Abstract
Connexin43 (Cx43) is a major cardiac gap junction channel protein required for normal electrical and contractile activity. Gap junction channel assembly, function and turnover are regulated by phosphorylation under both normal and disease conditions. The carboxyl terminus (CT) of Cx43 contains numerous amino acid residues that are phosphorylated by protein kinases. However, our knowledge of the specific residues and kinases involved is incomplete. The objective of this study was to identify amino acid residues in the Cx43-CT that are targets of the multi-functional protein kinase, Ca2+/calmodulin protein kinase II (CaMKII), an enzyme known to play critical roles in Ca2+ homeostasis, transcription, apoptosis and ischemic heart disease. We subjected fusion protein containing the Cx43-CT to phosphorylation by CaMKII in vitro, digestion with Lys-C and trypsin followed by enrichment for phosphorylated peptides using TiO2, and analysis in an LTQ XL Orbitrap with collision-induced dissociation and electron transfer dissociation. We deduced the sites of modification by interpreting tandem spectra from these “orthogonal” methods of gas phase peptide fragmentation. We have identified 15 serine residues, including one novel site, in the Cx43-CT that are phosphorylated by CaMKII, the activity of which may be important in regulating Cx43 in normal and diseased hearts.
Keywords: serine phosphorylation, Orbitrap, ETD, CID, C-terminal fusion protein
Introduction
Cardiovascular gap junctions are large specialized aggregates of intercellular channels responsible for cell-to-cell communication.1 Gap junction channels are formed by 2 docking hemichannels, each composed of 6 connexin subunits. Five different connexins are expressed in cardiac muscle and vasculature.1–3 Connexin43 (Cx43) is the predominant connexin expressed in working myocardium.2 Cx43 is not only responsible for electrical propagation throughout the chamber walls, and thus for normal electrical activation and subsequent ventricular contraction, but it is also a critical determinant of abnormal propagation, contractile dysfunction and arrhythmia secondary to pathophysiological remodeling during disease states.4,5
The expression, trafficking, distribution, turnover, permeability and gating of Cx43 are regulated primarily by phosphorylation.6,7 Eighteen serine residues in the carboxyl terminal tail of Cx43 have been reported as sites of protein phosphorylation,6,8–10 the majority of which have been identified as targets of a variety of kinases including mitogen-activated protein kinase (MAPK), protein kinase C (PKC), cyclin-dependent kinase p34cdc2, casein kinase (CK) and protein kinase A (PKA).6 Interestingly, during myocardial ischemia, a complex time-dependent pattern of both phosphorylation and dephosphorylation of four serine residues in the carboxyl terminus of Cx43 occurs. Specifically, S306 is dephosphorylated and S330 is phosphorylated early after ischemia; subsequently, S297 and S368 are dephosphorylated during the critical time when gap junction uncoupling occurs.8 Finally, S330 is also dephosphorylated during the later stages of ischemia.8 Correspondingly, Lampe et al. have observed an 8-fold reduction in phosphorylated (p)S325/S328/S330 in ischemic tissue.11
Details pertaining to the phosphorylation and dephosphorylation of the 18 serine residues in the carboxyl terminus of Cx43 are incomplete and, at times, contradictory. For example, phosphorylation of S368, the most widely studied phosphorylated residue, has been associated with reduced gap junction intercellular communication via a PKC-dependent mechanism in epithelial cells and fibroblasts;12 however, changes in gap junctional conductance in response to PKC activation depend, among other factors, on cell type and the state of phosphorylation of Cx43. Furthermore, the exact role of phosphorylation of S368 in ischemia and ischemic preconditioning is not clear because conflicting results have been reported. 8,13–16 Studies on alterations in the phosphorylation state of different amino acid residues may, in part, be limited by the availability, affinity and specificity of commercial antibodies directed toward phosphorylated residues in Cx43. Fortunately, recent advances in mass spectrometric analyses have contributed substantially to an improved ability to identify the modified amino acid residues in phosphorylated peptides.17
The purpose of the present study was to apply high-resolution tandem mass spectrometry to the carboxyl terminus of Cx43 to identify targets of the multifunctional protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII).18 CaMKII plays a central role in regulating a variety of cellular functions in the heart such as Ca2+ homeostasis, transcription and apoptosis.18,19 In addition, increased expression and/or activation of CaMKII have been shown to occur in cardiac disease states such as hypertrophy, heart failure, myocardial ischemia and infarction, and are thought to promote disease pathogenesis.20–24 Recently, we have found that CaMKII colocalizes with Cx43 at intercellular junctions in the infarct border zone.25 However, Cx43 has never, to our knowledge, been shown to be a target of CaMKII. De Pina-Benabou et al.26 hypothesized that CaMKII phosphorylation of the carboxyl terminus of Cx43 was the mechanism by which K+ produced an increase in coupling between spinal cord astrocytes, and they predicted four serine residues that could be potential phosphorylation targets for CaMKII based on the consensus sequence RXXSX. Although Cx43 has not been shown to be a substrate for CaMKII, this enzyme has been shown to phosphorylate Cx3227 in hepatocytes and Cx3628 in neurons. However, the specific CaMKII phosphorylation sites have not been identified in Cx32, and the phosphorylation sites identified in Cx36 do not share homology with Cx43.
The current study, therefore, was performed to test the hypothesis that the carboxyl terminus of Cx43 is subject to phosphorylation by CaMKII. We found that CaMKII phosphorylates a variety of reported residues, and one novel serine residue, in the carboxyl terminus of Cx43 in vitro. These results suggest that Cx43 may be a substrate for CaMKII in vivo. The data will be discussed in the context of remodeling of Cx43 during the pathogenesis of chronic ischemic heart disease.
Materials and Methods
Cx43 Fusion Protein
A DNA construct coding for a fusion protein containing amino acids 237–382 of the carboxyl terminus (CT) of rat Cx43 (GI:6978896) subcloned in frame with GST in pGEX-2T (GST-Cx43-CT, MW 42,122 g/mol) was grown in BL21-CodonPlus competent cells (Stratagene/Agilent Technologies, Santa Clara, CA). The fusion protein was purified using a B-PER GST Fusion Protein Purification Kit (Pierce/Thermo Scientific, Rockford, IL) and subsequently dialyzed against Tris-buffered saline for 48 hr. The sequence of the fusion protein was confirmed at the Washington University Protein and Nucleic Acid Chemistry Laboratories. The amino acid sequence of the Cx43-CT present in the fusion protein is shown in bold in Figure 1.
Figure 1.
