Abstract
We have identified a novel vertebrate homolog of the Drosophila gene dachshund, Dachshund2 (Dach2). Dach2 is expressed in the developing somite prior to any myogenic genes with an expression profile similar to Pax3, a gene previously shown to induce muscle differentiation. Pax3 and Dach2 participate in a positive regulatory feedback loop, analogous to a feedback loop that exists in Drosophila between the Pax gene eyeless (a Pax6 homolog) and the Drosophila dachshund gene. Although Dach2 alone is unable to induce myogenesis, Dach2 can synergize with Eya2 (a vertebrate homolog of the Drosophila gene eyes absent) to regulate myogenic differentiation. Moreover, Eya2 can also synergize with Six1 (a vertebrate homolog of the Drosophila gene sine oculis) to regulate myogenesis. This synergistic regulation of muscle development by Dach2 with Eya2 and Eya2 with Six1 parallels the synergistic regulation of Drosophila eye formation by dachshund with eyes absent and eyes absent with sine oculis. This synergistic regulation is explained by direct physical interactions between Dach2 and Eya2, and Eya2 and Six1 proteins, analogous to interactions observed between the Drosophila proteins. This study reveals a new layer of regulation in the process of myogenic specification in the somites. Moreover, we show that the Pax, Dach, Eya, and Six genetic network has been conserved across species. However, this genetic network has been used in a novel developmental context, myogenesis rather than eye development, and has been expanded to include gene family members that are not directly homologous, for example Pax3 instead of Pax6.
Keywords: Dach2, Eya2, Six1, Pax3, myogenesis, dachshund, somite development
Somites are segmentally organized mesodermal structures that are the embryonic precursors of the axial skeleton and of all skeletal muscle (for review, see Christ and Ordahl 1995). Somites form by budding off from the anterior end of the presegmental mesoderm (PSM) to form epithelial balls of tissue. Patterning signals from surrounding tissues induce different regions of the somite to acquire distinct fates: The dorsal somite develops into the dermamyotome, the precursor to the dermis and to the muscles; and the ventral somite gives rise to the sclerotome, the precursor of the axial skeleton and ribs (Christ and Ordahl 1995). Subsequent inductive signaling leads to further subdivision of cell fates within the somite.
The best studied aspect of this patterning and differentiation process is the specification of the myogenic cells. The establishment of muscle cell fate requires inductive signals both from axial tissues and from the dorsal ectoderm that overlays the somite (Christ and Ordahl 1995; Cossu et al. 1996). The progress of myogenic induction can be observed by following the expression of the paired-type transcription factor Pax3. In the chick embryo, Pax3 is initially expressed throughout the PSM (Williams and Ordahl 1994). However, early inductive influences restrict this expression such that when the epithelial somite buds off from the PSM, Pax3 expression is restricted to the dorsal aspect of the somite. Later in development, Pax3 transcripts are confined to the dermamyotome (Williams and Ordahl 1994). The myogenic derivatives of the dermamyotome develop from two distinct regions (Ordahl and Le Douarin 1992). The epaxial cells, which form the back and intercostal muscles, arise from the medial edge of the dermamyotome (Ordahl and Le Douarin 1992). These pass under the dermamyotome and then elongate to form a new ventral layer of differentiating and postmitotic cells, the myotome (Christ and Ordahl 1995; Denetclaw et al. 1997). Myotomal cells express Myf-5 and MyoD, muscle-specific basic–helix–loop–helix (bHLH) transcription factors, and this expression marks the initiation of the myogenic differentiation program (Ott et al. 1991; Pownall and Emerson 1992). The hypaxial muscle precursors, which will form the limb and ventral body wall muscles, arise from the lateral portion of the dermamyotome and migrate ventrolaterally to populate their target structures (Ordahl and Le Douarin 1992). These migrating cells continue to express Pax3 and only turn on MyoD and Myf-5 after they have reached their destination (Williams and Ordahl 1994). The expression of MyoD and Myf-5 in myogenic precursors is followed by the expression of Myogenin and MRF-4, which are downstream myogenic bHLH transcription factors in differentiating myoblasts, and by the expression of genes encoding sarcomeric proteins, such as Myosin Heavy Chain (MHC), during the terminal differentiation phase (Molkentin and Olson 1996).
Pax3 and the myogenic bHLH genes are not only markers of myogenic fate but also play important roles in directing cells to form muscle. Transfection of 10T1/2 fibroblasts with any of the members of the myogenic bHLH family of transcription factors can drive these cells to adopt a muscle cell fate (for review, see Weintraub et al. 1991). Pax3 also acts to induce the muscle differentiation program in vivo (Tajbakhsh et al. 1997) and when ectopically expressed in a variety of explanted embryonic tissues, including somites (Maroto et al. 1997), although transfection of 10T1/2 fibroblasts with Pax3 is not sufficient to induce these cells to adopt a myogenic fate (Maroto et al. 1997). This suggests that Pax3-induced myogenesis requires additional factors that are not present in the 10T1/2 fibroblasts but are present in somites.
On the basis of their expression patterns, several genes are candidates to be acting with Pax3 to direct muscle development. The putative transcriptional activators Eya1, Eya2, and Eya4 are all expressed in the dorsal epithelial somite (Xu et al. 1997; Mishima and Tomarev 1998; Borsani et al. 1999). As the somites mature, they become restricted to the medial and lateral aspects of the dermamyotome and are subsequently seen in the myotome and limb muscle precursors. Similarly, the homeodomain-containing transcription factors Six1 and Six4 are also expressed in the dorsal region of the developing somite and subsequently in the myotome and limb muscle precursors (Oliver et al. 1995a; Esteve and Bovolenta 1999).
Eya1, Eya2, Eya4, and Six1 and Six4 are homologous to the Drosophila genes eyes absent (eya) and sine oculis (so), respectively, and genetic studies in that organism suggest that they may function in common pathways. Both so and eya are expressed in the developing Drosophila eye and are required for normal eye formation (Bonini et al. 1993; Cheyette et al. 1994). In addition, eya has the ability to induce ectopic eyes when misexpressed (Bonini et al. 1997). The ability to induce ectopic eye formation is shared with the Drosophila gene eyeless (ey), which encodes a transcription factor of the Pax family, Pax6 (Quiring et al. 1994; Halder et al. 1995). Recent studies have begun to analyze how the functions of these various gene products are integrated in normal Drosophila eye development. For instance, eya and so have been shown to act synergistically downstream of ey to regulate the formation of ectopic eyes, and it has been demonstrated that their protein products physically interact (Pignoni et al. 1997). In addition, there are indications that a positive feedback loop exists such that eya and so regulate the expression of ey (Pignoni et al. 1997; Halder et al. 1998).
The fact that members of the Pax, Eya, and Six gene families have overlapping expression patterns in the developing somite raises the intriguing possibility that the Pax/Eya/Six regulatory network first identified in the context of the Drosophila eye may play an important role in vertebrate somitogenesis as well. If true, however, it would mean that the regulatory relationships are not limited to the direct homologs of the specific family members implicated in Drosophila eye development but extend to more divergent members of these gene families. For example, the only paired domain containing proteins implicated in cooperating with eya and so in Drosophila are ey and toy, which are both orthologs of the vertebrate gene Pax6 (Quiring et al. 1994; Halder et al. 1995; Bonini et al. 1997; Czerny et al. 1999). Pax6 is not, however, expressed in the developing somite (Walther and Gruss 1991). Several more distantly related Pax genes are expressed in the somite, and of these, only Pax3 and Pax7 are specific to the dermamyotome (Goulding et al. 1994; Williams and Ordahl 1994). Therefore, if Eya and Six genes are working with a Pax gene in the context of muscle development, it must be with one more distantly related to ey, for example, Pax3 or Pax7.
A fourth gene, dachshund (dac), has been shown to participate in the pathway regulating Drosophila eye development. dac encodes a novel nuclear protein that functions as a putative transcriptional activator and is both required for normal eye development and capable of initiating ectopic eye formation when misexpressed (Mardon et al. 1994; Shen and Mardon 1997). Genetic experiments established that dac and eya function synergistically to induce ectopic eyes (Chen et al. 1997). Furthermore, biochemical experiments have shown that Dac and Eya proteins physically interact (Chen et al. 1997). No direct synergy or protein interactions have been observed between Dac and So. Epistasis experiments have demonstrated that, like eya and so, dac functions downstream of ey and regulates ey in a positive feedback loop (Shen and Mardon 1997). Thus, Drosophila eye development is governed by a complex, integrated, regulatory network in which ey, eya, so, and dac all play key roles. Because Eya, Six, and Pax genes are all expressed during somite development and potentially play roles in vertebrate somitogenesis, we reasoned that vertebrate homologs of dac might also exist and participate in this same process.
