Abstract
Regulation of the magnitude and quality of immune responses is dependent on the integration of multiple signals which typically operate through positive and negative feedback loops. Cytokines that promote or limit T cell expansion and differentiation are often both present in the complex lymphoid environment where antigen-initiated T cell responses take place. The nature and strength of the cytokine signal received by the responding cell, as well as by surrounding regulatory cells, will determine the extent of clonal expansion and the progression towards effector and memory cell differentiation. The mechanisms that determine how much cytokine is produced and how cytokine activities are controlled by receptor expression and intracellular regulators of signaling are not fully understood. Here we discuss the opposing functions of two members of the common receptor gamma chain (γc) cytokines, IL-2 and IL-7 in the generation and regulation of immune responses in vivo.
Introduction
Cytokines can be produced by various cell populations and have been shown to augment or limit immune responses to pathogens and influence the autoimmune response. One family of cytokines, which uses the common receptor gamma chain (γc), a component of receptors for Interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15 and IL-21, has been classically defined as growth and survival factors. We will focus on two members of this family, IL-2 and IL-7, and the interplay of these two cytokines in the immune system during health and disease.
Traditional roles of IL-2
IL-2, originally termed T cell growth factor, is expressed by activated CD4+ T cells in response to mitogen or antigen stimulation (21), but can also be produced by various other cells of the immune system including activated CD8+ T cells (37). The IL-2 receptor is composed of three membrane-bound subunits, including a high-affinity receptor IL-2Rα (CD25), IL-2Rβ (CD122) and the γc chain (CD132). IL-2Rα is present as both a membrane and soluble protein (54, 77). Binding of IL-2 to IL-2Rα results in the formation of the tertiary receptor complex, followed by signaling through the tyrosine kinases Jak1 and Jak3, and Stat5-dependent downstream gene regulation (31, 47).
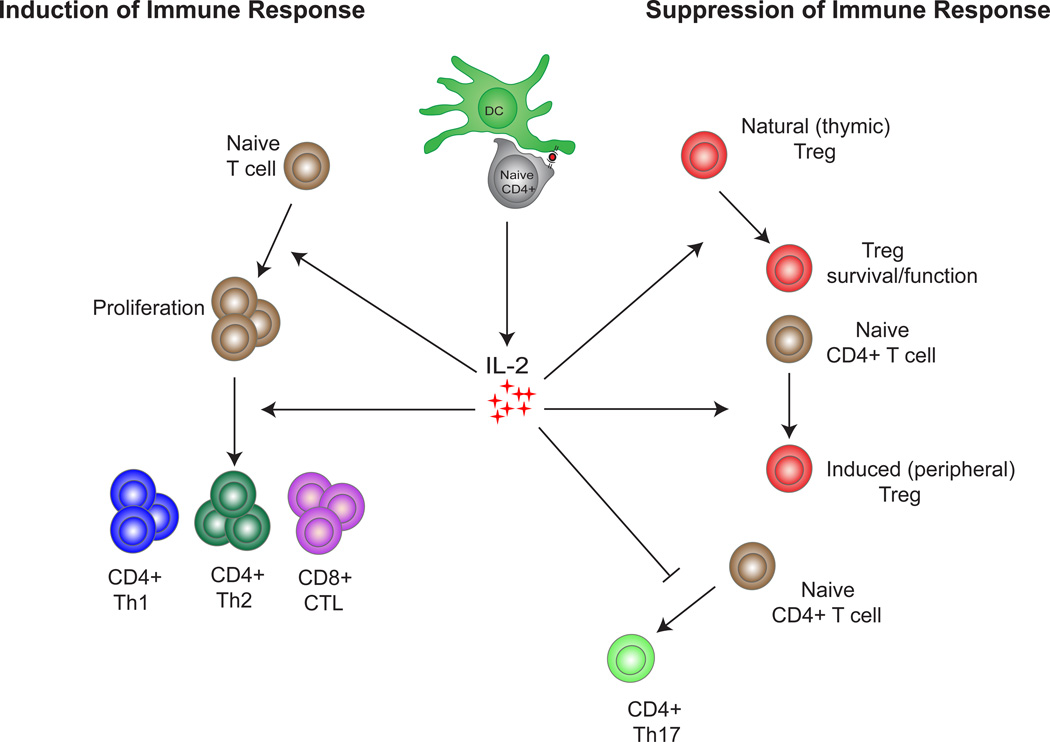
IL-2 was initially described to promote the proliferation and effector functions of CD4+ T cells during a primary immune response, and IL-2 produced by CD4+ T cells is also required for CD8+ T cell expansion and survival (91). It has also been shown to promote NK cell proliferation, induce cytolytic activitity in CD8+ T cells, and has been implicated in CD4+ T cell help for B cells (86). IL-2 can promote the generation of T effector cells including those of the Th1 and Th2 lineages, while inhibiting the differentiation of Th17 cells (38). In addition to influencing effector cell generation, the presence of IL-2 during the priming phase of CD4+ or CD8+ T cell differentiation is necessary for the development of long-lived memory cells (12, 17, 90) (Figure 1).
