Abstract
Transdifferentiation of stem cells into insulin-producing cells for the treatment of diabetes have shown promising but inconsistent results. We examined the potential for attracting bone marrow stem cells (BMSCs) to the pancreas using a chemokine, stromal cell derived factor-1 (SDF-1). SDF-1 treatment markedly increased the number of GFP labeled BMSCs in the pancreas, but surprisingly, the majority was observed in liver. The liver cells had typical pancreatic endocrine cell gene expression including insulin I, insulin II, PDX-1, somatostatin and glucagon. Combined treatment with SDF-1 and BMSC transplant reduced hyperglycemia and prolonged the long-term survival of diabetic mice, and a sub group had complete normoglycemia (<150 mg/dl), restored blood insulin levels, and normal glucose tolerance. Our results suggest that SDF-1 could potentially be used to improve the homing of stem cells and β-cell regeneration. The mechanism appears to involve an increase in insulin producing cells mainly in the liver.
Keywords: Bone marrow stem cells, Homing, SDF-1, Insulin, Diabetes, Liver
1. Introduction
A common feature in type 1 and type 2 diabetes is an inadequate mass of functional -cells, resulting from autoimmune destruction and/or adaptative dysfunction. In order to provide a comprehensive treatment and possible cure for diabetes, new therapeutic approaches need to be developed for restoration of the critical -cell mass. Pancreas and islet-cell transplantations have proven successful in replacing functional insulin-producing -cells. However, shortage of donor organs has limited their clinical application and thereby stimulated research in -cell regeneration from alternative sources. Among them, stem cells are particularly promising for islet neogenesis (Noguchi, 2010). However, the efficacy of stem cell therapy using current protocols is unsatisfactory. One of the problems may relate to insufficient homing of stem cells to the pancreas and the low efficiency of stem cell differentiation to -cells in vivo.
Generation of functional β-cells from embryonic stem cells (ESCs) (Blyszczuk et al., 2003; Hori et al., 2002; Kahan et al., 2003; Kania et al., 2003; Lumelsky et al., 2001; Miyazaki et al., 2004; Stoffel et al., 2004), bone marrow stem cells (BMSCs) (Ianus et al., 2003; Jahr and Bretzel, 2003; Oh et al., 2004; Tang et al., 2004) and progenitor cells isolated from tissues including pancreas (Bonner-Weir et al., 2000; Ramiya et al., 2000; Seaberg et al., 2004; Zulewski et al., 2001), liver (Yang et al., 2002) and spleen (Kodama et al., 2003) has been reported. The use of ESCs is not anticipated to become a clinical reality in the near future due to ethical concerns and problems with teratoma formation. Despite possible cell fusion events in certain tissues, there is convincing data demonstrating that adult stem cells can differentiate across the lineage barriers, a process termed “developmental plasticity”. Differentiation of autologous bone marrow or circulating stem cells appears to be the preferred therapeutic approach, as they are easily accessible in clinical practice and can preclude immunological disparities. BMSCs are classified into three types (Herzog et al., 2003): hematopoietic stem cells (HSC), which differentiate into mature blood cells; endothelial progenitor cells (EPC), which become endothelium; and mesenchymal stem cells (MSC), which differentiate into cells of mesenchymal tissues including fat, bone and cartilage. In addition, another population of multipotent adult progenitor cells (MAPCs) that initially co-purified with MSCs has been identified (Jiang et al., 2002).
The majority of studies on stem cells have focused on the strategy of in vitro differentiation of stem/progenitor cells into -cell phenotype followed by transplantation into diabetic subjects (Blyszczuk et al., 2003; Bonner-Weir et al., 2000; Hori et al., 2002; Jahr and Bretzel, 2003; Kahan et al., 2003; Kania et al., 2003; Lumelsky et al., 2001; Miyazaki et al., 2004; Oh et al., 2004; Ramiya et al., 2000; Seaberg et al., 2004; Stoffel et al., 2004; Tang et al., 2004; Yang et al., 2002; Zulewski et al., 2001). Hence, in vitro differentiation of insulin-expressing cells does not recapitulate the real events of -cell neogenesis in vivo, and are not truly -cells in functionality (Sipione et al., 2004). Moreover, this approach requires manipulation of culture conditions that vary tremendously with cell types and with laboratories. Therefore, attention has turned to in vivo regeneration of functional β-cells from bone marrow.
Studies have identified stem cell homing factors and -cell transcription factors (Noguchi, 2010; Ponte et al., 2007; Sordi, 2009). Among these, the interaction of stromal cell-derived factor-1 (SDF-1) and its receptor CXCR-4 stand out as a promising candidate for targeting stem cells to the pancreas. SDF-1 is a CXC chemokine expressed at high levels by bone marrow stromal cells (Nagasawa et al., 1994). It specifically interacts with the CXCR-4 chemokine receptor to induce the migration of monocytes, lymphocytes and endothelial cells (Bleul et al., 1996; Gupta et al., 1998). Substantial evidence confirmed SDF-1 as a major homing factor to target HSCs and MSCs (both expressing CXCR-4) to bone marrow (Ji et al., 2004; Lapidot and Petit, 2002; Peled et al., 1999; Petit et al., 2002; Wynn et al., 2004). Other studies revealed that SDF-1 participates in BMSC migration to specific organs, including heart (Askari et al., 2003), liver (Hatch et al., 2002), muscle (Ratajczak et al., 2003; Yamaguchi et al., 2003) and brain (Hill et al., 2004; Ji et al., 2004). It may also be involved in the body’s natural reparatory attempt upon tissue injury, as SDF-1 expression was transiently increased in infarcted myocardium (Askari et al., 2003; Pillarisetti and Gupta, 2001) and ischemic brain (Hill et al., 2004; Ji et al., 2004). Furthermore, SDF-1 inhibits apoptosis and enhances survival of hematopoietic and progenitor cells (Broxmeyer et al., 2003; Zhou et al., 2002). In the pancreas, SDF-1 and its receptor CXCR-4 are expressed in islets and are essential for the survival, proliferation and migration of ductal progenitor cells during pancreatic regeneration (Kayali et al., 2003). It is still unclear what environmental cues initiate mobilization and homing of adult BMSCs to normal and injured tissues.
In the present study, we determined whether intrapancreatic delivery of SDF-1protein would increase the homing and differentiation capability of transplanted BMSCs into functional β-cells in diabetic mice. The effects of combined SDF-1 and BMSC treatment were examined by glucose tolerance test, insulin and C-peptide measurements, immunohistochemistry and RT-PCR.
2. Materials and Methods
2.1. Animal handling and induction of hyperglycemia
Immune-deficient NOD/SCID mice 8–10 weeks of age (Jackson Laboratories, ME, USA) were given intraperitoneal (i.p.) injections of 50 mg/kg streptozotocin (STZ) for 5 consecutive days. STZ was prepared in a sodium citrate buffer (pH 4.5) and injected within 15 minutes of preparation. Mice were considered hyperglycemic at two consecutive fasting blood glucose measurements of >450 mg/dl. Animals were housed in micro-isolator cages on ventilated racks under sterile conditions. On day 10, the mice were irradiated at 300 rads prior to receiving SDF-1 and/or BMSC treatments. All procedures and treatments were approved by the Institutional Animal Care and Use Committee of University of South Florida.
