Abstract
Objectives
We investigated whether Acinetobacter baumannii isolates of veterinary origin shared common molecular characteristics with those described in humans.
Methods
Nineteen A. baumannii isolates collected in pets and horses were analysed. Clonality was studied using repetitive extragenic palindromic PCR (rep-PCR) and multilocus sequence typing (MLST). PCR and DNA sequencing for various β-lactamase, aminoglycoside-modifying enzyme, gyrA and parC, ISAba1 and IS1133, adeR and adeS of the AdeABC efflux pump, carO porin and class 1/2/3 integron genes were performed.
Results
Two main clones [A (n = 8) and B (n = 9)] were observed by rep-PCR. MLST indicated that clone A contained isolates of sequence type (ST) ST12 (international clone II) and clone B contained isolates of ST15 (international clone I). Two isolates of ST10 and ST20 were also noted. Seventeen isolates were resistant to gentamicin, 12 to ciprofloxacin and 3 to carbapenems. Isolates of ST12 carried blaOXA-66, blaADC-25, blaTEM-1, aacC2 and IS1133. Strains of ST15 possessed blaOXA-69, blaADC-11, blaTEM-1 and a class 1 integron carrying aacC1 and aadA1. ISAba1 was found upstream of blaADC (one ST10 and one ST12) and/or blaOXA-66 (seven ST12). Twelve isolates of different STs contained the substitutions Ser83Leu in GyrA and Ser80Leu or Glu84Lys in ParC. Significant disruptions of CarO porin and overexpressed efflux pumps were not observed. The majority of infections were hospital acquired and in animals with predisposing conditions for infection.
Conclusions
STs and the molecular background of resistance observed in our collection have been frequently described in A. baumannii detected in human patients. Animals should be considered as a potential reservoir of multidrug-resistant A. baumannii.
Keywords: MLST, MDR, PCR/ESI-MS, rep-PCR, T5000
Introduction
Acinetobacter baumannii is an opportunistic pathogen that is difficult to treat due to its ability to express multiple intrinsic and acquired mechanisms that make the organism frequently multidrug-resistant (MDR) to antibiotics.1 Production of acquired class A extended-spectrum β-lactamases (ESBLs), class B and/or class D carbapenemases (e.g. IMP and VIM types and OXA-23, -24/40 and -58), overexpression of chromosomal enzymes (i.e. ADCs and OXA-Ab) and efflux pumps (e.g. AdeABC), loss of outer membrane proteins (OMPs; e.g. CarO), altered penicillin binding proteins, production of aminoglycoside modifying enzymes (AMEs) or 16S RNA methylases and mutations in the quinolone resistance-determining region (QRDR) of the gyrA and parC genes are common mechanisms of resistance found in A. baumannii clinical isolates. The organism can also easily survive in hospital environments.2–4
A. baumannii is commonly responsible for nosocomial infections in immunocompromised and severely ill patients who undergo extended medical and invasive procedures. High morbidity and mortality rates have been reported during outbreaks observed in hospitals and long-term care facilities in different countries.1,5–7 On the basis of various typing analyses, three main clusters have been associated with the A. baumannii pandemic (i.e. European clone types I, II and III).3,8–10 More recently, a large multilocus sequence typing (MLST) analysis has shown that the majority of clinical A. baumannii isolates found in US military medical facilities and European hospitals were included in three lineages; the first contained the most predominant sequence type (ST) ST11 and its single-locus variants ST1/10/12/47, the second consisted of ST15/16/45/46 and the third most predominant ST was ST8/14. These three clusters correspond to the European clones II, I and III, respectively.11 These lineages are now more appropriately called ‘international clones’, as they are spreading not only in Europe, but also in other parts of the world.12
Infections due to A. baumannii have been rarely reported in animals and only a few studies have described this occurrence.3 In 2000, Vaneechoutte et al.13 identified seven A. baumannii isolates from jugular catheter tips placed in horses, but the organism was only indicated as responsible for local infection or colonization. In the same period at the University of Bern, Francey et al.14 described the clinical characteristics of several pets with various A. baumannii infections (i.e. urinary, respiratory, wound and bloodstream infections), reporting an overall attributable mortality of 47% [100% in the intensive care unit (ICU)]. At the same time it was noted that: (i) A. baumannii isolates collected in 1998–2000 from pets and horses were included in two main PFGE clones;15 (ii) these organisms may be responsible for rapidly progressing fatal systemic infections such as sepsis and fasciitis;16 and (iii) the majority of A. baumannii infections were hospital acquired.14,15
Since a detailed molecular analysis of isolates was not performed in the above investigations, data regarding A. baumannii of veterinary origin are still lacking.3 Herein, we report the first analysis of the antibiotic susceptibility profile, genetic determinants of resistance and clonality of a collection of A. baumannii isolates responsible for infections in pets and horses.
