Abstract
Background: Fat mass is thought to be protective against osteoporosis, primarily because of its weight-bearing effect. Few studies have evaluated the association between abdominal fat mass (AFM) and bone health beyond its weight-bearing effect.
Objective: We tested the hypothesis that higher body weight–adjusted AFM is associated with poor bone health.
Design: A cross-sectional study was conducted in 629 Puerto Rican adults aged 47–79 y. Bone mineral density (BMD) of the femoral neck, trochanter, total femur, and lumbar spine (L2-L4) were measured by using dual-energy X-ray absorptiometry (DXA). AFM and total fat mass (TFM) were assessed by using body-composition software from whole-body DXA scans. Osteoporosis and osteopenia were defined as T-scores ≤ −2.5 and −1.0 to −2.5 SD, respectively, at the respective bone site.
Results: After confounders were controlled for, body weight–adjusted AFM was inversely associated with BMD at all 4 bone sites in women and at the femoral neck in men. For TFM, small inverse associations were seen at the trochanter and total femur in women. In men, similar associations were seen at the 3 femur sites. In both sexes, the odds for osteoporosis or osteopenia at each of the femoral sites increased by 10−16% for every 100-g increase in body weight–adjusted AFM.
Conclusions: Higher AFM was associated with poor bone health in this Puerto Rican sample. Efforts to reduce abdominal obesity will not only reduce the risk of chronic disease but may also improve bone health. This trial is registered at clinicaltrials.gov as NCT01231958.
INTRODUCTION
Obesity and osteoporosis are 2 major public health concerns with high prevalence rates, the latter of which disproportionally affects older adults. Osteoporosis and low bone mass affect nearly 44 million US adults aged ≥50 y (1). By 2025, annual fractures and costs are expected to rise by 50% from $17 billion in 2005. The greatest increase in costs is estimated to be 175% for Hispanics (2), which suggests that this is a high-risk group. Likewise, the prevalence of obesity, especially abdominal obesity, remains disturbingly high among adults in the United States. Recent estimates from NHANES indicate that the prevalence of abdominal obesity among men and women has increased from 37.8% and 55.8% during 1999–2000 to 43.7% and 61.8% during 2007–2008 (3).
The prevailing view regarding the relation between fat and bone mass is that body fat protects against osteoporosis primarily because of its weight-bearing effect on the skeleton. With the recognition of fat as an endocrine organ, the effect of fat mass on bone may extend beyond its mechanical load on the skeleton. Comparing NHANES 1999–2002 data with NHANES III data, Looker et al (4) found a positive relation between BMI and BMD4 but concluded that the increasing rates of overweight among older women are not likely to lead to a significant reduction in the prevalence of osteoporosis. More recently, comparing NHANES III data with NHANES 2005–2006 data, Looker et al (5) found that the prevalence of osteoporosis at the femoral neck decreased but changes in BMI did not fully explain this decline. Whereas most of the research on the association between fat mass and BMD has focused on TFM, it is not clear how AFM is associated with bone mass. Abdominal obesity, assessed by using waist circumference, is associated with higher mortality independent of BMI (6). Furthermore, AFM is known to contribute to inflammation (7, 8), insulin resistance (9), dyslipidemia (10), metabolic syndrome (11), and hypertension (12). Given the established risks associated with AFM, it is not clear how AFM is associated with bone mass after its mechanical loading effect is controlled for, especially in an ethnic population.
Most research in Hispanics has focused on Mexican Americans, because of their majority as a Hispanic subgroup. However, Puerto Ricans are the largest Hispanic subgroup in the northeastern United States, and prior research indicates that they have established health disparities and a greater burden of chronic disease (13). The metabolic syndrome, characterized by abdominal obesity, is also high in this group (14). Yet, there is a paucity of research on bone health in this population. Because the health care costs of both osteoporosis and abdominal obesity, and the associated increase in chronic disease risk, are considerable, it is crucial to understand how AFM affects BMD independent of body weight. We therefore studied this important association in a group of 629 older Puerto Rican adults (n = 164 men, n =465 women), aged 47–79 y, living in the greater Boston area.
