Abstract
Background
Although plants are the main source of vitamin C in the human diet, we still have a limited understanding of how plants synthesise L-ascorbic acid (AsA) and what regulates its concentration in different plant tissues. In particular, the enormous variability in the vitamin C content of storage organs from different plants remains unexplained. Possible sources of AsA in plant storage organs include in situ synthesis and long-distance transport of AsA synthesised in other tissues via the phloem. In this paper we examine a third possibility, that of synthesis within the phloem.
Results
We provide evidence for the presence of AsA in the phloem sap of a wide range of crop species using aphid stylectomy and histochemical approaches. The activity of almost all the enzymes of the primary AsA biosynthetic pathway were detected in phloem-rich vascular exudates from Cucurbita pepo fruits and AsA biosynthesis was demonstrated in isolated phloem strands from Apium graveolens petioles incubated with a range of precursors (D-glucose, D-mannose, L-galactose and L-galactono-1,4-lactone). Phloem uptake of D-[U-14C]mannose and L-[1-14C]galactose (intermediates of the AsA biosynthetic pathway) as well as L-[1-14C]AsA and L-[1-14C]DHA, was observed in Nicotiana benthamiana leaf discs.
Conclusions
We present the novel finding that active AsA biosynthesis occurs in the phloem. This process must now be considered in the context of mechanisms implicated in whole plant AsA distribution. This work should provoke studies aimed at elucidation of the in vivo substrates for phloem AsA biosynthesis and its contribution to AsA accumulation in plant storage organs.
Background
In plants, L-ascorbic acid (AsA) is essential for photosynthetic activity via the detoxification of superoxide and hydrogen peroxide in chloroplasts in the absence of catalase [1]. AsA is also crucially involved in the regeneration of α-tocopherol and zeaxanthin and the pH-mediated modulation of PS II activity [2]. The critical importance of AsA in photosynthetic metabolism is emphasised by its abundance in chloroplasts where its concentration reaches 50 mM [2]. The constitutive role of AsA in photosynthesis explains its widespread distribution and generally high content in leaves [3,4]. These studies also revealed relatively low variability of AsA content between species, with coefficients of variation ranging between 59% in herbaceous plants (211 species) and 67% in woody plants (41 species). Conversely, the variability in the AsA content of non-photosynthetic tissues such as storage organs is generally much higher. Our estimates made from available data on fruits and vegetables from 65 species show an average vitamin C (AsA + dehydroascorbic acid [DHA]) content of 1.1 mg gFW-1 with a coefficient of variation of 334%. For example, a 10,000-fold difference in AsA content is found between the fruits of camu camu (Mirciaria dubia), which contains up to 30 mg gFW-1 vitamin C [5] and the medlar (Mespilus germanica) containing less than 3 μg gFW-1 [6]. There is no obvious taxonomic explanation for such differences and large variations in storage organ AsA content are also observed within individual species including cultivated species such as strawberry and blackcurrant [7,8]. There is also no clear physiological explanation for the vast variability of AsA content in plant storage organs, but our understanding of AsA functions in these tissues is still very limited. In addition to its general antioxidant functions, AsA has been implicated in cell division, cell wall metabolism, cell expansion, and plant-pathogen interactions [9].
Advances in our understanding of AsA biosynthesis in plants have been made recently. There is general consensus that the biosynthetic pathway proposed by the Smirnoff group [10], represents a major pathway in plants. Confirmation has been obtained from analyses of Arabidopsis thaliana AsA deficient mutants [11,12] and from identification and manipulation of several of the genes involved [13-15]. However, there are reports of AsA accumulation in lettuce over-expressing rat gulono-lactone oxidase [16] and in A. thaliana constitutively expressing a strawberry D-galacturonic acid reductase [17] suggesting alternative pathways may be operational. Additionally, AsA accumulation has been reported in maize and tobacco constitutively over-expressing the AsA recycling gene DHA reductase [18]. Thus, despite this renewed interest, the mechanisms controlling AsA accumulation in storage organs remain largely elusive. It is still unclear for example whether AsA accumulation in storage organs occurs as a result of in situ synthesis or import from photosynthetically active regions. There is evidence for developmentally regulated AsA accumulation in fruits with high rates soon after anthesis [8] and in potato tubers a large accumulation is observed soon after tuber induction [19].
The competence for AsA synthesis seems to be ubiquitous amongst plant cells and AsA accumulation has been observed in a wide variety of cultured cells and excised tissues or organs incubated with appropriate substrates (e.g. [10,15] and [19-23]). However, evidence for direct AsA biosynthesis in storage organs in planta is missing. A recent report [24] described uptake of radiolabelled AsA by Arabidopsis and Medicago leaves and its movement to sink tissues such as shoot and root tips, flowers, siliques and sink leaves, leading the authors to suggest that long-distance transport of AsA occurs in plants. The presence of AsA in the phloem was first reported some time ago [25], but its specific functions were not properly investigated. The purpose of the present paper is to provide additional information on phloem AsA content, specifically its origin and its implications for AsA accumulation in storage organs. By adopting a wide range of plant material and exploiting the most suitable model systems available we show that AsA is a widespread constituent of plant phloem sap, and that isolated phloem strands are competent for AsA biosynthesis from distant substrates. Moreover, we demonstrate that the intermediates of AsA biosynthesis D-mannose and L-galactose can be taken up by the phloem. The implications of these findings in relation to whole plant AsA distribution are discussed.
Results
Detection of AsA in plant vascular tissue
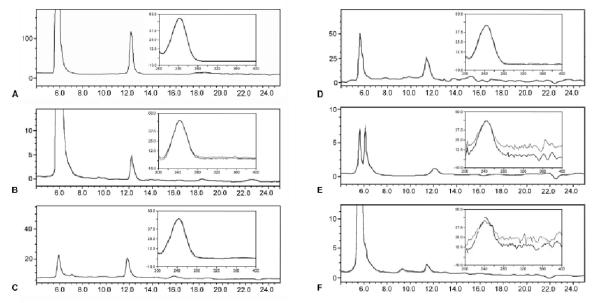
Aphid stylectomy was employed to isolate phloem exudates from a number of crop plants (barley, pea, potato, tobacco, turnip) in the absence of contamination from other tissues. When exudates were analysed by HPLC, AsA was detected in all cases (Fig. 1). We were unable to avoid evaporation of water from the exuded material and thus made no attempt to quantify AsA concentration in the exudates. However, it should be noted that in all cases AsA appeared as the dominant compound with absorption at 245 nm retained by the column.
Figure 1.