Tandem mass spectrometric coverage of Cx43-CT. The N-terminal, transmembrane, extracellular loop and intracellular loop domains of the protein are indicated in grey. The amino acids of the Cx43-CT present in the fusion protein used in this study are indicated in bold black or red (98% coverage). Amino acids in red were positively identified by tandem mass spectrometry. The 15 serine residues underscored and highlighted in yellow were phosphorylated by CaMKII in vitro.
In vitro Phosphorylation of GST-Cx43-CT by CaMKII
GST-Cx43-CT fusion protein was subjected to in vitro phosphorylation using CaMKII (New England Biolabs, Ipswich, MA) per the manufacturer’s instructions. Briefly, GST-Cx43-CT fusion protein (1–8 μg) was incubated with 1.2 μmol/L calmodulin, 2 mmol/L CaCl2, 210 μmol/L ATP and 1,500 U CaMKII in a final volume of 10.5 μL at 30°C for 24 h, after which the reaction was stopped on ice and phosphatase inhibitors were added to a final concentration of 1 mmol/L NaF and 2 mmol/L Na3VO4. Control reactions contained fusion protein, calmodulin, CaCl2, ATP and buffer, but no CaMKII. In one experiment we used CK1 (New England Biolabs, 1,500 U, 24 h) per the manufacturer’s instructions to test a protein kinase known to phosphorylate the Cx43-CT.11
Immunoprecipitation
Mouse ventricles flash-frozen in liquid N2 were pulverized and homogenized in lysis buffer containing 1.5% NP-40 (Surfact-Amps NP-40, Pierce) in phosphate-buffered saline (PBS, Diamedix), rotating for 30 min at 4°C.
Monoclonal anti-Cx43 (50 μg, Millipore/Chemicon) or mouse IgG (50 μg, Santa Cruz, negative control) antibody was crosslinked to 200 μL of protein G-bound magnetic Dynabeads (Invitrogen) using dimethyl pimelimidate (DMP, Pierce) and triethanolamine (Sigma) in silanized (10% dimethyldichlorosilyl, P.J. Cobert) tubes. The crosslinked beads were washed in PBS, citrate buffer (pH 5.0), sodium phosphate buffer (pH 8.1), sodium deoxycholate (0.5%) and Tween-20 (0.1%). Each set of antibody-crosslinked beads was incubated with protein from the right and left ventricles of one mouse heart, NaF (1 mM), Na3VO3 (2 mM) and protease inhibitor cocktail (1:100, Sigma) for 2 h at 4°C on a rotator. Immunoprecipitated protein was eluted with Rapigest (1.7%, Waters) and 8 M urea in 100 mM Tris buffer (pH 8.5) at 60°C for 5 min; the elution was repeated and the two volumes were combined. The eluent was dialyzed (Slide-A-Lyzer, Thermo) against 1 L of water for 2 h at 4°C followed by dialysis against 1 L of water overnight at 4°C.
Phosphopeptide Clean-up and Enrichment
In vitro phosphorylated GST-Cx43-CT fusion protein or immunoprecipitated full-length native Cx43 was precipitated using a 2-D Clean-Up Kit (GE Healthcare Waukesha, WI) and resolubilized in 20 μL of 8 M urea in 100 mM Tris, pH 8.5. Proteins were then reduced with 10 mM Tris(2-carboxyethyl)phosphine (TCEP, Thermo Fisher, Rockford, IL) at 25°C for 30 min followed by alkylation with 20 mM iodoacetamide (Sigma-Aldrich, St. Louis, MO) at 25°C for 30 min in the dark and quenched with 10 mM dithiothreitol (DTT, Thermo Fisher) at 25°C for 15 min. The proteins were digested with 1 μg endoproteinase Lys-C (Roche, Mannheim, Germany) at 37°C overnight, diluted 1:4 with 100 mM Tris pH 8.5, and further digested with 1 μg trypsin (Sigma-Aldrich) in 1 mM triethylammonium bicarbonate, pH 8.5 (TEAB, Sigma-Aldrich) at 37°C overnight. The peptide solutions were acidified with 2% trifluoroacetic acid (TFA) (Sigma-Aldrich). Phosphopeptide samples were enriched using TiO2 tips (Glygen, Columbia, MD) that were equilibrated in 0.1% TFA before drawing each sample onto the media. Tips were washed with 100 mM glutamic acid at pH 2.0 and the bound peptides eluted with 1.5% ammonium hydroxide. The eluates were desalted using carbon NuTips (Glygen), lyophilized, and resuspended in 20 mL of 1% formic acid and 1% acetonitrile.
To verify the activity of CaMKII in vitro, 1.5 μg of autocamtide (KKALHRQETVDAL, Calbiochem/EMD Chemicals, Gibbstown, NJ) was incubated with CaMKII for 24 h as described above to produce phospho-autocamtide (KKALHRQEpTVDAL). Commercially available phospho-autocamtide (AnaSpec, Fremont, CA) was run as a positive control.
Mass spectrometry
Autocamtide phosphorylation was verified using matrix-assisted laser desorption ionization (MALDI). Peptide solution was mixed 1:1 with α-cyano-4-hydroxycinnamic acid (CHCA, Sigma-Aldrich). For MALDI analyses using an ABI 4700 Proteomics Analyzer (Applied Biosystems, Framingham, MA) equipped with an Nd:YAG laser (355 nm, 3 to 7 ns pulses), the instrument was operated in reflector, positive-ion mode with an acceleration voltage of 20 kV. Spectra were averaged from 1000 laser shots at 200 Hz for each spot.
For analysis with a Thermo LTQ Orbitrap XL with electron transfer dissociation (ETD) and/or collision-induced dissociation (CID) (Thermo Fisher, San Jose, CA), samples were loaded and eluted using an autosampler and an Ultra 1D+ UPLC (Eksigent, Dublin, CA). The 75 μm diameter columns were pulled (Sutter Instruments, Novato, CA) and a 12-cm length was packed with Magic C18AQ reverse phase media (Michrom Bioresources, Auburn, CA).29 Columns were mounted in a Pico View nanospray source (New Objective, Woburn, MA) and eluted with a 60 min gradient from 2% – 60% acetonitrile with 0.1% formic acid. Fluoranthene anion was used for ETD.30 One full mass spectrometry acquisition was used to trigger 3 alternating scans each of CID and ETD. The Orbitrap parameters were: spray voltage, 2.0 kV; capillary temperature, 200°C; tandem mass spectrometry (MS2) selection threshold, 1000 counts; activation q, 0.25; activation time, 30 ms for CID and 100 ms for ETD. For ETD, isolation width was 3.0 m/z and reaction time was set as charge state dependent. Supplemental activation was enabled. MS2 data were centroided during acquisition.
