In response to antigen stimulation, PKCθ translocates to the T cell plasma membrane, becoming highly focused at the immunological synapse (IS). cis-Acting sequences that regulate IS retention are not known. It is shown that a catalytically competent PKCθ kinase domain is essential for IS retention but not for membrane translocation.
Abstract
Protein kinase Cθ (PKCθ) is a serine/threonine kinase that plays an essential role in antigen-regulated responses of T lymphocytes. Upon antigen stimulation, PKCθ is rapidly recruited to the immunological synapse (IS), the region of contact between the T cell and antigen-presenting cell. This behavior is unique among T cell PKC isoforms. To define domains of PKCθ required for retention at the IS, we generated deletion and point mutants of PKCθ. We used quantitative imaging analysis to assess IS retention of PKCθ mutants in antigen-stimulated T cell clones. Deletion of the kinase domain or site-directed mutation of a subset of known PKCθ phosphorylation sites abrogated or significantly reduced IS retention, respectively. IS retention did not correlate with phosphorylation of specific PKCθ residues but rather with kinase function. Thus PKCθ catalytic competence is essential for stable IS retention.
INTRODUCTION
Protein kinase C (PKC) enzymes comprise a family of serine/threonine kinases that regulate a broad array of cellular processes (Spitaler and Cantrell, 2004). Although T lymphocytes express multiple PKC enzymes, considerable evidence indicates that PKCθ is the most important isoform for transmission of activating signals from the T cell receptor (TCR) to nuclear targets. Expression of PKCθ is largely restricted to T lymphocytes, platelets, and skeletal muscle (Osada et al., 1992; Baier et al., 1993; Chang et al., 1993). PKCθ−/− mice display a selective T cell activation defect at the level of activator protein-1, nuclear factor-κB, and interleukin-2 induction, resulting in highly impaired T cell activation and proliferation (Sun et al., 2000), with the CD4+ T cell subset being more severely affected than CD8+ T cells (Kingeter and Schaefer, 2008).
It is intriguing that PKCθ is unique among T cell PKC isoforms, exhibiting a rapid, dramatic, and long-lasting membrane enrichment beneath the immunological synapse (IS)—the region of cell–cell contact between the responding T cell and the antigen-presenting cell (APC; Monks et al., 1997, 1998; Schaefer et al., 2004). Thus PKCθ is recruited to and retained at the membrane site of ligand engagement by the TCR and other transmembrane signaling molecules. The functional significance of this enrichment beneath the activated TCR is under debate, but data suggest that this localization pattern may control PKCθ activity and/or access to downstream targets (Bi et al., 2001) and contribute substantially to CD28-mediated costimulation (Yokosuka et al., 2008). Other recent data showed that PKCθ controls IS stability, suggesting that IS localization may directly regulate the structure of the IS and signal transmission from the IS (Sims et al., 2007).
Recent studies showed that initial membrane recruitment of PKCθ occurs at CD28 microclusters (Tseng et al., 2008; Yokosuka et al., 2008). Other studies suggested that lipid rafts, PI-3-kinase, and the adaptor molecule vav (Bi et al., 2001; Villalba et al., 2002) are also important determinants of PKCθ membrane recruitment. Similarly, phosphorylation of PKCθ residues Tyr-90 and Thr-219 was reported to contribute to PKCθ membrane translocation (Thuille et al., 2005; Melowic et al., 2007). Thus several mechanisms that regulate PKCθ membrane localization have been defined. However, as PKCθ is recruited to the plasma membrane in the same manner as other T cell PKCs (i.e., by diacylglycerol production), there is no obvious mechanism accounting for isoform-specific retention of PKCθ at the IS.
Of interest, although CD28 is essential for concentration of PKCθ within the central supramolecular activation cluster (c-SMAC) of the IS, CD28 is not required for IS enrichment per se (Huang et al., 2002). Indeed, a recent study suggested that membrane localization and stable IS retention of PKCθ are independently regulated. Specifically, when Jurkat T cells were stimulated with anti-CD3/anti-CD28 microspheres (Carrasco and Merida, 2004), recombinant constructs containing the PKCθ DAG-binding region initially translocated to the microsphere-proximal region of the plasma membrane but then rapidly dispersed around the entire plasma membrane. By contrast, full-length PKCθ was stably retained at the site of microsphere contact throughout the course of stimulation. These data thus suggest that functional elements outside of the C1 domains may be necessary for retention of the full PKCθ enzyme at the site of TCR engagement.
