidine incorporation of growth-factor-stimulated mouse mesangial cells. WM1 (A, solid black bars); F3M1 (A, B hatched bars); F3M2 (B, grey bars). Zero growth factor control was treated with vehicle alone. Each bar represents mean ± standard deviation of triplicate samples. Data representative of multiple independent assays. (C–E). Migration of MEFs and mesangial cells in a Neuroprobe chamber. Growth-factor-containing buffer was placed in the bottom well of the chamber. 5 × 104 MEFS or mesangial cells were placed in the top well and the cells were incubated for 5 hr. (C). Solid squares: PDGFR-α−/−/PDGFR-βF3/+ MEFs; solid circles: PDGFR-α−/−/PDGFR-βF3/F3 MEFs; solid triangles: PDGFR-α+/+/PDGFR-βF3/F3 MEFs. (D–E) Solid squares: Wild type mesangial cells (WM1); solid circles: F3 mesangial cells (F3M1 and F3M2, respectively). The number of migrated cells/well was counted in a field of C and D, 40× magnification or E, 20× magnification. Each data point represents the mean ± standard deviation of triplicate samples counted in 3–4 fields. Data are representative of multiple independent assays, except for MEFs, which were only assayed once.
Previously, we have shown that mutation of the PI3K binding sites disrupted PI3K signaling downstream from the receptor, including phosphorylation of phosphatidylinositol 4,5-bisphosphate and activation of the serine/threonine kinase Akt (Heuchel et al. 1999). To analyze the disruption of PLCγ signaling downstream from the F3 mutant receptor, we stimulated the mesangial cell lines with PDGF-BB and determined the extent of PLCγ phosphorylation. Immunoprecipitates of PLCγ were immunoblotted for phosphotyrosine residues (Fig. 2C,D). Both wild type and F3-bearing cells were capable of inducing phosphorylation of PLCγ, but the level of phosphorylation was reduced in the mutant cells. βγβ residual binding and phosphorylation of PLCγ is consistent with previous reports that PLCγ can bind to another tyrosine residue at site 1009 of the human PDGFR-β, albeit with lower affinity (Rönnstrand et al. 1992; Kashishian and Cooper 1993; Valius et al. 1993). To determine if the residual PLCγ binding and phosphorylation lead to PLCγ activation, we tested the cell lines in an inositol phosphates (IPs) formation assay. Wild type cells demonstrated IPs production in response to PDGF-BB stimulation, whereas none of the F3-mutant-receptor-bearing cells showed any appreciable IPs production (Fig. 2E). These results are in agreement with previous results that demonstrated that the mutation at site 1021 of the human PDGFR-β did not prevent PDGF-induced phosphorylation of PLCγ but did abolish inositol phosphate production (Valius et al. 1993). Together these findings demonstrate that PLCγ activation in response to PDGF-BB stimulation is disrupted in F3-mutant-receptor-bearing cells.
In vitro proliferation and migration
Because PLCγ and PI3K are required for proliferation and migration downstream from PDGFR-β, we assayed these cellular functions in the F3 cell lines. When tested for their ability to initiate DNA synthesis, F3 mutant mesangial cells and F3 mutant MEFs exhibited reduced to negligible proliferation when stimulated with PDGF-BB (Fig. 3A; data not shown). In contrast, wild type cells responded in a dose-dependent manner. This remained true even when the F3 cells were stimulated with a relatively high concentration of PDGF-BB (100 ng/mL). To verify that the F3 mutant cells were capable of initiating DNA synthesis, they were treated with two other stimuli that are known to induce DNA synthesis in mesangial cells, FGF and FBS (Shankland et al. 1997). All cell lines were found to proliferate in response to these stimuli. Therefore, the F3 mutant cells do not have a total incapacity to proliferate in these assays, but they do demonstrate a reduced response to PDGF-BB. In four independent experiments, the F3 cell proliferation in response to PDGF-BB was 3%–8% of the FBS-induced proliferation. In two other experiments, the observed F3 mutant cell proliferation was 17%–34% of the FBS-induced proliferation, whereas the wild type PDGF-BB-stimulated cells proliferated at 75%–160% of the FBS control (Fig. 3B).
When the mesangial cells were tested in migration assays, we observed a similar inability of the F3-receptor-bearing cells to respond to PDGF-BB (Fig. 3D,E). Conversely, cells bearing the wild type PDGFR-β migrated toward PDGF-BB in a dose-dependent manner. To determine if the inability of the F3 cells to migrate toward PDGF-BB was a general phenomenon of multiple cell types, we assayed primary MEFs for migration. To prevent interference from PDGFR-α, we crossed the F3 mice to mice bearing a PDGFR-α null allele. We obtained several primary cell lines with different genetic configurations (see Fig. 3 legend) and assayed them at passage 4. MEFs with at least one wild type (PDGFR-α or PDGFR-β) allele migrated in a dose-dependent manner toward PDGF-BB, but cells deficient for PDGFR-α and homozygous for the F3 mutation (PDGFR-βF3/F3) were incapable of migrating toward BB (Fig. 3C). It is interesting that cells homozygous mutant for PDGFR-βF3/F3 but expressing PDGFR-α migrated toward PDGF-BB because it has been suggested that PDGFR-α is inhibitory to migration in certain cell types (Yokote et al. 1996). Therefore, either PDGFR-α is not inhibitory in MEFs, or there may be cross-talk between PDGFR-α and PDGFR-βF3/F3. Taken together, the in vitro assays on F3 mutant cells suggest that either PI3K or PLCγ is required for both proliferation and migration in cultured cells.