2.2. Isolation and transplantation of GFP+ bone marrow stem cells
BMSCs from C57BL/6-GFP male transgenic mice 8–10 weeks old were isolated after euthanasia by CO2 inhalation. The hind legs were removed from the hip-bone and the limbs including femur and tibia bones placed in a 100 mm dish containing cold 1XHBSS + P/S. After rinsing the limbs with fresh 1XHBSS, the muscle tissue was removed by pulling with forceps from the lower to the upper leg. Complete stripping of the muscles was accomplished by cutting off with scissors and cleaning with gauze. Cartilage caps on bones were gently lifted off with gauze or scissors. The bones were then rinsed with fresh 1XHBSS + P/S and placed on ice inside a Petri dish until ready for flushing. Recovery of bone marrow cells was made by cutting an exit hole in one extreme of the bone followed by flushing with cold IMDM medium containing 2 % FBS in a 5 ml syringe and 25G needle. Cells were filtered through a 70 m mesh on a 50 ml tube and the marrow dispersed on surface of the mesh with a rubber plunger from a 1 ml syringe. The average yield of bone marrow cells using this technique was ~5 107 cells per mouse and ~10 106 BMSCs were administered intravenously via the tail vein of NOD/SCID diabetic mice after SDF-1 injection into the pancreas.
2.3. Injection of SDF-1 into the pancreas
Mice were anesthetized with a intra-peritoneal mixture of 100 mg/kg BW ketamine hydrochloride (Fort Dodge Animal Health, IA, USA) and 10 mg/kg BW of Xylazine (The Butler Co., OH, USA). A lateral incision on the left side of the abdominal cavity (1–1.5cm) was made to access the pancreas below the spleen. The pancreatic lobe was exposed and injected with 50 L of 1 µg rHuSDF-1α (ProSpec-Tany TechnoGene LTD, Rehovot, Israel) in sterile saline solution or sterile solution alone as control in a U-100 insulin syringe. Successful delivery was confirmed by a localized increase in the surface area at the site of injection. The peritoneum and abdominal muscles were sutured and mice returned to their cages after recovering from anesthesia. To rule out the possibility of pancreatitis due to this delivery method, CD45 staining was performed in pancreatic tissues at the end of experiments.
2.4. Measurement of blood glucose and body weight
Fasting blood glucose levels and body weights from all mice were measured once a week using a Freestyle® glucometer (Abbott Laboratories, IL, USA) and digital scale. Intraperitoneal glucose tolerance tests were performed on NOD/SCID diabetic mice from the following groups: BMSC and SDF-1 treated, STZ only, and untreated mice. Animals were fasted overnight and injected i.p. with 2mg/kg dextrose. A blood sample to determine the glucose concentration was collected from the tail vein at different time points.
2.5. Insulin and C-peptide analysis
Quantification of plasma insulin and C-peptide concentrations was accomplished by collecting a blood sample (>250 µl) from the retro-orbital vein of mice on days 0, 10 and 42 using a heparinized capillary tube and micro hematocrit centrifuge to spin down the samples. The plasma was then extracted for hormone determination. For tissue insulin levels, portions of the liver, spleen and pancreas were collected and placed in tubes containing 1 ml cold acid-ethanol buffer (180 mM HCl in 70% ethanol). After homogenization and incubation at 4 C overnight with constant agitation, the supernatant was harvested for hormone determination. All samples were stored at −80°C for radioimmunoassay (Cheng et al., 2007).
2.6. Immunohistochemistry
For insulin detection, 6 m pancreas and liver sections were blocked with 5% rabbit serum, incubated with guinea pig anti-insulin antibody (DAKO Corp., CA, USA) 1:200 or guinea pig serum 1:1000 as negative control, followed by incubation with TRITC-conjugated rabbit anti-guinea pig IgG (Sigma, MO, USA) 1:200. The slides were air dried and mounted in DAPI-containing fluorescence mounting media. In addition, a catalyzed signal amplification (CSA) kit (DAKO Corp., CA, USA) was used to detected insulin in tissues. The sections were blocked with protein block, 5% goat serum, streptavidin and biotin solutions, and incubated with guinea pig anti-insulin antibody (DAKO Corp., CA, USA) 1:3200 and 1:6400 or guinea pig serum 1:1000 as negative control. Biotinylated anti-guinea pig IgG (Vector, CA, USA) 1:1200 was applied to the sections. The slides were immersed in Mayer’s hematoxlin (DAKO Corp., CA, USA), air dried and mounted in DAPI-containing mounting media. For C-peptide detection, 6- m pancreas and liver sections were blocked with 5% donkey serum containing 1% BSA, incubated with goat anti-rat C-peptide serum (Linco Research, MO, USA) 1:200 or goat serum 1:1000 as negative control, followed by incubation with Alexa 555-donkey anti-goat IgG (Molecular Probes, OR, USA) 1:400. The slides were air dried and mounted in DAPI-containing fluorescence mounting media.
2.7. RT-PCR
Total RNA was extracted from the pancreas and liver tissues using the ToTALLY RNA kit (Ambion, TX, USA). The samples were digested with DNase I then reverse transcribed with M-MLV reverse transcriptase. Amplification was performed for 35 cycles with annealing at 55°C using the primers listed in Table 1. The amplicon was analyzed on 1% agarose gel prepared with 1X TAE buffer containing ethidium bromide for 60 min at 85 mV.
Table 1.
Primer sequences for RT-PCR analysis of pancreas, liver and spleen tissues
| Gene | Primer sequence | Molecular size |
|---|---|---|
| Insulin I | Forward, TTG CCC TCT GGG AGC CCA AA Reverse, CAG ATG CTG GTG CAG CAC TG |
253 bp |
| Insulin II | Forward, TCT TCC TCT GGG AGT CCC AC Reverse, CAG ATG CTG GTG CAG CAC TG |
259 bp |
| PDX-1 | Forward, CCT TCG GGC CTT AGC GTG TC Reverse, CGC CTG CTG GTC CGT ATT G |
396 bp |
| Somatostatin | Forward, CAG ACT CCG TCA GTT TCT GC Reverse, ACA GGA TGT GAA TGT CTT CCA |
262 bp |
| Glucagon | Forward, ATC ATT CCC AGC TTC CCA GA Reverse, CGG TTC CTC TTG GTG TTC AT |
161 bp |
| Elastase | Forward, GGG CAC AAA CAG ACC ATC AC Reverse, GGG ATG GGT AAG AAG GTG GT |
298 bp |
| Albumin | Forward, ATG CTC ATA CGA TGA GCA TGC Reverse, ATG GTG GCA GGC TGG GGT TG |
245 bp |
| β-actin | Forward ATG GAT GAC GAT ATC GCT Reverse ATG AGG TAG TCT GTC AGG T |
568 bp |
2.8. Statistical analysis
All data were analyzed using student’s t-test, except insulin and C-peptide concentrations were analyzed using analysis of variance with individual mean comparisons made by least significant difference test. The significance level was set at P<0.05.
