Abstract
Programmed cell death (PCD) is an integral part of plant development and of responses to abiotic stress or pathogens. Although the morphology of plant PCD is, in some cases, well characterised and molecular mechanisms controlling plant PCD are beginning to emerge, there is still confusion about the classification of PCD in plants. Here we suggest a classification based on morphological criteria. According to this classification, the use of the term ‘apoptosis' is not justified in plants, but at least two classes of PCD can be distinguished: vacuolar cell death and necrosis. During vacuolar cell death, the cell contents are removed by a combination of autophagy-like process and release of hydrolases from collapsed lytic vacuoles. Necrosis is characterised by early rupture of the plasma membrane, shrinkage of the protoplast and absence of vacuolar cell death features. Vacuolar cell death is common during tissue and organ formation and elimination, whereas necrosis is typically found under abiotic stress. Some examples of plant PCD cannot be ascribed to either major class and are therefore classified as separate modalities. These are PCD associated with the hypersensitive response to biotrophic pathogens, which can express features of both necrosis and vacuolar cell death, PCD in starchy cereal endosperm and during self-incompatibility. The present classification is not static, but will be subject to further revision, especially when specific biochemical pathways are better defined.
Keywords: apoptosis, autophagy, cell wall, hypersensitive response, necrosis, vacuolar cell death
Research on plant cell death has grown considerably in the past few years, owing to the importance of cell death for plant development and defense. Just as animal cells engage several mechanisms leading to death, the road to cell demise in plants can also vary. The long evolutionary distance and distinct cellular architecture between the two kingdoms may account for the differences between the mechanisms of plant and animal cell death. It is therefore appropriate to assess the relevance of animal cell death nomenclature1 to plants. At present, there is confusion in cell death terminology in plant biology, which drives our attempt to formulate a more logical classification. Although our molecular understanding of plant cell death regulation and execution is insufficient to create definitive classifications based on precise biochemical pathways, it is possible to begin classifying plant cell death scenarios based on morphological criteria, as was initially the case in animal cell death research2, 3 and is still used for the classification of cell death in animal science.1
This document attempts to provide a classification of plant cell death. We urge authors, reviewers and editors to follow this classification to facilitate communication between scientists and accelerate research in this field.
Absence of Apoptosis in Plants
Apoptosis is one of the three major types of cell death found in animals. Compared with the other two – autophagic cell death and necrosis – apoptosis is much better understood, both cytologically and biochemically.1, 4 Apoptosis is accompanied by rounding up of the cell, reduction of cellular volume, chromatin condensation, nuclear segmentation and very little ultrastructural modification of cytoplasmic organelles. Its hallmark is blebbing of the plasma membrane (which maintains its integrity until the final stages of apoptosis), followed by fragmentation of the cell into smaller parcels called apoptotic bodies. Finally, the apoptotic bodies are engulfed by phagocytes and degraded by lysosomal enzymes. This is critical to prevent subsequent induction of inflammation due to leakage of dead cell contents. The term ‘apoptosis' should be applied exclusively to cell death that manifests these morphological features. Although apoptosis is often associated with activation of caspases and oligonucleosomal fragmentation of DNA, these processes can also take place during non-apoptotic cell death, and are thus insufficient criteria for assignment.1
