Abstract
Microarray-based transcriptional profiling was used to determine the effect of progesterone in the cortical contusion (CCI) model. Gene ontology (GO) analysis then evaluated the effect of dose on relevant biological pathways. Treatment (vehicle, progesterone 10 mg/kg or 20 mg/kg given i.p.) was started 4 h post-injury and administered every 12 h post-injury for up to 72 h, with the last injection 12 hr prior to death for the 24 h and 72 h groups. In the CCI-injured vehicle group compared to non-injured animals, expression of 1,114, 4,229, and 291 distinct genes changed >1.5-fold (p<0.05) at 24 h, 72 h, and 7 days, respectively. At 24 h, the effect of low-dose progesterone on differentially expressed genes was <20% the effect of higher dose compared to vehicle. GO analysis identified a significant effect of low- and high-dose progesterone treatment compared to vehicle on DNA damage response. At 72 h, high-dose progesterone treatment compared to vehicle affected expression of almost twice as many genes as did low-dose progesterone. Both low- and high-dose progesterone resulted in expression of genes regulating inflammatory response and apoptosis. At 7 days, there was only a modest difference in high-dose progesterone compared to vehicle, with only 14 differentially expressed genes. In contrast, low-dose progesterone resulted in 551 differentially expressed genes compared to vehicle. GO analysis identified genes for the low-dose treatment involved in positive regulation of cell proliferation, innate immune response, positive regulation of anti-apoptosis, and blood vessel remodeling.
Key words: CCI injury model, gene expression, neurotrauma, progesterone
Introduction
The Centers for Disease Control and Prevention have stated that traumatic brain injury (TBI) is among the leading causes of acute and chronic disability in the United States, and that each year 1.7 million people there sustain a TBI, and 52,000 die (Centers for Disease Control, 2010). A recent study of hospitalization rates associated with active duty United States Army personnel found a 105% increase in TBI-associated hospitalization from 2000 to 2006 (Ivins, 2010). Because the primary causes of TBI are motor vehicle collisions, falls, and violence, all individuals are at risk of sustaining a TBI sometime during their lives (Langlois et al., 2006). Although more individuals survive TBI now than in the past, the survivors endure residual physical, cognitive, emotional and/or behavioral impairments from the cascade of central pathological responses resulting from TBI.
Brain injury in the United States, therefore, warrants investigation, because it is a major public health issue. However, there has been a problem with translation of effective pre-clinical treatments to the clinical setting (Narayan et al., 2002; Statler et al., 2001). The National Institute of Neurological Disorders and Stroke (NINDS) organized a workshop of experts to provide insights and recommendations based on lessons learned from previous clinical trials. The recommendations included: the use of at least two rodent models; that the pre-clinical model should reflect the severity of the injury found clinically; evaluation of both focal and diffuse injury; and evaluations of animal pharmacokinetics to establish the dosage regimen, ensure brain penetration, and establish dose–response curves and optimal dosing windows (Narayan et al., 2002).
The etiology of secondary brain injury is multi-factorial, with a host of likely inter-related processes including mitochondrial energy failure; excessive generation of reactive oxygen species; activation of destructive enzymes such as poly (ADP-ribose) polymerase (PARP), and the caspase family of proteases; membrane disruption; neuronal death; thrombosis caused by intravascular coagulation in small vessels; increased synaptic concentrations of excitatory amino acids; and activation of innate inflammatory responses (Schouten, 2007). Several reviews have suggested the need for pharmacological treatments that target multiple secondary factors, or combination treatment strategies (Faden and Stoica, 2007; Schouten, 2007).
A potential promising treatment for TBI is progesterone, which has been studied in pre-clinical models of TBI for several decades (Stein, 2005). For current reviews of the progesterone literature see Sayeed and Stein, 2009; Stein, 2008; Stein and Wright, 2010; Vink and Nimmo, 2009. A systemic review of progesterone treatment in experimental TBI concluded that progesterone reduced lesion volume in a dose-dependent manor if administered immediately following injury or not later than 6 h post-injury (Gibson et al., 2008). In male rats receiving progesterone at a dose of 4 mg/kg by intraperitoneal (i.p.) injection at 1, 6, and 24 h after cortical contusion injury (CCI), serum progesterone concentrations at 1, 6, and 48 h were shown to correlate with decreased cerebral edema (Wright et al., 2001). Serum concentrations ranged from 0 to 38 ng/mL and from 5 to 12 ng/mL at 1 and 6 h, respectively. This is consistent with the original observation that male rats had significantly more edema than female rats after cortical contusions (Roof et al., 1993). Mechanistically, it has been shown that progesterone (8 mg/kg, i.p.) attenuates the production of the pro-inflammatory cytokines, IL-1β and TNFα after TBI when given 1 h and 6 h after injury (He et al., 2004), although the effect of progesterone was limited to decreasing the peak expression at 3 h in the rat model with no effect at 8 and 24 h. Even with significant pre-clinical work with progesterone, there have been some concerns with dosing regimens. A dose–response study evaluated progesterone at either 8, 16, or 32 mg/kg administered at 1, 6, and 24 h post-CCI with repeated administrations every 24 h for an additional 4 days following CCI (Goss et al., 2003). The initial dose was administered i.p. and all subsequent injections were subcutaneous. It was found that the 8 and 16 mg/kg doses improved water maze performance compared to the 32 mg/kg dose, and that none of the doses significantly decreased lesion size (Goss et al., 2003). Similarly, a study in older rats also found that the 32 mg/kg dose resulted in significantly fewer beneficial effects on inflammatory factors than 8 mg/kg and 16 mg/kg at 48 h post-injury (Cutler et al., 2007).
There have also been two clinical studies demonstrating effectiveness of progesterone when administered later than 6 h post-TBI. The first, a double-blind placebo controlled pilot study of 100 patients with moderate-to-severe TBI, administered progesterone within 11 h of injury. Progesterone was administered with a loading dose of 0.71 mg/kg and then 0.5 mg/kg/h for a total of 3 days of treatment, resulting in average steady state progesterone concentration of 337±135 ng/mL (Wright et al., 2007). Patients receiving progesterone had a lower 30-day mortality compared to those receiving placebo treatment (Wright et al., 2005). In a second study of 159 severe TBI patients, progesterone administered at 1.0 mg/kg via intramuscular injection and then repeated every 12 h for 5 consecutive days had lower mortality and a more favorable outcome at 3 and 6 months follow-up time points (Xiao et al., 2008).
The overall objective of the present study was to use global transcriptional profiling analysis to determine the dose-dependent effects of progesterone on global gene expression patterns and profiles following CCI in the affected rat brain tissue. GO analysis was then used to evaluate the effect of dose on the relevant biological pathways. Prior to the gene expression studies, single- and multiple-dose pharmacokinetic studies were performed on uninjured animals to determine the dose regimens needed to provide average serum concentrations in the range of the concentrations reported in the experimental and clinical studies.
Methods
Animals
Male, Sprague Dawley rats (Harlan, Indianapolis, IN), 4 months of age at the time of the injury, were used in this study. All animal and surgical procedures were adhered to as described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Southern Illinois University Institutional Animal Care and Use Committee (IACUC) and the University of Washington's IACUC reviewed and approved all experimental procedures. Before and after injury, animals were housed in the university-maintained vivarium, with a 12-h light/dark schedule and a controlled environmental temperature of 22°C in standard housing cages with food and water available ad libitum.
Single and multiple dose pharmacokinetic studies
Male Sprague-Dawley rats (309±17 g) with surgically implanted jugular vein catheters were obtained from Harlan Laboratories (4 rats/study). For the single-dose studies, 5 mg/kg or 25 mg/kg doses of progesterone (Sigma-Aldrich Co, St. Louis, MO) dissolved in a peanut oil vehicle were administered by i.p. injection. Progesterone solutions were stored at −20o C until administration, as preliminary studies found that progesterone solutions were not stable at room temperature. Blood specimens were collected from the jugular catheter, immediately prior to the dose, and at 1, 6, and 24 h after the 5 mg/kg dose and at 1, 2, 4, 6, 8, and 24 h after the 25 mg/kg dose. Based on the results of the single-dose studies, multiple-dose studies were performed using progesterone doses of 10 mg/kg and 20 mg/kg administered by i.p. injection every 12 h for 72 h. Samples were collected immediately before the first and last dose and 1 h after each of the i.p. injections. Additional samples were collected at 1, 2 and 4 h after the last dose to estimate the elimination half-life (T1/2). Samples were collected in microtubes, separated using a centrifuge, and stored at −80 until assayed using an enzyme linked immunoassays (ELISA) (R&D Systems Inc, Minneapolis, MN, and VWR International, West Chester, PA). The terminal exponential rate constant (β) was used to determine T1/2 as 0.693/β.
Experimental injury model
All surgeries were performed under aseptic conditions. The cortical contusion injury (CCI) model utilized in the present study was based on previous studies and was intended to produce a moderately severe injury (Goffus et al. 2010; Quigley et al., 2009). Animals were anesthetized using a mixture of isofluorane (2–4%) and oxygen (0.8 L/min). When the animal became unresponsive (no ocular or pedal reflexes) the head was shaved and scrubbed with 70% alcohol followed by betadine and placed into a stereotaxic device. A midline incision was made in the skin as well as through the underlying fascia. A circular craniotomy (5.0 mm) was centered 2.4 mm posterior to and 2.4 mm lateral (left) to bregma. The contusion injury was created with the Benchmark™ stereotaxic impactor with a 4.0 mm diameter impactor tip (www.myneurolab.com, St. Louis, MO). A moderate injury was induced with an impact speed of 3.0 m/s and an impact depth of 2.5 mm (for model validation see Swan et al., 2011). The impact tip maintained contact with the brain tissue for 0.5 sec before retraction. Normal body temperature (37°C) during surgery and recovery was maintained with a warm water recycling bed and pump system (EZ Anesthesia, Palmer, PA). Rats receiving sham surgeries underwent identical surgical preparation as the injured animals but did not receive craniotomies (Cole et al., 2011) or injuries, and were then sutured and allowed to recover.
