Abstract
In rats, the long facial whiskers (mystacial macrovibrissae) are repetitively and rapidly swept back and forth during exploration in a behaviour known as ‘whisking’. In this paper, we summarize previous evidence from rats, and present new data for rat, mouse and the marsupial grey short-tailed opossum (Monodelphis domestica) showing that whisking in all three species is actively controlled both with respect to movement of the animal's body and relative to environmental structure. Using automatic whisker tracking, and Fourier analysis, we first show that the whisking motion of the mystacial vibrissae, in the horizontal plane, can be approximated as a blend of two sinusoids at the fundamental frequency (mean 8.5, 11.3 and 7.3 Hz in rat, mouse and opossum, respectively) and its second harmonic. The oscillation at the second harmonic is particularly strong in mouse (around 22 Hz) consistent with previous reports of fast whisking in that species. In all three species, we found evidence of asymmetric whisking during head turning and following unilateral object contacts consistent with active control of whisker movement. We propose that the presence of active vibrissal touch in both rodents and marsupials suggests that this behavioural capacity emerged at an early stage in the evolution of therian mammals.
Keywords: active sensing, touch, vibrissae, whisking, Monodelphis domestica
1. Introduction
Whiskers, or vibrissae, are prominent sinus hairs, found on nearly all mammals that act as specialized sensory organs for touch [1–4]. Rodents, such as rats and mice, have the ability to control the position and movement of their long facial whiskers (the mystacial microvibrissae) relative to the head [5]. This control is afforded by a specialized musculature, which includes dedicated muscles, one per whisker, that are intrinsic to the whisker pad, as well as muscles external to the pad that can move all of the whiskers together by shifting the pad or by transforming its shape [3,6–8]. The characteristic pattern of rat whisker movement, and also its principal component when viewed from above [9], is for all of the macrovibrissae (around 35 on each side of the snout) to move forward together (protract), and then be pulled back together (retract). This sweeping or scanning motion, known as ‘whisking’, is repeated successively, many times per second, for periods lasting several seconds at a time [7,10–12]. Typically, as the whiskers protract they also, but not always, fan out along the anterior–posterior axis [9,13] and, at the same time, rotate around the axis of the whisker shaft [12]. Rats and mice are capable of whisking at high frequencies (rates of over 20 Hz have been reported in mice [14,15]), and hence the whisking musculature of these animals contains a high proportion of type 2B muscle fibres that can support faster contractions than normal skeletal muscles [14].
Since rapid movement of the vibrissae consumes energy, and has required the evolution of specialized musculature, it can be assumed that whisking conveys some advantage to the animal. These observations motivate the proposal that whisking is a form of active sensing according to the definition used in this theme issue. That is, that animals whisk, and modulate their whisking behaviour in specific ways, in order to boost the acquisition of task-relevant sensory information [9,16]. To fully evaluate this hypothesis, two further steps are needed. First, we need to accurately describe the whisker and head movements of behaving animals under a range of different experimental conditions, including circumstances where the motivation of the animal can be reasonably well inferred (e.g. when it has been trained to perform some whisker-based tactile discrimination task). Second, we need to show that the types of whisker motion observed in animals, in these different conditions, positively enhance some relevant aspects of tactile sensing compared with either the absence of whisker motion, or to whisker motions that are unlike those that are naturally expressed. Progress is being made on both of these issues, described in more detail below. A parallel approach, that allows for direct measurement of the effects of sensor movement on vibrissal sensing, is to develop synthetic (robotic) whisker systems [17,18], then compare whisker control strategies similar to those seen in the rat, with strategies that differ in specific ways. A research programme directed at this challenge is described in a companion article in this theme issue [19]. Finally, an approach that allows us to explore the generality of active vibrissal sensing, and to investigate its appropriateness to the different adaptive zones exploited by whiskered animals, is comparative (cross-species) analysis, which is considered at length in this paper. However, before embarking on this wider investigation, we first briefly review some of the evidence so far gathered in relation to the active vibrissal touch in the rat.
(a). Active sensing strategies in rat whisking
Active sensing implies the ability to vary whisker control according to context. Conditioning studies have shown that rats can be trained to vary some of the key parameters of whisking, such as amplitude and frequency in a stimulus- or task-dependent manner [20–24]. Some persuasive evidence of the role of whisker control in sensing accuracy has been provided by Carvell & Simons [23] who showed that good and poor performers on two types of tactile discrimination tasks were distinguishable in terms of spatio-temporal characteristics of their whisker movements. Measures of whisker movement in that study also showed some consistent task-related differences in movement patterns between microgeometric (texture) and macrogeometric (shape) discrimination tasks. Zuo et al. [24] have shown that animals whose whiskers are trimmed are able to alter their whisker movements in order to improve their success on a texture discrimination task.
Further indicators that whisking is actively controlled are provided by studies of tactile exploration. High-speed video recordings show that the whisker movements of freely moving animals often diverge from the regular, bilaterally symmetric and synchronous motor patterns that are usually seen in head-restrained animals. Asymmetries, asynchronies and changes in whisk amplitude and timing have been noted, some of which appear likely to boost the amount of useful sensory information obtained by the animal [6,9,16,25]. Forms of whisking modulation that can be considered to be elements of active sensing control include the following.
First, it has been shown that during tactile search behaviour, whisking tends to be biased towards the direction in which the animal is turning its head [25]. That is, as the head moves in space, whisking movements appear symmetric around the future orientation of the head and are consequently asymmetric with respect to the current head orientation. We henceforth refer to this experimental observation as head-turning asymmetry (HTA). Interestingly, during rat neonatal development, the first whisking motions nearly always occur during turning, and on the side of the animal that is in the direction of the turn [26,27]. Thus, as the infant rat rotates, it begins to vibrate its whiskers in the direction in which it is moving, showing a similar anticipatory bias towards the area of free space into which the animal is moving. This might be considered to be an immature form of HTA.
Second, unilateral contact with a nearby surface tends to reduce whisking amplitude on the side of the snout ipsilateral to that contact and increase amplitude on the contralateral side [16]. We term this observation contact-induced asymmetry (CIA). CIA appears to function so as to prevent ipsilateral whiskers from bending hard against the contacted object, while increasing the number of contacts made with that object by whiskers on the contralateral side. CIA is observed in juvenile rats some time after the onset of bilateral whisking (typically delayed by a few days), suggesting that this form of control may be experience-dependent or rely on relatively late-maturing brain pathways [27].
Third, when the whiskers of the rat contact an unexpected object they cease to protract within a very short time period (typically within 15 ms) [9,16]. This rapid cessation of protraction (RCP) again serves to make whisker–surface contacts lighter than they might otherwise have been. In situations where there are unilateral contacts or where bilateral contacts are not simultaneous, RCP also appears to induce asynchronies in the relative timing of whisker movements on the two sides of the snout. Interestingly, when trained to make a specific discrimination, animals may modify this aspect of control and allow the whiskers to bend more strongly [28]. Further, when the whiskers touch a non-attended surface they may also show more pronounced bending [16], suggesting that RCP is not simply the consequence of some low-level reflex, but is related in some way to the current goals and focus of the animal.