Figure 1.
The opposing roles of IL-2 in the immune system: IL-2 production can induce an immune response by promoting the proliferation and generation of CD4+ Th1, CD4+ Th2 and CD8+ CTL effector cells. In contrast IL-2 can inhibit the immune response by promoting the survival and functionality of natural (thymic) Tregs, promoting the generation of induced (peripheral) Tregs and inhibiting the generation of CD4+ Th17 effector cells.
Surprisingly, deficiency in IL-2, CD25 or IL-2Rβ results in multi-organ inflammation and systemic autoimmunity in both mice and humans (67, 75, 89). In addition, several autoimmune-prone mouse strains demonstrate a loss of IL-2 expression or function (15). Genome-wide association studies have revealed a linkage between the IL-2 locus and several autoimmune diseases, including multiple sclerosis, type 1 diabetes (95), autoimmune thyroid disease, Graves’ disease, and rheumatoid arthritis (9, 23, 96). In the NOD model of type 1 diabetes, defective IL-2 production and signaling is implicated in the breakdown of self-tolerance (79). These studies demonstrate that dysregulated IL-2 production or signaling plays a role in the development of multiple autoimmune disorders. The mechanism of this unexpected function of IL-2 is described below.
Traditional roles of IL-7
IL-7 was originally characterized as a hematopoietic growth factor that was able to stimulate the proliferation of lymphoid progenitors (55), but has since been found to play a role as a T cell growth and survival factor for both memory and naïve T cell populations (70, 74, 78). IL-7 is produced by stromal cells primarily in lymphoid tissues (5, 28, 49) and is bound to and presented on the cell surface via extracellular matrix proteins (32). A circulating form of IL-7 is also produced and released by stromal cells in the bone marrow, lymph node, skin, gut and liver, which has been found to maintain T cell populations during homeostatic proliferation (64).
The IL-7 receptor (IL-7R) consists of two components: a unique IL-7Rα chain (CD127) and the γc chain (35). Upon receptor binding, Jak1 and Jak3, associate with IL7Rα and γc, respectively (56), and the two subunits heterodimerize and become phosphorylated, creating docking sites for both STAT5a/5b (39, 41).
IL-7 has been shown to play a role in the survival of CD4+ and CD8+ T cells, and this effect is attributed to regulation of the Bcl-2 family. IL-7 induces the production of the anti-apoptotic factor Bcl-2, and the inactivation of apoptotic factors Bad and Bax (29). IL-7R deficient mice experience severe lymphoenia and loss of T cell function and this defect can be rescued by the overexpression of Bcl-2 (2, 48). These data show that IL-7 is an important factor in the generation and maintenance of a functional effector response.
IL-2 in regulation of the immune response
IL-2 signaling has been shown to be important in both the initiation and regulation of immune responses. In these dual and opposing roles, IL-2 acts to balance immune response, both driving immune cell activation and subsequent contraction.