Glomerulonephritis
The observation that cultured mesangial cells from mutant mice showed defects in proliferation and migration raised the possibility that the mesangial response could be altered in disease. Previous studies have documented a critical role for PDGF and PDGFR-β in experimental mesangial proliferative nephritis in the rat induced by anti-Thy 1 antibody (Iida et al. 1991; Johnson et al. 1992, 1993). Although the anti-Thy 1 model does not exist in mouse, previous studies have documented mesangial cell activation in experimental glomerulonephritis induced with an anti-glomerular basement membrane (anti-GBM) antibody (Ophascharoensuk et al. 1998).
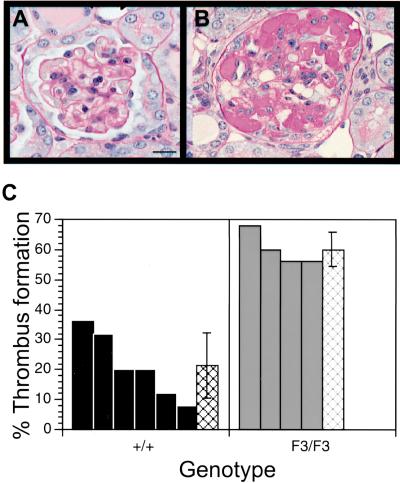
We therefore injected anti-GBM antibody into both wild type and F3 mutant mice. Both animal lines developed equivalent proteinuria and glomerular and tubulointerstitial renal injury as noted by light microscopy at day 12 (data not shown), suggesting that similar injury was induced. Both groups also showed an equivalent frequency of crescents, which is a proliferation and accumulation of cells outside the glomerular tuft but within Bowman's space. However, mutant mice had consistently fewer cells in the glomeruli relative to wild type mice, consistent with a reduction in the mesangial cell population (Table 1). The loss of cells within the glomerular tuft only occurred in the disease state as both mutant and wild type mice had similar numbers of nuclei when they were injected with control serum. Mutant mice also displayed a marked increase in capillary thrombi (Fig. 4). To investigate the identity of the cell types that were lost from the glomeruli, we stained the kidneys for α-smooth muscle actin (a smooth muscle cell marker) and PECAM (an endothelial cell marker). The number of PECAM positive glomeruli was similar between the diseased wild type and F3 kidneys. In contrast, there was a substantial reduction in the number of α-smooth muscle actin positive glomeruli in the F3 kidney when compared to the wild type kidney (data not shown). These data support either a selective loss or an inability to replace mesangial cells within the F3 mutant mice.
Table 1.
Number of nuclei per glomerulus counted to check for mesangial cell loss
|
|
NSS +/+
|
NSS F3/F3
|
GBM +/+
|
GBM F3/F3
|
|---|---|---|---|---|
| Nuclei/glomerulus | 36 ± 6 | 32 ± 6 | 38 ± 7 | 23 ± 6 |
| 35 ± 6 | 34 ± 5 | 28 ± 7 | 21 ± 7 | |
| 34 ± 4 | 31 ± 4 | 34 ± 8 | 24 ± 6 | |
| 31 ± 7 | 23 ± 6 | |||
| 29 ± 9 | ||||
| 26 ± 7 | ||||
| Overall mean | 35 ± 1 | 32 ± 1 | 31 ± 1 | 22 ± 1 |
The number of nuclei within each glomerulus counted with simultaneous check for presence of a thrombus. Thirty-five glomeruli with a diameter of 50–75 microns were counted from each mouse (40× magnification). Nuclei present in the surrounding Bowman's capsule were not included. (NSS normal sheep serum treatment; (GBM) anti-glomerular basement membrane antibody treatment. Mean ± standard deviation is listed for each individual mouse. Overall mean represents all of the data from each genotype group ± SEM.
Figure 4.
Quantitation of glomeruli-containing thrombi. (A,B) PAS-stained sections of anti-GBM-treated kidneys. (A) Glomerulus from a wild type diseased kidney with no thrombus. (B) Glomerulus from an F3 diseased kidney with thrombus formation. (C) Glomeruli were counted blindly for the presence or absence of a thrombus (as shown in A and B). Glomeruli 50–75 micron in diameter were included in the quantitation. Hatched bars indicate the average percentage of glomeruli that contained a thrombus ± standard deviation. No thrombi were observed in wild type or mutant mice that had been treated with normal sheep serum (NSS).
To investigate if the mesangial cell loss was a result of apoptosis, we did TUNEL labeling of the sections, but neither the wild type nor the mutant glomeruli had quantifiable numbers of apoptotic nuclei in the glomerulus at day 12 of the disease (data not shown). This may indicate that most of the cell loss in the glomeruli had already occurred by this time point. Therefore, we were not able to draw conclusions regarding the mechanism of loss of nuclei from the glomerulus, although it is likely that damaged mesangial cells were not efficiently replaced by new mesangial cells. Based on the data from the mesangial cell lines, the mechanism of cell loss from the glomerulus could possibly be reduced mesangial proliferation, the inability of mesangial precursor cells to migrate into the glomerulus, or a combination of defects in both mesangial cell migration and proliferation.
Chimeric analysis
Although VSMC formation and function were normal in the F3 mutant mice, our data on cultured mesangial cells suggested that the F3 mutant receptor does not transmit signals as efficiently as the wild type receptor. To investigate this apparent contradiction, we employed chimeric analysis to explore a potential impairment of the mutant receptor in vivo. Traditionally, chimeric analysis has been used to distinguish between the primary and secondary effects of null mutations, but we used the technique to determine the ability of the F3-mutant-receptor-bearing cells to compete against wild type PDGFR-β-bearing cells. Previously, chimeric analysis of the PDGFR-β null mutation has shown that PDGFR-β−/− cells contribute minimally to capillary pericytes, aortic SMC, cardiac muscle, skeletal muscle (Crosby et al. 1998), and kidney mesangial cells (Lindahl et al. 1998).