Fourth, rats appear to regulate the angular separation, or spread, between the whisker columns such that the whiskers are widely spaced when exploring across a floor or whisking in open space, but are more closely bunched when the animal is investigating an object or surface of interest [9]. This bringing together of the whiskers appears to occur through differential control of the angular velocities of the more anterior and posterior whisker columns. Thus, during protraction in free-space, the more anterior columns rotate faster than the posterior ones causing the whiskers to spread out. During focused exploration of a surface, on the other hand, the anterior and posterior columns move with similar velocities, so that low angular separation of the columns is maintained throughout the whisk [9]. This more foveated style of whisker movement may also be accompanied by a forward shift in the set-point (average angular position) of the whiskers [7]. These aspects of control will tend to increase the number of whiskers that are brought to bear on an object of interest.
We can summarize the active sensing role of the whisking modulations described above as follows. HTA ensures that the manner in which the whiskers explore the space surrounding the head is biased towards the direction of travel; the rat preferentially investigates the area it is moving into next. CIA, RCP and the ability to create more foveated patterns of whisking, all appear to be consistent with a strategy of making contact with surfaces of interest on as many whiskers as possible, while minimizing bending (impingement) of those whiskers such that contacts are made with a light touch. We have therefore proposed that these modulations collectively promote a minimal impingement/maximal contact (MIMC) active sensing strategy for environment exploration [16].
While the focus here is on whisker movement, it is also important to note that the control of head position is also critical in determining what contacts are made by the whiskers with nearby surfaces [9]. Thus, for instance, while locomoting slowly across a smooth floor, the head is usually tilted down to allow the whiskers to sample the ground plane directly ahead of the animal. When a raised object is encountered, the head tilts upwards so that the whiskers are now better positioned to sample in the vertical plane. On encountering a novel object with the macrovibrissae, the rat will also typically perform an orienting head movement that will allow directed exploration around the point of contact with the array of shorter, non-actuated microvibrissae on the chin and lips, and with other sensory systems located around the tip of the snout, while the macrovibrissae sample regions of space to either side of the point of interest.
(b). Which other animals whisk?
Bouts of periodic whisker motion are a defining feature of how the rat deploys its vibrissae during exploration, but do other whiskered mammals use their vibrissae in a similar way? Answering this question will help us to determine how whisking, and whisking modulation, contribute to vibrissal sensing and in what circumstances the ability to move the whiskers is particularly important.
All therian mammals (marsupial and placental mammals), except humans, have tactile hair at some point in their lives [4], and even humans have recently been found to have vestigial sinus hair muscles in their upper lips [29]. Pocock [2] examined example specimens from all of the principal mammalian orders, concluding that facial vibrissae were present in at least some species in all orders except the monetremes, and that species that lacked whiskers, or in which the whiskers were less evident, were usually the more specialized members of their order. He concluded that the possession of facial whiskers was a primitive mammalian trait. He also noted that the vibrissae tended to be more pronounced in aquatic or semi-aquatic predators, and in active scansorial (climbing) or burrowing species. Huber [3], on reviewing the facial musculature of the different mammalian orders, reached a similar conclusion—that early mammals will have possessed prominent, mobile facial vibrissae and that the emergence of the vibrissal system played an influential role in establishing a common ground-plan for the mammalian facial musculature and in driving the re-organization and expansion of the neopallium (cortex).
Whisking certainly appears to be widespread in rodents. Specifically, in addition to many species of rats and mice, whisking has been reported by Welker [5] in flying squirrels (Glaucomys), gerbils (Meriones), chinchillas (Chinchilla) and hamsters (Mesocricetus). We have also recently filmed whisking in the hazel dormouse (Muscardinus), the European field and water voles (Arvicolinae), the coypu (Myocastor) and the prehensile-tail porcupine (Coendu). However, while whisking is common in rodents, it is not universal. For instance, capybara (Hydrochoerus) and gophers (Geomys) do not appear to whisk [5], and guinea pigs (Cavia) appear to display only irregular and relatively short whisking bouts [14]. Current data, therefore, suggest that the presence of whisking in animals belong to at least three of the five rodent sub-orders recognized by Carleton & Musser [30].
Further research is needed to investigate the commonalities between rodents that display vigorous whisking and those that do not. While phylogenetic relationships are clearly important, body-size and lifestyle also appear to matter; for instance, vigorous whisking has been hypothesized to be more common in nocturnal animals of smaller size [6]. The circumstances in which whisking is expressed also need to be further investigated. In seeking to elicit whisking in a range of species, we have found that it is most easily observed when animals are exploring their local environment in an unhurried manner. Exploratory behaviour in new surroundings, and towards novel objects, similar to that described by Shillito [31] in the short-tailed vole, can be seen in a range of rodents and is typically accompanied by prolonged whisking bouts.
Despite the prevalence of movable whiskers in mammals, there are relatively few descriptions of periodic whisker motion in non-rodents, although it remains unclear to what extent whisking is absent in other mammalian orders or simply under-reported. Welker [5] described observations suggesting the absence of whisking in a number of terrestrial and aquatic carnivores with prominent vibrissae, while Wineski [6] classified animals as showing rapid movement (small rodents), sporadic movement (larger rodents, some carnivores, lagomorphs), or as having relatively stationary whiskers (canids, chiropterans, ungulates, higher primates, edentates and cetaceans). Prominent vibrissae appear to be a characteristic of many carnivores, particularly those that are aquatic or semi-aquatic (e.g. seals and otters) or nocturnal (e.g. cats and martens). That whisking is not present in these animals, despite the ability to move the whiskers, suggests that it is not specifically an adaptation for prey capture. Andrew [32] describes whisking as being absent in primates such as lemurs and lorises. Among non-rodents, rapid motion of the vibrissae has recently been described in the Etruscan shrew (Suncus etruscus), a member of the Soricomorpha (shrew-like mammals) [33,34]. Interestingly, this shrew, which is an efficient insectivorous predator, appears to whisk during the searching phase of its hunting behaviour and not during the attack phase (once a prey animal has been located). We have recently filmed rhythmic whisking, during locomotion and exploration of objects, in an afrosoricidan, the greater hedgehog tenrec (Setifer setosus); periodic whisker motion has also been noted in the sengi (Elephantulus) [32]. Rice [35] observed that whisking is present in two species of marsupial—the Brazilian short-tailed opossum (Monodelphis domestica), and the Virginia opossum (Didelphis marsupialis). That whisking is seen in rodents and at least some Soricomorpha, Afrosoricidae and marsupials suggests the presence of this behavioural capacity in a common ancestor (greater than 125 million years ago) of extant marsupial and placental mammals [36] as proposed by Huber [3].