Although some of the early studies on the regulatory functions of IL-2 suggested that it may limit immune responses by enhancing Fas-mediated activation-induced cell death (AICD), it is now clear that the primary defect in IL-2 deficient mice lies within the Treg compartment. Treg development in the absence of IL-2 is impaired (36, 93). A plethora of studies during the past decade have studied the importance of IL-2 to the generation, maintenance and functionality of Tregs.
IL-2 is required for the homeostasis and expansion of thymic-derived natural Tregs in the periphery, and for the generation, survival and function of induced (or adaptive) Tregs. Depletion of IL-2 or blockade of CD25 leads to the loss of Tregs and the subsequent development of systemic autoimmunity (72). We have shown a dual role for IL-2 using a model of self-antigen expression in which a monoclonal T cell population is transferred into recipient mice expressing a circulating form of the cognate antigen. The transferred cells become functionally anergic in lymphocyte sufficient hosts. In contrasts, these cells undergo uncontrolled activation in lymphocyte deficient hosts, resulting in spontaneously resolving systemic autoimmunity and the sequential development of both effector cell and Treg populations (33). In the absence of IL-2, early disease pathology was attenuated the subsequent generation of Tregs and therefore disease recovery was lost, consistent with a role of IL-2 in promoting both effector and regulatory T cell responses (Figure 1).
In addition to the effects on the generation and survival of Tregs, IL-2 maintains Treg homeostasis (20) and plays a role in their suppressive activity (6). Treatment of Tregs with IL-2 results in increased suppressive capacity in co-culture assays with effector CD4+ T cells. We have shown that when Treg apoptosis is inhibited in IL-2 deficient mice by eliminating the BH3-only protein Bim, lethal autoimmunity still occurs, and this is due to a critical role for IL-2 in the suppressive function of Tregs (6). IL-2 may work by enhancing the expression of the essential Treg-specific transcription factor FoxP3, and thus multiple FoxP3-dependent functions, and by promoting expression of CTLA-4, which is a major mediator of Treg suppressive activity (92). In addition, it has been demonstrated that FoxP3 is able to regulate miR155 expression and the subsequent control of SOCS1, a negative regulator of IL-2 signaling (44). Thus, IL-2 has multiple effects on Tregs that may influence both the survival and functionality of these cells.
The surprising finding that IL-2 has dual effects on immune responses generated interest in understanding not only its effects in vivo, but the kinetics of those effects in order to understand the biology of IL-2 and therefore target its therapeutic potential. Studies using STAT5 phosphorylation as an assay to mark cells responding to IL-2 demonstrated STAT5 phosphoryation in Tregs within hours of antigen stimulation while more prolonged or repeated exposure to antigen was necessary to induce STAT5 signaling in effector cells (57). These data demonstrated that the initial action of IL-2 is on the Treg compartment rather than the effector compartment, consistent with the constitutive high expression of the IL-2 receptor on Tregs. In the same studies, no other cell populations were shown to respond to IL-2, suggesting a quite limited spectrum of action of this cytokine in vivo.
Administration of rIL-2 bound to anti-IL-2 antibody (IL-2/IL-2mAb complexes) have been shown to enhance the natural activity of IL-2 (88). Careful dosing of IL-2 may also be effective in specifically targeting the desired cell population. In a mouse model where endogenous IL-2 production was absent, and hence could not confound the results, we found that high does of IL-2 complexes strongly stimulated effector T cells while low doses of IL-2 preferentially stimulated Tregs (HD and AKA unpublished data).
Published data using IL-2 complexes have found that low doses selectively stimulate regulatory T cells in vivo (8, 79), while high dose treatment result in effector T cell activation (79). Expansion of Tregs using low dose immune complexes has been shown to suppress autoimmune reactions in mouse models of myasthenia gravis (42), solid organ transplantation (59), EAE (88), and autoimmune diabetes (79).