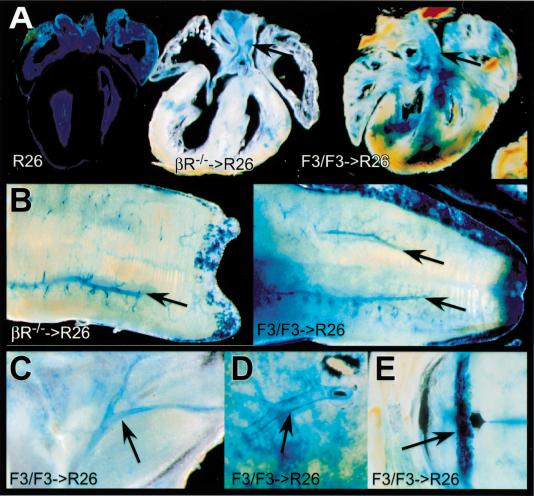
To determine the relative ability of PDGFRF3/F3-bearing cells to contribute to muscle lineages, we generated two independent homozygous F3 ES cell lines. Both yielded similar results. Homozygous mutant ES cells were injected into wild type, β-galactosidase-tagged ROSA26 (R26) blastocysts (Zambrowicz et al. 1997). We then allowed the embryos to develop and analyzed tissues from the resulting chimeras. Similar to what has been reported for PDGFR-β null cells, there was a strong selection against PDGFR-βF3/F3 cells in the layer of smooth muscle cells surrounding the vasculature. Figure 5 illustrates vibratome sections of the heart, tongue, lung, and brain. Even though many cell types in these tissues were predominantly PDGFR-βF3/F3 mutant cells, the VSMC in most tissues were wild type as judged by β-galactosidase staining along the blood vessels. The selection against F3-bearing cells was strikingly similar to the selection observed when chimeras were generated with PDGFR-β null cells (Fig. 5A,B). Both large vessels, such as the aorta, and smaller vessels like those in the choroid plexus showed an accumulation of wild type cells, even though most of the tissue was composed of the mutant F3 cells. A total of 12 extensively mutant chimeras were examined, and all showed similar selection of wild type cells in the vessels.
Figure 5.
Distribution of cells in PDGFR-βF3/F3 ➞ R26+ wild type chimeras and PDGFR-β−/− ➞ R26+ wild type chimeras. (A) Coronal vibratome sections of hearts. Left, R26+ wild type heart (100%); middle, PDGFR-β−/− heart (20% wt chimera); right, PDGFR-βF3/F3 heart (28% wt chimera). (B) Vibratome sectioned tongue. Left, PDGFR-β−/− ➞ R26+ wild type (20% wt chimera); right, PDGFR-βF3/F3 ➞ R26+ wild type (28% wt chimera). (C) Hind limb vasculature from PDGFR-βF3/F3 chimerica (15% wt chimera). (D) Close-up of pulmonary artery from PDGFR-βF3/F3 chimeric lung (28% wt chimera). (E) Choroid plexus from PDGFR-βF3/F3 chimera (29% wt chimera). Arrows in all panels indicate concentrations of wild type cells in vessels. Percent chimerism was determined by Southern blot analysis.
Another interesting point is that the F3 mutant and PDGFR-β null cells were capable of contributing to the skeletal muscle of the tongue and the cardiac muscle of the heart. Although we did not quantitate the relative contribution of cells in these tissues, it was clear that wild type cells were not the dominant cell type as determined by β-galactosidase staining. This is different from what has been previously reported for PDGFR-β null chimeras (Crosby et al. 1998). A possible explanation is that most of the chimeras generated had a greater than 60% contribution of mutant cells as determined by tail DNA. Therefore selection against PDGFR-β null cells in cardiac and skeletal muscle may only occur when there is an abundance of wild type cells present. It should be noted that although we did not see selection against mutant cells in the cardiac and skeletal muscle, we observed selection for wild type cells in smooth muscle cells of the trachea and intestine (data not shown).