To explore possible commonalities in active vibrissal sensing, we decided to directly compare whisking in rodents and marsupials. Within rodents, we examined rats (Rattus norwegicus) as the species in which whisking has been described in most detail, and the mouse (Mus musculus) as a near-relative of the rat, but of significantly smaller body-size. Some of the general characteristics of mouse whisking have previously been described [14,15,28], with evidence of active sensing modulation of whisking found in gap-crossing [15] and object localization tasks [28]. For marsupials, we chose to examine the grey short-tailed opossum (M. domestica). There are several reasons for the choice of this animal. First, this small pouchless marsupial is considered to be a useful species from the perspective of tracing the evolution of modern mammals. Fossil evidence suggests the presence of opossum-like animals in the early Cretaceous period (greater than 100 mya) [36]; hence M. domestica may have many of the traits possessed by a common ancestor of both modern marsupial and placental mammals. In particular, the opossum brain has a smaller number of cortical areas than most mammals [37], lacks a distinct motor cortex, and has relatively few corticospinal neurons [38]. Thus, it may be reasonably representative of an ancestral mammalian brain (motor cortex and the corticospinal tract are both new in mammals). Second, Frost et al. [38] showed that movements of the vibrissae in M. domestica can be induced by microstimulation of sites in somatosensory cortex, and that this is the only part of the motor system that appears to be sensitive to cortical stimulation. This finding implies that control of the vibrissal system may have preceded the evolution of cortical control over the motor periphery, and that an examination of the vibrissal movement in this species might therefore cast some light on the evolution of cortical regulation of movement. Finally, like all marsupials, M. domestica lacks a corpus callosum [39]. Since the active control strategies described above in rats often involve differential control of whisking behaviour on the two sides of the snout, they will necessarily involve transfer of information across the midline of the brain. In rats, the corpus callosum has a very substantial whisker representation [40], which could conceivably be involved in regulating the asymmetries and asynchronies seen rat exploratory whisking; hence it would be interesting to observe whether a mammal lacking this structure is capable of similar forms of bilateral active whisking control.
2. Methods
(a). Animals and behavioural methods
The animals studied were RCS dystrophic (n = 12) and sighted Hooded Lister (n = 7) rats (R. norwegicus), C57 (n = 13) mice (M. musculus) and 26 opossums (M. domestica). The experimental set-up was similar to that described in Grant et al. [9]. Briefly, a high-speed, high resolution (1024 × 1024) digital video camera was suspended above a transparent viewing arena illuminated from below. The animal was placed into the arena through a hinged lid and allowed to explore the space freely. In some cases, a rectangular object, such as a Perspex block, was placed in the arena before the animal in order to elicit object exploration. Recordings (‘clips’), of 0.6–6 s duration, were made opportunistically by the experimenter, at 250 or 500 fps, until the camera memory was full or the animal stopped exploring. From among hundreds of clips, ‘episodes’ (particular sections of clips) were chosen that appeared to meet the criteria for each analysis as outlined below.
(b). Whisker tracking and extraction of the naive mean angle
In each episode chosen for analysis, the animal's snout and whiskers were tracked semi-automatically using the BIOTACT Whisker Tracking Tool. The output of this operation, for each frame, is the orientation of the snout (direction of the midline), the position of the snout tip and a set of parametrized curves fitted to whisker-like objects located adjacent to the snout [41], from which (presumed) whisker-base angles (relative to the midline of the head) are derived. For opossums, the algorithm was tuned to ensure that only mystacial vibrissae were detected and not the prominent genal (cheek) vibrissae. Note that while automatic tracking usually finds a similar set of whiskers in adjacent frames, there was no specific tracking of whiskers across frames. Episodes for which the automatic detection of the whiskers failed, as judged by visual inspection, were excluded from further analysis, as were episodes where substantial head pitch or roll was present.
Our analyses focus on the movement of the entire macrovibrissal array on each side of the head, rather than attempting to quantify the movement of single whiskers or whiskers columns. To this end, we sought to summarize the rostro-caudal angular position of the whiskers on each side. Such a summary has been implemented in previous work using, for example, the mean angle of the most rostral and most caudal whiskers [25] or a mean across the five caudal-most columns [9]. Given the nature of the data produced by the automatic tracker, we follow [41] by using the mean angle across all detected mystacial whiskers, low-pass filtered to reduce noise (30 Hz, zero-phase third-order Butterworth). We refer to this metric as the naive mean angle (NMA)—naive, because it ignores the fact that whisker detection is imperfect and variable between frames and between sides of the head. A comparison of NMA derived from automatic tracking with summary measures derived from manual tracking of a handful of episodes is given in the electronic supplementary material. Briefly, NMA appears to capture temporal parameters of periodic whisking (frequency and phase) accurately, relative spatial parameters (amplitude) slightly less so, and absolute spatial parameters (set-point) least accurately (though still reasonably well). Episodes in which the NMA was judged, by visual inspection, to be a poor summary of the position of the whiskers were excluded from further analysis.
(c). Analysis methods
In order to provide a general characterization of whisking within a species, we selected a number of episodes of whisker motion that occurred when the animal was moving its whiskers periodically without contacting anything other than the smooth floor (i.e. with a minimum of sources of whisker movement modulation). We use the term regular whisking (RW) to describe the patterns of whisker movement observed under these circumstances.
To obtain estimates of whisker kinematics from such an episode, we fitted stationary Fourier-type series models constrained to have the same primary frequency, fW, on both sides; fW was also restricted to 2–30 Hz. A one-component model, denoted F1, had four further (unconstrained) parameters (besides fW), the phase and amplitude of the single sinusoidal component on each side. A two-component model denoted F2, had, correspondingly, eight further parameters besides fW. The second component on each side of F2 was constrained to have a frequency of 2fW—that is, to represent a harmonic of the first component. The model included a pre-pended filter (zero-phase third-order Butterworth, cutoff frequency fW/3) to remove the slow change in whisking set-point, so that only the periodic part of the motion remained to be fitted by the Fourier components. Models were fitted using multi-starting-point least mean square with some heuristic interventions (see the electronic supplementary for details). The frequencies of the primary components, bilateral peak-to-peak amplitude of the multi-component signal and inter-lateral phase difference between the primary components, are then used to describe the periodic whisker motion present in that episode. In §3, we report peak-to-peak amplitude in two ways. The first (model-based) is the peak-to-peak amplitude of the F2 model. For the second (data-based), we measured the peak-to-peak amplitude of the NMA data, as the maximum minus the minimum across the episode. Set-point is calculated as the simple average of NMA across the episode.
The amplitude and phase of the second harmonic of F2 can be used to quantify some of the variability between episodes in the shape of the whisking profiles. We report the amplitude of the second harmonic as a fraction of the amplitude of the first (denoted ‘normalized’), and its phase as the number of degrees of phase of the first harmonic by which the nearest peak of the second harmonic precedes the peak of the first (hence, this value varies between −90° and +90°). To summarize the relative phase of the second harmonic, we also compute the power (relative amplitude squared) for each sample, and sum this in bins regularly distributed across phase, to form the ‘normalized power density’. The relationship between the phase of the second harmonic on the two sides was assessed using simple linear regression.
In addition to estimating the parameters of RW motion we analysed, for each species, the effect of head-turning and of object contact on bilateral whisker asymmetry (i.e. testing for the presence of HTA and CIA active sensing control strategies as defined above). Further details of each of these analyses are given in §3 and in the electronic supplementary material.
3. Results
(a). Regular whisking kinematics
Thirty one, 44 and 52 episodes of RW were identified (here, and below, numbers are for rat, mouse and opossum, respectively) and used to provide our general characterization of whisking kinematics in each species. While whisker movements in many episodes were broadly periodic, some episodes did not lend themselves to the fitting of a (stationary) Fourier series model, reflecting the fact that whisker movement does not always consist of exclusively periodic motion.