3. Results
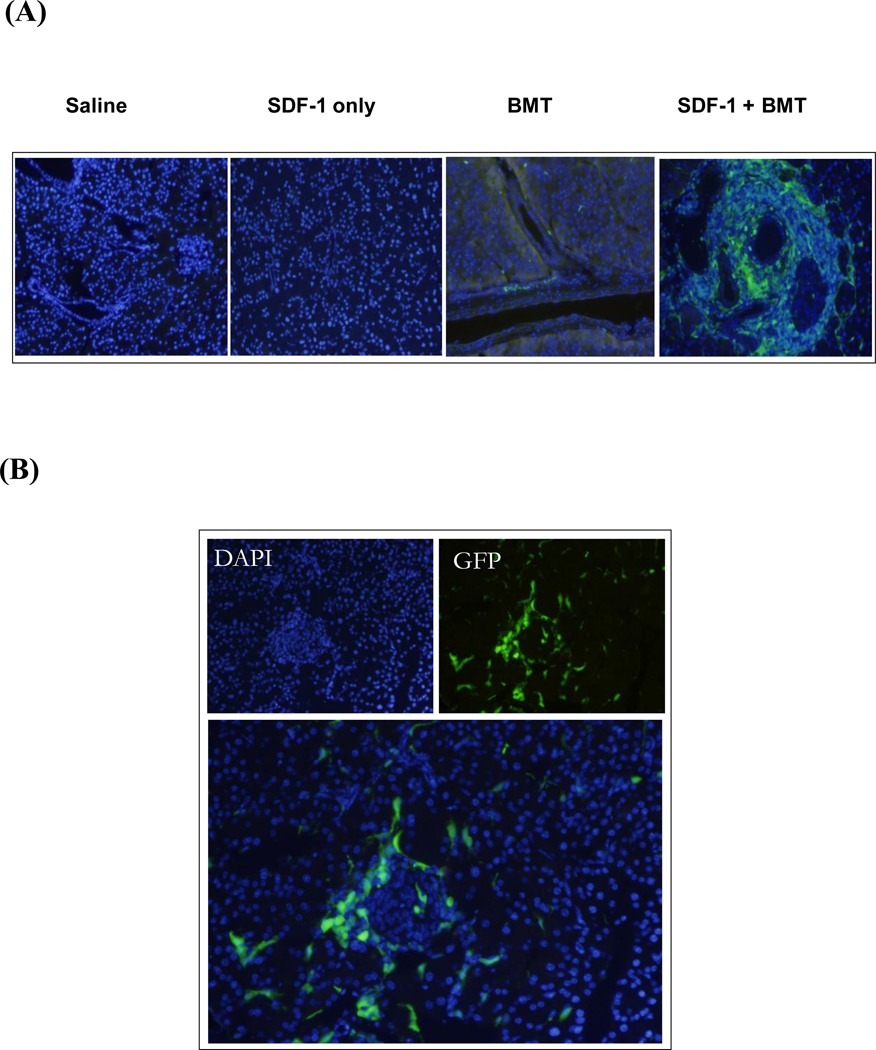
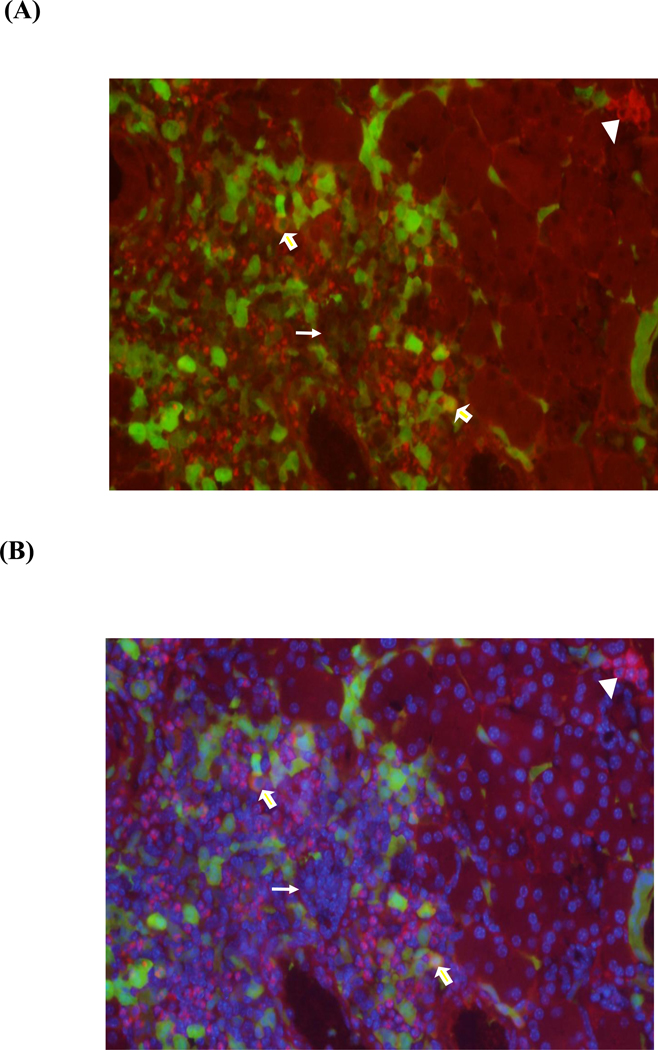
Stromal cell derived factor-1 is an important homing factor involved in stem cell migration and proliferation. Therefore, we hypothesized that SDF-1 injection into the pancreas would increase the recruitment of transplanted BMSCs into the organ. We selected stem cells from GFP transgenic mice as markers to establish proof of principle. Intrapancreatic injection of saline or SDF-1 alone in the absence of cell transplant did not result in green labeled cells in the pancreas (Fig. 1A). BMSC transplant (BMT) alone showed sparse GFP+ cells scattered in the pancreas, but SDF-1+BMT treatment greatly increased the number of GFP+ cells, especially in areas surrounding the blood vessels. Some of the BMSCs migrated into the islets as indicated by the presence of GFP+ cells (Fig. 1B). However, there was no evidence that they had become insulin producing cells. As shown in Figure 2, when the images were merged, there was little insulin-positive cells (red) derived from transplanted BMSCs (green). After quantification of 3800 insulin-positive cells in the pancreas, only 3 cells showed insulin co-localized with GFP label, indicating transplanted cells (Fig. 3A). Two of these cells are shown by the blocked arrows. This cluster of cells (arrowhead) is insulin-positive, and clearly of endogenous origin. This arrow shows an islet with DAPI staining (Fig. 3B), but it does not contain any β-cells, all ablated by STZ treatment. Interestingly, clusters of newly emerged highly insulin-positive cells with a phenotype atypical of traditional endocrine cells without the GFP marker were present (Fig. 3B). These results indicate that although transplanted BMSCs arrived at the pancreas very few became insulin-producing cells.
Figure 1. SDF-1-induced homing of bone marrow stem cells in the pancreas.
(A) Injection of SDF-1 protein into the pancreas increased the presence of transplanted BMSCs (BMT) compared to controls saline, SDF-1 or BMT alone. The presence of transplanted GFP+ cells was evident in the areas surrounding blood vessels. (B) Homing of BMSCs was also visible in the islets of Langerhans of SDF-1 treated mice.
Figure 2. Differentiation of bone marrow stem cells into β-cells.
Co-localization analysis of insulin (red) and transplanted BMSCs (green) revealed the presence of a small number of insulin+ cells derived from GFP+ cells in the pancreas of SDF-1 treated mice.
Figure 3. Atypical insulin cells in the pancreas of SDF-1 mice.