Results
Cloning of Dach2
To identify vertebrate homologs of Drosophila dachshund, a chick library was screened with a human EST clone that showed homology to dachshund. Two distinct classes of chick Dachshund clones were isolated, Dach1 (T. Heanue and C. Tabin, unpubl.) and Dach2. Independent studies identified a murine homolog of dachshund, referred to as Dach (Hammond et al. 1998; Caubit et al. 1999; Davis et al. 1999). Sequence comparison and expression analysis, which will be presented separately, suggest that Dach1 is the chicken homolog of mouse Dach and the original human EST, whereas Dach2 represents a novel vertebrate gene. Sequence analysis of Dach2 indicates that the predicted coding region of Dach2 is 1.8 kb, encoding 608 amino acids (Fig. 1A). Northern blot analysis and sequence analysis indicate that the Dach2 transcript size is ∼3.0 kb (Fig. 1B). Comparison of the predicted amino acid sequence of Dach2 with Drosophila Dac (Mardon et al. 1994) (Fig. 1A) shows two regions of high sequence conservation. These regions correspond to domains previously identified in comparisons between mouse Dach and Drosophila Dac, and denoted DD1/Dachbox-N and DD2/Dachbox-C (Hammond et al. 1998; Davis et al. 1999). The similarity between Dach2 and Drosophila Dac is 86% in the DD1/Dachbox-N domain and 70% in the DD2/Dachbox-C domain.
Figure 1.
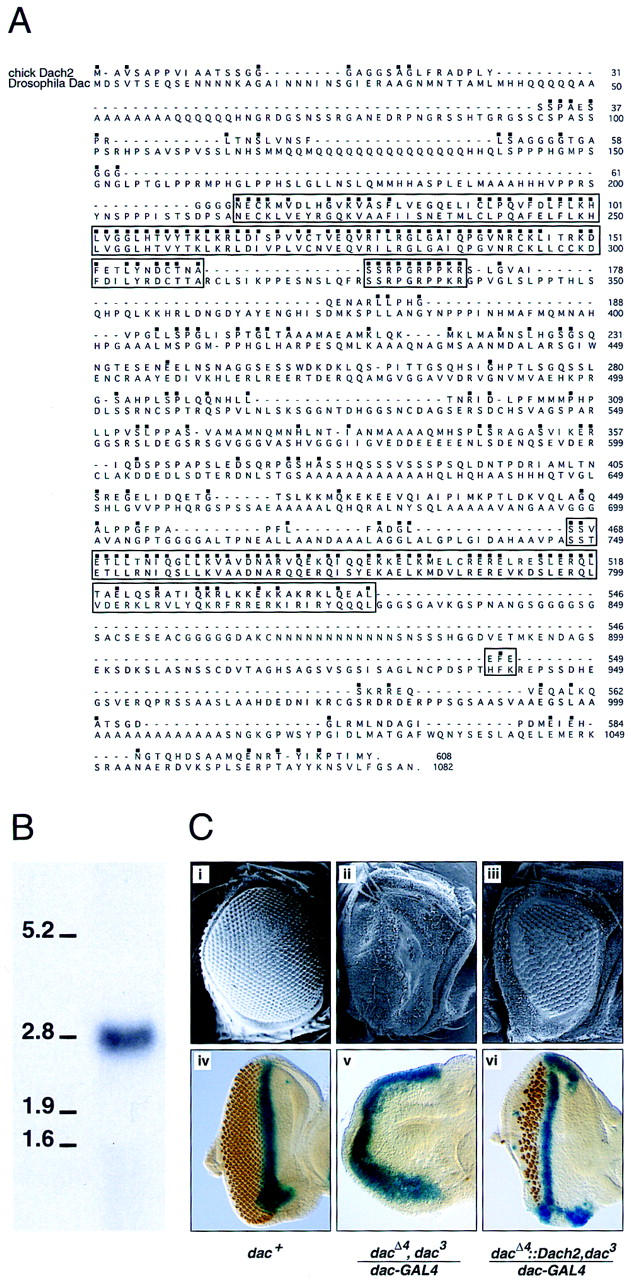
Dach2 is a vertebrate homolog of Drosophila dachshund. (A) Comparison of chick Dach2 and Drosophila dachshund putative amino acid sequences. Identical amino acids are marked with a dot over the amino acid. Two highly conserved regions are marked by a box. The amino-terminal box corresponds to DD1/Dachbox1, whereas the carboxy-terminal box corresponds to DD2/Dachbox2 (see text for details). Similarities in these two regions are 86% and 70%, respectively. (B) Northern blot on stage 22 embryo total RNA showing a single band, approximately 3 kb, hybridizing with a Dach2 probe. Compare with the 2.8-kb band in the RNA ladder. (C) Dach2 misexpression can compensate for the loss of dachshund in dac mutant lines. (i,iv) Wild-type eye; (ii,v) dac mutant eyes; (iii,vi) Dach2 expressed ectopically in a dac mutant background using Drosophila dac::Dach2 fusion transgene driven by dac–GAL4. (i–iii) Scanning electron microscopy images of Drosophila eyes. (iv–vi) Drosophila eye discs stained for dpp expression in blue (to mark the morphogenetic furrow) and Elav expression in orange (to mark photoreceptors).
These high levels of sequence conservation suggested that these might be conserved functional domains. Functional conservation between Dach2 and Drosophila Dac was tested by attempting to rescue Drosophila dac mutant phenotypes using the GAL4–UAS system (Brand and Perrimon 1993). To ensure that efficient translation of Dach2 took place in vivo, we constructed a Drosophila dac::Dach2 fusion transgene, encoding the first 31 amino acids of the Drosophila Dac protein fused to Dach2. We drove expression of the Drosophila dac::Dach2 fusion transgene, or the first 31 amino acids of Drosophila Dac alone, using a GAL4 driver that accurately reproduces the dac pattern of expression (dac–GAL4; G. Marden, unpubl.).
The normal fly eye consists of ∼800-unit eyes or ommatidia that are arranged in a precise hexagonal array (Fig. 1B,i). The adult eye develops in the larva from an epithelial monolayer termed the eye–imaginal disc. Photoreceptor differentiation proceeds in a wave of development from the morphogenetic furrow (Wolff and Ready 1991). decapentaplegic (dpp) marks the position of the furrow (Fig. 1B,iv) as it moves across the eye disc (Blackman et al. 1991). dac mutant adults develop with no eyes because of a failure of furrow initiation during larval stages (Mardon et al. 1994). When Dach2 is ectopically expressed in a dac null mutant background using the Drosophila dac::Dach2 fusion transgene driven by dac–GAL4, the mutant eye phenotype is rescued, resulting in morphogenetic furrow initiation and progression and ommatidia formation in both larvae in adults (Fig. 1B,iii,vi). The first 31 amino acids of Drosophila Dac by itself had no effect (Fig. 1B,ii,v). Targeted Drosophila dac::Dach2 expression driven by dac–GAL4 in a wild-type (dac+) background had no discernible effect on fly development (data not shown). These experiments show that the chick Dach2 gene encodes a functional protein capable of compensating for Dac function in the Drosophila eye. Therefore, because vertebrate Dach2 can apparently interact productively with the presumed target proteins of Drosophila Dac, the interacting domains of the proteins must have been maintained during the divergence of these two evolutionarily distant organsims. This suggests that the interactions themselves may be conserved in vertebrates.
Dach2 is expressed dynamically during somite development
To determine whether Dach2 is present in the developing somite, various stage chick embryos were analyzed by in situ hybridization (Fig. 2; data not shown). Expression profiles of Dach2 are distinct from those of Dach1 (data not shown), indicating that the RNA probes used are specific.
Figure 2.
