Abstract
The preparation and characterization of a mixed-valence π-cation radical derivative of an iron(III) oxochlorinato complex is reported. The new complex has been synthesized by the one-electron oxidation of a pair of [Fe(oxoOEC)(Cl)] molecules to form the dimeric cation [Fe(oxoOEC)(Cl)]+2. The cation has been characterized by an X-ray analysis, Mössbauer spectroscopy, UV-vis and near-IR spectroscopy, and magnetic susceptibility measurements from 6–300 K. The crystal structure shows that the two rings have a smaller overlap area that those of the formally related nickel and copper octaethylporphyrinateethylporphinate derivatives, reflecting the larger steric congestion at the periphery in part of the oxochlorin rings. The Mössbauer data is consistent with two equivalent iron(III) centers. The unpaired electron is delocalized over the two oxochlorin rings and mediates a strong antiferromagnetic interaction between the high-spin iron(III) centers.
Introduction
The electronic interactions between pairs of porphyrin rings with the π systems in close proximity play an important role in such diverse systems as photosynthesis1 and organic conductors.2 The photosynthetic proteins include the light-harvesting chlorophyll arrays3 and the photosynthetic reaction center (RC)4 special pair.5 The photoinduced formation of the one-electron oxidized reaction center special pair (the primary donor, often called P+) is the first step in the conversion of light energy to chemical energy. The presence of a strongly interacting pair of bacteriochlorophylls in photosynthetic bacterial reaction centers was originally inferred from spectroscopic measurements.6 The special pair of the reaction center from the purple bacterium Rhodopseudomonas viridis has two bacteriochlorophyll planes separated by about 3.3 Å in a “slipped” conformation, as shown by an X-ray structure determination.7 Structures of reaction centers from other bacteria show similar structures for their special pairs.8 The special pair has important physiochemical properties and an understanding of the structural basis for the interaction of the pair of bacteriochlorophylls is of interest. Two different, broad classes of bisporphyrin compounds have been studied as the photosynthetic reaction center models, i.e. covalently linked species9–17 and sandwich complexes.18–25
As an alternative to this approach for studying the photooxidized special pair of the reaction centers, we have shown that metallooctaethylporphyrinate derivatives can be oxidized to form π-cation radical derivatives in which only one electron is removed per two porphyrin rings rather than the expected one electron per porphyrin ring.26–28 We suggested “mixed-valence π-cation radical” as a descriptive name for this new class of synthetic porphyrin derivatives since the unpaired porphyrin electron is delocalized over the pair of porphyrin rings. The species were exceptionally stable and thus could allow for detailed physical characterization. We have attempted to extend the characterization of mixed-valence π-cations to derivatives of iron octaethylporphyrinates since a number of methods might be used to better understand the electron delocalization, spin-spin coupling and electronic communication. Unfortunately, all of the iron octaethylporphyrinate derivatives that we attempted to prepare were plagued by extreme insolubility and no derivative appropriate for characterization could be obtained.
We have now been able to prepare and characterize a related mixed-valence species based on the porphyrin ligand oxooctaethylchlorin (oxoOEC) that has one modified pyrrole ring. The new complex, [Fe(oxoOEC./2)(Cl)]2SbCl6, has been characterized by an X-ray analysis, UV-vis and near-IR spectroscopy, Mössbauer spectroscopy in both low and high applied magnetic field and magnetic susceptibility measurements between 6 and 300 K. The measurements are consistent with two high-spin iron(III) centers strongly coupled by the mediation of the unpaired electron that must be delocalized over both rings.
Experimental Section
General Information
H2OEP was synthesized by literature methods.29 Iron(II) chloride was purchased from Acros, and tris-(4-bromophenyl)aminium hexachloroantimonate was purchased from Aldrich. Dichloromethane and hexanes were distilled under argon from CaH2 and sodium/benzophenone, respectively. H2(oxoOEC) was synthesized by literature procedures.30–32 Insertion of iron into H2(oxoOEC) was accomplished by the reaction of the free base and iron(II) chloride in DMF.33 [Fe(oxoOEC.)(Cl)]SbCl6 was prepared by literature methods.34 Reactions involving the π-cation radical were performed under argon using a double manifold vacuum line, Schlenkware, and cannula techniques.
Synthesis of [Fe(oxoOEC./2)(Cl)]2SbCl6
[Fe(oxoOEC)(Cl)] (18.8 mg, 0.029 mmol) and tris(4-bromophenyl)aminium hexachloroantimonate (12 mg, 0.015 mmol) were placed in a 100-mL Schlenk flask and dichloromethane (~15 mL) was added. The solution was stirred for 1 h. Hexane was added to induce precipitation. The precipitate was filtered under argon and allowed to dry under vacuum for several hours. UV-vis/near-IR (CH2Cl2 solution): λmax (logε): 384 (4.7), 496 (3.9), 601 (4.0), 741 (3.4), 1417 (3.0) nm.
UV/visible/near-IR Spectroscopic Measurements
Absorption spectroscopy was recorded on a Perkin-Elmer Lambda 19 UV-vis/near-IR spectrometer. CH2Cl2 solutions of neutral Fe(oxoOEC)(Cl)] and the π-cation radical Fe(oxoOEC.)(Cl)]SbCl6 were each placed in one of the compartments of a Teflon-stoppered quartz mixing cell (total path length 0.87 cm). The experiments used the same concentrations for the two species. The “before reaction” spectrum was recorded from 300 to 3000 nm, the cell was then inverted, the two solutions was mixed thoroughly, and an “after reaction” spectrum was taken over the same spectral range.
X-Ray Structure Determinations
X-ray diffraction data were collected on a Nonius FAST area-detector diffractometer. Our detailed methods and procedure for small molecule X-ray data collection have been described previously.35 The structure was solved by direct methods.36 The structure was refined against F2 using SHELXL,36 in which all data collected were used including negative intensities. Hydrogen atoms of the porphyrin ligand and the solvent molecules were idealized with the standard SHELXL idealization methods. The absorption correction program DIFABS37 was applied. Brief crystal data are listed in Table 1. Complete details of the structure determination is available in the Supporting Information.
Table 1.