Materials and methods
Clinical isolates and susceptibility testing
A. baumannii isolates were collected from clinical samples of animals received by the Center for Zoonoses, Bacterial Animal Diseases and Antibiotic Resistance (ZOBA) of Bern (Switzerland) from May 2004 through August 2009. Species identification and antimicrobial susceptibility tests were routinely assessed using the Vitek 2 system (bioMérieux Inc.). Identification was confirmed with matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonik) and detection of the chromosomal blaOXA-Ab genes (formerly blaOXA-51-like; see below),4 whereas the MICs were determined by microdilution in Mueller–Hinton broth (BBL, Becton Dickinson) using Sensititre ESB1F plates (Trek Diagnostic Systems, Inc.). Susceptibility results were interpreted according to the current European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria.17 MICs were also determined in the presence of the efflux pump inhibitor phenyl-arginine-β-naphthylamide (PAβN; Sigma) at a final concentration of 40 mg/L.
Analysis of clonality
The genetic relatedness among the A. baumannii isolates was determined using the repetitive extragenic palindromic PCR (rep-PCR). Genomic DNA was obtained with a peqGOLD Bacterial DNA Kit (Peqlab). PCR amplification reactions were performed using the REP1 and REP2 degenerate primers and Expand Long Template Taq DNA Polymerase Mix (Roche) as described by Vila et al.18 Amplification conditions were as follows: 10 min at 94°C; 32 cycles of denaturation (1 min at 94°C), annealing (1 min at 43°C) and extension (6 min at 72°C); and a final extension of 12 min at 72°C. Rep-PCR products were separated by electrophoresis using the Agilent 2100 Bioanalyzer (Agilent Technologies), yielding profile bands for each strain. Band patterns were aligned using 2100 Expert software (Agilent Technologies) and interpreted using BioNumerics software version 5.1 (Applied Maths).
The T5000™ Biosensor System (Ibis Biosciences Inc., a subsidiary of Abbott), a PCR electrospray ionization mass spectrometry (PCR/ESI-MS) platform, was used to perform a form of MLST analysis of all A. baumannii isolates. As previously reported, the Acinetobacter genotyping kit uses eight primer pairs to target six housekeeping genes (i.e. efp, trpE, adk, mutY, fumC and ppa).11
Molecular methods
PCR and DNA sequence analyses for β-lactamase genes (i.e. blaADC, blaOXA-Ab and other acquired blaOXAs, blaTEM, blaSHV, blaCTX-M, blaPER, blaGES, blaVEB, blaIMP and blaVIM), AME genes (i.e. aphA6, aadA1, aadB, aacC1 and aacC2), ISAba1 and IS1133 insertion elements, regulatory genes of the AdeABC efflux pump (i.e. adeR and adeS), the carO porin gene, class 1/2/3 integrases and class 1 integron resistance gene(s) cassette were performed using primers and conditions previously published.19–23 Detection of the ISAba1 element upstream of the blaADC and blaOXA-Ab genes was carried out as described previously.24,25 Genetic analysis of the QRDR of gyrA and parC genes was also done.26 DNA traces were analysed using Sequencher 4.10.1 (Gene Codes Corporation) and translated into a protein sequence when necessary using the ExPASy Proteomic Server (www.expasy.ch/). The resulting protein sequences were compared with amino acid translated DNA reference sequences CP000863 for CarO, X82165 for GyrA, X95819 for ParC and AY426969 for AdeR and AdeS of A. baumannii.
Analytical isoelectric focusing (aIEF)
Detection of β-lactamases was performed by using aIEF as previously reported.27,28 One well-characterized Klebsiella pneumoniae isolate (i.e. VA367) producing TEM-1, KPC-2, SHV-11 and SHV-12 β-lactamases and one Escherichia coli strain (i.e. 9217/10) expressing CMY-2 β-lactamase were used as controls.28
Clinical data
Clinical records of animals developing infection due to A. baumannii and admitted to the Equine Clinic and the Small Animal Hospital (University of Bern, Switzerland) were examined retrospectively. The two clinics are situated approximately 200 m apart. The following data were recorded: age; underlying diseases; predisposing conditions of infection (e.g. use of corticosteroids, intravascular catheters and antibiotic use) when present for at least 1 week before the A. baumannii isolation; and antimicrobial agents administered during the infectious episode. History of hospitalizations and prior use of antibiotics were also taken into account. An infection that occurred at least 48 h after the admission of the animal to the veterinary hospital was defined as hospital acquired, otherwise it was defined as community acquired.