SUBJECTS AND METHODS
Participants
We used data from the Boston Puerto Rican Osteoporosis Study, an ancillary study to the Boston Puerto Rican Health Study, a prospective cohort study in older Puerto Ricans aged 45–75 y living in the greater Boston area. The design of the Boston Puerto Rican Health Study was described in detail elsewhere (14). Briefly, at baseline and at 2 y, bilingual interviewers visited the participants’ homes and administered questionnaires to collect information on socioeconomic status, health and health behaviors, acculturation, depressive symptoms, stress, social support, usual diet, and cognitive functioning. In addition, anthropometric, blood pressure, and physical performance measures were collected. Biological samples, including saliva, urine, and 12-h fasting blood, were collected by the phlebotomist in the participants’ homes on a day after the interview or as soon as possible thereafter. At the completion of the 2-y follow-up, participants reconsented to the osteoporosis study. An appointment was made for consenting participants to visit the Metabolic Research Unit at the Human Nutrition Research Center on Aging at Tufts University to undergo bone density and body-composition measurements, to have additional blood samples collected, and to complete additional questionnaires on osteoporosis medication use and sunlight exposure. Multiple attempts were made to complete this visit within 1 mo of the 2-y follow-up visit for the parent study. All questionnaires were administered by trained bilingual interviewers. By September 2010, 756 of a total of 1123 participants who completed 2-y follow-up visits consented to the osteoporosis study. Primary reasons for nonparticipation included not being interested in the osteoporosis study (n = 163), scheduling problems (n = 139), loss to follow-up (n = 33), and relocation out of Massachusetts (n = 15). Furthermore, 17 participants died since their 2-y follow-up interview. Women who declined participation were more likely to be older (61.3 compared with 59.3 y; P = 0.001) and have higher energy-adjusted intakes of alcohol (4.5 compared with 1.5 g/d; P = 0.05). Men who declined participation in the osteoporosis study were more likely to be older (61.6 compared with 58.4 y; P = 0.003), to have a lower BMI (28.6 compared with 30.2; P = 0.03), and to have a lower waist circumference (100 compared with 105 cm; P = 0.02). No other significant differences in sociodemographic or dietary variables were found. For analyses with femoral BMD measures as the outcome, we excluded one participant with a poor-quality hip scan. At the time of analysis, complete and cleaned data were available for 629 participants (164 men and 465 women). All study protocols were approved by the Institutional Review Board of Tufts Medical Center.
Methods
Outcome assessment
On the basis of recommendations from the International Society for Clinical Densitometry (15), we made an a priori decision to include only BMD measurements at the femoral neck, total hip, and posterior-anterior lumbar spine (L2-L4) in all our analyses. In addition, we also included the trochanter, because inclusion of this anatomic site provides a complete picture of the hip. We measured BMD (g/cm2) of the femoral neck, trochanter, total hip, and lumbar spine by DXA (Lunar model Prodigy scanner; General Electric) using standard procedures. The root mean square precisions of these measurements were 0.65% for total-hip BMD, 1.03% for the trochanter, 1.31% for the femoral neck, and 1.04% for the lumbar spine (16). For femur measurements, the right hip was scanned unless there was a history of hip fracture or joint replacement. During the study, the stability of DXA measurements was determined by scanning an external standard (aluminum spine phantom; Lunar Radiation Corp) every week. On the basis of the WHO definitions, osteoporosis and osteopenia were defined as T-score thresholds of ≥2.5 or 1.0 SD, respectively, below the healthy young adult mean at the respective bone site. We reviewed all scans with T-scores >4.0 to check for extraskeletal calcification or for the presence of nonanatomic parts in the DXA scan region.
Exposure assessment
TFM (kg) was assessed from whole-body scans. AFM (kg) was measured by using specialized regional body-composition software (ENCORE version 12.2) from whole-body DXA scans. The androidal or abdominal region of interest height was defined by the manufacturer as 20% from pelvis cut to neck cut. AFM was the weight of fat tissue in this region.