Brief crystallographic data and data collection parameters
| [Fe(oxoOEC•/2)(Cl)]2SbCl6 | |
| Formula | C36H44ClFeN4·0.5SbCl6·CH2Cl2 |
| FW | 892.2 |
| a, Å | 10.333(2) |
| b, Å | 12.555(3) |
| c, Å | 15.849(3) |
| α, deg | 83.64(3) |
| β, deg | 77.53(3) |
| γ, deg | 87.19(3) |
| V, Å3 | 1994.6(7) |
| Z | 2 |
| Space group | |
| Dc, g/cm3 | 1.486 |
| F(000) | 915 |
| μ, mm–1 | 1.146 |
| Radiation (λ, Å) | 0.71073 |
| Temperature, K | 127(2) |
| Final R indices [I > 2 σ(I)] | R1 = 0.0983; wR2 = 0.2397 |
| Final R indices [for all data] | R1 = 0.1633; wR2 = 0.2935 |
Physical Characterization
IR spectra were obtained on a Perkin-Elmer model 883 as KBr pellets. Mössbauer measurements were performed on a constant acceleration spectrometer from 4.2 K to 300 K with optional small field and in a 9 T superconducting magnet system (Knox College). Mössbauer velocity scales are referred to the centroid of the room temperature spectrum of a metallic iron foil. Magnetic susceptibility measurements were obtained on ground samples in the solid state over the temperature range 6−300 K on a Quantum Design MPMS SQUID susceptometer. All samples were immobilized in Dow Corning silicone grease. Measurements at two fields (2 and 20 kG) showed that no ferromagnetic impurities were present. χM was corrected for the underlying porphyrin ligand diamagnetism according to previous experimentally observed values;39 all remaining diamagnetic contributions (χdia) were calculated using Pascal's constants.40,41 All measurements included a correction for the diamagnetic sample holder and diamagnetic immobilizing agent. Magnetic susceptibility and Mössbauer spectroscopic measurements were taken on portions from the same sample preparation.
Results and Discussion
Structural Properties
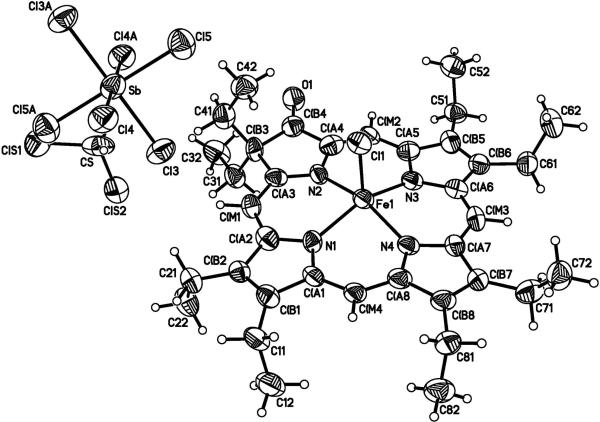
The molecular structure and the stoichiometry of the mixed-valence π-cation radical [Fe(oxoOEC./2)(Cl)]2SbCl6 has been determined by an X-ray structure analysis. Figure 1 shows a labeled ORTEP diagram of the crystallographically unique portion of the dimeric [Fe(oxoOEC./2)(Cl)]+2 cation and the associated half [SbCl6]− ion that has crystallographically required inversion symmetry.
Figure 1.
ORTEP diagram with the atom labeling scheme for the crystallographically unique portion of [Fe(oxoOEC./2)(Cl)]2SbCl6·2CH2Cl2. Thermal ellipsoids of all atoms are contoured at the 50% probability level. The [SbCl6]− counterion is located at the inversion center at 0,0,0 and only half of the atoms displayed in the anion are unique.
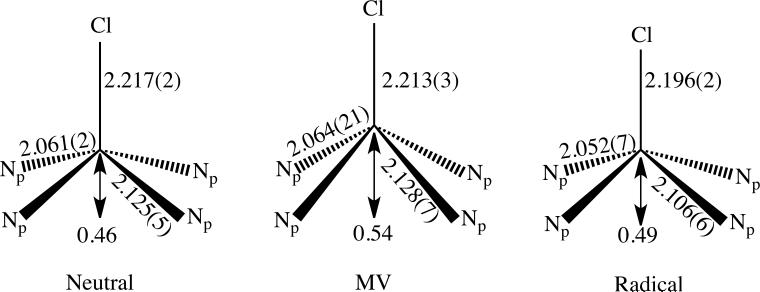
The equatorial Fe-N bond distances range from 2.047(8) to 2.124(8) Å; there are two types of Fe–N bond distances as observed in [Fe(oxoOEC)(Cl)],34 [Fe(oxoOEC.)(Cl)]SbCl6,34 and other metal β-oxoporphyrins.42–48 The metal to nitrogen distance is longer to the pyrrolinone ring than to the pyrroles: Fe–N(2) = 2.124(8) Å vs. the averaged Fe–Np distance = 2.060(18) Å, comparable to those found in [Fe(oxoOEC)(Cl)] 2.125(5) Å vs. 2.061(5) Å) and [Fe(oxoOEC.)(Cl)]SbCl6 (2.106(6) Å vs. 2.052(7) Å). Differences between the three species appear to be a consequence of differences in the macrocyclic conformation. The axial Fe–Cl distances and iron atom displacements are also similar, a complete comparison is shown in Figure 2. We can thus conclude that the oxidation must be primarily that of ring oxidation and not oxidation of an Fe(III) center.
Figure 2.
Schematic diagram comparing the FeN4Cl coordination group geometries of the neutral(left), mixed-valence radical (middle) and radical (right) derivatives of [Fe(oxoOEC)Cl]. All distances are in Å.