For analysis with an LTQ-FT Ultra (Thermo Fisher, San Jose, CA), samples were loaded onto a C18–75 μm diameter column using a NanoLC-1D (Eksigent). They were eluted with a 180 min analytical gradient from 2% – 50% acetonitrile containing 0.1% formic acid at 260 nL/min. The solution was sprayed into the mass spectrometer using a Pico View nanospray source (New Objective). One full MS acquisition was used to trigger 8 CID scans. The FT parameters were: spray voltage, 2.0 kV; capillary temperature, 200°C; MS2 selection threshold, 500 counts; activation q, 0.25; activation time, 30 ms. MS2 data were centroided during acquisition.
Alpha and beta casein (Sigma-Aldrich) and/or bovine serum albumin (BSA, Michrom Bioresources) were analyzed as controls in each experiment. In addition, casein was used to determine the optimal amount of protein to be used for detection of phosphopeptides.
Tryptic peptides from immunoprecipitated full-length native Cx43 were analyzed as above with the following exceptions. A 180 min gradient from 2% – 60% acetonitrile with 0.1% formic acid was used for separation. Both data dependent (discovery) and data-directed (inclusion list) methods were used to acquire mass spectra on the Thermo LTQ Orbitrap XL with ETD and CID.31 Parent mass lists for the directed experiment were created for all doubly, triply and quadruply charged ions corresponding to phosphopeptides identified in in vitro experiments.
Mascot database search and determination of phosphorylation sites
Thermo RAW files were processed using extract_msn (2007 version 4.0, Thermo Fisher, San Jose, CA) with a grouping tolerance of 0.8 Da, an intermediate scan setting of 1, and a minimum of 1 scan per group. The NCBI nonredundant database (version 20090105, restricted to mammals) was searched using MASCOT 2.2.06 (Matrix Science, Oxford, U.K.) with the following settings: enzyme, trypsin; MS tolerance, 10 ppm; MS/MS tolerance, 0.6 Da; maximum number of missed cleavages, 3; peptide charge of 1+, 2+ and 3+; variable modifications were carbamidomethylation of C, oxidation of M, phosphorylation of Y, T, and S, and deamidation of N-terminal Q to E. All identifications of phosphopetides were manually analyzed as described below.
Tryptic peptides containing S, T, or Y, including those with up to 3 missed cleavages, were predicted by in silico digestion (Protein Prospector MS-Digest, prospector.ucsf.edu). Theoretical MS2 spectra were generated (Protein Prospector MS-Product, prospector.ucsf.edu) for phosphopeptides that were identified in the Mascot search. When there were multiple possibilities for phosphorylation (e.g., more than one S, T, or Y in a singly-phosphorylated peptide, or more than two S, T, or Y in a doubly-phosphorylated peptide) a table of ions was made for each possible phosphopeptide. The product ion spectrum was manually compared to each table and the best match was identified. In cases where the elution profiles of phosphopeptides overlapped, they were separated using extracted ion chromatograms for diagnostic product ions. Tables for each identified phosphopeptide are included in the Supporting Information.
Results and Discussion
The amino acid sequence of carboxyl terminal domains varies widely across connexins and is thought to confer specific properties to the channels composed of different connexin subtypes.32 The Cx43-CT is a structurally disordered domain that fits the paradigm of an intrinsically flexible three-dimensional structure that provides for important cell signaling and regulatory activity.33,34 It has been shown that this region is involved in intra- and intermolecular interactions.35,36 Its post-translational modification by phosphorylation is involved in regulation of protein structure, subcellular distribution and function.6–8,11–16 Seventeen serine and two tyrosine residues in the Cx43-CT have been shown to be targets of various protein kinases under control, stimulated and pathological conditions, but phosphorylation of the Cx43-CT and identification of the modified amino acid residues by CaMKII have not been reported.
To identify sites of CaMKII phosphorylation in the Cx43-CT under controlled conditions in vitro, we first optimized the amount of fusion protein required for recovery of phosphopeptides and identification of phosphorylated amino acid residues using casein, a known phosphoprotein, at 3 different concentrations: 0.3 μg/20 μL, 1 μg/20 μL and 3 μg/20 μL. We identified 10 phosphorylation sites in casein (Figure S1, Supporting Information), and determined that 3 μg casein in 20 μL reaction volume yielded the best results.
To validate the in vitro conditions used for the CaMKII reactions, we incubated a peptide containing the threonine autophosphorylation site of CaMKII, nonphosphorylated autocamtide (KKALHRQETVDAL), with CaMKII in the presence of ATP, Ca2+ and calmodulin as described in Materials and Methods. We used nonphosphorylated autocamtide in the absence of CaMKII as a negative control, and phospho-autocamtide (KKALHRQEpTVDAL) as a positive control. The spectrum obtained from autocamtide subjected to in vitro phosphorylation demonstrated a phosphopeptide that was identical to that observed from phospho-autocamtide obtained from AnaSpec (Figure S2, Supporting Information). No phosphopeptide was detected in the reaction performed in the absence of CaMKII.
We next incubated GST-Cx43-CT fusion protein with CaMKII and subjected the reaction products to enzymatic digestion with Lys-C followed by trypsin and mass spectrometric analysis to identify the amino acid residues phosphorylated by CaMKII. Tandem mass spectrometry yielded 98% coverage of the Cx43-CT (Figure 1). Coverage was comparable for results obtained using CID and ETD; however, two phosphorylation sites were identified by ETD alone (see below). This is not surprising because ETD-derived alternative fragmentation patterns that complement CID-derived data have been reported previously.37 The sequence corresponding to amino acid residues 259–293, none of which was phosphorylated, was identified in only one of the experiments, possibly because we used TiO2 to enrich for phosphopeptides.38 The lack of phosphorylated residues observed in this sequence agrees with previous reports that S262, S279 and S282 are targets of protein kinases other than CaMKII.39,40
Four independent phosphorylation experiments were performed followed by digestion and tandem mass spectrometry. These data sets are presented in Table S1 (Supporting Information). No serine residues were phosphorylated in the negative control samples that were incubated in the presence of Ca2+, calmodulin and ATP, but in the absence of CaMKII (Figure 2, Table S1 in Supporting Information). Amino acid residues in Cx43-CT that were identified as being phosphorylated by CaMKII are reported in Table 1, and the diagnostic ions for these identifications are listed in Table 2. Several interesting observations can be made from these data.
Figure 2.