(A) Treatment of diabetic mice with SDF-1+BMT resulted in very few β-cells derived from transplanted cells. The blocked arrows show two examples. The cluster of cells (arrowhead) indicates the presence of insulin+ cells of endogenous origin with a phenotype atypical of β-cells. (B) Same image as in A, except with DAPI staining.
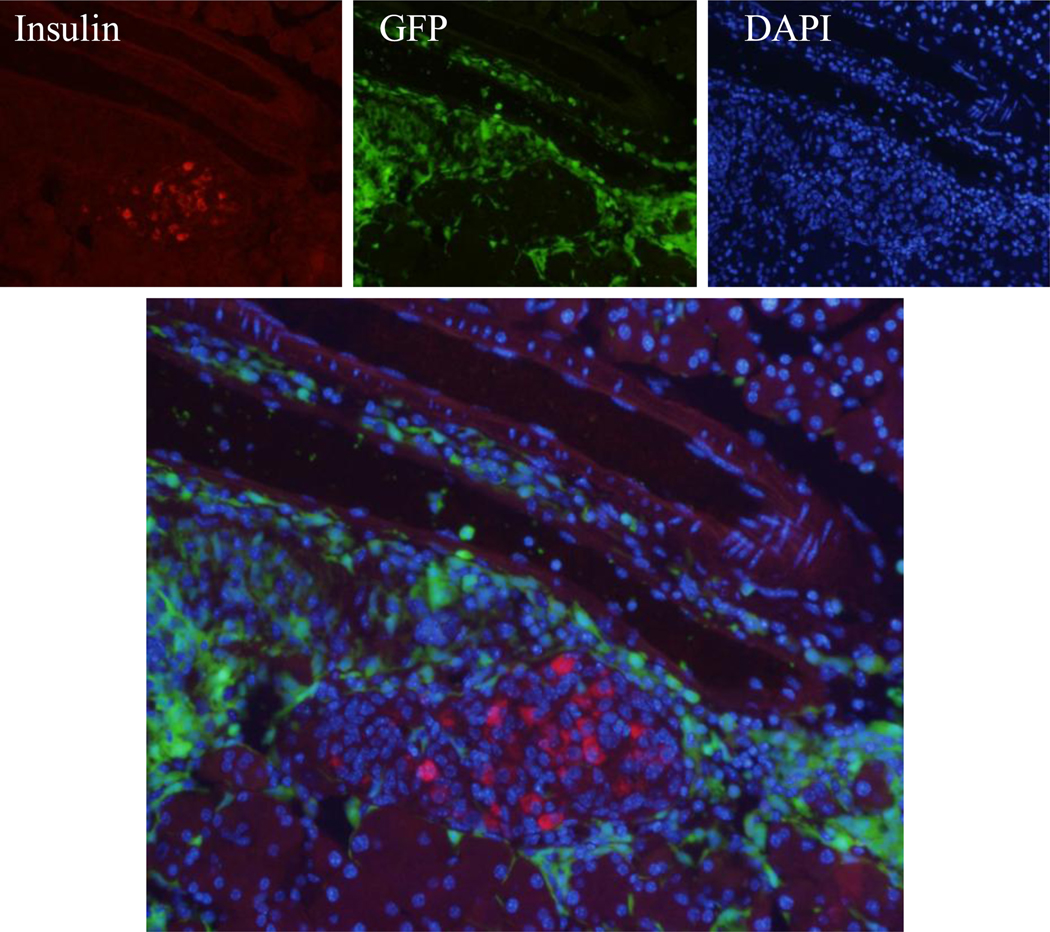
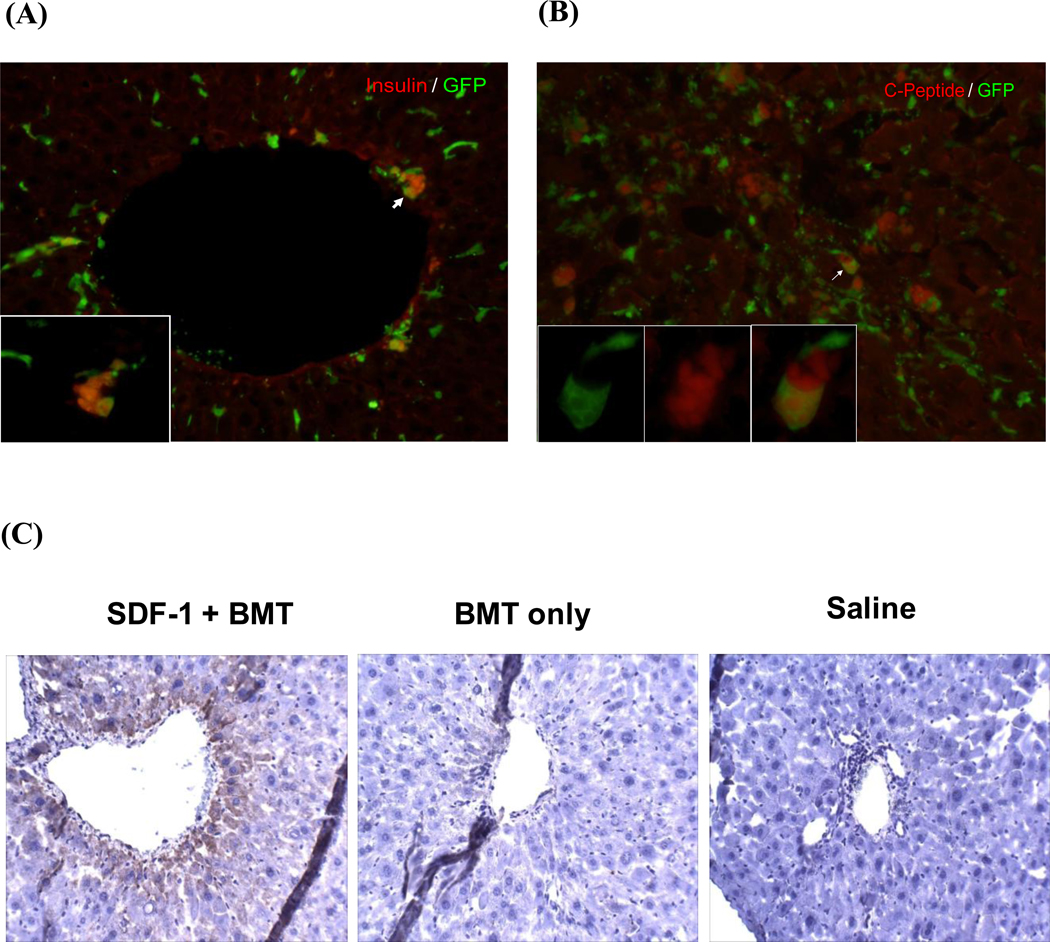
Interestingly, we observed a significant number of transplanted cells localized with the liver. Since pancreatic blood supply leads into the liver via the hepatic portal system, and there is evidence for the conversion of hepatic cells into insulin producing cells (Cao et al., 2004; Li et al., 2005; Sapir et al., 2005; Zalzman et al., 2003), we determined whether transplanted BMSCs migrated to the liver and became insulin producing cells. Immunohistochemistry analysis identified a significant number of insulin-positive cells with a small percentage GFP+ (Fig. 4A). To rule out the possibility of insulin uptake and ensure the cells we saw did produce insulin, we performed a C-peptide staining (Fig. 4B). We found that many cells were C-peptide positive, and once again, only a small percentage of them were GFP+. We confirmed the insulin staining with an insulin antibody using DAB as substrate (Fig. 4C). Insulin-positive cells were detected in the liver of SDF-1+BMT mice, but not in saline injected or BMT only groups.