IL-7: an environmental factor that promotes (autoreactive) memory cells
IL-7 shares a receptor component (γc chain) and signaling pathways (STAT5, PI3K) with IL-2 and exerts similar activities on T cells in vitro. For example, IL-2 and IL-7 are both capable of delivering powerful survival signals to T cells by inducing anti-apoptotic members of the bcl-2 family (2, 3, 48). However, genetic elimination of these cytokines leads to vastly different outcomes for the immune system in vivo. As was described, IL-2 deficiency leads to autoimmunity due to impaired Treg (7, 36, 76, 93). Lack of IL-7 signals results in lymphopenia and immunodeficiency (63, 84). IL-7 fulfills important functions in T cell development and this undoubtedly contributes to the paucity of T cells in the peripheral lymphoid organs of IL-7 or IL-7R deficient mice and in patients with X-linked severe combined immunodeficiency (X-SCID) (63, 84). Additionally, IL-7, in conjunction with TCR signals, is critically important for the survival of naïve T cells (70, 71, 78) and for the maintenance of CD4+ memory T cells (18, 34, 40).
IL-7 maintains peripheral T cell survival by regulating anti- and pro-apoptotic members of the bcl-2 family (10, 13, 58, 60). While this simple regulatory mechanism explains decreased levels of peripheral T cells in IL-7- or IL-7Rα-deficient mice, recent studies suggest that not all T cell populations are equally dependent on IL-7 signaling. For example, Th17 cells express high levels of IL-7Rα and treatment with anti-IL-7Rα antibodies dramatically improved Th17-dependent EAE (43). IL-7/IL-7Rα has also been implicated in other autoimmune diseases. IL-7 is highly expressed in the joints of rheumatoid arthritis patients (83) and anti-IL-7Rα antibody treatment inhibits the disease in animal models (24). Genome-wide association studies have revealed IL-7RA as a susceptibility gene for multiple sclerosis (22, 45), type 1 diabetes(69, 81)and primary biliary cirrhosis (51), further suggesting an important role for this cytokine in the pathogenesis of various autoimmune diseases. The underlying mechanisms for the role of IL-7 in autoimmune disease are largely unclear, but recent studies demonstrating that IL-7 enables anti-tumor and anti-viral T cells to escape cell-intrinsic and - extrinsic inhibitory mechanisms may provide the answer(61, 62). (61, 62) We speculate that some of the same inhibitory mechanisms, induced by exposure to persistent self antigens, operate to prevent autoimmunity and local increases in IL-7 levels may allow autoreactive T cells to overcome these control mechanisms, contributing to autoimmune pathology and tissue destruction. Blocking IL-7/IL-7R could hence be beneficial for treating autoimmune disease by reinforcing such inhibitory loops in autoreactive T cells (HD and AKA, unpublished data).
The cytokines IL-2 and IL-7, while exerting similar activities on T cells in vitro, exert opposing functions in the complex environment of an in vivo immune response. IL-2, by acting primarily on Tregs (57), suppresses T cell responses and protects against autoimmunity (7, 16, 26, 36, 76, 79, 93). IL-7, by counteracting inhibitory mechanisms in effector/memory cells and supporting memory cell survival, promotes immunity. Generally, IL-2Ra and IL-7Ra show a reciprocal expression pattern in both conventional T cells and Tregs. While the reason for this is currently not entirely clear, it suggests that the IL-2/IL-7 system acts as an alternating loop suppressing and promoting T cell responses in order to maintain the balance between immunity and tolerance. Disruption of the equilibrium may contribute to the development of immunopathologies and developing ways to (locally) restore the cytokine balance carries therapeutic potential
Adding to the complexity of the interplay between IL-2 and IL-7, the two cytokines have been shown to influence each other’s function. IL-2−/− T cells express higher levels of IL-7R (27) and blocking IL-2R CD25 chain induces increased IL-7 mediated homeostatic proliferation of T cells by increasing the formation of IL-7R and affecting its turnover rate (53). On the other hand IL-2 is positively involved in the formation of IL-7Rhi memory cells (18, 30). Additionally, IL-2 and IL-7 both play a role in the production of thymic Tregs and IL-7R signaling contributes to CD25+ Treg development and peripheral homeostasis (7). All those data indicate that in tissues such as the skin where both cytokines are present the balance between IL-2 and IL-7 as well as their receptor expression on T cells are likely to influence the outcome of immune responses. However the role of locally produced IL-7 in the development and maintenance of skin resident auto-reactive CD4+ T eff/mem cells as well as Tregs is still unclear. Additionally, the mechanisms by which IL-2 controls responses in the skin in Treg-sufficient mice have not yet been well defined. To address these issues, our lab has developed a mouse model that features inducible expression of a known self-antigen in the skin. Experiments using this system will be useful in providing information that defines the role of T cell subsets and cytokines production in the induction and maintenance of self-tolerance in peripheral tissues.
