Abstract
In Saccharomyces cerevisiae, proximity to a telomere affects both transcription and replication of adjacent DNA. In this study, we show that telomeres also impose a position effect on mitotic recombination. The rate of recombination between directly repeated tracts of telomeric C1–3A/TG1–3 DNA was reduced severely by proximity to a telomere. In contrast, recombination of two control substrates was not affected by telomere proximity. Thus, unlike position effects on transcription or replication, inhibition of recombination was sequence specific. Moreover, the repression of recombination was not under the same control as transcriptional repression (telomere position effect; TPE), as mutations in genes essential for TPE did not alleviate telomeric repression of recombination. The reduction in recombination between C1–3A/TG1–3 tracts near the telomere was caused by an absence of Rad52p-dependent events as well as a reduction in Rad1p-dependent events. The sequence-specific repression of recombination near the telomere was eliminated in cells that overexpressed the telomere-binding protein Rap1p, a condition that also increased recombination between C1–3A/TG1–3 tracts at internal positions on the chromosome. We propose that the specific inhibition between C1–3A/TG1–3 tracts near the telomere occurs through the action of a telomere-specific end-binding protein that binds to the single-strand TG1–3 tail generated during the processing of recombination intermediates. The recombination inhibitor protein may also block recombination between endogenous telomeres.
Keywords: Telomeres, recombination, yeast, position effect, telomere replication
In most organisms, telomeres consist of simple repetitive DNA. For example, each end of each Saccharomyces chromosome bears ∼300 bp of C1–3A/TG1–3 DNA. Telomeres are required for the stable maintenance and segregation of yeast chromosomes (Sandell and Zakian 1993). In most organisms, including yeast, telomeric DNA is replicated by telomerase, a telomere-specific reverse transcriptase (for review, see Greider 1995). Telomerase extends the G-strand of telomeric DNA using its RNA component as a template. Telomerase-independent pathways for telomere replication also exist. In yeasts (Lundblad and Blackburn 1993; McEachern and Blackburn 1995; Lendvay et al. 1996) and human cells in culture (Murnane et al. 1994; de Lange 1995; Rogan et al. 1995), telomere–telomere recombination can maintain telomeric DNA in the absence of telomerase. In some insects, recombination is probably the sole pathway for maintenance of telomeric DNA (Biessmann et al. 1996; Lopez et al. 1996).
The subtelomeric regions of chromosomes from many organisms, including yeast, consist of a variable array of middle repetitive DNA with the variability caused, at least in part, by homologous recombination among the repeats (Brown et al. 1990; Louis et al. 1994). In yeast, the number and identity of these middle repetitive elements vary, both from strain to strain and from chromosome to chromosome. In addition, in yeast, there are often interstitial tracts of telomeric DNA interspersed among the middle repetitive elements (Walmsley et al. 1984; Louis et al. 1994). Interstitial tracts of telomeric sequence exist in many other organisms, including mammals (Meyne et al. 1990; Cheung et al. 1994). In mammals, these tracts are not limited to subtelomeric regions of chromosomes and are believed to act as recombination hot spots (Park et al. 1992; Ashley and Ward 1993; Ashley 1994; Henderson 1995). In both yeast and mammals, short stretches of the telomere-like sequence poly(GT) increase recombination rates (Stringer 1985; Treco and Arnheim 1986; White et al. 1991). The preference for GT-rich DNA displayed in vitro by at least some strand transfer proteins may contribute to the elevated recombination rates of telomeric and telomere-like DNAs (Tracey et al. 1996, 1997).
In meiosis, telomeres themselves affect recombination. For example, molecular and cytological studies show reduced meiotic crossing-over in telomeric regions of grasshopper chromosomes (Miklos and Nankivell 1976). Most relevant for our studies, double-strand breaks, which initiate most meiotic recombination events, are absent in the terminal ∼25 kb of yeast chromosomes (Klein et al. 1996). In contrast, cytological and genetic evidence suggests that meiotic recombination occurs at elevated rates near some human telomeres (Ashley 1994; Kipling et al. 1996).
In mitotic cells, yeast telomeres affect the replication and transcription of nearby DNA. Proximity to a yeast telomere eliminates (Reynolds et al. 1989; Dubey et al. 1991; Zhu et al. 1992) or delays (Ferguson and Fangman 1992; Wellinger et al. 1993) activation of replication origins. Transcription of genes near telomeres is repressed in yeast (Gottschling et al. 1990) and other organisms (Levis et al. 1985; Nimmo et al. 1994; Horn and Cross 1995; Rudenko et al. 1995), a phenomenon called telomere position effect (TPE). In S. cerevisiae, transcriptional repression is not limited to telomeres, as interstitial tracts of C1–3A/TG1–3 DNA integrated onto the chromosome also repress transcription, even on circular chromosomes (Stavenhagen and Zakian 1994).
The telomeric C1–3A/TG1–3 repeats are organized into a non-nucleosomal protein–DNA structure, called the telosome (Wright et al. 1992). The major protein in the yeast telosome is the essential (Shore and Nasmyth 1987) duplex DNA-binding protein Rap1, present in 10–20 molecules per telomere (Conrad et al. 1990; Klein et al. 1992; Wright et al. 1992; Gilson et al. 1993; Wright and Zakian 1995). RAP1 is important for TPE and telomere length control (see Kyrion et al. 1992, 1993; Marcand et al. 1997).
Rap1p mediates its effects on telomeres at least in part through its interactions with other proteins. The carboxyl terminus of Rap1p interacts with Sir3p, Sir4p, Rif1p, and Rif2p (Hardy et al. 1992; Moretti et al. 1994; Wotton and Shore 1997). Sir2p interacts with Sir4p and Sir3p (Moazed et al. 1997) and hence indirectly with Rap1p. Sir2p, Sir3p, Sir4p, Rif1p, and Rif2p are telosomal proteins in vivo as is, Cdc13p (Bourns et al. 1998), a protein that binds single-strand TG1–3 DNA in vitro (Lin and Zakian 1996; Nugent et al. 1996). Sir2p, Sir3p, and Sir4p are essential for TPE (Aparicio et al. 1991) as well as for silencing at internal tracts of telomeric DNA (Stavenhagen and Zakian 1994) whereas Rif1p and Rif2p function cooperatively to limit telomere length (Wotton and Shore 1997). The phenotypes of cells limited for the essential Cdc13p suggest that it regulates access of both telomerase (Nugent et al. 1996) and nucleases (Garvik et al. 1995) to telomeric DNA. In wild-type cells, Rap1p and the three Sir proteins are concentrated in foci near the nuclear periphery that correspond to clusters of telomeres (Gotta et al. 1996, 1997; Palladino et al. 1993).
This paper presents a study of recombination between telomeric sequences at both subtelomeric loci and internal chromosomal sites. We found that recombination between C1–3A/TG1–3 tracts was decreased dramatically near the telomere, whereas recombination between two control sequences was not affected by telomere proximity. The reduction in recombination between C1–3A/TG1–3 tracts was caused in large part by the elimination of RAD52-dependent events although RAD1-dependent events were also reduced. Thus, yeast telomeres exert a position effect on recombination between C1–3A/TG1–3 sequences. Because this position effect did not require genes essential for TPE, telomere position effects on recombination were caused by a different mechanism than TPE.
Results
Direct-repeat recombination assay
Because of a long-term interest in the recombination behavior of telomeric DNA (Pluta and Zakian 1989; Wang and Zakian 1990), we devised a system to study recombination between two internal tracts of telomeric C1–3A/TG1–3 DNA as a function of the position of the tracts along the chromosome. In each experiment, both the rate of recombination and the structure of the recombination products were determined. As recombination was measured in haploid cells, the effects of different mutations on the recombination behavior of the tracts could be determined.
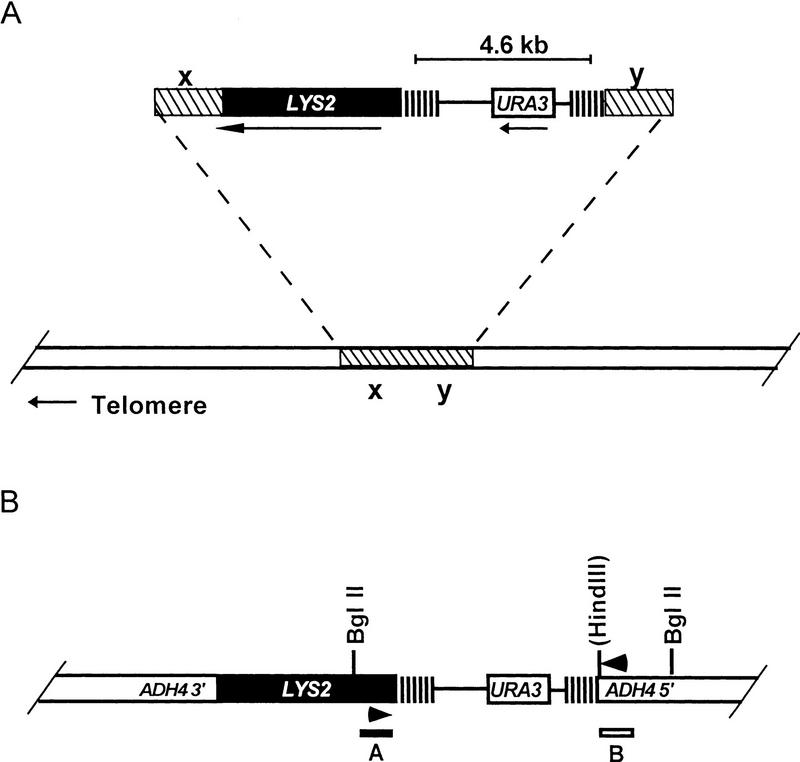
Three different classes of recombination substrates were constructed. Each of the three had two identical ∼300-bp tracts separated by a 4.6-kb segment of DNA that contained the URA3 gene (Fig. 1A). The three recombination substrates differed only in the identity of the sequence that comprised the ∼300 bp tracts. The three substrates contained ∼300 ± 25 bp of either C1–3A/TG1–3 DNA (Saccharomyces telomeric DNA), C4A2/T2G4 DNA (Tetrahymena telomeric DNA), or a unique sequence (a fragment from the Salmonella tetracycline-resistance gene). The base composition of the unique sequence tract was identical to that of C4A2/G4T2 DNA and similar to that of C1–3A/TG1–3. In contrast to the unique sequence tract, the C4A2/T2G4 and C1–3A/TG1–3 tracts had two features in common: Both are internally repetitive and both are substrates for C1–3A/TG1–3 addition in yeast in vivo (Dani and Zakian 1983; Szostak 1983). Although the internally repetitive C4A2/T2G4 and C1–3A/TG1–3 tracts can both align in multiple registers, C1–3A/TG1–3 DNA, an imperfect repeat, can also align with mismatches between base pairs. Therefore C1–3A/TG1–3 direct repeats can undergo both homologous and homeologous recombination. In addition, C1–3A/TG1–3 DNA is the only one of the three substrates that is expected to be bound by the yeast telomere-binding protein Rap1p in vivo (Berman et al. 1986; Conrad et al. 1990; Klein et al. 1992).
Figure 1.