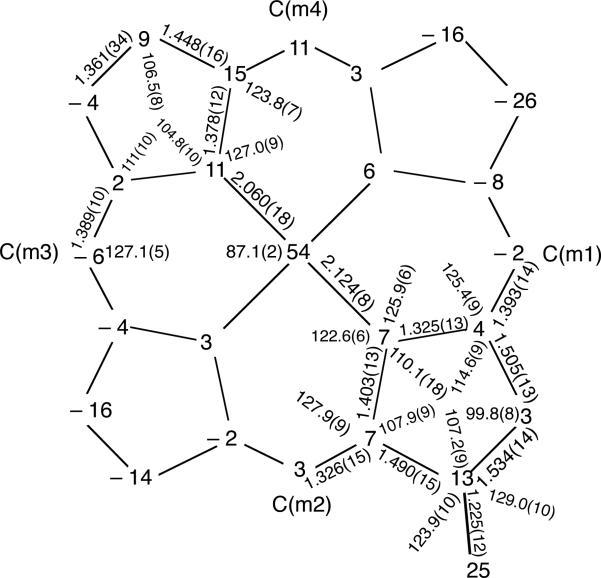
Figure 3 shows a formal diagram giving the perpendicular displacements of each atom from 24-atom mean plane. All three [Fe(oxoOEC)Cl] complexes of Figure 2 have a saddled conformation, but the magnitude of the saddle distortion decreases with increasing oxidation level. The mixed-valence complex has β-carbon atom displacements ranging from ±0.03–0.26 Å from the 24-atom mean plane and an absolute average value of 0.13(5) Å, the neutral complex has the β-carbon atom displacements of ±0.10–0.22 Å and an absolute average of 0.17(4) Å, while the β-carbon atoms are displaced by ±0.00–0.18 Å with an absolute average of 0.09(6) Å in the π-cation radical complex.
Figure 3.
Formal diagram giving the perpendicular displacements of each atom from the 24-atom mean plane (in Å × 102) for [Fe(oxoOEC./2)(Cl)]+2. All bond lengths and angles of the pyrrolinone ring are shown. The remaining bond lengths and angles are averages of the pyrrole rings. The estimated standard uncertainties are shown in parentheses; the uncertainties of the bond parameters reported for the pyrrolinone ring are those of the individual bond lengths and angles, while the numbers in parentheses of the remaining bond parameters represent the esd's calculated for the averaged bond lengths and angles of the pyrrole rings.
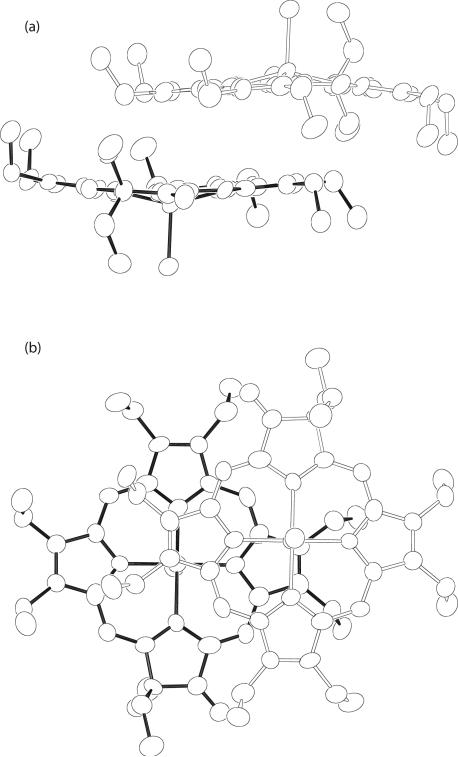
As might well be expected for this two porphyrin ring system, oxidized by a single electron, there is a significant pairwise interaction of the two β-oxo porphyrin rings. The interring geometry between the closest pair in [Fe(oxoOEC./2)(Cl)]+2 is shown in Figure 4. The inter-ring interaction parameters are listed in Table 2,49 along with those of Fe(oxoOEC)(Cl)] and [Fe(oxoOEC.)(Cl)]SbCl6 and other related iron derivatives. As shown in Table 2, the Fe···Fe distance, the lateral shift, and the mean plane separation all decrease upon oxidation of [Fe(oxoOEC)(Cl)] to the mixed-valence [Fe(oxoOEC./2)(Cl)]2SbCl6, which, however, is inverse of what is observed on oxidation of [Fe(oxoOEC)(Cl)] to the π-cation radical [Fe(oxoOEC.)(Cl)]-SbCl6. Thus the inter-ring interactions in the [Fe(oxoOEC)(Cl)] series follow the order: mixed-valence π-cation radical > neutral > π-cation radical, which is different from the observation in the solid-state structures of sterically unencumbered four- and five-coordinate metallooctaethyl-porphyrins,28 where the magnitude of the inter-ring interactions decrease in the order: π-cation radical > mixed-valence π-cation radical > neutral. Although there is less available data, a similar trend is seen for the meso-tetraalkyl derivatives (Table 2). The smaller overlap reflects that the oxoporphyrin derivatives, with their gem-dialkyl ring substituent(s), will naturally have more difficulty in achieving a strong ring-ring overlap. That a significant ring-ring overlap is observed in the mixed-valence [Fe(oxoOEC./2)(Cl)]2SbCl6 complex (Figure 4) is surely the result of the necessary delocalization of the single unpaired electron over the two rings.
Figure 4.
Edge-on (a) and top-down views (b) of inversion related dimeric units of [Fe(oxoOEC./2)(Cl)]+2. Note that the two views are rotated by 90° along the horizontal axis in the paper plane.
Table 2.
Comparisons of Geometry between Closest Pairs of Rings and Magnetic Properties in [Fe(oxoOEC)(Cl)], [Fe(oxoOEC•)(Cl)]SbCl6, and [Fe(oxo-OEC•/2)(Cl)]2SbCl6 and Related Species.