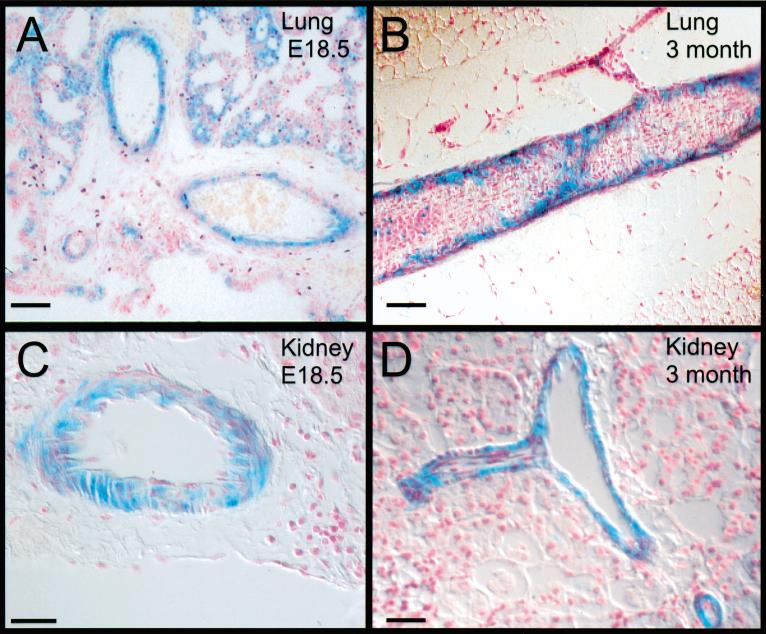
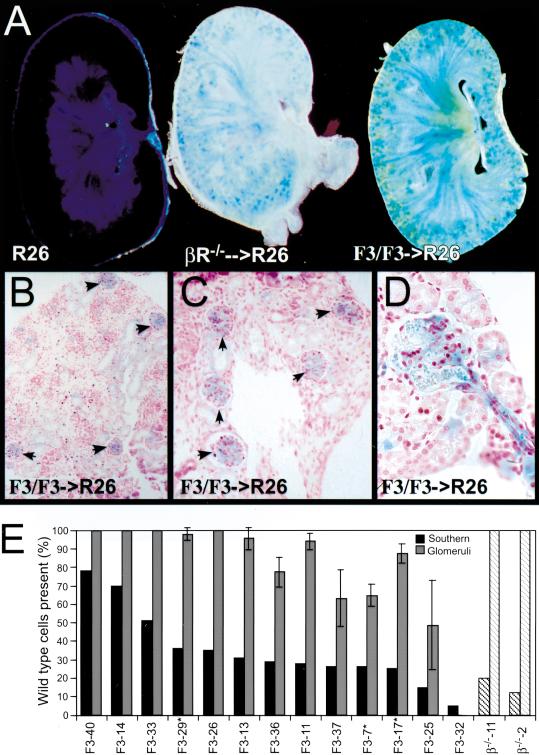
To provide resolution at a cellular level we analyzed 5-μM sections that confirm the whole mount results (Fig. 6). Sections of the lung and kidney showed that although many cell types including interstitial and endothelial cells were mutant, a distinct ring of wild type cells surrounded the vasculature. This selection against F3-bearing cells was observed in perinatal embryos (Fig. 6A,C) and in adult chimeras (Fig. 6B,D). Because quantitation of β-galactosidase positive cells in arteries and veins was difficult, we chose to concentrate our quantitation on mesangial cells, a localized VSMC type that clearly requires PDGFR-β. Sections through the chimeric kidneys showed that as in other tissues, the vasculature appeared to be outlined by wild type cells. In addition, both PDGFR-β−/− ➞ R26 and PDGF-RβF3/F3 ➞ R26 kidneys had a punctate distribution of wild type cells in the cortex of the kidney (Fig. 7A). These spots were the glomeruli of the kidney as shown in the 5-μM sections (Fig. 7B–D). To compare the number of mesangial cells that were wild type to those that were mutant, we quantitated the β-galactosidase positive glomeruli. This method of quantitation may overrepresent the number of glomeruli containing wild type mesangial cells because multiple cell types are present, and we cannot identify a mixture of mutant and wild type cells. Nonetheless, we propose that there was selection for wild type mesangial cells because F3 mutant cells contributed extensively to vascular endothelial cell and epithelial cell lineages in many other tissues of the chimeric embryos. As seen in Figure 7B–D, there is a clear preponderance of wild type cells within the chimeric glomeruli. Only in chimeras that were extensively (>95%) derived from mutant cells did we find a significant number of completely mutant derived glomeruli (Fig. 7E). In most other chimeras, the number of glomeruli that contained no wild type cells were drastically reduced, and in chimeras >50% wild type cell derived, there were no glomeruli that contained only mutant cells. Even when we examined kidneys from adult chimeras (marked by an asterisk), very few glomeruli were exclusively F3 mutant. This result suggests that once wild type cells were established within the glomerulus, the F3 mesangial cells did not replace them over time. Although the selection is not as complete as in the case of the PDGFR-β null derived chimeras, it is clear that in competition with wild type cells, F3-bearing cells contribute minimally to the mesangial cell compartment. Overall, the chimeric data demonstrate that cells bearing the F3 receptor are not as efficient as cells bearing the wild type receptor when placed in an environment such as the developing embryo. The chimeric data did not give a clear picture as to whether proliferation and/or migration may be the critical cellular function, but the cultured mesangial cells suggest that possibly both cellular functions are aberrant.
Figure 6.
Selection against F3-bearing cells in adults and neonates. (A,B) Lung section showing R26+ wild type cells in the vascular smooth muscle cell layers of the pulmonary arteries. (A) E18 chimeric embryo. (B) Adult chimera. (C,D) Arteries and arterioles in kidney of chimeric animals. (C) Arteriole from E18 chimeric embryo. (D) Arcuate artery from adult chimera. Note that the surrounding interstitial and tubule cells are mostly derived from F3 ES cells (not β-galactosidase positive) Bar: A,B, 50 microns; C,D, 25 microns.
Figure 7.
Mesangial cells in the chimeric animals are wild type. (A) Sagittal vibratome section through a R26+ and chimeric E18.5 kidneys. Left, R26+ wild type kidney; middle, PDGFR-β−/− kidney; right, PDGFR-βF3/F3 kidney. 300 micron thickness. (B,C) Presence of β-galactosidase positive cells in the glomerulus of E18.5 kidneys while the surrounding tissue contains randomly scattered β-galactosidase positive cells. (D) Close-up of glomerulus and afferent artery in an adult chimera. (E) Quantitation of glomeruli that contain wild type cells. Black bars represent the percentage of wild type cells in each chimera as determined by quantitation of genomic tail DNA by Southern blot. Grey bars are the average percentage ± standard deviation of glomeruli that contain β-galactosidase positive cells. Four nonconsecutive sections from each chimera were used in the calculation. * Indicates that kidneys were from adult mice; all other analyses were from E18.5 embryos. F3-40 is a chimera that was generated from the ES cell line that had the neo gene deleted. Hatched boxes, chimeras generated using PDGFR-β null cells. Data are organized in descending percent of wild type chimerism as determined from tail DNA.