Structures of recombination substrates. General structure of recombination substrates. The identity of x and y vary depending on the site of integration for each particular construct. (A) Using the homology provided by x and y, integrative transformation was used to target plasmids to a specific site on chromosome V-R or chromosome VII-L in the orientation shown. The parallel vertical lines represent a ∼300 bp tract of either C1–3A/TG1–3, C4A2/T2G4, or unique sequence DNA. For the C1–3A/TG1–3 and C4A2/T2G4 tracts, the G-rich strand runs 5′ → 3′ towards the telomere, in the same orientation as the endogenous telomere. The two copies of the tract are separated by 4.6 kb that contains the URA3 gene and sequences derived from the vector YIp5. Upon integration, LYS2 is always distal to the direct repeats. The arrows below the line indicate the direction of transcription of URA3 and LYS2. The diagram is not drawn to scale. (B) Genomic structure expected after recombination for substrates integrated ∼20 kb from the end of chromosome VII-L. Only the telomere proximal regions of the chromosome are depicted. Symbols used are the same as above. The HindIII site is present only in the control substrates. Arrowheads indicate positions of the PCR primers. The positions of the probes used in the Southern analysis are represented as bars (labeled A, LYS2, and B, ADH4) under the diagram of the chromosome.
Each recombination substrate was integrated at five different chromosomal loci in a haploid strain: These sites were ∼4 and 17 kb from the right telomere of chromosome V and ∼5, 20, and 200 kb from the left telomere of chromosome VII. In each case, LYS2 was placed distal to the recombination substrates (Fig. 1). Recombination rates were measured both in wild-type cells and in cells containing null mutations in genes that affect either TPE or recombination.
Recombination events were selected on plates containing 5-fluoro-orotic acid (FOA), which selects for cells lacking Ura3p (Boeke et al. 1987), and lacking lysine, which, by selecting for expression of LYS2, reduces transcriptional repression on URA3. In the system used here, a Ura− Lys+ cell can be generated by intrachromosomal recombination, interchromosomal recombination, or sister chromatid exchange. For the C1–3A/TG1–3 substrates, interchromosomal recombination can occur between one of the internal C1–3A/TG1–3 tracts on the marked chromosome and the telomere of another chromosome. This event transfers LYS2 to a different chromosome. Although they proceed by different mechanisms, intrachromosomal recombination and sister-chromatid exchange are expected to generate products of identical structure in which the recombinant chromosome has a single copy of the original tract at an internal site. Because these two classes of events, intrachromosomal recombination and unequal sister chromatid exchange, could not be distinguished from each other in our study, these two classes of events will be referred to collectively as excision events.
In the system used in this study, two events other than recombination can yield an FOAR cell, transcriptional silencing of URA3 and point mutations in URA3. To ensure that the reported rates were based solely on FOAR cells that arose from recombination, the absence of the URA3 gene was established by conventional Southern or PCR analysis on a subset of the recovered FOAR cells in all genetic backgrounds examined (see below). In each experiment, FOAR Lys+ events that were caused by transcriptional silencing or mutation of URA3 were subtracted before determining recombination rates.
Telomeres specifically repress recombination between tracts of C1–3A DNA
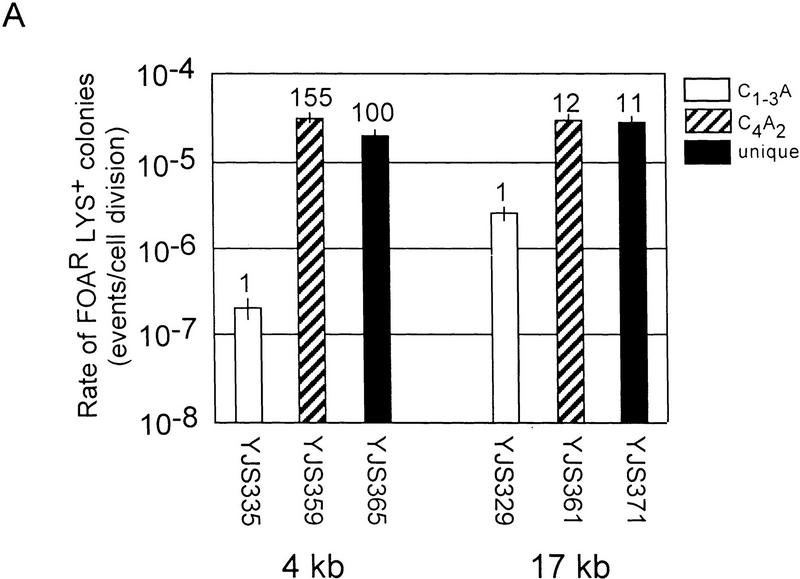
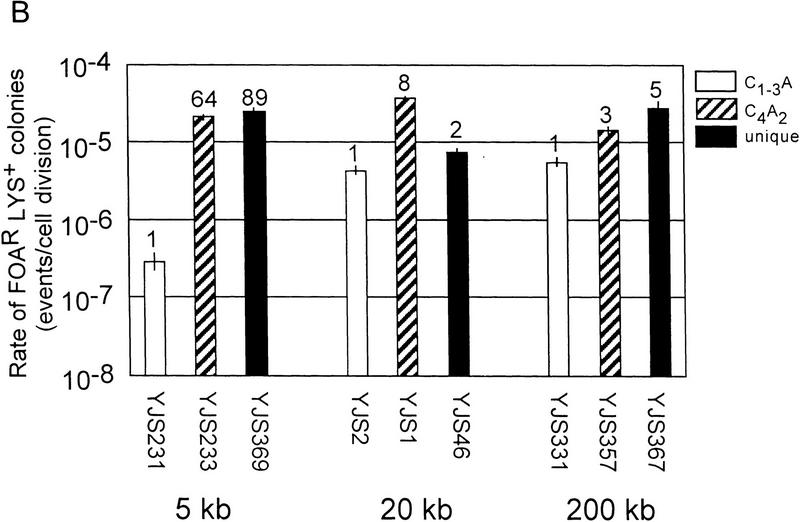
Recombination between either the C4A2/T2G4 or the unique sequence tracts, hereafter called the control substrates, occurred at similar rates at all five chromosomal loci (Fig. 2A,B). However, recombination between C1–3A/TG1–3 tracts was affected strongly by chromosomal position. Although recombination between C1–3A/TG1–3 tracts was lower than that of both controls at all five sites, by far the most dramatic difference among the three substrates was their recombination rates near the telomere. Recombination between C1–3A/TG1–3 tracts near the telomere occurred at rates 64- to 155-fold lower than the controls at the same site.
Figure 2.
Rates of recombination in a wild-type strain. (A) Rates of recombination between direct repeats at different distances from the telomere on chromosome V-R. Strain names and the distance of the repeats relative to the telomere are indicated below the bars on the x-axis. The y-axis indicates the recombination rates. The white columns are data for the C1–3A/TG1–3 substrates, the columns with diagonal lines are for C4A2/G4T2 substrates and the black columns are for the unique sequence substrate. The tops of the columns are the average median recombination rate from a minimum of four assays. The numbers above the columns represent the fold difference in recombination rate relative to the C1–3A/TG1–3 direct-repeat strain at the same location. The error bars show standard deviations (Lea and Coulson 1949). The rates for C1–3A/TG1–3 direct-repeat events are 2.0 × 10−7 (±0.6) events/cell division and 2.6 × 10−6 (±0.6) events/cell division for 4 and 17 kb from the telomere, respectively. (B) Rates of recombination between direct repeats at different distances from the telomere on chromosome VII-L. Interpretation of the graph is the same as in A. The rates for C1–3A/TG1–3 direct repeat events are 2.9 × 10−7 (±0.8) events/cell division, 4.4 × 10−6 (±0.7) events/cell division and 5.6 × 10−6 (±0.9) events/cell division for 5 and 20 kb and 200 kb from the telomere, respectively.
For example, on chromosome V-R (Fig. 2A), at both 4 and 17 kb from the telomere, recombination of the C4A2/T2G4 and unique sequence tracts occurred at very similar rates, ranging from 2.8 × 10−5 to 3.0 × 10−5 events/cell division. At 17 kb from the telomere, recombination between the C1–3A/TG1–3 tracts occurred at a rate ∼11 times lower than that seen for the two control substrates at the same locus. However, at 4 kb from the chromosome V-R telomere, recombination between the C1–3A/TG1–3 tracts was even lower, occurring at a rate 100 or 155 times lower than for the two control substrates. These differences in rates were caused by a 13-fold reduction in the rate of recombination between the C1–3A/TG1–3 tracts near the telomere, rather than to an increase in recombination rates of the control sequences near the telomere (Fig. 2A).
A similar pattern was seen on chromosome VII-L. The rate of recombination between the C1–3A/TG1–3 tracts was slightly lower, two- to eightfold, than the control substrates at both 20 and 200 kb from the telomere. However, recombination between the C1–3A/TG1–3 tracts 5 kb from the telomere occurred at a rate 64 or 89 times lower than the two control substrates at the same locus (Fig. 2B). Recombination between the C1–3A/TG1–3 tracts was ∼15- to 19-fold lower near the telomere than it was at the two internal sites on chromosome VII-L (Fig. 2B).
These data indicate that recombination between C1–3A/TG1–3 tracts was reduced by proximity to the telomere. Thus, the telomere imposed a position effect on recombination of C1–3A/TG1–3 DNA.
Structure of the chromosomes after recombination
For each strain and each recombination substrate, the resultant structure of chromosome V-R or chromosome VII-L was determined in at least 20 independent FOAR Lys+ colonies using Southern hybridization or PCR analysis (Table 1, e.g., see Fig. 5, below). This analysis demonstrated that FOAR colonies were generated by recombination, not transcriptional silencing nor mutation of URA3.
Table 1.
Summary of the products of chromosome VII-L excision events
| Strain
|
Sequence
|
Distance from telomere (kb)
|
No. of samples
|
Tract length (bp)
|
|||
|---|---|---|---|---|---|---|---|
| 100–200
|
200–300
|
300–400
|
400–500
|
||||
| YJS231 | C1–3A | 5 | 20 | 10% | 45% | 40% | 5% |
| YJS2 | C1–3A | 20 | 16 | 6% | 69% | 25% | 0 |
| YJS331 | C1–3A | 200 | 5 | 0 | 60% | 40% | 0 |
| YJS233 | C4A2 | 5 | 10 | 40% | 10% | 40% | 10% |
| YJS1 | C4A2 | 20 | 19 | 0 | 47% | 53% | 0 |
| YJS357 | C4A2 | 200 | 10 | 30% | 0 | 30% | 40% |
| YJS369 | Unique | 5 | 10 | 0 | 100% | 0 | 0 |
| YJS46 | Unique | 20 | 10 | 0 | 100% | 0 | 0 |
| YJS367 | Unique | 200 | 8 | 0 | 100% | 0 | 0 |
Data are displayed as the percentage of excision events that resulted in tract lengths of the designated size. The approximate length was determined by either genomic Southern analysis for strains YJS2, YJS233, YJS1, YJS357, YJS46 or colony PCR for strains YJS231, YJS2, YJS357, YJS369, YJS367 (Materials and Methods).
Figure 5.