| compound | Fe···Fea | Ct· · ·Cta,b | MPSa,c | LSa,d | μ effe | –2JFe–rf | –2JFe–Fef | –2Jr–rf | Df | ref |
|---|---|---|---|---|---|---|---|---|---|---|
| [Fe(oxoOEC)(Cl)] | 7.77 | 8.13 | 3.39 | 7.39 | 5.90 | NAg | –0.28 | NAg | 6 | 34 |
| [Fe(oxoOEC•)(Cl)]SbCl6 | 10.03 | 10.03 | 4.82 | 8.79 | 5.59 | –76 | –0.14 | –13 | 6 | 34 |
| [Fe(oxo-OEC•/2)(Cl)]2SbCl6 | 6.16 | 5.38 | 3.57 | 4.03 | 4.42 | –700 | –110 | NAg | 11.8 | this work |
| [Fe(TPrP•)(Cl)]SbCl6] | 4.51 | 3.65 | 3.35 | 1.44 | 5.02 | –225 | 0.5 | –36 | 7 | 53 |
| [Fe(TEtP•)(Cl)]SbCl6 | 5.26 | 4.58 | 3.25 | 3.22 | 5.07 | –193 | 0.4 | –24 | 11 | 53 |
| [Fe(OEP•)(Cl)]C104 | 4.11 | 3.25 | 3.24 | 0.2 | – | – | – | – | – | 51 |
| [Fe(OEP•)(Cl)]SbCl6h | – | – | – | – | 5.52 | –90 | 1 | –278 | 3 | 51 |
| [Fe(OEP•)(Br)]SbCl6h | – | – | – | – | 5.50 | –118 | 3 | –348 | 1 | 51 |
| [Fe(TPP•)(Cl)]SbCl6h | – | – | – | – | 4.80 | –100 | – | –16 | 3 | 55 |
| [Fe(TTP•)(Cl)]SbCl6 | 5.39 | 4.70 | 3.68 | 3.13 | – | – | – | – | – | 56 |
| [Fe(TEtP)(Cl)] | 5.56 | 5.04 | 3.34 | 3.79 | 5.88 | NAg | 0.45 | NAg | 7 | 57 |
| [Fe(TPrP)(Cl)] | 6.44 | 5.64 | 3.66 | 4.29 | 5.90 | NAg | 0.17 | NAg | 7 | 57 |
Values in Å.
Ct is the center of the 24-atom porphyrin ring.
MPS = mean plane separation.
LS = lateral shift of two porphyrin rings.
Values in μb at 300K.
Values in cm–1.
Not applicable.
Structure not measured.
Electronic Spectra
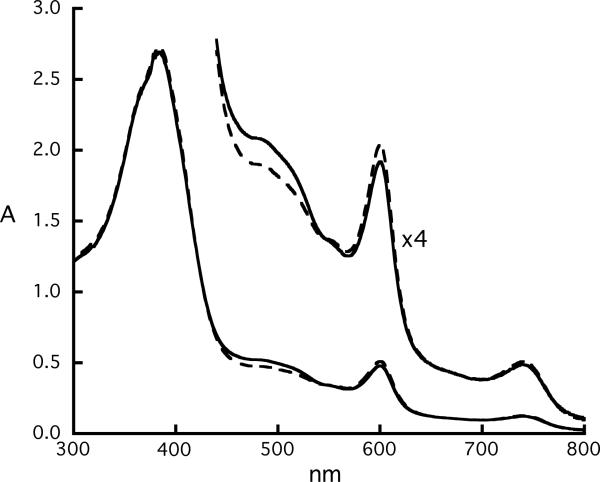
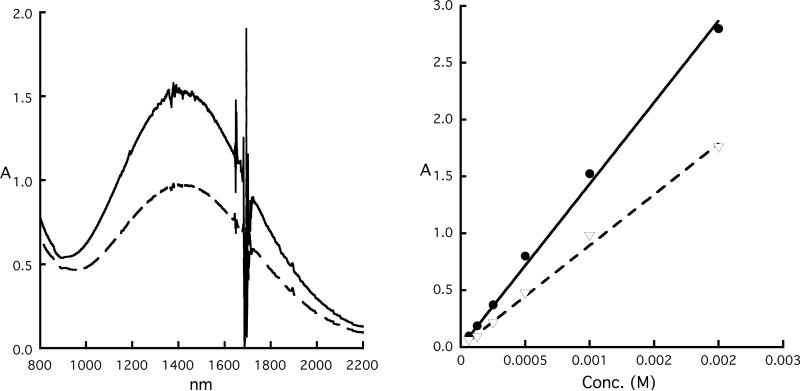
A concentration-dependent spectral study of the mixed-valence π-cation radical [Fe(oxoOEC./2)(Cl)]2SbCl6 was carried out over the concentration range of 6.3×10−5 to 1.0×10−3 M. These spectra were compared with the spectra obtained from equivalent concentrations of the neutral and π-cation radical species. Representative spectra for the UV-vis and near-IR regions are shown in Figures 5 and 6, respectively. The UV-vis spectrum of the mixed-valence species, represented by the dashed line, shows only very small changes compared to the “before reaction” spectrum. This observation, perhaps surprising, has also been observed in other mixed-valence π-cation radicals.27 In the near-IR region, the absorption maximum of the mixed-valence species (dashed line) occurs at 1417 nm and does not shift relative to the near-IR band observed for the π-cation radical, [Fe(oxoOEC.)(Cl)]SbCl6 (solid line). Most interestingly, both near-IR bands displays only linear concentration-dependent behavior (Figure 6, right ), indicating the presence of only one species—either a monomer or a dimer—over the complete investigated concentration range. Despite these similarities, the near-IR bands of the two complexes have distinct absorption coefficients, the values of the absorption coefficient (ε) are 1028 and 1649 M−1·cm−1 for the mixed valence and the π-cation radical complexes, respectively.
Figure 5.
UV-vis spectra (300–800 nm) of equimolar solutions (concentration = 6.3×10−5) of [Fe(oxoOEC)(Cl)] and [Fe(oxoOEC.)(Cl)]SbCl6 held in the two compartments of a mixing cuvette (“before reaction” spectrum (solid line)). “After reaction” spectrum (dashed line) is obtained after the two solutions are mixed, which is essentially identical with that given by pure of [Fe(oxoOEC./2)(Cl)]2SbCl6.
Figure 6.
(Left) UV-vis spectra of equimolar solutions of [Fe(oxoOEC)(Cl)] and [Fe(oxoOEC.)(Cl)]SbCl6 held in the two compartments of a mixing cuvette (“before reaction” spectrum, solid line). “After reaction” spectrum (dashed line) is obtained after the two solutions are mixed, which is essentially identical with that pure of [Fe(oxoOEC./2)(Cl)]2SbCl6. (Right) Plot of the absorbance of “before reaction”(•) and “After reaction”(▽) as functions of concentrations.