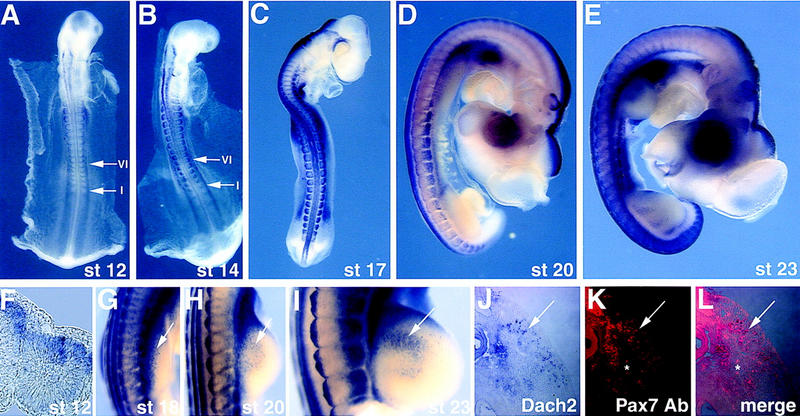
Dach2 is expressed dynamically during somite development and in myoblast precursors migrating into the limb buds. (A–E,G–I) Whole-mount RNA in situ hybridization: (A) stage (st) 12 and (B) stage 14, arrows point to the newly formed somite I and the more mature somite VI; (C) stage 17; (D) stage 20; (E) stage 23; (G) stage 18 forelimb; (H) stage 20 forelimb; (I) stage 23 forelimb. Arrows in G–I point to myoblast precursors migrating into the forelimbs. (F) Section in situ hybridization on a transverse section of a stage 11 embryo at the level of somite I. (J–L) Transverse section through a stage 20 forelimb visualized for Dach2 RNA distribution, seen as blue (J), and Pax7 protein distribution, seen as red (I). The two images are merged in K, and cells expressing Dach2 and Pax7 overlap and are purple. Red blood cells autoflouresce, and some examples are indicated above and below the asterix (*) in K and L.
Somites develop in a rostral to caudal sequence; thus, the degree of maturation of a somite depends on both the age of the embryo and the location of the somite within the embryo (Christ and Ordahl 1995). Early somites are morphologically uniform and are unpatterned along the medial–lateral axis; however, some molecular differences are apparent along the dorsal–ventral axis (Williams and Ordahl 1994; Ebensperger et al. 1995). At this stage, Dach2 is expressed throughout the medial–lateral extent of the somite but restricted to the dorsal region (Fig. 2A,B,F; see arrow to somite I in B). Pax3 expression is similarly restricted to the dorsal region of these early somites, throughout their medial–lateral extent (Williams and Ordahl 1994).
As the somite matures, morphological changes in the dorsal region of the somite give rise to a visibly recognizable dermamyotome (Christ and Ordahl 1995). Dach2 is expressed throughout the medial–lateral extent of the dorsal somite and at higher levels in the lateral regions (Fig. 2A,B; see arrows to somite VI). At this same somite level, myotomal precursor cells at the medial edge of the dermamyotome begin to express MyoD and down-regulate Pax3, resulting in a more lateral restriction of Pax3 expression (Williams and Ordahl 1994).
After the somites become patterned along both the dorsal–ventral and medial–lateral axes, the migratory population of the lateral dermamyotome begins to invade the limb bud and body wall (Christ and Ordahl 1995). Dach2 is expressed at high levels in the medial and lateral dermamyotome of somites at the limb level at this stage and in a punctate pattern in the proximal region of the emerging limb bud (Fig. 2C,G). The myoblasts in the limb assemble into dorsal and ventral muscle masses. These muscle masses proliferate for several days before beginning the process of differentiation and before expressing myogenic markers (Christ and Ordahl 1995). During this process, Dach2 expression is seen in a cluster of cells extending towards the distal limb bud in both dorsal and ventral streams (Fig. 2D,E,H,I; data not shown). Cells expressing Dach2 also express Pax7 protein, an established marker of muscle precursors in the limb (Yamamoto et al. 1998) (Fig. 2J–L).
Expression of Dach2 overlaps with Pax3, Eya2, and Six1
Expression of Dach2 in the dorsal somite and in the migrating hypaxial myoblast precursors is similar to that reported for Pax3, Eya2, and Six1 (Williams and Ordahl 1994; Oliver et al. 1995a; Xu et al. 1997; Mishima and Tomarev 1998). To determine whether these four genes might be expressed in the same populations of cells, we compared their expression domains on adjacent sections (Fig. 3). In early epithelial somites, Dach2 is expressed dorsally as well as in the dorsal neural tube and in the intermediate mesoderm (Fig. 3A). Pax3 expression overlaps the Dach2 expression domain in the dorsal somite and in the dorsal neural tube (Fig. 3B). Eya2 is expressed throughout the somite, with higher levels dorsally (Fig. 3C). Six1 expression is detectable throughout the somite, with higher levels dorsally (Fig. 3D). Neither Eya2 nor Six1 are expressed in the neural tube (Fig. 3C,D). Thus, the expression of the four genes overlaps in the dorsal compartment of the somite.
Figure 3.
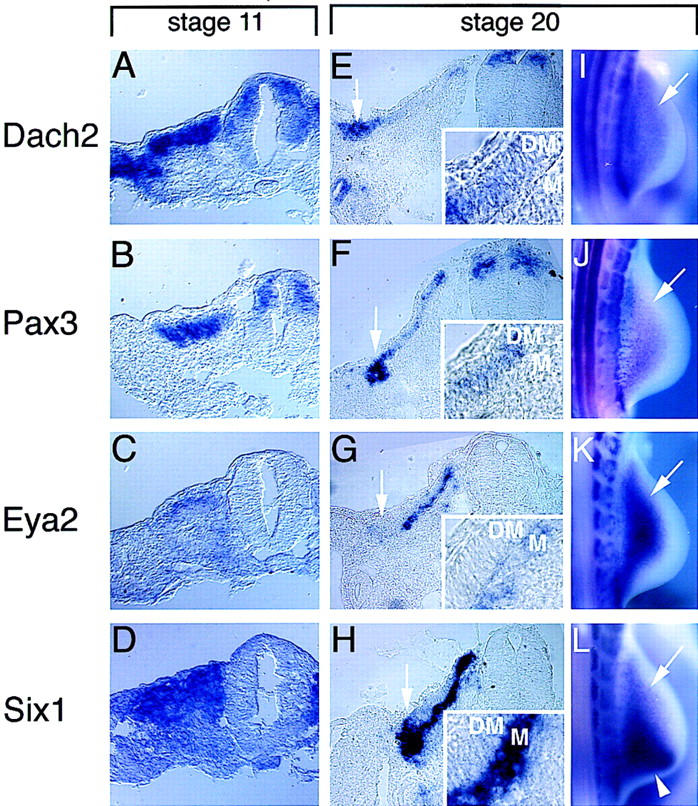
Expression patterns of Dach2, Pax3, Eya2, and Six1 overlap in the developing somite and in limb muscle precursors. (A–H) Nonradioactive section in situ hybridizations on adjacent sections: (A–D) stage 11, adjacent transverse sections at the level of somite V; (E–H) stage 20, adjacent transverse sections at the anterior edge of the forelimb. Arrows in E–H point to the hypaxial myoblast precursors at the lateral edge of dermamyotome. (Insets) Magnifications of the dermamyotome (DM) and myotomal (M) layers of sections shown in E–H. (I–L) Whole-mount in situ hybridization of stage 20 embryos, hindlimbs. Arrows point to myoblast precursors migrating into the hindlimbs. (A,E,I) Dach2 expression. (B,F,J) Pax3 expression. (C,G,K) Eya2 expression, Eya2 probe gives weaker signals relative to other probes used. (D,H,L) Six1 expression. Arrowhead in L points to an additional domain of Six1 expression in the posterior limb mesenchyme.
In more mature somites, both Dach2 and Pax3 are detected throughout the dermamyotome, with elevated levels at the medial edge and even higher levels at the lateral edge, whereas Dach2 shows an additional domain of expression in the nephrogenic ducts (Fig. 3E,F). Throughout most of the somite at this stage, Eya2 and Six1 are restricted to the differentiating myotomal layer that lies ventral to the Pax3 and Dach2 expression domains (Fig. 3G,H). The expression of all four genes overlap in the hypaxial myoblast precursors (Fig. 3E–H, see arrows). The common expression of these genes in the hypaxial derivatives is maintained as the undifferentiated myoblast precursors migrate into the limb buds and the lateral body wall (Fig. 3I–L). To verify that the expression of these genes in the limb buds is attributable to the migrating myoblasts, double staining experiments were performed using an antibody against Pax7, which like Pax3 is a definitive marker for the myoblast population in the limb (Yamamoto et al. 1998). Cells in the limb expressing Dach2, Pax3, Eya2, and Six1 are, in each case, also expressing Pax7 (Fig. 2J–L; data not shown).
Thus, Pax3, Dach2, Eya2, and Six1 are coexpressed in cells prior to muscle differentiation, and their overlapping expression continues in derivatives where the cells are maintained in an undifferentiated state. These genes are therefore candidates to be acting together upstream of the myogenic regulatory factors to regulate early phases of skeletal myogenesis.