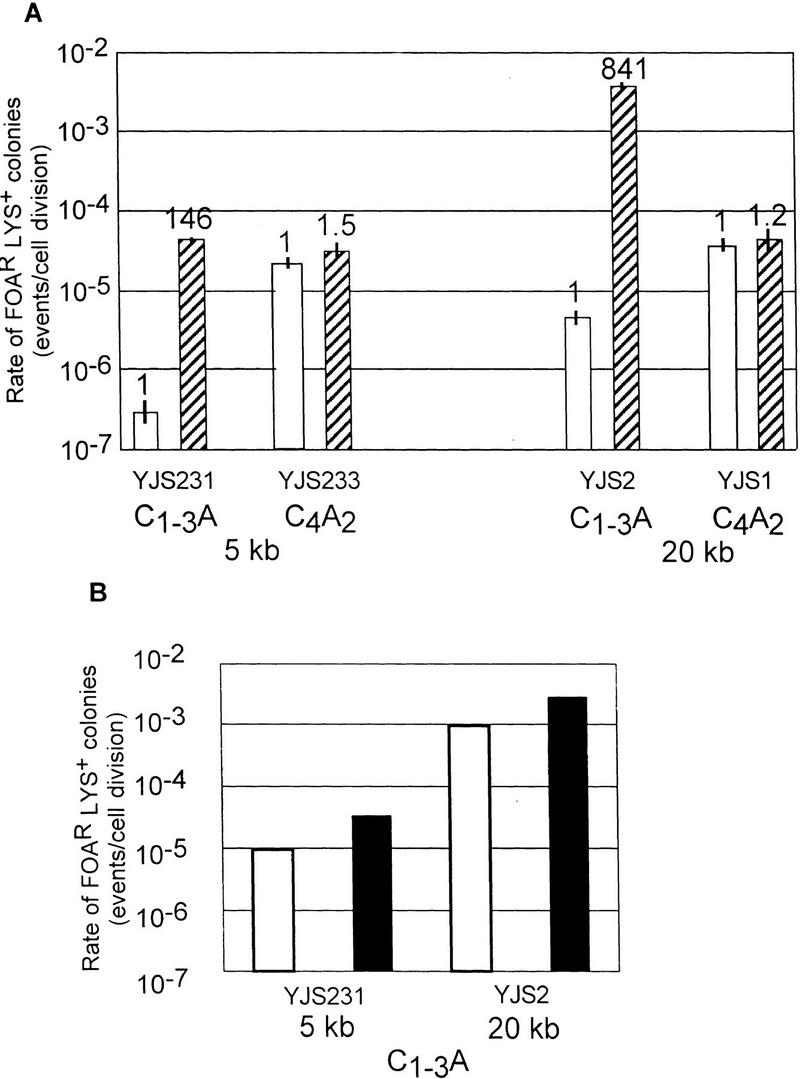
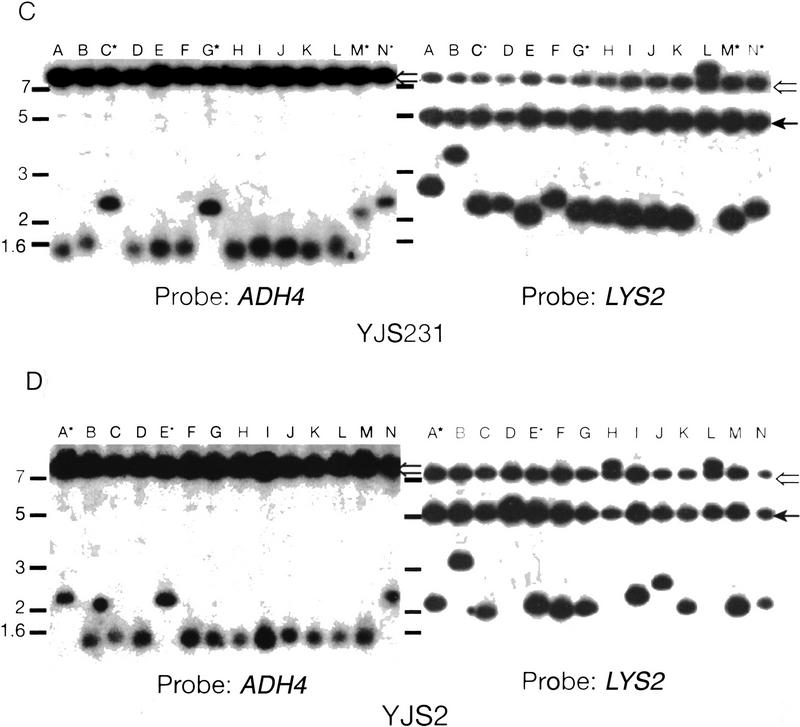
Recombination in strains overexpressing Rap1p. (A) Rates of recombination between direct repeats in strains over expressing RAP1. Description of the graph is the same as in Fig. 3. The white columns represent the values for strains with wild-type levels of Rap1p and the striped columns represent cells containing FATRAP, which express high levels of Rap1p. (B) The rates of excision or interchromosomal C1–3A/TG1–3 direct-repeat recombination events at 5 and 20 kb from the telomere are depicted separately. (Open bars) Excision events; (solid bars) interchromosomal events. The different events were determined by Southern analysis (see below). (C) Southern analysis of FOARLys+ events from YJS231 (tract 5 kb from chromosome VII-L telomere) transformed with FATRAP (see Materials and Methods). Genomic DNA was digested with BglII and run on a 1% agarose gel. Molecular mass standards (kb) are indicated at left. The probe is indicated at the bottom. The filter was first hybridized to the ADH4 (probe B), the probe was removed, and filters were then reprobed with LYS2 DNA (probe A). (Lanes A–N) Different independent recombination events. Hybridizing high molecular mass bands are caused by cross-hybridization of the probe to the FATRAP vector sequences (open arrow) and to the endogenous LYS2 gene (solid arrow). All other bands seen are products of the recombination reaction. Lanes marked with an asterisk have a 2.0–2.6 kb hybridizing band that hybridizes to both the LYS2 and ADH4 probes, indicative of an excision event. (D) Southern analysis of FOARLys+ events from YJS2 (tracts 20 kb from chromosome VII-L telomere) transformed with FATRAP. Symbols are the same as in C.
Recombination between direct repeats is expected to leave one copy of the repeat tract at an internal site on the chromosome bearing LYS2. Southern analysis was done by probing restriction-enzyme-digested-genomic DNA, BglII–HindIII for the control strains and BglII for the C1–3A/TG1–3 strains, with DNA fragments derived from the 5′-end of the LYS2 gene (Fig. 1B; probe A) and the 3′-end of the ADH4 gene (Fig. 1B; probe B). Events that occurred by either intrachromosomal recombination or sister chromatid exchange produced a fragment that hybridized to both probe A and B (Fig. 5C,D, below). Colony PCR was done using oligonucleotides complementary to region A and region B (see Fig. 1B; Materials and Methods).
The size of the remaining tract is presented for each of the three recombination substrates at each of the three loci on chromosome VII-L (Table 1). Recombination between two unique sequence tracts is expected to leave a tract identical in size to the 280-bp starting tract. This result was seen for 28 of 28 colonies examined. As expected, recombination between either C1–3A/TG1–3 or C4A2/T2G4 tracts resulted in chromosomes with remaining tracts of variable size in different recombination events (Table 1). The fact that tracts of variable length were recovered demonstrated that, as expected, both the C1–3A/TG1–3 and C4A2/T2G4 tracts were able to align in multiple registers prior to completion of recombination. By this same criterion, proximity to the telomere affected the frequency but not the alignment of the C1–3A/TG1–3 tracts.
The structure of LYS2 in the recombination products also provides information on the mechanism of recombination. Because interchromosomal recombination transfers LYS2 to a different chromosome, recombinants produced by this path will have restriction fragments of novel sizes and will not yield a PCR product with the primers used. No interchromosomal recombination events were detected in the ∼150 recombination products examined (Table 1), except in experiments in which Rap1p was overexpressed (see below, Fig. 5). These data suggest that for the three substrates in each chromosomal location and in a variety of genetic backgrounds, most recombination events occurred by either intrachromosomal recombination or sister chromatid exchange.
Telomeric repression of recombination between C1–3A/TG1–3 tracts is not alleviated by mutations in genes required for TPE
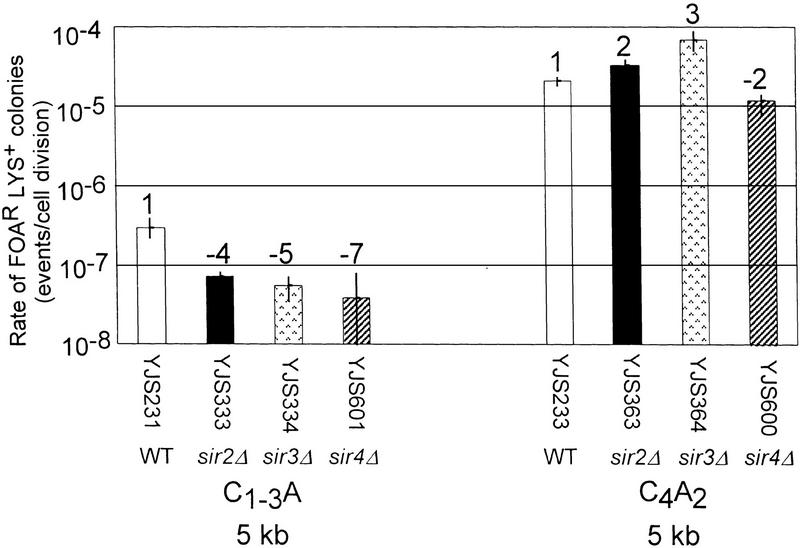
Mutations in SIR2, SIR3, or SIR4, eliminate position effects on transcription at the silent mating type loci (Rine and Herskowitz 1987), the telomere (Aparicio et al. 1991), and internal tracts of C1–3A/TG1–3 DNA (Stavenhagen and Zakian 1994). Intrachromosomal recombination at the rDNA locus is increased 19-fold in a sir2Δ strain (Gottlieb and Esposito 1989). To determine if the SIR genes are required for the repression of recombination between C1–3A/TG1–3 tracts near the telomere, the rate of recombination in strains containing null mutations in each of the three SIR genes was determined (Fig. 3). If the SIR genes were required for repression of recombination, then recombination between C1–3A/TG1–3 tracts should occur at an elevated rate in sirΔ strains compared to wild-type cells.
Figure 3.
Rates of recombination in strains that lack transcriptional repression at telomeres. Rates were determined for recombination between C1–3A/TG1–3 (left) or C4A2/T2G4 (right) tracts 5 kb from the chromosome VII-L telomere in wild-type, sir2Δ, sir3Δ, and sir4Δ strains. Interpretation of the graph is the same as in Fig. 2A except that the average median recombination rate is based on a minimum of two assays. The numbers above the columns represent the fold difference in recombination rate for the same substrate relative to the wild-type strain at the same location.
Contrary to this expectation, recombination between C1–3A/TG1–3 tracts near the telomere was even lower in the three sirΔ strains than in wild-type cells (four- to sevenfold reduction, depending on strain; Fig. 3A). Recombination between C4A2/T2G4 tracts was affected modestly in sirΔ strains (two- or threefold increase or twofold reduction depending on sirΔ strain.) As a result, the differences in recombination rates near the telomere were exacerbated in sirΔ strains (225- to 965-fold lower recombination rates for C1–3A/TG1–3 tracts vs. C4A2/T2G4 tracts). Elimination of Sir proteins also had modest inhibitory effects on C1–3A/TG1–3 tracts at 20 kb [2.6 × 10−6(±0.6) events/cell division]. Similar recombination rates were found when sirΔ strains were grown in the presence of lysine, indicating that transcription through the telomere did not mask the activity of the Sir proteins (data not shown). As reduced recombination between C1–3A/TG1–3 tracts did not require Sir proteins, the telomere′s effect on recombination of C1–3A/TG1–3 tracts is not under the same genetic control as the position effects that cause transcriptional repression at telomeres (Aparicio et al. 1991) or at internal tracts of C1–3A/TG1–3 DNA (Stavenhagen and Zakian 1994).