To define PKCθ functional domains that are required for retention at the IS, we used retroviral infection of an antigen-responsive T cell clone with green fluorescent protein (GFP)– or yellow fluorescent protein (YFP)–tagged mutants of PKCθ. We then stimulated these clones using natural TCR ligands in a biologically relevant context (i.e., cognate peptide presented by living antigen-presenting cells). Finally, quantitative imaging was used to assess the efficiency of PKCθ retention in many individual antigen-stimulated T cells, allowing mutants to be statistically compared with wild-type (WT) PKCθ.
RESULTS
The PKCθ C-terminal region is essential for retention at the IS
PKCθ is a member of the “novel” subclass of PKC enzymes having an N-terminal, C2-like domain that cannot bind calcium, followed by two diacylglycerol-binding C1 domains. The C-terminal portion of the enzyme contains the kinase domain (KD), turn motif, and hydrophobic motif (Supplemental Figure S1A). Previous work reported that the isolated PKCθ C1 domains fail to be retained at the IS following stimulation with anti-CD3/anti-CD28 microspheres (Carrasco and Merida, 2004). To confirm that this result could be generalized to T cells stimulated with antigen-loaded APCs, we infected D10 T cells with retroviruses encoding full-length PKCθ (WT) and the PKCθ C1a and C1b domains (C1) fused to GFP or YFP, respectively. In addition, to allow discrimination between possible contributions of the C2 domain and the KD to IS retention, we made a third D10 cell line that expressed the PKCθ N-terminal region, including both the C2 and C1 domains, fused to YFP (C2 + C1) (Supplemental Figure S1A). The resulting cell lines were stimulated with CH12 B cells with no specific antigen (No Ag) or with conalbumin-loaded CH12 cells (+Antigen). D10/CH12 conjugates were fixed at 30-min poststimulation and imaged. Note that for these PKCθ constructs, as well as the other PKCθ mutants used in this study, expression levels were modest, with the levels of fluorescent protein-tagged variants ranging from 0.78 to 15.8 times the levels of endogenous PKCθ (Supplemental Figure S1, B and C).
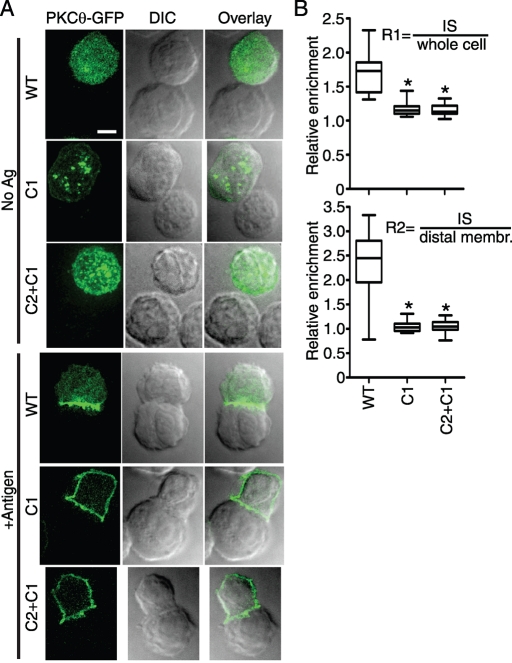
As shown in Figure 1A, in the absence of stimulatory antigen, PKCθ was uniformly distributed throughout the T cell, whereas the C1 and C2+C1 constructs were concentrated at punctate intracellular structures, previously shown to consist of Golgi and other intracellular membranes (Carrasco and Merida, 2004). In response to specific antigen stimulation of the D10 TCR, both the C1 and C2 + C1 constructs redistributed to the plasma membrane, with no apparent IS retention at 30-min postconjugation. In contrast, the WT PKCθ construct was stably maintained at the IS under the same stimulation conditions.
FIGURE 1:
The C-terminal fragment containing the KD is required for PKCθ IS retention. (A) The indicated D10 T cell lines were stimulated with CH12 cells loaded with no antigen (No Ag) or 250 μg/ml conalbumin (+Antigen). PKCθ-GFP constructs were visualized by digital deconvolution epifluorescence microscopy, and conjugates were imaged using DIC microscopy. Bar, 5 μm. (B) Relative retention of PKCθ-GFP constructs at the IS was quantified for 20–45 conjugates per cell line, and relative IS retention was calculated using the two ratios R1 and R2 (see Materials and Methods). *p < 0.001 in comparisons of PKCθ mutants to WT (one-way analysis of variance, Tukey's multiple comparison test).
To quantify PKCθ IS retention for statistical analyses, we used two approaches (see Materials and Methods for further details). First, we calculated the ratio (R1) of GFP intensity at the IS divided by GFP intensity in the entire cell. Second, we calculated the ratio (R2) of GFP intensity at the IS divided by plasma membrane GFP intensity away from the IS. Both R1 and R2 were highly significantly different when comparing WT to the C1 or C2 + C1 constructs (Figure 1B; p < 0.001). Moreover, as suggested by the images in Figure 1A, the mean value of R2 was ∼1.0 for the C1 and C2 + C1 constructs, indicating that the plasma membrane enrichment per unit area is identical within and outside of the IS. Thus the PKCθ C-terminus, containing the complete KD, is required for IS retention in response to antigen stimulation of the TCR.