Drug administration
Each animal received a regimen of progesterone or vehicle (peanut oil) administered by i.p. injection based on the calculated pharmacokinetics of progesterone in the rat described as follows. Injections were administered starting at 4 h post-CCI and repeated every 12 h post-CCI for up to 72 hrs. For animals killed at 24 and 72 h post-CCI, the final injection was 12 h prior to death in order to evaluate the effect of chronic dosing and not acute dosing of progesterone on gene expression. Animals were randomly assigned to one of four groups: (a) Intact sham, (b) CCI-injured progesterone (10 mg/kg, i.p), (c) CCI-injured progesterone (20 mg/kg, i.p), and (d) CCI-injured vehicle (1.0 ml/kg, i.p). Groups numbers were 5 rats/group and assignment was not disclosed until all progesterone analyses were completed.
Tissue harvest
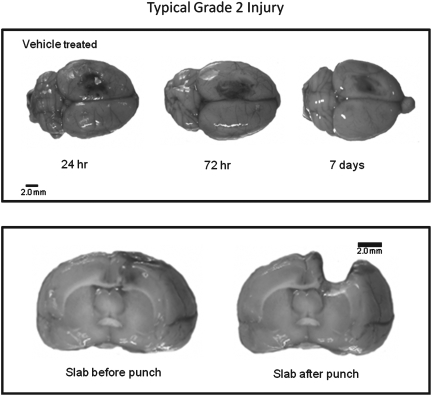
At specified time point's post-CCI (24 h, 72 h, and 7 days) the animals were overdosed with a mixture of CO2 (80%) and O2 (20%). The rats were then decapitated and the brains were rapidly extracted. To maintain quality control and to assure that all of the brains were injured, each brain was assigned a rating score (1=no visual sign of trauma; 2=bruised and swollen cortex; 3=no remaining cortex or extensive damage). Only brains with a score (grade) of 2 were used in the subsequent analyses and representative examples are provided in Figure 1. The brain was then cut into a 4.0- mm coronal slab containing the injury site in a brain matrix (Braintree Scientific, Inc., Braintree, MA) and was placed onto an RNAase free cold plate and a 5.0-mm biopsy punch was used to collect the injury site and surrounding cortical tissue (Hoane et al., 2006). The tissue punch included all injured cortical tissue and a small strip of pericontusional tissue, with the ventral extent of the punch extending to the corpus callosum. Tissue punches were placed into microcentrifuge tubes, snap frozen, and then stored at −80°C. All samples were shipped by overnight carrier to the University of Washington on dry ice.
FIG. 1.
Histology plate. Shown in the top panel are representative dorsal images depicting normal (grade 2) levels of damage at various time points post-CCI. The bottom panel shows a cortical slab before and after tissue punching to remove the core of the injury for molecular analysis.
Processing of samples for microarray analysis
Integrity of RNA samples was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA) which is the method of choice and the recognized standard in the field. RNA integrity was judged by observing distinct and sharp 18S and 28S ribosomal RNA peaks that were baseline separated. RNA quantity was determined by measuring OD260 with a Thermo Scientific NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Inc.; Wilmington, DE). The NanoDrop instrument was also used to determine purity of RNA samples by measuring OD260/280 and OD260/230 ratios. Only samples passing this stringent quality control were processed further. Processing of the RNA samples was performed according to the Affymetrix GeneChip Whole Transcript Sense Target labeling protocol. Briefly, double-stranded cDNA was synthesized with random hexamers tagged with a T7 promoter sequence. The double-stranded cDNA was subsequently used as a template and amplified by T7 RNA polymerase producing many copies of antisense cRNA. In the second cycle of cDNA synthesis, random hexamers were used to prune reverse transcription of the cRNA from the first cycle to produce single-stranded DNA in the sense orientation. In order to reproducibly fragment the single-stranded DNA and improve the robustness of the assay, dUTP was incorporated in the DNA during the second-cycle, first-strand reverse transcription reaction. This single-stranded DNA sample was then treated with a combination of uracil DNA glycosylase (UDG) and apurinic/apyrimidinic endonuclease 1 (APE 1) that specifically recognizes the unnatural dUTP residues and breaks the DNA strand. DNA was labeled by terminal deoxynucleotidyl transferase (TdT) with the Affymetrix® proprietary DNA Labeling Reagent that is covalently linked to biotin. The biotin-labeled DNA fragments were hybridized to Affymetrix GeneChip Rat Gene 1.0 ST arrays, washed, and stained with fluorescent anti streptavidin biotinylated antibody. Following an additional wash step, the arrays were scanned with an Affymetrix GeneChip® 3000 scanner. Image generation and feature extraction was performed using Affymetrix GeneChip Command Console Software.
Microarray data analysis
Raw microarray data were processed and analyzed with tools from Bioconductor (Gentleman et al., 2004). We normalized the data using the Robust Multichip Average (RMA) method from the Bioconductor Affy package. The quantile normalization step of the RMA normalization was performed at the probeset level. Using the normalized data, we identified genes with significant evidence for differential expression using the limma package (Smyth, 2004). The limma methodology calculates a p-value for each gene using a modified t test in conjunction with an empirical Bayes method to moderate the standard errors of the estimated log fold changes. This method of detecting differentially expressed genes draws strength across genes for more robust and accurate detection of differentially expressed genes. Such an adjustment has repeatedly been shown to avoid an excess of false positives when identifying differentially expressed genes (Allison et al., 2006). Using the p-values from limma, we used the Bioconductor package qvalue (Dabney and Storey, 2006; Tusher et al., 2001) to estimate the false discovery rate associated with our list of differentially expressed genes. This methodology allows us to address the problem of multiple hypotheses testing without resorting to an excessively conservative approach that controls the familywise error, such as a Bonferroni correction.
We carried out Gene Ontology category analysis via the cumulative hypergeometric distribution method to determine enhanced Gene Ontology categories (Camon et al., 2004) using the bioconductor package GOstats (Gentleman et al., 2005). We used differentially expressed genes (more than 1.5 fold up or down regulated, p<0.05) for this analysis to identify Gene Ontology categories by evidence of overrepresentation of significant genes.
Validation of data obtained with microarrays using fluorogenic 5′-nuclease-based assay and quantitative reverse transcription polymerase chain reaction (RT-PCR)
Quantitative TaqMan-based RT-PCR (qPCR) analysis has a greater dynamic range for changes in gene expression levels compared to microarray-based analysis. Therefore, we used qPCR to validate expression changes of genes of interest that had been identified by microarray analysis. Briefly, reverse transcription was performed according to the manufacturer's established protocol using total RNA and the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA.). For gene expression measurements, 2 μL of cDNA were included in a PCR reaction (12 μL final volume) that also consisted of the ABI inventoried TaqMan® Gene Expression Assays mix and the TaqMan Gene Expression Master Mix according to the manufacturer's protocol (Applied Biosystems Inc., Foster City, CA). Amplification and detection of PCR amplicons were performed with the ABI PRISM 7900 system (Applied Biosystems Inc., Foster City, CA) with the following PCR reaction profile: 1 cycle of 95°C for 10 min., 40 cycles of 95°C for 30 sec, and 60°C for 1 min. GAPDH amplification plots derived from serial dilutions of an established reference sample were used to create a linear regression formula in order to calculate expression levels, and β-actin and GAPDH gene expression levels were utilized as an internal control to normalize the data. Supplemental Table 1 lists the PCR oligonucleotide primers and probes that were used for quantitative TaqMan-based RT-PCR analysis.
Results
Pharmacokinetic studies
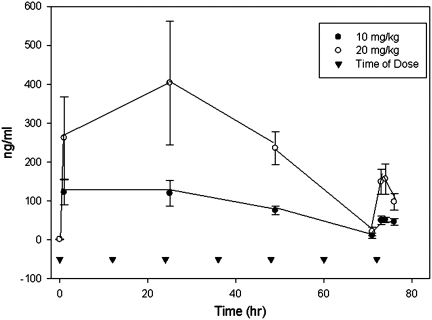
The single dose of progesterone at 5 mg/kg and 25 mg/kg resulted in peak progesterone concentrations at 1 h of 63±16 ng/mL and 396±70 ng/mL and a T1/2 of 2.8±1.5 h and 1.8±0.3 h, respectively. With multiple dosing, progesterone concentrations obtained 1 h post-injection decreased with time (Fig. 2). In contrast to the single-dose study, where the 1 h time point was the highest concentration measured, for multiple dosing the 2 h time-point on day 3 was higher than the earlier time point in 7 of 8 animals. The time dependent decrease in the 1 h post-dose concentrations appeared to be caused by a prolonged i.p. absorption that occurred upon multiple dosing. There was also an accumulation of vehicle in the peritoneal cavity, which was clearly visible at time of death. This is a novel effect and has not been previously reported. Based on the results of this pharmacokinetic study, multiple progesterone dosing (every 12 h) of 10 mg/kg and 20 mg/kg was used in the CCI gene expression study to represent the progesterone concentrations found in the previous experimental and clinical studies.
FIG. 2.
Mean plasma concentrations (and standard deviations) of progesterone following administration of 10 mg/kg or 20 mg/kg i.p. at time 0, 12 h, 24 h, 36h, 48 h, 60 h, and 72 h.