Assessment of covariates
At the 2-y follow-up visit, information on age, sex, education, and smoking status was collected by questionnaire. Physical activity was assessed by using a modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey (17, 18). Usual intakes of calcium (mg/d), alcohol (g/d), and total energy (kcal/d) were assessed by using a semiquantitative food-frequency questionnaire that was specifically developed and validated for the Puerto Rican population (19). At the osteoporosis study visit, we administered a short questionnaire to assess osteoporosis prescription medication use (yes or no), including use of bisphosphonates, calcitonin, calcium, vitamin D, and cod liver oil. Because BMD is known to vary by season in the New England area (20, 21), we created a 4-level categorical variable for season of BMD measurement as follows: July, August, and September were coded as summer; October, November, and December as fall; January, February, and March as winter; and April, May, and June as spring. Standing height was measured with a stadiometer (Seca). Weight was measured with a digital scale (model Alpha Seca). Fasting blood samples (12 h) were drawn from participants by a certified phlebotomist during the morning of the osteoporosis study visit. Blood was collected into evacuated tubes containing EDTA, and plasma was separated by immediately centrifuging at 3421 × g at 4°C for 15 min. Plasma 25-hydroxyvitamin D (ng/mL) was measured by using a 125I radioimmunoassay kit procedure (DiaSorin Inc) as specified by the manufacturer's procedural documentation (68100E). The intra- and interassay CVs were 10.8% and 9.4%, respectively.
Statistical analyses
All statistical analyses were performed by using SAS version 9.2 (SAS Institute Inc). Formal hypothesis testing was 2-sided, and the nominal type I error rate was 0.05. Because distribution of central (abdominal) fat mass is sex-specific, we stratified all analyses by sex. Because body weight and AFM may be highly collinear, inclusion of both variables in a regression model may introduce multicollinearity and make the model unstable. Therefore, we first regressed AFM on body weight and saved the residuals. These residuals represent the variation in AFM that is independent of body weight. We then added the mean body weight to each of these residuals to arrive at body weight–adjusted AFM (22). Body weight–adjusted AFM was used as the primary exposure in all our analyses. Participants were divided into quartiles of body weight adjusted AFM, separately for men and women. We calculated age-adjusted means for lifestyle, socioeconomic, anthropometric, and health characteristics across increasing quartiles of body weight–adjusted AFM by using PROC GLM. Similarly, dietary intakes were examined across quartiles by using ANOVA with adjustment for age and energy intake. We assessed significance across quartiles of body weight–adjusted AFM using linear (for continuous variables) or logistic (for categorical variables) regression. Tests for linear trend were conducted by assigning each participant the median grams of body weight–adjusted AFM for each quartile category and treating this value as a continuous measure in a regression model.
We used the general linear models procedure to model associations between body weight–adjusted AFM (continuous and categorical) and BMD (continuous) of the femoral neck, trochanter, total hip, and lumbar spine. We adjusted for age (y), current smoking status (y), education (<9th grade, 9th–12th grade/GED, some college/college or graduate school), alcohol intake (g/d), calcium intake (mg/d), total energy intake (kcal/d), season of BMD measurement (spring, summer, fall, or winter), physical activity score, plasma 25-hydroxyvitamin D (ng/mL) concentration, and osteoporosis medication use (y). To adjust for confounding due to skeletal size and the mechanical loading of body weight, we additionally adjusted for height and body weight. For all linear models, we checked the assumptions of normality, linearity, and homogeneity by examining plots of residuals compared with predicted values and normal probability plots of residuals. Final models were checked for outliers and influential points by using scatter plots. All analyses were adjusted for multiple comparisons by using Dunnett's adjustment with the lowest quartile as the reference group. To compare the magnitude of the effect sizes of AFM on BMD with those of TFM on BMD, we repeated all our analyses by replacing body weight–adjusted AFM with body weight–adjusted TFM as the main exposure variable. We used logistic regression to model the odds of either osteoporosis or osteopenia for each 100-g increase in body weight–adjusted AFM. Goodness of fit was assessed by using the Hosmer-Lemeshow test.