For each episode we first fitted an F1 model with a single sinusoidal component on each side. Estimates of whisking frequency, fW, derived from this model fell into a narrow range for rat, but were strongly bimodal in mouse, with peaks in the ranges 9–14 Hz and 20–26 Hz. Review of the individual traces and fits suggested that, rather than indicating whisking in two distinct frequency bands during different episodes, the latter peak was more likely to be indicating that a second harmonic in the data was sufficiently strong to be preferentially fitted by the model in these episodes. Reasoning that F1 therefore was not an adequate description of the data, we then fitted F2 models for all episodes, which included a second component at twice the primary frequency. Estimates of fW from F2 fell exclusively into a relatively narrow range in both rat (7–10 Hz) and mouse (9–13 Hz) episodes. In opossum, frequency displayed a broader peak, ranging from 3 to 11 Hz, and therefore the distributions returned by the two models were not clearly distinguished. All results presented below are derived from F2 (results from F1 are included in the electronic supplementary material, along with details of the semi-automatic model fitting process).
Episodes in which the final (F2) model explained less than 33 per cent of the variance in NMA (1, 7, 4 per species), as well as those less than two whisk periods in length after model fitting (6, 7, 13), were excluded from further analysis. This left 24, 30 and 35 episodes for the three species (mean duration across all species 540 ms). Examples of good F2 model fits are given in figure 1. In general, rat and opossum data allowed for convincing modelling (median explained variance 80 and 76%, respectively), whereas fits for mouse were poorer (median 62%). In addition, we estimated locomotion speed as the total distance travelled by the snout tip during the episode divided by the episode duration.
Figure 1.
Examples of the better fitting models included in the analysis, for (a) rat, (b) mouse and (c) opossum. Pale grey is raw NMA, blue/green are left/right smooth NMA, magenta is the set-point trend, and red is the bilateral sinusoidal model. Scale bars, 50 ms.
Summary statistics of whisking frequency and peak-to-peak amplitude averaged across the two sides (both data-based and model-based measures), interlateral phase difference and locomotion speed obtained from our analyses of RW in each species are given in table 1; plots of their distributions are shown in figure 2. A comparison between the kinematic variables measured from each individual in each species set is included in the electronic supplementary material—broadly, the results for individual animals are consistent with the results pooled over each species. Below, we note some of the most interesting characteristics of these data.
Table 1.
Cross-species comparison of whisking kinematics. Values shown are ‘mean (standard deviation)’. There was a significant overall difference in measures of regular whisking kinematics after controlling for differences in locomotion speed (MANCOVA: F10,164 = 16.806, p < 0.001). Differences for specific measures are computed using MANCOVA, with locomotion speed as a covariate, except for locomotion speed itself (ANOVA). Effect-size measure is partial η-squared, post hoc comparisons used a Bonferroni test.
| species | n | whisking frequency (Hz) | amplitude data-based (°) | amplitude model-based (°) | interlateral phase difference (°) | set-point (°) | locomotion speed (mm s−1) |
|---|---|---|---|---|---|---|---|
| rat (R) | 24 | 8.55 (0.62) | 43.19 (7.65) | 29.19 (7.14) | 1.67 (11.28) | 100.63 (9.21) | 96.65 (9.21) |
| mouse (M) | 30 | 11.35 (0.95) | 31.25 (11.64) | 16.21 (6.49) | 1.69 (29.96) | 112.53 (6.85) | 132.00 (8.24) |
| opossum (O) | 35 | 7.32 (1.91) | 36.04 (9.53) | 18.84 (5.10) | −1.38 (11.34) | 94.42 (9.01) | 71.66 (7.63) |
| F2,89 | 49.311 | 8.858 | 31.334 | 0.076 | 20.501 | 14.47 | |
| p | <0.001 | <0.001 | <0.001 | 0.927 | <0.001 | <0.001 | |
| effect size | 0.537 | 0.172 | 0.424 | 0.002 | 0.325 | 0.252 | |
| post hoc | M > R > O | R > M,O | R > M,O | n.a. | M > R,O | M > R,O |
Figure 2.
Kinematic parameters of regular whisking (RW). Distribution of kinematic parameters during RW (rat, mouse and opossum, from top to bottom). The mean of each distribution is marked as a vertical line.
Whisking frequency in both rat and mouse was relatively invariant across episodes; on average, mouse whisking is faster than that of rat (by approx. 35%), as might be expected from previous analyses [14]. Frequency in opossum was much more broadly distributed, covering the range observed in rat as well as including plenty of examples of much slower movement, leading to a somewhat slower average overall.
Peak-to-peak amplitude estimates derived from the data were typically 50–100% higher than those obtained from the model, primarily reflecting the fact that they include the movement in set-point during the episode (e.g. figure 1), and are reasonably similar to estimates from previous reports (e.g. [14]). We also provide the model-based estimate because it focuses on the amplitude of the periodic motion, ignoring lower frequency changes in set-point; the value of this metric in rat (about 30°) is consistent with a previous report [22]. Whisking amplitude based on either estimate was quite variable within each species; across species, mean estimates were significantly higher in rat, though the highest values of amplitude in all three species, using either estimate, were fairly similar.
Interlateral phase difference was relatively low (almost exclusively less than 20°) in rat and opossum, indicating strongly synchronous bilateral whisking. Some larger magnitude values were found in mouse; however, the lower model fitting scores (median explained variance 62%) and lower amplitude of whisking in mouse would both be expected to lead to noisier estimates of this parameter. Further investigation is required to establish whether bilateral synchrony is significantly different in these species. A recent description of whisker movements in head-fixed mice performing a localization task [28] does suggest that the bilateral movement can become decoupled in these animals.
The whisking set-point (mean angle throughout episode) was higher in mouse than in rat or opossum, indicating that mouse whiskers were on average more protracted than the other two species.
Locomotion speed varied significantly across species, mice being the fastest movers, then rats, then opossums. Whisking behaviour is likely to be affected by locomotion, although the relationship between body movement and whisking has yet to be adequately described. Note that the significant species differences in whisking kinematics reported in table 1 are after controlling for the variation in locomotion speed.
The second harmonic components of F2 are further analysed in figure 3. In both rat and opossum, the second harmonic was relatively weak (almost exclusively less than 30% of the amplitude of the first-model component), indicating that the whisker movements in our recordings of these species, obtained during free movement across a smooth floor, are fairly simple waveforms. However, in mouse, variability in movement shape was very much a feature of the data, and this is reflected in the high amplitude (almost exclusively greater than 30%) of the second harmonic in the F2 model. As shown, the phase and amplitude of the second harmonic, relative to the first, dictate the shape of the individual whisks. Note that in episodes with a very strong second harmonic, the data could be alternatively interpreted as whisking at the frequency of the second harmonic, as reflected in the frequency distributions generated by F1 (see the electronic supplementary material). No strong relationship was found between the phases of the second harmonics on the two sides in mouse, either in the full set (n = 30, r2 = 0.06, p = 0.21) or in a focused set including only the examples for which the mean second harmonic amplitude was above the median of the full set (n = 18, r2 = 0.01, p = 0.69).
Figure 3.