CCI and treatment gene expression study
Body weight at the time of injury (361±24 g) was evaluated using a one-way ANOVA, with group (low-dose progesterone, high-dose progesterone, and vehicle) as the between-subjects factor. The analysis revealed that there was no statistically significant effect of group [F(3, 36)=2.09, p>0.05]. The visual assessment of tissue injury severity was evaluated using a χ2 test, with group (low- and high-dose treatment and vehicle) as factors. The analysis revealed that there were no statistically significant differences in injury severity among the groups [χ2 (2, n=45)=0.53, p>0.77] with all groups having the same average severity of injury (mean=2.0, SD=0.0). These data suggest that no general neuroprotective effect was observed with progesterone treatment; however, further investigation is warranted and may show a differential effect.
The microarray data passed all the standard and advanced quality control metrics. The number of differentially expressed genes (>1.5-fold change, p<0.05) at 24 h, 72 h, and 7 days is presented in Table 1. At 24 h, for the CCI-injured vehicle group compared to uninjured (sham) animals, 1,114 distinct genes showed significant changes in expression with 709 upregulated and 405 downregulated. The effect of low dose progesterone on differentially expressed genes was <20% of the effect of the high dose compared to vehicle. At 24 h post-CCI, GO analysis, which shifts the emphasis from evaluation of single genes to evaluation of pathways, networks, and functions, identified a significant effect of low-dose and high-dose progesterone treatment compared to vehicle on DNA damage response (Table 2). Examples of genes differentially expressed at 24 h are presented in Table 3. Several of the differentially expressed genes encoded proteins involved in transport and signaling pathways. Although the expression of the number of statistically significant genes was considerably less for the low dose than for the high dose, many of the same genes were also affected by low dose, but to a lesser, not statistically significant extent.
Table 1.
The Total Number of Genes that Were Differentially Expressed (>1.5-fold Up or Down, p<0.05) at 24 h, 72 h, and 7 Days Are Shown. In Addition, the Number of Genes that were Down- and Upregulated Are also Shown for Each Time Point. The Array Contains Probes for Transcripts that Lack Annotations, Gene Symbol Assignments, or Entrez Gene IDs. For Some Transcripts/Genes, the Array Contains Multiple Probe Sets. The Numbers in Table 1 Refer to Transcripts Derived from Unique Genes for which Entrez Gene IDs Were Available
| |
24 h |
72 h |
7 days |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Down | Up | Total | Down | Up | Total | Down | Up | Total | |
| Low/Sham | 525 | 666 | 1191 | 1757 | 2933 | 4690 | 65 | 538 | 603 |
| High/Sham | 485 | 645 | 1130 | 1965 | 2971 | 4936 | 80 | 376 | 456 |
| Vehicle/Sham | 405 | 709 | 1114 | 1502 | 2727 | 4229 | 58 | 223 | 291 |
| Low/Vehicle | 8 | 6 | 14 | 15 | 25 | 40 | 168 | 383 | 551 |
| High/Vehicle | 11 | 76 | 87 | 18 | 57 | 75 | 11 | 3 | 14 |
| Low/High | 11 | 3 | 14 | 30 | 3 | 33 | 66 | 290 | 356 |
Table 2.
Gene Ontology Analysis: Effect of Progesterone Dose
|
24 h |
Low/Vehicle |
High/Vehicle |
||||||
|---|---|---|---|---|---|---|---|---|
| GO ID | GO term | Annotated | Expecteda | Significant | p-value | Expecteda | Significant | p-value |
| 0006261 | DNA-dependent DNA replication | 57 | 0.03 | 2 | 0.00031 | 0.18 | 2 | 0.0132 |
| 0000724 | Double-strand break repair via homologous recombination | 21 | 0.01 | 1 | 0.0100 | 0.06 | 2 | 0.0019 |
| 0006978 | DNA damage response, signal transduction by P53 class mediator resulting in induction of apoptosis | 8 | 0.00 | 1 | 0.00382 | 0.02 | 1 | 0.0243 |
| 0032728 | Positive regulation of interferon-β production | 11 | 0.01 | 1 | 0.00525 | 0.03 | 1 | 0.0333 |
| 0045088 | Regulation of innate immune response | 65 | 0.03 | 1 | 0.03067 | 0.20 | 1 | 0.1817 |
| 0014054 | Positive regulation of GABA secretion | 5 | 0.00 | 0 | 1.0000 | 0.02 | 1 | 0.0153 |
| 0010940 | Positive regulation of necrotic cell death | 6 | 0.00 | 0 | 1.0000 | 0.02 | 1 | 0.0183 |
| 0014050 | Negative regulation of glutamate secretion | 8 | 0.00 | 0 | 1.0000 | 0.02 | 1 | 0.0243 |
| 0071456 | Cellular response to hypoxia | 14 | 0.01 | 0 | 1.0000 | 0.04 | 1 | 0.0422 |
| 72 h | ||||||||
| 0006953 | Acute phase response | 43 | 0.09 | 5 | 2.3E-08 | 0.19 | 6 | 2.9E-08 |
| 0006954 | Inflammatory response | 300 | 0.61 | 6 | 2.8E-05 | 1.31 | 14 | 3.2E-11 |
| 0006935 | Chemotaxis | 133 | 0.27 | 1 | 0.23968 | 0.58 | 6 | 2.4E-05 |
| 0050715 | Positive regulation of cytokine secretion | 24 | 0.05 | 1 | 0.04807 | 0.10 | 1 | 0.09987 |
| 0071347 | Cellular response to interleukin-1 | 9 | 0.02 | 0 | 1.0000 | 0.04 | 2 | 0.00066 |
| 0032652 | Regulation of interleukin-1 production | 22 | 0.05 | 0 | 1.0000 | 0.10 | 2 | 0.00411 |
| 0034612 | Response to tumor necrosis factor | 31 | 0.06 | 0 | 1.0000 | 0.14 | 2 | 0.00806 |
| 0032757 | Positive regulation of interleukin-8 production | 10 | 0.02 | 0 | 1.0000 | 0.04 | 1 | 0.04287 |
| 0042542 | Response to hydrogen peroxide+ | 88 | 0.18 | 2 | 0.01393 | 0.38 | 3 | 0.00671 |
| 0007263 | Nitric oxide mediated signal transduction | 13 | 0.03 | 1 | 0.02632 | 0.06 | 2 | 0.00142 |
| 0007218 | Neuropeptide signaling pathway | 86 | 0.18 | 3 | 0.00071 | 0.38 | 3 | 0.00630 |
| 0001516 | Prostaglandin biosynthetic process | 19 | 0.04 | 1 | 0.03824 | 0.08 | 1 | 0.07991 |
| 0019369 | Arachidonic acid metabolic process | 17 | 0.03 | 0 | 1.0000 | 0.07 | 2 | 0.00245 |
| 0006915 | Apoptosis | 1055 | 2.16 | 6 | 0.01837 | 4.61 | 12 | 0.00183 |
| 0043066 | Negative regulation of apoptosis | 423 | 0.87 | 4 | 0.01038 | 1.85 | 5 | 0.03729 |
| 0045765 | Regulation of angiogenesis | 92 | 0.19 | 0 | 1.0000 | 0.40 | 5 | 5.0E-05 |
| 0031645 | Negative regulation of neurological system | 36 | 0.07 | 2 | 0.00245 | 0.16 | 5 | 4.6E-07 |
| 7 days | ||||||||
| 008284 | Positive regulation of cell proliferation | 466 | 11.27 | 27 | 2.6E-05 | 0.22 | 0 | 1.0000 |
| 0045087 | Innate immune response | 158 | 3.82 | 14 | 3.0E-05 | 0.08 | 0 | 1.0000 |
| 0002674 | Negative regulation of acute inflammation | 8 | 0.19 | 3 | 0.00072 | 0 | 0 | 1.0000 |
| 0050728 | Negative regulation of inflammatory response | 38 | 0.92 | 4 | 0.01298 | 0.02 | 1 | 0.01803 |
| 0032722 | Positive regulation of chemokine production | 16 | 0.39 | 3 | 0.00621 | 0.1 | 0 | 1.0000 |
| 0032647 | Regulation of interferon alpha production | 6 | 0.15 | 2 | 0.0082 | 0 | 0 | 1.0000 |
| 0045078 | Positive regulation of interferon-gamma production | 9 | 0.22 | 2 | 0.01875 | 0 | 0 | 1.0000 |
| 0032757 | Positive regulation of interleukin-8 production | 10 | 0.24 | 2 | 0.02307 | 0 | 0 | 1.0000 |
| 0001974 | Blood vessel remodeling | 25 | 0.60 | 3 | 0.02174 | 0.1 | 0 | 1.0000 |
| 0045768 | Positive regulation of anti-apoptosis | 30 | 0.73 | 3 | 0.03513 | 0.1 | 0 | 1.0000 |
| 0043524 | Negative regulation of neuron apoptosis | 70 | 1.69 | 2 | 0.50753 | 0.03 | 1 | 0.0330 |
| 0043154 | Negative regulation of caspase activity | 27 | 0.65 | 1 | 0.48387 | 0.01 | 1 | 0.01284 |
| 0014049 | Positive regulation of glutamate secretion | 7 | 0.17 | 0 | 1.0000 | 0 | 1 | 0.00334 |
The GO analysis takes the total number of differentially expressed genes in a given contrast into account when it calculates “expected” and “p-values”. topGO analysis calculates statistical significance using three different methods, the classic, elimination and weighted methods. The p-values shown in this table are based on the classic method.
Table 3.