Phosphorylation of Thr-538 is an important determinant of PKCθ IS retention
The data in Figure 1 suggest that the activity of the KD and/or specific peptide sequences within the PKCθ C-terminal fragment is required for IS retention. Several phosphorylation sites within the PKCθ KD are known to contribute to stability of the protein and/or kinase activity (Liu et al., 2002; Park et al., 2009; Newton, 2010). These residues include Thr-538 in the activation loop, Ser-676 in the turn motif, and Ser-695 in the hydrophobic domain (Supplemental Figure S1A). We therefore mutated these residues (individually) to alanine to test the hypothesis that KD catalytic activity is a determinant of IS retention of PKCθ. We also mutated two residues outside of the KD, Tyr-90 and Ser-219 (Supplemental Figure S1). Tyr-90 is a conserved residue within the C2 domain that has been reported to be phosphorylated by the src kinase, p56lck, in conjunction with TCR stimulation (Liu et al., 2000). Thr-219 is a recently described autophosphorylation site located between the two PKCθ C1 domains. Both Tyr-90 and Thr-219 sites were reported to contribute to membrane translocation of PKCθ (Thuille et al., 2005; Melowic et al., 2007).
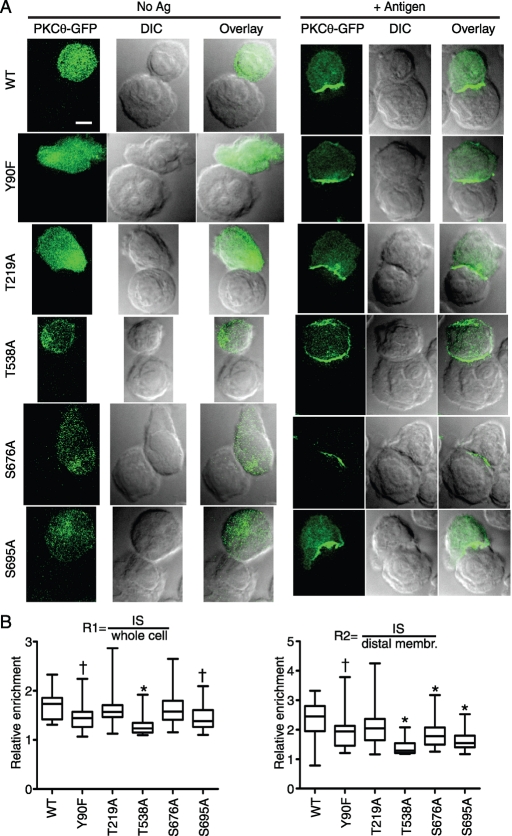
As shown in Figure 2A, the T538A mutation had the most profound effect on PKCθ IS retention, with microscopy analysis revealing substantial amounts of PKCθ present outside of the T cell/APC contact region. This distribution was similar to the enrichment pattern observed with the C1 and C2 + C1 mutants. Quantification of IS retention showed a highly significant effect (p < 0.001) of the T538A mutation versus WT for both the R1 and R2 comparisons. For R2, the WT mean was 2.4 versus the T538A mean of 1.4. Thus the T538A mutant retained some degree of retention at the IS, in contrast to the C1 and C2 + C1 mutants (which had an R2 mean of 1.0; see Figure 1B). Imaging of the other C-terminal mutants (S695A in the hydrophobic domain, and S676 in the turn motif) generally did not suggest membrane enrichment of PKCθ outside of the IS (Figure 2A). However, image quantification of many cells revealed a significant difference of both S676A and S695A from WT in the R2 comparison (means of 1.7 and 1.8, respectively; p < 0.001 for both), although only S695A was significantly different from WT in the R1 comparison (p < 0.05). Thus the hierarchy of effects of these kinase-domain phosphorylation-site mutants on IS retention is T538A > S695A > S676A. Of interest, this observation closely mirrors the hierarchy of effects of these mutations on PKCθ kinase activity, with T538A having only 1% of WT kinase activity, S695A having 20% of WT activity, and S676A showing no effect on kinase activity (Liu et al., 2002). These data therefore suggest that kinase activity is a major determinant of IS retention for PKCθ.
FIGURE 2:
Mutation of the PKCθ activation-loop phosphorylation site (T538A) severely impairs PKCθ IS retention. (A) D10 T cell lines expressing the indicated PKCθ point mutants were stimulated and imaged as in Figure 1A. Bar, 5 μm. (B) Relative retention and statistical significance were determined as in Figure 1B. †p < 0.05; *p < 0.001.