Characteristics of the second harmonic. (a) Second harmonic normalized amplitude is usually weak in rat (red) and opossum (blue), but usually strong in mouse (green). Normalized power density graphs show that the bulk of the second harmonic power in mouse falls between 0° and 60° prior to the peak of the primary—owing to weak harmonics in rat and opossum, phase is poorly defined. (b) The location of the second harmonic in phase-amplitude space determines the shape of the whisker movement. (c) Six examples taken from the data (blue) with overlaid F2 model (red), from different regions of phase-amplitude space (numbers inset to each panel correspond to numbers in lower left scattergram, showing the parameters of the second harmonic of the model for that example). (Panels to right) Model whisk profile (red) is formed from the primary (thick grey line) and secondary (thin grey line) components. (c) Panel (1, rat) is an example of a weak second harmonic, typical for rat and opossum, resulting in a fairly sinusoidal profile. Panels (2, opossum) and (3–6, mouse) show the shape of the whisk with the second harmonic in different parts of phase-amplitude space. Panel (6) shows a very strong second harmonic, swamping the first, such that the whisking could be interpreted as being high frequency (approx. 20 Hz); however, the best-fit model in this example has a primary frequency of approximately 10 Hz and a secondary at approximately 20 Hz. Scale barsare 50 ms and 10°.
(b). Head-turning asymmetry
Towal & Hartmann [25] reported that rats motivated to search in free space for a water reward displayed a very reliable HTA, such that the whiskers led head-turning movements (were symmetric around the future head orientation) by approximately the duration of one whisk (115 ms, matching the mean whisking frequency reported above). To assess whether HTA is expressed under our conditions of unconstrained exploratory behaviour, we reprised their analysis exactly, save for the use of automatic tracking.
For this analysis, 17, 29 and 48 episodes were identified for rat, mouse and opossum, respectively, wherein the animal was executing head turns and its whiskers were not contacting anything other than the floor. Estimates of bilateral asymmetry were calculated on a frame-by-frame basis as the difference (left–right) between the instantaneous NMAs on the two sides of the snout. Values close to zero indicate similar whisk amplitudes on the two sides of the snout, and positive/negative values biases towards the left/right sides of the animal. This value was compared with the angular velocity (positive values indicating anticlockwise) of the head at the same instant. Figure 4 displays the results, pooled within each species. Note that each panel is directly comparable with fig. 6a of Towal & Hartmann [25] for rat, but for the differences in experimental paradigm.
Figure 4.
Head-turning asymmetry. (a) Instantaneous bilateral asymmetry in NMA plotted against angular head velocity for rat (top), mouse (middle) and opossum (bottom). Solid lines show linear regression, dashed lines indicate strength of relationship reported by Towal & Hartmann [25]. A similar trend is observed in all species (tendency to explore in the direction of head turning), but is weaker than in the original study that employed a tactile search paradigm. (b) Three frames from an episode of HTA in rat showing the asymmetry in the whisking pattern through a head-turn.
All species expressed robust HTA (R ≥ 0.40, p < 0.001) of the expected polarity (i.e. turning to the left predicted greater protraction on the right, see inset example in figure 4), but the magnitude of the response was consistently less strong in all species than that reported previously (coefficient of linearity is 28 to 35 compared with 115 in Towal & Hartmann [25]). Thus, while HTA is clearly expressed under conditions of free exploration, and in all tested species, the modulation may be less strong than in the tactile search paradigm employed in the original study. The apparently lesser strength of HTA modulation in free exploration may reflect a real reduction owing to the less focused nature of the task, or it might be due to confounding with other effects that were less pronounced in the particular condition targeted by Towal & Hartmann. For instance, contacts with the floor may be a significant driver of whisking behaviour (in the previously reported condition, there was no floor present). Our main finding, however, is that all three species express this effect robustly.
(c). Contact-induced asymmetry
In previous work in our laboratory [16], we reported that the location of a nearby wall (vertical plane obstacle) was a strong predictor of whisking asymmetry—that is, a difference in bilateral whisking amplitude with increased amplitude, as measured through recordings of mystacial muscle electromyogram, on the side of the snout furthest from the wall and reduced amplitude on the side closest to the wall. That study also showed that an obstacle directly ahead tended to excite protraction bilaterally. We reprised this analysis of CIA across the three species examined in the current study, but using automated whisker tracking in place of EMG.
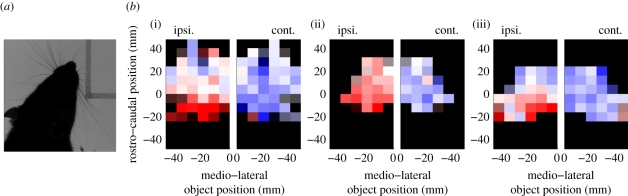
Twenty four, 23 and 44 clip regions were identified for rat, mouse and opossum, respectively, in which the animal was interacting with one corner of a smooth rectangular object with the object ahead of the animal and no other nearby obstructions, other than the floor, that could contact the whiskers. The location of the object was summarized by manual identification of its corner; this location was then transformed into a ‘head-centric’ x–y coordinate system with the origin at the snout tip and the animal facing upwards (along the positive y-axis). Thus, the object ‘moves around’ in the head-space of the animal. The NMA measure for each side of the snout was low-pass filtered (zero-phase 500 ms boxcar) to remove the periodic component and leave only an estimate of set-point. This number was then normalized by subtracting from this signal the mean set-point for the species during RW (table 1) to give the instantaneous relative set-point for each side of the snout. Thus, we obtained two numbers for each frame, with positive/negative indicating more/less protracted whisker positions on a given side than was typical for RW episodes recorded for that species. These numbers were then pooled across all episodes for each species. Finally, assuming that the animals did not express any behavioural bias to one side or the other, samples with the object on the right-hand side of the head were flipped, so that the object location was always on the left-hand side—this allows us to consider movements of the two whisker arrays classified as either ipsilateral or contralateral to the obstruction. Ipsilateral object location was then binned on a regular grid, and the average relative set-point calculated for each bin; the results are displayed in figure 5. Our main finding is that all tested species robustly displayed the expected basic pattern of CIA—reduced protraction ipsilaterally, increased contralaterally. Increased protraction, for objects directly ahead, reported in rats by Mitchinson et al. [16] was apparent in opossum, less so in rat, and not evident in mouse.
Figure 5.
Contact-induced asymmetry. (a) One frame from an episode of CIA in rat showing the asymmetry in the whisking pattern in the presence of an obstruction. (b) For each species ((i) rat, (ii) mouse and (iii) opossum) there are two grids. The left-hand grid shows results for the side nearest (ipsilateral) to the object, the right-hand grid shows movement on the opposite (contralateral) side. The colour of each cell indicates average relative set-point for the ipsilateral/contralateral whisker array for an ipsilateral object in the indicated 8 mm×8 mm region relative to the tip of the snout which is at [0,0]. Red indicates that on average the whiskers are less protracted than in RW for an obstacle in that location, blue that the whiskers are more protracted, and white that the set-point is similar to average values. Darker shades indicate limited (n < 10) data points for that cell with black indicating no data. Colours are fully saturated for relative set-point of ±20°. Note that for each grid decreasing values along the x-axis always denote object positions further away from the snout on the ipsilateral side. Plots for all species show reduced ipsilateral protraction (lower set-point) and increased contralateral protraction (higher set-point), consistent with previous results in rat alone [16].