Genes Differentially Expressed at 24 h after TBI (Fold Change, *p<0.05)
| Affymetrix transcript ID | Gene symbol | Gene description | Vehicle sham | Low vehicle | High vehicle | Low high | GO: Biological process |
|---|---|---|---|---|---|---|---|
| 10930616 | ATP8 | ATPase subunit 8 | 1.28 | 2.28* | 2.49* | 0.91 | Ion transport // ATP synthesis coupled proton transport // response to hyperoxia |
| 10750551 | Epha6 | Eph receptor A6 | 1.14 | 0.66* | 1.00 | 0.66* | Protein amino acid phosphorylation // transmembrane receptor protein tyrosine kinase signaling pathway |
| 10741765 | LOC287167 | Globin, alpha | 0.81 | 1.38 | 1.52* | 0.91 | Oxygen transporter activity |
| 10771134 | Grinl1a | Glutamate receptor, ionotropic, N-methyl D-aspartate-like 1A | 1.38 | 1.22 | 1.71* | 0.71 | Transcription // maintenance of ER location |
| 10911309 | Gtf2a2 | General transcription factor IIA, 2 | 0.62* | 0.78 | 0.60* | 1.29 | Transcription initiation from RNA polymerase II promoter |
| 10784054 | Gzmb | Granzyme B | 1.18 | 1.24 | 1.55* | 0.80 | Proteolysis // induction of apoptosis by granzyme // cytolysis |
| 10715200 | Hells | Helicase, lymphoid specific | 0.51* | 0.66* | 0.62* | 1.08 | DNA binding // chromatin binding // helicase activity // ATP binding |
| 10929656 | Kcnj13 | Potassium inwardly-rectifying channel, subfamily J, member 13 | 1.24 | 0.58* | 0.64* | 0.91 | Ion transport // potassium ion transport |
| 10736634 | Rnf135 | Ring finger protein 135 | 0.68 | 0.57* | 0.59* | 0.97 | Small GTPase mediated signal transduction |
| 10865817 | Senp18 | Sentrin 18 | 1.34 | 1.22 | 1.88* | 0.65* | Proteolysis |
| 10922909 | Slc9a2 | Solute carrier family 9 (sodium/hydrogen exchanger), member 2 | 1.13 | 0.61* | 1.03 | 0.59* | Sodium ion transport // regulation of pH |
| 10781273 | Stc1 | Stanniocalcin 1 | 0.72 | 1.02 | 1.51* | 0.67 | Ossification // cellular calcium ion homeostasis |
| 10864170 | Trh | Thyrotropin releasing hormone | 0.35* | 1.15 | 1.82* | 0.63* | Response to hypoxia |
| 10792456 | Tpte | Transmembrane phosphatase with tensin homology | 1.33 | 0.86 | 0.66* | 1.30 | dephosphorylation |
By 72 h post-CCI, the number of differentially expressed genes in the CCI-injured vehicle group compared to the sham group was 4,229 with 2,727 up- and 1,502 downregulated. Based on gene expression data, high-dose progesterone treatment compared to vehicle affected expression of almost twice as many genes (75) as did low dose progesterone (40). Relative to the vehicle group, administration of both low and high doses of progesterone affected expression of genes involved in regulating inflammatory response, apoptosis, response to hydrogen peroxide, nitric oxide mediated signal transduction, neuropeptide signaling pathway, prostaglandin synthetic process, and negative regulation of neurological system process (Table 2). GO analysis identified several biological categories that differed between the low and high dose progesterone. The GO analysis identified a significant effect of high-dose progesterone treatment on genes involved in chemotaxis, arachidonic acid metabolic process, interleukin 1 and 8 production, and regulation of angiogenesis. Examples of specific genes that were differentially expressed between the low dose/vehicle, high dose/vehicle and low/high dose groups are given in Table 4. For example, both high and low doses significantly decreased matrix metallopedptiase 8 (MMP-8), whereas only high doses significantly increased MMP-9. Both high and low doses increased prostaglandin-endoperoxide synthase 2, also referred to as cyclooxygenase 2 (COX-2). High-dose progesterone also significantly increased interleukin-1 receptor antagonist (IL1rn) and syndecan (Sdc1). In contrast, without progesterone treatment, there was decreased expression of IL1rn, CALCA, CCR1, PTGS2, MMP-8, MMP-9, and Sdc1 in the CCI-vehicle group compared to sham.
Table 4.
Genes Differentially Expressed at 72 h after TBI (Fold Change, *p<0.05)
| Affymetrix transcript ID | Gene symbol | Gene description | Vehicle sham | Low vehicle | High vehicle | Low high | GO biological process |
|---|---|---|---|---|---|---|---|
| 10744425 | Alox15 | Arachidonate 15-lipoxygenase | 0.74 | 1.02 | 2.57* | 0.34* | Inflammatory response |
| 10725051 | Calca | Calcitonin/calcitonin-related polypeptide, alpha | 0.41* | 1.27 | 1.71* | 0.75 | Endothelial cell proliferation |
| 10921163 | Ccr1 | Chemokine (c-c motif) receptor 1 | 0.39* | 1.43 | 1.66* | 0.86 | Inflammatory response |
| 10814286 | Fabp4 | Fatty acid binding protein 4, adipocyte | 1.14 | 1.08 | 1.66* | 0.65* | Cytokine production/transportor activity |
| 10834109 | Il1rn | Interleukin 1-receptor antagonist | 0.40* | 1.44 | 1.91* | 0.75 | Inflammatory response |
| 10908861 | Ldha | Lactate dehydrogenase A | 0.89 | 1.84* | 1.57* | 1.17 | Response to hypoxia |
| 10907913 | MMP8 | Matrix metallopeptidase 8 | 0.19* | 0.46* | 0.65* | 0.72 | Metalloendopeptidase activity |
| 10842239 | MMP9 | Matrix metallopeptidase 9 | 0.48 | 1.44 | 1.51* | 0.95 | Metalloendopeptidase activity |
| 10764551 | Ptgs2 | Prostaglandin-endoperoxide synthase 2 | 0.56* | 1.43 | 1.93* | 0.74 | Prostaglandin biosynthetic process |
| 10883530 | Sdc1 | Syndecan 1 | 0.45* | 1.20 | 1.63* | 0.74 | Inflammatory response |
| 10864170 | Trh | Thyrotropin-releasing hormone | 0.87 | 1.51* | 1.31 | 1.15 | Neuropeptide hormone activity |
By 7 days, there was a significant decrease in the effects of CCI with only 291 differentially expressed genes in the vehicle-treated animals compared to sham animals. There was only a modest difference in high-dose progesterone compared to vehicle with only 14 differentially expressed genes. In contrast, low-dose progesterone resulted in 551 differentially expressed genes compared to vehicle with 383 genes increased and 168 genes decreased. GO analysis (Table 2) identified genes involved in negative regulation of inflammatory response as statistically significant for both the high- and low-dose treatments relative to the vehicle treatment. In addition, for the low-dose treatment, genes involved in positive regulation of cell proliferation, innate immune response, positive regulation of anti-apoptosis, and blood vessel remodeling were identified.
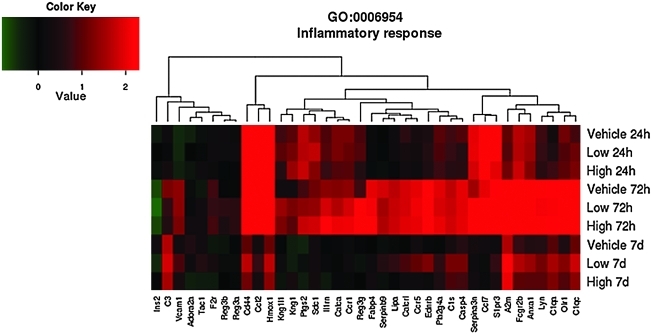
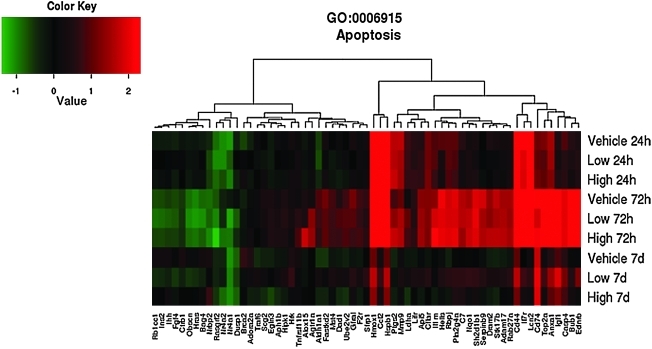
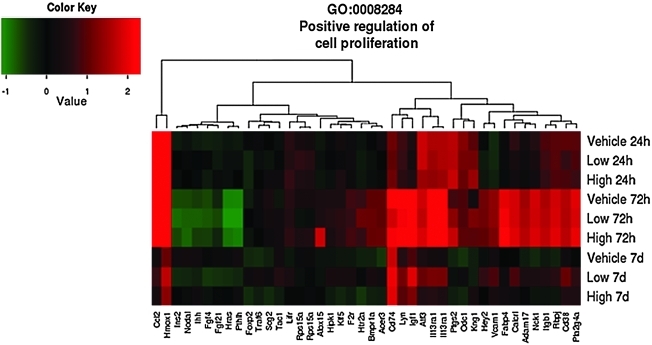
As shown in the GO heat maps, the effects of high-dose progesterone were not significantly different than those of vehicle at 7 days for the genes involved in inflammatory response (p=0.14) (Fig. 3), apoptosis (p=0.41) (Fig. 4) and positive regulation of cell proliferation (p=1) (Fig. 5). Examples of genes that were affected by the difference in progesterone dose are given in Table 5 and include genes associated with both apoptosis and anti-apoptosis and oxidative stress, in addition to other pathways identified in the GO analysis.
FIG. 3.