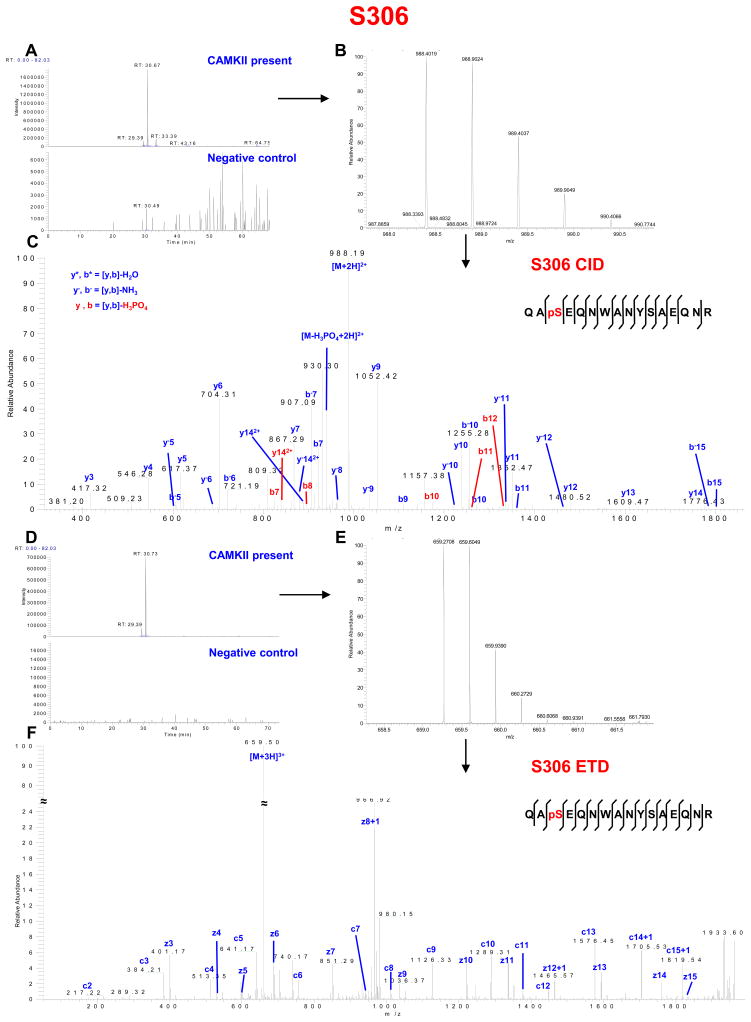
CaMKII phosphorylation of Cx43-CT at S306. (A) Extracted chromatogram of m/z 988.3930–988.3990. (B) MS spectrum of m/z 988.4019 represents doubly charged peptide QASEQNWANYSAEQNR (one phosphorylation site). (C) CID MS2 spectrum indicates phosphorylation on S306. (D) Extracted chromatogram of m/z 659.2617–659.2717. (E) MS spectrum of m/z 659.2708 represents triply charged peptide QASEQNWANYSAEQNR (one phosphorylation site). (F) ETD MS2 spectrum indicates phosphorylation on S306. See Supplemental Table S2 for complete list of theoretical (ProteinProspector) and observed fragment ions.
Table 1.
Serine residues in Cx43-CT phosphorylated by CaMKII with previously published kinase information where available.
| Phosphosite (# of expts) | Published or Novel | Previous Kinase Association | Role |
|---|---|---|---|
| S244 (3) | Novel, unreported site | -- | ? |
| S255 (3) | a, b, c, d | MAPKa; cyclin B/p34cdc2 b,c | ↓ Poe |
| S257 (3) | d | ? | ? |
| S296 (3) | d | ? | ? |
| S297 (2) | d | ? | ? |
| S306 (4) | d, f | ? | Maintained couplingf |
| S314 (2) | g, h | -- | ? |
| S325 (3)/S328 (2)/S330 (2) | d, i | CK1i | Gap junction formation; P2 formi |
| S364 (3) | j, k | PKAj,k | cAMP-enhanced GJ assemblyk |
| S365 (1) | l, m | PKA?l,m | Protection from ↓ gj; P1 formm |
| S369 (2) | d, l | PKA?l | No effect on GJICl |
| S372 (3) | d, n | PKCn | Maintained electrical couplingn |
| S373 (3) | d, l, o | PKA?l; Akto | No effect on GJICl; Cx43 binding to 14-3-3o |
, Warn-Cramer et al.40 (1996);
, Kanemitsu et al.39 (1998);
, Lampe et al.51 (1998);
, Axelsen et al.8 (2006);
, Cottrell et al.52 (2003);
, Procida et al.41 (2009);
, Munton et al.9 (2007);
, Brill et al.10 (2009);
, Cooper and Lampe42 (2002);
, Shah et al.53 (2002);
, TenBroek et al.54 (2001),
, Yogo et al.55 (2002);
, Solan et al.56 (2007);
, Sáez et al.57 (1997);
Park et al.58 (2007); Po = gap junction channel open probability
Table 2.
Phosphoserine-containing peptides from Cx43-CT in vitro phosphorylation by CaMKII.