4. Discussion
(a). Describing the movement of vibrissal arrays
The characteristic sweeping motion of mammalian whisking can be measured in an intact whisker field by automated processing of high-speed digital video sequences [41], which extracts the instantaneous mean angle of the macrovibrissal array. Using data from RW episodes in two species of rodent and one marsupial, we have shown (figures 1–3) that the change over time in this measure can be described with reasonable accuracy using a two-component Fourier series composed of a fairly invariant fundamental frequency (in rat and mouse) and its second harmonic. For mouse data, this two-component series improved the accuracy of fit by 19 per cent (median R2) compared with fitting a series based on a single frequency; smaller improvements in fit (4 and 10%, respectively) were found for whisking in rat and opossum, reflecting the simpler characteristics of the recorded motions.
Mouse whisking has been described as occurring at high frequencies with a mean of around 20 Hz [14,15]; however, whisking at lower frequencies around or below 10 Hz has also been reported [42–44]. Whisking in rat has been described as falling into two different frequency bands by Berg & Kleinfeld [7] and characterized as ‘exploratory’ (range 5–15 Hz) and ‘foveal’ whisking (15–25 Hz), the latter reported as occurring specifically when animals were trained to crane across a gap to investigate a food tube. At the same time, rodent whisking patterns are often reported to be highly variable [6,22,28,45]. One form of variability is where multiple (typically two) peaks are observed to occur within a single forward–backward sweep; these ‘double pumps’ have been described in both hamsters [6] and rats [45]. A specific pattern that we have observed in mouse whisking data (figure 3, traces 4 and 5) and that has also been described for hamster [6], is an alternating sequence of larger and smaller pumps. Traces of this type of secondary oscillation can also be seen in other published mouse whisking profiles (see fig. 3 of [15]) suggesting that this is not an artefact of our behavioural task or of our tracking algorithm.
When we modelled the mouse whisker angle series using a single component model (F1), the distribution of frequency of the motion was strongly bimodal (see the electronic supplementary material for results), with the upper peak at about twice the frequency of the lower peak, a location consistent with our interpretation of the high-frequency data as second harmonics. However, when mouse whisking was modelled using a two component model (F2), we identified a continuum of profiles that moves through the amplitude-phase space of the second harmonic. Smooth, almost sinusoidal, whisking lies at one extreme (primary frequency dominant) and whisking that appears to be at double-frequency at the other (second harmonic dominant). In between, the alternating sequences of larger and smaller pumps in figure 3 are captured as a Fourier series consisting of synchronized oscillations of two harmonics with a primary:secondary amplitude ratio of around 3 : 2. Other points in the space generate similarly rich kinematic patterns.
Given that the two-component modelling approach provides a mechanism for the generation of whisking at the fundamental frequency, at twice that frequency, and in various mixed profile patterns consistent with observed data, it seems parsimonious to posit that modes with different underlying frequencies are not present in our mouse data. Indeed, the success of the F2 model suggests to us that in both mouse and rat distinct frequency ‘modes’ and different types of movement profile could be described parsimoniously as having the same origins. Thus, the ‘underlying’ whisking frequency may remain relatively invariant (in a band of a few hertz) in both species, with examples of higher-frequency motion being reinterpreted as owing to a strong second harmonic, rather than a change in the fundamental frequency. On the basis of this interpretation, it would be interesting, for example, to investigate whether rat foveal whisking shows any evidence of an underlying fundamental frequency in the exploratory whisking range. Data from Carvell & Simons [22], in a similar task where the rat is reaching across a gap to investigate a texture, show significant power at the second harmonic above a primary frequency of 8 Hz (compare fig. 7a from [22] with our supplementary figure A9) consistent with the possibility of a two-component whisking pattern in rats. Complex movement profiles, such as double pumps, may also be explicable as arising from blends of fundamental frequency and second harmonic at specific phase offsets.
We have so far not observed high-frequency whisking, or whisking with a large second harmonic component, in opossum (the apparently broad range of whisking frequencies expressed in opossum might, in any case, make whisking in different ‘bands’ difficult or impossible to distinguish). In this context, and in order to explore whether the second harmonic component is a later development in evolutionary terms, it would be useful to investigate opossum whisking behaviour in similar circumstances to those that elicit foveal whisking in the rat.
As a descriptive tool, the two-component Fourier series allows a good characterization of the visibly rich patterns of whisking seen in different species using a model with only a small number of parameters. As such it provides an alternative to the ‘whisk cycle’ as a descriptive element with which to characterize vibrissal behaviour, and which has proved to be problematic for the analysis of the many whisking profiles in which the ‘boundaries’ between whisk events are hard to discern. One of the benefits of such a description may be to assist efforts to identify the pattern generator mechanisms underlying periodic vibrissal motion. For instance, in mice this analysis might prompt us to look for component systems that oscillate in the lower frequency band and that are then modulated to form drive signals that accentuate the second harmonic.
We noted no differences between the behaviour of our RCS and Hooded Lister animals; furthermore, no evidence of differences in quantitative kinematic metrics between rat strains has so far been reported in the wider literature [7,9,22]. Nonetheless, we note the possibility that both quantitative and qualitative metrics, including those reported here, may vary with animal strain.
(b). Whisking as a form of active touch
This theme issue presents the case that active sensing is not simply about movement of the sensor, but about control of the sensor in a manner that will boost task-relevant information. Although further evidence needs to be gathered, a picture is beginning to emerge about the nature of active sensing control in whisking animals.
A fundamental question is why animals whisk. Though the circumstances in which whisking occurs are in need of further characterization, it is possible to say the following.
Moving the whiskers allows more degrees of freedom for sensor positioning, the possibility of sampling a larger volume of space with a given density of whiskers, and the ability to control the velocity with which the whiskers contact the surface. Periodic whisker movements allow control of the frequency of contacts and the possibility of many distinct contacts per second. Whisking animals appear to be able to exert significant control over the area of space selected for exploration by the whiskers, making it wider or narrower (control of amplitude and spread), more anterior or more posterior (control of set-point), dependent on context. Thus, when whisking to locate objects in free space animals make large amplitude sweeps, when whisking across a gap they shift the set-point of the whisk forwards, when turning they bias exploration in the direction of movement, and when they contact an object unilaterally, the whiskers on the contralateral side sweep round to gather more information about the detected object.
Not all animals with prominent whiskers show periodic whisker motion. The benefits of whisking are probably enhanced by the need to operate in low or zero light while negotiating complex terrain; hence the apparent importance of whiskers and whisking in many species of nocturnal climbing animals. The benefits appear to be reduced, though not entirely eliminated (witness the coypu), in larger animals whose heads are more distant from the ground. Periodic motion of the whiskers does not appear to be critical in the final stages of prey capture. It is notable that whisker movement emerges alongside forward ambulation [27]. This reinforces the view that whisking is fundamentally a strategy for exploring nearby space, identifying properties of immediate relevance to the animal, such as the presence of surfaces that can support locomotion, and selecting locations that warrant further investigation by orienting the multi-sensory zone surrounding the tip of the snout.