IL-2 and IL-7 in skin-specific immune responses
There is growing interest in the idea that tissues acquire the ability to regulate potentially damaging immune responses. One way this control might be achieved is by regulating the balance between harmful and protective lymphocytes in the tissue, and this balance may be controlled by the production of cytokines such as IL-2 and IL-7. Keratinocyte-derived IL-7 is an essential component of the epidermal cytokine milieu and serves as a growth factor for dendritic epidermal T cells (DETC). DETCs are a member of the epithelial tissue-type gamma delta T-cell family that play a crucial role in inflammation, wound healing, and tumor surveillance (46). Therefore it is not surprising that IL-7 production by keratinocytes has been implicated in several skin pathologies such as cutaneous T cell lymphoma (65, 94). Various experimental mouse models also emphasize the localized function of IL-7, in these models, over-expression of IL-7 resulted in a skin phenotype only when IL-7 was produced by cells present in the skin (66, 82) and not when IL-7 expression was targeted to the lymphoid compartment (52, 68).
There are multiple potential sources for IL-2 in the skin, primarily skin-infiltrating T cells (50). IL-2 has been shown to have a role in controlling “organ-specific” inflammation in the skin of scurfy (Sf) mice (deficient in functional Tregs) (73). IL-2−/− Sf double mutant mice were devoid of Tregs but did not develop skin and lung inflammation as control Sf mice did. Interestingly, inflammation in the liver, pancreas and colon remained.
Regulatory and memory T cells in the skin
The skin is a site where local cells can be studied in some detail. Tregs and memory T cells are the two major T cell populations present in the skin under pathologic conditions (19) (14). It has been shown that 95% of all the skin resident T cells in human skin under normal conditions are T effector memory cells expressing skin homing addressins (14). The expression of E-selectin and P-selectin ligands (such as CLA), CCR4 and/or CCR10 results in skin homing properties of T cells (11, 80). These skin resident T eff/mem cells are well placed to respond to antigen that penetrates the barrier of the skin as all the elements necessary for a memory response including T cells and APCs, which are present in the skin and capable of inducing a secondary response. Knowing that T eff/mem cells are dependent on IL-7 and the fact that this cytokine is constitutively produced by keratinocytes in the skin leads us to speculate that keratinocytes provide an ideal microenvironment for T eff/mem to reside cells forming an improved immunological barrier.
Manipulation of the IL-2 and IL-7 pathways for therapeutic benefit
Given the well-studied roles of the IL-2 and IL-7 pathways in experimental animal models, ongoing efforts focus on exploiting these pathways to treat human disease. The potential clinical applicability of either augmenting or inhibiting signals mediated by IL-2 or IL-7 is vast and includes cancer, autoimmunity, organ transplantation and HIV.
IL-2 was first used to boost immune responses in patients with advanced cancer. Although some benefit has been reported in numerous studies (4), the utility of the agent was limited by toxicities largely resulting from the production of inflammatory cytokines. Treated patients also showed an increased number of blood FoxP3+ T cells, but it is not clear if these cells had any impact on the clinical outcome (1). The cytokine has also been used to boost immune responses in HIV-infected patients. A recent trial of combination anti-retroviral therapy + IL-2 showed an increase in the number of circulating T cells but no clinical benefit (1). The phenotype of the blood T cells was not determined.