HPLC traces of plant phloem exudates obtained by aphid stylectomy. Stylets were excised from aphids feeding on Hordeum vulgare (barley) (B), Pisum sativum (pea) (C), Solanum tuberosum (potato) (D), Nicotiana tabacum (tobacco) (E) and Brassica rapa ssp. Oleifera (turnip) (F) using a microcautery device. The exuded phloem sap was collected and diluted into ice-cold 5% H3PO3 containing 5 mM TCEP. The figure shows chromatograms (A245) of phloem exudates and insets show the absorbance spectrum from the left and right sides of the peak with identical retention time to authentic AsA (A). The horizontal axis represents retention time (min) and the vertical axis detector response (milli-absorbance units).
In order to obtain more substantial amounts of exudate to allow quantification of AsA, we focussed our attention onto Cucurbitaceae fruits, from which relatively high volumes of vascular exudates (approximately 1 ml Kg-1 of fruit) could be obtained. Table 1 shows the AsA concentration in vascular exudates from fruits of a number of Cucurbitaceae species in relation to that of soluble carbohydrates. Values in the region of 0.49 to 1.44 mg gFW-1 (2.8 to 8.2 mM) were obtained, whilst the total carbohydrate concentration ranged from 10.95 to 23.28 mg gFW-1.
Table 1.
Total carbohydrate and AsA content of Curcubitaceae fruit vascular exudates
| Species | Carbohydrate (mg gFW-1) | AsA (mg gFW-1) | AsA (mM) |
| Cucumis sativus | 10.95 ± 0.13 | 1.02 ± 0.05 | 5.8 ± 0.33 |
| Cucurbita maxima | 22.48 ± 0.09 | 1.44 ± 0.04 | 8.2 ± 0.20 |
| C. moschata | 21.98 ± 0.80 | 0.49 ± 0.03 | 2.8 ± 0.15 |
| C. pepo (courgette) | 17.96 ± 0.34 | 0. 87 ± 0.05 | 4.9 ± 0.26 |
| C. pepo (squash) | 20.46 ± 0.53 | 0.81 ± 0.02 | 4.6 ± 0.13 |
| C. maxima x C. moschata | 23.28 ± 0.66 | 0.63 ± 0.02 | 3.6 ± 0.09 |
Mean ± S.E. n = 3 Samples were extracted in 5% H3PO3 (w/v) containing 5 mM TCEP and carbohydrate concentration estimated according to Dubois et al. (1956). AsA concentration was estimated by HPLC.
AsA localisation in the vascular tissue was confirmed by histochemical analysis of slices excised from courgette fruits and celery petiole (also used for metabolic studies, see below) using methanolic AgNO3 solutions. This method exploits the specific ability of AsA to reduce Ag+ at low temperature resulting in the formation of metallic silver deposits [26]. In both courgette fruit and celery petiole, metallic silver deposits were abundant in the vascular bundles with the staining in this tissue clearly limited to the phloem elements in the case of celery (Fig. 2). In addition, staining was evident in the tissue surrounding developing seeds in the courgette fruit and in the storage parenchyma in the celery petiole. No formation of metallic silver deposits were observed in control specimens pre-treated with CuSO4, to induce AsA oxidation. The penetration of AgNO3 in parenchyma cells was confirmed by preincubation of tissues with 25 mM L-galactose which resulted in intense staining of parenchymal tissues (data not shown)
Figure 2.

Localisation of AsA with methanolic AgNO3 in courgette and celery tissues. The left side of each image shows sections stained immediately after excision and the right side control sections which were pre-incubated in 5% (w/v) CuSO4 to oxidise endogenous AsA. Transverse section of courgette fruit (Ai) shows intense staining in the region of the outer vascular ring (arrows). Staining was also observed in the region surrounding developing seeds. Detail of the outer vascular area confirms that staining was confined to vascular regions (Aii). Staining was absent in control sections pre-incubated with 5% CuSO4. In the celery petiole (Bi) intense staining was associated with vascular bundles (arrows) and storage parenchyma (tail-less arrows). Detail of the vascular region (Bii) shows intense staining associated with the phloem but not the xylem.
AsA biosynthesis in the phloem
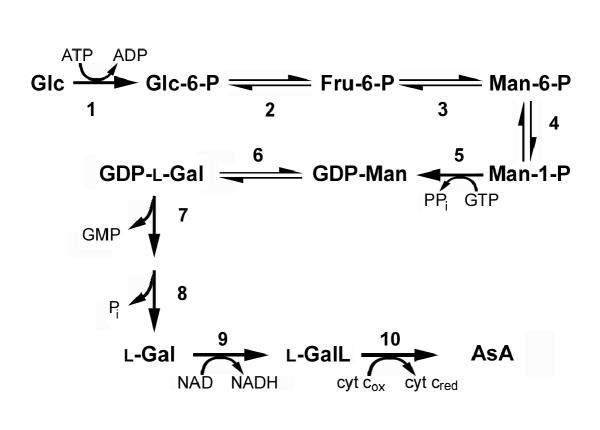
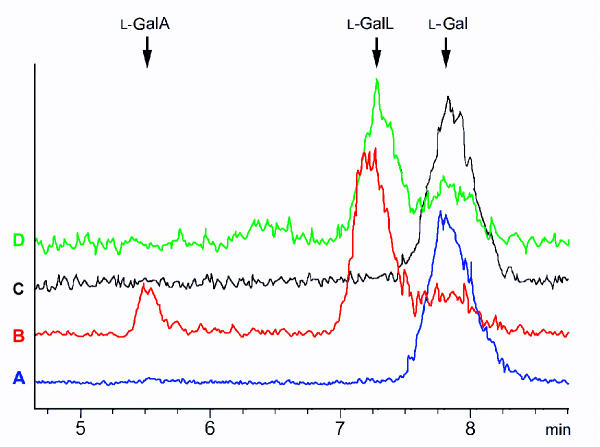
The relatively large quantities of vascular exudates obtained from excision of courgette fruit enabled us to investigate the presence of the enzymes of the AsA biosynthetic (Smirnoff-Wheeler) pathway (Fig. 3). We detected activities of all the previously identified enzymes of the AsA biosynthetic pathway [10] in the vascular exudates with the exception of the terminal (mitochondrial) enzyme L-galactono-1,4-lactone dehydrogenase (Table 2). Similarly, no conversion of L-galactono-1,4-lactone to AsA was detected in isolated vascular exudate (data not shown). Both vascular exudates and homogenised fruit tissue were competent for the enzymic hydrolysis of GDP-L-[1-14C]galactose although we were unable to test for the activity of L-galactose-1-phosphate phosphatase due to unavailability of substrate. In many cases, the enzyme activities in exudates were higher than in whole fruit tissue. In some cases e.g. phosphomannose isomerase, phosphomannose mutase and GDP-D-mannose pyrophosphorylase, activity was only detected in the vascular exudate. Incubation of L-[1-14C]galactose directly in collected vascular exudate without any additions resulted in the formation of L-[14C]galactono-1,4-lactone (Fig. 4). This conversion was prevented by desalting of the exudate and could be restored by the addition of NAD to desalted exudate.