Analysis of mutations outside of the kinase domain revealed that Y90F caused a reduction in IS retention relative to WT (R2 mean, 1.9; p < 0.05 for both R1 and R2). As the Y90F mutant was reported to have impaired in vitro kinase activity (Melowic et al., 2007), the observed reduction in IS retention might be attributable to the reduced kinase activity of this variant. In contrast, analysis of the T219A mutation showed no significant difference from WT in either the R1 or R2 comparisons (R2 mean, 2.4). Previous data showed that the Thr219A mutation does not impair PKCθ kinase activity (Thuille et al., 2005). Thus our analysis of five known PKCθ phosphorylation sites established a strong correlation between IS retention and PKCθ kinase activity, with impaired kinase activity correlating with poor IS retention.
The phosphomimetic mutation T538E does not impair PKCθ IS retention
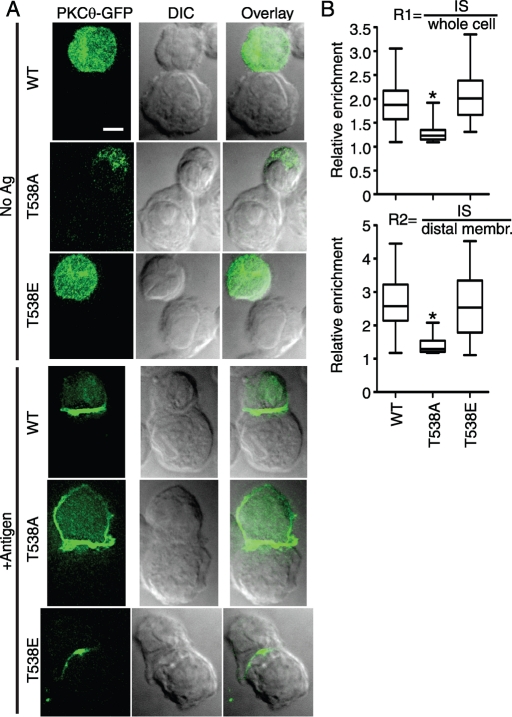
Among the tested phosphorylation site mutants, T538A had the most profound effect, with very poor retention at the IS (Figure 2). To provide further evidence that phosphorylation of Thr-538 contributes to IS retention, we analyzed the T538E mutant, in which the charged Glu mimics the effect of phosphorylation at this site. Previous data showed that T538E has only a modest effect on PKCθ kinase activity (threefold reduction), in contrast to the severe kinase impairment resulting from the T538A mutation (>100-fold reduction in activity; Liu et al., 2002). As shown in Figure 3A, IS retention of the T538E mutant was indistinguishable from the result for WT PKCθ. Quantification of IS retention in the population of stimulated cells (Figure 3B) showed that there was no significant difference in IS retention between the T538E mutant and WT (R2 means, 2.7 and 2.6, respectively). These data suggest either that phosphorylation of Thr-538 is directly responsible for IS retention of PKCθ or that Thr-538 phosphorylation indirectly promotes IS retention by activating the kinase function of PKCθ.
FIGURE 3:
T538E phosphomimetic mutation enables WT levels of IS retention. (A) D10 T cell lines expressing the indicated PKCθ point mutants were stimulated and imaged as in Figure 1A. Bar, 5 μm. (B) Relative retention and statistical significance were determined as in Figure 1B. *p < 0.001.
