Summary
Chronic feeding on high-calorie diets causes obesity and type 2 diabetes mellitus (T2DM), illnesses that affect hundreds of millions. Thus, understanding the pathways protecting against diet-induced metabolic imbalance is of paramount medical importance. Here we show that mice lacking SIRT1 in steroidogenic factor 1 (SF1) neurons are hypersensitive to dietary obesity owing to maladaptive energy expenditure. Also, mutant mice have increased susceptibility to develop dietary T2DM due to insulin resistance in skeletal muscle. Mechanistically, these aberrations arise, in part, from impaired metabolic actions of the neuropeptide orexin-A and the hormone leptin. Conversely, mice overexpressing SIRT1 in SF1 neurons are more resistant to diet-induced obesity and insulin resistance due to increased energy expenditure and enhanced skeletal muscle insulin sensitivity. Our results unveil important protective roles of SIRT1 in SF1 neurons against dietary metabolic imbalance.
Introduction
Feeding on high-calorie (HC) diets and sedentary lifestyle are thought to play critical pathogenic roles in the development of obesity and type 2 diabetes mellitus (T2DM) (Vianna and Coppari, 2011; Wisse et al., 2007). Chronic hypercaloric feeding causes energy and glucose/insulin imbalance in mammalian organisms, including nonhuman primates (McCurdy et al., 2009; Surwit et al., 1988), thus supporting a cause-effect relationship between HC diets and metabolic diseases. Because some subjects are more resistant than others from developing diet-induced metabolic dysfunctions (Enriori et al., 2007) heterogeneities in the robustness of homeostatic mechanisms against dietary obesity and T2DM must exist. The behavioral and autonomic adaptations against dietary obesity are partially understood; these include reduced food intake and increased energy expenditure, respectively (Dhillon et al., 2006; Ramadori et al., 2010; Tong et al., 2008). On the other hand, the protective mechanism(s) against dietary T2DM is unknown. Considering that hundreds of millions of people are affected by obesity and/or T2DM (both of which greatly increase the risk of cardiovascular disease and cancer) a better understanding of the key molecules and cell-types that coordinate homeostatic responses against increased body adiposity and glucose/insulin imbalance is of paramount scientific and medical importance.
Neurons within the hypothalamus detect changes in circulating amounts of hormones and substrates and relay this information into adaptive outputs aimed at maintaining normal energy and glucose/insulin homeostasis (Coppari et al., 2005; Hill et al., 2010; Lam et al., 2005; Parton et al., 2007; Pocai et al., 2005; Ramadori et al., 2010; Vianna and Coppari, 2011). Among these cells, neurons within the ventromedial hypothalamic nucleus (VMH) have been shown to be of particular importance. Indeed, loss of leptin receptor (LEPR) in steroidogenic factor 1 (SF1)-expressing neurons [a group of cells found only in the VMH within the brain (Ikeda et al., 1993; Luo et al., 1994)] significantly predisposes to developing obesity (Dhillon et al., 2006). Similarly, reduced phosphoinositide 3-kinase (PI3K) signaling in SF1 neurons (induced by SF1-neuron-specific deletion of p110α, a catalytic subunit of PI3K) impairs autonomic responses to hypercaloric feeding and consequentially engenders hypersensitivity to diet-induced obesity (Xu et al., 2010). VMH neurons are also thought to govern glucose/insulin homeostasis because: i) the VMH is enriched in glucose-sensing neurons (Oomura et al., 1964); ii) VMH-centered electrical stimulation or leptin administration stimulates glucose uptake in peripheral tissues (Minokoshi et al., 1999; Sudo et al., 1991); iii) SF1-neuron-specific deletion of LEPR causes insulin resistance (Bingham et al., 2008); iv) enhanced LEPR signaling in SF1 neurons (induced by SF1-neuron-specific deletion of suppressor of cytokine signaling 3, a negative regulator of LEPR signaling) augments insulin sensitivity (Zhang et al., 2008); and v) the actions of orexins [also known as hypocretins; two neuropeptides, orexin-A and orexin-B, secreted from neurons within the lateral hypothalamic area (LHA)] on VMH neurons have been linked to increased insulin sensitivity in skeletal muscle (Shiuchi et al., 2009). Interestingly, VMH neurons project excitatory synapses to hypothalamic arcuate nucleus (ARH) proopiomelanocortin (POMC) neurons (Sternson et al., 2005); a group of neurons that has been shown to coordinate defensive mechanisms against diet-induced obesity (Ramadori et al., 2010) and glucose-induced hyperglycemia (Parton et al., 2007). Together, these findings suggest that VMH SF1 neurons are important rheostats of metabolic function that by sensing changes in extracellular amounts of metabolic cues (e.g.: leptin, orexin, glucose) coordinate homeostatic responses aimed at maintaining normal body weight and glucose/insulin homeostasis.
SIRT1 is a metabolic-sensor protein that requires oxidized nicotinamide adenine dinucleotide (NAD+) as a cofactor for its enzymatic activity (Imai et al., 2000). SIRT1 deacetylates diverse proteins (e.g.: histones, transcription factors, and cofactors) and therefore influences several biological programs (Ramadori and Coppari, 2010). An emerging and converging view is that SIRT1 (the orthologs of which are found in several species) has been conserved throughout evolution due to its robust protective actions (Haigis and Sinclair, 2010). For example, SIRT1 is crucial for appropriate adaptations to fasting. Indeed, SIRT1 activity increases in several tissues (including the hypothalamus) following a decrease in energy availability (Cohen et al., 2004; Ramadori et al., 2008). In this context, SIRT1 triggers lipid mobilization from adipose tissue (Picard et al., 2004), a switch from glucose to lipid oxidation in skeletal muscle (Gerhart-Hines et al., 2007) and liver (Purushotham et al., 2009), and an increase in hepatic glucose production (Rodgers et al., 2005), all of which are crucial defense mechanisms to diminished energy availability. SIRT1 has also been shown to exert protective actions against Alzheimer's disease, amyotrophic lateral sclerosis (Donmez et al., 2010; Kim et al., 2007a; Qin et al., 2006), and axonal degeneration (Araki et al., 2004). In addition, we have recently reported that SIRT1 in POMC neurons is a critical molecular component of autonomic mechanisms against dietary obesity (Ramadori et al., 2010).
SIRT1 is abundantly expressed in VMH (Ramadori et al., 2008). Thus, we hypothesized that SIRT1 in neurons within this hypothalamic structure is a key molecular component of protective pathways against excessive body weight gain and glucose/insulin imbalance. In this study, we directly tested this hypothesis by investigating the metabolic consequences of either loss or overexpression of SIRT1 only in SF1 neurons in mice.
Results
Somatic deletion of SIRT1 in SF1 neurons
Cre-mediated deletion of the loxP-flanked exon 4 of Sirt1 (Sirt1loxP) produces a Sirt1 null allele (Cheng et al., 2003). Thus, to generate mice lacking SIRT1 in SF1 neurons the Sirt1loxP allele was bred to the Sf1-Cre transgene that expresses functional Crerecombinase only in SF1 cells (Dhillon et al., 2006; Tong et al., 2007; Xu et al., 2010). To determine if Sf1-Cre mice homozygous for the Sirt1loxP allele (hereafter referred to as Sf1-Cre; Sirt1loxP/loxP mice) lack SIRT1 only in SF1 cells we first carried out genotyping PCR analysis of several tissues. The Cre-deleted Sirt1 allele was detected only in hypothalamus, pituitary, testis, ovary, and adrenal gland of Sf1-Cre; Sirt1loxP/loxP mice (Figure S1A). SIRT1 deletion in these sites is expected as SF1 is expressed only in VMH, pituitary (gonadotropes), testis (Leydig cells), ovaries (corpus luteum and theca and granulosa cells) and adrenal cortex (Ikeda et al., 1993; Luo et al., 1994). SIRT1 deletion in SF1-expressing peripheral tissues was inconsequential as fertility capacity (i.e.: the average age of first delivery, litter size, time between deliveries), estrous cycle, gonadal weight, and serum testosterone levels were all undistinguishable between mutants and controls (Table S1 and data not shown). Also, the basal circadian fluctuations and stress-induced levels of serum corticosterone were normal in mutants (Table S1).