These near-IR features are in contrast to those from metallooctaethylporphyrin π-cation radicals–[M(OEP./2)]+2 and [M(OEP.)]2+2 (M2+ = Ni, Cu, Pd, Zn, vanadyl).27 The near-IR bands of the mixed-valence species, [M(OEP./2)]+2, are red-shifted relative to the near-IR bands observed for the π-cation radical, [M(OEP.)]2+2, the absorptions from both species are strongly concentration dependent, which are associated with the following equilibria:
| (1) |
| (2) |
The magnitudes of the equilibrium constants for the reactions (1) and (2) were found to range from ~1600 to ~20000 M-1, and from ~50 to ~1100 M-1, respectively.
The concentration-independent near-IR absorptions seem to be related to the Fe(III) center in the radical porphyrin complexes. The related π-cation radical-Cu(oxoOEC.)]SbCl6 shows two broad, concentration-dependent near-IR bands observed at 1285 and 1548 nm.42 Moreover, very recently we reported that two broad, near-IR absorption bands are concentration-independent in the [Fe(TEtP.)(Cl)]SbCl6 (Et = ethyl) and [Fe(TPrP.)(Cl)]SbCl6] (Pr = Propyl) radicals.53 In addition, the concentration-independent behavior appears to have no relationship with the strength of the intermolecular interaction. The crystal structures reveal these two species form cofacial π–π dimers with lateral shifts of 1.44 and 3.22 Å, respectively, for [Fe(TPrP.)(Cl)]SbCl6] and [Fe(TEtP.)(Cl)]SbCl6, whereas [Fe(oxoOEC.)(Cl)]SbCl6 and [Fe(oxoOEC./2)(Cl)]2SbCl6, exhibit weaker intermolecular interactions (Table 2). The likely origin of the near-IR bands in the iron species is that of d-d transitions, but the current study does not attempt to present an unequivocal assignment of the near-IR bands.
Mössbauer Spectra
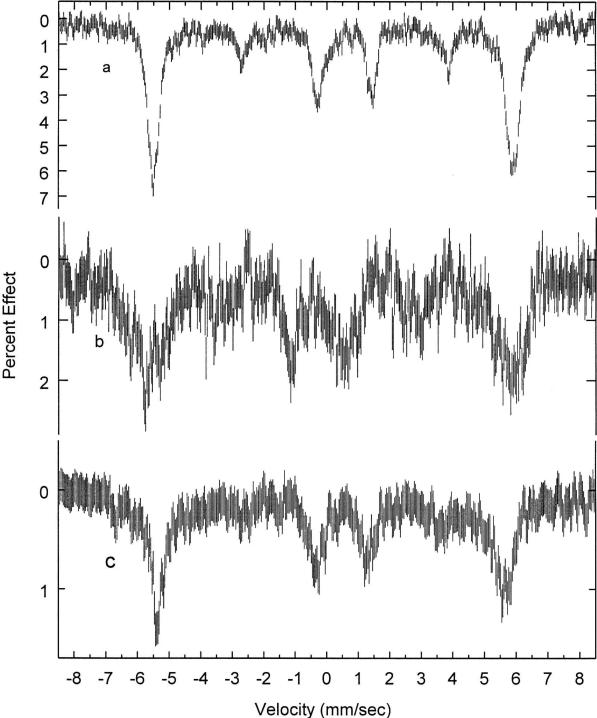
Mössbauer spectra of [Fe(oxoOEC./2)(Cl)]2SbCl6 were recorded at various temperatures ranging from 4.2 to 298 K. Least-squares fitting of the spectra to Lorentzian line shapes clearly indicates that each spectrum is comprised of a single, asymmetric quadrupole-split doublet whose relative intensities vary with temperature. Table 3 summarizes the Mössbauer fitting parameters. The isomer shift and quadrupole splitting values are in the range of those observed for five-coordinate high-spin iron(III) porphyrins (ΔEQ = 0.4–1.0 mm/s; δ = 0.25–0.43 mm/s).51,52 The spectra have also been measured at several applied magnetic fields ranging from 1 to 9 T and are compared with the spectra obtained for the two related species in Figure 7. The comparison of the quadrupole splitting values of the neutral and radical complexes at 4.2 K, as well as the lack of a second signal demonstrates the purity of the sample. Moreover, the unique value of the quadrupole splitting constant demonstrates that a distinctly new compound has in fact been prepared. The broad lines in the magnetic spectra and the line widths of the “average-valence” doublet, larger than the instrumental line widths of ~0.25 mm/s, are possibly reflective of the complex magnetic coupling (vide infra).
Table 3.
Comparison of Mössbauer Parameters for [Fe(oxoOEC)(Cl)], [Fe(oxoOEC•)(Cl)]SbCl6 and [Fe(oxo-OEC•/2)(Cl)]2SbCl6.
| compound | T(K) | δ(mm/s)a | ΔEq (mm/s)b | Γ (mm/s)c | ref |
|---|---|---|---|---|---|
| [Fe(oxo-OEC•/2)(Cl)]2SbCl6 | 298 | 0.25 | 0.69 | 0.43, 0.93 | this work |
| 200 | 0.38 | 0.76 | 0.58, 1.15 | this work | |
| 100 | 0.43 | 0.80 | 0.56, 1.04 | this work | |
| 15.5 | 0.43 | 0.77 | 0.60, 0.74 | this work | |
| 4.2 | 0.35 | 0.77 | this work | ||
| [Fe(oxoOEC)(Cl)] | 4.2 | 0.47 | 0.83 | 34 | |
| [Fe(oxoOEC•)(Cl)]SbCl6 | 4.2 | 0.56 | 0.70 | 34 |
Isomer shift relative to metallic iron
Quadrupole splitting
Full width at half-maximum.
Figure 7.
Mössbauer spectra of the complexes [Fe(oxoOEC)(Cl)] (a), [Fe(oxoOEC.)(Cl)]SbCl6 (b) and [Fe(oxoOEC./2)(Cl)]2SbCl6 (c) recorded at 4.2 K in an 9.0 T applied filed.