Just as augmenting the IL-2 pathway can enhance immune responses, targeted inhibition of this pathway results in immunosuppression. Clinically, this has been exploited in solid organ transplantation using monoclonal antibodies targeting CD25. When used as induction therapy for preventing acute rejection in recipients of kidney transplants, IL-2 receptor antagonists reduce the incidence of graft rejection, with less side effects (i.e., infection with opportunistic pathogens and malignancy) when compared to standard regimens (87). Similar results were observed in liver transplantation, where anti-IL-2 receptor therapy has been shown to reduce the rates of both acute rejection and new onset diabetes within one year of transplant (85). There is great interest in determining the effect of this treatment on Tregs.
One of the potentially interesting applications of IL-2 therapy is to boost Treg numbers and function to treat inflammatory diseases. This possibility is challenging because of the known dual actions of the cytokine (on effector/memory and Treg cells) and the need to control its action in vivo. One possible approach is to vary the does of IL-2, perhaps in the form of immune complexes, to target it preferentially to Tregs. Another possibility is to combine IL-2 therapy with agents that inhibit effector responses, such as an mTOR inhibitor (rapamycin), which is required for the generation of effector T cells but may be dispensable for Tregs (25).
A major challenge in the treatment of inflammatory diseases may be the presence of pathogenic memory T cells, which are likely resistant to conventional immune modulator therapy and tolerance strategies. Since IL-7 is a known survival factor for memory T cells, targeting this cytokine may be beneficial for inflammatory diseases. We and others have initiated attempts to inhibit IL-7 signaling in pre-clinical models of autoimmune diseases.
In summary, published clinical trials have provided ‘proof-of-principle’ data showing that manipulation of IL-2 and IL-7 pathways can be exploited for therapeutic benefit in several human diseases. We are currently on the cusp of exciting translational approaches that may enhance the benefits that have already been observed. Activation of Tregs through administration of IL-2/IL-2mAb complexes and depletion of memory T cells via inhibition of IL-7 signaling may provide important new tools in our armamentarium to treat autoimmunity.
Highlights.
IL-2 is involved in the generation of an immune response, driving the proliferation and generation of effector T cells.
IL-2 is involved in the regulation of immune response, mainly in the generation and functionality of Tregs.
IL-7 is important in the generation of naïve T cells and the maintenance of memory cells.
Treg and Teff/mem cells in the skin are important for the immune response to skin-restricted antigens.
The possibility of using IL-2 to boost Treg numbers and responses is a target for potential therapeutic interventions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2 bcl-xL) but not pro-apoptotic (bax bcl-xS) gene expression. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 4.Antony GK, Dudek AZ. Interleukin 2 in cancer therapy. Curr Med Chem. 2010;17:3297–3302. doi: 10.2174/092986710793176410. [DOI] [PubMed] [Google Scholar]
- 5.Barata JT, Cardoso AA, Boussiotis VA. Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk Lymphoma. 2005;46:483–495. doi: 10.1080/10428190400027852. [DOI] [PubMed] [Google Scholar]
- 6.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 9.Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, Gough SC. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves' disease using a multilocus test and tag SNPs. Clin Endocrinol (Oxf) 2007;66:508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, Orinska Z, Bulfone-Paus S, Scheffold A. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 13.Chetoui N, Boisvert M, Gendron S, Aoudjit F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology. 130:418–426. doi: 10.1111/j.1365-2567.2009.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauphinee MJ, Kipper SB, Wofsy D, Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981;127:2483–2487. [PubMed] [Google Scholar]
- 16.Dooms H, Abbas AK. Revisiting the role of IL-2 in autoimmunity. Eur J Immunol. 40:1538–1540. doi: 10.1002/eji.201040617. [DOI] [PubMed] [Google Scholar]
- 17.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol. 2004;172:5973–5979. doi: 10.4049/jimmunol.172.10.5973. [DOI] [PubMed] [Google Scholar]