Effects of traumatic brain injury and progesterone treatment on expression of genes involved in inflammatory response. Genes belonging to the inflammatory response gene ontology category (GO:0006954) whose expression changed more than 1.5-fold (up or down, p<0.05) in at least one of the three comparisons “Low progesterone treatment versus vehicle treatment”, “High progesterone treatment versus vehicle treatment”, or “Low progesterone treatment versus high progesterone treatment,” are displayed. The rows show the average expression of the vehicle, and low and high progesterone experimental groups at 24 h, 72 h, and 7 days. Each column shows the expression of a single gene. Red indicates that expression was higher compared to the average of the uninjured sham group, green indicates the opposite, and black means no change. Abbreviations: A2m, alpha-2-macroglobulin; Adora2a, adenosine A2a receptor; Anxa1, annexin A1; C1qa, complement component 1, q subcomponent, alpha polypeptide; C1qc,complement component 1, q subcomponent C chain. group IVA (cytosolic, calcium-dependent); C1s, complement component 1, s subcomponent; C3, complement component 3; Calca, calcitonin/calcitonin-related polypeptide, alpha; Calcr l, calcitonin recptor-like; Casp4, caspase 4, apoptosis-related cystine peptidase; Ccl12, chemokine (C-C motif) ligand 12; Ccl17, chemokine (C-C motif) ligand17; Ccr1, chemokine (C-C) receptor 1; Ccr5, chemokine (C-C motif) receptor 5; Cd44, Cd44 molecule; Ednrb, endothelin receptor type b; F2r, coagulation factor II (thrombin) receptor; Fabp4, fatty acid binding protein 4, adipocyte; Fcgr2b, FC fragment of IgG, low affinity llb, receptor (CD32); Hmox1, heme oxygenase (decycling) 1; Il1rn, interleukin 1 receptor antagonist; Ins2, insulin 2; Kng1l1, kininogen 1-like 1; Kng1, kininogen 1; Lipa, lipase A, lysomsomal acid, cholesterol esterase; Lyn, v-yes-1 Yamagtuchi sarcoma viral related oncogene homolog; Olr1, oxidized low density lipoprotein (lectin-like) receptor 1; Pla2g4a, phospholipase A2, group IVA (cytosolic, calcium-dependent); Ptgs2, prostaglandin-endoperoxide synthase 2; Reg3a, regenerating islet-derived 3 alpha; Reg3b, regenerating islet-derived 3 beta; Reg3g, regenerating islet-derived 3 gamma; S1pr3, serine (or cystine) peptidase inhibitor, clade A, member 3N; Sdc1, syndecan1; Tac1, tachykinin 1.
FIG. 4.
Effects of traumatic brain injury and progesterone treatment on expression of genes involved in apoptosis. Genes belonging to the apoptosis gene ontology category (GO: 0006915) whose expression changed more than 1.5-fold (up or down, p<0.05) in at least one of the three comparisons “Low progesterone treatment versus vehicle treatment”, “High progesterone treatment versus vehicle treatment”, or “Low progesterone treatment versus high progesterone treatment,” are displayed. The rows show the average expression of the vehicle and low and high progesterone experimental groups at 24 h, 72 h, and 7 days. Each column shows the expression of a single gene. Red indicates that expression was higher compared to the average of the uninjured sham group, green indicates the opposite, and black means no change. Abbreviations: Adam17, ADAM metallopeptidase domain 17; Adora2a, adenosine A2a receptor; Agtr1a, angiotensin II receptor, type 1; Aldh1a1, aldehyde dehydrogenase 1 family, member A1; Alox15, arachidonate 15-lipoxygenase; Anxa1, annexin A1; Aph1b, anterior pharynx defective 1 homolog B (C. elegans); Api5, apoptosis inhibitor 5; Bag4, BCL2-associated athanogene 4; Brca2, breast cancer 2, early onset; Bub1, budding uninhibited by benzimidazoles 1 homolog (S. cerevisiae); C7, complement component 7; Casp4, caspase 4, apoptosis-related cysteine peptidase; Ccl2, chemokine (C-C motif) ligand 2; Cd44, CD44 molecule (Indian blood group); Cd74, CD74 molecule, major histocompatibility complex, class II invariant chain; Cflar, CASP8 and FADD-like apoptosis regulator; Ctrb1, chymotrypsinogen B1; Dad1, defender against cell death 1; Dram2, transmembrane protein 77; Dusp1, dual specificity phosphatase 1; Ednrb, endothelin receptor type B; Egln3, egl nine homolog 3 (C. elegans); F2r, coagulation factor II (thrombin) receptor; Fastkd2, FAST kinase domains 2; Fgf4, fibroblast growth factor 4; Gfra1, GDNF family receptor alpha 1; Hells, helicase, lymphoid-specific; Hipk1, homeodomain interacting protein kinase 1; Hmox1, heme oxygenase (decycling) 1; Hras, v-Ha-ras Harvey rat sarcoma viral oncogene homolog; Hrk, harakiri, BCL2 interacting protein (contains only BH3 domain); Hspb1, heat shock 27kDa protein 1, Igf1, insulin-like growth factor 1 (somatomedin C); Ihh, Indian hedgehog; Il1rn, interleukin 1 receptor antagonist; Il7r, interleukin 7 receptor; Ins2, insulin 2; Lcn2, lipocalin 2; Ldha, lactate dehydrogenase A; Lifr, leukemia inhibitory factor receptor alpha; Mmp9, matrix metallopeptidase 9; Mst4, serine/threonine protein kinase MST4; Nqo1, NAD(P)H dehydrogenase, quinone 1; Nr4a2, nuclear receptor subfamily 4, group A, member 2; Nrbp2, nuclear receptor binding protein 2; Obscn, obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF; Pla2g4a, phospholipase A2, group IVA (cytosolic, calcium-dependent); Ptgs2, prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase); Rab27a, RAB27A, member RAS oncogene family; Rasgrf2, Ras protein-specific guanine nucleotide-releasing factor 2; Rb1cc1, RB1-inducible coiled-coil 1; Rbpj, recombination signal binding protein for immunoglobulin kappa J region; Scg2, secretogranin II (chromogranin C); Serpinb9, serpin peptidase inhibitor, clade B (ovalbumin), member 9; Sh2d1b1, SH2 domain containing 1B; Sfrp1, secreted frizzled-related protein 1; Stk17b, serine/threonine kinase 17b; Tnfrsf11b, tumor necrosis factor receptor superfamily, member 11b; Top2a, topoisomerase (DNA) II alpha 170kDa; Traf6, TNF receptor-associated factor 6; Ube2v2, ubiquitin-conjugating enzyme E2 variant 2.
FIG. 5.
Effects of traumatic brain injury and progesterone treatment on expression of genes involved in positive regulation of cell proliferation. Genes belonging to the positive regulation of cell proliferation gene ontology category (GO: 0008284) whose expression changed more than 1.5-fold (up or down, p<0.05) in at least one of the three comparisons “Low progesterone treatment versus vehicle treatment”, “High progesterone treatment versus vehicle treatment”, and “Low progesterone treatment versus high progesterone treatment” are displayed. The rows show the average expression of the vehicle and low and high progesterone experimental groups at 24 h, 72 h, and 7 days. Each column shows the expression of a single gene. Red indicates that expression was higher compared to the average of the uninjured sham group, green indicates the opposite, and black means no change. Abbreviations: Adam17, ADAM metallopeptidase domain 17; Alox15, arachidonate 15-lipoxygenase; Bmpr1a, bone morphogenetic protein receptor, type 1A; Calcrl, calcitonin receptor-like; Ccl12, chemokine (C-C motif) ligand 12; Cd38, Cd38 molecule; F2r, coagulation factor (thrombin) receptor; Fabp4, fatty acid binding protein 4, adipocyte; Fgfr4, fibroblast growth factor receptor 4; Foxp2, forkhead box P2; Hey2, hairy/enhancer of split related to YRPW motif 2; Hipk1, homeodomain interacting protein kinase 1; Hras, Harvey rat sarcoma virus oncogene; Htr2a, 5-hydroxytryptamine (serotonin) receptor 2A; igsf1, immunoglobulin superfamily, member 1; ihh, Indian hedgehog; il13ra1, interleukin 13 receptor, alpha 1; Ins2, insulin 2; itgb1, integrin beta 1 (fibronectin receptor beta); Klf5, Kruppel-like factor 5; Lifr, leukemia inhibitory factor receptor alpha; Lyn, v-yes-1 Yamaguchi sarcoma viral related oncogene homolog; Nck1, NCK adaptor protein 1; Nodal, nodal homolog (mouse); Odc1, ornithine decarboxylase 1; Ptgs2, prostaglandin-endoperoxide synthase 2; Pla2g4a, phospholipase A2, Pthlh, parathyroid hormone-like hormone; Rbpj, recombination signal binding protein for immunoglobulin kappa J region; Rps15a, ribosomal protein S15a; Stat1, signal transducer and activator of transcription 1; Scg2; secetogranin II (chromogranin c); Tac1, tachykinin 1; Traf6, Tnf receptor-associated factor 6; Vcam1, vascular cell adhesion molecule 1.
Table 5.