Magnetic Properties
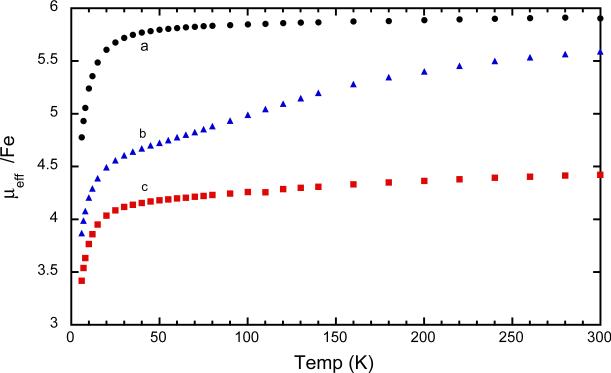
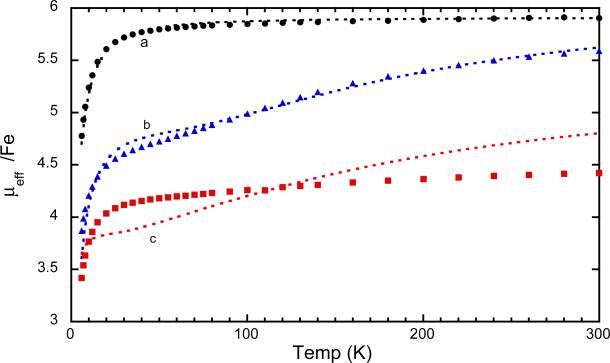
The magnetic susceptibility of [Fe(oxoOEC./2)(Cl)]2SbCl6 has been measured between 6–300 K. The values can be compared with the previously reported susceptibilities of the related species to shed light on the effects of differing radical states on the interactions between the iron and radical centers. The magnetic moments at room temperature for the neutral (parent) species, [Fe(oxoOEC)(Cl)], is 5.9 μB, precisely that expected for a high-spin iron(III) complex. The room temperature magnetic moments for the radical, [Fe(oxoOEC.)(Cl)]SbCl6, is 5.59 μB per iron and that for the mixed-valence radical, [Fe(oxoOEC./2)(Cl)]2SbCl6, is 4.42 μB. The moments for both radical species and especially the mixed-valence species are notably less than the spin-only moments and consistent with a significantly increasing magnetic interactions in the series. The complete set of temperature-dependent moments, measured from 6 to 300 K, are displayed in Figure 8. The conclusions based on the comparison of the room temperature magnetic moments are seen to extend over the entire temperature range.
Figure 8.
Magnetic susceptibilities of [Fe(oxoOEC)(Cl)] (a, black), [Fe(oxoOEC.)(Cl)]SbCl6 (b, blue) and [Fe(oxoOEC./2)(Cl)]2SbCl6 (c, red) recorded from 6 K to 300 K.
We attempted to obtain a quantitative description of the spin-spin interactions that lead to observed magnetic susceptibilities of [Fe(oxoOEC./2)(Cl)]2SbCl6.54 We have been only partly successful at obtaining a quantitative fit. The sometime difficulty of obtaining such fits, especially on the systems with more than two spin centers, can be seen from the results previously obtained on the related neutral and radical complexes. The magnetic susceptibility data for [Fe(oxoOEC)(Cl)] could be fit using the Hamiltonian
with zero-field splitting parameter of D = 6 cm-1 and an Fe···Fe interaction of 2JFe−Fe = −0.28 cm-1 with S and S′ the two Fe spins.
Fitting the experimental magnetic susceptibility for [Fe(oxoOEC.)(Cl)]SbCl6 required a model that includes four terms in the total Hamiltonian: a zero-field splitting parameter, intramolecular magnetic coupling between the iron spin and the π-cation radical spin (2JFe−r), intermolecular coupling of the two iron spins (2JFe−Fe), and intermolecular coupling of the two radical spins (2Jr−r). The Hamiltonian of such a system is:
where D is the zero-field splitting parameter, S and S′ are the two Fe spins, and s and s′ are the radical spins. It must be noted that D, 2JFe-Fe, and 2Jr-r are strongly correlated in their effects on calculated susceptibilities.
The use of a similar Hamiltonian for [Fe(oxoOEC./2)(Cl)]2SbCl6, given below, led to a fit that is in qualitative agreement with the experimental data. In this Hamiltonian only one radical spin needs to be accounted for and we allow a symmetric interaction with this spin with the two iron centers. The Hamiltonian is thus
A comparison of the fits for the experimental magnetic data for the series of the mono β-oxoporphyrin derivatives is given in Figure 8; the variables used in the fits for the three complexes are given in the top three lines of Table 2. Also given in the table are the fitting parameters reported for a number of additional iron(III) π-cation radicals and their neutral precursors. Although the fits for [Fe(oxoOEC./2)(Cl)]2SbCl6 are far from ideal, a comparison of the plots and the fitting parameters given in Table 2 clearly show that the single unpaired electron of the mixed-valence π-cation mediates significantly stronger coupling between the two irons than any of the other species listed in the table. The mixed-valence complex, with the single porphyrin ring electron, plainly has new and distinct magnetic exchange properties.
Supplementary Material
Figure 9.
Magnetic susceptibilities of [Fe(oxoOEC)(Cl)] (a, black), [Fe(oxoOEC.)(Cl)]SbCl6 (b, blue) and [Fe(oxoOEC./2)(Cl)]2SbCl6 (c, red) recorded from 6 K to 300 K. The dashed lines are from the fits reported in Table 2. Data for the first two complexes are taken from reference 34.
Acknowledgments
We thank the National Institutes of Health for support of this research under Grant GM-38401 to WRS. We also thank the Scripps Foundation and Knox College for their support in funding for the Mössbauer spectrometer and the 57Co source. Funds for the purchase of the FAST area detector diffractometer was provided through NIH Grant RR-06709 to the University of Notre Dame.
Footnotes
Supporting Information Available. Tables S1-S6, giving complete crystallographic details, atomic coordinates, anisotropic temperature factors, fixed hydrogen atom positions and complete listings of bond distances and angles. The crystallographic information file (CIF) is also available. Ordering information is given on any current masthead page.