Figure 3.
The Smirnoff-Wheeler AsA biosynthetic pathway. Plants synthesize AsA from glucose via phosphorylated hexose intermediates. 1. hexokinase (E.C. 2.7.1.1), 2. phosphoglucose isomerase (E.C. 5.3.1.9), 3. phosphomannose isomerase (E.C. 5.3.1.8), 4. phosphomannose mutase (E.C. 5.4.2.8), 5. GDP-mannose pyrophosphorylase (E.C. 2.7.7.22), 6. GDP-mannose-3,5-epimerase (E.C. 5.1.3.18), 7. GDP-L-galactose pyrophosphatase, 8. L-galactose-1-phosphate phosphatase, 9. L-galactose dehydrogenase, 10. L-galactono-1,4-lactone dehydrogenase (E.C. 1.3.2.3). Glc, D-glucose; Fru, D-fructose; Man, D-mannose; L-Gal, L-galactose; L-GalL, L-galactono-1,4-lactone. Where no E.C. numbers are listed they have yet to be assigned.
Table 2.
Activity of Smirnoff Pathway Enzymes in Courgette Vascular Exudate and Tissue
| Activitya | Phloem | Tissue |
| HK (D-Glc) | 23.0 ± 1.73 | 22.5 ± 2.89 |
| HK (D-Frc) | 29.7 ± 1.33 | 24.8 ± 3.58 |
| HK (D-Man) | 4.0 ± 0.40 | 4.0 ± 0.58 |
| PGI | 1861.3 ± 15.18 | 1194.7 ± 173.38 |
| PMI | 94.2 ± 5.20 | N.D.b |
| PMM | 1.5 ± 0.11 | N.D.b |
| GDPM PPase | 22.8 ± 1.56 | N.D.b |
| GDPM-3,5-epimerase | 0.07 ± 0.012 | 0.12 ± 0.017 |
| GDP-L-gal PPPase | 0.012 + 0.002 | 0.010 ± 0.002 |
| L-GalDH | 28.0 ± 0.46 | 7.0 ± 1.27 |
| L-GalLDH | N.D.b | 1.98 ± 0.18 |
a Activity is expressed in nmol min-1 gFW-1 of tissue ± S.E., n = 3 b N.D. = Activity not detected Courgette fruit exudate or tissue was prepared and desalted as described in the text. Enzymes listed are: HK, hexokinase (E.C. 2.7.1.1) with D-glucose (D-Glc), D-fructose (D-Frc) or D-mannose (D-Man) as substrate; PGI, phosphoglucose isomerase (E.C. 5.3.1.9); PMI, phosphomannose isomerase (E.C. 5.3.1.8); PMM, phosphomannose mutase (E.C. 5.4.2.8); GDPM PPase, GDP-D-mannose pyrophosphorylase (E.C. 2.7.7.22); GDPM-3,5-epimerase, GDP-D-mannose-3,5-epimerase (E.C. 5.1.3.18); GDP-L-gal PPPase, GDP-L-galactose pyrophosphatase; L-GalDH, L-galactose dehydrogenase; L-GalLDH, L-galactono-1,4-lactone dehydrogenase (E.C. 1.3.2.3). Where no E.C. numbers are supplied they have yet to be assigned.
Figure 4.
Synthesis of L-[14C]galactono-1,4-lactone from L-[1-14C]galactose in courgette fruit exudate. Radiochromatograms obtained from exudate incubated with L-[1-14C]galactose for 30 min at 30°C. The reaction was stopped by the addition of H3PO3 prior to removal of precipitate and analysis by HPLC with online flow scintillation detection. Individual chromatograms show reactions containing L-[1-14C]galactose and heated (100 °C) exudate (A), fresh exudate (B), desalted exudate (C) and desalted exudate with addition of 0.5 mM NAD (D). The elution times of authentic L-[1-14C]galactose (L-Gal), L-[1-14C]galactonolactone (L-GalL) and L-[1-14C]galactonic acid (L-GalA) are shown.
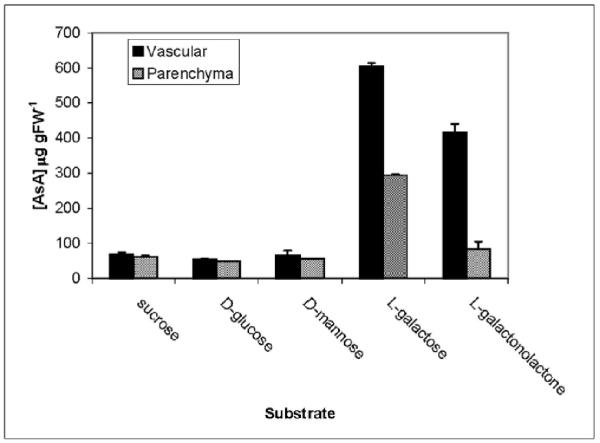
Functional characterisation of the AsA biosynthetic pathway in the phloem in vivo was carried out using isolated phloem strands from celery petioles, an established model system for phloem metabolism [27]. Phloem strands or storage parenchyma discs were incubated for 18 h with a range of AsA precursors. AsA accumulation was observed in both tissues upon incubation with L-galactose but the phloem strands accumulated ca. twice the amount of AsA compared with the parenchyma (Fig. 5). Incubation with L-galactono-1,4-lactone resulted in substantial AsA accumulation in the phloem strands only. AsA accumulation was not observed when samples were incubated with sucrose, D-glucose or D-mannose. However, the capacity of both tissues to synthesise AsA from these substrates was confirmed by incubation with D-[U-14C]glucose or D-[U-14C]mannose. Radiolabel accumulation into AsA was observed from both these substrates as well as from L-[1-14C]galactose (Table 3). With all labelled substrates, phloem strands showed a much higher total uptake and a significantly higher proportion of label incorporation into L-[14C]AsA compared with parenchyma discs.
Figure 5.