Genes Differentially Expressed at 7 days after TBI (Fold Change, *p<0.05)
| Affymetrix transcript ID | Gene symbol | Gene description | Vehicle sham | Low vehicle | High vehicle | Low high | GO: Biological process |
|---|---|---|---|---|---|---|---|
| 10788822 | Bag4 | BCL2-associated athanogene 4 | 0.82 | 0.63* | 0.90 | 0.70 | Apoptosis |
| 10907825 | Casp4 | Caspase 4 | 0.66* | 1.51* | 1.18 | 1.28 | Inflammation/Apoptosis |
| 10777232 | CD38 | CD38 molecules | 0.86 | 1.82* | 1.22 | 1.48 | Response to hypoxia |
| 10847761 | CD44 | CD44 molecule | 0.49* | 1.60* | 1.10 | 1.45 | Regulation of cell growth/blood vessel maturation |
| 10923580 | Cflar | CASP8 and FADD-like apoptosis regulator | 1.11 | 1.66* | 1.12 | 1.49 | Anti-apoptosis |
| 10817419 | Ctsk | Cathepsin K | 0.99 | 1.53* | 1.12 | 1.37 | Intramembranous ossification/proteolysis |
| 10712623 | Fgf4 | Fibroblast growth factor 4 | 0.94 | 0.65* | 0.87 | 0.75 | Positive regulation of cell proliferation/ |
| 10806122 | Hmox1 | Heme oxgenase (decycling) 1 | 0.47* | 1.53* | 1.19 | 1.29 | Angiogenesis/response to hypoxia |
| 10727008 | Ins2 | Insulin 2 | 0.85 | 0.63* | 0.86 | 0.73 | Glucose metabolism/positive regulation of cytokine secretion |
| 10894695 | Igf1 | Insulin-like growth factor 1 | 0.54* | 2.08* | 1.28 | 1.63* | Anti-apoptosis |
| 10812021 | Itgb1 | Integrin beta 1 (fibronectin receptor beta) | 0.84 | 1.67* | 1.07 | 1.56* | Tissue homeostasis/cell-matrix adhesion |
| 10712171 | Ifitm1 | Interferon-induced transmembrane protein 1 | 0.40* | 1.86* | 1.33 | 1.40 | None identified |
| 10726679 | Ifitm2 | Interferon-induced transmembrane protein 2 | 0.79 | 1.90* | 1.30 | 1.46 | None identified |
| 10754983 | Il13ra1 | Interleukin 13 receptor, alpha 1 | 0.83 | 1.89* | 1.15 | 1.64* | Positive regulation of immunoglobulin production |
| 10714745 | Il33 | Interleukin 33 | 1.04 | 1.66* | 1.15 | 1.44 | Cytokine activity |
| 10821851 | Il7r | Interleukin 7 receptor | 1.00 | 1.72* | 1.21 | 1.41 | Negative regulation of T cell mediated cytotoxicity |
| 10751988 | Kng1 | Kininogen 1 | 1.25 | 1.01 | 1.58* | 0.64 | Positive regulation of endothelial cell proliferation |
| 10821741 | Lifr | Leukemia inhibitory factor receptor alpha | 1.15 | 1.57* | 1.20 | 1.31 | Positive regulation of cell proliferation |
| 10729780 | Lipa | Lipase A, lysosomal acid, cholesterol esterase | 1.02 | 1.64* | 1.06 | 1.55* | Cytokine production |
| 10810867 | Nqo1 | NAD(P)H dehydrogenase, quinone 1 | 0.96 | 1.58* | 1.08 | 1.46 | Response to oxidative stress |
| 10919548 | Nck1 | NCK adaptor protein 1 | 1.35 | 1.87* | 1.20 | 1.57* | Positive regulation of T cell proliferation |
| 10830075 | Nodal | Nodal homolog (mouse) | 0.82 | 0.62* | 0.79 | 0.78 | Positive regulation of cell proliferation |
| 10883785 | Odc1 | Ornithine decarboxylase 1 | 1.43 | 1.60* | 1.08 | 1.47 | Positive regulation of cell proliferation |
| 10866970 | Pthlh | Parathyroid hormone- like hormone | 0.83 | 0.63* | 0.93 | 0.68 | Skeletal system development |
| 10912255 | Plod2 | Procollagen lysine, 2-oxoglutarate 5-dioxygenase 2 | 0.89 | 1.53* | 1.08 | 1.41 | Response to hypoxia |
| 10838100 | Traf6 | Tnf receptor-associated factor 6 | 1.37 | 1.67* | 1.11 | 1.50* | Positive regulation of T-cell cytokine response |
| 10826249 | Vcam1 | Vascular cell adhesion molecule 1 | 0.83 | 1.52* | 1.07 | 1.42 | Cell adhesion |
Validation of microarray data
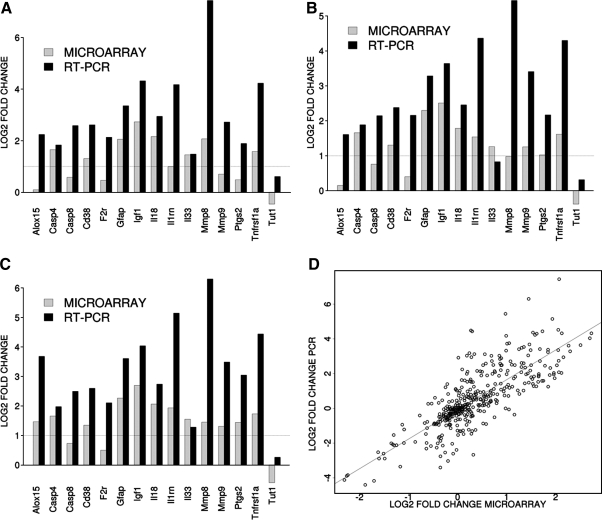
To validate the gene expression changes, 15 genes were selected from specific pathways of interest. In Figure 6A–C, the results are shown as expression data normalized to GAPDH expression for the 72-h time point for vehicle/sham, low/sham, and high/sham. In Figure 5D, data obtained from all groups, time points, and contrast are included. The qPCR findings were highly correlated with the microarray data (r2=0.63, p=4.61e-90).
FIG. 6.
TaqMan-based RT-PCR validation of the microarray data for the selected genes: Casp 4, caspase 4; Casp 8, caspase 8; Cd 38, CD38 molecule, F2r, coagulation factor II (thrombin) receptor; Gfap, glial fibrillary acidic protein; Igf1, insulin-like growth factor 1; Il18, interleukin 18; Il1rn, interleukin 1 receptor antagonist; Il33, interleukin 33; Mmp8, matrix metallopeptidase 8; Mmp9, matrix metallopeptidase 9; Ptgs2, prostaglandin-endoperoxide synthase 2; Tnfrsf1a, tumor necrosis factor receptor superfamily, member 1a; Tut1; terminal uridylyl transferase 1. The RT-PCR data were normalized to the housekeeping gene GAPDH. In order to compare the microarray data to the RT-PCR data, the microarray data for each gene was divided by the GAPDH data as it was measured by the microarray analysis. A–C: The gray bars show the microarray data and the black bars display the RT-PCR data for the contrast. Panels A, B and C show the data from the 72-h time point for the contrasts vehicle versus sham, low prostaglandin versus sham and high prostaglandin versus sham, respectively. Panel D shows the overall correlation between the microarray data and the RT-PCR for all contrasts.
Discussion
Although the neuroprotective effect of progesterone has been established in experimental models of TBI and spinal cord injury, the mechanism of the effect has not been well characterized. The observed effects of progesterone on inflammation reduction, swelling, and apoptosis have been proposed to be caused by regulation of gene transcription, the modulation of neurotransmitter receptors, and the activation of signaling cascades (Schumacher et al., 2007). Progesterone has been shown previously to modulate the activity of neurotransmitters, including positive modulation of γ-aminobutyric acid type A (GABAA) receptors, primarily by allopregnanolone, the active metabolite of progesterone (Belelli et al., 2002; Majewska et al., 1986). Progesterone has also been shown to protect against lipid membrane peroxidation in an experimental TBI model (Roof et al., 1997) consistent with the antioxidant effects found in the present study.
The use of microarray analysis to delineate gene expression patterns and profile changes is a powerful tool that can be used to evaluate the potential effect of treatment, in addition to increasing understanding of the mechanism of the treatment effect. There have been several studies evaluating the effect of TBI on the time-dependent expression of specific genes (Bazan et al., 1995; Hayes et al., 1995; Marciano et al., 2002) and more recently, using microarray technology, expression of thousands of genes can be measured (Dash et al., 2004; Di Pietro et al., 2010; Natale et al., 2003; Raghavendra Rao et al., 2003; Rall et al., 2003; Truettner et al., 2005). The majority of the these studies evaluated gene expression only up to 24 h post-injury with the exception of that by Natale and associates who used microarray-based technology to evaluate gene expression 4, 8, 24, and 72 h post-injury (Natale et al., 2003). Genes involved in inflammation, transcription regulation, and cell adhesion/extracellular matrix accounted for the largest number of expression changes. Other functional classes included oxidative stress, apoptosis/cell cycle, proteolysis, and red blood cell related.
The effects of low- and high-dose progesterone at 24 h on gene expression were similar; although considerably less expression was observed with the lower dose compared to vehicle. However, at 72 h and 7 days, there were substantial differences between low- and high-dose progesterone treatments. The significant effects of high-dose progesterone compared to low-dose progesterone at 72 h included both potentially positive and negative effects on inflammation. Consistent with the previous studies, there was no significant effect of progesterone on the pro-inflammatory cytokines, IL-1β and TNFα at 24 h, (He et al., 2004) or at 72 hr or 7 days. Both low- and high-dose progesterone increased IL-1 receptor antagonist (IL-1ra)with a significant effect only with high doses. Endogenous IL-1ra is produced by macrophages and activates monocytes in response to exposure to endotoxcin and IL-1. IL-1ra binds to both type I and type II IL-1 receptors, partially blocking cellular responses mediated by IL-1α and IL-1β (Loddick et al., 1997). In the rat fluid percussion injury (FPI) model, 10 μg of intracerebroventricular IL-1ra, administered 15 min and 2, 4, 6, 8, 24, and 48 h after injury significantly reduced lesion size (Toulmond and Rothwell, 1995). An increase in the IL-1ra expression has been proposed as a mechanism for the anti-inflammatory and neuroprotective effect of estrogen in hippocampal slice studies (Choi et al., 2008). High-dose progesterone significantly increased chemokine (c-c motif) receptor 1 (CCR1), which is expressed on the neurophil surface and interacts primarily with IL-8, an inflammatory cytokine. Inflammation is an important characteristic of the brain pathology of Alzheimer's disease (Galasko and Montine, 2010), and in this regard CCR1 is an early and specific marker of Alzheimer's disease, with the number of CCR1-positive plagues correlated with the extent of dementia (Halks-Miller et al., 2003).