References and Notes
- 1.Okamura MY, Feher G, Nelson N. Photosynthesis: Energy Conversion by Plants and Bacteria. Vol. Vol. 1. Academic Press; New York: 1982. p. 195. [Google Scholar]
- 2.Hoffman BM, Ibers JA. Acc. Chem. Res. 1983;16:15. [Google Scholar]
- 3.a Kühlbrandt W, Wang DN, Fujiyoshi Y. Nature (London) 1994;367:614. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]; b McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ, Isaacs NW. Nature (London) 1995;374:517. [Google Scholar]
- 4.Abbreviations used in this paper: RC, photosynthetic reaction center; PS, photosystem; P, the bacteriochlorophyll special pair; OEP, dianion of octaethylporphyrin; OEP., the π-cation radical of OEP; TPP, dianion of tetraphenylporphyrin; oxoOEC (oxooctaethylchlorin), dianion of 3,3,7,8,12,13,17,18-octaethyl-(3H)-porphin-2-onato(2-); (oxoOEC•), the π-cation radical of oxoOEC; TEtPP, dianion of meso-tetraethylporphyrin; TPrPP, dianion of meso-tetrapropylporphyrin.
- 5.Deisenhofer J, Norris JR, editors. The Photosynthetic Reaction Center. Vol. 2. Academic Press; New York: 1993. [Google Scholar]
- 6.a McElroy JD, Feher G, Mauzerall DC. Biochim. Biophys. Acta. 1969;172:180. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]; b Norris JR, Uphaus RA, Crespi HL, Katz JJ. Proc. Natl. Acad. Sci. U.S.A. 1971;68:625. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Norris JR, Scheer H, Katz JJ. Ann. N.Y. Acad. Sci. 1975;244:260. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]; d Feher G, Hoff AJ, Isaacson RA, Ackerson LC. Ann. N.Y. Acad. Sci. 1975;244:239. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]; e Davis MS, Forman A, Hanson LK, Thornber JP, Fajer J. J. Phys. Chem. 1979;83:3325. [Google Scholar]
- 7.a Deisenhofer J, Epp O, Miki K, Huber R, Michel H. J. Mol. Biol. 1984;180:385. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]; b Deisenhofer J, Epp O, Miki K, Huber R, Michel H. Nature. 1985;318:618. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]; c Deisenhofer J, Epp O, Sinning I, Michel H. J. Mol. Biol. 1995;246:429. doi: 10.1006/jmbi.1994.0097. [DOI] [PubMed] [Google Scholar]
- 8.a Allen JP, Feher G. Proc. Natl. Acad. Sci. U.S.A. 1984;81:4795. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chang C-H, Tiede D, Tang J, Smith U, Norris J, Schiffer M. FEBS. 1986;205:3951. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]; c El-Kabbani O, Chang C-H, Tiede D, Norris J, Schiffer M. Biochemistry. 1991;30:5361. doi: 10.1021/bi00236a006. [DOI] [PubMed] [Google Scholar]; d Chirona AJ, Lous EJ, Huber M, Allen JP, Schenck C, Paddock ML, Feher G, Rees DC. Biochemistry. 1994;33:4584. doi: 10.1021/bi00181a020. [DOI] [PubMed] [Google Scholar]; e Ermler U, Fritzsch G, Buchanan S, Michel H. Structure. 1994;2:925. doi: 10.1016/s0969-2126(94)00094-8. [DOI] [PubMed] [Google Scholar]
- 9.Senge MO, Vicente MG, Gerzevske KR, Forsyth TP, Smith KM. Inorg. Chem. 1994;33:5625. [Google Scholar]
- 10.Pascard C, Guilhem J, Chardon-Noblat S, Sauvage J-P. New J. Chem. 1993;17:331. [Google Scholar]
- 11.Le Mest Y, L'Her M, Hendricks NH, Kim K, Collman JP. Inorg. Chem. 1992;30:853. [Google Scholar]
- 12.Osuka A, Nagata T, Maruyama K. Chem. Lett. 1991:481. [Google Scholar]
- 13.Osuka A, Nakajima S, Nagata T, Maruyama K, Toriumi K. Angew. Chem., Int. Ed. Engl. 1991;30:582. [Google Scholar]
- 14.Rodrigues J, Kirmaier C, Johnson MR, Friesner RA, Holten D, Sessler JL. J. Am. Chem. Soc. 1991;113:1652. [Google Scholar]
- 15.Sessler JL, Johnson MR, Creager SE, Fettinger JC, Ibers JA. J. Am. Chem. Soc. 1990;112:9310. [Google Scholar]
- 16.Cowan JA, Sanders JKM, Beddard GS, Harrison RJ. J. Chem. Soc., Chem. Commun. 1987:55. [Google Scholar]
- 17.Dubowchik GM, Hamilton AD. J. Chem. Soc., Chem. Commun. 1986:1391. [Google Scholar]
- 18.Buchler JW, Eäasser K, Kihn-Botulinski M, Scharbert B. Angew. Chem. 1986;98:257. [Google Scholar]
- 19.Girolami GS, Milam SN, Suslick KS. J. Am. Chem. Soc. 1988;110:2011. [Google Scholar]
- 20.Buchler JW, Scharber BJ. Am. Chem. Soc. 1988;110:4272. [Google Scholar]
- 21.Donohoe RJ, Duchowski JK, Bocian DF. J. Am. Chem. Soc. 1988;110:6119. doi: 10.1021/ja00226a028. [DOI] [PubMed] [Google Scholar]
- 22.Duchowski JK, Bocian DF. J. Am. Chem. Soc. 1990;112:3312. doi: 10.1021/ja00226a028. [DOI] [PubMed] [Google Scholar]
- 23.Perng J-H, Duchowski JK, Bocian DF. J. Phys. Chem. 1990;94:6684. [Google Scholar]