Effect of exogenous substrates on AsA coxntent of celery tissues. Isolated phloem strands or storage parenchyma discs were incubated for 18 h in 20 mM MES pH 5.5, 300 mM mannitol, 5 mM MgCl2, 2 mM KCl, 1 mM CaCl2, 1 mM CaSO4 (Daie, 1987) and 25 mM of the appropriate substrate. AsA was extracted and total AsA quantified by HPLC after reduction of DHA with 5 mM TCEP. Data represent mean ± S.E., n = 3.
Table 3.
Conversion of 14C-precursors to AsA in celery tissues
| Substrate Tissue |
d-[U-14C]glucose | d-[U-14C]mannose | l-[1-14C]galactose | |||
| Parenchyma | Vascular | Parenchyma | Vascular | Parenchyma | Vascular | |
| % Incorporation to AsAa | 1.4 ± 0.06 | 3.1 ± 0.23 | 2.7 ± 0.06 | 5.9 ± 0.23 | 19.9 ± 0.92 | 49.8 ± 0.46 |
| Uptake (μCi gFW-1)a | 0.65 ± 0.07 | 3.61 ± 0.05 | 0.32 ± 0.02 | 3.49 ± 0.05 | 0.09 ± 0.01 | 0.67 ± 0.02 |
| Uptake (nmol gFW-1)a | 2.1 ± 0.23 | 11.6 ± 0.17 | 1.0 ± 0.06 | 11.4 ± 0.17 | 1.6 ± 0.12 | 12.2 ± 0.23 |
a Values are presented as mean ± S.E., n = 3. Samples were prepared and incubated as described in the text. After incubation samples were extracted with 5% (w/v) PCA containing 5 mM TCEP and AsA purified as described in materials and methods. Radiolabel distribution to AsA was quantified by HPLC using online flow scintillation detection.
Uptake of AsA and other precursors by the leaf phloem
The ability of source leaf phloem to take up L-[1-14C]AsA, or a number of substrates related to AsA metabolism ([1-14C]DHA, D-[U-14C]mannose, L-[1-14C]galactose and [U-14C]sucrose as a reference) was tested in leaf discs of Nicotiana benthamiana, a model system for phloem uptake studies as it allows good resolution of major and minor veins [28]. Although [U-14C]sucrose and D-[U-14C]mannose appeared to be taken up more readily by the discs, the pattern of radioactivity distribution was similar with all precursors used, showing clear labelling of major and minor veins (Fig. 6). Except for L-[1-14C]ascorbic acid, no significant metabolism of applied substrates was observed during the incubation period. With L-[1-14C]ascorbic acid, ca. 80% of the radioactivity was present as [14C]DHA at the end of the experiment as determined by HPLC with flow scintillation analysis (data not shown).
Figure 6.
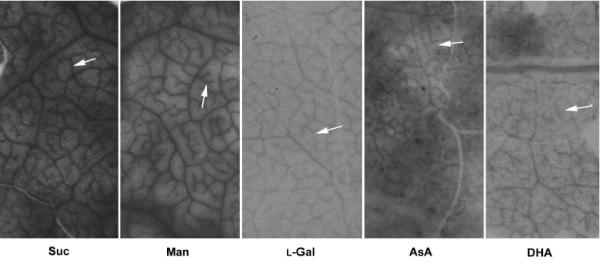
Uptake of AsA related metabolites by Nicotiana benthamiana leaf discs. N. benthamiana source leaf discs were prepared as described in Materials and Methods and incubated with 25 mM MES pH 5.5, 20 mM CaCl2 and the appropriate labelled substrate (at a final S.A. of 18.5 kBq mmol-1) for 3 h in the light (200 μmol m-2 s-1). Leaf discs were washed, dried and lyophilised prior to exposure to Kodak Biomax X-ray film for 1 week. With all substrates tested, labelling is observed in both the major and minor (arrows) leaf veins. Labelling intensity appeared much higher with sucrose and mannose compared with the other substrates.
Discussion
Detection of AsA in the phloem
We have used stylectomy to access the phloem sap of a number of crop plants (barley, pea, potato, tobacco, turnip) and AsA was detected without exception. This finding corroborates earlier reports [24,25] and indicates that AsA is a widespread constituent of plant phloem. The AsA concentration in the samples obtained by stylectomy was not established in this or in previous studies but values of 0.49 to 1.45 mg gFW-1 (2.8 to 8.2 mM) were obtained from vascular exudates of mature fruits of Cucurbitaceae species (Table 1). Such exudates have been used extensively to study phloem metabolism [29-32] and have been shown to have properties typical of phloem saps such as high pH and inorganic ions, amino acid and organic acid levels similar to those of phloem saps from other species. The possibility of large dilution from xylem sap or water channels has also been dismissed [32] and the AsA concentration detected in vascular exudates from Cucurbitaceae species is comparable with the values reported by Ziegler [25] for other plant species. Additional evidence for the presence of AsA in the phloem tissue was obtained using a histochemical approach based on the ability of AsA to reduce cold, acidified, ethanolic AgNO3 and generate metallic silver deposits [26]. In both courgette fruit and celery petioles, (i.e. the plant organs used for experimental investigation on AsA biosynthesis) strong staining was observed in the vascular region with metallic deposit specifically localised to the phloem tissue in the case of celery (Fig. 2). Although this technique is not specific for AsA, its selectivity is substantially improved at low temperature and pH where other reducing agents are ineffective [26,33]. Additionally, pre-treatment of plant specimens with CuSO4 completely prevented the formation of metallic silver deposits (Fig. 2). The relatively more intense staining of the vascular region in relation to the surrounding parenchymous areas may be explained if AsA concentration in the cytosol was substantially higher than in the vacuole [34]. Intense staining of non-vacuolated (meristematic) cells has been previously reported with this technique [35].
Synthesis of AsA in the phloem
The presence of AsA synthesising enzymes was investigated in vascular exudates from mature fruits of courgette, a system validated for the isolation and identification of phloem resident proteins [36]. With the exception of the putative L-galactose 1-phosphate phosphatase [10] and L-galactono-1,4-lactone dehydrogenase, the activities of all AsA biosynthetic enzymes were detected in the vascular exudates. This includes the enzyme activity responsible for the hydrolysis of GDP-L-galactose, a key rate-limiting step for AsA biosynthesis in plants (Hancock and Viola, in preparation). To our knowledge this represents the first direct assay of GDP-L-galactose hydrolysis in extracts of plant tissues. The enzyme activities in the exudates appeared substantial by comparison with whole tissue extracts and for some of the enzymes activity was detectable solely in the exudates.