The effect of hpr1Δ on recombination between tracts of C1–3A/TG1–3 DNA
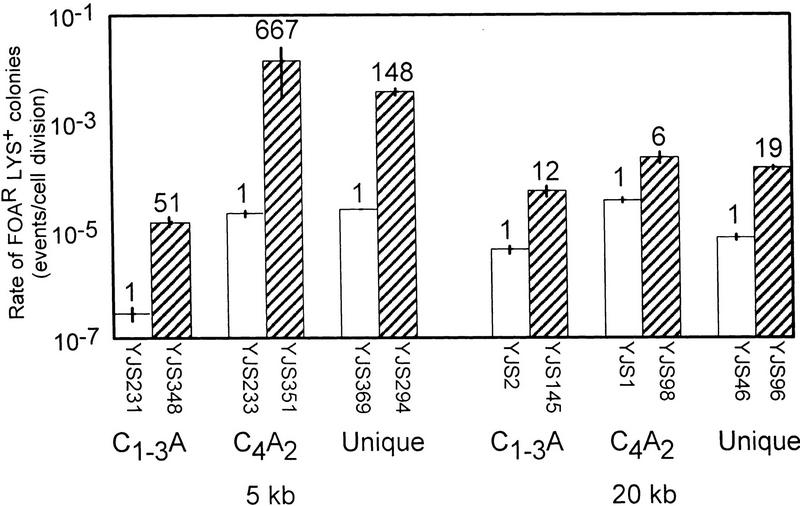
Lack of Hpr1p is associated with a large increase in direct-repeat recombination but has little or no effect on gene conversion or reciprocal exchange (Aguilera and Klein 1989). Using direct-repeat assay systems based on either 2.16- or 0.75-kb tracts of unique sequence DNA, the rate of excision events increases 950- and 270-fold, respectively, in an hpr1Δ strain (Santos-Rosa and Aguilera 1994). To determine whether Hpr1p is involved in the repression of recombination between C1–3A/TG1–3 tracts near a telomere, HPR1 was deleted in strains with the C1–3A/TG1–3 recombination substrate or the control substrates at 5 and 20 kb from the chromosome VII-L telomere, and the rate of generating FOAR Lys+ colonies was measured in each strain (Fig. 4).
Figure 4.
Rates of recombination in an hpr1Δ strain. Rates of recombination were determined for wild-type (open bars) and hpr1Δ (hatched bars) versions of the same strain with the recombination substrate located either 5 (left) or 20 (right) kb from the chromosome VII-L telomere (see Materials and Methods). Interpretation of the graph is the same as in Fig. 2A, however the scale for the y-axis is different than in preceding figures. The numbers above the columns represent the fold difference in recombination rate for the same substrate relative to the wild-type strain at the same location. The rates for direct repeat recombination in hpr1Δ strains 5 kb from the telomere are 1.5 × 10−5 (±0.2) events/cell division, 1.4 × 10−2 (±1.1) events/cell division, and 3.7 × 10−3 (±0.5) events/cell division for C1–3A/TG1–3, C4A2/T2G4, and unique sequence, respectively. The rates for direct repeat recombination in hpr1Δ strains 20 kb from the telomere are 5.3 × 10−5 (±1.1) events/cell division, 2.2 × 10−4 (±0.5) events/cell division, and 1.4 × 10−4 (±0.1) events/cell division for C1–3A/TG1–3, C4A2/T2G4, and Unique sequence, respectively.
Recombination of all three substrates at both 5 and 20 kb from the telomere was increased in an hpr1Δ background compared to the recombination rate of the same substrate at the same location in a wild-type cell (Fig. 4). However, for all three substrates, the increase was more dramatic near the telomere than at 20 kb from the telomere. This difference was especially marked for the two control substrates. For example, compared to wild-type, recombination of the unique sequence substrate in the hpr1Δ strain was increased 148-fold near the telomere but only 19-fold at 20 kb. The difference was even more dramatic for the C4A2/T2G4 direct repeats, which showed an ∼670-fold difference at 5 kb but only a sixfold difference at 20 kb. As a consequence, in the hpr1Δ strain, both control substrates recombined at a much higher rate near the telomere than at 20 kb. This result is the first unequivocal example of Hpr1p having markedly different effects on recombination rates as a function of the substrate’s position within the genome. Thus, the magnitude of Hpr1’s effect on recombination can be influenced profoundly by both the sequence and the chromosomal context of the recombining DNA.
Like the two control substrates, the recombination rate between C1–3A/TG1–3 tracts at both 5 and 20 kb from the telomere was higher in the hpr1Δ strain than in wild-type cells (51- and 12-fold elevations, respectively; Fig. 4). Because the rate of recombination between C1–3A/TG1–3 tracts was increased to a greater extent near the telomere, recombination between C1–3A/TG1–3 tracts occurred at roughly similar rates at 5 and 20 kb from the telomere in the hpr1Δ strain (threefold higher rate at 20 kb in the hpr1Δ strain vs. 15-fold in wild type, Fig. 4). However, in the hpr1Δ strain, recombination near the telomere was still much less frequent between C1–3A/TG1–3 tracts than between the control tracts, occurring at rates that were ∼250 (unique)- or ∼900 (C4A2/G4T2)-fold lower than the controls at the same site. Thus, although Hpr1p reduced recombination of all three substrates at both chromosomal sites, it was not responsible for the sequence-specific reduction in recombination between C1–3A/TG1–3 tracts near the telomere. Rather, this position effect was exacerbated in hpr1Δ cells.
Recombination between C1–3A/TG1–3 tracts near the telomere is RAD52-independent
Recombination between direct repeats resulting in the excision of intervening DNA can occur by different recombination pathways (for review, see Klein 1995). For example, a pop-out deletion event can proceed via a RAD52-dependent pathway, involving parallel alignment of the direct repeats on the same chromosome, followed by a crossover event (Petes et al. 1991). Single-strand annealing (SSA), an alternative nonconservative pathway for recombination between direct repeats which, like pop-out events, eliminates the DNA between the repeats, is RAD1 dependent (Lin et al. 1984; Fishman-Lobell et al. 1992). SSA events between naturally occurring tandem repeats such as those found at the rDNA are RAD52 independent (Zamb and Petes 1981). To begin to understand the mechanism of recombination between direct repeats near the telomere, recombination between the C1–3A/TG1–3 tracts and the control substrates was studied in rad1Δ, rad52Δ, and rad1Δ rad52Δ strains either at 5 or 20 kb from the telomere on chromosome VII-L (Table 2).
Table 2.
Effects of RAD52 and RAD1 on recombination between direct repeats
| Strain
|
Distance from telomere (kb)
|
Tract sequence
|
RAD allele
|
Rate (s.d.)a
|
radΔ/RAD
|
|---|---|---|---|---|---|
| YJS231 | 5 | C1–3A/TG1–3 | wild type | 2.9 × 10−7 (±0.80) | — |
| YJS288 | 5 | C1–3A/TG1–3 | rad52Δ | 3.0 × 10−7 (±0.68) | 1.0 |
| YJS393 | 5 | C1–3A/TG1–3 | rad1Δ | 1.8 × 10−8 (±0.75) | 0.06 |
| YJS399 | 5 | C1–3A/TG1–3 | rad52Δrad1Δ | <1.8 × 10−8* | <0.06 |
| YJS233 | 5 | C4A2/T2G4 | wild type | 2.1 × 10−5 (±0.30) | — |
| YJS290 | 5 | C4A2/T2G4 | rad52Δ | 2.6 × 10−6 (±0.66) | 0.1 |
| YJS392 | 5 | C4A2/T2G4 | rad1Δ | 1.2 × 10−5 (±0.17) | 0.6 |
| YJS369 | 5 | unique | wild type | 2.5 × 10−5 (±0.20) | — |
| YJS292 | 5 | unique | rad52Δ | <1.0 × 10−7 | <0.01 |
| YJS2 | 20 | C1–3A/TG1–3 | wild type | 4.4 × 10−6 (±0.70) | — |
| YJS142 | 20 | C1–3A/TG1–3 | rad52Δ | 1.7 × 10−6 (±0.42) | 0.4 |
| YJS146 | 20 | C1–3A/TG1–3 | rad1Δ | 9.0 × 10−7 (±2.40) | 0.2 |
| YJS402 | 20 | C1–3A/TG1–3 | rad52Δrad1Δ | <1.0 × 10−7* | <0.02 |
| YJS1 | 20 | C4A2/T2G4 | wild type | 3.6 × 10−5 (±0.30) | — |
| YJS90 | 20 | C4A2/T2G4 | rad52Δ | 1.6 × 10−5 (±0.18) | 0.4 |
| YJS390 | 20 | C4A2/T2G4 | rad1Δ | 5.8 × 10−6 (±1.10) | 0.16 |
| YJS46 | 20 | unique | wild type | 7.3 × 10−6 (±1.00) | — |
| YJS88 | 20 | unique | rad52Δ | 3.5 × 10−7 (±1.10) | 0.05 |
The rate of FOARLys+ colonies was measured and calculated as described in Materials and Methods.
Two (*) 10-colony fluctuation tests on the rad52Δrad1Δ strains at 5 kb from the telomeres resulted in 5/10 and 7/10 colonies for which 0 recombination events were recovered. Similarly for rad52rad1 strains 20 kb from the telomere fluctuation assays resulted in 7/10 and 3/10 colonies in which 0 recombination events were recovered. These fluctuation tests resulted in a median value of zero and are shown as rates less than a maximum value.
At 5 kb from the telomere, recombination of the control substrates occurred almost exclusively by a RAD52-dependent pathway (Table 2). In contrast, the few recombination events that occurred between the C1–3A/TG1–3 tracts 5 kb from the telomere were not eliminated in the rad52Δ strain but rather were RAD1 dependent. At 20 kb from the telomere, as at 5 kb, recombination between the unique sequence tracts was RAD52 dependent. However, for both the C1–3A/TG1–3 and C4A2/T2G4 substrates 20 kb from the telomere, recombination was reduced substantially but not eliminated in both the rad52Δ and rad1Δ strains. Thus, for these internally repetitious substrates at 20 kb from the telomere, both pathways contributed to the observed rate of recombination. The level of recombination between C1–3A/TG1–3 tracts at both 5 and 20 kb from the telomere was below the limits of detection in the rad1Δ rad52Δ strain, suggesting that Rad52p- and Rad1p-dependent recombination accounted for virtually all recombination events. This result also confirmed that recombination was the predominant mode for generating FOAR colonies in this system. Taken together, these data suggest that the rarity of recombination between C1–3A/TG1–3 tracts near the telomere is explained in large part by the absence of RAD52-dependent events, either because these events did not occur or because they could not be recovered near the telomere. However, RAD1-mediated recombination events were also repressed ∼10-fold near the telomere (compare recombination rate for C1–3A/TG1–3 tracts and C4A2/T2G4 tracts near the telomere in the rad52Δ strains; Table 2).
The effect of RAP1 on C1–3A direct-repeat recombination
Rap1p, the major telomere-binding protein in yeast, or proteins that bind to the telomere via interactions with Rap1p are excellent candidates for restricting the access of telomeric sequences to recombination enzymes. Since RAP1 is an essential gene, it was not possible to determine the effects of eliminating Rap1p on recombination. As an alternative approach, high levels of Rap1p were expressed by introduction of a high copy plasmid harboring the RAP1 gene, FATRAP, into the appropriate strains. As determined previously, cells carrying this plasmid have 3–5 times more Rap1p than wild-type cells, have longer and more heterogeneous length telomeres, and show elevated rates of chromosome loss and mitotic recombination at nontelomeric sites (Conrad et al. 1990). If Rap1p were limiting for recombination between C1–3A/TG1–3 tracts near the telomere, in the simplest model, this recombination might be reduced even further in cells overexpressing Rap1p.