Plant cells do not exhibit ‘classic' apoptosis for the following reasons. First, rigid cell walls preclude the necessity for breakdown of the plant cells into apoptotic bodies. Second, there are no phagocytic cells. A considerable number of articles describing ‘plant apoptosis' or ‘apoptotic-like programmed cell death (PCD)' have nevertheless been published. Critical analysis of this literature reveals three major points that indicate misuse of the term ‘apoptosis'. First, chromatin condensation and DNA fragmentation are often quoted as apoptotic features. However, neither is specific to apoptosis, because they can also be observed during necrosis and autophagic death.5, 6, 7 Second, stress treatments often induce shrinkage of the plant protoplast, but not of the cell itself, which can be morphologically reminiscent of apoptotic cell shrinkage. However, animal cells that shrink during apoptosis maintain their plasma membrane integrity to form apoptotic bodies,8 whereas plant protoplasts that shrink in response to stress usually have damaged plasma membranes and do not fragment further into discrete bodies.9 Third, increased caspase-like proteolytic activities (in most cases unlinked to specific proteases) in dying plant cells have been used as an argument for the existence of plant apoptosis. This is an insufficient criterion because activation of caspases per se does not always lead to apoptosis in animal cells.7 Furthermore, the activation of plant proteases that possess caspase-like activity has not been shown to lead to apoptotic morphology.10, 11, 12
Definition of ‘Vacuolar' Plant Cell Death
Plants have elaborate vacuolar systems that, in contrast to animal lysosomes, can occupy most of the plant cell volume.13 Similar to the roles of lysosomes in animals, plants also use lytic vacuoles to recycle parts of their cells during normal development and during nutritional stress.14 These lytic vacuoles acquire an important function in one major class of plant cell death, which we recommend be termed ‘vacuolar cell death'.15
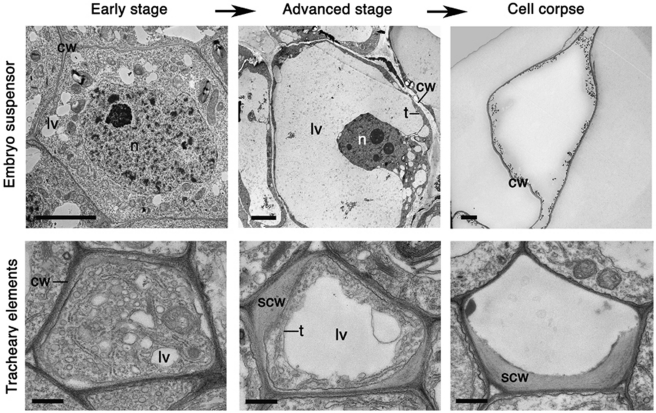
Vacuolar cell death is often manifested by a gradual decrease in the volume of the cytoplasm and a concomitant increase in the volume occupied by lytic vacuoles (Figure 1). Engulfment of the cytoplasm by lytic vacuoles with subsequent cargo degradation is a major mechanism of cell dismantling during vacuolar cell death. Electron micrographs often show invaginations in the vacuolar membrane (tonoplast) and fusion of vesicles with the vacuole, followed by uptake and degradation of portions of the cytoplasm in the vacuolar lumen. This process resembles micro- or macro-autophagy.16, 17, 18, 19, 20 The final step in the execution of vacuolar cell death is rupture of the tonoplast, and a massive release of vacuolar hydrolases. These rapidly destroy the entire protoplast or in some cases even the entire cell including the cell wall. Other morphological events during vacuolar cell death include formation of actin cables, nuclear envelope disassembly and, in some examples, nuclear segmentation. The remaining mitochondria and other organelles, as well as the plasma membrane, remain morphologically intact until rupture of the tonoplast (Table 1; Figure 1). A robust approach to diagnose vacuolar cell death would combine electron microscopy (EM) with the analysis of autophagic activity, requirement for vacuolar processing enzymes (VPE) and cytoskeletal changes (Table 2).
Figure 1.
Vacuolar cell death. Electron micrographs of programmed cell death (PCD) in the Norway spruce embryo-suspensor cells17 (top panels) and Arabidopsis tracheary elements20 (bottom panels). cw, cell wall; lv, lytic vacuole; n, nucleus; scw, secondary cell wall; t, tonoplast. Scale bars, 5 μm (embryo suspensor) and 500 nm (tracheary elements). Pictures of Norway spruce embryo-suspensor cells were kindly provided by Dr. Lada Filonova and Dr. Elena Minina (Swedish University of Agricultural Sciences, Uppsala, Sweden) and those of Arabidopsis tracheary elements by Dr. Utku Avci (University of Georgia, Complex Carbohydrate Research Center, Athens, GA, USA)
Table 1. Morphological features of the two major classes of plant cell death.