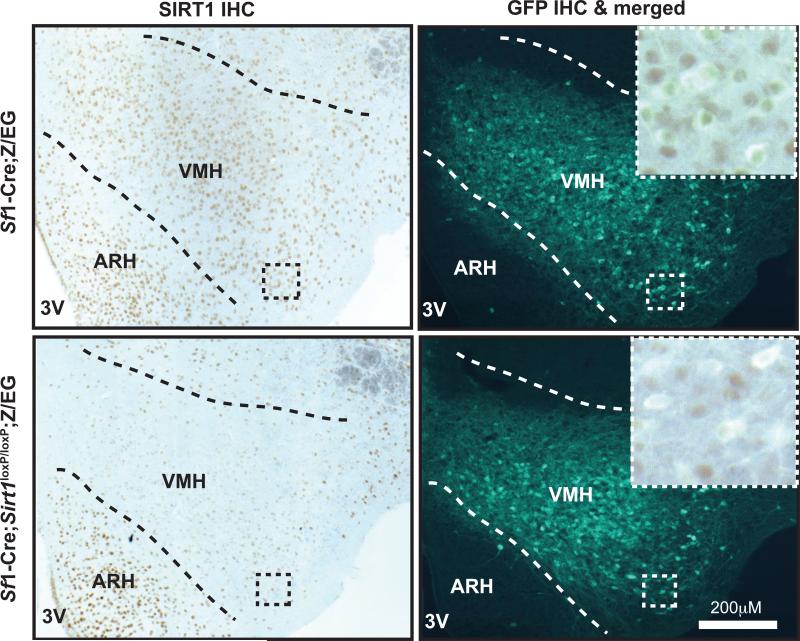
To further investigate SIRT1 deletion in the central nervous system (CNS), the amount of full length Sirt1 mRNA was measured and found to be reduced in hypothalamus of Sf1-Cre; Sirt1loxP/loxP mice (Figure S1B). These data suggest that SIRT1 is absent only in SF1 neurons. To directly test this possibility, immunohistochemistry (IHC) analyses of brains from Sirt1loxP/loxP (control) and mutant mice were performed using SIRT1-specific antibodies (Ramadori et al., 2010). In line with our previous results (Ramadori et al., 2008), numerous SIRT1-positive cells were detected in the VMH of controls (Figure S1C, upper panel). Noteworthy, co-localization IHC assays indicated that ~97% of SF1 neurons express SIRT1 in controls (Figure 1, upper panel). Conversely, only few SIRT1-positive cells were detected in the VMH of Sf1-Cre; Sirt1loxP/loxP mice (Figure S1C, lower panel). The result that some SIRT1-expressing cells were still retained in VMH of mutant mice is expected as SF1 neurons constitute a large component but not the entire VMH (Dhillon et al., 2006). Also, in Sf1-Cre; Sirt1loxP/loxP mice the distribution of SIRT1-expressing cells was unchanged in VMH's neighboring nuclei as for example the ARH and the dorsomedial hypothalamic nucleus (Figure S1C, lower panel). Importantly, co-localization IHC assays indicated that only ~9% of SF1 neurons express SIRT1 in mutants (Figure 1, lower panel).
Figure 1. Deletion of SIRT1 is restricted to SF1 neurons.
Representative photomicrographs of brain slices from Sirt1loxP/loxP;Z/EG (control) or Sf1-Cre; Sirt1loxP/loxP;Z/EG mice stained for SIRT1 and GFP. The Z/EG allele was introduced to allow the expression of GFP selectively in SF1 neurons, as previously described (Dhillon et al., 2006). Dark-brown staining and green fluorescence represent SIRT1 and GFP immunoreactivity, respectively. Higher magnification of the boxed region is in the top left corner of the photomicrograph. Note that while almost all SF1 neurons express SIRT1 in control brains (530 out of 546 SF1 neurons were found to be SIRT1-positive) very few express SIRT1 in Sf1-Cre; Sirt1loxP/loxP; Z/EG mice (52 out of 576 SF1 neurons were found to be SIRT1-positive). Abbreviations: third ventricle (3V); hypothalamic arcuate (ARH) and ventromedial (VMH) nuclei; immunohistochemistry (IHC). Dashed lines indicate VMH boundaries. See also Figure S1.
Because SIRT1 exerts neuroprotective actions (Donmez et al., 2010; Kim et al., 2007a; Qin et al., 2006), we explored whether SIRT1 deficiency affects the survival of SF1 neurons. Results shown in Figures S1B and S1D indicate that the amounts and anatomical distribution of Sf1 mRNA are normal in hypothalamus of mutants. No genotype differences in hypothalamic amounts of apoptotic or neurodegenerative markers were also observed (Figures S1B, S1E and S1F). Collectively, these results demonstrate that Sf1-Cre; Sirt1loxP/loxP mice lack SIRT1 specifically in SF1 neurons and that this mutation does not cause death or degeneration of SF1 neurons.
Lack of SIRT1 in SF1 neurons causes hypersensitivity to dietary obesity
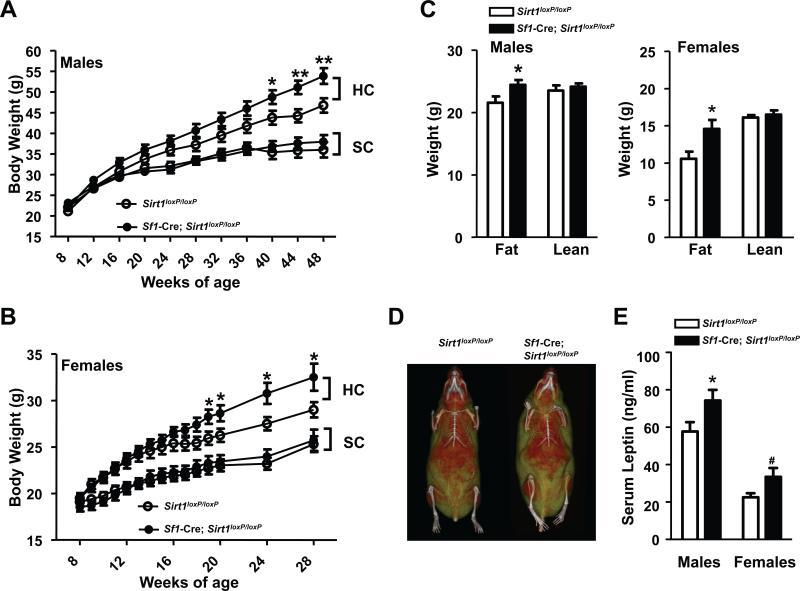
Sf1-Cre; Sirt1loxP/loxP mice were born at the expected Mendelian ratio, developed into adulthood similarly to their littermate controls, and displayed normal body weights when fed ad libitum on a standard chow (SC) diet (Figures 2A and 2B). Body fat and lean mass were assessed by magnetic resonance imaging (MRI) and found to be unchanged in SC-fed mutants (data not shown). Because body length was also normal (Table S1), our results indicate that SIRT1 in SF1 neurons is dispensable for normal body weight in the context of normocaloric diet feeding. Conversely, when fed on a HC diet Sf1-Cre;Sirt1loxP/loxP mice displayed increased propensity to develop obesity (Figures 2A and 2B). MRI and micro-computed tomography imaging analyses indicated that the increased body weight is solely due to increased fat mass (Figures 2C and 2D). In line with increased adiposity, circulating levels of the adipocyte-secreted hormone leptin were also elevated (Figure 2E). Energy imbalance was not secondary to changes in circulating sex and/or thyroid hormones, and/or corticosterone levels (Table S1), and/or altered expression of Sf1 and/or key hypothalamic neuropeptides known to regulate energy balance (Figure S1B). Serum non-esterified fatty acids were also unchanged (Table S1). Collectively, these results demonstrate that SIRT1 in SF1 neurons is a key molecular component of defense mechanisms against dietary obesity in both, male and female mice.