The FATRAP plasmid was introduced into strains carrying either C1–3A/TG1–3 or C4A2/T2G4 tracts at both 5 and 20 kb from the telomere of chromosome VII-L. The presence of the FATRAP plasmid had no effect on recombination in control strains (Fig. 5A). However, its presence led to a dramatic increase in the generation of FOAR Lys+ colonies in both strains with C1–3A/TG1–3 tracts (Fig. 5A). Recombination rates were increased 146-fold at 5 kb and 841-fold at 20 kb such that recombination between the C1–3A/TG1–3 tracts was either comparable (at 5 kb) or greater (at 20 kb) than recombination between the control tracts at the same locus. A control plasmid carrying an out-of-frame deletion derivative of the RAP1 gene, FATRAPΔBBF (Conrad et al. 1990), did not affect recombination rates in any of the strains, demonstrating that the effects were caused by expression of Rap1p (data not shown).
It had been proposed previously that the increase in heterogeneity of telomere length associated with excess Rap1p might be the result of interchromosomal recombination between telomeric and/or internal tracts of telomeric DNA (Conrad et al. 1990). As inferred from structural analysis (Table 1), interchromosomal recombination was not detected in wild-type or mutant cells. To determine whether interchromosomal recombination occurred in the presence of excess Rap1p, Southern blot analysis was used to determine the structure of recombinant chromosomes in independent FOAR Lys+ colonies derived from strains containing the FATRAP plasmid (Fig. 5C,D). As described earlier, both intrachromosomal recombination and sister chromatid exchange produce a BglII fragment that will hybridize to both the A (LYS2) and the B (ADH4) probes. In contrast, interchromosomal recombination will transfer LYS2 to another chromosome such that the A and B probes hybridize to different-sized restriction fragments.
In 21% and 26% of the FOARLys+ colonies examined at 5 and 20 kb, respectively, the predicted product for an excision event was observed (Fig. 5C,D; data not shown; summarized in Fig. 5B). In the remaining recombinants, interchromosomal recombination occurred as inferred from the hybridization of the A probe to different-sized bands on the Southern blot (Fig. 5C,D). Furthermore, in each of these samples the B probe hybridized to a ∼1.5-kb band, the expected size of a terminal restriction fragment if de novo telomere formation occurred at the proximal C1–3A/TG1–3 tract on chromosome VII-L. In one sample (lane M, Fig. 5C), a mixed population of cells was detected, containing both an ADH4 hybridizing terminal fragment and a band that hybridized to both the A and B probes.
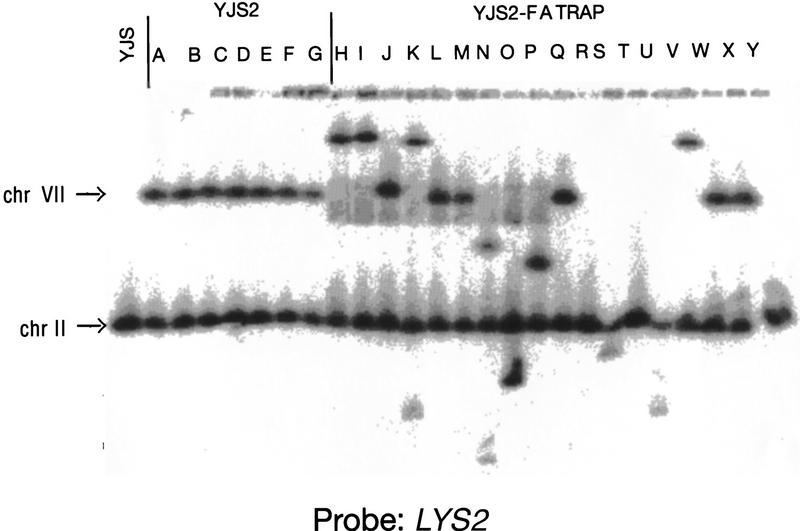
Pulsed-field gel analysis was done to confirm that the altered size of the LYS2 hybridizing fragments was caused by interchromosomal recombination. In the starting strain with the recombination substrate integrated 20 kb from the telomere on chromosome VII-L, a LYS2 probe should hybridize to both chromosome VII and to chromosome II, the normal chromosomal location of LYS2. As expected, seven of seven recombinants obtained in the wild-type strain had the structure expected for an excision event (i.e., LYS2 hybridized to both chromosomes VII and II; Fig. 6, lanes A–G). In contrast, in 13 of 18 recombinants from cells expressing high levels of Rap1p, LYS2 did not hybridize to chromosome VII (Fig. 6, lanes H–Y). In 3 of 18 recombinants, LYS2 hybridized to a single chromosome, similar in size to chromosome II (Fig. 6, lanes R,T, and Y). In two recombinants, LYS2 did not hybridize to chromosome VII but hybridized to two new chromosomes (in addition to chromosome II; Fig. 6, lanes K,N), suggesting that recombination occurred after DNA replication. In eight recombinants, LYS2 hybridized to chromosome II and to a second chromosome other than chromosome VII (Fig. 6, lanes H–J,O,P,S,U,V).
Figure 6.
Analysis of chromosomes from FOARLYS+ cells generated in the presence of excess Rap1p. Chromosomes were isolated from the parent strain (YJS), a strain containing the C1–3A/TG1–3 direct repeat recombination assay 20 kb from the telomere on chromosome VII-L (YJS2) and the same strain expressing excess Rap1p (YJS2–FATRAP) (see Materials and Methods). Chromosomes were separated using a 1.5% agarose gel using a CHEF gel apparatus. The filter was hybridized with probe A. Chromosome II, which contains the endogenous LYS2 locus (chr II), and chromosome VII, where the LYS2 gene and C1–3A/TG1–3 direct repeats were integrated (chr VII), are indicated.
In summary, Rap1p overexpression virtually eliminated the sequence-specific repression of recombination between tracts of C1–3A/TG1–3 DNA near the telomere, so far the only gene or condition found to do so. Overexpression of Rap1p also greatly increased recombination between C1–3A/TG1–3 tracts at internal sites. As a consequence, in Rap1p overexpressing cells, recombination between C1–3A/TG1–3 tracts at 20 kb was ∼25-fold higher than the control at 20 kb and ∼90-fold higher than for C1–3A/TG1–3 tracts near the telomere.
Discussion
In this paper we show that recombination between tracts of C1–3A/TG1–3 DNA was inhibited substantially when the tracts were near a telomere. This reduction was caused by both to an absence of RAD52-dependent recombination events and to a reduction in the number of RAD1-dependent events (Table 2). Inhibition was general, occurring at both 4 kb from the right telomere of chromosome V and at 5 kb from the left telomere of chromosome VII (Fig. 2). In contrast, the recombination rate for two control substrates was not affected by proximity to the telomere, occurring at essentially the same rate whether the tracts were ∼5, ∼20, or ∼200 kb from a telomere (Fig. 2). These results are consistent with an earlier study which found that the recombination rate between direct repeats of LEU2 segments was not affected by proximity to the telomere (Prado and Aguilera 1995).
Intrachromosomal recombination between telomeric DNA is a mechanism for the rapid shortening of elongated telomeres (Li and Lustig 1996). Telomere rapid deletion (TRD) events occur at a high rate in yeast cells containing abnormally long telomeres (Li and Lustig 1996). TRD events are repressed 10-fold by Hpr1p (Li and Lustig 1996), similar to the events detected in this study (Fig. 4). Unlike recombination events between C1–3A/TG1–3 direct repeats, TRD events are regulated negatively by SIR3 and are RAD1-independent (Li and Lustig 1996). TRD has been proposed as a mechanism for maintaining telomere length using nonhomologous telomeres as a yardstick to determine how much telomeric DNA to delete (Li and Lustig 1996). The relationship between TRD and C1–3A/TG1–3 direct-repeat recombination is unclear with regard to both the initiation of these events and the exact pathway of recombination.
In mitotic yeast cells, telomere-adjacent DNA is both transcriptionally repressed and late replicating (for review, see Zakian 1996). Given these data, it is not surprising that telomeres affect mitotic recombination rates. However, position effects on transcription, replication, and recombination typically act on most nearby DNA. In contrast, the reduced mitotic recombination near yeast telomeres was seen only for recombination between C1–3A/TG1–3 tracts. Moreover, this position effect did not depend on Sir proteins (Fig. 3), which are essential for transcriptional silencing at telomeres (Aparicio et al. 1991). In yeast meiosis, double-strand breaks (DSBs), which are thought to initiate recombination, are rare near telomeres (Klein et al. 1996; Baudat and Nicolas 1997). Lack of Sir4p does not increase the number of DSBs near telomeres in meiosis (Baudat and Nicolas 1997), suggesting that the DSB-resistant nature of subtelomeric DNA is also not mediated by the factors that are responsible for the closed chromatin structure associated with TPE. Any model to explain the reduction in mitotic recombination near telomeres must explain why this effect is sequence specific.
The yeast telosome is comprised of the duplex DNA-binding protein Rap1p, the single-strand TG1–3 binding protein Cdc13p, and at least five other proteins, Sir2p, Sir3p, Sir4p, Rif1p, and Rif2p, that associate with the telomere via protein-protein interactions (Bourns et al. 1998). None of the telosomal proteins is expected to bind either of the two control substrates. We propose that internal tracts of C1–3A/TG1–3 DNA, like telomeres themselves (Wright et al. 1992), can adopt a Rap1p-mediated telosome-like chromatin structure. This proposal is supported by the demonstration that Rap1p, Sir proteins, and Rif proteins bind internal tracts of C1–3A/TG1–3 DNA (Bourns et al. 1998) as well as by the ability of these tracts to act as Rap1p-dependent transcriptional silencers (Stavenhagen and Zakian 1994). We further hypothesize that the internal tracts are more likely to form this non-nucleosomal chromatin structure when they are near a telomere. This prediction is supported by the demonstration that the silencing activity of internal C1–3A/TG1–3 tracts increases as the tracts get closer to a telomere (Stavenhagen and Zakian 1994). According to this model, the telosome-like chromatin structure inhibits the generation or processing of recombination intermediates. Regional differences in chromatin structure have also been proposed to affect HO-endonuclease-induced recombination and nucleotide excision repair at the silent mating type loci (Verhage et al. 1994; Sugawara et al. 1995).
Rap1p is the main telomere-binding protein in yeast and as such is present at high concentrations at natural telomeres (see Introduction). High levels of Rap1p increased dramatically recombination between tracts of C1–3A/TG1–3 near the telomere and resulted in high levels of interchromosomal recombination. The identification of different recombination products suggests that, in the presence of excess Rap1p, internal tracts of telomeric DNA may recombine via multiple recombination pathways. In addition, high levels of Rap1p eliminated the sequence-specific repression of recombination between C1–3A/TG1–3 tracts near the telomere (Fig. 5). The effects of Rap1p on recombination between C1–3A/TG1–3 tracts were not caused by a global effect on recombination as the recombination rates of the control substrates were not affected (Fig. 5A).
Why does excess Rap1p eliminate the sequence-specific effect of the telomere on recombination? One possibility is that excess Rap1p disrupts the normal telosome structure and prevents the telomere from blocking recombination. Alternatively, the recombination inhibitor might be a Rap1p-interacting protein that is titrated from the telomere by excess Rap1p. This model is argued against by the fact that elimination of the Rap1p-interacting Sir proteins resulted in even lower rates of recombination (Fig. 3). Moreover, elimination of both Rif1p and Rif2p, two Rap1p-interacting proteins that act synergistically to restrict telomere length (Wotton and Shore 1997), led to a modest ∼threefold increase in C1–3A/TG1–3 recombination near the telomere (J.B. Stavenhagen, unpubl.).