Dach2 is regulated by signals from the ectoderm
The striking similarity in the expression patterns of Pax3 and Dach2 suggested that their expression might be regulated by the same signals. Dorsal somite fate and the expression of Pax3 are dependent on signals from both the overlying ectoderm and the dorsal neural tube (Fan and Tessier-Lavigne 1994; Maroto et al. 1997; Reshef et al. 1998; Tajbakhsh et al. 1998). In the dorsomedial somite, expression of Pax3 is regulated by the neural tube and is independent of the ectoderm (Dietrich et al. 1997). In contrast, in the dorsolateral somite, Pax3 expression is dependent on the ectoderm. When a barrier is placed between the somite and the ectoderm, Pax3 expression is down-regulated (Dietrich et al. 1997). To test whether Dach2 expression in the dorsolateral somite is similarly regulated, we performed in ovo barrier experiments to separate somites from the influence of the overlying ectoderm. At stage 10–11, a barrier was placed under the ectoderm and over the PSM and somites I–III on one side of the embryo. After 24–36 hr, embryos were analyzed for gene expression. As previously reported, we observe down-regulation of lateral Pax3 expression in the somites covered by the barrier (Fig. 4A,E) (Dietrich et al. 1997). Likewise, Dach2 shows a dramatic down-regulation of dorsolateral expression when the somites are blocked from contacting the ectoderm (Fig. 4B,F). As previously noted, lateral MyoD expression is also down-regulated in the presence of such a barrier (Fig. 4C,G) (Dietrich et al. 1997). In contrast, expression of the control ventral somitic marker Pax1 (Ebensperger et al. 1995) is expanded dorsally (Fig. 4D,H). Moreover, analysis of these embryos in transverse sections shows a morphologically normal somite (Fig. 4E–H; data not shown).
Figure 4.
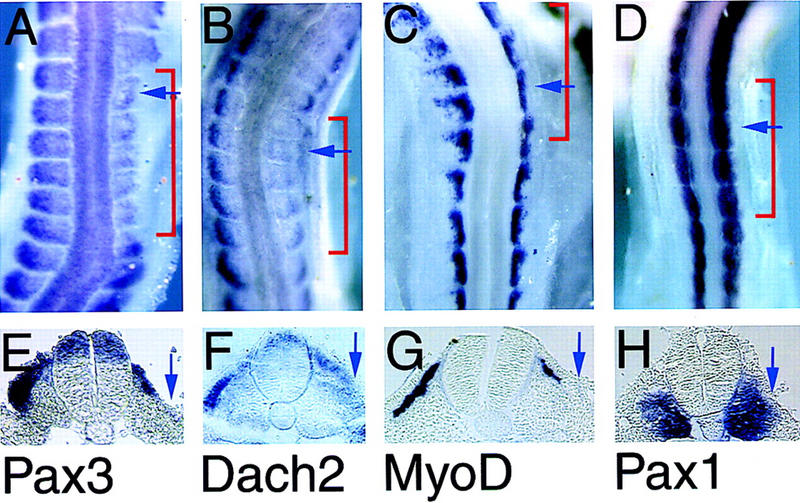
Pax3, Dach2, and MyoD are regulated by signals from the ectoderm. (A–D) Whole-mount in situ hybridizations of embryos 24–36 hr after barrier placement between the somite and the dorsal ectoderm. Embryos at stage 16–19. The region covered by the barrier is indicated with a red bracket. (E,F) Paraffin sagital sections of the embryos shown in A–D at the position indicated by the blue arrows in A–D. (A,E) Pax3; (B,F) Dach2; (C,G) MyoD; (D,H) Pax1. Blue arrows in E–H point to the lateral edge of the somite where expression of Pax3, Dach2, and MyoD is lost and where Pax1 expression is expanded.
Pax3 and Dach2 positively regulate each other's expression
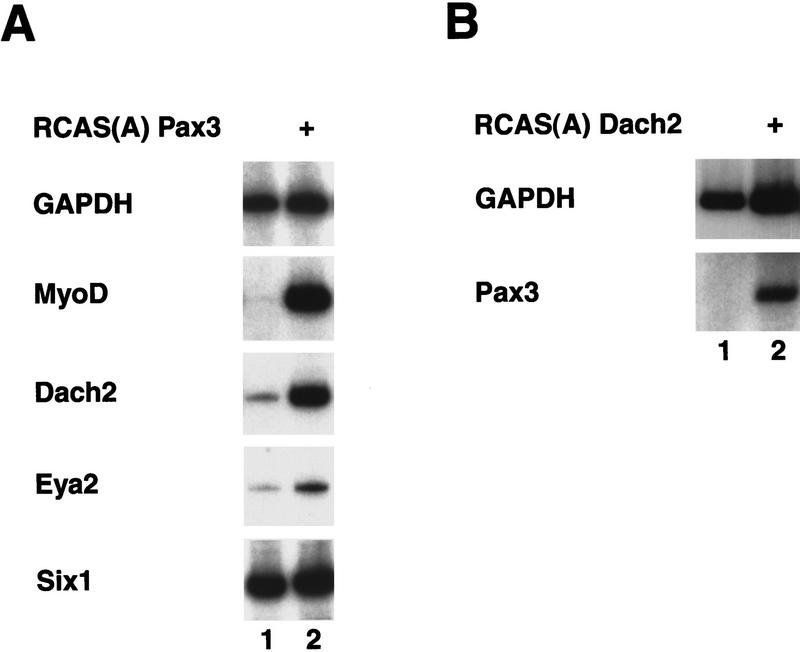
The similarities in Pax3 and Dach2 expression and their mutual dependence on ectodermal signals could reflect either independent regulation of the two genes or a more complex, integrated regulation. In Drosophila eye formation, ey (a Drosophila Pax6 homolog; Quiring et al. 1994) acts in a positive feedback loop with dac (Shen and Mardon 1997). To test for a similar relationship between Pax3 and Dach2, we used an in vitro somite culture system that we have previously shown faithfully recapitulates in vivo somite differentiation without the confounding influence of adjacent inductive tissues (Maroto et al. 1997). Somites were explanted into culture and infected with a retrovirus containing either Pax3 or Dach2, and target gene expression was analyzed by RT–PCR after 5 days of culture.
As observed previously (Maroto et al. 1997), retroviral misexpression of Pax3 in somites results in an induction of myogenic gene expression (Fig. 5A, lane 2), showing that Pax3 regulates muscle differentiation in the somitic tissue. Dach2 is expressed at a low level in uninfected somite cultures (Fig. 5A, lane 1); however, cultures exposed to a Pax3 retrovirus show strong up-regulation of Dach2 expression (Fig. 5A, lane 2). Thus, Pax3 can positively regulate the expression of Dach2. In addition, Pax3 misexpression leads to weak Eya2 up-regulation (Fig. 5A, lane 2), although Six1 expression is detected at the same high level in the presence or absence of Pax3 (Fig. 5, lanes 1,2).
Figure 5.
Misexpression of Pax3 and Dach2 in somite explant culture reveals a positive regulatory feedback loop between these two genes. (A) Misexpression of Pax3. (Lane 1) Somites cultured alone; (lane 2) somites cultured with Pax3 retrovirus (+). Cultures analyzed by RT–PCR for GAPDH, MyoD, Dach2, Eya2, and Six1. (B) Misexpression of Dach2 retrovirus. (Lane 1) Somites cultured alone; (lane 2) somites cultured with Dach2 retrovirus (+). Cultures analyzed by RT–PCR for GAPDH and Pax3. (+) The addition of a retrovirus to the culture.
To test for a possible reciprocal regulation of Pax3 by Dach2, we infected similar somite cultures with a retrovirus containing Dach2. Pax3 is not expressed at all in uninfected cultures. Retroviral Dach2 misexpression in somites leads to a clear induction of Pax3 (Fig. 5B, lane 2) when compared with the control culture (Fig. 5B, lane 1). In some experiments, weak up-regulation of myogenic genes was also seen (data not shown); however, these results were not consistently observed. These indicate that a positive regulatory feedback loop operates between Pax3 and Dach2 in the context of the developing somite.