The kinase-inactivating mutant K409R blocks IS retention of PKCθ independent of Thr-538 phosphorylation
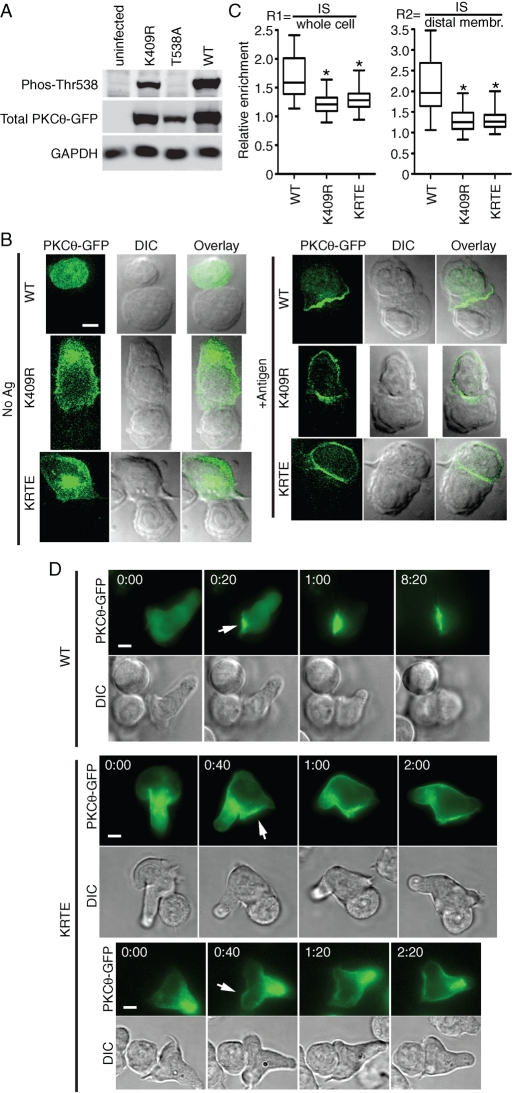
To determine the mechanism by which phosphorylation of Thr-538 contributes to IS retention, we first examined the phosphorylation of this residue in D10 T cells. As previously reported for Jurkat and primary human CD4+ T cells (Liu et al., 2002; Freeley et al., 2005; Thuille et al., 2005), PKCθ Thr-538 is constitutively phosphorylated in D10 T cells, with no increase in phosphorylation in response to TCR stimulation (Supplemental Figure S2). To test the hypothesis that kinase activity, rather than Thr-538 phosphorylation, is a major determinant of PKCθ IS retention, we made an additional PKCθ mutant, K409R (KR), which severely impairs kinase activity by disrupting ATP binding by the KD. Western blotting with a phospho-specific (P-Thr-538) antibody revealed that the KR mutant has approximately wild-type levels of phosphorylation of Thr-538 (Figure 4A; quantification of these data revealed that the WT/KR mutant ratio was 4.0 for P-Thr-538 and 4.5 for total PKCθ). As expected, the T538A mutant did not react with the P-Thr-538 antibody.
FIGURE 4:
Kinase activity is the critical determinant of PKCθ IS retention. (A) Whole-cell lysates from the indicated cell lines were separated by SDS–PAGE and analyzed by Western blotting with anti–phospho-Thr-538-PKCθ, anti–(total) PKCθ, and anti-GAPDH. (B) D10 T cell lines expressing the indicated PKCθ point mutants were stimulated and imaged as in Figure 1A. Bar, 5 μm. (C) Relative retention and statistical significance were determined as in Figure 1B. (D) Live-cell images of D10 T cell lines contacting antigen-loaded CH12 cells. Translocation of WT (top) and KRTE (middle and bottom) PKCθ-GFP is shown at selected times post-CH12 contact. Arrows indicate initial IS translocation. Displayed data were taken from Supplemental Movies S01 (top; frames 11, 12, 14, and 26), S02 (middle; frames 1, 3, 4, and 7), and S03 (bottom; frames 2, 4, 6, and 9). *p < 0.001.
The finding that the KR mutant was efficiently phosphorylated at Thr-538 was somewhat surprising, based on data showing lack of activation-loop phosphorylation of a similar kinase-inactive mutant of PKCε (K437M; Cameron et al., 2009). Further analysis of phosphorylation of the PKCθ constructs showed the expected phosphorylation of WT PKCθ at Ser-676 (Freeley et al., 2005). However, there was no detectable phosphorylation of Ser-676 for the KR, T538A, and S676A mutants (Supplemental Figure S3), consistent with data suggesting that phosphorylation of the PKC C-terminal turn motif requires an active KD (Cameron et al., 2009; Newton, 2010). Thus the kinase-inactive KR mutant of PKCθ is phosphorylated at the activation loop (Thr-538) to approximately WT levels in D10 T cells, whereas the C-terminal turn motif (S676) is not detectably phosphorylated.
In imaging analysis, the KR mutant exhibited poor retention at the IS, showing a redistribution pattern very similar to the T538A mutation (Figure 4B). The R2 mean value for the KR mutant was 1.3 versus the WT R2 mean of 2.2 (Figure 4C). We also generated a double mutant, K409R,T538E (KRTE), to test the possibility that phosphorylation at Thr-538, rather than an active KD, is responsible for IS retention. As shown in Figure 4, B and C, the IS retention pattern of the KRTE mutant was indistinguishable from that of the KR mutant (R2 mean, 1.3), and both the KR and KRTE mutants were significantly different from WT in the R1 and R2 comparisons (p < 0.001 for both). Together, the Western blotting and imaging data in Figure 4 strongly suggest that kinase activity, and not phosphorylation of Thr-538, is the crucial determinant of IS retention.