Results and discussion
A. baumannii isolates and analysis of genetic relatedness
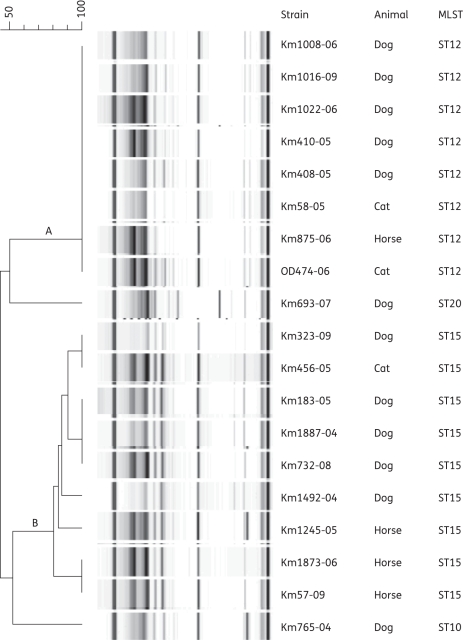
Nineteen A. baumannii isolates collected from dogs (n = 12), horses (n = 4) and cats (n = 3) were analysed. As shown in Figure 1, the majority of A. baumannii were grouped into two clones [i.e. clone A (n = 8) and clone B (n = 9)] that share <60% band pattern homology, whereas two isolates were not related to the above clones. Further MLST analysis by PCR/ESI-MS indicated that clone A contained isolates of ST12 (international clone II) and clone B included isolates of ST15 (international clone I); the remaining two isolates were ST10 (a single-locus variant of ST12) and ST20.11
Figure 1.
Results of rep-PCR and MLST analyses for the 19 A. baumannii isolates responsible for infection in animals (12 dogs, 4 horses and 3 cats). rep-PCR identified two main clones (A and B) that shared <60% band homology. Isolates included in clone A showed 100% band homology, whereas those included in clone B demonstrated slightly different band patterns. By using MLST, isolates included in clone A were ST12 (international clone II), whereas those in clone B were ST15 (international clone I). Two A. baumannii isolates were ST10 (a variant related to ST12) and ST20.
Since 2002 A. baumannii isolates of ST10, ST12 and ST15, along with ST11, ST14 and ST24, have been frequently reported as being responsible for bloodstream infection and skin colonization in military and civilian patients admitted to US medical facilities.11,19,29 In a recent analysis, Perez et al.5 described the spread and the clinical impact of carbapenem-resistant A. baumannii isolates of ST10 and ST12 across a six-hospital healthcare system in northeast Ohio. It should also be noted that A. baumannii isolates belonging to international clones I and II are found all over the world, including the countries surrounding Switzerland.8,30–32
Overall, our data indicate that two lineages of A. baumannii isolates that are spreading worldwide among patients are also found among hospitalized animals in Switzerland (see clinical information in Table S1, available as Supplementary data at JAC Online). We speculate that these two clonal lineages might be identical to those observed in pets hospitalized at our institution in 1998–2000 and that subsequently disappeared after thorough cleaning and disinfection of the ICU.15 Unfortunately those A. baumannii isolates were not available for comparison using rep-PCR and MLST. Furthermore, we were not able to provide information regarding the clinical and/or travel history of the animal owners or the geographic origin of the pets and horses.
Phenotypic and molecular characterization of drug resistance genes
Table 1 summarizes the antibiotic phenotypes and the genetic basis of resistance of the 19 A. baumannii isolates. Overall, 17 isolates were resistant to gentamicin, 12 were resistant to ciprofloxacin and 3 were non-susceptible to carbapenems.17
Table 1.