RESULTS
Fat mass around the abdominal area in Puerto Rican women in the highest quartile was nearly 1.4 times that in the lowest quartile of body weight–adjusted AFM (Table 1). Median AFM values in quartiles 1, 2, 3, and 4 were 2.84, 3.25, 3.55, and 3.92 kg, respectively. Women in the highest compared with those in the lowest quartile of body weight–adjusted AFM were more likely to have lower height and higher waist circumference, less likely to be physically active, had lower educational status, and had a lower total household income than did women with the least body weight–adjusted AFM. Body weight–adjusted AFM in Puerto Rican men in the highest quartile of AFM was nearly 1.5 times that in men in the lowest quartile (Table 2). Median values of body weight–adjusted AFM in increasing higher quartiles were 2.38, 2.86, 3.27, and 3.64 kg, respectively. Similar to their female counterparts, these men were more likely to have lower height and higher waist circumference than men in the lowest quartile of body weight–adjusted AFM. These men were also older and were less likely to be physically active than were men with the lowest body weight–adjusted AFM.
TABLE 1.
Characteristics of Puerto Rican women across quartiles of body weight–adjusted abdominal fat mass1
| Quartile of abdominal fat mass |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Abdominal fat mass (kg) | 2.84 (1.84–3.06)2 | 3.25 (3.07–3.38) | 3.55 (3.39–3.67) | 3.92 (3.68–5.09) | |
| No. of subjects | 116 | 116 | 117 | 116 | |
| Age (y) | 60.2 ± 0.73 | 60.6 ± 0.7 | 60.7 ± 0.7 | 61.1 ± 0.7 | 0.39 |
| Current smoker (%)4 | 19.0 | 15.5 | 17.4 | 14.2 | 0.40 |
| Alcohol intake (g/d)5 | 1.47 ± 0.63 | 2.12 ± 0.64 | 1.84 ± 0.66 | 1.58 ± 0.64 | 0.96 |
| Calcium intake (g/d)5 | 972 ± 47 | 1024 ± 48 | 967 ± 49 | 951 ± 47 | 0.62 |
| Plasma 25(OH)D (ng/mL)4 | 18.7 ± 0.7 | 20.3 ± 0.7 | 19.1 ± 0.7 | 18.8 ± 0.7 | 0.82 |
| BMI (kg/m2)4 | 33.7 ± 0.6 | 31.6 ± 0.66 | 31.9 ± 0.6 | 34.9 ± 0.6 | 0.22 |
| Weight (kg)4 | 83.3 ± 1.6 | 76.2 ± 1.6 | 74.7 ± 1.6 | 81.9 ± 1.6 | 0.37 |
| Height (m)4 | 1.57 ± 0.01 | 1.55 ± 0.017 | 1.53 ± 0.017 | 1.56 ± 0.018 | <0.0001 |
| Waist circumference (cm)4 | 101 ± 1 | 101 ± 1 | 101 ± 1 | 110 ± 17 | <0.0001 |
| Physical activity score4 | 31.4 ± 0.4 | 30.9 ± 0.4 | 31.5 ± 0.4 | 30.2 ± 0.4 | 0.08 |
| Education (%)4 | |||||
| <9th grade | 38.6 | 59.68 | 53.06 | 55.66 | 0.02 |
| 9th–12th grade/GED | 35.3 | 29.2 | 34.8 | 30.3 | 0.58 |
| At least some college | 26.2 | 11.28 | 12.28 | 14.16 | 0.02 |
| Osteoporosis medication use (%)4 | 50.5 | 52.6 | 48.7 | 51.7 | 0.99 |
| Total household income (US$/y)4 | 19,561 ± 1869 | 18,543 ± 1826 | 16,051 ± 1826 | 13,678 ± 1851 | 0.02 |
GED, General Education Development; 25(OH)D, 25-hydroxyvitamin D.
Median; range in parentheses (all such values).
Mean ± SEM (all such values).
Adjusted for age by ANOVA (PROC GLM; SAS Institute).
Adjusted for age and energy intake by ANOVA (PROC GLM).
Significantly different from quartile 1: 6P < 0.05, 7P < 0.0001, 8P < 0.01.
TABLE 2.