| Class of cell death | Features | Notes |
|---|---|---|
| Vacuolar | Accumulation of autophagosomes and small lytic vacuoles Formation of actin cables Nuclear envelope disassembly Normal turgor and intact organelles until rupture of the tonoplasta Formation of large lytic vacuoles Rupture of tonoplast Empty-walled cell corpsea | The extent of autophagic cell dismantling before the rupture of tonoplast can vary depending on the system |
| Necrosis | Swelling of mitochondria Early rupture of plasma membrane Shrinkage of the protoplast Cell corpse remains largely unprocessed | This type of cell death is distinguished from accidental or injury-induced cellular demise due to physical destruction of cellular integrity. An important feature is the required participation of cellular components |
Note: HR cell death often combines all above features of necrosis and most features of vacuolar cell death.
Not found during HR
Table 2. Biochemical and cell biological features of the two major classes of plant cell death and their detection methods.
| Class of cell death | Features | Detection methods |
|---|---|---|
| Vacuolar | Autophagic activity | MDC and lysotracker staining Atg8-GFP expression Immunoblotting detection of Atg8 lipidation |
| Acidification of vacuoles | Lysotracker staining | |
| Reorganisation of cytoskeleton | Staining of F-actin by specific dyes or antibody | |
| Activation of VPE | Colorimetric/fluorogenic substrate-based assays in live cells and cell lysates | |
| Necrosis | MMP | JC-1 or TMRE staining of mitochondria IF microscopy or immunoblotting detection of cytochrome c release |
| Respiratory decline | Oxygen consumption measurement (polarography) | |
| Drop in ATP level | Luminometric assay of intracellular ATP concentration | |
| ROS and RNS accumulation | In situ detection and spectrofluorometric measurement using ROS- and/or RNS-sensitive probes |
Note: All the above features of vacuolar cell death and necrosis can be found during HR cell death
Execution of vacuolar cell death may be a slow process that can take several days until the rupture of the tonoplast that accomplishes protoplast clearance.18, 19, 20, 21 Depending on the system, the cell wall can be largely degraded, as for example during aerenchyma formation, leaf perforations in the lace plant and petal senescence22, 23, 24 or can remain intact, for example, during xylem differentiation in vascular plants or leaf remodelling in Monstera (Figure 1).24, 25, 26 Examples of vacuolar cell death are found during embryo, organ and tissue morphogenesis and senescence, and include, in addition to those mentioned above, the formation of embryo-suspensor, pollen, ovary, ducts and laticifers.19
Knockout of ATG genes was shown to accelerate Arabidopsis leaf senescence,27, 28 and ATG5 has recently been found to be required for vacuolar cell death of Arabidopsis tracheary elements.29 More extensive work is still needed to determine whether or not ATG-dependent autophagic pathways are required for the execution of vacuolar cell death.
Definition of ‘Necrotic' Plant Cell Death
Necrosis of animal cells is defined morphologically by the lack of apoptotic or autophagic features, and positively by the frequent occurrence of an initial gain in cell volume, swelling of various organelles, early rupture of the plasma membrane and loss of intracellular content.1, 30 Although it is no longer considered to be an unprogrammed process, necrosis remains poorly characterised at the biochemical and genetic levels, so there are as yet no molecular markers for it. In animal systems, necrosis is often preceded by an increase in cytosolic calcium ion concentration ([Ca2+]cyt), lipid degradation and activation of calpain family proteases. Mitochondria and lysosomes have been implicated in the downstream events. Mitochondrial changes include uncoupling of respiration, the production of reactive oxygen species (ROS) and nitrogen species (RNS), a drop in ATP level and mitochondrial membrane permeabilisation (MMP). Lysosomal events include ROS production and permeabilisation of the lysosomal membrane causing release of active cathepsin proteases to the cytosol.1, 31
Cell death with many of the above characteristics occurs widely in plants. It is induced by a range of abiotic stresses and by successful recognition of a pathogen during the hypersensitive response (HR). It is also found in the cells challenged by necrotrophic pathogens (they are called necrotrophic because they kill host cells to derive nutrients). However, in the case of the HR, necrotic features are often accompanied by the features of vacuolar cell death (see below).