- 18.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J Exp Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 21.Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 22.Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 23.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 24.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, van Roon JA. Blockade of the IL-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T-cell activity and proinflammatory mediators. Arthritis Rheum. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 25.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer KK, Wolslegel K, Dooms H, Abbas AK. Targeting T cell-specific costimulators and growth factors in a model of autoimmune hemolytic anemia. J Immunol. 2007;179:2844–2850. doi: 10.4049/jimmunol.179.5.2844. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kameyama K, Nemoto Y, Kanai T, Shinohara T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Totsuka T, Hibi T, Watanabe M. IL-2 is positively involved in the development of colitogenic CD4+ IL-7R alpha high memory T cells in chronic colitis. Eur J Immunol. 2010;40:2423–2436. doi: 10.1002/eji.200939764. [DOI] [PubMed] [Google Scholar]
- 31.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K, Matsubara H, Sogoh S, Kita Y, Sakata T, Nishitani Y, Watanabe S, Hamaoka T, Fujiwara H. Role of glycosaminoglycans in the regulation of T cell proliferation induced by thymic stroma-derived T cell growth factor. J Immunol. 1991;146:2618–2624. [PubMed] [Google Scholar]
- 33.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 36.Kramer S, Schimpl A, Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J Exp Med. 1995;182:1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristensen NN, Christensen JP, Thomsen AR. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J Gen Virol. 2002;83:2123–2133. doi: 10.1099/0022-1317-83-9-2123. [DOI] [PubMed] [Google Scholar]
- 38.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Leonard WJ. Role of Jak kinases and STATs in cytokine signal transduction. Int J Hematol. 2001;73:271–277. doi: 10.1007/BF02981951. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 42.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40:1577–1589. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, Lu L, Li R, Pan H, Song M, Liu A, Hong J, Lu H, Zhang JZ. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 44.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundmark F, Duvefelt K, Iacobaeus E, Kockum I, Wallstrom E, Khademi M, Oturai A, Ryder LP, Saarela J, Harbo HF, Celius EG, Salter H, Olsson T, Hillert J. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 46.Macleod AS, Havran WL. Functions of skin-resident gammadelta T cells. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1 −/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 49.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 50.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB, Ducker SJ, Muriithi AW, Wheater EF, Hammond CJ, Dawwas MF, Jones DE, Peltonen L, Alexander GJ, Sandford RN, Anderson CA. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 43:329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mertsching E, Burdet C, Ceredig R. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int Immunol. 1995;7:401–414. doi: 10.1093/intimm/7.3.401. [DOI] [PubMed] [Google Scholar]
- 53.Monti P, Brigatti C, Heninger AK, Scirpoli M, Bonifacio E. Disengaging the IL-2 receptor with daclizumab enhances IL-7-mediated proliferation of CD4(+) and CD8(+) T cells. Am J Transplant. 2009;9:2727–2735. doi: 10.1111/j.1600-6143.2009.02825.x. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura H, Komatsu K, Ayaki M, Kawamoto S, Murakami M, Uoshima N, Yagi T, Hasegawa T, Yasumi M, Karasuno T, Teshima H, Hiraoka A, Masaoka T. Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Allergy Clin Immunol. 2000;106:S45–S50. doi: 10.1067/mai.2000.106774. [DOI] [PubMed] [Google Scholar]
- 55.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 56.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 57.O'Gorman WE, Dooms H, Thorne SH, Kuswanto WF, Simonds EF, Krutzik PO, Nolan GP, Abbas AK. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 59.Park YH, Koo SK, Kim Y, Kim HM, Joe IY, Park CS, Kim SC, Han DJ, Lim DG. Effect of in vitroexpanded CD4(+)CD25(+)Foxp3(+) regulatory T cell therapy combined with lymphodepletion in murine skin allotransplantation. Clin Immunol. 2010;135:43–54. doi: 10.1016/j.clim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009;15:528–536. doi: 10.1038/nm.1953. [DOI] [PubMed] [Google Scholar]