However, this may again reflect the vacuolisation of parenchyma cells resulting in dilution of enzyme activities below our levels of detection. We also show that vascular exudates contain, in addition to the required enzyme activities, pyridine nucleotides required for the conversion of L-[1-14C]galactose into L-[14C]galactono-1,4-lactone (Fig. 4). The further conversion of L-galactono-1,4-lactone into AsA would require the activity of L-galactono-1,4-lactone dehydrogenase, the only membrane-bound enzyme of the AsA biosynthetic pathway (located in the inner mitochondrial membrane [37]). It is generally acknowledged that mitochondria are not abundant in the phloem sap however it is expected that, in vivo, metabolites such as L-galactono-1,4-lactone may be exchanged between the sieve elements and the companion cells via specialised plasmodesmata [38]. Thus in vivo AsA biosynthesis within the phloem tissue (i.e. the sieve element-companion cell [SE-CC] complex) may involve enzymic cooperation between the sieve tubes and the mitochondria rich companion cells.
The competence of the phloem tissue for the in vivo conversion of L-galactose or L-galactono-1,4-lactone to AsA was demonstrated in isolated phloem strands from celery petioles (Fig. 5). Additionally incubation of the phloem strands with D-[U-14C]glucose or D-[U-14C]mannose resulted in substantial labelling of AsA clearly demonstrating that this tissue possesses a fully operational AsA biosynthetic (Smirnoff-Wheeler) pathway (Fig. 3). Moreover, AsA synthesis and partitioning to AsA from labelled precursors (including L-[1-14C]galactose) was significantly higher in phloem strands compared with storage parenchyma tissue, although differences in rates of precursor uptake were observed between the tissues used. The overall conclusion from the different experimental approaches used is that the phloem unexpectedly represents a tissue with a highly active AsA biosynthetic capacity.
Origin of phloem AsA
How can we reconcile our finding of phloem AsA biosynthesis with the hypothesis of long distance AsA transport in plants put forward by Franceschi and Tarlyn [24]? These authors reported that application of 14C-AsA to leaf flaps in Medicago sativa and A. thaliana resulted in its translocation to sink tissues. We also found that that supply of L-[1-14C]AsA (and [1-14C]DHA) to source leaf discs of Nicotiana benthamiana resulted in accumulation of radiolabel in both major and minor veins. However, care needs to be used in interpreting experiments where exogenous substrates are introduced into the plant transport system via abraded or damaged leaves. Evidence of phloem translocation (via mass flow) of xenobiotics supplied to leaves and their unloading in sinks is available from experiments with carboxyfluorescein diacetate and other phloem tracers [e.g. [39]]. Work with carboxyfluorescein diacetate also demonstrates that phloem leakage of charged molecules (e.g. AsA) during translocation to sink tissues is minimal. An alternative possibility is that phloem AsA biosynthesis may be directly relevant to long-distance transport of AsA. For example, the SE-CC is known to contain sucrose synthase which is involved in sucrolytic cleavage to sustain energy production [40]. Sucrolytic intermediates could thus be used to sustain AsA biosynthesis within the SE-CC complex via the Smirnoff-Wheeler pathway. Micro-tubers obtained from isolated potato internodes cultured in vitro in the dark with sucrose as the sole carbon source accumulated AsA in concentrations similar to those observed in tubers obtained from field-grown plants [19]. Potato tubers are phloem-rich organs with a network of phloem strands internal and external to the vascular ring [39] and AsA distribution in potato tubers closely follows the phloem distribution (Viola et al., unpublished). However, our finding that both D-[U-14C]mannose and L-[1-14C]galactose were readily taken up by N. benthamiana leaf discs (resulting in a labelling intensity of major and minor veins similar to that of [U-14C]sucrose in the case of the former) also raises the possibility that more direct intermediates of AsA biosynthesis may be provided to the phloem by the leaf mesophyll. In a following paper we show that L-galactose supply to source potato leaves results in AsA accumulation in developing tubers (Tedone et al., in preparation).
Conclusions
We show here that AsA presence in the phloem of higher plants is widespread and that the SE-CC complex of courgette and celery is competent for the synthesis of AsA from a range of substrates. Further work is required to establish precisely the nature of the substrate for phloem AsA biosynthesis in vivo and to establish quantitatively the contribution of phloem biosynthesis to the overall AsA content in storage organs. Although AsA transporters will be required for the transfer of AsA into storage organs involving apoplastic unloading steps, phloem biosynthesis may represent a significant direct source of AsA for those storage organs (e.g. potato tubers) where phloem unloading follows a symplastic route [39].
Methods
Plant Material and Preparation
Celery (Apium graveolens L.) and courgette (Curbita pepo L.) were purchased locally. Other plants were grown in unheated glasshouses under conditions of natural light in a standard mixture of peat, sand, limestone, vermiculite and celcote containing sincrostart and sincrocel 6 fertilisers. Plants were hand watered daily. Celery vascular bundles and parenchyma discs were prepared as described [27]. Vascular exudates were collected from courgettes and other Cucurbitaceae by positive displacement pipette from mature cut fruit and directly transferred to the appropriate ice-cold buffer for either enzyme activity or AsA determinations (see below). Phloem exudates from other plants were collected directly from severed stylets of Myzus persicae feeding on source leaf petioles. Prior to HPLC injection, phloem exudate was diluted into ice-cold 5% metaphosphoric acid with or without 5 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP).
Chemicals and labelled substrates
D-[U-14C]glucose (S.A. 11.47 MBq mmol-1), D-[U-14C]mannose (S.A. 11.36 MBq mmol-1), [U-14C]sucrose (S.A. 17.09 MBq mmol-1) and L-[1-14C]AsA (S.A. 0.52 MBq mmol-1) were obtained from Amersham Pharmacia Biotech, Little Chalfont, UK. L-[1-14C]galactose (S.A. 2.04 MBq mmol-1) was obtained from American Radiolabelled Chemicals, St. Louis, USA. [14C]DHA was obtained by incubating 0.037 MBq L-[1-14C]AsA with 10 μg ascorbate oxidase (E.C. 1.10.3.3; Roche, Lewes, UK) in 50 mM sodium phosphate buffer pH 5.6 (final volume 100 μl). L-[1-14C]galactonic acid was synthesised by incubation of 0.037 MBq L-[1-14C]galactose with 1U fucose dehydrogenase (E.C. 1.1.1.122; Sigma, Dorset, UK) and 0.5 mM NADP in 50 mM glycine-KOH buffer pH 9.5 (final volume 100 μl). L-[1-14C]galactonolactone was obtained by incubation of 0.037 MBq L-[1-14C]galactose with 1U fucose dehydrogenase and 0.5 mM NADP in 50 mM MOPS buffer pH 7.8 (100 μl). GDP-L-[1-14C]galactose was a gift from Dr. Ken Lawrie, GaxoSmithKline Pharmaceuticals, Marlow, UK and was synthesised according to the method of Baisch and Ohrlein [41] from L-[1-14C]galactose [ARC, St. Louis, USA]. The final product had a specific activity 0.37 MBq mmol-1, radiochemical purity of >95% as estimated by anion exchange HPLC and overall purity >90% as estimated by 1H and 13C NMR.