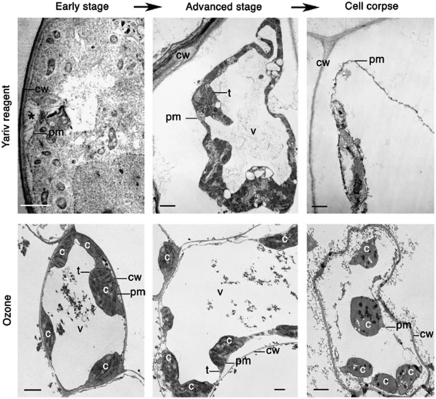
Cytological hallmarks that distinguish plant necrosis from vacuolar cell death include mitochondrial swelling, the absence of the growing lytic vacuoles and an early rupture of the plasma membrane leading to shrinkage of the protoplast (Table 1; Figure 2).9, 19, 32, 33 Because there are no lytic vacuoles that clear the cytoplasm during necrosis, the corpses of necrotic cells remain largely unprocessed. A shrunken protoplast is one of the most easily detected features of plant necrotic cells (Figure 2). Time-course analysis of animal necrosis has revealed that the initial gain in cell volume (swelling) as a result of ion pump failure is followed by cell shrinkage.30 Plant cells have a cell wall that should counteract swelling of the protoplast at early stages of necrosis, which would therefore escape detection.32 However, an early loss of plasma membrane integrity can result in readily detectable protoplast shrinkage.
Figure 2.
Necrotic cell death. Electron micrographs of Yariv reagent-induced death in the Arabidopsis cell culture53 (top panels) and ozone-induced death of the palisade cells in bean plants54 (bottom panels). Asterisks denote detachment of plasma membrane form the cell wall at early stage of cell death. c, chloroplast; cw, cell wall; pm, plasma membrane; t, tonoplast; v, vacuole. Scale bars, 2 μm. Pictures of Yariv reagent-induced cell death were kindly provided by Dr. Allan Showalter (Ohio University, Athens, OH, USA) and reproduced with permission from Gao and Showalter.53 Pictures of ozone-induced cell death were kindly provided by Dr. Franco Faoro (University of Milan, Milan, Italy)
Necrosis is typically an acute cell death response that develops rapidly and takes from several minutes (toxic treatments) to up to a day, as seen in the HR. A recommended approach to diagnose plant necrosis is by combining EM analysis with the assessment of mitochondrial dysfunction (MMP and decreased levels of both oxygen consumption and ATP production) and both ROS and RNS accumulation (Table 2).
Mixed and Atypical Modalities of Plant Cell Death
HR with some features of vacuolar cell death
It has been long known that a programmed, localised cell death connected with the HR occurs at the site of successful recognition of biotrophic pathogens. Whether this cell death is the cause of restricted pathogen replication or a consequence thereof has been debated for decades.34 The nature of the HR cell death with respect to its morphology has also been debated.35, 36, 37, 38, 39 Most recently, HR cell death and pathogen replication restriction have been de-coupled by manipulation of metacaspase expression, showing that, for at least the pathogens tested, the elimination of the host cell death response does not lead to pathogen proliferation.40 HR cell death usually exhibits all characteristics of plant cell necrosis (Tables 1 and 2). However, HR cell death is at the same time often accompanied by the growth of lytic vacuoles and tonoplast rupture, which can require VPE from the vacuole in some cases.10, 41 In addition, increased autophagic activity before HR cell death is apparently controlled by ATG genes,42 although the precise role of autophagy may differ depending on the particular HR cell death pathway being studied.43, 44 Although autolytic components appear to be important for the HR cell death in some cases that have been studied, collapse of lytic vacuoles during the HR does not lead to complete clearance of the protoplast, as it does in vacuolar PCD.39, 45
When discussing the relationship of the HR cell death to its correlated cytological features, and ultimately to the restriction of pathogen success, it is important to consider where the pathogens proliferate: for example, bacterial pathogens proliferate in the apoplast, outside the cell, while viruses proliferate within cells. Thus, vacuolar collapse can be effective to restrict viral pathogens,10 while discharge of defense proteins into the apoplast, accompanied by fusion of the tonoplast and plasma membrane, slows bacterial pathogens outside the cells.11
Shrunken protoplast and intact plasma membrane during victorin-induced cell death