| Sequence | Start-end | Charge | Theoretical mass |
Observed mass |
Phosphorylation site localized |
MASCOT score (CID) |
MASCOT score (ETD) |
Diagnostic ions (CID) |
Diagnostic ions (ETD) |
|---|---|---|---|---|---|---|---|---|---|
| GRSDPYHATTGPLSPSK | 242–258 | 3 | 1849.836 | 1849.841 | S244 | 43 | 37 | b2, b3 | c2, c3; z14, z15 |
| GRSDPYHATTGPLSPSK | 242–258 | 3 | 1849.836 | 1849.821 | S255 | 23 | 42 | b*14; y3, y4 | c13, c15 |
| GRSDPYHATTGPLSPSK | 242–258 | 3 | 1849.836 | 1849.840 | S257 | 37 | 45 | b2+15, b2+16 | c15, c16; z2 |
| NNSSC(Carbamidomethyl)RNYNK | 294–303 | 2 | 1335.503 | 1335.500 | S296 | 39 | N/A | y7, y8, y2+7, y2+8 | N/A |
| LVTGDRNNSSC(Carbamidomethyl)R | 288–299 | 3 | 1457.608 | 1457.613 | S296 | N/A | 61 | N/A | c8, c9; z3, z4 |
| LVTGDRNNSSC(Carbamidomethyl)RNYNK | 288–303 | 3 | 1976.847 | 1976.852 | S297 | 36 | N/A | b2+ 9; y2+ 7 (a) | N/A |
| LVTGDRNN(Deamidated)SSC(Carbamidomethyl)R | 288–299 | 2 | 1458.592 | 1458.597 | S297 | N/A | 30 | N/A | c9, c11 |
| QASEQNWANYSAEQNR | 304–319 | 2 | 1974.786 | 1974.782 | S306 | 30 | N/A | y13, y14 | N/A |
| QASEQNWANYSAEQNR | 304–319 | 3 | 1974.786 | 1974.783 | S306 | N/A | 33 | N/A | c2, c3; z13, z14 |
| QASEQNWANYSAEQNR | 304–319 | 3 | 1974.786 | 1974.782 | S314 | 46 | 85 | b10; y5, y6 | c10, c11 |
| MGQAGSTISNSHAQPFDFPDDNQNAK | 320–345 | 3 | 2856.181 | 2856.194 | S325 | 39 | 55 | b6; y2+20, y2+21 | c5, c6 |
| MGQAGSTISNSHAQPFDFPDDNQNAK | 320–345 | 3 | 2856.181 | 2856.191 | S328 | N/A | 59 | N/A | c8, c9; z17, z2+18 |
| MGQAGSTISN(Deamidated)SHAQ(Deamidated)PFDFPDDNQNAK | 320–345 | 3 | 2858.166 | 2858.149 | S330 | 33 | N/A | y15, y16 | N/A |
| MGQAGSTISNSHAQPFDFPDDN(Deamidated)QNAK | 320–345 | 3 | 2857.165 | 2857.188 | S330 | N/A | 60 | N/A | c10, c11; z15, z16 |
| VAAGHELQPLAIVDQRPSSR | 347–366 | 3 | 2223.116 | 2223.102 | S364 | 24 | 35 | y2, y4 | c+117, c+118; z+12, z+13 |
| VAAGHELQPLAIVDQRPSSR | 347–366 | 3 | 2223.116 | 2223.107 | S365 | 22 | N/A | y2 | N/A |
| ASSRPRPDDLEI | 371–382 | 2 | 1434.650 | 1434.641 | S372 | 25 | N/A | y2+ 10, y2+11 | N/A |
| ASSRASSRPRPDDLEI | 367–382 | 3 | 1835.853 | 1835.857 | S372 | N/A | 36 | N/A | c5, c6; z10, z11 |
| ASSRPRPDDLEI | 371–382 | 2 | 1434.650 | 1434.641 | S373 | 25 | N/A | y9, y10 | N/A |
| ASSRASSRPRPDDLEI | 367–382 | 3 | 1915.819 | 1915.826 | S369, S373 | N/A | 30 | N/A | c6, c7; z+19, z+110, z+113, z+114 |
Loss of H3PO4
One previously identified phosphorylation site, S306, was identified in each of the 4 experiments (Figure 2, Table S2 in Supporting Information). This serine is dephosphorylated in response to global ischemia in Langendorff perfused rat hearts.8 Dephosphorylation of S306 has recently been shown to contribute to reduced coupling.41 CaMKII and other protein kinases were tested by Axelsen et al.8 in in vitro phosphorylation assays using synthetic peptides containing this site; however, they could not identify the kinase(s) responsible for this phosphorylation. The discrepancy between their results and ours is likely due to their use of a short peptide containing amino acid residues 291–313 (with cysteine 298 substituted with an alanine)8 instead of a protein containing the full length of the carboxyl terminus of Cx43 as substrate for the CaMKII in vitro phosphorylation reaction. We can only speculate that the presence of C298 or a more extensive surrounding sequence may allow the protein to adopt a conformation required for phosphorylation of S306 by CaMKII.
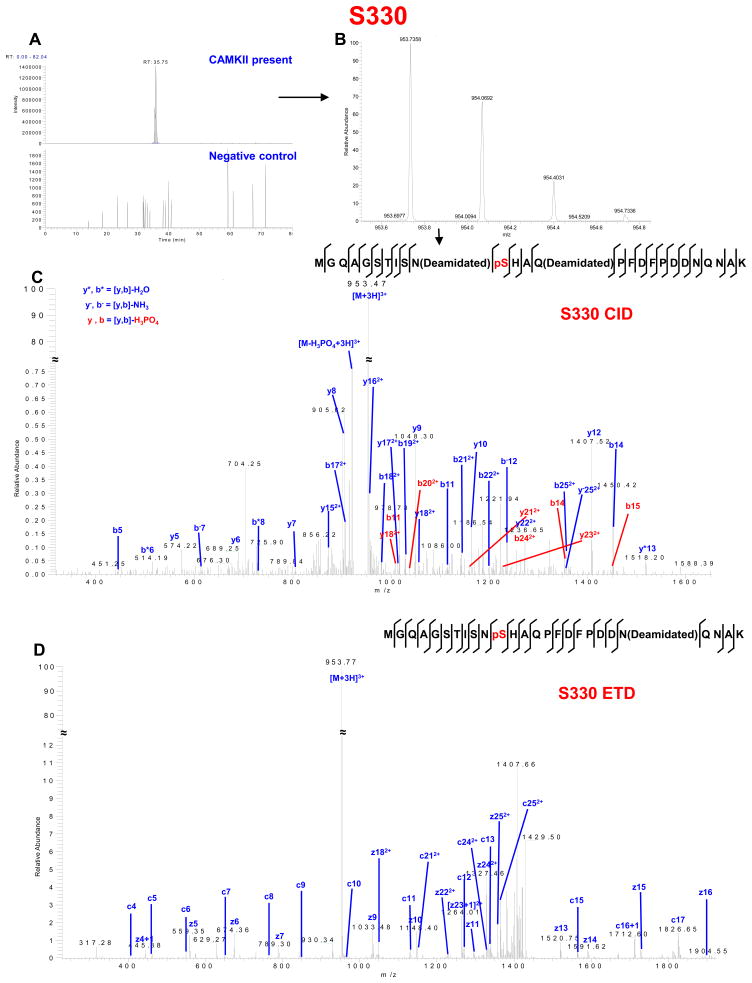
Another substrate for CaMKII was the previously identified serine residue at position 330 (Figure 3, Table S3 in Supporting Information). S330 is one of three serine residues (S325/S328/S330) that have been shown by Lampe et al.11 to be phosphorylated in Cx43 localized at intercalated disks, and that are involved in the electrophoretic mobility shift of Cx43 to the slowest migrating phosphorylated form (P2) in immunoblots.7 S330 is phosphorylated after 7 min of global ischemia, and subsequently dephosphorylated between 30 and 45 min of ischemia.8 Cooper and Lampe found that S325/S328/S330 are phosphorylated by CK1.42 Interestingly, we observed phosphorylation of S325/S328/S330 by CaMKII (Figure 3 and Figure S3 in Supporting Information), but not by CK1. While we could not detect phosphorylation of S325/S328/S330 by CK1, we found that this kinase phosphorylated S306 (Figure S4, Supporting Information) and S296 (not shown). Additional studies will be required to determine whether CaMKII and CK1 compete for target sites on Cx43 in vivo. One example in which these two kinases share target sites is in the Alzheimer’s disease protein, tau.43–46
Figure 3.