Dach2, Eya2, and Six1 synergistically regulate myogenic gene expression
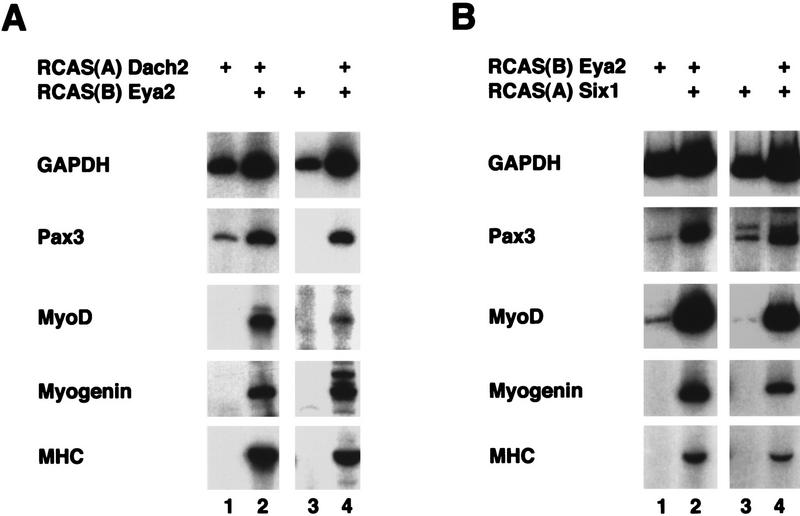
In Drosophila, dac, eya, and so act synergistically to regulate eye development (Chen et al. 1997; Pignoni et al. 1997). Whereas dac and eya can each induce ectopic eye formation, the two genes together induce ectopic eyes at a frequency that is much greater than the sum of the rates of eye induction produced by the individual genes (Chen et al. 1997). Ectopic eyes are also induced at a higher rate when eya is coexpressed with so (Pignoni et al. 1997). Our results demonstrate that the vertebrate homologs of these genes are coexpressed in muscle precursor populations prior to muscle differentiation, suggesting that they may function together to regulate myogenesis. To test whether synergistic relationships, similar to those seen in Drosophila eye, exist between Dach2, Eya2, and Six1 within the context of myogenesis, we misexpressed these genes in somite culture.
When Dach2 is misexpressed in somite culture, low level Pax3 expression is induced, but no myogenic gene expression is detected (Fig. 6A, lane 1; see also Fig. 5B, lane 2). Misexpression of Eya2 in somites resulted in either trace or undetectable levels of Pax3 and MyoD expression and no expression of Myogenin or MHC (Fig. 6A, lane 3, and Fig. 6B, lane 1). However, when Dach2 and Eya2 are misexpressed in combination, elevated levels of Pax3 are detected, and MyoD, Myogenin, and MHC expression are induced (Fig. 6A, lanes 2,4). Like Eya2, Six1 shows only weak Pax3 and MyoD inducing ability and does not induce Myogenin or MHC (Fig. 6B, lane 3). In contrast, when Eya2 and Six1 are misexpressed together, a dramatic up-regulation of Pax3, MyoD, Myogenin, and MHC is seen (Fig. 6B, lanes 2,4). No synergistic up-regulation of Pax3 or myogenic genes was observed when Dach2 and Six1 were misexpressed in combination (data not shown). These results demonstrate that Dach2 and Eya2, as well as Eya2 and Six1, act synergistically to regulate the expression of Pax3 and the process of myogenic differentiation.
Figure 6.
Misexpression of Dach2 and Eya2, and Eya2 and Six1 in somite explant culture results in synergistic regulation of Pax3 and myogenic marker genes. (A) Misexpression of Dach2 and Eya2 in somite explant culture. (Lane 1) Somites cultured with RCAS(A) Dach2 alone; (lanes 2,4) somites cultured with RCAS(A) Dach2 and RCAS(B) Eya2; (lane 3) somites cultured with RCAS(B) Eya2 alone. Cultures analyzed by RT–PCR for GAPDH, Pax3, MyoD, Myogenin, and MHC. (B) Misexpression of Eya2 and Six1 in somite explant culture. (Lane 1) Somites cultured with RCAS(B) Eya2 alone; (lanes 2,4) somites cultured with RCAS(B) Eya2 and RCAS(A) Six1; (lane 3) somites cultured with RCAS(A) Six1 alone. Cultures analyzed by RT–PCR for GAPDH, Pax3, MyoD, Myogenin, and MHC. (+) The addition of a retrovirus to the culture.
Dach2 and Eya2, and Eya2 and Six1, physically interact
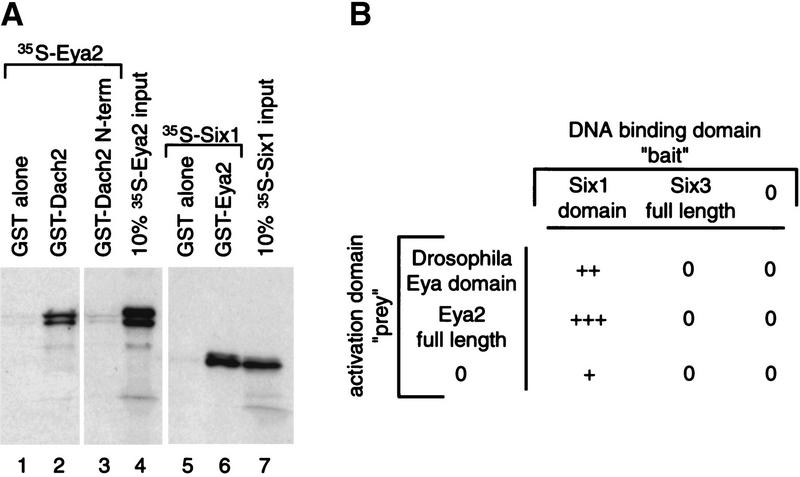
One possible mechanism for the synergistic action of Dach2 with Eya2 and Eya2 with Six1 is that the proteins function in a physical complex to regulate myogenesis. Consistent with this idea, the Drosophila homologs of these pairs of proteins have been shown to interact: Dac with Eya and Eya with So (Chen et al. 1997; Pignoni et al. 1997). To test whether similar physical interactions occur between the vertebrate proteins, we performed GST pull-down interaction assays.
GST–Dach2 and GST–Eya2 proteins were tested for their ability to interact with 35S-labeled Eya2 and Six1 proteins, respectively. GST–Dach2 fusion protein efficiently pulled down 35S-labeled Eya2 (Fig. 7A, lane 2), whereas control experiments showed that GST alone did not pull down any 35S-labeled Eya2 (Fig. 7A, lane 1) nor did GST fused to the first 170 amino acids of Dach2 (Fig. 7A, lane 3). This finding indicates that the amino 170 amino acids of Dach2 are not sufficient to interact with Eya2 and suggests that the interaction of Dach2 with Eya2 requires the carboxyl terminus of the Dach2 protein. In a similar assay, GST–Eya2 fusion protein was found to interact with 35S-labeled Six1 protein (Fig. 7A, lane 6), whereas the control GST alone showed no interaction with Six1 protein (Fig. 7A, lane 5). The GST pull-down experiment was also used to assay potential Dach2 and Six1 interactions; however, no interactions were detected (data not shown). This finding is consistent with our failure to detect synergistic gene regulation by Dach2 and Six1 in somite cultures (data not shown).
Figure 7.
Interaction assays to reveal interactions between Dach2 and Eya2, and Eya2 and Six1 proteins. (A) GST pull-down interaction assays. (Lanes 1–4) Analysis of Dach2–Eya2 interactions. GST alone (lane 1), GST–Dach2 full-length protein (lane 2), and GST–Dach2 amino-terminal fragment (N-term) (lane 3) were tested for their ability to pull down radiolabeled Eya2 protein. (Lanes 5–7) Analysis of Eya2–Six1 interactions. GST alone (lane 5) and GST–Eya2 full-length protein (lane 6) were tested for their ability to pull down radiolabeled Six1 protein. Strength of the interactions can be assessed by comparing the amount of protein pulled down in the test lanes (lane 1–3 and 5–6, respectively) to the amount in the 10% input lane (lanes 4 and 7, respectively). (B) Yeast two-hybrid interaction assays. Cotransformants were analyzed for their ability to show lacZ color reaction. Strength of interactions were judged by the amount of time passed before a color reaction was observed: (0) no staining after 4 hr; (+++) staining in <60 min.
A second method for determining the ability of proteins to physically interact is the GAL4 yeast two-hybrid system. In this system, “baits” are constructed as fusions with a GAL4 DNA-binding domain capable of binding to a UAS target sequence upstream of a lacZ reporter. “Preys” are constructed as fusions with the GAL4 transcriptional activation domain. Thus, in vivo interactions between bait and prey will result in lacZ transcription.
We were unable to use this approach to verify that Dach2 interacts with Eya2 because both of these genes induce lacZ expression by themselves when expressed in a bait construct (data not shown). In contrast, we were able to use the GAL4 yeast two-hybrid system to confirm the Eya2/Six1 interaction seen with GST pull-downs. Transformation of Six1 bait construct (containing the Six domain of Six1; Oliver et al. 1995b) with either Drosophila Eya domain prey construct (Chen et al. 1997) or Eya2 prey construct led to very strong lacZ expression, relative to the prey constructs transformed alone (Fig. 7B). To test the specificity of this interaction, we performed the same experiment with Six3. Six3 is another member of the Six family (Oliver et al. 1995b), but it is the direct homolog of Drosophila optix, not of so (Toy et al. 1998). However, no activation of lacZ expression was seen when a Six3 bait construct was transformed with the Eya2 prey construct (Fig. 7B).