Discussion
The activation of PI3K and PLCγ downstream from PDGF receptors is key for proliferation and migration in many cultured cells. We have investigated the roles of these two effectors downstream from the PDGFR-β in the mouse. Despite evidence from mutational analysis in cells in culture, loss of the binding of these molecules to PDGFR-β does not significantly disrupt receptor function in mice, especially when compared to the phenotype of PDGFR-β−/− mice. Only when we removed the mutant cells from the animal or created cellular challenges in vivo were defects evident. Although the data from our cultured cells demonstrated that migration and proliferation in response to F3 activation were substantially affected, the in vivo data support an attenuation rather than ablation of cellular responses. Mice bearing the F3 mutant receptor develop normally with apparently intact VSMC. However, the VSMC of chimeric animals was almost exclusively wild type, suggesting that wild type cells were more efficient than mutant cells at populating the VSMC compartment. Other evidence of a deficiency in signaling was obtained when the mice were subjected to experimental glomerulonephritis. These data reinforce the idea that although the F3 receptor mutant is adequate for development and survival, it is not equivalent to the wild type receptor. Unfortunately, our analysis of the F3 mice does not allow us to pinpoint the independent roles of PI3K and PLCγ. We can only determine that either individually or in combination they are important for vascular smooth muscle cell function.
Several explanations exist for why there may be different requirements for PI3K or PLCγ downstream from PDGFR-β in the mouse versus tissue culture cells. The simplest is that the remaining signaling pathways of the receptor somehow compensate for the loss of PI3K or PLCγ. There is no compensation by upregulation of receptor protein because expression levels of the mutant PDGFR-β are very similar to wild type levels. Another way cells could generate a quantitatively greater signal is by sensitizing the downstream targets such that less initial signal from PDGFR-β is required to elicit the desired cellular responses. Signaling networks are highly complex and obviously not linear, but thus far there is little evidence that Src, SHP-2, and RasGAP can replace PLCγ and PI3K in activities such as release of intracellular Ca2+ and activation of PKC and Akt.
Possibly another growth factor receptor can act as a surrogate for binding PI3K and PLCγ. Many receptor tyrosine kinases transmit signals for proliferation and migration using these same signaling molecules. The most likely candidate for this type of assistance is PDGFR-α, which is capable of dimerization with the β receptor, binds the PDGFR-β ligand (PDGF-BB), and signals through a similar set of adaptor proteins. Even though PDGFR-α is the most likely candidate to compensate for loss of signal transduction, our data suggest that PDGFR-α was not upregulated in F3 mice. Although several cell types have been reported to express PDGFR-α and PDGFR-β simultaneously (Ataliotis and Mercola 1997), previous analyses of PDGFR-α in the kidney (Seifert et al. 1998) and our cultured mesangial cells demonstrate that mesangial cells from the mouse do not have abundant levels of PDGFR-α. Therefore, PDGFR-α may not be completely responsible for the maintenance of mesangial cells in the F3 mutant mice. One must also remember that if PDGFR-α could rescue PDGFR-β-type signals, it should be able to do so in the PDGFR-β null mice, and this is certainly not the case in pericytes and mesangial cells. We are left with the argument that another molecule may help in the activation of PI3K and PLCγ.
In vivo, cells must receive and respond to signals through many different pathways simultaneously. Therefore, multiple receptors from different protein families may exist in the same microdomain of the membrane. The localized interactions in these clusters are unclear, but there are examples of PDGFR-β cooperating with other RTKs or integrins to enhance or fortify a signaling response (Sundberg and Rubin 1996; Bruckner et al. 1997; Schneller et al. 1997; Woodard et al. 1998; DeMali et al. 1999). Clearly, these interactions can occur, but testing for their significance in vivo is a difficult task. Further investigations must be done to determine if receptor cooperation is responsible for the development and survival of the F3 mice. Possibly by looking for genetic interactions or enhancement of cellular function in vitro, we can identify key signals that cooperate with those of PDGFR-β.
Compensatory or redundant signals are probably responsible for the differences in the behavior of mesangial cells in vivo and ex vivo, but the chimeric analysis shows that these signals are less effective than wild type PDGFR-β signals. A surprising finding was that the selection against F3-bearing cells in the chimeric animals was not subtle. In fact, the selection was similar to that observed in the chimeras containing PDGFR-β−/− cells. F3 cells failed to participate in many VSMC compartments in the chimeras (both large vessels and capillaries). Therefore, VSMC progenitors may be sensitive to loss of PI3K or PLCγ signals from the PDGFR-β at this stage. Once established, the wild type cells presumably maintained their dominance; they were in place to receive additional cues that would direct proliferation and migration along newly forming vessels. Only in the chimeras that were >95% mutant cell derived, were there mutant cells that contributed noticeably to the vasculature.