Molecular characterization of drug resistance genes and antibiotic susceptibility profile of the 19 A. baumannii isolates responsible for infection in pets and horses
| Molecular analysis of resistance genesa |
MIC (mg/L)b |
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain, STs | IC | aIEF (pI) | blaOXA-Ab | blaADC | blaTEM | ISAba1 | IS1133 | IntI | aacC1 | aadA1 | aacC2 | aadB | GyrA | ParC | CarO | CTX | CAZ | FEP | TZP | IPM | MEM | AMK | GEN | TOB | CIP | SXT | TET | PolB |
| Km1008-06, ST12 | II | 9.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 4 | 4 | 4 | ≤4 | ≤0.5 | ≤1 | 4 | ≥32 | 4 | ≥4 | ≥320 | ≥16 | 1 |
| Km1016-09, ST12 | II | 9.5, 9.0, 6.5 | OXA-66 | ADC-25 | — | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 4 | 2 | 4 | 32 | 8 | 8 | ≤2 | ≥32 | ≤1 | ≥4 | ≥320 | ≥16 | 1 |
| Km1022-06, ST12 | II | 9.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 4 | 2 | 4 | ≤4 | ≤0.5 | ≤1 | 4 | ≥32 | 4 | ≥4 | ≥320 | ≥16 | 1 |
| Km408-05, ST12 | II | 9.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 8 | 4 | 8 | 16 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≤1 | ≥4 | 40 | ≥16 | 1 |
| Km58-05, ST12 | II | 9.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 4 | 4 | 8 | 8 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≤1 | ≥4 | 40 | ≥16 | 1 |
| OD474-06, ST12 | II | 9.5, 6.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 64 | 64 | 16 | 32 | 1 | ≤1 | ≤2 | ≥32 | ≤1 | ≥4 | 40 | ≥16 | 0.5 |
| Km410-05, ST12 | II | 9.5, 6.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | +c,d | + | − | − | − | + | − | Ser83Leu | Ser80Leu | = | ≥128 | 64 | 16 | ≥128 | 4 | 4 | ≤2 | ≥32 | ≤1 | ≥4 | 160 | ≥16 | 1 |
| Km875-06, ST12 | II | 9.5, 5.4 | OXA-66 | ADC-25 | TEM-1 | + | − | − | − | − | + | − | Ser83Leu | Ser80Leu | = | 8 | 4 | 8 | 8 | ≤0.5 | ≤1 | 4 | ≥32 | 4 | ≥4 | ≥320 | ≥16 | 1 |
| Km765-04, ST10 | II | 9.5, 6.5 | OXA-66 | ADC-25 | TEM-1 | +d | − | − | − | − | − | − | Ser83Leu | Ser80Leu | = | ≥128 | 64 | 16 | ≥128 | 2 | ≤1 | 4 | ≤4 | ≤1 | ≥4 | ≥320 | ≥16 | 1 |
| Km693-07, ST20 | NI | 9.5 | OXA-95 | ADC-26 | TEM-1 | − | − | − | − | − | − | − | = | = | = | 8 | 4 | 2 | ≤4 | ≤0.5 | ≤1 | ≤2 | ≤4 | ≤1 | ≤1 | ≤ 20 | ≤1 | ≤0.25 |
| Km323-09, ST15 | I | 9.5, 9.0, 6.5, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | + | +e | + | + | − | − | Ser83Leu | Ser80Leu | Ile74Leu, Glu217Lys | 8 | 4 | 8 | 32 | 8 | 4 | ≤2 | ≥32 | ≤1 | ≥4 | ≤ 20 | ≤1 | 0.5 |
| Km732-08, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | − | Ser83Leu | Ser80Leu | Glu217Lys | 4 | 2 | 4 | ≤4 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≤1 | ≥4 | ≥320 | ≥16 | 1 |
| Km1492-04, ST15 | I | 9.0 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | − | Ser83Leu | Glu84Lys | Ser214Thr | 16 | 8 | 16 | 32 | ≤0.5 | ≤1 | 8 | ≥32 | 2 | ≥4 | ≥320 | ≥16 | 1 |
| Km1245-05, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | − | = | = | Glu217Lys | 8 | 4 | 8 | ≤4 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≤1 | ≤1 | 80 | 2 | 1 |
| Km183-05, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | − | = | = | Glu217Lys | 8 | 4 | 4 | 8 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≤1 | ≤1 | ≥320 | ≥16 | 1 |
| Km1887-04, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | − | = | = | Glu217Lys | 8 | 4 | 4 | ≤4 | ≤0.5 | ≤1 | 4 | ≥32 | ≤1 | ≤1 | ≥320 | ≥16 | 1 |
| Km456-05, ST15 | I | 9.0 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | + | = | = | Glu217Lys | 2 | 1 | 4 | ≤4 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≥16 | ≤1 | 160 | ≥16 | 1 |
| Km1873-06, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | + | − | +e | + | + | − | + | = | = | Glu217Lys | 4 | 2 | 8 | ≤4 | ≤0.5 | ≤1 | ≤2 | ≥32 | ≥16 | ≤1 | ≥320 | ≥16 | 1 |
| Km57-09, ST15 | I | 9.0, 5.4 | OXA-69 | ADC-11 | TEM-1 | − | − | +e | + | + | − | + | = | = | Glu217Lys | 4 | 4 | 4 | ≤4 | ≤0.5 | ≤1 | 8 | ≥32 | ≥16 | ≤1 | ≥320 | ≥16 | 1 |
IC, international clone; NI, not included among the three international clones; +, positive; −, negative; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; TZP, piperacillin/tazobactam; IPM, imipenem; MEM, meropenem; AMK, amikacin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline; PolB, polymyxin B.