Figure 2. SIRT1 in SF1 neurons is required for normal defenses against diet-induced obesity in male and female mice.
(A) Body weight curves of standard chow (SC)-fed Sirt1loxP/loxP (n=21) and Sf1-Cre, Sirt1loxP/loxP (n=21) males, high-calorie (HC)-fed Sirt1loxP/loxP (n=16) and Sf1-Cre, Sirt1loxP/loxP (n=24) males, (B) SC-fed Sirt1loxP/loxP (n=16) and Sf1-Cre, Sirt1loxP/loxP (n=13) females, HC-fed Sirt1loxP/loxP (n=14) and Sf1-Cre,Sirt1loxP/loxP (n=14) females. (C) Body composition, (D) representative micro-computed tomography images, and (E) serum leptin levels of Sirt1loxP/loxP and Sf1-Cre, Sirt1loxP/loxP mice; data shown in C, D, and E were gathered from 28-week-old females and 48-week-old males (n=13-17). In (D) red and yellow/green colors represent lean and fat mass, respectively; females are shown. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test. #P=0.05; *P<0.05; **P<0.01; #P=0.05. See also Table S1.
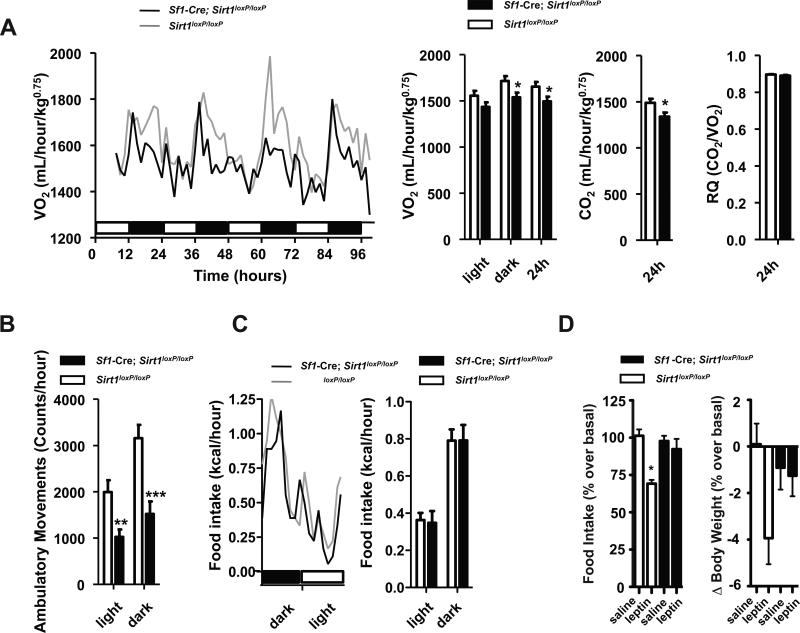
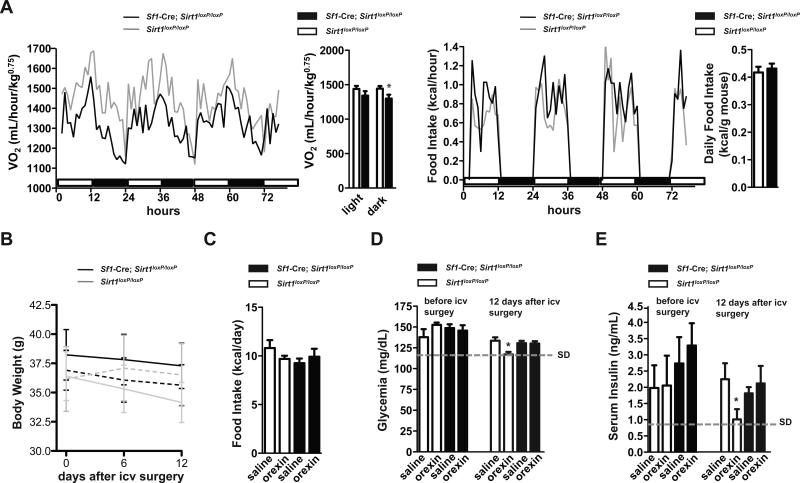
Body weight is kept normal when energy intake equals energy expenditure over time (Vianna and Coppari, 2011). Autonomic (e.g.: increased energy expenditure) and behavioral (e.g.: reduced food intake) changes are critical defense maneuvers against excessive body weight gain brought on by HC diet feeding (Dhillon et al., 2006; Ramadori et al., 2010). Thus, to identify the defective mechanisms underpinning the hypersensitivity to dietary obesity observed in mice lacking SIRT1 in SF1 neurons the autonomic and behavioral adaptations to hypercaloric feeding were assessed. Energy expenditure and food intake during the 4 days before and after the switch from SC to HC diet were similar between groups (Figures S2A and S2B). However, after 8 weeks on the HC diet O2 consumption, CO2 production, and ambulatory movements were all diminished (while food intake was still normal) in Sf1-Cre; Sirt1loxP/loxP mice (Figures 3A-C and S2C). At the time the aforementioned metabolic parameters were assessed there were no genotype differences in body weight, fat mass and leptinemia [Sirt1loxP/loxP and Sf1-Cre; Sirt1loxP/loxP mice; mean values ± s.e.m.; n=12 each group. Body weight (g): 23.07 ± 0.84 and 23.90 ± 1.06 (n.s.); fat mass (g): 5.57 ± 0.57 and 6.48 ± 0.84 (n.s.); serum leptin (ng/mL): 14.39 ± 2.14 and 15.55 ± 2.28 (n.s.); n.s. = not statistically significant] thus ruling out the possibility that the hypometabolic rate seen in mutants is secondary to obesity. In fact, these results indicate that reduced energy expenditure underlies the hypersensitivity to dietary obesity caused by lack of SIRT1 in SF1 neurons. Altered metabolic homeostasis was not accompanied with changes in carbohydrates vs. lipids oxidation rates as respiratory quotient was normal in mutants (Figure 3A).
Figure 3. Normophagia, reduced energy expenditure, and leptin resistance in mice lacking SIRT1 in SF1 neurons.
(A) O2 consumption, CO2 and heat production, respiratory quotient (RQ), (B) ambulatory movements, and (C) food intake were measured before body weight diverged in 16-week-old Sirt1loxP/loxP and Sf1-Cre, Sirt1loxP/loxP females fed on a high-calorie (HC) diet for 8 weeks (n=12). Data were collected using the Columbus Instruments Comprehensive Lab Animal Monitoring System (CLAMS). Time 0 represents the beginning of the dark cycle in a 12-hour dark/light cycle environment. Black and white boxes represent dark and light cycles, respectively. (D) Food intake and body weight difference following 2 days of intraperitoneal injections of leptin (daily dose = 3g of leptin/kg) or saline in 16-week-old Sirt1loxP/loxP and Sf1-Cre, Sirt1loxP/loxP HC-fed females (n=6). Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test and one-way ANOVA (Tukey's post test) . *P<0.05, **P<0.01, ***P<0.01. See also Figure S2.