Characteristics of Puerto Rican men across quartiles of body weight–adjusted abdominal fat mass1
| Quartile of abdominal fat mass |
|||||
| 1 | 2 | 3 | 4 | P-trend | |
| Abdominal fat mass (kg) | 2.38 (1.63–2.68)2 | 2.86 (2.69–3.05) | 3.27 (3.06–3.45) | 3.64 (3.46–4.96) | |
| No. of subjects | 41 | 41 | 41 | 41 | |
| Age (y) | 58.2 ± 1.23 | 59.6 ± 1.2 | 59.7 ± 1.2 | 62.0 ± 1.2 | 0.03 |
| Current smoker (%)4 | 40.2 | 24.1 | 29.9 | 30.9 | 0.45 |
| Alcohol intake (g/d)5 | 11.9 ± 5.6 | 9.4 ± 5.4 | 9.7 ± 5.5 | 4.0 ± 5.3 | 0.34 |
| Calcium intake (g/d)5 | 898 ± 70 | 995 ± 68 | 838 ± 69 | 1052 ± 66 | 0.34 |
| Plasma 25(OH)D (ng/mL)4 | 17.3 ± 1.0 | 18.5 ± 1.0 | 15.7 ± 1.0 | 17.8 ± 1.0 | 0.74 |
| BMI (kg/m2)4 | 29.6 ± 0.8 | 28.7 ± 0.8 | 30.7 ± 0.8 | 31.0 ± 0.8 | 0.12 |
| Weight (kg)4 | 86.9 ± 2.7 | 80.6 ± 2.6 | 84.4 ± 2.6 | 84.9 ± 2.6 | 0.77 |
| Height (m)4 | 1.72 ± 0.01 | 1.67 ± 0.016 | 1.66 ± 0.017 | 1.65 ± 0.017 | <0.0001 |
| Waist circumference (cm)4 | 103 ± 2 | 101 ± 2 | 105 ± 2 | 108 ± 2 | 0.04 |
| Physical activity score4 | 33.4 ± 0.8 | 32.4 ± 0.8 | 31.0 ± 0.8 | 30.6 ± 0.88 | 0.009 |
| Education (%)4 | |||||
| <9th grade | 50.3 | 51.5 | 42.6 | 44.4 | 0.46 |
| 9th–12th grade/GED | 36.0 | 38.9 | 45.0 | 34.9 | 0.89 |
| At least some college | 13.9 | 9.6 | 12.4 | 18.0 | 0.59 |
| Osteoporosis medication use (%)4 | 19.8 | 26.9 | 22.6 | 16.7 | 0.70 |
| Total household income (US$/y)4 | 21,768 ± 3455 | 17,357 ± 3439 | 16,768 ± 3485 | 19,274 ± 3458 | 0.56 |
GED, General Education Development; 25(OH)D, 25-hydroxyvitamin D.
Median; range in parentheses (all such values).
Mean ± SEM (all such values).
Adjusted for age by ANOVA (PROC GLM; SAS Institute).
Adjusted for age and energy intake by ANOVA (PROC GLM).
Significantly different from quartile 1: 6P < 0.01, 7P < 0.0001, 8P < 0.05.
After differences in confounders and in the mechanical loading effect of body weight and height were controlled for, body weight–adjusted AFM was negatively associated with BMD in both men and women (Table 3). These associations were significant at all 4 bone sites in women. In men, significant negative associations were observed only at the femoral neck. In women, body weight–adjusted TFM (kg) was significantly and negatively associated with BMD at the trochanter and total femur. In men, body weight–adjusted TFM was negatively associated with BMD at all 3 hip sites, but not at the lumbar spine. Effect sizes for body weight–adjusted TFM were much lower than those for body weight–adjusted AFM.
TABLE 3.