The results of our GST pull-down and yeast two-hybrid assays demonstrate conservation of protein interaction domains from flies to vertebrates and suggest that these conserved interactions might be responsible for their synergistic regulation of Pax3 and myogenesis.
Discussion
Conserved domains in Dach2
The predicted Dach2 amino acid sequence is highly similar to Drosophila Dac (Mardon et al. 1994) and to mouse Dach (Hammond et al. 1998; Caubit et al. 1999; Davis et al. 1999) in two domains denoted DD1/Dachbox-N and DD2/Dachbox-C (Hammond et al. 1998; Davis et al. 1999). These domains of the Dach proteins show some sequence similarities to domains in the proto-oncogene c-ski and the c-ski-like gene sno (Hammond et al. 1998; Davis et al. 1999). Mammalian c-ski is required for normal muscle development (Berk et al. 1997), and both c-ski and chicken SnoN have the ability to induce myogenic differentiation (Boyer et al. 1993; Zheng et al. 1997a). Ski and Sno are thought to function as dimers, and although they do not directly contact DNA, they are thought to act through alternate participation in either repressor or activator complexes (Nomura et al. 1999). The helical domain of Ski/Sno is required for dimerization, and is required for the complete activity of the v-ski oncogene to transform fibroblasts and induce myogenic differentiation (Nagase et al. 1993; Zheng et al. 1997b). The predicted tertiary structure of the DD2 domain of Dach2 protein indicates that this domain could, like Ski and Sno, form an α-helical coiled-coil structure (data not shown). The sequence and protein structure similarities between Dach2 and Ski/Sno raises the intriguing possibility that these proteins are acting in similar ways to regulate myogenesis.
Cross-regulation between Pax3 and Dach2
The expression profile of Dach2 is identical to that of Pax3 during somitogenesis (Williams and Ordahl 1994). We have shown in barrier experiments that control of lateral Dach2 expression is regulated by the dorsal ectoderm. Because Dach2 transcripts are already present in the somites at the time of barrier placement, the experiments demonstrate a requirement for the ectoderm for maintenance of Dach2 expression, perhaps mediated by Pax3. The dorsal ectoderm may also be responsible for the initial induction of Dach2 expression.
Several secreted factors present in the dorsal ectoderm are candidates for the ectodermal signal that induces and maintains expression of Pax3 and Dach2. Several Wnt genes, including Wnt4 and Wnt6 (Parr et al. 1993), are present at the correct time and place to be transmitting this signal. Moreover, these and other Wnt genes have been shown to induce Pax3, MyoD, and Myf-5 expression in cultured somites (Munsterberg et al. 1995; Fan et al. 1997; Maroto et al. 1997; Tajbakhsh et al. 1998). It is interesting to note that in the Drosophila leg imaginal disc, dac gene expression is regulated, in part, by wg (the Drosophila homolog of vertebrate Wnt genes; Lecuit and Cohen 1997).
Not only are Dach2 and Pax3 under similar regulatory control by the ectoderm, but these two genes also participate in a positive regulatory feedback loop. Because Pax3 is expressed in the PSM prior to Dach2 expression (Williams and Ordahl 1994; data not shown), it is possible that the feedback loop is initiated by Pax3.
Our analysis of the cross-regulation between Pax3 and Dach2 was performed in vitro. We were unable verify these results in vivo (data not shown), consistent with previous negative results with Pax3 misexpression in vivo (A.B. Lassar, unpubl.). This is likely due to the buffering influence of other factors present in vivo. An alternative approach to working in vitro would be to remove some of the confounding influences in vivo. For example, the dorsal ectoderm can be physically separated from the underlying somite with a barrier before performing misexpression experiments, thus removing the normal regulatory influence of this tissue. Preliminary experiments, using such conditions, suggest that the regulatory interactions between Pax3 and Dach2 can indeed be observed in vivo (data not shown).
It is clear that additional regulators of Dach2 expression must exist elsewhere in the embryo because Dach2 is expressed in domains that do not overlap with Pax3 expression, including the developing urogenital system. However, another Pax gene, Pax2, is expressed in the nephrogenic duct and is a plausible candidate for regulating Dach2 expression (Dressler et al. 1990). Moreover, mouse Dach2 (isolated in a screen using the chick Dach2 probe; G. Mardon, unpubl.) is expressed normally in Pax3 (Splotch; Epstein et al. 1991) mutant mouse embryos (T. Heanue and C. Tabin, unpubl.), indicating that Pax3 is not the sole regulator of Dach2. However, it is possible that Pax7, which is up-regulated in the dermamyotome of Splotch mutants (Borycki et al. 1999), compensates for the loss of Pax3 expression.
The regulatory feedback loop between Pax3 and Dach2 is analogous to the regulatory relationship seen between ey and dac in Drosophila. However, Pax3 is not the vertebrate ortholog of ey (Quiring et al. 1994). The fact that over evolutionary time, two Pax genes apparently substituted for one another in regulating a Dach gene could be based on recognition of a common Pax binding site upstream of the Dach gene.
Dach2 and Eya2, and Eya2 and Six1 act synergistically
In addition to Dach2 and Pax3, we identified Eya2 and Six1 as important regulators of myogenesis. We have shown that Pax3, Dach2, Eya2, and Six1 expression patterns overlap in the early dorsal somite and in limb muscle precursors. Both of these cell populations are composed of undifferentiated muscle precursor cells (Christ and Ordahl 1995), and neither population has begun to express markers of progressive muscle differentiation such as MyoD, Myf-5, Myogenin, or MHC (Christ and Ordahl 1995). Pax3, Dach2, Eya2, and Six1 are expressed in a manner consistent with their working cooperatively, possibly setting the stage for myogenic differentiation. However, once further myogenic differentiation occurs (e.g., after myotome formation), the genes display different expression patterns and may function with other partners and on other targets.
Pax3 can induce myogenesis in cultured somites (Maroto et al. 1997). However, misexpression of Dach2 on its own in somite cultures only rarely induced low level myogenic gene expression (data not shown). This result could mean that Dach2 is a weak muscle inducer or that induction of myogenic genes was an indirect effect of inducing Pax3, which in turn induced MyoD. In either case, the lower level of induction of myogenic genes by Dach2 relative to Pax3 parallels the different potencies of the Drosophila homologs dac and ey to induce ectopic eyes: ey has a greater ability to induce ectopic eye formation than dac (Shen and Mardon 1997).
The ability of dac to induce ectopic eyes is potentiated when dac is misexpressed in combination with eya (Chen et al. 1997), and the ability of eya to induce ectopic eyes is also potentiated by so (Pignoni et al. 1997). We have found that the vertebrate homologs of dac, eya, and so have similar abilities to regulate myogenesis in a synergystic manner. For instance, Dach2 and Eya2 synergistically regulate both Pax3 and myogenic gene expression, and indeed the proteins physically interact. However, because neither protein is capable of binding to DNA (Bonini et al. 1993; Mardon et al. 1994), this complex presumably interacts with a protein that binds to DNA. One possible candidate is Six1, which is present in explanted somites in the absence of any extrinsic factors (see Fig. 5A, lane 1). However, it has yet to be demonstrated that these three proteins are acting as a single protein complex, and they could alternatively have other partners.
Eya2 and Six1 also synergize to regulate the expression of target genes and physically interact. Interaction between Eya and Six proteins has also been observed in recent independent studies (Ohto et al. 1999). Although Six1 is normally present in somite cultures, endogenous levels are apparently not high enough to induce Pax3 or the myogenic genes when Eya2 is misexpressed alone. However, when Eya2 and Six1 are ectopically expressed together, levels of Six1 are considerably higher, allowing for induction of Pax3 and myogenic genes.
Dach2, Eya2, Six1 action in myogenesis
Dach2, Eya2, and Six1 function in complexes to regulate Pax3, MyoD, Myogenin, and MHC. However it is still not known whether any of these genes are direct transcriptional targets of these complexes. Recent findings have shown that Six1 and Six4 are able to bind upstream of the Myogenin promoter, thereby regulating Myogenin gene expression (Spitz et al. 1998). More recently, it has been shown that Six and Eya proteins act synergistically to regulate this promoter (Ohto et al. 1999). Thus, at least one myogenic bHLH transcription factor is a direct target of Six and Eya proteins and is, therefore, a potential target of various Eya–Six or Dach–Eya–Six transcriptional complexes. Future experiments will determine the precise roles these proteins play in regulating the expression of the downstream myogenic regulatory genes.