With the exception of L-fucose dehydrogenase and phosphomannose isomerase, which were purchased from Sigma, Dorset, UK, all enzymes were purchased from Roche, East Sussex, UK.
In Situ Staining of AsA using Acidic Silver Nitrate
Transverse sections (approximately 1 mm) of either celery petiole or courgette fruit were cut by hand and washed briefly in distilled water then stained and fixed as described by Chinoy [26]. Control reactions were undertaken in which AsA in the tissue was first oxidised by exposure to aqueous 5% CuSO4 as described [26].
Tissue Incubation, Metabolite Extraction and HPLC Analysis
Celery tissues were incubated in buffer A consisting of 20 mM MES pH 5.5, 300 mM mannitol, 5 mM MgCl2, 2 mM KCl, 1 mM CaCl2 and 1 mM CaSO4 [27] in petri dishes with rotary shaking of 100 rpm. For experiments with unlabelled precursors, the appropriate compound was added to a final concentration of 25 mM and the incubation continued for 18 h. For labelling experiments, 400 mg tissue were incubated in 650 μl buffer containing 111 kBq of either D-[U-14C]glucose, D-[U-14C]mannose or L-[1-14C]galactose in sealed vessels with 100 rpm rotary shaking for 4 h. For experiments with unlabelled precursors, AsA was extracted by grinding the celery tissue in liquid N2 followed by the addition of 5 volumes of 5% (w/v) metaphosphoric acid and further homogenisation. Cell debris was removed by centrifugation (16000 g, 1°C, 5 min). The supernatant was used directly for analysis of reduced AsA or incubated with 5 mM TCEP (1 h, 4°C) prior to analysis for measurement of total AsA by HPLC [22]. Recoveries of authentic AsA added to tissue samples immediately prior to extraction exceeded 90%. In radiolabelling experiments the tissue was removed from the incubation medium and washed three times for 5 min in 5 ml of buffer A. The tissue was then blotted, ground in liquid N2 and homogenised in 5 volumes of 5% perchloric acid (PCA) containing 5 mM TCEP. After standing in ice for 30 min, cell debris was removed by centrifugation (16000 g, 5 min, 1°C) and a pH indicator (BDH 4080 indicator, 20 μl ml-1) was added to the supernatant. The pH range of the sample was adjusted to 6–7 by the drop-wise addition of a solution of 5 M K2CO3. Insoluble KClO3 salts were removed by centrifugation (16000 g, 5 min, 1°C), the supernatant was divided into 500 μl aliquots and applied to SAX SPE cartridges (100 mg, acetate counter ion, Alltech, Carnforth, UK). After washing the column with 4 ml H2O, L-[14C]AsA was released with 4 ml 300 mM formic acid. After lyophilisation and resuspension in 200 μl H2O, L-[14C]AsA was further purified and quantified by HPLC as previously described [22] with the exception that radioactivity was detected and quantified using a Packard 150TR radiodetector in the homogenous mode (500 μl flow cell) with postcolumn addition of Ultima Flo M scintillant at 3 ml min-1. Under these conditions, recovery of authentic L-[1-14C]AsA added to the tissue samples immediately prior to extraction exceeded 85%. Total carbohydrate determination in Cucurbitaceae fruit exudates was carried out using the phenol-sulphuric acid method [42].
Extraction and Determination of Enzyme Activities
Hexose kinase (HK), phosphoglucose isomerase (PGI), phosphomannose isomerase (PMI), phosphomannose mutase (PMM), GDP-L-galactose pyrophosphatase (GDP-L-gal PPPase) and L-galactose dehydrogenase activities (L-GalDH) were extracted from courgette fruit tissue by grinding in a mortar and pestle in ice cold 50 mM HEPES pH 8.0, 5 mM MgCl2, 5 mM DTT, 1 mM EDTA, 1 mM EGTA, 1 mM benzamidine hydrochloride and 0.5 mM PMSF (1:3 w/v). Cell debris was removed by centrifugation (10000 g, 10 min, 1°C) and sample supernatants were desalted on Sephadex G25 PD10 columns (Amersham Biosciences, UK) equilibrated with the same buffer prior to use. GDP-D-mannose pyrophosphorylase activity was extracted and desalted as described above with the exception that the buffer used throughout consisted of 100 mM tris pH 7.5, 15 mM 2-mercaptoethanol and 0.5 mM PMSF as described by Keller et al. [13]. GDP-D-mannose-3,5-epimerase activity was extracted and desalted using 100 mM tris pH 7.6, 5 mM DTT and 1 mM EDTA [43].
L-Galactono-1,4-lactone dehydrogenase (L-GalLDH) activity was extracted as described [44] with the exception that mitochondria were collected by centrifugation at 30000 g. In all cases, vascular exudates were treated exactly as whole tissue with the exception that exudates were diluted directly into extraction buffer and were not ground in a mortar and pestle.
HK activity was determined in a reaction mixture consisting of enzyme extract, 50 mM HEPES pH 8.0, 5 mM MgCl2, 2 mM ATP, 0.5 mM NAD and 1 U NAD-dependent glucose-6-phosphate dehydrogenase (from Leuconostoc mesnteroides) in a final volume of 1 ml. The reaction was undertaken at 30°C and started by the addition of 2 mM D-glucose. Reaction kinetics were followed by measurement of NAD reduction at 340 nm in a Hitachi U-3010 spectrophotometer. Control reactions were undertaken using boiled extract. HK activity was also measured using fructose as substrate in which case the reaction mixture additionally contained 1 U PGI or mannose as substrate where the mixture contained 1 U PMI in addition to PGI.