Another possibility is that proteins that bind to the very end of the chromosome might inhibit recombination between C1–3A/TG1–3 tracts. One candidate is the yeast homolog of mammalian Ku proteins, a heterodimer in which one subunit is encoded by HDF1 (Feldmann and Winnacker 1993) and the other by YKU80/HDF2 (Boulton and Jackson 1996). Hdf2p binds telomeres in vivo (Gravel et al. 1998), whereas the absence of Ku proteins disrupts the subnuclear positioning of telomeres (Laroche et al. 1998) and increases the intratelomeric recombination (Polotnianka et al. 1988) that results in rapid shortening of long telomeres (Li and Lustig 1996). Because Sir4p associates with Hdf1p in vivo (Tsukamoto et al. 1997), eliminating Sir proteins might increase the amount of Hdf1p available for telomere binding.
Yet another possibility is that the single-strand TG1–3 binding protein Cdc13p inhibits recombination between C1–3A/TG1–3 tracts. A 3′-single-stranded tail generated by a 5′-3′-exonuclease is thought to be an intermediate in both the RAD1-dependent SSA and RAD52-dependent models of recombination (White and Haber 1990; Ozenberger and Roeder 1991; Fishman-Lobell et al. 1992). If a break occurs between two C1–3A/TG1–3 tracts at any chromosomal locus, processing of this break by a 5′-3′-exonuclease will produce a molecule with a 3′ single-stranded TG1–3-tail. It is thought that RAD52 interacts with a single-stranded DNA intermediate via the single-stranded DNA-binding protein Rfa1p (Firmenich et al. 1995). Cdc13p might compete with Rfa1p for binding, and its binding would block Rfa1p-enhanced recombination. As the binding of Cdc13p is telomere limited in vivo (Bourns et al. 1998), the probability of a processed internal C1–3A/TG1–3 tract binding Cdc13p is expected to increase and recombination to decrease with proximity to the telomere.
What is the relevance of these data to telomere behavior on normal chromosomes? It is easy to imagine that the telomeric recombination inhibitor would also prevent recombination between natural telomeres in wild-type cells. For example, if Cdc13p or a Rif protein is the inhibitor and if its presence also prevents recombination between chromosomal telomeres, it would explain why telomeres are longer in cells limited for these proteins (Hardy et al. 1992; Grandin et al. 1997; Wotton and Shore 1997). Because telomere–telomere recombination might occasionally generate telomere–telomere fusions, inhibition of this recombination in wild-type cells might reduce the generation of dicentric chromosomes. However, telomere–telomere recombination might be advantageous when telomeres are short, providing a telomerase-independent mechanism of telomere lengthening. If the recombination inhibitor has reduced affinity for short telomeres, its absence might promote RAD52-dependent recombination between C1–3A/TG1–3 tracts. This type of process could explain the presence and RAD52 dependency of terminal tracts of telomeric DNA in cells that lack telomerase (Lundblad and Blackburn 1993); Singer and Gottschling 1994; McEachern and Blackburn 1995). If this model is correct, mutations that specifically increase recombination between C1–3A/TG1–3 tracts near the telomere might identify gene products important for a recombination pathway for maintenance of telomeric DNA.
Materials and methods
Yeast manipulations
Media and plates for growth of yeast were made using standard procedures. Both liquid and solid media growth were carried out at 30°C. Transformations were done by LiAc transformation (Stavenhagen and Zakian 1994). Yeast strains were sporulated in 0.5% KoAc at room temperature. Tetrad dissection was performed using a Singer dissection apparatus. For all dissected tetrads, strains were derived from four spore tetrads that showed the expected 2:2 segregation pattern.
Construction of direct-repeat recombination strains
The plasmids pYPVN, pTPV, and pUSR were used to integrate C1–3A/TG1–3, C4A2/T2G4, or unique sequence tracts, ∼20 kb from the telomere on chromosome VII-L to construct strains YJS2, YJS1, and YJS46, respectively (Stavenhagen and Zakian 1994; Table 3). Plasmid pUSR was constructed by digesting pYPV–TPV–LYS with BglII. The resulting 5.5-kb fragment was digested subsequently with BamHI and then ligated to the other 6-kb BglII fragment to create plasmid pPV–Dis. Plasmid pPV–Dis was cut with EcoRI and blunt-ended with Klenow. A HincII–EcoRV fragment from the tetracycline resistance (Tc) gene of pBR322 was cloned into this site to create a 276-bp direct repeat with a sequence from the Tc gene already present in the plasmid. To integrate C1–3A/TG1–3 tracts on chromosome VII-L, 5 kb from the telomere (YJS231), pYPVN was digested with SmaI–SalI and gel isolated. A HindIII–PvuII fragment containing an 81-bp yeast telomeric tract from pTCA–1X was ligated to the SmaI–SalI fragment and the resulting fragment was used to transform strain YJS5 (Stavenhagen and Zakian 1994). Integration of C4A2T2G4 tracts at the same locus (YJS233) was carried out with plasmid pTPV, using the same protocol. The resultant fragment was used to transform YJS4 (Stavenhagen and Zakian 1994). For integration of USR, 5 kb from the telomere at VII-L (YJS369), a single FOARLys+ colony from YJS233 was transformed with an HpaI fragment from pUSR to generate a Ura+Lys+ cell. To integrate the direct repeat substrates ∼200 kb from the telomere on chromosome VII-L, a 2.4-kb ClaI fragment flanking LYS5 from plasmid pSC5 (Rajnarayan et al. 1992) was cut with XbaI and ligated into the XbaI site of pYPVN or cut with EcoRI and ligated into the EcoRI site of pTPVN. The resulting plasmids were linearized with ClaI and used to transform strain YPH499 (Sikorski and Hieter 1989), resulting in the strains YJS331 and YJS357, respectively. For YJS367 a single FOARLys+ colony from YJS357 was transformed with a HpaI fragment from pUSR and a Ura+Lys+ colony was selected. To integrate the C1–3A/TG1–3 direct repeats, 4 kb from the telomere on chromosome V–R (YJS335), a 2.8-kb fragment from plasmid B6–10H (Ferguson et al. 1991) was ligated into pYPVN. The resulting plasmid, pYPVN–VR–4, was digested with PvuII and ligated to the PvuII–HindII fragment from pTCA–IX. The ligation reaction was digested with NotI and transformed into YPH499. For YJS365 and YJS359, a single FOARLys+ colony from YJS335 was transformed with an HpaI fragment from pUSR and a EcoRI–HpaI fragment from pTPV, respectively. For integration of the C1–3A/TG1–3 direct repeats, ∼17 kb from the V–R telomere (YJS329), a 2.4-kb HindIII fragment from B6–10H was cut with XbaI and ligated into the XbaI site of pYPVN. The resulting plasmid, pYPVN–VR–17, was cut with HindIII and transformed into YPH499. For YJS371 and YJS361 a single FOARLys+ colony from YJS329 was transformed with a HpaI fragment from pUSR and a EcoRI–HpaI fragment from pTPV, respectively.
Table 3.
Summary of strains
| Strain
|
Genotype
|
Source
|
|---|---|---|
| YJS(YPH499) | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 | Sikorski and Heiter (1989) |
| YPH500 | MATα version of YPH499 | Sikorski and Heiter (1989) |
| YJS37 | YPH500; hpr1Δ∷HIS3 | this study |
| YJS53 | YPH500; rad52Δ∷LEU2 | this study |
| YJS110 | YPH500; rad1Δ | this study |
| YJS2 | YJS; adh4∷pYPVN (20 kb from telomere VII-L) | Stavenhagen and Zakian (1994) |
| YJS1 | YJS; adh4∷pTPVN (20 kb from telomere VII-L) | Stavenhagen and Zakian (1994) |
| YJS46 | YJS; adh4∷pUSR (20 kb from telomere VII-L) | this study |
| YJS231 | YJS; pYPVN-TEL-VIL-L (5 kb from telomere VII-L) | this study |
| YJS233 | YJS; pTPVN-TEL-VIL-L (5 kb from telomere VII-L) | this study |
| YJS369 | YJS; pUSR-TEL-VIL-L (5 kb from telomere VII-L) | this study |
| YJS331 | YJS; lys5∷pYPVN (∼200 kb from telomere VII-L) | this study |
| YJS357 | YJS; lys5∷pTPVN (∼200 kb from telomere VII-L) | this study |
| YJS367 | YJS; lys5∷pUSR (∼200 kb from telomere VII-L) | this study |
| YJS335 | YJS; pYPVN-TEL-V-R (4 kb from telomere V-R) | this study |
| YJS359 | YJS; pTPVN-TEL-V-R (4 kb from telomere V-R) | this study |
| YJS365 | YJS; pUSR-TEL-V-R (4 kb from telomere V-R) | this study |
| YJS329 | YJS; pYPVN-TEL-V-R (17 kb from telomere V-R) | this study |
| YJS361 | YJS; pTPVN-TEL-V-R (17 kb from telomere V-R) | this study |
| YJS371 | YJS; pUSR-TEL-V-R (17 kb from telomere V-R) | this study |
| YJS602 | YJS; pYPVN-TEL-VII-L sir1∷HIS3 | this study |
| YJS333 | YJS; pYPVN-TEL-VII-L sir2∷HIS3 | this study |
| YJS334 | YJS; pYPVN-TEL-VII-L sir3∷LEU2 | this study |
| YJS601 | YJS; pYPVN-TEL-VII-L sir4∷HIS3 | this study |
| YJS603 | YJS; pTPVN-TEL-VII-L sir1∷HIS3 | this study |
| YJS363 | YJS; pTPVN-TEL-VII-L sir2∷HIS3 | this study |
| YJS364 | YJS; pTPVN-TEL-VII-L sir3∷LEU2 | this study |
| YJS600 | YJS; pTPVN-TEL-VII-L sir4∷HIS3 | this study |
| YJS348 | YJS; pYPVN-TEL-VII-L hpr1∷HIS3 | this study |
| YJS351 | YJS; pTPVN-TEL-VII-L hpr1∷HIS3 | this study |
| YJS294 | MATa; YJS; pUSR-TEL-VII-L hpr1∷HIS3 | this study |
| YJS145 | YJS; adh4∷pYPVN hpr1∷HIS3 | this study |
| YJS98 | YJS; adh4∷pTPVN hpr1∷HIS3 | this study |
| YJS96 | YJS; adh4∷pUSR hpr1∷HIS3 | this study |
| YJS288 | YJS; pYPVN-TEL-VII-L rad52∷LEU2 | this study |
| YJS393 | YJS; pYPVN-TEL-VII-L rad1Δ | this study |
| YJS399 | YJS; pYPVN-TEL-VII-L rad1rad52∷LEU2 | this study |
| YJS290 | YJS; pTPVN-TEL-VII-L rad52∷LEU2 | this study |
| YJS392 | MATa; YJS; pTPVN-TEL-VII-L rad1Δ | this study |
| YJS292 | YJS; pUSR-TEL-VII-L rad52∷LEU2 | this study |
| YJS147 | MATa; YJS; adh4∷pYPVN rad52∷LEU2 | this study |
| YJS146 | YJS; adh4∷pYPVN rad1Δ | this study |
| YJS402 | MATa; YJS; adh4∷pYPVN rad1Δrad52∷LEU2 | this study |
| YJS90 | YJS; adh4∷pTPVN rad52∷LEU2 | this study |
| YJS390 | YJS; adh4∷pTPVN rad1Δ | this study |
| YJS88 | YJS; adh4∷pUSR rad52∷LEU2 | this study |
The YJS53 and YJS110 strains were made using the plasmids pSM20 (rad52:LEU2; gift of D. Schild, Lawrence Berkeley Laboratory, CA) and pLK23 (rad1) (Kadyk and Hartwell 1993), respectively. Initially, the mutations were made in strain YPH500 (Sikorski and Hieter 1989) by transformation and then mated into the appropriate direct repeat strain. For both rad52Δ and rad1Δ strains the correct transformants were first tested for UV sensitivity and then confirmed by genomic Southern analysis. After mating, diploids were sporulated. For rad1Δ, tetrads were dissected and spores showing Ura+Lys+ and UV-sensitive phenotypes were selected for further analysis. Direct-repeat strains containing the rad52Δ allele were derived from a random spore analysis by selection for Ura+ Lys+ Leu+ spores. For the rad1Δ rad52Δ double mutants, tetrads were dissected and Ura+ Lys+ Leu+ spores were examined by genomic Southern analysis to determine if the rad1Δ allele was present.