(c). The evolution of active vibrissal touch
Based on a comparative analysis of mammalian facial musculature, Huber [3] proposed that the evolution of mobile vibrissae shaped not only the evolution of the face muscles, but also the organization of the trigeminal complex, and played an influential role in the early evolution of cortex. The data presented in the current paper lend support to this view. In particular, it seems unlikely—given the evidence of periodic whisker motion in a diverse range of species including rodents, Afrotheria and marsupials—that whisking emerged late on in evolution in a convergent fashion across multiple animal classes. Rather, the principle of parsimony would suggest that this was a primary mode of interaction with the world for an early common ancestor of therian mammals. If this common ancestor whisked, it seems likely that it also actively controlled its whisking movements in the fashion described above as common to rat, mouse and opossum, biasing exploration in the direction of travel and towards surfaces of interest. The vibrissal system of murid rodents is likely to be more sophisticated than that of opossums and the manner in which it is so may well be due to the role of motor cortex and of the corpus callosum, both of which have large whisker representations and are absent in the opossum. Interestingly though, our observations of opossum whisking suggest that neither structure is critical to the communication of information between the two halves of the brain that support CIA in bilateral whisking. That is, the commissural connections at the thalamic or brainstem level of the marsupial brain appear to be sufficient for the whiskers on one side of the snout to know something of what those on the other side are sensing and respond accordingly.
Further behavioural, anatomical and neurophysiological investigations of the marsupial whisker system should cast valuable light on how our early mammalian ancestors used their vibrissae to explore the world, and how this activity shaped and influenced the evolution of mammalian brains and bodies. What we might already say, however, is that alongside thermoregulation, the birth of live young and the expansion of cortex, the emergence of active vibrissal touch was a further critical milestone along the evolutionary path to modern therian mammals.
Acknowledgements
The authors are grateful to Antonello Mallamachi and Marco Stebel for providing access to the opossum colony at the University of Trieste animal facility, to Hazel Ryan and the Wildwood Trust, Canterbury, UK for allowing access to various native UK rodent species and the coypu, and to Washington zoo for allowing filming of the coendu and tenrec. We would like to thank members of the Active Touch Laboratory at Sheffield University for their advice and help, particularly Charles Fox, for assisting in the development of analysis methods, and Mathew Evans and Stuart Wilson for help in collecting comparative data. Video analysis was performed using the BIOTACT Whisker Tracking Tool which was jointly created by the International School of Advanced Studies in Trieste, the University of Sheffield and the Weizmann Institute in Rehovot under the auspices of the FP7 BIOTACT project (ICT 215910), which also funded the study. We are particularly grateful for the contribution of Goren Gordon to programming parts of the tracking tool. Kendra Arkley was supported by an EPSRC doctoral training award.
References
- 1.Vincent S. B. 1912. The function of the vibrissae in the behaviour of the white rat. Behav. Monogr. 1, 1–82 [Google Scholar]
- 2.Pocock R. I. 1914. On the facial vibrissae of mammalia. Proc. Zool. Soc. Lond. 84, 889–912 10.1111/j.1469-7998.1914.tb07067.x (doi:10.1111/j.1469-7998.1914.tb07067.x) [DOI] [Google Scholar]
- 3.Huber E. 1930. Evolution of the facial musculature and cutaneous field of the trigeminus. 1. Q. Rev. Biol. 5, 133–188 10.1086/394355 (doi:10.1086/394355) [DOI] [Google Scholar]
- 4.Ahl A. S. 1986. The role of vibrissae in behavior—a status review. Vet. Res. Commun. 10, 245–268 10.1007/BF02213989 (doi:10.1007/BF02213989) [DOI] [PubMed] [Google Scholar]
- 5.Welker C. I. 1964. Analysis of sniffing in the albino rat. Behaviour 22, 223–244 10.1163/156853964X00030 (doi:10.1163/156853964X00030) [DOI] [Google Scholar]
- 6.Wineski L. E. 1983. Movements of the cranial vibrissae in the golden-hamster (Mesocricetus auratus). J. Zool. 200, 261–280 10.1111/j.1469-7998.1983.tb05788.x (doi:10.1111/j.1469-7998.1983.tb05788.x) [DOI] [Google Scholar]
- 7.Berg R. W., Kleinfeld D. 2003. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J. Neurophysiol. 89, 104–117 10.1152/jn.00600.2002 (doi:10.1152/jn.00600.2002) [DOI] [PubMed] [Google Scholar]
- 8.Haidarliu S., Simony E., Golomb D., Ahissar E. 2010. Muscle architecture in the mystacial pad of the rat. Anat. Rec. (Hoboken). 293, 1192–1206 [DOI] [PubMed] [Google Scholar]
- 9.Grant R. A., Mitchinson B., Fox C., Prescott T. J. 2009. Active touch sensing in the rat: anticipatory and regulatory control of whisker movements during surface exploration. J. Neurophysiol. 101, 862–874 10.1152/jn.90783.2008 (doi:10.1152/jn.90783.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zucker E., Welker W. I. 1969. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 12, 138–156 10.1016/0006-8993(69)90061-4 (doi:10.1016/0006-8993(69)90061-4) [DOI] [PubMed] [Google Scholar]
- 11.Bermejo R., Friedman W., Zeigler H. P. 2005. Topography of whisking II: interaction of whisker and pad. Somatosens. Mot. Res. 22, 213–220 10.1080/08990220500262505 (doi:10.1080/08990220500262505) [DOI] [PubMed] [Google Scholar]
- 12.Knutsen P. M., Biess A., Ahissar E. 2008. Vibrissal kinematics in 3D: tight coupling of azimuth, elevation and torsion across different whisking modes. Neuron 59, 35–42 10.1016/j.neuron.2008.05.013 (doi:10.1016/j.neuron.2008.05.013) [DOI] [PubMed] [Google Scholar]
- 13.Sachdev R. N., Sato T., Ebner F. F. 2002. Divergent movement of adjacent whiskers. J. Neurophysiol. 87, 1440–1448 [DOI] [PubMed] [Google Scholar]
- 14.Jin T. E., Witzemann V., Brecht M. 2004. Fiber types of the intrinsic whisker muscle and whisking behavior. J. Neurosci. 24, 3386–3393 10.1523/JNEUROSCI.5151-03.2004 (doi:10.1523/JNEUROSCI.5151-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voigts J., Sakmann B., Celikel T. 2008. Unsupervised whisker tracking in unrestrained behaving animals. J. Neurophysiol. 100, 504–515 10.1152/jn.00012.2008 (doi:10.1152/jn.00012.2008) [DOI] [PubMed] [Google Scholar]
- 16.Mitchinson B., Martin C. J., Grant R. A., Prescott T. J. 2007. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc. R. Soc. B 274, 1035–1041 10.1098/rspb.2006.0347 (doi:10.1098/rspb.2006.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson M. J., Pipe A. G., Melhuish C., Mitchinson B., Prescott T. J. 2007. Whiskerbot: a robotic active touch system modeled on the rat whisker sensory system. Adapt. Behav. 15, 223–240 10.1177/1059712307082089 (doi:10.1177/1059712307082089) [DOI] [Google Scholar]