A particular cell death, evoked by the fungal toxin victorin, is important because it has evolved to use the host HR as a means to kill cells, which are then ‘digested' by the necrotrophic pathogen. Furthermore, similar to classic pathogen-induced HR, victorin sensitivity is dependent on an NB-LRR immune receptor.46 Although victorin-induced cell death in oat plants exhibits hallmarks of necrosis such as protoplast shrinkage and MMP, the shrunken protoplast is surrounded by an intact plasma membrane and the tonoplast retains its integrity.47 This suggests that initiation of the HR-related cell death can sometimes occur without the loss of membrane integrity.48
Mixture of vacuolar and necrotic hallmarks during self-incompatibility response
During self-incompatibility (SI) response in Papaver, an incompatible pollen tube is stopped by interactions with the pistil S-determinant that trigger a network of signalling events that converge to mediate PCD.49 SI cell death exhibits some characteristics of vacuolar cell death, including alterations to the actin cytoskeleton, organelle engulfment and loss of vacuolar integrity. SI also has features of necrosis including swelling of the mitochondria and increase in [Ca2+]cyt.49, 50
A long time gap between cell death and corpse processing in cereal starchy endosperm
The cereal endosperm consists of the starchy endosperm surrounded by the aleurone cell layer. Cells of the starchy endosperm accumulate storage reserves and die during seed maturation, but their corpses remain unprocessed until germination. Upon seed germination, aleurone cells secrete hydrolytic enzymes that break down and mobilise the reserves accumulated in the dead starchy endosperm.51, 52
Recommendations to Authors, Reviewers and Editors of Scientific Journals
Arbitrary and sometimes contradictory usage of terminology has been a problem in the field of plant cell death research. Here we have grouped together morphological characteristics that distinguish two major classes of cell death occurring in plants (Table 1). On the basis of this simplistic grouping, we suggest that terms ‘vacuolar cell death' and ‘necrotic cell death' (or ‘necrosis') are used when referring to corresponding classes of cell death in plants. Because the HR cell death with autolytic features, victorin-induced cell death, and both starchy endosperm and SI cell death do not neatly fall into these two categories, we suggest that they are left as separate cell death modalities. The present classification is of course not static, but will be subjected to further revisions, especially when specific biochemical pathways and molecular identity of mediators for plant PCD are better defined.
We recommend that plant cell death researchers abandon terms such as ‘apoptosis' or ‘apoptotic-like'. We think such terminology is incorrect and misleading, because the features often cited are also found in other types of PCD, whereas the bona fide cytological characteristics of apoptosis (formation of apoptotic bodies and phagocytosis) are absent in plants.
Adequate choices of analytical methods are required to correctly diagnose a type of plant cell death (Table 2). Being a classic, cytological method, EM analysis provides excellent descriptive data on the changes in the dying cell for the initial classification of the particular morphotype. We encourage the use of EM in showing the temporal details of the cell death under study, such as the structure of organelles, formation of autophagosome-like structures, nuclear events and early detachment of plasma membrane from the cell wall (beginning of protoplast shrinkage).
We would furthermore advise that experiments with protoplasts are not used in isolation, but are supported by tests having a direct relationship to the physiologically relevant cell death in the model system.
Several of the recommendations formulated in the classification of animal cell death1 are also relevant to studies of plant cell death. For example, the term ‘dead cell' should only be used for cells that are shown to be dead by specific staining, such as fluorescein diacetate or Evan's blue. We thus encourage the use of quantification of cell death along with the necessary statistical treatments to show significance for the reported data.