Because leptin's action on SF1 neurons is required for appropriate body weight balance (Dhillon et al., 2006), we tested the integrity of leptin sensitivity and found that the anorectic and body-weight-suppressing effects of the hormone were blunted in Sf1-Cre; Sirt1loxP/loxP mice (Figure 3D). These results suggest that SIRT1 deletion in SF1 neurons causes leptin resistance; a defect that, in part, may underlie the energy imbalance. Together, our data demonstrate that lack of SIRT1 in SF1 neurons significantly predisposes to dietary obesity owing to reduced energy expenditure.
SIRT1 in SF1 neurons regulates skeletal muscle insulin sensitivity
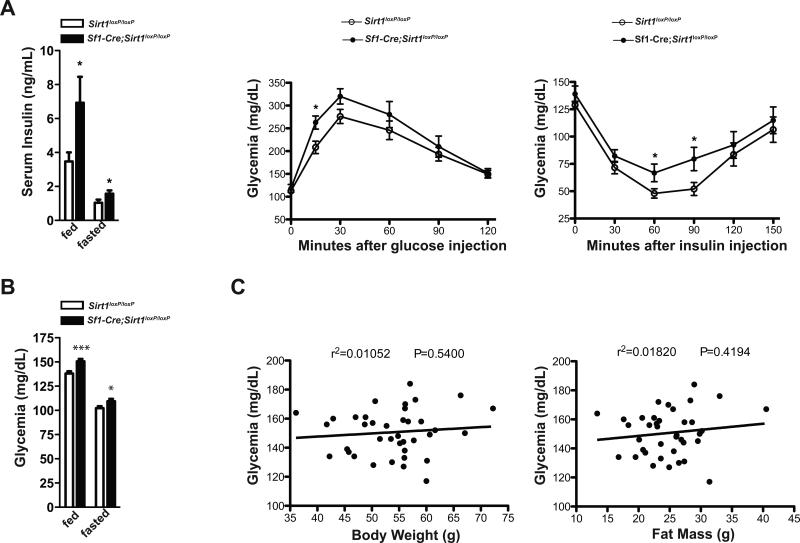
SF1 neurons have been suggested to coordinate glucose metabolism (Minokoshi et al., 1999; Shiuchi et al., 2009; Tong et al., 2007). Thus, we set to determine the direct contribution of SIRT1 in SF1 neurons on glucose/insulin homeostasis in the context of either normo- or hyper-caloric diet feeding. No obvious aberrations in glycemia, insulinemia, glucose tolerance and insulin sensitivity were noted in SC-fed mutants (data not shown). On the other hand, when challenged with HC diet mutants displayed increased susceptibility to develop T2DM. Indeed, as shown in Figures 4A and S3A, HC-fed mutants were hyperinsulinemic, glucose intolerant and insulin resistant even before their body weight, fat mass, and circulating leptin levels diverged from HC-fed controls (Figures S3B and S3C). Sf1-Cre; Sirt1loxP/loxP HC-fed mice also developed hyperglycemia (Figures 4B and S3D). Although the onset of hyperglycemia coincided with the one of obesity the former is likely not a consequence of the latter because no significant correlations between glycemia and body weight or fat mass of Sf1-Cre; Sirt1loxP/loxP HC-fed mice were noted (Figures 4C and S3D).
Figure 4. Loss of SIRT1 in SF1 neurons causes impaired glucose/insulin homeostasis in a body-adiposity-independent manner.
(A) Serum insulin level after 3 hours of food removal (fed values) or 14 hours of fasting (fasted values), glucose tolerance test (GTT) and insulin tolerance test (ITT) in 28-week-old Sirt1loxP/loxP (n=12) and Sf1-Cre, Sirt1loxP/loxP (n= 17) males fed on a high-calorie (HC) diet. (B) Glycemia after 3 hours of food removal (fed values) or 14 hours of fasting (fasted values), GTT and ITT in 48-week-old Sirt1loxP/loxP (n=34) and Sf1-Cre, Sirt1loxP/loxP (n=38) males fed on a HC diet. HC-fed mice were fed on a SC diet up to 8 weeks of age and then switched and maintained on a HC diet. (C) Correlation between glycemia and fat mass or body weight. GTT: dose of glucose injected intraperitoneally/mouse weight = 1g/kg. ITT: dose of insulin injected intraperitoneally/mouse weight = 1.25U/kg and 0.75U/kg in males and females, respectively. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test. Correlation analyses were performed by using the Spearman rank-correlation test. *P<0.05, ***P<0.001. See also Figure S3.
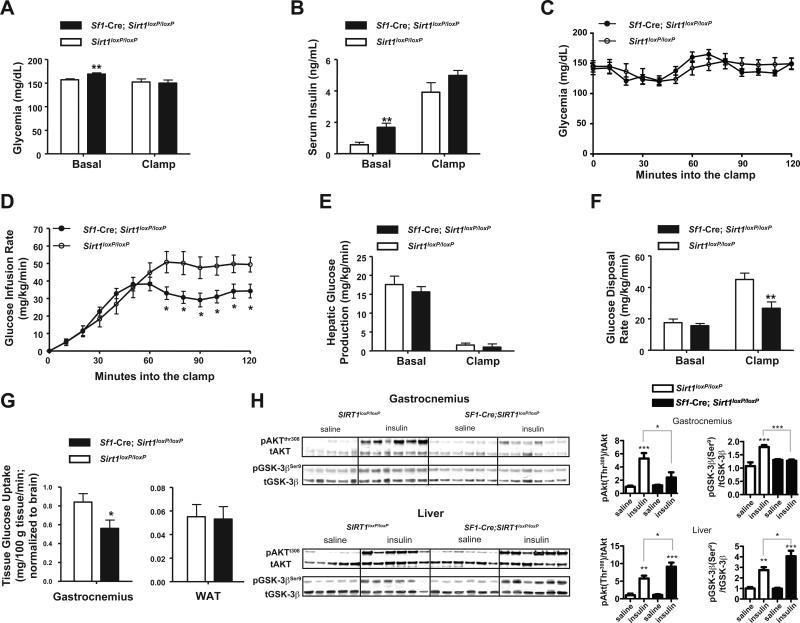
To gather insights into the mechanisms underlying the altered glucose/insulin homeostasis, hyperinsulinemic/euglycemic clamp studies were performed. As expected, in basal conditions, circulating insulin and glucose levels were elevated in Sf1-Cre;Sirt1loxP/loxP compared to Sirt1loxP/loxP HC-fed mice (Figures 5A and 5B). During the clamp, serum insulin levels were experimentally increased and maintained similar between groups (Figure 5B). In agreement with the aforementioned data pinpointing insulin resistance, glucose infusion rate (GIR) needed to clamp euglycemia (Figures 5A and 5C) was greatly reduced in Sf1-Cre; Sirt1loxP/loxP HC-fed mice compared to controls (Figure 5D). Basal hepatic glucose production (HGP) and glucose disposal rate (GDR) were not different between groups (Figures 5E and 5F). During the clamp, insulin suppressed HGP to a similar extent in both groups (Figure 5E); a result that suggested insulin resistance in extra-hepatic tissue(s). Strikingly, insulin-induced GDR was significantly hampered in Sf1-Cre; Sirt1loxP/loxP HC-fed mice (Figure 5F). Because insulin-induced glucose disposal is mainly accounted for by glucose uptake in skeletal muscle and adipose tissue, the amounts of exogenously-administered radiolabeled glucose in these tissues were measured at completion of the clamp experiment. As shown in Figure 5G, insulin-stimulated glucose uptake was normal in adipose tissue but significantly reduced in gastrocnemius of Sf1-Cre; Sirt1loxP/loxP HC-fed mice.