CaMKII phosphorylation of Cx43-CT at S330. (A) Extracted chromatogram of m/z 953.7332–953.7428. (B) MS spectrum of m/z 953.7358 represents triply charged peptide MGQAGSTISNSHAQPFDFPDDNQNAK (one phosphorylation site, two deamidated sites). (C) CID MS2 spectrum indicates phosphorylation on S330. (D) ETD MS2 spectrum indicates phosphorylation on S330. See Supplemental Table S3 for complete list of theoretical (ProteinProspector) and observed fragment ions.
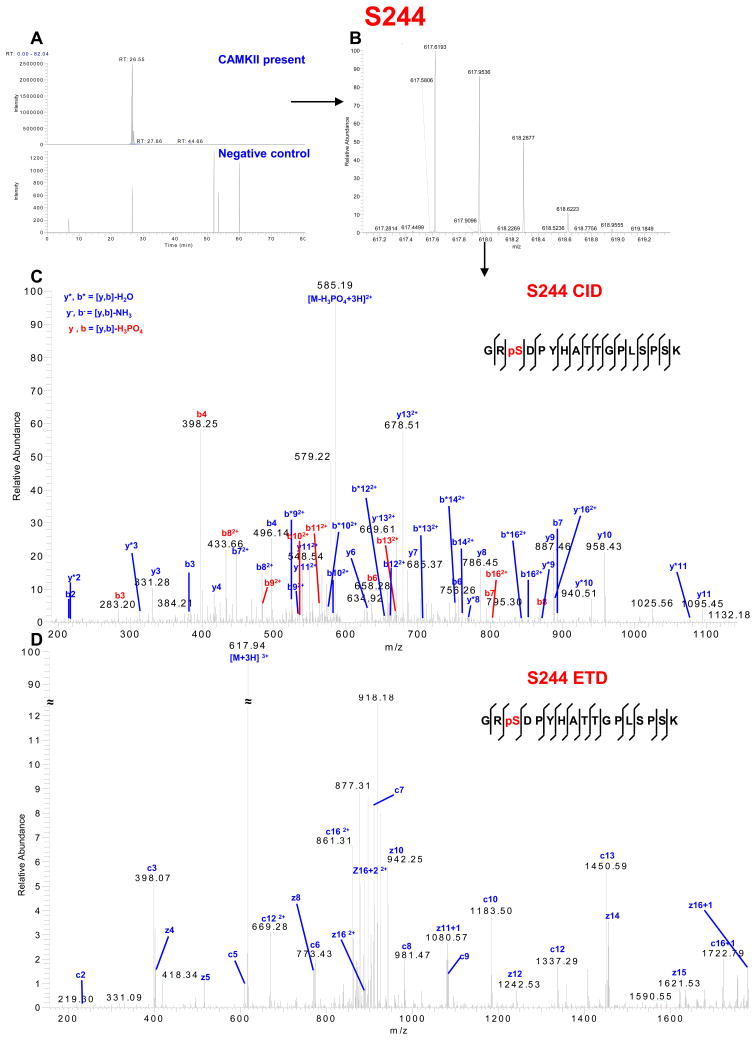
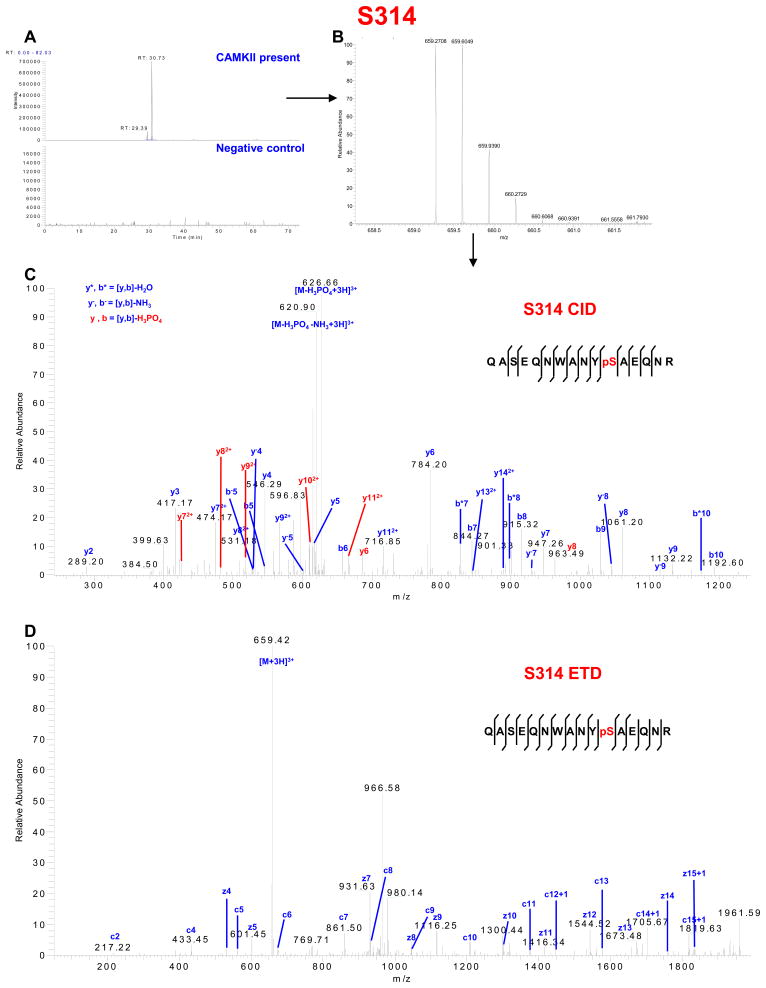
Importantly, we found one novel serine phosphorylation site that has not been reported previously, S244, and a second site, S314, that has recently been described in mouse brain synaptosomal preparations9 and human embryonic stem cells10 (Table 1 and Table S1 in Supporting Information). The spectra identifying these residues are shown in Figures 4 and 5 and Tables S4 and S5 in Supporting Information, respectively. We also identified S257, S296 and S297 (Figures S5 and S6 and Tables S4 and S6 in Supporting Information), which have not been linked previously to a specific kinase, as phosphorylation sites for CaMKII. Moreover, phosphorylation of S255, S364, S365, S369, S372 and S373 (Figures S5, S7 and S8 and Tables S4, S7 and S8 in Supporting Information) which has been reported previously to be directly or indirectly associated with other protein kinases was also identified in the GST-Cx43-CT fusion protein after in vitro phosphorylation by CaMKII.