To formally demonstrate that inactivation of the kinase domain interferes with PKCθ IS retention, we performed live-cell imaging analysis of D10 T cell lines expressing WT PKCθ or the KRTE mutant. Whereas WT PKCθ became rapidly enriched at the IS, the KRTE mutant was poorly recruited, exhibiting less intense enrichment at the point of T cell/APC contact than the WT enzyme. In addition, whereas WT PKCθ was stably retained at the IS, the KRTE mutant was observed around the majority of the plasma membrane within 1–2 min of the initial translocation (Figure 4D and Supplemental Movies S01–S03). These data further support our conclusion that a functional kinase domain is necessary for stable IS retention.
DISCUSSION
Previous data showed that antigen-mediated TCR signaling triggers translocation of PKCθ, but not other PKC isoforms, to the IS (Monks et al., 1997). Of interest, more recent data showed that IS recruitment and IS retention are mechanistically separable phenomena, with initial IS recruitment requiring only the PKCθ C1 domains. However, whereas the isolated C1 domain fragment rapidly diffuses from the IS across the entire plasma membrane, the full PKCθ enzyme is stably retained at the IS (Carrasco and Merida, 2004). These studies suggested that one or more functional domains outside of the C1A-C1B region are responsible for PKCθ IS retention.
Through the analysis of multiple PKCθ mutants, we showed that TCR-stimulated retention of PKCθ at the IS requires the presence of a signaling-competent KD. Deletion of the C-terminal fragment that includes the KD, turn motif, and hydrophobic motif completely abolished stable retention of PKCθ at the IS. The kinase-inactivating T538A and K409R point mutations dramatically reduced IS retention of PKCθ, although a slight tendency for IS retention remained (Figures 2–4). This weak retention may be the result of low-level kinase activity of these mutants (Liu et al., 2002; Thuille et al., 2005). Such an interpretation is consistent with modest suppression of IS retention by the Y90F and S695A mutations, which have moderately impaired kinase activity (Liu et al., 2002; Melowic et al., 2007). In addition, we observed that phosphorylation of Thr-538 was not impaired in the PKCθ K409R mutant, and that IS retention of the kinase-dead K409R protein is not enabled by the T538E mutation. These data strongly support our contention that phosphorylation of Thr-538 does not directly mediate IS retention.
The observed high correlation between IS retention and kinase activity is also consistent with the unimpaired IS retention of the T219A mutant, which has WT levels of kinase activity (Thuille et al., 2005). In contrast to our results, another group reported that the T219A mutation renders PKCθ unable to translocate to the plasma membrane in response to superantigen stimulation of Jurkat T cells (Thuille et al., 2005). Although the reason for the discrepancy between our results and this previous study is unclear, there were a number of differences in the experimental design that could have been a factor, including the use of superantigen stimulation of the Jurkat T cell line in that study versus conventional antigen stimulation of the D10 T cell clone in this work.
Our live-cell analyses showed that IS recruitment of the KRTE mutant is less efficient than WT. In addition, whereas the WT enzyme remained continuously enriched at the IS, the enrichment of the KRTE mutant spanned the majority of the plasma membrane soon after initial translocation at the IS. Together with the previously reported behavior of the isolated PKCθ C1 domains (Carrasco and Merida, 2004), these data suggest that PKCθ rapidly diffuses away from the site of TCR engagement in the absence of a functional kinase domain.
For this study, we used the D10 T cell line, which is a Th2 clone that expresses ICOS but not CD28. Studies showed that c-SMAC focusing of PKCθ requires signals from CD28 (Huang et al., 2002; Tseng et al., 2008; Yokosuka et al., 2008). We are aware of no data demonstrating that ICOS plays an analogous role in Th2 cells, and we have observed only slight c-SMAC enrichment of PKCθ in the D10 clone (unpublished data). Thus our data define PKCθ sequences required for TCR-dependent retention at the IS, without the potentially confounding influence of CD28 signals that enable focusing at the c-SMAC. Further studies in CD28-positive T cells will be required to address whether TCR-dependent IS retention and CD28-dependent c-SMAC focusing are equally dependent on the PKCθ KD.
Together, our data very strongly suggest that PKCθ requires an active KD in order to maintain stable IS retention. The KD is thus the first identified PKCθ functional element that specifically controls retention at the IS. The target of PKCθ phosphorylation that is responsible for IS retention remains to be defined. The simplest possibility is that the phosphorylation target is PKCθ, itself, although phosphorylation of a partner protein cannot yet be ruled out. The autophosphorylation of PKCθ has been characterized primarily by using bacterially produced recombinant PKCθ (Liu et al., 2002; Thuille et al., 2005). Thus it is quite possible that functionally important PKCθ autophosphorylation sites, which are only phosphorylated efficiently during in vivo expression in T cells, remain unidentified. Autophosphorylation could either directly create a binding site for a partner protein or indirectly create a binding site by stabilizing a particular conformation of PKCθ. If intramolecular autophosphorylation is indeed the mechanism effecting IS retention, an implication is that only active kinase molecules are stably retained at the IS. In this manner, active PKCθ may be selectively retained at the IS, where PKCθ function is clearly of particular importance (Sims et al., 2007). Further analysis of phosphorylation sites of PKCθ and its binding partners, using protein purified directly from activated T cells, will likely be required to fully elucidate the mechanism of PKCθ IS retention.