- 24.Kim HJ, Whang D, Kim J, Kim K. Inorg. Chem. 1992;31:3882. [Google Scholar]
- 25.Girolami GS, Gorlin PA, Suslick KS. Inorg. Chem. 1994;33:626. [Google Scholar]
- 26.Scheidt WR, Cheng B, Haller KJ, Mislanker A, Rae AD, Reddy KV, Song H, Orosz RD, Reed CA, Cubiernik F, Marchon JC. J. Am. Chem. Soc. 1993;115:1181. [Google Scholar]
- 27.Brancato-Buentello KE, Kang S-J, Scheidt WR. J. Am. Chem. Soc. 1997;119:2839. [Google Scholar]
- 28.Scheidt WR, Brancato-Buentello KE, Song H, Reddy KV, Cheng B. Inorg. Chem. 1996;35:7500. [Google Scholar]
- 29.Milgram BC, Eskildsen K, Richter SM, Scheidt WR, Scheidt KA. J. Org. Chem. 2007;72:3941. doi: 10.1021/jo070389+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inhoffen HH, Nolte W. Liebigs Ann. Chem. 1969;725:167. doi: 10.1002/jlac.19697250121. [DOI] [PubMed] [Google Scholar]
- 31.Chang CK. Biochemistry. 1980;19:1971. doi: 10.1021/bi00550a037. [DOI] [PubMed] [Google Scholar]
- 32.Chang CK, Sotiriou C. J. Org. Chem. 1985;50:4989. [Google Scholar]
- 33.Adler AD, Longo FR, Kampas F, Kim J. J. Inorg. Nucl. Chem. 1970;32:2443. [Google Scholar]
- 34.Neal TJ, Kang S-J, Turowska-Tyrk I, Schulz CE, Scheidt WR. Inorg. Chem. 2000;39:872. doi: 10.1021/ic991052w. [DOI] [PubMed] [Google Scholar]
- 35.Scheidt WR, Turowska-Tyrk I. Inorg. Chem. 1994;33:1314. [Google Scholar]
- 36.Sheldrick GM. Acta Crystallogr. Sect A. 2008;A64:112. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 37.The process is based on an adaptation of the DIFABS38 logic to area detector geometry by Karaulov: Karaulov, A. I.; School of Chemistry and Applied Chemistry, University of Wales, College of Cardiff, Cardiff CF1 3TB, UK, personal communication.
- 38.Walker NP, Stuart D. Acta Crystallogr., Sect. A. 1983;A39:158. [Google Scholar]
- 39.Sutter TPG, Hambright P, Thorpe AN, Quoc N. Inorg. Chim. Acta. 1992;195:131. [Google Scholar]
- 40.Selwood PW. Magnetochemistry. Interscience; New York: 1956. Chapter 2. [Google Scholar]
- 41.Earnshaw A. Introduction to Magnetochemistry. Academic; London: 1968. Chapter 1. [Google Scholar]
- 42.Neal TJ, Kang S-J, Schulz CE, Scheidt WR. Inorg. Chem. 1999;38:4294. doi: 10.1021/ic991052w. [DOI] [PubMed] [Google Scholar]
- 43.a Stolzenberg AJ, Glazer PA;, Foxman BM. Inorg. Chem. 1986;25:983. [Google Scholar]; b Connick PA, Haller KJ, Macor KA. Inorg. Chem. 1993;32:3256. [Google Scholar]
- 44.Turowska-Tyrk I, Kang S-J, Scheidt WR. J. Porphyrins and Phthalocyanines. 2011 doi: 10.1142/S1088424611003112. in press. DOI No: 10.1142/S1088424611003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi M, Mass O, Boyle PD, Tang Q, Diers JR, Bocian DF, Holten D, Lindsey JS. J. Mol. Struct. 2010:27. [Google Scholar]
- 46.Hiroto S, Hisaki I, Shinokubo H, Osuka A. J. Am. Chem. Soc. 2008;130:16172. doi: 10.1021/ja807659h. [DOI] [PubMed] [Google Scholar]
- 47.Chang CK, Barkigia KM, Hanson LK, Fajer J. J. Am. Chem. Soc. 1986;108:1352. [Google Scholar]
- 48.Barkigia KM, Chang CK, Fajer J, Renner MW. J. Am. Chem. Soc. 1992;114:1701. [Google Scholar]
- 49.Scheidt WR, Lee YJ. Struct. Bonding (Berlin) 1987;64:1–70. [Google Scholar]
- 50.a Lü J-M, Rosokha SV, Kochi JK. J. Am. Chem. Soc. 2003;125:12161. doi: 10.1021/ja0364928. [DOI] [PubMed] [Google Scholar]; b Maguères PL, Lindeman SV, Kochi JK. J. Chem. Soc., Perkin Trans. 2. 2001:1180. [Google Scholar]; c Kochi JK, Rathore R, Maguères PL. J. Org. Chem. 2000;65:6826. doi: 10.1021/jo000570h. [DOI] [PubMed] [Google Scholar]; d Mest YL, L'Her M, Hendricks NH, Kim K, Collman JP. Inorg. Chem. 1992;31:835. [Google Scholar]
- 51.Schulz CE, Song H, Mislankar A, Orosz RD, Reed CA, Debrunner PG, Scheidt WR. Inorg. Chem. 1997;36:406. [Google Scholar]
- 52.Sams JR, Tsin TB. In: The Porphyrins. Dolphin D, editor. Vol. 4. Academic Press; New York: 1978. pp. 425–478. [Google Scholar]
- 53.Li M, Neal TJ, Wyllie GRA, Schulz CE, Scheidt WR. Inorg. Chem. 2010;49:8078. doi: 10.1021/ic101099z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The magnetic susceptibility data were obtained at two different fields (2 and 29 kG) and on two distinct samples)
- 55.Lang G, Boso B, Erler BS, Reed CA. J. Chem. Phys. 1986;84:2998. [Google Scholar]
- 56.Gans P, Buisson G, Duee E, Marchon J-C, Erler BS, Scholz WF, Reed CA. J. Am. Chem. Soc. 1986;108:1223. [Google Scholar]
- 57.Li M, Neal TJ, Ehlinger N, Schulz CE, Scheidt WR. J. Porphyrins and Phthalocyanines. 2010;14:115. doi: 10.1142/S1088424610001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.