PGI activity was determined in a reaction mixture consisting of enzyme extract, 50 mM HEPES pH 8.0, 5 mM MgCl2, 0.5 mM NAD, 5 μM ZnSO4 and 1 U glucose-6-phosphate dehydrogenase in a final volume of 1 ml. The reaction was undertaken at 30°C and started by the addition of 2 mM D-fructose-6-phopshate. Reaction kinetics were measured as described for hexokinase. PMI activity was measured as for PGI but the reaction mixture additionally contained 1 U PGI and was started by the addition of D-mannose-6-phosphate. PMM activity was assayed by including 1 U PMI in addition to PGI and starting the reaction with D-mannose-1-phosphate. In all cases control reactions were undertaken using boiled enzyme preparations.
GDP-D-mannose pyrophosphorylase activity was measured as described [13] with the exception that 2 mM NaF was included in the reaction mixture and control reactions lacked inorganic pyrophosphate. The reaction was linear with time for up to 2 h. GDP-D-mannose-3,5-epimerase activity was determined as described [43]. GDP-L-galactose pyrophosphatase activity was determined in a reaction mixture consisting of enzyme extract, 50 mM HEPES pH 8.0 and 5 mM MgCl2in a volume of 0.5 ml. The reaction was started by the addition of 1.11 kBq GDP-L-[1-14C]galactose. The reaction was stopped by the addition of 500 μl 10% (w/v) activated charcoal followed by brief vortexing. The charcoal was removed by centrifugation (16000 g; 10 min; 1°C) and radioactivity in the supernatant estimated by scintillation counting (Packard Tri-Carb 2000CA).
L-GalDH activity was measured in a reaction mixture consisting of enzyme extract, 50 mM HEPES pH 8.0, 5 mM MgCl2 and 0.5 mM NAD. The reaction was started by the addition of 2 mM L-galactose. Reaction conditions and kinetic measurements were as described for hexokinase. L-Galactose dehydrogenase activity was also analysed in crude fruit exudate by addition of L-[1-14C]galactose. Samples were incubated at 30°C and the reaction stopped by addition of exudate to an equal volume of 5% (w/v) H3PO3.
Precipitated material was removed by centrifugation and formation of L-[1-14C]galactonic acid monitored by HPLC on a Metacarb 87C 300 × 7.8 mm column (MetaChem Technologies Inc., Torrance, CA) maintained at 70°C with a mobile phase of ultrapure water flowing at 1 ml min-1. HPLC equipment used was as described in Hancock et al. [22] with the exception that radioactivity was detected and quantified using a Packard 150TR radiodetector in the homogenous mode (500 μl flow cell) with postcolumn addition of Ultima Flo M scintillant at 3 ml min-1.
L-GalLDH activity was assayed as described by Oba et al. [44] with the exception that the buffer additionally contained 0.03% triton X-100 and 0.1 mM KCN to prevent reoxidation of cytochrome C by cytochrome C oxidase [37]. In another deviation from the published protocol, the reaction was started by addition of L-galactono-1,4-lactone to 5 mM. The value for molar extinction coefficient of cytochrome c at 550 nm used was 25300.
Source Leaf Loading and Autoradiography
Loading of source leaves with 14C-assimilates was undertaken as described by Turgeon and Gowan [45]. Leaves were removed from turgid plants and moistened with buffer B (25 mM MES pH 5.5, 20 mM CaCl2). The abaxial surface was gently abraded with carbarundum powder and 16 mm discs cut using a sharp cork borer. Leaf discs were floated on buffer B under light (200 μmol m-2 sec-1) for 15 h at 25°C with gentle rotary shaking. Finally, discs were transferred to 10 ml fresh buffer B containing 1 mM [U-14C]sucrose, D-[U-14C]mannose, L-[1-14C]galactose, L-[1-14C]AsA or [1-14C]DHA at a final S.A. of 18.5 kBq mmol-1. Incubation was continued under light for a further 3 h and subsequently, leaf discs were removed and washed five times (20 ml, 5 min) in buffer B. Small samples of incubation buffer were taken for HPLC analysis to ensure no metabolism had occurred at the beginning and end of the incubation period. Discs were surface dried and lyophilised between filter paper held flat between metal plates secured with G-clamps. Dried leaves were exposed to Kodak Biomax single sided X-ray film for 1 week at -80°C prior to development.
Authors' contributions
RDH undertook the biochemical investigations assisted by DMR, was responsible for data preparation and assisted in writing the manuscript. SH undertook the histochemical staining, performed the uptake studies in N. benthamiana leaf discs and prepared the figures. RV conceived the study and drafted the manuscript.
Acknowledgments
Acknowledgements
The authors wish to thank Dr. Ken Lawrie, GlaxoSmithKline Pharmaceuticals for the gift of GDP-L-[1-14C]galactose. The Scottish Executive Environment and Rural Affairs Department funded this work.
Contributor Information
Robert D Hancock, Email: rhanco@scri.sari.ac.uk.
Diane McRae, Email: dmcrae@scri.sari.ac.uk.
Sophie Haupt, Email: shaupt@scri.sari.ac.uk.
Roberto Viola, Email: rviola@scri.sari.ac.uk.