Figure 4. Detection of β-cells derived from bone marrow stem cells in the liver.
(A) Immunohistochemistry of liver tissue revealed a significant number of insulin+ cells with many derived from transplanted BMSCs. An example is shown by the arrow. (B) Co-localization of C-peptide (red) and GFP (green) demonstrated the presence of insulin producing cells derived from transplanted cells (yellow). (C) Immunostaining with DAB substrate confirmed the presence of insulin+ cells in the liver of SDF-1+BMT mice, but not in controls saline or BMT alone.
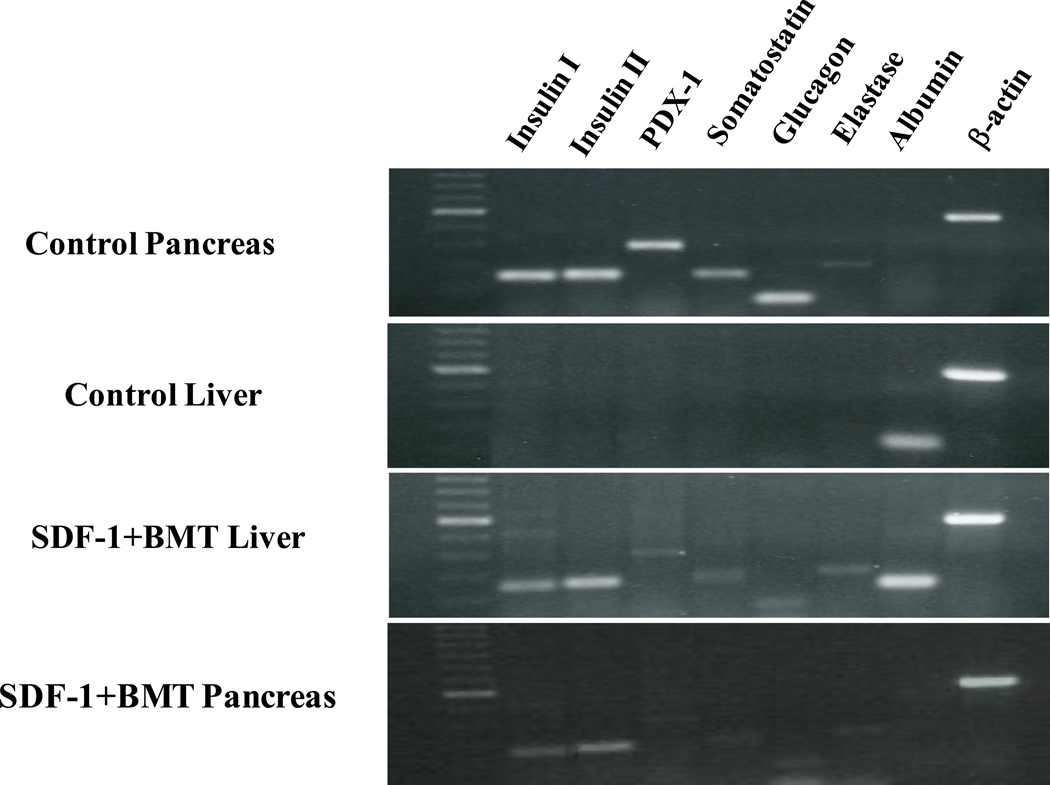
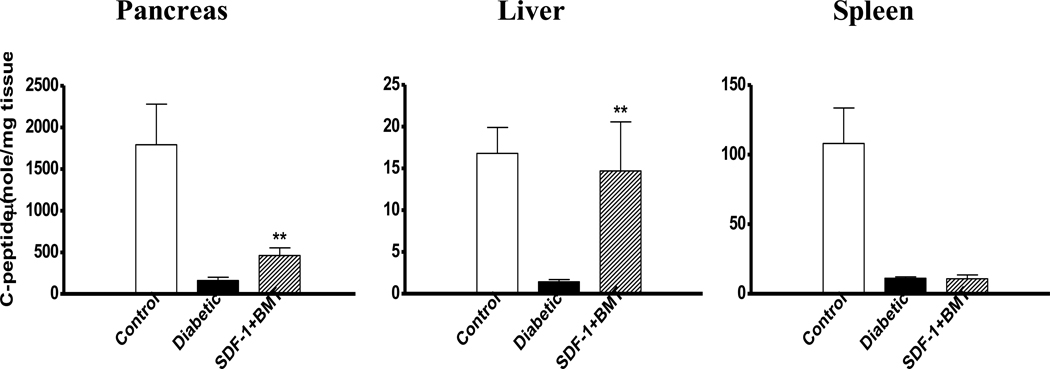
To provide another measure of insulin production, we performed RT-PCR on the RNA extracted from pancreas and liver (Fig. 5). The control pancreas expressed all the pancreas-specific genes, e.g. insulin I, insulin II, PDX-1, somatostatin, glucagon, and elastase. They did not express albumin, which is liver-specific. These results were also confirmed in pancreas of diabetic mice treated with SDF-1+BMT. Oppositely, a control liver expressed albumin, but none of the pancreas-specific genes. When mice were treated with SDF-1+BMT, the liver showed expression of all of the pancreas-specific genes examined. Having confirmed insulin production in the liver, we measured the C-peptide levels in the tissue and compared with pancreas and spleen (Fig. 6). All three tissues from diabetic mice had the lowest C-peptide levels relative to control non-diabetic mice. Treatment with SDF-1+BMT resulted in a small but significant increase in C-peptide in the pancreas, while liver values increased to levels similar to those of control non-diabetic mice. Spleen C-peptide in this group remained low. These results provide further evidence that liver may produce its own insulin.
Figure 5. Expression of pancreas-specific genes in the liver.
Total RNA isolated from pancreas and liver of SDF-1+BMT mice and transcribed into cDNA. RT-PCR was performed with specific primers for insulin I and II, PDX-1, somatostatin, glucagon, elastase, albumin and β-actin. Pancreas and liver tissues from untreated mice were used as controls. Note the expression of pancreas-specific genes with combined SDF-1 and BMT.
Figure 6. Determination of C-peptide levels in different tissues.
Insulin production in pancreas, liver and spleen of control non-diabetic, diabetic, and diabetic mice treated with SDF-1 and BMT. All tissues from diabetic mice without treatment had the lowest C-peptide content. Combined SDF-1+BMT significantly increased C-peptide levels in the pancreas, while its concentration in the liver was even higher and comparable to control non-diabetic mice (**P<0.05 compared to diabetic mice).