The loss of mesangial cells in the experimental glomerulonephritis supports the hypothesis of a reduced ability of the F3 cells to respond to cues induced by damage. During glomerulonephritis in wild type mice, mesangial cells are in a constant flux, where damaged cells are replaced by newly recruited cells (Hugo et al. 1997). In the F3 mutant mice the loss of mesangial cells appeared to be greater than the rate by which they are replaced. This situation resulted in a net loss of cells and a compromised capillary system. Preliminary results from experimental glomerulonephritis studies on the F2 (PI3K disrupted signaling) mutant mice suggest that fewer mesangial cells are lost in these mice. These data support the hypothesis that the additional loss of the PLCγ pathway results in a more severe disease outcome (M.D. Tallquist, unpubl.). We could not determine if the major defect in the F3 mesangial cells was proliferation or migration, but based on the cultured cells both cellular functions are potentially disrupted. In the nondiseased F3 animals, no defects were observed in any glomeruli; therefore, either the normal turnover of mesangial cells is minimal or replacement rate of mesangial cells is below the threshold of capillary disruption. Taken together, both the chimeric data and glomerulonephritis indicate a reduced capacity of VSMC bearing the F3 mutant receptor to respond to surrounding cues as effectively as the wild type cells.
Recent analyses of immediate early gene expression by mutant PDGFR-β are consistent with the findings reported here. These analyses examined a panel of PDGFR-β Y ➞ F mutants (Fambrough et al. 1999). Rather than yielding a qualitative shift in gene expression, stimulation of the mutant receptors exhibited a quantitative change. We have demonstrated that in the F3 mutant mice, the F3 receptor can apparently instruct an adequate cellular response but not as efficiently as the wild type receptor. Although we did not examine the IEG induction by the F3 mutant receptor, it is tempting to speculate that cells with the F3 receptor would also have decreased IEG induction. Perhaps these reduced levels (along with other cellular responses directed by PI3K or PLCγ) translate to the dampened proliferation and/or migration in the F3 mice.
Materials and methods
Mice
The desired receptor alterations were introduced into the PDGFR-β locus by engineering point mutations into the arms of homology as described previously (Heuchel et al. 1999). The PI3K mutations were identical to those already described, whereas the PLCγ mutation was engineered by site-directed mutagenesis. The PLCγ binding site at Y1020 was changed to an isoleucine; this particular codon usage allowed the introduction of an EcoRV restriction site permitting verification of the mutation by Southern blot analysis. The mutated fragment sequences were verified by sequencing before introduction into ES cells. ES cells were screened initially by PCR, then positive clones were verified by Southern blot analysis. Internal and external probes were used on multiple digests to verify correct targeting and absence of concatemers. Two independent ES cells were transmitted through the germline, giving rise to two mouse lines. Sequence analysis of PCR-amplified genomic DNA from homozygous mice verified that all mutations were present in the mutant mouse lines. Most analyses were accomplished using mice on a mixed C57/Bl6 129/Sv background. Exceptions to the genetic background are mentioned where appropriate. In vivo deletion of PGKneo flanked by loxP sites was accomplished by mating the F3 mice to a germline Cre deleter mouse, MORE (Tallquist and Soriano 2000). No difference in phenotype has been observed between neo positive and neo negative alleles. Our inference that neo was not disrupting gene expression was also supported when we crossed F3 homozygous mice to mice bearing the null allele to generate mice transheterozygous for the F3 allele (with neo present) and the null allele. Again, there was no apparent phenotype even with a single copy of the F3 allele.
Cell lines and antibodies
Mutant and wild type MEFs were derived from embryonic day 9 (E9) embryos of PDGFR-α+/− PDGFR-βF3/+ intercrosses, and genotype was determined by PCR as described previously (Soriano 1997; Heuchel et al. 1999). The PDGFR-α null allele was included to avoid stimulation of that receptor by PDGF-BB in assays for cellular functions. Embryos were dissociated by gentle trituration using a 200-μL pipette tip and then plated on gelatin-coated plates. When confluent, the cells were passaged. Migration assays were accomplished between passage 4 and 6 of the MEF cells.
Mutant and wild type mesangial cells were derived by passing 6–8 minced kidneys through a series of mesh screens (250 μM, 125 μM, and 90 μM). Wild type and F3 mutant glomerular filtrates were directly plated on gelatin-coated plates, indicated by WM1 and F3M1 cell lines, respectively. For the second F3 mesangial cell line, F3M2, individual glomeruli were picked after the filtration event and then plated. The resulting cells (epithelial and mesangial) were plated and allowed to grow for four weeks. After the mesangial cells became predominant in the culture, they were passaged every 4–7 days. During the initial passages of the mesangial cells there was not a sufficient number of cells to complete our analysis; therefore, we chose to generate stable lines rather than use primary cells. Although past reports indicate that mesangial cells dedifferentiate after serial passaging, the mesangial cell lines were suitable for our analysis because we wanted cells expressing PDGFR-β and not PDGFR-α. Mesangial cell passages 24–30 were used in both the migration and proliferation assays.
Immunoblot analyses for PDGFR-α and PDGFR-β were accomplished using the 80.8 and 30A rabbit polyclonal antibodies, respectively, kindly provided by A. Kazlauskas. The anti-phosphotyrosine antibody was 4G10, a mouse monoclonal, and anti-phospholipase Cγ-1 was a mixed mouse monoclonal (Upstate Biotechnology).
Assay for release of inositol phosphates
Protocol was performed as described previously (Eriksson et al. 1995). Briefly, six well dishes containing 1.5 × 105 cells were incubated for 36 hr with 2 μCi/well of myo-[3H]inositol in DMEM media containing 0.1% calf serum. Cells were then washed three times with DMEM containing 20 mM HEPES and 20 mM LiCl. The last wash was incubated for 15 min at 37°C. The media was replaced with 2 mL of PDGF-BB-containing media (50 ng/mL) and incubated for 10 min at 37°C. A total of 3 mL of ice cold acidified methanol (100:1, methanol:HCl) was added to the wells. The cells were scraped and placed into tubes containing 1.5:3, water:CHCl3. The samples were mixed vigorously, and the aqueous phase was separated by centrifugation. Two volumes of water were added to the water soluble phase, and the aqueous phase was applied to an AG 1-X8 column (BioRad). The column was washed with 15 mL of 5 mM sodiumtetraborate–60 mM sodium formate. IPs were eluted with 3 mL 0.1 M formic acid–1.0 M ammonium formate. The radioactivity in both the eluted material and the organic phase was subjected to quantitation by scintillation counting. Data are expressed as fold over the uninduced water phase. Two independent assays were performed.