MATERIALS AND METHODS
PKCθ mutants and D10 T cell lines
The murine PKCθ-GFP construct was previously described (Schaefer et al., 2004). The C1 and C2 + C1 constructs consisted of PKCθ sequences encoding amino acids 145–303 and 2–303, respectively. The C1 and C2 + C1 sequences were further modified by the addition of N-terminal FLAG (MDYKDDDDKEF) and C-terminal YFP (Citrine variant; Griesbeck et al., 2001) tags. The point mutations Y90F, T219A, K409R, T538A, T538E, S676A, S695A, and K409R + T538E (KRTE) were introduced into the PKCθ-GFP cDNA using a QuickChange Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). All mutants were cloned into the retroviral expression vector pEneo (Schaefer et al., 2001). Retroviral infection of D10 T cells and selection of stable cell lines were as previously described (Schaefer et al., 1999). The D10 T cell clone (Kaye et al., 1983) and the CH12 B cell line were maintained as described (Schaefer et al., 2004).
Flow cytometry and microscopy
Flow cytometry analysis was performed on D10 T cells D10 cell lines stably expressing PKCθ-GFP (or YFP) retroviral constructs during log phase growth ([1–3] × 105 cells/ml). Cell lines were analyzed using a BD Biosciences (San Diego, CA) LSRII flow cytometer, with all fluorescence data collected in the FL1 channel. Median fluorescence intensity was quantified using FlowJo software (TreeStar, Ashland, OR).
To form T cell/B cell conjugates, 1.5 × 105 D10 T cells expressing the indicated PKCθ constructs were added to 1.5 × 105 CH12 B cells loaded overnight with no antigen or with 250 μM conalbumin, as previously described (Schaefer et al., 1999). At 22-min postconjugation, the D10/CH12 conjugate mixture was pipetted onto washed poly-d-lysine–coated coverslips (3 mg/ml) and placed in a humidified 5% CO2 incubator at 37°C for 8 min. Cells were then fixed and mounted (at 30-min postactivation) as described (Schaefer et al., 1999). The 30-min time point was chosen to allow ample time for plasma membrane equilibration of PKCθ constructs, which are not stably retained at the IS (Carrasco and Merida, 2004), as well as eliminating from the analysis transient and unstable PKCθ translocation events that have been shown to occur when upstream signaling to PKCθ is disrupted (Yokosuka et al., 2008) (and could theoretically occur with some or all of the PKCθ mutants in this study).
Fluorescence and differential interference contrast (DIC) images were acquired on a Zeiss (Jena, Germany) Axiovert 200M inverted microscope by using a 100× Plan-Apochromat 1.4–numerical aperture oil objective. The microscope was controlled by a TILL monochromator-based imaging system, driven by TILLvisION 4.0 software (TILL Photonics, Planegg, Germany). Data were collected as z-stacks of 50 images with 0.3-μm steps between images and digitally deconvolved using a constrained iterative algorithm. The five images representing the central 1.5 μm (in the z-dimension) of each conjugate were used to generate (maximal intensity) projection images and for subsequent quantification. All image analysis was performed with TILLvisION 4.0 software.
For each conjugate, the DIC image was used to manually define regions of interest representing the whole cell, the T cell membrane at the IS (the region of physical contact between the T cell and CH12 B cell), and the T cell membrane away from the IS (the membrane region not in contact with the CH12 B cell). Fluorescence intensity per unit area was then calculated for each region, and ratios R1 and R2 were determined. R1 was calculated by dividing IS intensity by whole-cell intensity and serves as a relative measure of IS intensity. R2 was calculated by dividing IS intensity by membrane intensity away from the IS. Thus R2 most directly measures the degree to which membrane-associated PKCθ remains concentrated at the IS.
Live-cell image series were acquired on the same TILL/Zeiss system, using chambered coverslip maintained at 37°C. D10/CH12 conjugates were imaged as z-stacks of 30 images using 0.5-μm steps and 300-ms exposures. Frames were acquired every 20 s. Movies show GFP fluorescence (green) and DIC (blue) as an overlay image on the left and GFP fluorescence on the right.