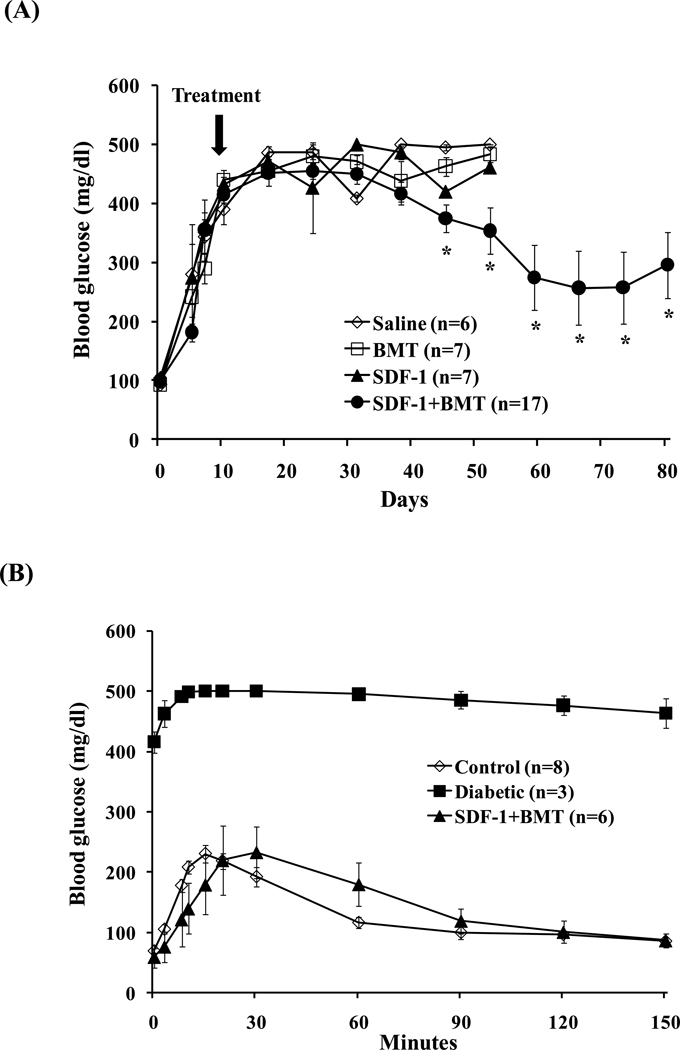
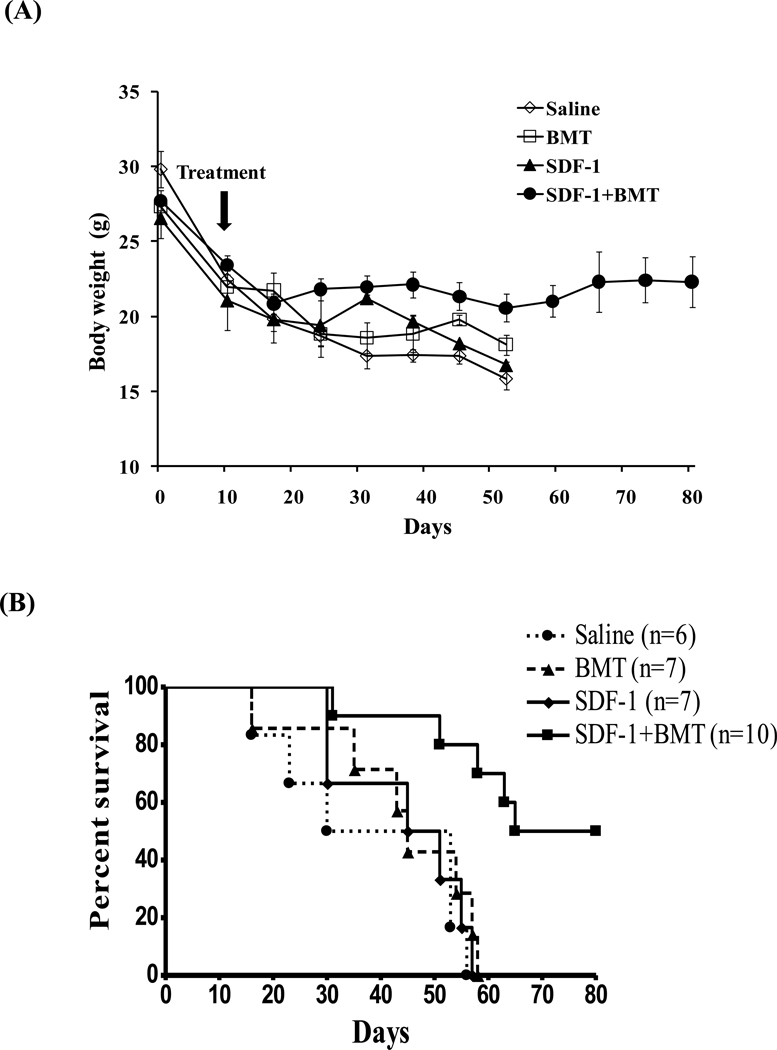
Finally, we tested whether combined SDF-1 and BMT treatments would reduce hyperglycemia in diabetic mice. The fasting blood glucose (BG) levels rose quickly to above 400 mg/dl in all groups after STZ injections. On day 10 after STZ, one of the four treatments was given: 1) Saline alone, 2) BMT alone, 3) SDF-1 alone, 4) SDF-1+ BMT (Fig. 7A). None of the three groups given treatments alone had significant effects on BG. As a consequence, sick mice had to be sacrificed and died by day 58. In contrast, SDF-1+BMT significantly reduced hyperglycemia from day 45, which continued to decrease until at least day 80. In this group, 6 out of 17 animals had their glycemia completely normalized to lower than 150 mg/dl. Therefore, we performed a glucose tolerance test in these 6 mice to determine their responses to glucose overload (Fig. 7B). The control non-diabetic mice responded to glucose overload normally with a peak BG at 15 min after i.p. injection, and returned to baseline at 120 min. Diabetic mice had a constant BG levels above 400 mg/dl. The 6 mice that received SDF-1+BMT had normalized BG, and responded to glucose overload in a similar manner as control non-diabetic mice. The results demonstrate that glucose responsiveness was restored in these animals. The remaining 11 mice that received SDF-1+BMT survived with BG levels between 200–300 mg/dl. In addition, we determined whether the treatments impacted the body weights of the mice as an indicator of their health status. All animals lost weight after induction of diabetes (Fig. 8A). The groups that received saline, SDF-1 or BMT alone continued to lose weight to below 18 g and died or became sick and were sacrificed. By contrast, the SDF-1+BMT group maintained their body weights between 20–28 g. The survival rates are shown in Figure 8B. Mice that received SDF-1+BMT lived much longer compared to saline controls or treatment alone, which died within 58 days. There were two animals that remained normoglycemic and healthy at day 135 (data not shown). Several animals were sacrificed for other assays, and therefore they were not included in this graph.
Figure 7. Effects of bone marrow stem cells and SDF-1 on hyperglycemia and responses to glucose overload.
(A) Combined SDF-1 and BMT significantly decreased hyperglycemia after day 40 compared to controls BMT, SDF-1 or saline alone. Data represents mean ± s.e.m. (n=6–17 mice/group; *P<0.05). (B) Glucose tolerance test performed in 6 mice that were normoglycemic after BMT and SDF-1 treatment. The responses to glucose overload were similar to control non-diabetic mice. Data represents mean ± s.e.m. (n=3–8 mice/group).
Figure 8. Effects of bone marrow stem cell transplant and SDF-1 on body weight and survival rates.
(A) Treatment with SDF-1+BMT prevented further weight loss after induction of diabetes compared to controls saline, SDF-1 or BMT alone. Data represents mean ± s.e.m. (n=6–17 mice/group; *P<0.05). (B) Mice from control groups died within 58 days, while SDF-1+BMT animals lived much longer. Data represents the mean from 6–10 mice/group.