References
- Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. doi: 10.1006/anbo.1996.0175. [DOI] [Google Scholar]
- Jones E, Hughes RE. Foliar ascorbic acid in some angiosperms. Phytochem. 1983;22:2493–2499. doi: 10.1016/0031-9422(83)80147-2. [DOI] [Google Scholar]
- Jones E, Hughes RE. A note on the ascorbic acid content of some trees and woody shrubs. Phytochem. 1984;23:2366–2367. doi: 10.1016/S0031-9422(00)80554-3. [DOI] [Google Scholar]
- Justi KC, Visentainer JV, de Souza NE, Matsushita M. Nutritional composition and vitamin C stability in stored camu-camu (Myrciaria dubia) pulp. Archivos Latinoamericanos De Nutricion. 2000;50:405–408. [PubMed] [Google Scholar]
- Rodriguez MAR, Oderiz MLV, Hernandez JL, Lozano JS. Determination of vitamin-C and organic-acids in various fruits by HPLC. J Chromatogr Sci. 1992;30:433–437. doi: 10.1093/chromsci/30.11.433. [DOI] [PubMed] [Google Scholar]
- Sone K, Mochizuki T, Noguchi Y. Variations in ascorbic acid content among strawberry cultivars and their harvest times. J Japan Soc Hort Sci. 1999;68:1007–1014. [Google Scholar]
- Viola R, Brennan RM, Davies HV, Sommerville L. L-Ascorbic acid accumulation in berries of Ribes nigrum L. J Hort Sci Biotechnol. 2000;75:409–412. [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: Biosynthesis and function. Critical Rev Biochem Mol Biol. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Springer F, Renz A, Kossmann J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999;19:131–141. doi: 10.1046/j.1365-313X.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- Tabata K, Oba K, Suzuki K, Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J. 2001;27:139–148. doi: 10.1046/j.1365-313x.2001.01074.x. [DOI] [PubMed] [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J. 2002;30:541–533. doi: 10.1046/j.1365-313X.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- Jain AK, Nessler CL. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol Breeding. 2000;6:73–78. doi: 10.1023/A:1009680818138. [DOI] [Google Scholar]
- Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- Chen Z, Young TE, Ling J, Chang S-C, Gallie DR. Increasing vitamin C content of plants through enhanced recycling. Proc Natl Acad Sci USA. 2003;100:3525–3530. doi: 10.1073/pnas.0635176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola R, Vreugdenhil D, Davies HV, Sommerville L. Accumulation of L-ascorbic acid in tuberising stolon tips of potato (Solanum tuberosum L.) J Plant Physiol. 1998;152:58–63. [Google Scholar]
- Yoneyama T, Yasuda M, Sato S, Takebe M. 13CO2 Feeding studies on the metabolism of carbohydrates, ascorbate and oxalate in spinach (Spinacia oleracea L.) Soil Sci Plant Nutr. 1997;43:1147–1151. [Google Scholar]
- Davey MW, Gilot C, Persiau G, Ostergaard J, Han Y, Bauw GC, van Montagu MC. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol. 1999;121:535–543. doi: 10.1104/pp.121.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RD, Galpin JR, Viola R. Biosynthesis of l-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiol Lett. 2000;186:245–250. doi: 10.1016/S0378-1097(00)00155-5. [DOI] [PubMed] [Google Scholar]
- De Pinto MC, Tommasi F, De Gara L. Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco bright yellow 2. Plant Physiol Biochem. 2000;38:541–550. doi: 10.1016/S0981-9428(00)00773-7. [DOI] [Google Scholar]
- Franceschi VR, Tarlyn NL. l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. Nature of transported substances. In: Zimmermann MH, Milburn JA, editor. In Encyclopedia of Plant Physiology New Series. Vol. 1. Berlin: Springer-Verlag; 1975. pp. 59–100. [Google Scholar]
- Chinoy NJ. On the specificity of alcoholic, acidic silver nitrate reagent for the histochemical localization of ascorbic acid. Histochemie. 1969;20:105–107. doi: 10.1007/BF00268703. [DOI] [PubMed] [Google Scholar]
- Daie J. Sucrose uptake in isolated phloem of celery is a single saturable transport system. Planta. 1987;171:474–482. doi: 10.1007/BF00392294. [DOI] [PubMed] [Google Scholar]
- Eschrich W, Fromm J. Evidence for two pathways of phloem loading. Physiol Plant. 1994;90:699–707. doi: 10.1034/j.1399-3054.1994.900411.x. [DOI] [Google Scholar]
- Richardson PT, Baker DA, Ho LC. Assimilate transport in cucurbits. J Exp Bot. 1984;35:1575–1581. [Google Scholar]
- Alosi MC, Melroy DL, Park RB. The regulation of gelation of phloem exudate from Cucurbita fruit by dilution, glutathione, and glutathione-reductase. Plant Physiol. 1988;86:1089–1094. doi: 10.1104/pp.86.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz C, Juenger M, Schad M, Kehr J. Evidence for the presence and activity of a complete antioxidant defence system in mature sieve tubes. Plant J. 2002;31:189–197. doi: 10.1046/j.1365-313X.2002.01348.x. [DOI] [PubMed] [Google Scholar]
- Fiehn O. Metabolic networks of Cucurbita maxima phloem. Phytochem. 2003;62:875–886. doi: 10.1016/S0031-9422(02)00715-X. [DOI] [PubMed] [Google Scholar]
- Chinoy NJ, Sanjeevan AG. On the specificity of alcoholic silver nitrate reagent for the histochemical localizatin of ascorbic acid. A reappraisal. Histochemistry. 1978;56:275–282. doi: 10.1007/BF00495989. [DOI] [PubMed] [Google Scholar]
- Rautenkranz AAF, Li L, Mächler F, Märtinoia E, Oertli JJ. Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgare L. cv Gerbel) leaves. Plant Physiol. 1994;106:187–193. doi: 10.1104/pp.106.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Berlyn GP. Chemical investigations on multiple bud formation in tissue cultures of Pinus coulteri. Can J Bot. 1983;61:575–585. [Google Scholar]
- Haebel S, Kehr J. Matrix-assisted laser desorption/ionization time of flight mass spectrometry peptide mass fingerprints and post source decay: a tool for the identification and analysis of phloem proteins from Cucurbita maxima Duch. separated by two-dimensional polyacrylamide gel electrophoresis. Planta. 2001;213:586–593. doi: 10.1007/s004250100523. [DOI] [PubMed] [Google Scholar]
- Siendones E, Gonzalez-Reyes JA, Santos-Ocana C, Navas P, Cordoba F. Biosynthesis of ascorbic acid in kidney bean. L-Galactono-γ-lactone dehydrogenase is an intrinsic protein located at the innner mitochondrial membrane. Plant Physiol. 1999;120:907–912. doi: 10.1104/pp.120.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel AJE, Ehlers K, Knoblauch M. Sieve elements caught in the act. Trends Plant Sci. 2002;7:126–132. doi: 10.1016/S1360-1385(01)02225-7. [DOI] [PubMed] [Google Scholar]
- Viola R, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell. 2001;13:385–398. doi: 10.1105/tpc.13.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Baisch G, Ohrlein R. Convenient chemoenzymatic synthesis of β-purine-diphosphate sugars (GDP-fucose-analogues) Bioorg Med Chem. 1997;5:383–391. doi: 10.1016/S0968-0896(96)00258-1. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Davey MW, Boerjan W. A high-performance liquid chromatography radio method for determination of L-ascorbic acid and guanosine 5'-diphosphate-L-galactose, key metabolites of the plant vitamin C pathway. Anal Biochem. 2001;294:161–168. doi: 10.1006/abio.2001.5165. [DOI] [PubMed] [Google Scholar]
- Oba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T. Purification and properties of L-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic acid biosynthesis, from sweet potato roots. J Biochem. 1995;117:120–124. doi: 10.1093/oxfordjournals.jbchem.a124697. [DOI] [PubMed] [Google Scholar]
- Turgeon R, Gowan E. Phloem loading in Coleus blumei in the absence of carrier-mediated uptake of export sugar from the apoplast. Plant Physiol. 1990;94:1244–1249. doi: 10.1104/pp.94.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]