The sir2Δ, sir3Δ, and sir4Δ strains were made using plasmids pJR531 (Kimmerly and Rine 1987), AR78 (Braunstein et al. 1993), and pMM10.7 (gift of J. Broach, Princeton University) (Stavenhagen and Zakian 1994), respectively. For the sirΔ strains, transformants were verified by marker selection, loss of mating ability, and by genomic Southern analysis. Plasmid pABX4 (hpr1::HIS3 gift of H. Klein) (Aguilera and Klein 1989) was used to make YJS37 (hpr1Δ). The correct transformant was confirmed by genomic Southern analysis. YJS37 was mated to the direct repeat strains and Ura+Lys+His+ spores were selected for further analysis. Both FATRAP and FATRAPΔBB plasmids were described previously (Conrad et al. 1990).
Recombination assays
Recombination events were selected on medium containing FOA, which selects for loss of URA3 function, and lacking lysine, which selects for the presence of LYS2. The number of recombination events was indistinguishable for cells plated on medium lacking or containing lysine (data not shown). Rates were determined by fluctuation analysis (Luria and Delbruck 1943). All fluctuation assays were done using at least 10 independent colonies (20 colonies for analysis of wild-type rates for all three substrates at the five loci tested). For each strain two independent transformants were examined. Strains were grown on YC–Lys plates for 3–5 days. Individual colonies were isolated and picked as plugs into 0.5 ml of ddH2O. Aliquots from each colony were tested for the number of FOARLys+ colonies by spreading a fraction of the total colony onto plates lacking lysine and containing the drug 5-FOA (Boeke et al. 1987). The colonies that grew were replica plated subsequently to plates lacking uracil to ensure that FOAR colonies were caused by recombination, not transcriptional silencing. A second aliquot from each colony was pooled and serially diluted. A viable cell count was done by plating a fraction of the serial dilutions onto either YC or YC–Lys plates, depending on which media was used for the pregrowth prior to the fluctuation test. Rates and standard deviations were calculated using the method of the median (Lea and Coulson 1949) with the following modification. Standard deviations were determined using the average median rate, from a minimum of four assays, and adding all the colonies tested to obtain a value for total number of colonies. For each strain or condition, genomic Southern blot or PCR analysis was carried out on a subset of the FOAR cells to establish that URA3 was eliminated. In some strains, the recombination rate was low enough that some FOAR cells were caused by point mutation in URA3. In these cases, the FOAR cells caused by mutation were eliminated from the calculation for the rate of recombination.
DNA manipulations
All restriction digests were done using standard protocols and enzymes (New England Biolabs). Yeast DNA preparations and genomic Southern blots were done as described previously (Stavenhagen and Zakian 1994). Specifically, genomic DNA was digested with BglII and HindIII. The DNA was separated subsequently on a 1% agarose gel and transferred to nylon membrane (Amersham Hybond-N+). The appropriate band was visualized by hybridization with a random primed BglII–HindIII fragment from the 5′-end of the LYS2 gene. PCR reactions were done in a total volume of 10 μl using Taq polymerase (Promega) in standard reaction buffer with the following modifications: 2 mm MgCl2, 200 mm dNTPs, 0.5 mm primers (JS04: 5′-GAA GATCTGGGTTAGTCAAATGGCAGGC-3′ and JS05: 5′-CGGGATCCCTGGCAAAACTA TTGAAGAG-3′). A fraction of each yeast colony was picked using a plastic pipetman tip and placed at the bottom of the reaction tube into 5 μl containing the primers. This was covered with an ampligem wax pellet (Perkin-Elmer). On top of the hardened wax was layered the remaining reaction ingredients. The cycling protocol for PCR amplification was as follows: 1× (94°C for 2 min), 5× (94°C for 30 sec, 45°C for 1 sec, 72°C for 3 min), 35× (94°C for 30 sec, 55°C for 1 min, 72°C for 3 min). Reaction products were analyzed on a 1.2% agarose gel.
Pulse-field gel electrophoresis
DNA was prepared using an imbedded cell lysis protocol (Carle and Olson 1985). Thirty milliliters of cells was grown in YC–Lys media to late log phase (O.D. ∼10.0). Cells were pelleted and washed twice in 50 mm EDTA at pH 7.5. The final pellet was resuspended in 1 ml 50 mm EDTA at pH 7.5 and placed at 4°C. Subsequently, 0.75 ml of the resuspended cells were mixed with 0.25 ml of solution 1 (1 m sorbitol, 0.1 m Na citrate, 60 mm EDTA at pH 7.0, 1 mg/myl Zymolase, 5% β-mercaptoethanol). During gentle vortexing 1.7 ml of warm 1% low-melt agarose (125 mm EDTA at pH 7.5) was added to the mixture and then poured immediately into a 2-ml Petri dish and allowed to solidify at room temperature. Once solidified the plate was covered with 2 ml of solution 2 (0.5 m EDTA at pH 9.0, 10 mm Tris at pH 8.0, 7.5% β-mercaptoethanol) and incubated at 37°C overnight in a sealed box. The first overlay was removed and replaced with 1.5 ml of solution 3 (10 mm Tris at pH 8.0; 0.5 m EDTA at pH 9.0; 1% Sarkosyl; 1 mg/ml proteinase K) and incubated at 50°C overnight. Solution 3 was removed and the plates overlayed with 0.5 m EDTA at pH 9.0 and stored at 4°C. Plugs were cut out of the agarose using a glass cover slip and placed in the wells of a 1.5% agarose gel in 0.5× TBE. Chromosome separation was done using a Bio-Rad CHEF-DR II pulsed field gel electro phoresis (PFGE) system run for 24 hr at 200 V with a 90-sec switch time. After electrophoresis, the gel was treated for 30 min in ∼40 μg/ml RNase and stained subsequently with EtBr and photographed. The gel was treated with 0.25 m HCl for 15 sec prior to Southern blotting (see above).
Acknowledgments
We thank Catherine Freudenreich, Andressa Ivessa, and Shu-Chun Teng for critical reading of the manuscript. We thank Taryn Phippen for help with some of the experiments. In addition, we thank past and present members of the Zakian laboratory for many helpful discussions and suggestions during the course of this work. This work was supported by National Institutes of Health (NIH) grants GM26938 and GM43265 to V.A.Z. and an NIH postdoctoral fellowship to J.B.S. (GM13307).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL stavenha@neelix.udayton.edu; FAX (937) 229-2021.
References
- Aguilera A, Klein HL. Genetic and molecular analysis of recombination events in Saccharomyces cerevisiae occurring in the presence of the hyper-recombination mutation hpr1. Genetics. 1989;122:503–517. doi: 10.1093/genetics/122.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- Ashley T. Mammalian meiotic recombination: A reexamination. Hum Genet. 1994;94:587–593. doi: 10.1007/BF00206950. [DOI] [PubMed] [Google Scholar]
- Ashley T, Ward DC. A ‘hot spot’ of recombinaton coincides with an interstitial telomeric sequence in the Armenian hamster. Cytogenet Cell Genet. 1993;62:169–171. doi: 10.1159/000133464. [DOI] [PubMed] [Google Scholar]
- Baudat F, Nicolas A. Clustering of meiotic double-strand breaks on yeast chromosome III. Proc Natl Acad Sci. 1997;94:5213–5218. doi: 10.1073/pnas.94.10.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J, Tachibana CY, Tye B-K. Identification of a telomere-binding activity from yeast. Proc Natl Acad Sci. 1986;83:3713–3717. doi: 10.1073/pnas.83.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H, Donath J, Walter MF. Molecular characterization of the Anopheles gambiae 2L telomeric region via an integrated transgene. Insect Mol Biol. 1996;5:11–20. doi: 10.1111/j.1365-2583.1996.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Flouroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Boulton SJ, Jackson SP. Identification of a Saccharomyces cerevisiae Ku80 homologue: Roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 1996;24:4639–4648. doi: 10.1093/nar/24.23.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourns BD, Alexander MK, Smith AM, Zakian VA. Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol Cell Biol. 1998;18:5600–5608. doi: 10.1128/mcb.18.9.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes & Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Brown WRA, MacKinnon PJ, Villasante A, Spurr N, Buckle VJ, Dobson MJ. Structure and polymorphism of human telomere-associated DNA. Cell. 1990;63:119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Carle GF, Olson MV. An electrophoretic karyotype for yeast. Proc Natl Acad Sci. 1985;82:3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WY, Money TA, Abbo S, Devos KM, Gale MD, Moore G. A family of related sequences associated with (TTTAGGG)n repeats are located in the interstitial regions of wheat chromosomes. Mol & Gen Genet. 1994;245:349–354. doi: 10.1007/BF00290115. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Wright JH, Wolf AJ, Zakian VA. RAP1 protein interacts with yeast telomeres in vivo: Overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63:739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Dani GM, Zakian VA. Mitotic and meiotic stability of linear plasmids in yeast. Proc Natl Acad Sci. 1983;80:3406–3410. doi: 10.1073/pnas.80.11.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Telomere dynamics and genome instability in human cancer. In: Blackburn EH, Greider CW, editors. Telomeres. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. pp. 265–293. [Google Scholar]
- Dubey DD, Davis LR, Greenfeder SA, Ong LY, Zhu JG, Broach JR, Newlon CS, Huberman JA. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol Cell Biol. 1991;11:5346–5355. doi: 10.1128/mcb.11.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Winnacker EL. A putative homologue of the human autoantigen Ku from Saccharomyces cerevisiae. J Biol Chem. 1993;268:12895–12900. [PubMed] [Google Scholar]
- Ferguson BM, Fangman WL. A position effect on the time of replication origin activation in yeast. Cell. 1992;68:333–339. doi: 10.1016/0092-8674(92)90474-q. [DOI] [PubMed] [Google Scholar]
- Ferguson BM, Brewer BJ, Reynolds AE, Fangman WL. A yeast origin of replication is activated late in S phase. Cell. 1991;65:507–515. doi: 10.1016/0092-8674(91)90468-e. [DOI] [PubMed] [Google Scholar]
- Firmenich AA, Elias-Montserrat E-A, Berg P. A novel allele of Saccharomyces cerevisiae RFA1 that is deficient in recombination and repair and suppressible by RAD52. Mol Cell Biol. 1995;15:1620–1631. doi: 10.1128/mcb.15.3.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser SM. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser SM. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol. 1996;134:1349–1363. doi: 10.1083/jcb.134.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy BK, Grunstein M, Gasser SM. Localization of Sir2p: The nucleolus as a compartment for silent information regulators. EMBO J. 1997;16:3243–3255. doi: 10.1093/emboj/16.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A nw role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Grandin N, Reed SI, Charbonneau M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes & Dev. 1997;11:512–527. doi: 10.1101/gad.11.4.512. [DOI] [PubMed] [Google Scholar]
- Gravel, S., M. Larrivee, P. Labrecque, and R. Wellinger. 1998. Yeast Ku as a regulator of chromosomal DNA end-structure. Science (in press). [DOI] [PubMed]
- Greider CW. Telomerase biochemistry and regulation. In: Blackburn EH, Greider CW, editors. Telomeres. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. pp. 35–68. [Google Scholar]
- Hardy CF, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes & Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- Henderson E. Telomere DNA structure. In: Blackburn EH, Greider CW, editors. Telomeres. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. pp. 11–34. [Google Scholar]