Materials and methods
Cloning of Dach2
A human retinal cDNA (IMAGE Consortium cDNA clone, ID 381801; Lennon et al. 1996) was identified as sharing homology to Drosophila dachshund by a TBLASTN screen of the Drosophila dachshund sequence against the dbEST database and was obtained from Research Genetics, Inc. (Huntsville, AL). A stage 12–15 embryonic chick cDNA library cloned in λZAPII was screened with a 600-bp SmaI–EcoRI fragment of 381801. Filters were hybridized in 20% formamide, 10% dextran sulfate, 2× SSC, and 1% SDS at 42°C overnight and washed in 2× SSC, 1% SDS at 52°C. Positive clones were sequenced and fell into two classes, one more closely related to the EST clone representing Dach1 (T. Heanue and C. Tabin, unpubl.) and a second representing Dach2. Several overlapping clones were used to construct a full-length Dach2 clone, Dach2-L. Dach2-L was found to encode an ∼1.8-kb open reading frame, 50 bp of 5′ UTR, and 1.1 kb of 3′ UTR. This sequence has been submitted to GenBank (accession no. AF198349). No upstream stops were identified 5′ to the putative ATG; however, the nucleotide sequence surrounding the ATG is found to be a strong context Kozak sequence: GCCatgG (Kozak 1996). Northern blot analysis was performed using standard methods and using stage 22 embryo total RNA and a 800-bp 3′ fragment of Dach2-L as a probe. This analysis revealed a Dach2 transcript size of ∼3 kb, further indicating that the complete coding region has been identified.
Fly genetics
Drosophila crosses were performed at 25°C on standard media. The Drosophila dac::Dach-2 fusion transgene encodes the first 31 amino acids of Drosophila Dac and the last 556 amino acids of Dach2 (named pUAS–DD31::CD556). This was constructed using a 700-bp EcoRI–SacII fragment from Drosophila dac cDNA p2-2 and a 1.9-kb SacII fragment from Dach2 cDNA pcd2c cloned into an EcoRI–SacII digested pUAST vector (Brand and Perrimon 1993). The 700-bp EcoRI–SacII fragment from Drosophila dac, which produces a truncated protein containing only the first 31 amino acids of Drosophila Dac, was used as a negative control (named pUAS–DD31). Flies were transformed using standard techniques (Spradling and Rubin 1982; Rubin and Spradling 1983). Five independent transformants for each construct were analyzed by driving expression using either dpp–GAL4 (Staehling-Hampton and Hoffmann 1994) or dac–GAL4 (to be described elsewhere). No ectopic expression phenotypes were observed for either construct when driven by dac–GAL4, and pUAS–DD31 had no phenotype when driven by dpp–GAL4. In contrast, dpp–GAL4 driven pUAS–DD31::CD556 results in leg truncations reminiscent of that caused by misexpression of full-length Drosophila dac (Chen et al. 1997). Rescue of dac null mutant animals was performed as follows: dac3, UAS–DD31::CD556/CyO, Kr-GFP;dpp-lacZ/+ flies were crossed to dac–GAL4/CyO, Kr-GFP;dpp-lacZ/+ flies and dac3, UAS–DD31::CD556/dac–GAL4;±dpp-lacZ animals were selected as non-GFP larvae or non-CyO adults. Similar experiments were performed with the truncated form of the Drosophila Dac protein alone except that the transgene was located on the X chromosome.
Scanning electron microscopy and immunohistochemistry
Samples were prepared for scanning electron microscopy as described previously (Kimmel et al. 1990). Imaginal discs were dissected and stained with anti-ELAV (Robinow and White 1991) as described previously (Mardon et al. 1994). dpp expression was detected using a β-galactosidase reporter construct specific for imaginal discs (Blackman et al. 1991).
Chick embryos
Fertilized White Leghorn chicken embryos were obtained from SPAFAS (Norwich, CT). Embryos were staged according to Hamburger and Hamilton (1951). Somites were staged according to established nomenclature (Christ and Ordahl 1995).
Whole-mount and section RNA and Ab in situ hybridization
Whole-mount in situ hybridization and section in situ hybridization with nonradioactive and [33P]UTP probes were performed as described previously (Riddle et al. 1993; Vortkamp et al. 1996; Bao and Cepko 1997). Probe templates were Dach2 (cd2c, SalI digest, T3 polymerase), Pax3 (CHPax3, BamHI, T3), Eya2 (cEya2, EcoRI, T3), Six1 (chLZ54/x, SacI, T3), MyoD (pCMDmyoD, HindIII, T7), and Pax1 (QP1, HindIII, T7). For double labeling experiments with Pax7, embryos processed by whole-mount in situ hybridization were paraffin-sectioned. Slides were washed with PBS, blocked with 5% goat serum/PBS for 1 hr, and then incubated in mouse monoclonal Pax7 antibody (Developmental Studies Hybridoma Bank), diluted 1:10, at 4°C overnight. After PBS washes, slides were incubated in Cy3 goat anti-mouse, diluted 1:200, for 1 hr at room temperature, and finally washed again in PBS.
Embryo surgery for barrier placement
Embryo surgeries were performed essentially as described (Dietrich et al. 1997). Fifteen-micron-thick cellophane barriers were inserted under the ectoderm overlapping the paraxial mesodermal tissues at the level of PSM or from somites number I–VI (Christ and Ordahl 1995) of stage 10–12 embryos. Embryos were incubated for an additional 24–36 hr, fixed in 4% paraformaldehyde, and analyzed by whole-mount RNA in situ hybridization. After photographing, the embryos were paraffin sectioned (10 μm thick) and rephotographed.
RCAS virus construction
Generation of viral constructs and production of high titer virus followed the protocols described previously (Logan and Tabin 1998). The Pax3 viral construct was described previously (Maroto et al. 1997). cDNAs encoding the entire open reading frames of chick Dach2, chick Eya2, and mouse Six1 were cloned in-frame with the initiator ATG of the pSLAX-13 shuttle vector, and transferred as ClaI fragments to both the RCAS(A) and RCAS(B) retroviral vectors. Retroviral titers ranged from 4 × 108 to 1 × 109 CFU/ml.
Explant culture
Embryonic tissues were isolated and cultured as described (Munsterberg et al. 1995). Coculture of paraxial tissues with various RCAS constructs was performed as described (Maroto et al. 1997). When RCAS type B envelope was used, 8 μg/ml of polybrene was added to the medium to increase infection. Medium (500 ml) was added to the collagen cultures after overnight incubation with 35 μl of medium plus virus. The cultures were incubated for 5 days and analyzed by RT–PCR.
RT–PCR analysis
RT–PCR analysis was performed essentially as described (Munsterberg et al. 1995; Maroto et al. 1997). After production of cDNA by reverse transcriptase, single PCR reactions were performed with appropriate primer pairs for the designated genes. After individual PCR reactions were run, radiolabeled PCR transcripts were visualized by gel electrophoresis and autoradiography. Each PCR cycle was 93°C for 30 sec, 60°C for 35 sec, and 72°C for 1 min. Pax3, Dach2, and Six1 were amplified in the presence of 5% formamide with an annealing temperature of 50°C for Dach2, 52°C for Pax3, and 55°C for Six1. Twenty-five cycles were used to assay GAPDH, and 30–33 cycles to assay other genes. The primers used for PCR amplification were as described (Munsterberg et al. 1995; Maroto et al. 1997) and as follows: Dach2, 5′-CGCCATTTCTTTTTGCTGAT and 3′-CGCCTGTTCCACTTGTTCTC (308 bp); Eya2, 5′-ACATAGAAGGCAACAGTAAAG and 3′-TGGGATGGCTGAAGGGCTGAT (497 bp); Six1, 5′-TTCGGCTTCACGCAGGAGCAG and 3′-CCTCCGCCGCCCGGTCCCGCT (500 bp). The specificity of the PCR reactions was verified for these primers by restriction mapping of the PCR products.