- 18.Prescott T. J., Pearson M. J., Mitchinson B., Sullivan J. C. W., Pipe A. G. 2009. Whisking with robots: from rat vibrissae to biomimetic technology for active touch. IEEE Robot. Autom. Mag. 16, 42–50 10.1109/MRA.2009.933624 (doi:10.1109/MRA.2009.933624) [DOI] [Google Scholar]
- 19.Pearson M. J., Mitchinson B., Sullivan J. C., Pipe A. G., Prescott T. J. 2011. Biomimetic vibrissal sensing for robots. Phil. Trans. R. Soc. B 366, 3085–3096 10.1098/rstb.2011.0164 (doi:10.1098/rstb.2011.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermejo R., Harvey M., Gao P., Zeigler H. P. 1996. Conditioned whisking in the rat. Somatosen. Mot. Res. 13, 225–233 10.3109/08990229609052578 (doi:10.3109/08990229609052578) [DOI] [PubMed] [Google Scholar]
- 21.Gao P., Ploog B. O., Zeigler H. P. 2003. Whisking as a ‘voluntary’ response: operant control of whisking parameters and effects of whisker denervation. Somatosens. Mot. Res. 20, 179–189 10.1080/08990220310001623031-411 (doi:10.1080/08990220310001623031-411) [DOI] [PubMed] [Google Scholar]
- 22.Carvell G. E., Simons D. J. 1990. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 10, 2638–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvell G. E., Simons D. J. 1995. Task- and subject-related differences in sensorimotor behavior during active touch. Somatosens Mot. Res. 12, 1–9 10.3109/08990229509063138 (doi:10.3109/08990229509063138) [DOI] [PubMed] [Google Scholar]
- 24.Zuo Y., Perkon I., Diamond M. E. 2011. Whisking and whisker kinematics during a texture classification task. Phil. Trans. R. Soc. B 366, 3058–3069 10.1098/rstb.2011.0161 (doi:10.1098/rstb.2011.0161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Towal R. B., Hartmann M. J. 2006. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J. Neurosci. 26, 8838–8846 10.1523/JNEUROSCI.0581-06.2006 (doi:10.1523/JNEUROSCI.0581-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landers M., Haidarliu S., Philip Zeigler H. 2006. Development of rodent macrovibrissae: effects of neonatal whisker denervation and bilateral neonatal enucleation. Somatosens. Mot. Res. 23, 11–17 10.1080/08990220600700784 (doi:10.1080/08990220600700784) [DOI] [PubMed] [Google Scholar]
- 27.Grant R. A., Mitchinson B., Prescott T. J. In press The development of whisker control on rats in relation to locomotion. Dev. Psychobiol. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor D. H., Clack N. G., Huber D., Komiyama T., Myers E. W., Svoboda K. 2010. Vibrissa-based object localization in head-fixed mice. J. Neurosci. 30, 1947–1967 10.1523/JNEUROSCI.3762-09.2010 (doi:10.1523/JNEUROSCI.3762-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamatsu Y., Tsukahara K., Hotta M., Shimada K. 2007. Vestiges of vibrissal capsular muscles exist in the human upper lip. Clin. Anat. 20, 628–631 10.1002/ca.20497 (doi:10.1002/ca.20497) [DOI] [PubMed] [Google Scholar]
- 30.Carleton M. D., Musser G. G. 2005. Order Rodentia. In Mammal species of the world: a taxonomic and geographic reference (eds Wilson D. E., Reeder D. M.), pp. 745–752, 3rd edn Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 31.Shillito E. E. 1963. Exploratory behaviour in the short-tailed vole Microtus agrestis. Behaviour 21, 145–154 10.1163/156853963X00149 (doi:10.1163/156853963X00149) [DOI] [Google Scholar]
- 32.Andrew R. J. 1963. The origin and evolution of the calls and facial expressions of the primates. Behaviour 20, 1–109 10.1163/156853963X00220 (doi:10.1163/156853963X00220) [DOI] [Google Scholar]
- 33.Anjum F., Turni H., Mulder P. G., van der Burg J., Brecht M. 2006. Tactile guidance of prey capture in Etruscan shrews. Proc. Natl Acad. Sci. USA 103, 16 544–16 549 10.1073/pnas.0605573103 (doi:10.1073/pnas.0605573103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munz M., Brecht M., Wolfe J. 2010. Active touch during shrew prey capture. Front. Behav. Neurosci. 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice F. L. 1995. Comparative aspects of barrel structure and development. In Cerebral cortex, vol. 2: The barrel cortex of rodents (eds Jones E. G., Diamond I. T.). New York, NY: Plenum Press [Google Scholar]
- 36.Ji Q., Luo Z. X., Yuan C. X., Wible J. R., Zhang J. P., Georgi J. A. 2002. The earliest known eutherian mammal. Nature 416, 816–822 10.1038/416816a (doi:10.1038/416816a) [DOI] [PubMed] [Google Scholar]
- 37.Wong P., Kaas J. H. 2009. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica). Brain Behav. Evol. 73, 206–228 10.1159/000225381 (doi:10.1159/000225381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost S. B., Milliken G. W., Plautz E. J., Masterton R. B., Nudo R. J. 2000. Somatosensory and motor representations in cerebral cortex of a primitive mammal (Monodelphis domestica): a window into the early evolution of sensorimotor cortex. J. Comp. Neurol. 421, 29–51 (doi:10.1002/(SICI)1096-9861(20000522)421:1<29::AID-CNE3>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 39.Karlen S. J., Krubitzer L. 2007. The functional and anatomical organization of marsupial neocortex: evidence for parallel evolution across mammals. Prog. Neurobiol. 82, 122–141 10.1016/j.pneurobio.2007.03.003 (doi:10.1016/j.pneurobio.2007.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alloway K. D., Smith J. B., Beauchemin K. J., Olson M. L. 2009. Bilateral projections from rat MI whisker cortex to the neostriatum, thalamus, and claustrum: forebrain circuits for modulating whisking behavior. J. Comp. Neurol. 515, 548–564 10.1002/cne.22073 (doi:10.1002/cne.22073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkon I., Kosir A., Itskov P. M., Tasic J. F., Diamond M. E. 2011. Unsupervised quantification of whisking and head movement in freely moving rodents. J. Neurophysiol. 105, 1950–1962 10.1152/jn.00764.2010 (doi:10.1152/jn.00764.2010) [DOI] [PubMed] [Google Scholar]
- 42.Woolsey T. A., Durham D., Harris R., Simons D. J., Valentino K. 1981. Somatosensory development. In The development of perception: psychobiological perspectives (eds Aslin R. N., Pisani D. B.), pp. 259–292 New York, NY: Academic Press [Google Scholar]
- 43.Kiryakova S., et al. 2010. Recovery of whisking function promoted by manual stimulation of the vibrissal muscles after facial nerve injury requires insulin-like growth factor 1 (IGF-1). Exp. Neurol. 222, 226–234 10.1016/j.expneurol.2009.12.031 (doi:10.1016/j.expneurol.2009.12.031) [DOI] [PubMed] [Google Scholar]
- 44.Sohnchen J., et al. 2010. Recovery of whisking function after manual stimulation of denervated vibrissal muscles requires brain-derived neurotrophic factor and its receptor tyrosine kinase B. Neuroscience 170, 372–380 10.1016/j.neuroscience.2010.06.053 (doi:10.1016/j.neuroscience.2010.06.053) [DOI] [PubMed] [Google Scholar]
- 45.Towal R. B., Hartmann M. J. 2008. Variability in velocity profiles during free air whisking behavior of unrestrained rats. J. Neurophysiol. 100, 740–752 10.1152/jn.01295.2007 (doi:10.1152/jn.01295.2007) [DOI] [PubMed] [Google Scholar]