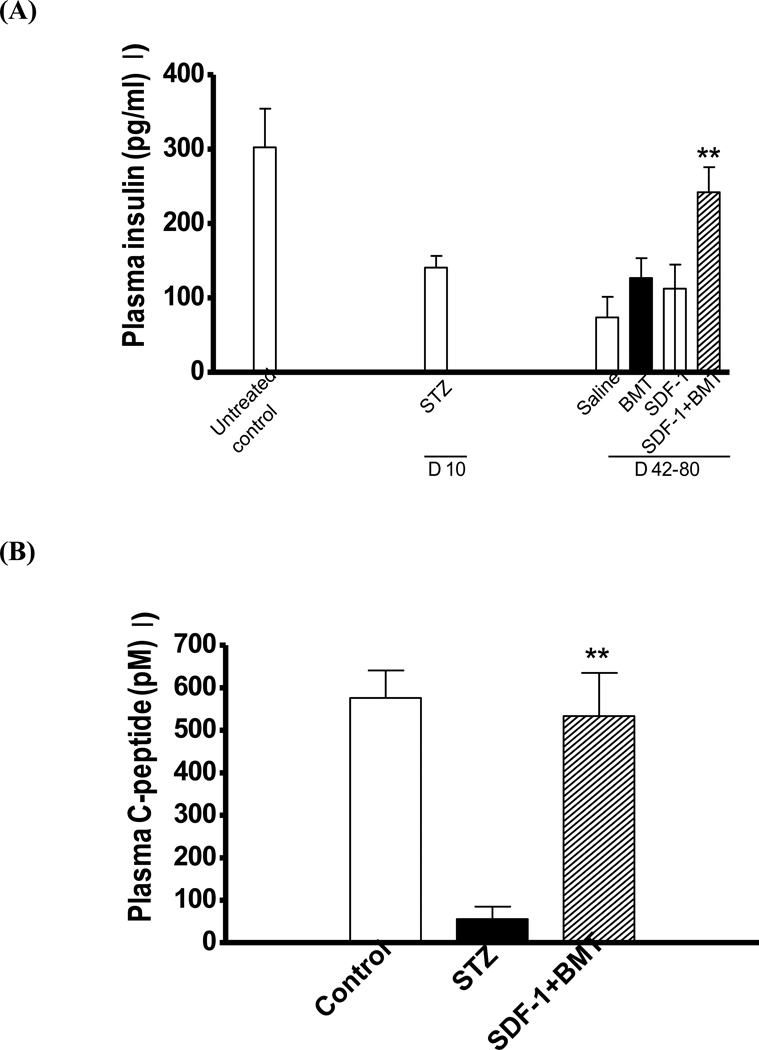
Insulin produced by β-cells is critical for glucose homeostasis because it promotes its uptake in different tissues and reduces blood glucose concentration. Insulin production can be determined by C-peptide levels, which is a byproduct of insulin synthesis. Hence, we quantified both insulin and C-peptide concentrations in the plasma of treated mice (Fig. 9A). Induction of diabetes with STZ caused a decrease in insulin concentration compared to controls non-STZ animals. Treatment with saline, SDF-1 or BMT alone did not cause significant changes in insulin concentration compared to STZ mice prior to intervention. However, a significant increase in plasma insulin was observed in SDF-1+BMT animals. Since combined treatment increased insulin concentrations, we compared the levels of C-peptide to the ones prior to SDF-1+BMT to confirm insulin production. Indeed, a significant increase in C-peptide levels was observed in SDF-1+BMT mice compared to STZ prior to treatment and was similar to control non-diabetic mice (Fig. 9B).
Figure 9. Restoration of blood insulin and C-peptide levels with bone marrow stem cell and SDF-1 treatment.
(A) A significant increase in plasma insulin levels occurred in SDF-1+BMT treated animals compared to diabetic mice prior to BMSC transplant and/or pancreatic injections. Treatment with saline, SDF-1 or BMT alone failed to increase plasma insulin levels after induction of diabetes. Data represents mean + s.e.m. (n=6–10 mice/group; **P<0.05 compared to STZ group). (B) There was also a significant increase in C-peptide levels in plasma of SDF-1+BMT mice compared to diabetic mice. These results indicate insulin production similar to non-diabetic controls (n=6–10 mice/group; **P<0.05 compared to STZ group).
4. Discussion
In the present study, we examined the effect of SDF-1 injection into the pancreas to improve the homing of BMSCs and potentially β-cell differentiation for the treatment of diabetes. We found that SDF-1 significantly improved the efficiency of BMSCs in reducing hyperglycemia in diabetic mice. Normalized animals lived longer, had normal insulin levels and were responsive to glucose tolerance tests. There was very low frequency of BMSC-derived β-cells in the pancreas, but we observed clusters of newly emerged insulin+ cells with a phenotype atypical of endocrine cells. The results also indicated that liver appeared to produce most of the insulin with expression of pancreas-specific genes.
Our results may help explain why previous efforts to transplant stem cells into the pancreas have failed and why the liver should be the target for bone marrow stem cells. Treatment of diabetic mice with either human or mouse BMSCs alone have shown promising results in the regeneration of β-cells (Hasegawa et al., 2007; Lee et al., 2006). Despite reducing blood glucose levels and restoring islet cells, reversal of hyperglycemia in diabetics with BMSCs alone has been inconsistent. Furthermore, it is suggested that homing of donor bone marrow cells is essential for regeneration of functional islet cells (Hasegawa et al., 2007). Contrary to most reports, we did not observe an effect of BMSC transplant alone on hyperglycemia and insulin production, despite the presence of a small number of GFP+ cells in the pancreas. The reason might be due to the limited number of BMSCs recruited to this organ, which was insufficient to regenerate an adequate number of functional β-cells. This is in line with the idea that homing may be critical for the success of BMSC transplants in diabetics. Pancreatic injection of the SDF-1 protein to improve homing resulted in 6 out of 17 mice (35%) with their blood glucose and insulin levels normalized. These animals responded to glucose overload in a similar manner as to control non-diabetic mice with prolonged survival and stable body weight. There were also a greater number of GFP+ cells in the pancreas compared to BMSC transplant without SDF-1. Despite an increase in the number of GFP+ cells, insulin production remained low. It is possible that by altering the delivery system using gene therapy with viral vectors to make pancreatic cells express SDF-1 will increase homing and cure rates. Recent studies show that damage to the pancreas increases SDF-1 mRNA levels, which functions in the recruitment of stem cells (Huang et al., 2010).
An interesting observation was the presence of insulin+ cells with atypical characteristics of endocrine cells. Normal β-cells have insulin staining on the cell surface while these new insulin+ cells had insulin staining only in the cytoplasm and appear to be smaller in size. However, these cells did not express the GFP marker suggesting that they were not transplanted cells. One possibility is that SDF-1 induced the recruitment of endogenous stem cells (e.g. bone marrow or pancreatic stem cells). It is well established that MSCs from bone marrow are capable of migrating to different sites (Houghton et al., 2004). Another possibility is that SDF-1 may have induced transdifferentiation of other cell types (e.g. endothelial cells) within the pancreas to give origin to the atypical insulin+ cells observed in our study. Lavazais and colleagues (Lavazais et al., 2007) proposed that endothelial cells are capable of differentiating into functional β-cells. In another study, it was shown that BMSCs give origin to pancreatic endothelial cells (Hess et al., 2003). Hence, the insulin+ cells detected in the pancreas of SDF-1 + BMT mice could have originated from endothelial cells or endogenous BMSCs. Similarly to other findings, transplanted BMSCs were preferentially localized in the areas of the pancreatic ducts and islets of Langerhans (Gao et al., 2003; Ramiya et al., 2000).