Antibodies and Western blotting
The following anti-PKCθ antibodies were used: mouse anti–(total) PKCθ (clone 27; BD Biosciences), rabbit anti–(total) PKCθ (Cell Signaling Technology, Beverly, MA), rabbit anti–phospho-Thr-538 (Cell Signaling Technology), and rabbit anti–phospho-Ser-676 (Invitrogen, Carlsbad, CA). Other antibodies included mouse anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH; clone 6C5; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-IκBα (Cell Signaling Technology), mouse anti–α-tubulin (clone DM1A; Sigma-Aldrich, St. Louis, MO), and rabbit anti–α-tubulin (Santa Cruz Biotechnology). Preparation of whole-cell lysates and Western blotting were as previously described (Langel et al., 2008).
Supplementary Material
Acknowledgments
This work was supported by grants to B.C.S. from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI057481), the Center for Neuroscience and Regenerative Medicine, the Sidney Kimmel Foundation for Cancer Research, and the Dana Foundation.
Abbreviations used:
- APC
antigen-presenting cell
- IS
immunological synapse
- KD
kinase domain
- TCR
T cell receptor
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-11-0916) on July 27, 2011.
REFERENCES
- Baier G, Telford D, Giampa L, Coggeshall KM, Baier-Bitterlich G, Isakov N, Altman A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993;268:4997–5004. [PubMed] [Google Scholar]
- Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- Cameron AJ, Escribano C, Saurin AT, Kostelecky B, Parker PJ. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat Struct Mol Biol. 2009;16:624–630. doi: 10.1038/nsmb.1606. [DOI] [PubMed] [Google Scholar]
- Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JD, Xu Y, Raychowdhury MK, Ware JA. Molecular cloning and expression of a cDNA encoding a novel isoenzyme of protein kinase C (nPKC). A new member of the nPKC family expressed in skeletal muscle, megakaryoblastic cells, and platelets. J Biol Chem. 1993;268:14208–14214. [PubMed] [Google Scholar]
- Freeley M, Volkov Y, Kelleher D, Long A. Stimulus-induced phosphorylation of PKC theta at the C-terminal hydrophobic-motif in human T lymphocytes. Biochem Biophys Res Commun. 2005;334:619–630. doi: 10.1016/j.bbrc.2005.06.136. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Porcelli S, Tite J, Jones B, Janeway CA., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983;158:836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingeter LM, Schaefer BC. Loss of protein kinase C theta, Bcl10, or Malt1 selectively impairs proliferation and NF-kappa B activation in the CD4+ T cell subset. J Immunol. 2008;181:6244–6254. doi: 10.4049/jimmunol.181.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel FD, Jain NA, Rossman JS, Kingeter LM, Kashyap AK, Schaefer BC. Multiple protein domains mediate interaction between Bcl10 and MALT1. J Biol Chem. 2008;283:32419–32431. doi: 10.1074/jbc.M800670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Graham C, Li A, Fisher RJ, Shaw S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem J. 2002;361:255–265. doi: 10.1042/bj3610255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Witte S, Liu YC, Doyle M, Elly C, Altman A. Regulation of protein kinase Ctheta function during T cell activation by Lck-mediated tyrosine phosphorylation. J Biol Chem. 2000;275:3603–3609. doi: 10.1074/jbc.275.5.3603. [DOI] [PubMed] [Google Scholar]
- Melowic HR, Stahelin RV, Blatner NR, Tian W, Hayashi K, Altman A, Cho W. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Ctheta. J Biol Chem. 2007;282:21467–21476. doi: 10.1074/jbc.M700119200. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S, Mizuno K, Saido TC, Suzuki K, Kuroki T, Ohno S. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer BC, Kappler JW, Kupfer A, Marrack P. Complex and dynamic redistribution of NF-kappaB signaling intermediates in response to T cell receptor stimulation. Proc Natl Acad Sci USA. 2004;101:1004–1009. doi: 10.1073/pnas.0307858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer BC, Mitchell TC, Kappler JW, Marrack P. A novel family of retroviral vectors for the rapid production of complex stable cell lines. Anal Biochem. 2001;297:86–93. doi: 10.1006/abio.2001.5327. [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Ware MF, Marrack P, Fanger GR, Kappler JW, Johnson GL, Monks CR. Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity. 1999;11:411–421. doi: 10.1016/s1074-7613(00)80116-8. [DOI] [PubMed] [Google Scholar]
- Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Spitaler M, Cantrell DA. Protein kinase C and beyond. Nat Immunol. 2004;5:785–790. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- Sun Z, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- Thuille N, et al. Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCtheta in T lymphocytes. EMBO J. 2005;24:3869–3880. doi: 10.1038/sj.emboj.7600856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M, Bi K, Hu J, Altman Y, Bushway P, Reits E, Neefjes J, Baier G, Abraham RT, Altman A. Translocation of PKC[theta] in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J Cell Biol. 2002;157:253–263. doi: 10.1083/jcb.200201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.