Figure 5. Loss of SIRT1 in SF1 neurons causes insulin resistance in skeletal muscle.
(A-G) Euglycemic-hyperinsulinemic clamps were preformed in Sirt1loxP/loxP (n=9) and Sf1-Cre, Sirt1loxP/loxP (n=8) 28-week old conscious males that have been fed on a high-calorie (HC) diet for 20 weeks. Mice were surgically implanted with a right jugular vein catheter 5-7 days prior to study. Mice were fasted for 4 hours and blood samples were obtained from tail vein. 2 weeks prior to the clamp assay body weight was undistinguishable between mutants and controls. At the time clamps were performed,Sf1-Cre, Sirt1loxP/loxP mice displayed a slight increase in body weight compared to controls (P=0.04). (A) Basal and clamp glycemia and (B) serum insulin levels. (C) Glycemia and (D) glucose infusion rate during the clamp. (E) Basal and clamp hepatic glucose production and (F), glucose disposal rate. (G) Tissue-specific insulin stimulated glucose uptake. (H) Immunoblot and quantification of Akt (Thr308) and GSK-3β (Ser9) phosphorylation status relative to total AKT (tAKT) or total GSK-3β (tGSK-3β) in gastrocnemius muscle and liver 20 minutes after an intraperitoneal bolus of insulin (3U/kg) or saline in Sirt1loxP/loxP and Sf1-Cre, Sirt1loxP/loxP standard chow (SC)-fed 28-week old males (n=5-6 per group). Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test and one-way ANOVA (Tukey's post test). *P <0.05; **P <0.01; ***P <0.001. In (H) P values are saline- vs. insulin-treated mice of the same genotype, unless otherwise indicated. See also Figure S4.
Next, to independently assess in vivo insulin sensitivity biochemical approaches were employed. Phosphorylation of AKT and glycogen synthase kinase 3 (GSK3; a target of AKT) are well-established events following insulin-induced activation of the PI3K signaling cascade (Manning and Cantley, 2007). Thus, the statuses of phosphorylated AKT (p-AKT) and GSK3 (p-GSK3) have been widely used as measures of the ability of insulin to activate its receptor. Insulin administration greatly elevated p-AKT/AKT and p-GSK3/GSK3 ratios in liver, gastrocnemius and soleus of Sirt1loxP/loxP controls (Figures 5H and S4). Interestingly, p-AKT/AKT and p-GSK3/GSK3 ratios were higher in liver (possibly a compensatory mechanism to maintain normoglycemia) but significantly reduced in gastrocnemius and soleus of insulin-injected Sf1-Cre; Sirt1loxP/loxP mice compared to controls (Figures 5H and S4). Noteworthy, the blunted ability of insulin to activate PI3K-AKT signaling in skeletal muscle is body-weight- and HC-diet-independent as this defect was manifest in SC-fed mutants that had normal body weight (Figures 2A, 5H and S4). Importantly, SF1-neuron-specific SIRT1 overexpression led to enhanced skeletal muscle insulin sensitivity (see below). Collectively, our results demonstrate SIRT1 in SF1 neurons regulates insulin sensitivity in skeletal muscle and that this circuitry protects against dietary diabetes.
SIRT1 in SF1 neurons is required for the normal metabolic actions of Orexin-A
A peculiar characteristic of mice lacking SIRT1 in SF1 neurons is the hypometabolic rate being more pronounced during the dark than the light cycles (Figure 3A). Dark periods are times at which feeding activities are highest in mice (Figure 3C). To better understand the central defects underlying metabolic imbalance in Sf1-Cre; Sirt1loxP/loxP mice we explored whether the nocturnal hypometabolic rate is consequence of impaired feeding-induced energy expenditure. Interestingly, the nocturnal deficit in O2 consumption still persisted in mutant mice that had access to food only at lights-on times (Figure 6A); thus suggesting that other defect(s) is present. The activity of orexin neurons increases in wake periods that are longest during dark cycles in rodents (Estabrooke et al., 2001). Also, VMH-centered orexin administration has been shown to rapidly enhance insulin sensitivity in skeletal muscle (an effect the physiological relevance of which is unknown) (Shiuchi et al., 2009). These notions prompted us to test whether orexin's metabolic effects are altered in Sf1-Cre; Sirt1loxP/loxP mice. To test this hypothesis, Sf1-Cre; Sirt1loxP/loxP and Sirt1loxP/loxP HC-fed mice were subjected to chronic intracerebroventricular (icv) administration of orexin-A. As shown in Figures 6B and 6C, no significant differences in body weight and food intake between Sf1-Cre; Sirt1loxP/loxP and Sirt1loxP/loxP HC-fed mice that either received icv orexin-A or placebo were observed. Remarkably, icv orexin-A administration normalized hyperglycemia and hyperinsulinemia brought on by HC diet feeding in controls but failed to exert this action in Sf1-Cre; Sirt1loxP/loxP HC-fed mice (Figures 6D and 6E). These data indicate that SIRT1 in SF1 neurons is required for normal anti-diabetic actions of orexin-A.
Figure 6. SIRT1 in SF1 neurons is required for the normal metabolic actions of Orexin-A.
(A) O2 consumption and food intake were measured in Sirt1loxP/loxP (n=12) and Sf1-Cre, Sirt1loxP/loxP (n=12) HC-fed 17-week old females after they were maintained for 3 weeks in a lights-on restricted feeding regimen. Data were collected using the Columbus Instruments Comprehensive Lab Animal Monitoring System (CLAMS). Time 0 represents 6 hours after the beginning of the light cycle in a 12-hour dark/light cycles environment. HC-fed mice were fed on a SC diet up to 8 weeks of age and then switched and maintained on a HC diet. (B) Body weight and (C) daily average of food intake of Sirt1loxP/loxP and Sf1-Cre, Sirt1loxP/loxP 20-week old HC-fed males 12 days into icv delivery of either placebo (n=7-8 each genotype; dashed lines) or orexin-A (n=7 each genotype; solid lines). (D) Glycemia and (E) serum insulin were assessed before and after 12 days of treatment. In (D) and (E) dashed lines represent average glycemia and serum insulin levels in age-matched standard diet (SD)-fed Sirt1loxP/loxP males, respectively. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test or using one-way ANOVA (Tukey's post test).*P<0.05. See also Figure S6.