aCarO, GyrA and ParC proteins are reported with their amino acid substitutions, otherwise they are reported as equal (=) to the reference sequences (i.e. CP000863, X82165 and X95819, respectively). The remaining resistance genes tested (see the Molecular methods section) are not reported because they were not detected in any of the A. baumannii isolates.
bInterpretation of MICs according to 2011 EUCAST criteria (S, susceptible):17 TZP (criteria not available); CTX, CAZ and FEP (susceptibility testing not recommended); IPM and MEM (S, ≤2 mg/L); AMK (S, ≤8 mg/L); GEN and TOB (S, ≤4 mg/L); CIP (S, ≤1 mg/L); SXT (S, ≤2/38 mg/L); TET (susceptibility testing not recommended); and PolB (S, ≤2 mg/L). Data regarding AMK, TOB, SXT, TET and PolB were obtained using the Vitek 2 system.
cISAba1 was upstream of blaOXA-Ab.
dISAba1 was upstream of blaADC.
eThe class I integron carried a 2.6 kb cassette with the aacC1-orfX-orfX′-aadA1 gene structure.
All A. baumannii isolates were susceptible to amikacin, indicating that they did not produce 16S rRNA methylases.33 The two isolates of ST10 and ST20 were susceptible to aminoglycosides due to the lack of AME genes. In contrast, isolates of ST12 possessed aacC2 (usually associated with IS1133), whereas those of ST15 carried a class 1 integron with a resistance gene cassette of 2.6 kb containing aacC1 and aadA1. Furthermore, three isolates of ST15 also resistant to tobramycin harboured aadB (Table 1). These genetic signatures of AMEs are fully consistent with the aminoglycoside resistance patterns observed in our isolates.34 We also noted that these specific AME genes were previously reported in MDR A. baumannii isolates of human origin.2,19,23 In particular, the 2.6 kb class 1 integron resistance cassette was reported in several analyses performed in different countries,23,35–38 whereas the aacC2 gene associated with IS1133 may be part of a residual class 1 integron with a truncated integrase gene as previously described in one A. baumannii detected in South Africa.39
Twelve A. baumannii isolates of different STs were resistant to ciprofloxacin due to amino acid substitutions in the GyrA and ParC proteins. In particular, 11 of the 12 isolates contained both Ser83Leu in GyrA and Ser80Leu in ParC substitutions, whereas strain Km1492-04 possessed the Ser83Leu in GyrA along with Glu84Lys in ParC (Table 1). While substitutions at Ser83 of GyrA and Ser80 of ParC are frequently detected among quinolone-resistant A. baumannii, substitutions at Glu84 of ParC are more rarely reported.26,40–42 We hypothesize that the high prevalence (i.e. 63.2%) of quinolone resistance in different lineages of A. baumannii could be due to the frequent use of enrofloxacin and marbofloxacin in veterinary medicine (as also observed in our clinical cases; see Table S1).43
The carO gene was found intact in all A. baumannii isolates (i.e. insertion elements were not observed). When compared with the reference sequence CP000863, the CarO porin of isolates belonging to ST10, ST12 and ST20 did not show amino acid substitutions, whereas isolates belonging to ST15 showed one or two amino acid variations (Table 1). These substitutions are most likely the result of normal allelic variations between clone types and probably have little effect on the function of the OMP. With regard to the AdeABC efflux pump, all 19 A. baumannii possessed the adeR gene, whereas the adeS was not amplified from isolates of international clone II (n = 9). The lack of adeS was previously linked to increased susceptibility to aminoglycosides due to its essential action for the expression of the adeABC operon.22 Analysis of 19 adeR and 10 adeS translated amino acid sequences did not reveal substitutions previously associated with increased resistance (i.e. Pro116Leu for AdeR and Thr153Met for AdeS).22 In addition, the MIC values of tested antibiotics did not show a significant decrease when the efflux inhibitor PAβN was added (data not shown). Overall, the above results indicate that all A. baumannii isolates most likely have functional CarO porins and did not possess overexpressed efflux systems such as the AdeABC pump.