Conclusion
We recognise two major classes of cell death occurring in plant biology: (i) vacuolar cell death and (ii) necrotic cell death. Vacuolar cell death occurs during plant tissue and organ formation and elimination, although necrosis is typically found under abiotic stress, some forms of the HR-related cell death and cell death induced by necrotrophic pathogens. A few examples of cell death cannot be ascribed to either major class and therefore classified as separate modalities. This category includes HR cell death with autolytic features and victorin-induced cell death, as well as cell death occurring in starchy cereal endosperm and during SI response. Further studies using tools of genetics, biochemistry and cell biology are required to understand molecular mechanisms underlying variability of plant cell death morphology.
Glossary
- GFP
green fluorescent protein
- EM
electron microscopy
- IF microscopy
immunofluorescent microscopy
- HR
hypersensitive response
- JC-1
JC-1 mitochondrial membrane potential detection kit based on 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- MDC
monodansylcadaverine
- MMP
mitochondrial membrane permeabilisation
- PCD
programmed cell death
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SI
self-incompatibility
- TMRE
tetramethyl-rhodamine ethyl ester
- VPE
vacuolar processing enzyme
The authors declare no conflict of interest.
Footnotes
Edited by V De Laurenzi
References
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death. Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PGH. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–206. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jäättelä M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez R, Sancho-Martinez SM, Novoa JML, Lopez-Hernandez FJ. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 2010;17:1665–1671. doi: 10.1038/cdd.2010.96. [DOI] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2005;44:321–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, et al. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science. 2004;305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, et al. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 2009;23:2496–2506. doi: 10.1101/gad.1825209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichkova NV, Shaw J, Galiullina RA, Drury GE, Tuzhikov AI, Kim SH, et al. Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO J. 2010;29:1149–1161. doi: 10.1038/emboj.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntz K. Protein dynamics and proteolysis in plant vacuoles. J Exp Bot. 2007;58:2391–2407. doi: 10.1093/jxb/erm089. [DOI] [PubMed] [Google Scholar]
- Jones AM. Programmed cell death in development and defense. Plant Physiol. 2001;125:95–97. doi: 10.1104/pp.125.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP. Programmed cell death during plant growth and development. Cell Death Differ. 1997;4:649–661. doi: 10.1038/sj.cdd.4400297. [DOI] [PubMed] [Google Scholar]
- Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S. Two waves of programmed cell death occur during formation of development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci. 2000;113:4399–4411. doi: 10.1242/jcs.113.24.4399. [DOI] [PubMed] [Google Scholar]
- Bozhkov PV, Filonova LH, Suarez MF. Programmed cell death in plant embryogenesis. Curr Top Dev Biol. 2005;67:135–179. doi: 10.1016/S0070-2153(05)67004-4. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Avci U, Petzold HE, Ismail IO, Beers EP, Haigler CH. Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J. 2008;56:303–315. doi: 10.1111/j.1365-313X.2008.03592.x. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Lonsdale JE, Fath A, Jones RL. Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell. 1999;11:1033–1046. doi: 10.1105/tpc.11.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Regulation of cell death in flower petals. Plant Mol Biol. 2000;44:303–318. doi: 10.1023/a:1026540524990. [DOI] [PubMed] [Google Scholar]
- Gunawardena AHLAN. Programmed cell death and tissue remodeling in plants. J Exp Bot. 2008;59:445–451. doi: 10.1093/jxb/erm189. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:299–345. doi: 10.1146/annurev.arplant.47.1.299. [DOI] [PubMed] [Google Scholar]
- Courtois-Moreau CL, Pesquet E, Sjödin A, Muñiz L, Bollhöner B, Kaneda M, et al. A unique program for cell death in xylem fibers of Populus stem. Plant J. 2009;58:260–274. doi: 10.1111/j.1365-313X.2008.03777.x. [DOI] [PubMed] [Google Scholar]
- Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J Biol Chem. 2002;277:33105–33114. doi: 10.1074/jbc.M204630200. [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, et al. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 2002;129:1181–1193. doi: 10.1104/pp.011024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SI, Cho HJ, Jung JH, Yoshimoto K, Park OK. 2010. The RabGTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in Arabidopsis. Plant J. 2010;64:151–164. doi: 10.1111/j.1365-313X.2010.04315.x. [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. Does the plant mitochondrion integrate cellular stress and regulate programmed cell death. Trends Plant Sci. 2000;5:225–230. doi: 10.1016/s1360-1385(00)01605-8. [DOI] [PubMed] [Google Scholar]
- Scott I, Logan DC. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 2008;177:90–101. doi: 10.1111/j.1469-8137.2007.02255.x. [DOI] [PubMed] [Google Scholar]
- Kiraly Z, Barna B, Ersek T. Hypersensitivity as a consequence, not cause, of plant resistance to infection. Nature. 1972;239:456–458. [Google Scholar]
- Jones AM, Dangl JL. Logjam at the Styx: programmed cell death in plants. Trends Plant Sci. 1996;1:114–119. [Google Scholar]
- Greenberg JT. Programmed cell death in plant–pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- Richberg MH, Aviv DH, Dangl JL. Dead cells do tell tales. Curr Opin Plant Biol. 1998;1:480–485. doi: 10.1016/s1369-5266(98)80039-3. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know. J Exp Bot. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- Coll NS, Vercammen D, Smidler A, Clover C, van Breusegem F, Dangl JL, et al. Arabidopsis type I metacaspases control cell death. Science. 2010;330:1393–1397. doi: 10.1126/science.1194980. [DOI] [PubMed] [Google Scholar]
- Rojo E, Martin R, Carter C, Zouhar J, Pan S, Plotnikova J, et al. VPEγ exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol. 2004;14:1897–1906. doi: 10.1016/j.cub.2004.09.056. [DOI] [PubMed] [Google Scholar]
- Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NHT, Mattson O, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137:773–783.193. doi: 10.1016/j.cell.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP, McDowell JM. Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol. 2001;4:561–567. doi: 10.1016/s1369-5266(00)00216-8. [DOI] [PubMed] [Google Scholar]
- Lorang J, Cuesta-Marcos A, Hayes PM, Wolpert TJ. Identification and mapping of adult-onset sensitivity to victorin in barley. Mol Breeding. 2010;26:545–550. [Google Scholar]
- Curtis MJ, Wolpert TJ. The victorin induced mitochondrial permeability transition precedes cell shrinkage and biochemical markers of cell death and shrinkage occurs without loss of membrane integrity. Plant J. 2004;38:244–259. doi: 10.1111/j.1365-313X.2004.02040.x. [DOI] [PubMed] [Google Scholar]
- Krzymowska M, Konopka-Postupolska D, Sobczak M, Macioszek V, Ellis BE, Hennig J. Infection of tobacco with different Pseudomonas syringae pathovars leads to distinct morphotypes of programmed cell death. Plant J. 2007;50:253–264. doi: 10.1111/j.1365-313X.2007.03046.x. [DOI] [PubMed] [Google Scholar]
- Bosch M, Franklin-Tong VE. Self-incompatibility in Papaver: signalling to trigger PCD in incompatible pollen. J Exp Bot. 2008;59:481–490. doi: 10.1093/jxb/erm195. [DOI] [PubMed] [Google Scholar]
- Geitmann A, Franklin-Tong VE, Emons AC. The self-incompatibility response in Papaver rhoeas pollen causes early and striking alterations to organelles. Cell Death Differ. 2004;11:812–822. doi: 10.1038/sj.cdd.4401424. [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R. Programmed cell death in cereal aleurone. Plant Mol Biol. 2000;44:255–266. doi: 10.1023/a:1026584207243. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. Programmed cell death during endosperm development. Plant Mol Biol. 2000;44:283–301. doi: 10.1023/a:1026588408152. [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM. Yariv reagent induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999;19:321–331. doi: 10.1046/j.1365-313x.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- Faoro F, Iriti M. Plant cell death and cellular alterations induced by ozone: key studies in Mediterranean conditions. Environ Pollut. 2009;157:1470–1477. doi: 10.1016/j.envpol.2008.09.026. [DOI] [PubMed] [Google Scholar]