SIRT1 overexpression in SF1 neurons protects against dietary metabolic imbalance
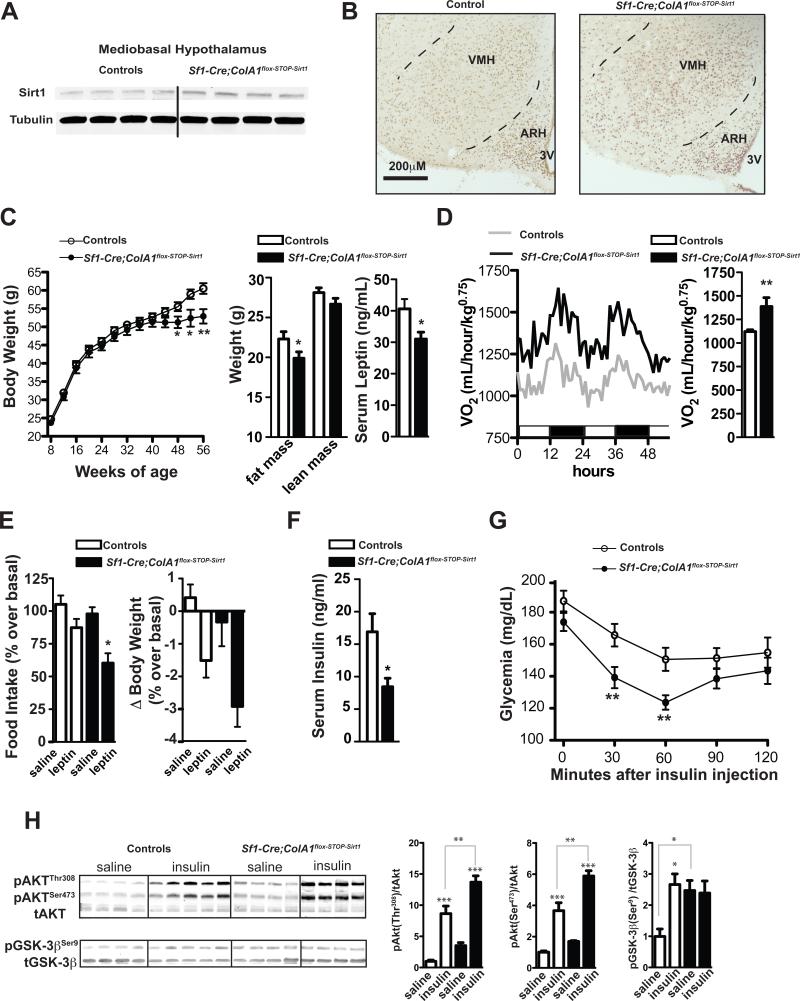
Results from the aforementioned loss-of-function mutation experiments demonstrate that SIRT1 in SF1 neurons is required for normal adaptive mechanisms against dietary obesity and diabetes. To directly test whether a SF1-neuron-specific SIRT1 gain-of-function mutation is sufficient to protect against diet-induced metabolic imbalance we engineered and analyzed mice overexpressing SIRT1 only in SF1 neurons. These mutants were generated by crossing the Sf1-Cre transgene to the ColA1flox-STOP-Sirt1 allele that has been created by insertion of a loxP-flanked-STOP Sirt1 coding sequence cassette into the 3’ end of the ColA1 locus (Firestein et al., 2008). ColA1flox-STOP-Sirt1 mice do not overexpress SIRT1 unless Cre-recombinase is present (Oberdoerffer et al., 2008). Accordingly, in Sf1-Cre; ColA1flox-STOP-Sirt1 mice the Cre-recombined ColA1flox-STOP-Sirt1 allele was detected only in sites known to express SF1 as for example the VMH but not the ARH (Figure S5A). SIRT1 overexpression in SF1-expressing peripheral tissues was inconsequential as fertility capacity, gonadal weight, and basal circadian fluctuations and stress-induced levels of serum corticosterone were normal in Sf1-Cre; ColA1flox-STOP-Sirt1 mice (Table S1 and data not shown). To assess if the Cre-recombined ColA1flox-STOP-Sirt1 allele resulted in increased SIRT1, the amount of SIRT1 was measured and found to be two-fold higher in mediobasal hypothalamus (but unchanged in other brain areas) of Sf1-Cre; ColA1flox-STOP-Sirt1 mice compared to controls (Figure 7A and data not shown). Importantly, SIRT1 expression was selectively increased in the VMH of Sf1-Cre; ColA1flox-STOP-Sirt1 mice (Figure 7B) thus suggesting that these mice have augmented SIRT1 content in SF1 neurons.
Figure 7. SIRT1 overexpression in SF1 neurons protects against diet-induced obesity and glucose/insulin imbalance.
(A) SIRT1 and α-tubulin (used as loading control) protein levels were assessed by immunoblot in hypothalamus of Sf1-Cre; ColA1flox-STOP-Sirt1 mice or controls. (B) Representative photomicrographs of brain slices from control or Sf1-Cre; ColA1flox-STOP-Sirt1 mice stained for SIRT1 (dark-brown staining represents SIRT1 immunoreactivity). (C) Body weight curves, body composition, and serum leptin level and (D) oxygen consumption of high-calorie (HC)-fed Sf1-Cre; ColA1flox-STOP-Sirt1 males (n=12) and controls (n=25). Body composition and serum leptin contents were measured in 48-week-old mice. (E) Food intake and body weight difference following 2 days of intraperitoneal injections of leptin (daily dose = 3g of leptin/kg) or saline in 52-week-old HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1 and control males (n=5). (F) Serum insulin level after 3 hours of food removal, (G) insulin tolerance test (ITT) in 48-week-old HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1 (n=12) and control mice (n=17). ITT: dose of insulin injected intraperitoneally/mouse weight = 1.25U/kg. (H) Immunoblot and quantification of Akt(Ser479), Akt (Thr308) and GSK-3β(Ser9) phosphorylation status relative to total AKT (tAKT) or total GSK-3β (tGSK-3β) in gastrocnemius muscle 10 minutes after an intraperitoneal bolus of insulin (5U/kg) or saline in 14-weeks-old HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1and control mice. Sf1-Cre; ColA1flox-STOP-Sirt1 mice were generated by mating Sf1-Cre and ColA1flox-STOP-Sirt1 mice. This breeding also generated Sf1-Cre and wild-type mice. Because no differences in body weight, food intake, energy expenditure, glycemia, insulinemia, ITT and GTT were noted between Sf1-Cre and wild-type HC-fed mice data from these two groups were pooled together and used as controls. Error bars represent s.e.m. Statistical analyses were done using two-tailed unpaired Student's t test and one-way ANOVA (Tukey's post test). *P<0.05, **P<0.01, ***P<0.001. Abbreviations: third ventricle (3V); hypothalamic arcuate (ARH) and ventromedial (VMH) nuclei. Dashed lines indicate VMH boundaries. In (H) P values are saline- vs. insulin-treated mice of the same genotype, unless otherwise indicated. See also Figure S5.
Oppositely to the consequence of loss of SIRT1 in SF1 neurons, overexpression of SIRT1 in SF1 neurons protects against dietary obesity as HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1 mice had reduced body weight, fat mass, and serum leptin level compared to controls fed on the same diet (Figure 7C). The improved energy balance was not due to reduced food intake but brought on by increased energy expenditure (Figures 7D and S5B). The hypermetabolic rate was not a consequence of changes in locomotor activity as ambulatory movements were normal in Sf1-Cre; ColA1flox-STOP-Sirt1 mice (Figure S5C). However, enhanced expression of Pgc1-α and SERCA2a in skeletal muscle suggests that increased uncoupled respiration may underlie, at least in part, the enhanced metabolic rate displayed by Sf1-Cre; ColA1flox-STOP-Sirt1 mice (Figure S5D). Of note, while SIRT1 deficiency in SF1 neurons causes leptin resistance (Figure 3D) SIRT1 overexpression in SF1 neurons leads to hypersensitivity to the hormone. In fact, leptin's ability to suppress body weight and food intake was enhanced in HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1 mice compared to controls (Figure 7E).
SF1-neuron-specific SIRT1 overexpression also led to striking protective actions against diet-induced glucose/insulin imbalance as HC-fed Sf1-Cre; ColA1flox-STOP-Sirt1 mice had reduced circulating insulin levels, enhanced insulin sensitivity and glucose tolerance compared to controls (Figures 7F, 7G, and S5E). These improvements were likely caused by enhanced skeletal muscle insulin sensitivity because basal and insulin-induced p-AKT/AKT and p-GSK3/GSK3 ratios were increased in gastrocnemius (but unchanged in liver) of these mutants (Figures 7H and S5F).