Further molecular and biochemical analyses showed that the A. baumannii isolates produced several β-lactamases. In particular, isolates of ST12 carried blaOXA-66, blaADC-25 and blaTEM-1 (only Km1016-09 was blaTEM-negative), whereas those of ST15 possessed blaOXA-69, blaADC-11 and blaTEM-1. The expression of the bla genes found in our collection was in part supported by the aIEF results (i.e. TEM-1, pI of 5.4; OXA-Ab, pI of ∼6.5; and ADCs, pI of ∼9.0). Other bla genes encoding for acquired ESBLs (e.g. PER, VEB and GES types) or carbapenemases (e.g. OXA-23, -24/40 and -58) were not detected. It should also be noted that in using the ESB1F microdilution panel, synergy between clavulanate and cefotaxime or ceftazidime was not observed, indicating the plausible absence of other ESBLs (data not shown).
Two A. baumannii isolates (i.e. Km410-05 and Km765-04) possessed the ISAba1 element upstream of blaADC (Table 1). As previously reported,24 ISAba1 augments the expression level of ADCs, and this is consistent with the higher MIC values for extended-spectrum cephalosporins and piperacillin/tazobactam observed in these two isolates. ISAba1 is also usually able to increase the expression level of the chromosomal OXA-Ab carbapenemase when upstream of this bla gene.25 However, only two (i.e. Km410-05 and Km1016-09) of the seven A. baumannii possessing this IS element had MICs for imipenem and/or meropenem (i.e. MICs of 4–8 mg/L) in the resistant range. This is partially in contrast with other reports where the MICs for carbapenems were more significantly increased (e.g. MICs >8 mg/L).23,25,40 We speculate that in our A. baumannii isolates this phenomenon is due to the absence of further mechanisms of resistance (e.g. porin loss and increased expression of efflux pumps). In fact, isolates of human origin usually possess multiple mechanisms of resistance that contribute to carbapenem resistance.23,40
Clinical data of infected animals
As shown in Table S1, infections due to A. baumannii in animals were commonly hospital acquired and involved various body sites (with a slight preponderance of wound infections and abscesses). About half of infected animals had a previous history of hospitalization, whereas the majority of them had underlying diseases and risk factors that could favour opportunistic infections such as those due to A. baumannii. Three cases of systemic infection (i.e. those due to Km765-04, Km1492-04 and Km474-06) resulted in death. We also noted that several cases of hospital-acquired A. baumannii infection occurred within a short period of time (e.g. five cases of ST12 during February–April 2005), suggesting a potential role for nosocomial transmission through the environment and/or transmission by hospital staff (Table S1).
Conclusions
This is the first molecular analysis of A. baumannii responsible for infections in pets and horses using established methods previously used for human isolates. Our strains did not possess any known acquired carbapenemase gene, loss of porin CarO or overexpressed efflux system, but the molecular backgrounds of resistance to aminoglycosides and quinolones and the hyperproduction of chromosomal β-lactamases had patterns common to those described in A. baumannii of human origin. More importantly, the two international clones persistently found in animals admitted at our institutions during 2004–09 are often reported in civilian and military patients hospitalized all over the world. The spread of such A. baumannii isolates in companion animals is very concerning because in humans these clonal lineages are associated with multiple mechanisms of resistance, including those against ‘last-line’ antibiotics such as carbapenems and colistin.5,29 Since the majority of animal cases were hospital acquired and in subjects with common predisposing conditions for infection compared with those reported in humans (e.g. prolonged use of antibiotics), we advance that in the near future A. baumannii isolates of veterinary origin could acquire further mechanisms of resistance and evolve into pathogens that are more difficult to treat. Larger screening and epidemiological studies should be planned to investigate the impact of animals on the spread of MDR A. baumannii isolates among humans.