One unexpected finding was the presence of GFP+ cells in the liver with the characteristics of pancreatic β-cells. These cells were not only capable of producing insulin, but expressed several pancreas-specific genes. Our data suggested that liver insulin was mainly responsible for the reduction in hyperglycemia in SDF-1 + BMT mice since the levels of C-peptide was much higher in this organ compared to pancreas or spleen. One of the goals of our study was to increase BMSC homing in the pancreas; however, we found a significant number of transplanted cells in the liver, whereas none was detected in the liver from BMT only mice (data not shown). It is likely that diffusion of the SDF-1 protein occurred within the pancreas and was transported via the hepatic portal system to the liver. This would explain the presence of GFP+ cells in the liver and is supported by reports that SDF-1 is also capable of recruiting BMSCs to the liver (Hatch et al., 2002; Kollet et al., 2003). Despite the identification of GFP+ and insulin+ cells in pancreas and liver, the number of cells containing both markers was relative small. Initial studies by Ianus and colleagues (2003) in mice revealed that transplanted bone marrow cells are capable of differentiating into functional β-cells. However, others show that transplanted bone marrow cells migrate to the pancreas and reverse hyperglycemia without differentiating into β-cells (Choi et al., 2003; Hess et al., 2003; Mathews et al., 2004). These studies suggest that transplanted bone marrow cells stimulate pancreatic cell proliferation and tissue regeneration with the presence of newly formed β-cells. Our study is in agreement with these findings since we identified a number of insulin+ in both pancreas and liver without the GFP marker. In this scenario, SDF-1 facilitated β-cell regeneration by recruiting BMSCs. Furthermore, liver stem cells and hepatocytes are prime candidates for the treatment of hyperglycemia and β-cell regeneration because they are derived from the same embryonic layer as pancreas (endoderm) and have similar glucose sensing mechanisms (reviewed in Yang, 2005). In fact, liver stem cells when cultured under high-glucose conditions or after genetic manipulation are capable of producing a β-cell phenotype (Cao et al., 2004; Kaneto et al., 2005; Gefen-Halevi et al., 2010).
In conclusion, treatment with SDF-1 can attract BMSCs to the pancreas and liver. However, the liver appears to be the preferential site for β-cell regeneration due to the increased number of insulin+ cells capable of reducing hyperglycemia in STZ-induced diabetic mice. As a result, the treated had blood glucose levels reduced to normal or near normal levels and prolonged life. A gene therapy using SDF-1 gene delivered in a vector might be the key to homing enough BMSCs to the liver to provide a constant source of insulin in diabetic patients.
Highlights.
>SDF-1 is a stem cell homing factor. >We examined bone marrow stem cell homing in the pancreas using SDF-1. >Stem cells were successfully recruited by SDF-1. >Treatment with bone marrow stem cells and SDF-1 improved diabetes
Acknowledgments
This work was supported by grants from the American Heart Association (0435243N), National Institute of Health (R01 HL067248), and a private donation from John Horst, Tampa, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, Hangoc G, Shaheen M, Li X, Clapp DW. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J. Leukoc. Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- Cao LZ, Tang DQ, Horb ME, Li SW, Yang LJ. High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells. Diabetes. 2004;53:3168–3178. doi: 10.2337/diabetes.53.12.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JB, Uchino H, Azuma K, Iwashita N, Tanaka Y, Mochizuki H, Migita M, Shimada T, Kawamori R, Watada H. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia. 2003;46:1366–1374. doi: 10.1007/s00125-003-1182-9. [DOI] [PubMed] [Google Scholar]
- Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Gefen-Halevi S, Rachmut IH, Molakandov K, Berneman D, Mor E, Meivar-Levy I, Ferber S. NKX6.1 promotes PDX-1-induced liver to pancreatic β-cells reprogramming. Cell Reprogram. 2010;12:655–664. doi: 10.1089/cell.2010.0030. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Ogihara T, Yamada T, Ishigaki Y, Imai J, Uno K, Gao J, Kaneko K, Ishihara H, Sasano H, Nakauchi H, Oka Y, Katagiri H. Bone marrow (BM) transplantation promotes beta-cell regeneration after acute injury through BM cell mobilization. Endocrinology. 2007;148:2006–2015. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kucia M, Hussain LR, Wen Y, Xu H, Yan J, Ratajczak MZ, Ildstad ST. Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells. Transplantation. 2010;89:677–685. doi: 10.1097/TP.0b013e3181c9dc7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant. Proc. 2003;35:2140–2141. doi: 10.1016/s0041-1345(03)00747-4. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kahan BW, Jacobson LM, Hullett DA, Ochoada JM, Oberley TD, Lang KM, Odorico JS. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- Kania G, Blyszczuk P, Czyz J, Navarrete-Santos A, Wobus AM. Differentiation of mouse embryonic stem cells into pancreatic and hepatic cells. Methods Enzymol. 2003;365:287–303. doi: 10.1016/s0076-6879(03)65021-4. [DOI] [PubMed] [Google Scholar]
- Kayali AG, Van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G, Flodstrom-Tullberg M, Zhang YQ, Sarvetnick N. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J. Cell Biol. 2003;163:859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Lavazais E, Pogu S, Sai P, Martignat L. Cytokine mobilization of bone marrow cells and pancreatic lesion do not improve streptozotocin-induced diabetes in mice by transdifferentiation of bone marrow cells into insulin-producing cells. Diabetes Metab. 2007;33:68–78. doi: 10.1016/j.diabet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Horb ME, Tosh D, Slack JM. In vitro transdifferentiation of hepatoma cells into functional pancreatic cells. Mech. Dev. 2005;122:835–847. doi: 10.1016/j.mod.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- Mathews V, Hanson PT, Ford E, Fujita J, Polonsky KS, Graubert TA. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic beta-cell injury. Diabetes. 2004;53:91–98. doi: 10.2337/diabetes.53.1.91. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor 2. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H. Production of pancreatic beta-cells from stem cells. Curr. Diabetes Rev. 2010;6:184–190. doi: 10.2174/157339910791162934. [DOI] [PubMed] [Google Scholar]
- Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of Functional CXCR4 by Muscle Satellite Cells and Secretion of SDF-1 by Muscle-Derived Fibroblasts is Associated with the Presence of Both Muscle Progenitors in Bone Marrow and Hematopoietic Stem/Progenitor Cells in Muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben-Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der KD. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia. 2004;47:499–508. doi: 10.1007/s00125-004-1349-z. [DOI] [PubMed] [Google Scholar]
- Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–S45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- Stoffel M, Vallier L, Pedersen RA. Navigating the pathway from embryonic stem cells to beta cells. Semin. Cell Dev. Biol. 2004;15:327–336. doi: 10.1016/j.semcdb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly express functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Liver stem cell-derived β-cell surrogates for treatment of type 1 diabetes. Autoimmunity Reviews. 2005;5:409–413. doi: 10.1016/j.autrev.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J. Biol. Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]