[ 3H]thymidine uptake
Cells were plated at a density of 5 × 104/well in DMEM plus 10% FBS overnight. The cells were washed three times with PBS and placed in DMEM with 0.1% calf serum and insulin, transferrin, and selenium supplement (ITS, Sigma) for 24 hours. Growth factors, serum, or vehicle were then added, and the cells were incubated for 18 hr. Human recombinant PDGF-BB was a kind gift of C. Hart, Zymogenetics. Human recombinant PDGF-AA and FGF-4 were purchased from Boehringer Mannheim and R&D Systems, respectively. The medium was then replaced with 0.5 mL DMEM (5% calf serum) containing 1 μCi [3H]thymidine/mL. After 4 hr, cells were washed with ice cold PBS, then with 5% trichloroacetic acid and lysed with 0.25 N NaOH. The acid-precipitable material was harvested and quantitated in the presence of scintillation fluid. Data represent the average of triplicate wells.
Migration assay
Migration toward growth factor was carried out as previously described (Heuchel et al. 1999). Briefly, 5 × 104 cells in 0.1% BSA/DMEM were added to the top chamber of a NeuroProbe 48 well chemotaxis chamber (NeuroProbe, Gaithersburg, MD). The polycarbonate membranes (8-μm pores) were coated with collagen type I (Collaborative Biomedical Products, Bedford, MA). The lower compartment contained 0.1% BSA/DMEM with or without growth factor (see above). Chambers were incubated for 4–5 hr at 37°C, after which adherent cells were fixed and stained with HemaStain 3 (Sigma). The number of cells that had migrated to the lower surface of each well was counted under 20× magnification. Each treatment was run in triplicate.
Glomerulonephritis
Six-week-old mice generated from 129/C57 hybrid intercrosses were used in these studies. Six homozygous wild type and 6 F3 homozygous mutant mice were injected on two consecutive days with sheep anti-rabbit glomerular basement membrane antibody (Ophascharoensuk et al. 1998). Three mice of each genotype were injected with normal sheep serum as negative controls. Kidneys from mice were analyzed 12 days after the second injection of antibody. During the course of the experiment two F3 mutant mice died; therefore, we were not able to analyze these mice and tissues. Proteinuria levels were determined for all mice, and only the mice injected with the anti-GBM antibody had increased proteinuria. Sections for nuclei and thrombi quantitation were PAS stained. The number of nuclei/glomerulus were counted under 40× magnification, blindly. Glomeruli counted were 50–75 microns in diameter from a total of 35 glomeruli for each mouse. Simultaneously, the presence or absence of a thrombus was recorded. Immunohistochemistry was performed according to manufacturers' protocols with the exception that α-smooth muscle actin antibody was used at a 1:7000 dilution. α-Smooth muscle actin antibody (clone 1A4, Sigma) and PECAM antibody (CD31, Pharmingen) were used for the analysis.
Chimeric analysis
ES cell lines were isolated from blastocysts recovered from PDGFR-βF3/+ × PDGFR-βF3/F3 intercross matings on the 129 background. Two independent ES cell lines were generated, and the homozygous mutant line genotypes were verified by Southern blot. Chimeric mice were generated by injecting ROSA26+/− (Zambrowicz et al. 1997) blastocysts with 13–15 PDGFR-βF3/F3 mutant ES cells. Most embryos were isolated 16 days after the day of injection (equivalent to embryonic day 18) by Cesarean section. Tissues were fixed for 30 min in 2% formaldehyde, 0.2% glutaraldehyde in PBS. Then 200–300-μm vibratome sections were generated and fixed for an additional 30 min. The thick sections were then stained overnight for β-galactosidase activity (MacGregor et al. 1995). Staining controls were accomplished on both β-galactosidase-expressing (R26-positive) and β-galactosidase-nonexpressing (R26-negative) embryos. Background β-galactosidase was observed in developing bone and the intestines, but all other areas were devoid of positive staining. Photographs were taken before the tissues were further processed for paraffin embedding and subsequent 5-μm sections. β-Galactosidase-positive glomeruli were quantitated by counting glomeruli from 4 nonconsecutive sections using (percent of wild type glomeruli) = (number of glomeruli-containing β-galactosidase-positive cells)/(total number of glomeruli) × 100.
Acknowledgments
The authors thank Srinath Sampath, Jared Ragland, and Renee Hoch for their help in this project during their rotations. Many thanks to Jon Cooper and our lab colleagues for critical review of the manuscript. We would also like to thank Andrius Kazlauskas for PDGFR-α, PDGFR-β, and PLCγ antibodies; and Charlie Hart for PDGF-BB. Karin Weismann provided superb technical assistance. This work was supported by grants to P. Soriano from NICHD (HD24875 and HD25326) and to M.D. Tallquist from the American Cancer Society (PF-98-149-01).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL psoriano@fhcrc.org; FAX (206) 667-6522.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.844700.