- Horn D, Cross GA. A developmentally regulated position effect at a telomeric locus in Trypanosoma brucei. Cell. 1995;83:555–561. doi: 10.1016/0092-8674(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Kadyk LC, Hartwell LH. Replication-dependent sister chromatid recombination in rad1 mutants of Saccharomyces cerevisiae. Genetics. 1993;133:469–487. doi: 10.1093/genetics/133.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly WJ, Rine J. Replication and segregation of plasmids containing cis-acting regulatory sites of silent mating-type genes in Saccharomyces cerevisiae are controlled by the SIR genes. Mol Cell Biol. 1987;7:4225–4237. doi: 10.1128/mcb.7.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D, Wilson HE, Thomson EJ, Lee M, Perry J, Palmer S, Ashworth A, Cooke HJ. Structural variation of the pseidoautosomal region between and within inbred mouse strains. Proc Natl Acad Sci. 1996;93:171–175. doi: 10.1073/pnas.93.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HL. Genetic control of intrachromosomal recombination. BioEssays. 1995;17:147–159. doi: 10.1002/bies.950170210. [DOI] [PubMed] [Google Scholar]
- Klein F, Laroche T, Cardenas ME, Hofmann JF, Schweizer D, Gasser SM. Localization of RAP1 and topoisomerase II in nucleic and meiotic chromosomes of yeast. J Cell Biol. 1992;117:935–948. doi: 10.1083/jcb.117.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Zenvirth D, Dror V, Barton AB, Kaback DB, Simchen G. Patterns of meiotic double-strand breakage on native and artificial yeast chromosomes. Chromosoma. 1996;105:276–284. doi: 10.1007/BF02524645. [DOI] [PubMed] [Google Scholar]
- Kyrion G, Boakye KA, Lustig AJ. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G, Liu K, Liu C, Lustig AJ. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes & Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- Laroche T, Martin SG, Gorham HC, Pryde FE, Louis EJ, Gasser SM. Mutation of Ku protein homologues disrupts the subnuclear organization of yeast telomeres. Curr Biol. 1998;8:653–656. doi: 10.1016/s0960-9822(98)70252-0. [DOI] [PubMed] [Google Scholar]
- Lea DE, Coulson CA. The distribution of the numbers of mutants in bacterial populations. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R, Hazelrigg T, Rubin GM. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985;229:558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Li B, Lustig AJ. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes & Dev. 1996;10:1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- Lin F-L, Perle K, Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: Role for DNA ends in the recombination process. Mol Cell Biol. 1984;4:1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Zakian VA. The Saccharomyces CDC13 protein is a single-strand TG1–3 telomeric DNA binding protein in vitro that affects telomere behavior in vivo. Proc Nat Acad Sci. 1996;93:13760–13765. doi: 10.1073/pnas.93.24.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CC, Nielsen L, Edstrom JE. Terminal long tandem repeats in chromosomes from Chironomous pallidivittatus. Mol Cell Biol. 1996;16:3285–3290. doi: 10.1128/mcb.16.7.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis EJ, Naumova ES, Lee A, Naumov G, Haber JE. The chromosome end in yeast: Its mosaic nature and influence on recombinational dynamics. Genetics. 1994;136:789–802. doi: 10.1093/genetics/136.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est-senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Luria SE, Delbruck M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- McEachern MJ, Blackburn EH. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature. 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OG, Wiley JE, Wurster-Hills DH, Yates TL, Moyzis RK. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 1990;99:3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- Miklos GLG, Nankivell RN. Telomeric satellite DNA functions in regulating recombination. Chromosoma. 1976;56:143–167. doi: 10.1007/BF00293113. [DOI] [PubMed] [Google Scholar]
- Moazed D, Kistler A, Axelrod A, Rine J, Johnson A. Silent information regulator protein complexes in Saccharomyces cerevisiae: A SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci. 1997;94:2186–2191. doi: 10.1073/pnas.94.6.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes & Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- Murnane JP, Sabatier L, Marder BA, Morgan WF. Telomere dynamics in an immortal human cell line. EMBO J. 1994;13:4953–4962. doi: 10.1002/j.1460-2075.1994.tb06822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo ER, Cranston G, Allshire RC. Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J. 1994;13:3801–3811. doi: 10.1002/j.1460-2075.1994.tb06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent CI, Hughes TR, Lue NF, Lundblad V. Cdc13p: A single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- Ozenberger BA, Roeder GS. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol Cell Biol. 1991;11:1222–1231. doi: 10.1128/mcb.11.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser SM. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75:543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- Park VM, Gustashaw KM, Wathen TM. The presence of interstitial telomeric sequences in the constitutional chromosome abnormalities. Am J Human Genet. 1992;50:914–923. [PMC free article] [PubMed] [Google Scholar]
- Petes TD, Malone RE, Symington LS. Recombination in yeast. In: Broach JR, Pringle JR, Jones EW, editors. The molecular and cellular biology of the yeast Saccharomyces: Genome dynamics, protein synthesis and energetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1991. pp. 407–521. [Google Scholar]
- Pluta AF, Zakian VA. Recombination occurs during telomere formation in yeast. Nature. 1989;337:429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Polotnianka RM, Li J, Lustig AJ. The yeast ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998;8:831–834. doi: 10.1016/s0960-9822(98)70325-2. [DOI] [PubMed] [Google Scholar]
- Prado F, Aguilera A. Role of reciprocal exchange, one-ended invasion crossover and single-strand annealing on inverted and direct repeat recombination in yeast: Different requirements for the RAD1, RAD10, and RAD52 genes. Genetics. 1995;139:109–123. doi: 10.1093/genetics/139.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnarayan S, Vaughn JC, Bhattacharjee JK. Physical and biochemical characterization of the cloned LYS5 gene required for alpha-aminoadipate reductase activity in the lysine biosynthetic pathway of Saccharomyces cerevisiae. Curr Genet. 1992;21:13–16. doi: 10.1007/BF00318647. [DOI] [PubMed] [Google Scholar]
- Renauld H, Aparicio OM, Zierath PD, Billington BL, Chhablani SJ, Gottschling DE. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and SIR3 dosage. Genes & Dev. 1993;7:1133–1145. doi: 10.1101/gad.7.7a.1133. [DOI] [PubMed] [Google Scholar]
- Reynolds AE, McCarroll RM, Newlon CS, Fangman WL. Time of replication of ARS elements along yeast chromosome III. Mol Cell Biol. 1989;9:4488–4494. doi: 10.1128/mcb.9.10.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan EM, Bryan TM, Hukku B, Maclean K, Chang AC-M, Moy EL, Englezou A, Warneford SG, Dalla-Pozza L, Reddel RR. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of li-fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Blundell PA, Dirks-Mulder A, Kieft R, Borst P. A ribosomal DNA promoter replacing the promoter of a telomeric VSG gene expression site can be efficiently switched on and off in T. brucei. Cell. 1995;83:547–553. doi: 10.1016/0092-8674(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Zakian VA. Loss of a yeast telomere: Arrest, recovery and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Aguilera A. Increase in incidence of chromosome instability and non-conservative recombination between repeats in Saccharomyces cerevisiae hpr1 strains. Mol Gen Genet. 1994;245:224–236. doi: 10.1007/BF00283271. [DOI] [PubMed] [Google Scholar]
- Shore D, Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987;51:721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer MS, Gottschling DE. TLC1, the template RNA component of the Saccharomyces cerevisiae telomerase. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- Stavenhagen JB, Zakian VA. Internal tracts of telomeric DNA act as silencers in Saccharomyces cerevisiae. Genes & Dev. 1994;8:1411–1422. doi: 10.1101/gad.8.12.1411. [DOI] [PubMed] [Google Scholar]
- Stringer JR. Recombintion between poly[d(GT)-d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985;5:1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Ivanov EL, Fishman-Lobell J, Ray BL, Wu X, Haber JE. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature. 1995;373:84–86. doi: 10.1038/373084a0. [DOI] [PubMed] [Google Scholar]
- Szostak JW. Replication and resolution of telomeres in yeast. Cold Spring Harbor Symp Quant Biol. 1983;47:1187–1194. doi: 10.1101/sqb.1983.047.01.134. [DOI] [PubMed] [Google Scholar]
- Tracey RB, Baumohl JK, Kowalczykowski SC. The preference for GT-rich DNA by the yeast RAD51 protein defines a set of universal pairing sequences. Genes & Dev. 1997;11:3423–3431. doi: 10.1101/gad.11.24.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey RB, Chedin F, Kowalczykowski SC. The recombination hot spot chi is embedded within islands of preferred DNA pairing sequences by the Escherichia coli RecA protein. Cell. 1996;90:205–216. doi: 10.1016/s0092-8674(00)80328-1. [DOI] [PubMed] [Google Scholar]
- Treco D, Arnheim N. The evolutionary conserved repetitive sequence d(GT-AC)n promotes reciprocal exchange and generates unusual recombination tetrads during yeast meiosis. Mol Cell Biol. 1986;6:3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Kato J-I, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–903. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- Verhage R, Zeeman A-M, de Groot N, Gleig F, Bang DD, van de Putte P, Brouwer J. The RAD7 and RAD16 genes are essential for pyrimidine dimer removal from silent mating type loci, are also required for the repair of the nontranscribed strand of an active gene in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:6135–6142. doi: 10.1128/mcb.14.9.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley RM, Chan CSM, Tye B-K, Petes TD. Unusual DNA sequences associated with the ends of yeast chromosomes. Nature. 1984;310:157–160. doi: 10.1038/310157a0. [DOI] [PubMed] [Google Scholar]
- Wang S-S, Zakian VA. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990;345:456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]
- Wellinger RJ, Wolf AJ, Zakian VA. Origin activation and formation of single-strand TG1–3 tails occur sequentially in late S phase on a yeast linear plasmid. Mol Cell Biol. 1993;13:4057–4065. doi: 10.1128/mcb.13.7.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:633–670. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MA, Wierdl M, Detloff P, Petes TD. DNA-binding protein RAP1 stimulates meiotic recombination at the HIS4 locus in yeast. Proc Natl Acad Sci. 1991;88:9755–9759. doi: 10.1073/pnas.88.21.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes & Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- Wright JH, Gottschling DE, Zakian VA. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes & Dev. 1992;6:197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- Wright JH, Zakian VA. Protein–DNA interactions in soluble telosomes from Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:1454–1460. doi: 10.1093/nar/23.9.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian VA. Structure, function and replication of Saccharomyces cerevisiae telomeres. In: Campbell A, Anderson W, Jones EW, editors. Annual Review of Genetics. Palo Alto, CA: Annual Reviews; 1996. pp. 141–172. [DOI] [PubMed] [Google Scholar]
- Zamb TJ, Petes TD. Unequal sister-strand recombination within yeast ribosomal DNA does not require the RAD52 gene product. Curr Genet. 1981;3:125–132. doi: 10.1007/BF00365716. [DOI] [PubMed] [Google Scholar]
- Zhu J, Newlon CS, Huberman JA. Localization of a DNA replication origin and termination zone on chromosome III of Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4733–4741. doi: 10.1128/mcb.12.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]