- 62.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, Depinho RA, Basta S, Ohashi PS, Mak TW. IL-7 Engages Multiple Mechanisms to Overcome Chronic Viral Infection and Limit Organ Pathology. Cell. 144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponchel F, Cuthbert RJ, Goeb V. IL-7 and lymphopenia. Clin Chim Acta. 412:7–16. doi: 10.1016/j.cca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Qin JZ, Zhang CL, Kamarashev J, Dummer R, Burg G, Dobbeling U. Interleukin-7 and interleukin-15 regulate the expression of the bcl-2 and c-myb genes in cutaneous T-cell lymphoma cells. Blood. 2001;98:2778–2783. doi: 10.1182/blood.v98.9.2778. [DOI] [PubMed] [Google Scholar]
- 66.Rich BE, Campos-Torres J, Tepper RI, Moreadith RW, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177:305–316. doi: 10.1084/jem.177.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 68.Samaridis J, Casorati G, Traunecker A, Iglesias A, Gutierrez JC, Muller U, Palacios R. Development of lymphocytes in interleukin 7-transgenic mice. Eur J Immunol. 1991;21:453–460. doi: 10.1002/eji.1830210230. [DOI] [PubMed] [Google Scholar]
- 69.Santiago JL, Alizadeh BZ, Martinez A, Espino L, de la Calle H, Fernandez-Arquero M, Figueredo MA, de la Concha EG, Roep BO, Koeleman BP, Urcelay E. Study of the association between the CAPSL-IL7R locus and type 1 diabetes. Diabetologia. 2008;51:1653–1658. doi: 10.1007/s00125-008-1070-4. [DOI] [PubMed] [Google Scholar]
- 70.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 71.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 72.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma R, Sharma PR, Kim YC, Leitinger N, Lee JK, Fu SM, Ju ST. IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient scurfy mice: implication to multiorgan inflammation and control of skin and lung inflammation. J Immunol. 2011;186:1268–1278. doi: 10.4049/jimmunol.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2003;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki H, Zhou YW, Kato M, Mak TW, Nakashima I. Normal regulatory alpha/beta T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor beta in vivo. J Exp Med. 1999;190:1561–1572. doi: 10.1084/jem.190.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Symons JA, Wood NC, Di Giovine FS, Duff GW. Soluble IL-2 receptor in rheumatoid arthritis. Correlation with disease activity, IL-1 and IL-2 inhibition. J Immunol. 1988;141:2612–2618. [PubMed] [Google Scholar]
- 78.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tietz W, Allemand Y, Borges E, von Laer D, Hallmann R, Vestweber D, Hamann A. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- 81.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uehira M, Matsuda H, Hikita I, Sakata T, Fujiwara H, Nishimoto H. The development of dermatitis infiltrated by gamma delta T cells in IL-7 transgenic mice. Int Immunol. 1993;5:1619–1627. doi: 10.1093/intimm/5.12.1619. [DOI] [PubMed] [Google Scholar]
- 83.van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52:1700–1710. doi: 10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
- 84.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang XF, Li JD, Peng Y, Dai Y, Shi G, Xu W. Interleukin-2 receptor antagonists in liver transplantation: a meta-analysis of randomized trials. Transplant Proc. 2010;42:4567–4572. doi: 10.1016/j.transproceed.2010.09.169. [DOI] [PubMed] [Google Scholar]
- 86.Watson J, Aarden LA, Shaw J, Paetkau V. Molecular and quantitative analysis of helper T cell-replacing factors on the induction of antigen-sensitive B and T lymphocytes. J Immunol. 1979;122:1633–1638. [PubMed] [Google Scholar]
- 87.Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010:CD003897. doi: 10.1002/14651858.CD003897.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 90.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 92.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 93.Wolf M, Schimpl A, Hunig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(−) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 94.Yamanaka K, Clark R, Rich B, Dowgiert R, Hirahara K, Hurwitz D, Shibata M, Mirchandani N, Jones DA, Goddard DS, Eapen S, Mizutani H, Kupper TS. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107:2440–2445. doi: 10.1182/blood-2005-03-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeitlin AA, Simmonds MJ, Gough SC. Genetic developments in autoimmune thyroid disease: an evolutionary process. Clin Endocrinol (Oxf) 2008;68:671–682. doi: 10.1111/j.1365-2265.2007.03075.x. [DOI] [PubMed] [Google Scholar]