References
- Alimandi M, Heidaran MA, Gutkind JS, Zhang J, Ellmore N, Valius M, Kazlauskas A, Pierce JH, Li W. PLC-gamma activation is required for PDGF-βR-mediated mitogenesis and monocytic differentiation of myeloid progenitor cells. Oncogene. 1997;15:585–593. doi: 10.1038/sj.onc.1201221. [DOI] [PubMed] [Google Scholar]
- Ataliotis P, Mercola M. Distribution and functions of platelet-derived growth factors and their receptors during embryogenesis. Int Rev Cytol. 1997;172:95–127. doi: 10.1016/s0074-7696(08)62359-1. [DOI] [PubMed] [Google Scholar]
- Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Amer J Phys. 1997;273:F931–938. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF. Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet. 1998;18:385–388. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Whiteford CC, Ulug ET, Kazlauskas A. Platelet-derived growth factor-dependent cellular transformation requires either phospholipase Cγ or phosphatidylinositol 3 kinase. J Biol Chem. 1997;272:9011–9018. doi: 10.1074/jbc.272.14.9011. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Balciunaite E, Kazlauskas A. Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J Biol Chem. 1999;274:19551–19558. doi: 10.1074/jbc.274.28.19551. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Nanberg E, Rönnstrand L, Engstrom U, Hellman U, Rupp E, Carpenter G, Heldin CH, Claesson-Welsh L. Demonstration of functionally different interactions between phospholipase C-γ and the two types of platelet-derived growth factor receptors. J Biol Chem. 1995;270:7773–7781. doi: 10.1074/jbc.270.13.7773. [DOI] [PubMed] [Google Scholar]
- Fambrough D, McClure K, Kazlauskas A, Lander ES. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell. 1999;97:727–741. doi: 10.1016/s0092-8674(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Heuchel R, Berg A, Tallquist M, Ählen K, Reed RK, Rubin K, Claesson-Welsh L, Heldin CH, Soriano P. Platelet-derived growth factor β receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3′ kinase signaling. Proc Natl Acad Sci USA. 1999;96:11410–11415. doi: 10.1073/pnas.96.20.11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki M, Sakaue H, Ogawa W, Kasuga M, Shimokado K. Phosphatidylinositol 3-kinase-independent signal transduction pathway for platelet-derived growth factor-induced chemotaxis. J Biol Chem. 1996;271:29342–29346. doi: 10.1074/jbc.271.46.29342. [DOI] [PubMed] [Google Scholar]
- Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest. 1997;100:786–794. doi: 10.1172/JCI119592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, Seifert R, Alpers CE, Gronwald RG, Phillips PE, Pritzl P, Gordon K, Gown AM, Ross R, Bowen-Pope DF, et al. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci USA. 1991;88:6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Raines EW, Floege J, Yoshimura A, Pritzl P, Alpers C, Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Floege J, Couser WG, Alpers CE. Role of platelet-derived growth factor in glomerular disease. J Amer Soc Neph. 1993;4:119–128. doi: 10.1681/ASN.V42119. [DOI] [PubMed] [Google Scholar]
- Kashishian A, Cooper JA. Phosphorylation sites at the C-terminus of the platelet-derived growth factor receptor bind phospholipase C γ 1. Mol Biol Cell. 1993;4:49–57. doi: 10.1091/mbc.4.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A. Receptor tyrosine kinases and their targets. Curr. Opin Gen & Dev. 1994;4:5–14. doi: 10.1016/0959-437x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, Zetter BR. Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes & Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellström M, Bostrom H, Li H, et al. PDGF-C is a new protease-activated ligand for the PDGF α-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997a;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Karlsson K, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997b;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellström M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rβ signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- MacGregor GR, Zambrowicz BP, Soriano P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development. 1995;121:1487–1496. doi: 10.1242/dev.121.5.1487. [DOI] [PubMed] [Google Scholar]
- Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RG. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Intl. 1998;54:416–425. doi: 10.1046/j.1523-1755.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Rönnstrand L, Mori S, Arridsson AK, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin CH. Identification of two C-terminal autophosphorylation sites in the PDGF β-receptor: Involvement in the interaction with phospholipase C-γ. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert RA, Alpers CE, Bowen-Pope DF. Expression of platelet-derived growth factor and its receptors in the developing and adult mouse kidney. Kidney Intl. 1998;54:731–746. doi: 10.1046/j.1523-1755.1998.00046.x. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Pippin J, Flanagan M, Coats SR, Nangaku M, Gordon KL, Roberts JM, Couser WG, Johnson RJ. Mesangial cell proliferation mediated by PDGF and bFGF is determined by levels of the cyclin kinase inhibitor p27Kip1. Kidney Intl. 1997;51:1088–1099. doi: 10.1038/ki.1997.151. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF β-receptor mutant mice. Genes & Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- ————— The PDGF α receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Rubin K. Stimulation of β1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF β-receptors. J Cell Biol. 1996;132:741–752. doi: 10.1083/jcb.132.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist M, Soriano P. Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Valius M, Kazlauskas A. Phospholipase C-γ 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor's mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Valius M, Bazenet C, Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor β subunit and are required for binding of phospholipase C γ and a 64-kilodalton protein, respectively. Mol & Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Ann Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- Wennström S, Siegbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF β-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111:469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- Yokote K, Mori S, Siegbahn A, Rönnstrand L, Wernstedt C, Heldin CH, Claesson-Welsh L. Structural determinants in the platelet-derived growth factor α-receptor implicated in modulation of chemotaxis. J Biol Chem. 1996;271:5101–5111. doi: 10.1074/jbc.271.9.5101. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zent R, Ailenberg M, Silverman M. Tyrosine kinase cell signaling pathways of rat mesangial cells in 3-dimensional cultures: Response to fetal bovine serum and platelet-derived growth factor-BB. Exp Cell Res. 1998;240:134–143. doi: 10.1006/excr.1998.4008. [DOI] [PubMed] [Google Scholar]