An increased expression of prostaglandin endoperoxide synthase 2 (PTGS2), also known as COX-2 can be either pro-inflammatory or anti-inflammatory (Choi et al., 2009). COX-2 is localized in neurons and is mainly induced in response to inflammatory stimuli. Animal studies have shown that during the early phase of inflammation, COX-2 is pro-inflammatory, but during the later phases of inflammation dominated by mononuclear cells, COX-2 appears to have anti-inflammatory effects by generating an alternate set of anti-inflammatory prostaglandins (Gilroy et al., 1999). The syndecan proteins participate in the inflammatory mediated cytoskeletal organization, cell binding, and signaling. High-dose progesterone increased the expression of Sdc1, which expresses a protein that is a transmembrane heparin sulfate proteoglycan and is upregulated by IL-6 (Sneed et al., 1994).
High-dose progesterone increased arachidonate 15-lipoxygenase (Alox15 or 12/15 LOX) expression, which reportedly led to the generation of unstable lipid products from arachidonate (Kuhn and O'Donnell, 2006). These products have been shown to stimulate expression of the pro-inflammatory cytokines, IL-6 and TNF, in mouse adipocytes and macrophages (Fairfax et al., 2010). Alox15 is considered to have a pro-inflammatory effect and may play a key role in the acute inflammatory response. Short-term administration of atorvastatin in a model of cerebral ischemia, has been shown to downregulate the expression of Alox 15 and reduce brain damage (Cui et al., 2010). In this study, high dose progesterone also increased the expression of the fatty acid binding protein 4 (Fabp4) and calcitonin/calcitonin related polypeptide (Calca) which are associated with endothelial function. Fabp4 has been shown to be inversely related to endothelial function in type II diabetic patients (Aragones et al., 2010). Calca, is a potent vasodilator and immune cell modulatory (Brain et al., 1985). Calca secretion activates glial cells that release nitric oxide and initiate an inflammatory response (Li et al., 2008; McKallip et al., 2002). Low dose progesterone treatment was associated with an increased expression of heme oxgenase 1 (Hmox1), an enzyme that catalyzes the conversion of heme into carbon monoxide and biliverdin, has antioxidant capacity and can act as a potent anti-inflammatory protein in the presence of oxidative injury (Yachie et al., 1999).
By 7 days post-CCI injury and 4 days after the completion of the progesterone treatment, low dose progesterone treatment still demonstrated significant effects on gene expression compared to vehicle. In contrast, the effect of high dose progesterone was much less pronounced. For low dose progesterone, significant effects included increased expression of anti-apoptotic genes (Cflar, Igf1) and decreased expression of genes associated with apoptosis (Bag4). Igf1, a neutrophic factor, has been shown to be neuroprotective in models of brain and spinal cord injury (Perez-Martin et al., 2010; Rubovitch et al., 2011). Progesterone has also been shown to up-regulate IgF1 in glial cells (Chesik and De Keyser, 2010). Low dose progesterone increased the expression of caspase-4. There were no other significant effects on the caspase family at any of the time points evaluated. However, the understanding of the function of caspase-4 is limited. The caspase family is subdivided into two sub-groups, those that are activated during apoptosis (caspase-2, -3, -7, -8, -9, -10) and those that undergo activation by the inflammatory response (caspase-1, -4, -5, -11) and are implicated in the maturation of cytokines, (Creagh et al., 2003). Previous studies of progesterone initiated 1 hr post- injury resulted in an increased expression of anti-apoptotic molecules, Bcl-2 and Bcl-XL (Yao et al., 2005) and decreased expression of Bax, Bad (Yao et al., 2005) and caspase-3, pro-apoptotic molecules (Djebaili et al., 2004) that were not differentially expressed in the current study.
Low-dose progesterone resulted in an increased expression of CD38 and CD44. CD38 is expressed in the brain and can catalyze the formation of cyclic ADP-ribose from nicotinamide adenine dinucleotide (NAD+), a reaction that is essential for the regulation of intracellular Ca2+ (Malavasi et al., 2008). In studies of closed head injury in mice, it has been suggested that CD38 plays a role in recovery, in part because of the effect of CD38 on the microglia response following injury (Levy et al., 2009). CD44 is a cell surface glycoprotein that participates in a wide variety of cell–cell interactions, cell adhesion, and migration (Yasuda et al., 2002). Studies in CD44 knockout mice suggest that CD44 may also be involved in apoptosis.
In contrast to our results, in an ischemic rodent stroke model, progesterone administration (8 mg/kg subcutaneously) at 1, 6, 24, and 48 h decreased the expression of MMP-9 (Ishrat et al., 2010). In the current TBI study, both low- and high-dose progesterone increased expression of MMP-9 by ∼50%, however, it was only statistically significant at the high dose. MMP-9 has been shown to be increased in rodent models of TBI (Hayashi et al., 2009; Jia et al., 2008) and ischemic stroke (Ishrat et al., 2010), and in the cerebral spinal fluid (CSF) of patients (Grossetete et al., 2009). The increase in MMP-9 was found to be proportional to injury severity in the previous animal models. In contrast, MMP-8 expression was decreased by 54 and 35% by low- and high-dose progesterone, respectively. However, much less is known about MMP-8 in neurotrauma. MMP-8, also known as collagenase-2 or neutrophil collagenase, is associated with a wide range of inflammatory diseases and is a mediator of both acute and chronic inflammation (Van Lint and Libert, 2006). MMP-8 is stored in neurophils as a pro-enzyme and is therefore present at the initial stages of an inflammatory reaction (Van Lint et al., 2006).
In conclusion, the results of the pharmacokinetic study showed that progesterone administration at doses of 10 mg/kg and 20 mg/kg administered every 12 h produces progesterone concentration in the range found in experimental and clinical studies of progesterone in TBI. The present gene expression study supports the hypothesis that progesterone has significant effects on inflammatory responses and apoptosis. The current findings, demonstrating progesterone's ability to regulate the DNA damage response, cell proliferation, and blood vessel remodeling following TBI, are novel and have not been previously described. The decreased dose-dependent effect of progesterone on functional recovery and inflammatory markers in experimental TBI models (Cutler et al., 2007; Goss et al., 2003) is consistent with our findings of a decreased effect on gene expression at the higher dose of progesterone 7 days post-TBI. Currently, it is not known whether high-dose progesterone is more beneficial than low-dose progesterone at 7 days. An understanding of the biological role of many of the genes differentially expressed during TBI is incomplete, therefore limiting an inclusive interpretation of the treatment and dose effect of progesterone. Further studies are needed in the CCI and other TBI models, comparing the low- and high-dose progesterone treatments using more well-established behavioral and histological markers.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institutes of Health/National Institute of Child, Health and Development (R01 HD061944-01). ). This work was also supported in part by the UW NIEHS sponsored Center for Ecogenetics & Environmental Health (P30ES07033).
Author Disclosure Statement
No competing financial interests exist.
References
- Allison D.B. Cui X. Page G.P. Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat. Rev. Genet. 2006;7:55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- Aragones G. Ferre R. Lazaro I. Cabre A. Plana N. Merino J. Heras M. Girona J. Masana L. Fatty acid-binding protein 4 is associated with endothelial dysfunction in patients with type 2 diabetes. Atherosclerosis. 2010;213:329–331. doi: 10.1016/j.atherosclerosis.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Bazan N.G. Rodriguez de Turco E.B. Allan G. Mediators of injury in neurotrauma: intracellular signal transduction and gene expression. J. Neurotrauma. 1995;12:791–814. doi: 10.1089/neu.1995.12.791. [DOI] [PubMed] [Google Scholar]
- Belelli D. Casula A. Ling A. Lambert J.J. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Brain S.D. Williams T.J. Tippins J.R. Morris H.R. MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Camon E. Magrane M. Barrell D. Lee V. Dimmer E. Maslen J. Binns D. Harte N. Lopez R. Apweiler R. The Gene Ontology Annotation (GOA) Database: sharing knowledge in Uniprot with gene ontology. Nucleic Acids Res. 2004;32:D262–266. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Injury Prevention and Control: Traumatic Brain Injury. 2011. http://www.cdc.gov/traumaticbraininjury/statistics/html. [Aug 29;2011 ]. http://www.cdc.gov/traumaticbraininjury/statistics/html
- Chesik D. De Keyser J. Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neurosci. Lett. 2010;468:178–182. doi: 10.1016/j.neulet.2009.10.051. [DOI] [PubMed] [Google Scholar]
- Choi J.S. Kim S.J. Shin J.A. Lee K.E. Park E.M. Effects of estrogen on temporal expressions of IL-1beta and IL-1ra in rat organotypic hippocampal slices exposed to oxygen-glucose deprivation. Neurosci Lett. 2008;438:233–237. doi: 10.1016/j.neulet.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Choi S.H. Aid S. Bosetti F. The distinct roles of cyclooxygenase-1 and −2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.T. Yarnell A. Kean W.S. Gold E. Lewis B. Ren M. McMullen D.C. Jacobowitz D.M. Pollard H.B. O'Neill J.T. Grunberg N.E. Dalgard C.L. Frank J.A. Watson W.D. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh E.M. Conroy H. Martin S.J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 2003;193:10–21. doi: 10.1034/j.1600-065x.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Cui L. Zhang X. Yang R. Wang L. Liu L. Li M. Du W. Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res. 2010;1325:164–173. doi: 10.1016/j.brainres.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Cutler S.M. Cekic M. Miller D.M. Wali B. VanLandingham J.W. Stein D.G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Dabney A. Storey J. Bioconductor's qvalue package. 2006. http://bioconductor.org/packages/1.8/bioc/vignettes/qvalue/inst/doc/qvalue.pdf. [Sep;2010 ]. http://bioconductor.org/packages/1.8/bioc/vignettes/qvalue/inst/doc/qvalue.pdf