GST pull-down
GST pull-down interaction assays were performed essentially as described (Pearse et al. 1999). Full-length Dach2 and Eya2 were cloned into the bacterial expression vector pGEX-KG vector to fuse GST to the Dach2 and Eya2 proteins. Also, a 5′ fragment of Dach2 corresponding to the first 170 amino acids was cloned into pGEX-KG. Recombinant proteins were purified from induced cultures and bound to glutathione resin as described. Full-length 35S-labeled radioactive test proteins were generated using the TnT Rabbit Reticulocyte Lysate System (Promega) using T7 polymerase for the Eya2 template pSlax–Eya2AS and using T3 polymerase for the Six1 template pSlax–Six1. Proteins were analyzed on SDS-PAGE gels prior to performing interaction assays. Stringent conditions for interaction assays followed those used previously to test interactions between the homologous Drosophila proteins (Chen et al. 1997). 35S–Labeled Eya2 and Six1 proteins (50,000 TCA-precipitable cpm) were incubated with 50 μl of a 50:50 slurry of glutathione resin containing bound GST, GST::Dach2, GST::Dach2-N-term, or GST::Eya2 in binding buffer [20 mm HEPES–KOH at pH 7.7, 150 mm NaCl, 0.1% NP-40, 10% glycerol, and 1× Complete protease inhibitors (Boehringer)] for 2 hr at 4°C. Resins were washed four times in 1 ml of binding buffer before elution by boiling in loading buffer and loading onto SDS-PAGE gels. After running, staining, and fixing, the gels were treated with Enlightening (NEN Life Sciences) to enhance the radioactive signal. Radioactive test proteins were visualized by audioradiography and were detectable after 6 hr. Intensity of the bands was compared with a 10% input lane.
Yeast two-hybrid
The MATCHMAKER Gal4 two-hybrid system (Clontech Laboratories, Inc.) was used for yeast interaction assays. Full-length chick Eya2 and the Drosophila Eya interaction domain were cloned into the GAL4 activation domain vector (pACT2). The Six domain of mouse Six1 (from the fourth amino acid of the protein to the second amino acid of the homeodomain) and full-length Six3 were cloned into the GAL4 DNA-binding domain vector (pAS2-1). The Six domain has been shown to be the interaction domain of the Drosophila So protein (Pignoni et al. 1997). Small scale LiAc cotransformations of the plasmid DNAs into Y190 cells were performed as outlined in the Clonetech protocols. β-Galactosidase colony-lift filter assays were performed on double transformants as described and incubated at 30°C. Strong positives were visible after 30–60 min, whereas weaker positives showed staining after 3 hr.
Acknowledgments
We thank Nikki Davis, Richard Pearse, and Miguel Maroto for technical assistance and Malcolm Logan, Gabrielle Kardon, Richard Pearse, Kyle Vogan, and Pascal Maire, for critical comments on the manuscript. This work was supported by a grant from the NIH to C.J.T. Work by R.R. was supported by grants to A.B.L from the National Science Foundation and the NIH. Work by G.M. was supported by the National Eye Institute, the Baylor Mental Retardation Research Center, the Retina Research Foundation, and the Moran Foundation. G.O. was partially supported by NIH grants EY12162 and GM58462 and by the American Lebanese Syrian Associated Charities of St. Jude's Children's Research Hospital.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL tabin@rascal.med.harvard.edu; FAX (617) 432-7595.
References
- Bao ZZ, Cepko CL. The expression and function of Notch pathway genes in the developing rat eye. J Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes & Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991;111:657–666. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: Genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Bui QT, Gray-Board GL, Warrick JM. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- Borsani G, DeGrandi A, Ballabio A, Bulfone A, Bernard L, Banfi S, Gattuso C, Mariani M, Dixon M, Donnai D, Metcalfe K, Winter R, Robertson M, Axton R, Brown A, van Heyningen V, Hanson I. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum Mol Genet. 1999;8:11–23. doi: 10.1093/hmg/8.1.11. [DOI] [PubMed] [Google Scholar]
- Borycki AG, Li J, Jin F, Emerson CP, Epstein JA. Pax3 functions in cell survival and in pax7 regulation. Development. 1999;126:1665–1674. doi: 10.1242/dev.126.8.1665. [DOI] [PubMed] [Google Scholar]
- Boyer PL, Colmenares C, Stavnezer E, Hughes SH. Sequence and biological activity of chicken snoN cDNA clones. Oncogene. 1993;8:457–466. [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Caubit X, Thangarajah R, Theil T, Wirth J, Nothwang HG, Ruther U, Krauss S. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev Dyn. 1999;214:66–80. doi: 10.1002/(SICI)1097-0177(199901)214:1<66::AID-DVDY7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila [see comments] Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M. twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- Davis, R.J., W. Shen, T.A. Heanue, and G. Mardon. 1999. Mouse Dach, a homolog of Drosophila dachshund, is expressed in the developing retina, brain, and limbs. Dev. Genes Evol. (in press) [DOI] [PubMed]
- Denetclaw WF, Jr, Christ B, Ordahl CP. Location and growth of epaxial myotome precursor cells. Development. 1997;124:1601–1610. doi: 10.1242/dev.124.8.1601. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Lumsden A. Control of dorsoventral pattern in the chick paraxial mesoderm. Development. 1997;124:3895–3908. doi: 10.1242/dev.124.19.3895. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Ebensperger C, Wilting J, Brand-Saberi B, Mizutani Y, Christ B, Balling R, Koseki H. Pax-1, a regulator of sclerotome development is induced by notochord and floor plate signals in avian embryos. Anat Embryol (Berl) 1995;191:297–310. doi: 10.1007/BF00534682. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. cSix4, a member of the six gene family of transcription factors, is expressed during placode and somite development. Mech Dev. 1999;85:161–165. doi: 10.1016/s0925-4773(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Fan CM, Lee CS, Tessier-Lavigne M. A role for WNT proteins in induction of dermomyotome. Dev Biol. 1997;191:160–165. doi: 10.1006/dbio.1997.8713. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermamyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila [see comments] Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hammond KL, Hanson IM, Brown AG, Lettice LA, Hill RE. Mammalian and Drosophila dachshund genes are related to the Ski proto-oncogene and are expressed in eye and limb. Mech Dev. 1998;74:121–131. doi: 10.1016/s0925-4773(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Kimmel BE, Heberlein U, Rubin GM. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes & Dev. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Kozak M. Interpreting cDNA sequences: Some insights from studies on translation. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Lennon G, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. Consortium: An integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Logan M, Tabin C. Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses. Methods. 1998;14:407–420. doi: 10.1006/meth.1998.0595. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Mishima N, Tomarev S. Chicken Eyes absent 2 gene: Isolation and expression pattern during development. Int J Dev Biol. 1998;42:1109–1115. [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes & Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Nagase T, Nomura N, Ishii S. Complex formation between proteins encoded by the ski gene family. J Biol Chem. 1993;268:13710–13716. [PubMed] [Google Scholar]
- Nomura T, Khan MM, Kaul SC, Dong HD, Wadhwa R, Colmenares C, Kohno I, Ishii S. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes & Dev. 1999;13:412–423. doi: 10.1101/gad.13.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995a;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995b;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Ott MO, Bober E, Lyons G, Arnold H, Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991;111:1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Pearse RV, II, Collier LS, Scott MP, Tabin CJ. Vertebrate homologs of Drosophila suppressor of fused interact with the Gli family of transcriptional regulators. Dev Biol. 1999;212:323–336. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development [published erratum appears in Cell 1998 Feb 20, 92: (4) following 585] Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Emerson CP., Jr Sequential activation of three myogenic regulatory genes during somite morphogenesis in quail embryos. Dev Biol. 1992;151:67–79. doi: 10.1016/0012-1606(92)90214-2. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans [see comments] Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes & Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983;11:6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mardon G. Ectopic eye development in Drosophila induced by directed dachshund expression. Development. 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci. 1998;95:14220–14225. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann FM. Ectopic decapentaplegic in the Drosophila midgut alters the expression of five homeotic genes, dpp, and wingless, causing specific morphological defects. Dev Biol. 1994;164:502–512. doi: 10.1006/dbio.1994.1219. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax- 3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- Toy J, Yang JM, Leppert GS, Sundin OH. The optx2 homeobox gene is expressed in early precursors of the eye and activates retina-specific genes. Proc Natl Acad Sci. 1998;95:10643–10648. doi: 10.1073/pnas.95.18.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein [see comments] Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: The morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci. 1997;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Gotoh Y, Tamura K, Tanaka M, Kawakami A, Ide H, Kuroiwa A. Coordinated expression of Hoxa-11 and Hoxa-13 during limb muscle patterning. Development. 1998;125:1325–1335. doi: 10.1242/dev.125.7.1325. [DOI] [PubMed] [Google Scholar]
- Zheng G, Teumer J, Colmenares C, Richmond C, Stavnezer E. Identification of a core functional and structural domain of the v-Ski oncoprotein responsible for both transformation and myogenesis. Oncogene. 1997b;15:459–471. doi: 10.1038/sj.onc.1201205. [DOI] [PubMed] [Google Scholar]
- Zheng G, Blumenthal KM, Ji Y, Shardy DL, Cohen SB, Stavnezer E. High affinity dimerization by Ski involves parallel pairing of a novel bipartite alpha-helical domain. J Biol Chem. 1997a;272:31855–31864. doi: 10.1074/jbc.272.50.31855. [DOI] [PubMed] [Google Scholar]