In agreement with previous findings (Dhillon et al., 2006), body weight, food intake, glycemia, insulinemia, insulin sensitivity and glucose tolerance were all undistinguishable between Sf1-Cre and wild-type HC-fed mice (data not shown). Therefore, the improved and worsen metabolic profiles of Sf1-Cre; ColA1flox-STOP-Sirt1 and Sf1-Cre; Sirt1loxP/loxP HC-fed mice, respectively, are not the consequence of the Sf1-Cre transgene per se. Collectively, our data demonstrate that SIRT1 overexpression in SF1 neurons exerts robust protective actions against diet-induced obesity and glucose/insulin imbalance in part owing to enhanced leptin sensitivity and improved insulin action in skeletal muscle.
Discussion
More than 1 billion people suffer from increased body weight and/or type 2 diabetes mellitus (T2DM) (http://www.who.int/mediacentre/factsheets). Both, genetic/epigenetic predisposition and environmental factors contribute to this epidemic. To date, effective, long-lasting, and safe anti-obesity drugs are lacking (Vianna and Coppari, 2011). Anti-T2DM therapies are available; yet, responses to these treatments are refractory and they do not prevent serious morbidities (O'Rahilly et al., 2005). Thus, a better understanding of the mechanisms protecting against obesity and T2DM is key to the fight against these diseases. Here, we report that loss of the protein deacetylase SIRT1 in steroidogenic factor 1 (SF1)-expressing neurons predisposes to, whereas overexpression of SIRT1 in SF1 neurons protects against, diet-induced energy and glucose/insulin imbalance.
The overlaps between CNS pathways controlling food intake and hedonic/mood circuitries combined with the complicatedness to reduce appetite and/or enhance energy expenditure without cardiovascular adverse effects have been major roadblocks to the development of effective and safe anti-obesity drugs. Indeed, the use of drugs able to curtail food intake often causes detrimental effects on mood and vice versa (Despres et al., 2005; Di Marzo, 2008; Tardieu et al., 2003). Also, several anti-obesity drugs have been shown to have serious harmful consequences on the cardiovascular system (Connolly et al., 1997; James et al., 2010). In this study we report that SIRT1 in ventromedial hypothalamic nucleus (VMH) SF1 neurons does not regulate food intake but instead coordinates energy expenditure adaptations against excessive body weight gain (Figures 2, 3, and 7). Normophagia concomitant with hypometabolic rate in the context of chronic HC diet feeding is also a consequence of SIRT1 deletion in another group of extra-VMH neurons (i.e.: POMC neurons) (Ramadori et al., 2010). Thus, results from this (Figures 2, 3, 7 and S5) and our previous study (Ramadori et al., 2010) indicate a divergence between the roles of SIRT1 in SF1 and POMC neurons on food intake vs. energy expenditure. Collectively, our data suggest that harnessing SIRT1 function in POMC and SF1 neurons may represent an effective approach to reduce body weight without causing detrimental behavioral changes.
An interesting aspect of our study is that it unveils the existence of a previously unrecognized form of diabetes that is neurogenic T2DM. Although the onsets of hyperglycemia and increased body weight coincided (Figures 4B and S3D), hyperinsulinemia, glucose intolerance, and insulin resistance were all manifest in Sf1-Cre; Sirt1loxP/loxP mice before their body weight and adiposity differed from controls (Figures 4A, S3A, S3B, and S3C). Therefore, glucose/insulin imbalance likely is a primary defect caused by lack of SIRT1 in SF1 neurons and not secondary to obesity. This contention is further bolstered by the following observations: i) the impaired insulin sensitivity in skeletal muscle was detected in SC-fed mutant mice the body weight and adiposity of which were not different from controls (Figures 2, 5H and S4); ii) the insulinemia- and glycemia-lowering actions of icv orexin-A administration were blunted in mice lacking SIRT1 in SF1 neurons albeit their body weights were not different from controls (Figures 6B, 6D and 6E); iii) there were no correlations between glycemia and body weight or fat mass in diabetic and obese Sf1-Cre; Sirt1loxP/loxP HC-fed mice (Figures 4B, 4C, and S3D), and iv) increased adiposity does not always lead to glucose/insulin imbalance (Kim et al., 2007b). Indeed, HC-fed mice lacking SIRT1 in POMC neurons display normal glucose/insulin homeostasis despite the fact that they have a similar degree of obesity as the one seen in HC-fed Sf1-Cre; Sirt1loxP/loxP mice (Ramadori et al., 2010).
Our results suggest that SIRT1 in SF1 neurons directly controls skeletal muscle insulin sensitivity as SIRT1 deletion in SF1 neurons impairs, whereas SIRT1 overexpression in SF1 neurons enhances, insulin action selectively in skeletal muscle (Figures 5H, 7H and S4). Changes in neuroendocrine circulating factor(s) levels and/or the activity of the autonomic nervous system may underlie this regulation. We favor the latter possibility because data shown in Figure 6 indicate that orexin-A's anti-diabetic actions are severely impaired in mutant mice and it has been shown that VMH-centered orexin-A delivery enhances insulin sensitivity in skeletal muscle via increased sympathetic tone in this tissue (Shiuchi et al., 2009). Future studies are required to establish the underpinnings of the SIRT1-in-SF1-neuron-to-skeletal-muscle circuitry. Regardless to the mechanisms, our loss- and gain-of-function studies unveiled SIRT1 in SF1 neurons as a critical molecule of homeostatic pathways safeguarding glucose/insulin homeostasis and as such SIRT1 in SF1 neurons may represent an ideal target of anti-diabetic drugs.
Orexin-A signaling is impaired in mice lacking SIRT1 in SF1 neurons (Figure 6). There are at least three not mutually exclusive possibilities that could explain this defect. Orexin-A has been shown to affect the biophysical properties of VMH SF1 neurons (Shiuchi et al., 2009). Thus, one possible explanation is that the direct effects of this neuropeptide on membrane potential and firing rate of mutant SF1 neurons are altered. To test this idea, we employed hypothalamic slice preparations and measured electrophysiological properties of SF1 neurons containing or devoid of SIRT1. As shown in Figure S6A, we found that SF1 neurons are heterogeneous in respect to their biophysical responsiveness to orexin-A as ~33% or ~55% of SF1 neurons displayed excitatory or inhibitory outcomes to the neuropeptide, respectively. A similar percentage of SF1 neurons lacking SIRT1 was either activated or inhibited by orexin-A (Figure S6B). Membrane potential and firing rates at basal and after orexin-A administration were also similar between SF1 neurons containing or devoid of SIRT1 (Figures S6A and S6B). Thus, imperfections in other pathways lying downstream of orexin receptors signaling in SF1 and/or non-SF1 neurons may underlie the diminished orexin-A's anti-diabetic actions observed in Sf1-Cre; Sirt1loxP/loxP mice. Largely due to the lack of reliable readouts for orexin receptors intracellular signaling and the widespread CNS distribution of orexin neurons projections (Chemelli et al., 1999) these two possibilities could not be resolved in this study.
Interestingly, deletion of SIRT1 in SF1 neurons leads to reduced, whereas overexpression of SIRT1 in SF1 neurons causes increased, sensitivity to leptin's anti-obesity actions (Figures 3E and 7E). Furthermore, deletion of SIRT1 in SF1 neurons causes insulin resistance in the skeletal muscle while overexpression has the opposite effect (Figures 5H and 7H). These results suggest that the metabolic phenotypes engendered by either loss or overexpression of SIRT1 in SF1 neurons are caused by changes in similar pathways. However, while locomotor activity was reduced in Sf1-Cre;Sirt1loxP/loxP mice this parameter was unchanged in Sf1-Cre; ColA1flox-STOP-Sirt1 mutants (Figures 3B and S5C). Also, expression of SERCA2a in skeletal muscle was increased in Sf1-Cre; ColA1flox-STOP-Sirt1 but unchanged in Sf1-Cre; Sirt1loxP/loxP mice (Figure S5D and data not shown). Therefore, our data also indicate that mechanisms affected by the loss-are unaltered by the gain-of-function mutation and vice versa.