Funding
This work was supported in part by the Veterans Affairs Merit Review Program (R. A. B.), the National Institutes of Health (grant AI072219-05 and AI063517-07 to R. A. B.), the Geriatric Research Education and Clinical Center VISN 10 (R. A. B.) and the Institute of Veterinary Bacteriology of Bern (grant 35-539).
Transparency declarations
R. A. B. has received research and speaking invitations from various pharmaceutical companies. None of these pose a conflict of interest with the present work. Other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We thank Andreas Thomann and Isabelle Brodard for technical assistance. We are grateful to the Center for Zoonoses, Bacterial Animal Diseases and Antibiotic Resistance (ZOBA) of Bern for providing the A. baumannii strains.
References
- 1.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–81. doi: 10.1056/NEJMra070741. doi:10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 2.Perez F, Hujer AM, Hujer KM, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–84. doi: 10.1128/AAC.01464-06. doi:10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. doi:10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:24–38. doi: 10.1128/AAC.01512-08. doi:10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65:1807–18. doi: 10.1093/jac/dkq191. doi:10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37:715–22. doi: 10.1016/j.ajic.2009.01.008. doi:10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hulten EA, et al. Antibiotic resistance determinants in Acinetobacter spp and clinical outcomes in patients from a major military treatment facility. Am J Infect Control. 2009;38:63–5. doi: 10.1016/j.ajic.2009.05.007. doi:10.1016/j.ajic.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dessel H, Dijkshoorn L, van der Reijden T, et al. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol. 2004;155:105–12. doi: 10.1016/j.resmic.2003.10.003. doi:10.1016/j.resmic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Turton JF, Gabriel SN, Valderrey C, et al. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin Microbiol Infect. 2007;13:807–15. doi: 10.1111/j.1469-0691.2007.01759.x. doi:10.1111/j.1469-0691.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 10.Dijkshoorn L, Aucken H, Gerner-Smidt P, et al. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–25. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ecker JA, Massire C, Hall TA, et al. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J Clin Microbiol. 2006;44:2921–32. doi: 10.1128/JCM.00619-06. doi:10.1128/JCM.00619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diancourt L, Passet V, Nemec A, et al. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. doi:10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaneechoutte M, Devriese LA, Dijkshoorn L, et al. Acinetobacter baumannii-infected vascular catheters collected from horses in an equine clinic. J Clin Microbiol. 2000;38:4280–1. doi: 10.1128/jcm.38.11.4280-4281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francey T, Gaschen F, Nicolet J, et al. The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med. 2000;14:177–83. doi: 10.1892/0891-6640(2000)014<0177:trobaa>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Boerlin P, Eugster S, Gaschen F, et al. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet Microbiol. 2001;82:347–59. doi: 10.1016/s0378-1135(01)00396-0. doi:10.1016/S0378-1135(01)00396-0. [DOI] [PubMed] [Google Scholar]
- 16.Brachelente C, Wiener D, Malik Y, et al. A case of necrotizing fasciitis with septic shock in a cat caused by Acinetobacter baumannii. Vet Dermatol. 2007;18:432–8. doi: 10.1111/j.1365-3164.2007.00624.x. doi:10.1111/j.1365-3164.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 17.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 1.3. 2011. [Google Scholar]
- 18.Vila J, Marcos MA, Jimenez de Anta MT. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus-A. baumannii complex. J Med Microbiol. 1996;44:482–9. doi: 10.1099/00222615-44-6-482. doi:10.1099/00222615-44-6-482. [DOI] [PubMed] [Google Scholar]
- 19.Hujer KM, Hujer AM, Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–23. doi: 10.1128/AAC.00778-06. doi:10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazel D, Dychinco B, Webb VA, et al. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob Agents Chemother. 2000;44:1568–74. doi: 10.1128/aac.44.6.1568-1574.2000. doi:10.1128/AAC.44.6.1568-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel L, Le Thomas I, Naas T, et al. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:622–32. doi: 10.1128/aac.44.3.622-632.2000. doi:10.1128/AAC.44.3.622-632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchand I, Damier-Piolle L, Courvalin P, et al. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother. 2004;48:3298–304. doi: 10.1128/AAC.48.9.3298-3304.2004. doi:10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bratu S, Landman D, Martin DA, et al. Correlation of antimicrobial resistance with β-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York City. Antimicrob Agents Chemother. 2008;52:2999–3005. doi: 10.1128/AAC.01684-07. doi:10.1128/AAC.01684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heritier C, Poirel L, Nordmann P. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin Microbiol Infect. 2006;12:123–30. doi: 10.1111/j.1469-0691.2005.01320.x. doi:10.1111/j.1469-0691.2005.01320.x. [DOI] [PubMed] [Google Scholar]