- Dash P.K. Kobori N. Moore A.N. A molecular description of brain trauma pathophysiology using microarray technology: an overview. Neurochem. Res. 2004;29:1275–1286. doi: 10.1023/b:nere.0000023614.30084.eb. [DOI] [PubMed] [Google Scholar]
- Di Pietro V. Amin D. Pernagallo S. Lazzarino G. Tavazzi B. Vagnozzi R. Pringle A. Belli A. Transcriptomics of traumatic brain injury: gene expression and molecular pathways of different grades of insult in a rat organotypic hippocampal culture model. J. Neurotrauma. 2010;27:349–359. doi: 10.1089/neu.2009.1095. [DOI] [PubMed] [Google Scholar]
- Djebaili M. Hoffman S.W. Stein D.G. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Stoica B. Neuroprotection: challenges and opportunities. Arch. Neurol. 2007;64:794–800. doi: 10.1001/archneur.64.6.794. [DOI] [PubMed] [Google Scholar]
- Fairfax B.P. Vannberg F.O. Radhakrishnan J. Hakonarson H. Keating B.J. Hill A.V. Knight J.C. An integrated expression phenotype mapping approach defines common variants in LEP, ALOX15 and CAPNS1 associated with induction of IL-6. Hum. Mol. Genet. 2010;19:720–730. doi: 10.1093/hmg/ddp530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D. Montine T.J. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark. Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R. Carey V. Bates D. Bolstad B. Dettling M. Dudoit S. Ellis B. Gautier L. Ge Y. Gentry J. Hornik K. Hothorn T. Huber W. Iacus S. Irizarry R. Leisch F. Li C. Maechler M. Rossini A. Sawitzki G. Smith C. Smyth G. Tierney L. Yang J. Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R. Carey V. Huber W. Irizarry R. Dudoit S. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer Science-Business Media, Inc; New York: 2005. Iimma: Linear models for microarray data; pp. 397–420. [Google Scholar]
- Gibson C.L. Gray L.J. Bath P.M. Murphy S.P. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Gilroy D.W. Colville–Nash P.R. Willis D. Chivers J. Paul–Clark M.J. Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Goffus A.M. Anderson G.D. Hoane M. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid. Med. Cell Longev. 2010;3:145–152. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss C.W. Hoffman S.W. Stein D.G. Behavioral effects and anatomic correlates after brain injury: a progesterone dose–response study. Pharmacol. Biochem. Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Grossetete M. Phelps J. Arko L. Yonas H. Rosenberg G.A. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halks–Miller M. Schroeder M.L. Haroutunian V. Moenning U. Rossi M. Achim C. Purohit D. Mahmoudi M. Horuk R. CCR1 is an early and specific marker of Alzheimer's disease. Ann. Neurol. 2003;54:638–646. doi: 10.1002/ana.10733. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Kaneko Y. Yu S. Bae E. Stahl C.E. Kawase T. van Loveren H. Sanberg P.R. Borlongan C.V. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009;1280:172–177. doi: 10.1016/j.brainres.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Hayes R.L. Yang K. Raghupathi R. McIntosh T.K. Changes in gene expression following traumatic brain injury in the rat. J. Neurotrauma. 1995;12:779–790. doi: 10.1089/neu.1995.12.779. [DOI] [PubMed] [Google Scholar]
- He J. Evans C.O. Hoffman S.W. Oyesiku N.M. Stein D.G. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Gilbert D.R. Holland M.A. Pierce J.L. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci. Lett. 2006;408:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Ishrat T. Sayeed I. Atif F. Hua F. Stein D.G. Progesterone and allopregnanolone attenuate blood–brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp. Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B.J. Hospitalization associated with traumatic brain injury in the active duty US Army: 2000–2006. NeuroRehabilitation. 2010;26:199–212. doi: 10.3233/NRE-2010-0556. [DOI] [PubMed] [Google Scholar]
- Jia F. Pan Y.H. Mao Q. Liang Y.M. Jiang J.Y. Matrix metalloproteinase-9 expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. J. Neurotrauma. 2008;27:1059–1068. doi: 10.1089/neu.2009.1067. [DOI] [PubMed] [Google Scholar]
- Kuhn H. O'Donnell V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland–Brown W. Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Levy A. Bercovich–Kinori A. Alexandrovich A.G. Tsenter J. Trembovler V. Lund F.E. Shohami E. Stein R. Mayo L. CD38 facilitates recovery from traumatic brain injury. J. Neurotrauma. 2009;26:1521–1533. doi: 10.1089/neu.2008.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Vause C.V. Durham P.L. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick S.A. Wong M.L. Bongiorno P.B. Gold P.W. Licinio J. Rothwell N.J. Endogenous interleukin-1 receptor antagonist is neuroprotective. Biochemical and biophysical research communications. 1997;234:211–215. doi: 10.1006/bbrc.1997.6436. [DOI] [PubMed] [Google Scholar]
- Majewska M.D. Harrison N.L. Schwartz R.D. Barker J.L. Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Malavasi F. Deaglio S. Funaro A. Ferrero E. Horenstein A.L. Ortolan E. Vaisitti T. Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Marciano P.G. Eberwine J.H. Ragupathi R. Saatman K.E. Meaney D.F. McIntosh T.K. Expression profiling following traumatic brain injury: a review. Neurochem. Res. 2002;27:1147–1155. doi: 10.1023/a:1020973308941. [DOI] [PubMed] [Google Scholar]
- McKallip R.J. Do Y. Fisher M.T. Robertson J.L. Nagarkatti P.S. Nagarkatti M. Role of CD44 in activation-induced cell death: CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. Int. Immunol. 2002;14:1015–1026. doi: 10.1093/intimm/dxf068. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale J.E. Ahmed F. Cernak I. Stoica B. Faden A.I. Gene expression profile changes are commonly modulated across models and species after traumatic brain injury. J. Neurotrauma. 2003;20:907–927. doi: 10.1089/089771503770195777. [DOI] [PubMed] [Google Scholar]
- Perez–Martin M. Cifuentes M. Grondona J.M. Lopez–Avalos M.D. Gomez–Pinedo U. Garcia–Verdugo J.M. Fernandez–Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur. J. Neurosci. 2010;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Quigley A. Tan A.A. Hoane M.R. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–148. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra Rao V.L. Dhodda V.K. Song G. Bowen K.K. Dempsey R.J. Traumatic brain injury-induced acute gene expression changes in rat cerebral cortex identified by GeneChip analysis. J. Neurosci. Res. 2003;71:208–219. doi: 10.1002/jnr.10486. [DOI] [PubMed] [Google Scholar]
- Rall J.M. Matzilevich D.A. Dash P.K. Comparative analysis of mRNA levels in the frontal cortex and the hippocampus in the basal state and in response to experimental brain injury. Neuropathol. Appl. Neurobiol. 2003;29:118–131. doi: 10.1046/j.1365-2990.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Duvdevani R. Stein D.G. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Roof R.L. Hoffman S.W. Stein D.G. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol. Chem. Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Rubovitch V. Edut S. Sarfstein R. Werner H. Pick C.G. The intricate involvement of the Insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol. Dis. 2011;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Sayeed I. Stein D.G. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Schouten J.W. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr. Opin. Crit. Care. 2007;13:134–142. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- Schumacher M. Guennoun R. Stein D.G. De Nicola A.F. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Smyth G.K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:1–25. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Sneed T.B. Stanley D.J. Young L.A. Sanderson R.D. Interleukin-6 regulates expression of the syndecan-1 proteoglycan on B lymphoid cells. Cell Immunol. 1994;153:456–467. doi: 10.1006/cimm.1994.1042. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Jenkins L.W. Dixon C.E. Clark R.S. Marion D.W. Kochanek P.M. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J. Neurotrauma. 2001;18:1195–1206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- Stein D.G. The case for progesterone. Ann. N. Y. Acad. Sci. 2005;1052:152–169. doi: 10.1196/annals.1347.011. [DOI] [PubMed] [Google Scholar]
- Stein D.G. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.G. Wright D.W. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin. Investig. Drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- Swan A.A. Chandrashekar R. Beare J. Hoane M.R. Preclinical efficacy testing in middle-aged rats: nicotinamide, a novel neuroprotectant, demonstrates diminished preclinical efficacy after controlled cortical impact. J. Neurotrauma. 2011;28:431–440. doi: 10.1089/neu.2010.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmond S. Rothwell N.J. Interleukin-1 receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- Truettner J.S. Suzuki T. Dietrich W.D. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res. Mol. Brain Res. 2005;138:124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Tusher V.G. Tibshirani R. Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P. Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17:217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Vink R. Nimmo A.J. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6:28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D.W. Bauer M.E. Hoffman S.W. Stein D.G. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J. Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- Wright D.W. Kellermann A.L. Hertzberg V.S. Clark P.L. Frankel M. Goldstein F.C. Salomone J.P. Dent L.L. Harris O.A. Ander D.S. Lowery D.W. Patel M.M. Denson D.D. Gordon A.B. Wald M.M. Gupta S. Hoffman S.W. Stein D.G. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. 402 e391–392. [DOI] [PubMed] [Google Scholar]
- Wright D.W. Ritchie J.C. Mullins R.E. Kellermann A.L. Denson D.D. Steady-state serum concentrations of progesterone following continuous intravenous infusion in patients with acute moderate to severe traumatic brain injury. J. Clin. Pharmacol. 2005;45:640–648. doi: 10.1177/0091270005276201. [DOI] [PubMed] [Google Scholar]
- Xiao G. Wei J. Yan W. Wang W. Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A. Niida Y. Wada T. Igarashi N. Kaneda H. Toma T. Ohta K. Kasahara Y. Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.L. Liu J. Lee E. Ling G.S. McCabe J.T. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J. Neurotrauma. 2005;22:656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- Yasuda M. Nakano K. Yasumoto K. Tanaka Y. CD44: functional relevance to inflammation and malignancy. Histol. Histopathol. 2002;17:945–950. doi: 10.14670/HH-17.945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.