Putative SIRT1-activating compounds (STACs) have been shown to exert beneficial actions against metabolic imbalance in animal models of obesity and T2DM (Baur et al., 2006; Lagouge et al., 2006; Milne et al., 2007; Ramadori et al., 2009). Genetically-induced SIRT1 overexpression has also been shown to improve metabolic defects in similar animal models (Banks et al., 2008; Pfluger et al., 2008). However, due to uncertainties regarding STACs specificity (Beher et al., 2009; Pacholec et al., 2010) and ubiquitous nature of SIRT1 gain-of-function experiments (Banks et al., 2008; Pfluger et al., 2008) the relevance of SIRT1 in mediating STACs actions and in which cell-type(s) enhanced SIRT1 would eventually lead to improved metabolic imbalance have not been established yet. The current model however is that enhanced SIRT1 activity in peripheral sites (e.g.: skeletal muscle and adipose tissue) is a mechanistic link between STACs administration and improved metabolism (Banks et al., 2008; Baur et al., 2006; Lagouge et al., 2006; Milne et al., 2007). Interestingly, based on our results that SF1-neuron-specific SIRT1 gain-of-function mutation protects against diet-induced metabolic defects (Figures 7 and S5) this paradigm will need to be amended to include SIRT1 in SF1 neurons as a novel molecular target for anti-obesity and -T2DM compounds.
In summary, the present study has uncovered critical protective roles of SIRT1 in VMH SF1 neurons against dietary obesity and diabetes. These results represent an important step forward in our understanding of the mechanisms safeguarding metabolic homeostasis.
Experimental Procedures
Generation of Sf1-Cre; Sirt1loxP/loxP and Sf1-Cre; ColA1flox-STOP-Sirt1 mice
Mice were housed in groups of 4-5 with food (either a standard chow rodent diet or the high-calorie (HC) diet (D12331 from Research Diets, New Brunswick, NJ, USA) and water available ad libitum in light- and temperature-controlled environments unless otherwise specified. Care of mice was within the Institutional Animal Care and Use Committee (IACUC) guidelines, and all the procedures were approved by the University of Texas Southwestern Medical Center IACUC. HC-fed mice were fed on a standard chow diet up to 8 weeks of age and then switched and maintained on a HC diet.
Immunohistochemistry and in-situ hybridization histochemistry analyses
SIRT1 immunohistochemistry was performed on brain sections from mice using SIRT1-specific antibodies as previously described (Ramadori et al., 2010).
Body composition, oxygen consumption and locomotor activity analyses
Body fat and lean mass were determined using the EchoMRI-100 system (Echo Medical Systems, Houston, TX, USA). Metabolic rate and physical activity were measured using a comprehensive lab animal monitoring system (Columbus Instruments, Columbus, OH, USA).
Hyperinsulinemic-Euglycemic Clamp Study
Hyperinsulinemic-euglyclemic clamps were performed as previously described (Hill et al., 2010).
Central orexin administration
A cannula was positioned stereotaxically into the cerebral lateral ventricles (-0.34mm from bregma; ±1mm lateral; -2.3mm from skull) and a small osmotic minipump (model 1004, Alzet, Cupertino, CA, USA) implanted subcutaneously was attached via a catheter to the cannula for intracerebroventricular infusion.
Electrophysiological Study
Whole-cell patch-clamp recordings were made from SF1 neurons of Sf1-Cre; Sirt1loxP/loxP; Z/EG or Sf1-Cre; Z/EG (controls) mice. During the recordings neurons were maintained in hypothalamic slice preparations and data analysis were performed as previously described (Dhillon et al., 2006).
Statistical analysis
Data sets were analyzed for statistical significance using PRISM (GraphPad, San Diego, CA) for a two-tail unpaired Student's t test when two groups were compared or one-way ANOVA (Tukey's post test) when three or more groups were compared.
Supplementary Material
Highlights.
Loss of SIRT1 in SF1 neurons predisposes to dietary obesity and diabetes
Enhanced SIRT1 in SF1 neurons protects against diet-induced metabolic imbalance
SIRT1 in SF1 neurons controls insulin sensitivity in skeletal muscle
SIRT1 in SF1 neurons is required for normal anti-diabetic actions of orexin-A
Acknowledgements
We thank Kristen Wertz for technical assistance, Drs. Joyce Repa (UTSW Medical Center, USA) for quantitative real time PCR primers, Yoshiyuki Horio (Sapporo Medical School, Japan) for the SIRT1 antibodies, Frederick Alt and Raul Mostoslavsky (Harvard Medical School, USA) for the Cre-conditional Sirt1 null mice, Kevin Williams (UTSW Medical Center, USA) for helping with electrophysiological studies, Joel Elmquist (UTSW Medical Center, USA) and Bradford Lowell (Harvard Medical School, USA) for critical reading of the manuscript, and Dr. Aktar Ali and Laura Brule for metabolic assessments at the Mouse Metabolic Phenotyping Core at UTSW Medical Center (supported by PL1 DK081182-01 and 1UL1RR024923-01). This work was supported by the Paul F. Glenn Foundation for Medical Research (D.A.S.), the American Heart Association (Scientist Development Grant to R.C. and Postdoctoral Fellowship to G.R.), by the Italian Ministry of University and Research (PRIN 2005 to R.C.), and by National Institutes of Health Grants (DK080836 to R.C., RL1DK081185 to E.D.B., AG028730 and AG027916 to D.A.S.). D.A.S. is a Senior Fellow of the Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science (New York, NY. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chemical biology & drug design. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science (New York, NY. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. The New England journal of medicine. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr., et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. The New England journal of medicine. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nature reviews. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 Suppresses beta-Amyloid Production by Activating the alpha-Secretase Gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell metabolism. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The EMBO journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annual review of pathology. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Molecular endocrinology (Baltimore, Md. 1993;7:852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England journal of medicine. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. The EMBO journal. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. The Journal of clinical investigation. 2007b;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science (New York, NY. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. The Journal of clinical investigation. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- O'Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science (New York, NY. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal Activities of the Ventromedial and Lateral Hypothalamic Areas of Cats. Science (New York, NY. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. The Journal of biological chemistry. 2010 doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. The Journal of biological chemistry. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Coppari R. Pharmacological manipulations of CNS sirtuins: potential effects on metabolic homeostasis. Pharmacol Res. 2010;62:48–54. doi: 10.1016/j.phrs.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Gautron L, Fujikawa T, Vianna CR, Elmquist JK, Coppari R. Central Administration of Resveratrol Improves Diet-Induced Diabetes. Endocrinology. 2009 doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nature neuroscience. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. The American journal of physiology. 1991;261:E298–303. doi: 10.1152/ajpendo.1991.261.3.E298. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Tardieu S, Micallef J, Gentile S, Blin O. Weight gain profiles of new anti-psychotics: public health consequences. Obes Rev. 2003;4:129–138. doi: 10.1046/j.1467-789x.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, et al. Synaptic Glutamate Release by Ventromedial Hypothalamic Neurons Is Part of the Neurocircuitry that Prevents Hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008 doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, Coppari R. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152:11–18. doi: 10.1210/en.2010-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science (New York, NY. 2007;318:928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, Lee CE, Choi MJ, Lauzon DA, Dhillon H, et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010;12:88–95. doi: 10.1016/j.cmet.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.