Figure 4.
CaMKII phosphorylation of Cx43-CT at a novel site, S244. (A) Extracted chromatogram of m/z 617.6179–617.6203. (B) MS spectrum of m/z 617.6193 represents triply charged peptide GRSDPYHATTGPLSPSK (one phosphorylation site). (C) CID MS2 spectrum indicates phosphorylation on S244. (D) ETD MS2 spectrum indicates phosphorylation on S244. See Supplemental Table S4 for complete list of theoretical (ProteinProspector) and observed fragment ions.
Figure 5.
CaMKII phosphorylation of Cx43-CT at S314. (A) Extracted chromatogram of m/z 659.2613–659.2717. (B) MS spectrum of m/z 659.2708 represents triply charged peptide QASEQNWANYSAEQNR (one phosphorylation site). (C) CID MS2 spectrum indicates phosphorylation on S314. (D) ETD MS2 spectrum indicates phosphorylation on S314. See Supplemental Table S5 for complete list of theoretical (ProteinProspector) and observed fragment ions.
In summary, we identified a total of 15 putative serine phosphorylation sites for CaMKII in the carboxyl terminus of Cx43, bringing the total number of reported phosphorylation sites in the carboxyl terminus of Cx43 to 21 (19 Ser and 2 Tyr). Interestingly, two of these sites, S328 and S369, were identified by ETD alone. In each case, the phosphorylated serine was part of a multiply phosphorylated peptide fragment that was not identified by CID. This is an example in which combined use of CID and ETD as complementary fragmentation techniques provides enhanced identification of post-translationally modified amino acid residues. Because no phosphopeptides were identified in the control, CaMKII-free reactions (Figures 2–5 and Figures S3 and S5–S8 in Supporting Information), it is unlikely that phosphorylation of these residues was non-enzymatic or nonspecific.
In one experiment, we identified phosphorylation of S306, S372 and S373 in full-length native Cx43 immunoprecipitated from murine ventricular myocardium (Figures S9 and S10 and Tables S9–S11). Additional studies will be required to determine whether the serine residues in Cx43-CT identified in the present study, both in vitro and in vivo, are phosphorylated by CaMKII in vivo. Our data suggest that some residues may be targets for multiple protein kinases, implying that modulation of gap junction assembly and/or intercellular communication may depend on the combined levels of activity of the different protein kinases under different pathophysiological conditions. It also remains to be determined not only which protein kinase pathways are activated in the initial phases of ischemic injury that result in phosphorylation of Cx43, but also which pathways are activated subsequently that lead to dephosphorylation of Cx43 during ischemia and subsequent rephosphorylation in post-ischemic tissue.
Conclusions and Limitations
Mass spectrometry is a powerful technique that has allowed for identification of several phosphorylation sites, including a novel site at S244, that are targets of an important, biologically relevant protein kinase, CaMKII. In the present study, we identified four putative CaMKII serine phosphorylation sites (S296, S365, S369 and S373) that had been postulated by Payne et al.47 and confirmed by Pearson et al.48 as CaMKII phosphorylation sites based on an RXXS consensus sequence. Two CaMKII sites identified in our study (S244 and S306) conform to a KXXS sequence, which is also a consensus sequence for PKC.49 Utilization of ETD may enhance identification of phosphopeptides, particularly when multiple potential phosphorylation sites are present in a given peptide. It should be noted that conditions were used in the present study to drive the phosphorylation reaction to completion, and that steady state phosphorylation of Cx43 by CaMKII was not meant to represent transient signaling activity of CaMKII. Thus, residues observed to be phosphorylated in the present study exist in different structural environments and are not likely to be phosphorylated by CaMKII in vivo under the same physiological or pathophysiological condition or time frame. Finally, because CaMKII activity levels exhibit dynamic changes during acute and chronic ischemia, the mass spectrometric analyses performed in our study should be applied to full-length protein isolated from native cardiac tissue subjected to various pathophysiological conditions to elucidate whether and to what extent CaMKII regulates the phosphorylation state of Cx43 in diseased myocardium. For example, analysis of Cx43 extracted from CaMKII transgenic mice subjected to myocardial infarction50 would reveal how activation and inhibition of CaMKII influences phosphorylation of Cx43 associated with cardiac dysfunction or arrhythmogenesis during myocardial ischemia in vivo.
Supplementary Material
Synopsis.
Gap junctions are critical membrane specializations that contain clusters of intercellular channels required for normal cardiac electrical and contractile activity. The present report identifies 15 serine residues, including one novel site, in the gap junction channel protein connexin43 that are phosphorylated by CaMKII in vitro, suggesting that similar to other protein kinases, CaMKII may phosphorylate, and thereby regulate, connexin43.
Acknowledgments
The authors would like to thank Ms. Petra Gilmore in the Proteomics Core Facilities for expert sample preparation and TiO2 protocol development and Mr. Hao Zhang for assistance and guidance with the set-up and maintenance of the LTQ-Orbitrap. This work was supported by National Centers for Research Resources NIH Grant 2P41RR000954, UL1 RR024992, and NIH/NHLBI Grant HL066350 (to K.A.Y.).