- 25.Turton JF, Ward ME, Woodford N, et al. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006;258:72–7. doi: 10.1111/j.1574-6968.2006.00195.x. doi:10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 26.Hujer KM, Hujer AM, Endimiani A, et al. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol. 2009;47:1436–42. doi: 10.1128/JCM.02380-08. doi:10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterson DL, Rice LB, Bonomo RA. Rapid method of extraction and analysis of extended-spectrum β-lactamases from clinical strains of Klebsiella pneumoniae. Clin Microbiol Infect. 2001;7:709–11. doi:10.1046/j.1469-0691.2001.00348.x. [PubMed] [Google Scholar]
- 28.Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the eastern USA. J Antimicrob Chemother. 2009;63:427–37. doi: 10.1093/jac/dkn547. doi:10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wortmann G, Weintrob A, Barber M, et al. Genotypic evolution of Acinetobacter baumannii strains in an outbreak associated with war trauma. Infect Control Hosp Epidemiol. 2008;29:553–5. doi: 10.1086/588221. doi:10.1086/588221. [DOI] [PubMed] [Google Scholar]
- 30.Higgins PG, Dammhayn C, Hackel M, et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–8. doi: 10.1093/jac/dkp428. doi:10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 31.D'Arezzo S, Capone A, Petrosillo N, et al. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy) Clin Microbiol Infect. 2009;15:347–57. doi: 10.1111/j.1469-0691.2009.02668.x. doi:10.1111/j.1469-0691.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohlenberg A, Brummer S, Higgins PG, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in a German university medical centre. J Med Microbiol. 2009;58:1499–507. doi: 10.1099/jmm.0.012302-0. doi:10.1099/jmm.0.012302-0. [DOI] [PubMed] [Google Scholar]
- 33.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45:88–94. doi: 10.1086/518605. doi:10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–71. doi: 10.1016/j.drup.2010.08.003. doi:10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott Y, O'Mahony R, Leonard N, et al. Characterization of a 2.6 kbp variable region within a class 1 integron found in an Acinetobacter baumannii strain isolated from a horse. J Antimicrob Chemother. 2005;55:367–70. doi: 10.1093/jac/dkh543. doi:10.1093/jac/dkh543. [DOI] [PubMed] [Google Scholar]
- 36.Nemec A, Dolzani L, Brisse S, et al. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J Med Microbiol. 2004;53:1233–40. doi: 10.1099/jmm.0.45716-0. doi:10.1099/jmm.0.45716-0. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan VB, Rajamohan G, Pancholi P, et al. Genetic relatedness and molecular characterization of multidrug resistant Acinetobacter baumannii isolated in central Ohio, USA. Ann Clin Microbiol Antimicrob. 2009;8:21. doi: 10.1186/1476-0711-8-21. doi:10.1186/1476-0711-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kansakar P, Dorji D, Chongtrakool P, et al. Local dissemination of multidrug-resistant Acinetobacter baumannii clones in a Thai hospital. Microb Drug Resist. 2011;17:109–19. doi: 10.1089/mdr.2010.0062. doi:10.1089/mdr.2010.0062. [DOI] [PubMed] [Google Scholar]
- 39.Segal H, Thomas R, Gay Elisha B. Characterization of class 1 integron resistance gene cassettes and the identification of a novel IS-like element in Acinetobacter baumannii. Plasmid. 2003;49:169–78. doi: 10.1016/s0147-619x(03)00011-8. doi:10.1016/S0147-619X(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 40.Adams-Haduch JM, Paterson DL, Sidjabat HE, et al. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother. 2008;52:3837–43. doi: 10.1128/AAC.00570-08. doi:10.1128/AAC.00570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamouda A, Amyes SG. Novel gyrA and parC point mutations in two strains of Acinetobacter baumannii resistant to ciprofloxacin. J Antimicrob Chemother. 2004;54:695–6. doi: 10.1093/jac/dkh368. doi:10.1093/jac/dkh368. [DOI] [PubMed] [Google Scholar]
- 42.Vila J, Ruiz J, Goni P, et al. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother. 1997;39:757–62. doi: 10.1093/jac/39.6.757. doi:10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 43.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54:321–32. doi: 10.1093/jac/dkh332. doi:10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.