Footnotes
Supporting Information Available: This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 2.van Veen TAB, van Rijen HVM, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res. 2001;51:217–229. doi: 10.1016/s0008-6363(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 3.Kreuzberg MM, Willecke K, Bukauskas FF. Connexin-mediated cardiac impulse propagation: connexin 30.2 slows atrioventricular conduction in mouse heart. Trends Cardiovasc Med. 2006;16:266–272. doi: 10.1016/j.tcm.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saffitz JE, Schuessler RB, Yamada KA. Mechanisms of remodeling of gap junction distributions and the development of anatomic substrates of arrhythmias. Cardiovasc Res. 1999;42:309–317. doi: 10.1016/s0008-6363(99)00023-1. [DOI] [PubMed] [Google Scholar]
- 5.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjølbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, Panse C, Schlapbach R, Mansuy IM. Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics. 2007;6:283–293. doi: 10.1074/mcp.M600046-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Brill LM, Xiong W, Lee KB, Ficarro SB, Crain A, Xu Y, Terskikh A, Snyder EY, Ding S. Phosphoproteomic analysis of human embryonic stem cells. Cell Stem Cell. 2009;5:204–213. doi: 10.1016/j.stem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita S, Kurihara H, Watanabe M, Okada T, Sakai T, Amano A. Alterations of phosphorylation state of connexin 43 during hypoxia and reoxygenation are associated with cardiac function. J Histochem Cytochem. 2006;54:343–353. doi: 10.1369/jhc.4A6611.2005. [DOI] [PubMed] [Google Scholar]
- 15.Hund TJ, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Protein kinase Cε mediates salutary effects on electrical coupling induced by ischemic preconditioning. Heart Rhythm. 2007;4:1183–1193. doi: 10.1016/j.hrthm.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naitoh K, Yano T, Miura T, Itoh T, Miki T, Tanno M, Sato T, Hotta H, Terashima Y, Shimamoto K. Roles of Cx43-associated protein kinases in suppression of gap junction-mediated chemical coupling by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2009;296:H396–H403. doi: 10.1152/ajpheart.00448.2008. [DOI] [PubMed] [Google Scholar]
- 17.Boersema PJ, Mohammed S, Heck AJR. Phosphopeptide fragmentation and analysis by mass spectrometry. J Mass Spectrom. 2009;44:861–878. doi: 10.1002/jms.1599. [DOI] [PubMed] [Google Scholar]
- 18.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 19.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Heller Brown J, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagemann D, Bohlender J, Hoch B, Krause EG, Karczewski P. Expression of Ca2+/calmodulin-dependent protein kinase II δ-subunit isoforms in rats with hypertensive cardiac hypertrophy. Mol Cell Biochem. 2001;220:69–76. doi: 10.1023/a:1010899724222. [DOI] [PubMed] [Google Scholar]
- 21.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The δ isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoch B, Meyer R, Hetzer R, Krause E-G, Karczewski P. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 23.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. β1-Adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1377–H1386. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, Yamada KA, Rudy Y. Role of activated CaMKII in abnormal calcium homeostasis and INa remodeling after myocardial infarction: insights from mathematical modeling. J Mol Cell Cardiol. 2008;45:420–428. doi: 10.1016/j.yjmcc.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Pina-Benabou MH, Srinivas M, Spray DC, Scemes E. Calmodulin kinase pathway mediates the K+-induced increase in gap junctional communication between mouse spinal cord astrocytes. J Neurosci. 2001;21:6635–6643. doi: 10.1523/JNEUROSCI.21-17-06635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saez JC, Nairn AC, Czernik AJ, Spray DC, Hertzberg EL, Greengard P, Bennett MVL. Phosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur J Biochem. 1990;192:263–273. doi: 10.1111/j.1432-1033.1990.tb19223.x. [DOI] [PubMed] [Google Scholar]
- 28.Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Höher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schieltz DM, Washburn MP, Hays LG. Proteins and proteomics. Chapter 8 Cold Spring Harbor Laboratory Press; 2003. Analysis of Complex Protein Mixtures Using Nano-LC Coupled to MS/MS. [Google Scholar]
- 30.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat Biotech. 2010;28:710–721. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 32.Yeager M. Structure of cardiac gap junction intercellular channels. J Struct Biol. 1998;121:231–245. doi: 10.1006/jsbi.1998.3972. [DOI] [PubMed] [Google Scholar]
- 33.Dunker AK, Obradovic Z. The protein trinity linking function and disorder. Nat Biotechnol. 2001;19:805–806. doi: 10.1038/nbt0901-805. [DOI] [PubMed] [Google Scholar]
- 34.Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V, Sorgen PL. Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl terminal and cytoplasmic loop domains. J Biol Chem. 2009;284:34257–24271. doi: 10.1074/jbc.M109.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of connexin43 channels. Cardiovasc Res. 2004;62:268–275. doi: 10.1016/j.cardiores.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Hervé J-C, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Sobott F, Watt SJ, Smith J, Edelmann MJ, Kramer HB, Kessler BM. Comparison of CID versus ETD based MS/MS fragmentation for the analysis of protein ubiquitination. J Am Soc Mass Spectrom. 2009;20:1652–1659. doi: 10.1016/j.jasms.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Jensen SS, Larsen MR. Evaluation of the impact of some experimental procedures on different phosphopeptide enrichment techniques. Rapid Commun Mass Spectrom. 2007;21:3635–3645. doi: 10.1002/rcm.3254. [DOI] [PubMed] [Google Scholar]
- 39.Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43: during mitosis. Cell Growth Diff. 1998;9:13–21. [PubMed] [Google Scholar]
- 40.Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- 41.Procida K, Jørgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, Nielsen MS, Braunstein TH. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm. 2009;6:1632–1638. doi: 10.1016/j.hrthm.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- 43.Singh TJ, Wang JZ, Novak M, Kontzekova E, Grundke-Iqbal I, Iqbal K. Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett. 1996;387:145–148. doi: 10.1016/0014-5793(96)00485-1. [DOI] [PubMed] [Google Scholar]
- 44.Gupta RP, Abou-Donia MB. Tau phosphorylation by diisopropyl phosphorofluoridate (DFP)-treated hen brain supernatant inhibits its binding with microtubules: role of Ca2+/calmodulin-dependent protein kinase II in tau phosphorylation. Arch Biochem Biophys. 1999;365:268–278. doi: 10.1006/abbi.1999.1165. [DOI] [PubMed] [Google Scholar]
- 45.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/s0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 46.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007;282:23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 47.Payne ME, Schworer CM, Soderling TR. Purification and characterization of rabbit liver calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1983;258:2376–2382. [PubMed] [Google Scholar]
- 48.Pearson RB, Woodgett JR, Cohen P, Kemp BE. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985;260:14471–14476. [PubMed] [Google Scholar]
- 49.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 50.Zhang R, Khoo MSC, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song L-S, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 51.Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- 52.Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src-and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284:C511–C520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah MM, Martinez AM, Fletcher WH. The connexin43 gap junction protein is phosphorylated by protein kinase A and protein kinase C: in vivo and in vitro studies. Mol Cell Biochem. 2002;238:57–68. doi: 10.1023/a:1019902920693. [DOI] [PubMed] [Google Scholar]
- 54.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulose cells. FEBS Lett. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- 56.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sáez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 58.Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates connexin43 on ser373, a “mode-1” binding site for 14-3-3. Cell Commun Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.