Association between body weight–adjusted AFM (kg) and TFM (kg) and BMD1
| Femoral neck BMD | Trochanter BMD | Total femur BMD | Lumbar spine BMD | |||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Women (n = 462)2 | ||||||||
| AFM3 | −0.056 | (−0.080, −0.032) | −0.042 | (−0.066, −0.018) | −0.047 | (−0.074, −0.020) | −0.040 | (−0.073, −0.008) |
| TFM3 | −0.002 | (−0.006, 0.001) | −0.006 | (−0.010, −0.002) | −0.007 | (−0.011, −0.003) | −0.004 | (−0.009, 0.001) |
| Men (n = 164) | ||||||||
| AFM3 | −0.056 | (−0.106, −0.006) | −0.048 | (−0.096, 0.000) | −0.049 | (−0.100, 0.003) | −0.011 | (−0.075, 0.054) |
| TFM3 | −0.008 | (−0.015, −0.001) | −0.010 | (−0.017, −0.004) | −0.010 | (−0.017, −0.003) | −0.006 | (−0.015, 0.003) |
Note that sample sizes for each analysis fluctuate around the reported value (approximate n) because of missing data for some covariates. β-Coefficients (and 95% CIs) were calculated by using ANCOVA (PROC GLM; SAS Institute). AFM, abdominal fat mass; BMD, bone mineral density; TFM, total fat mass.
n = 463 for lumbar spine.
Adjusted for age (y), current smoking status (yes or no), education (<9th grade, 9th–12th grade/General Education Development, some college/college or graduate school), alcohol intake (g/d), energy intake (kcal/d), season of BMD measurement (spring, summer, fall, or winter), osteoporosis prescription medication use (yes or no), physical activity score (%), calcium intake (mg/d), plasma 25-hydroxyvitamin D status (ng/mL), weight (kg), and height (m).
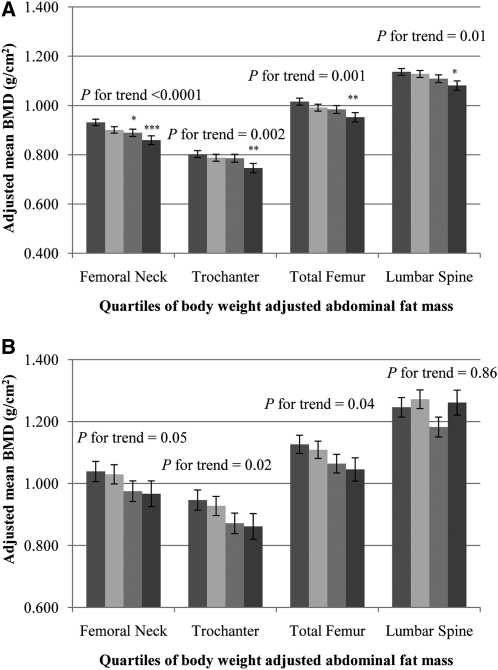
Among Puerto Rican women, BMD in the highest quartile of body weight–adjusted AFM for all 4 bone sites was significantly lower than BMD in the lowest quartile (P-trend < 0.01) (Figure 1A). In Puerto Rican men, BMD at the 3 hip sites was lower across increasing quartiles of body weight–adjusted AFM (P-trend < 0.05). No association was seen at the lumbar spine (Figure 1B).
FIGURE 1.
Adjusted mean (±SEM) BMD by quartiles 1–4 (from left to right) of body weight–adjusted abdominal fat mass in women (A) and in men (B). Data were adjusted for age (y), current smoking status (yes or no), education (<9th grade, 9th–12th grade, General Education Development, some college, college, or graduate school), alcohol intake (g/d), energy intake (kcal/d), season of bone mineral density measurement (spring, summer, fall, or winter), osteoporosis prescription medication use (yes or no), physical activity score (%), calcium intake (mg/d), plasma 25-hydroxyvitamin D status (ng/mL), body weight (kg), and height (m) by using ANCOVA (PROC GLM; SAS Institute). Adjustment for multiple comparisons was performed by using Dunnett's adjustment. A: quartile 1, n = 116, median (range) = 2.84 (1.84–3.06); quartile 2, n = 116, median (range) = 3.25 (3.07–3.38); quartile 3, n = 117, median (range) = 3.55 (3.39–3.67); quartile 4, n = 116, median (range) = 3.92 (3.68–5.09). *,**,***Significantly different from quartile 1: *P < 0.05, **P < 0.01, ***P < 0.001. : quartile 1, n = 41, median (range) = 2.38 (1.63–2.68); quartile 2, n = 41, median (range) = 2.86 (2.69–3.05); quartile 3, n = 41, median (range) = 3.27 (3.06–3.45); quartile 4, n = 41, median (range) = 3.64 (3.46–4.96). BMD, bone mineral density.
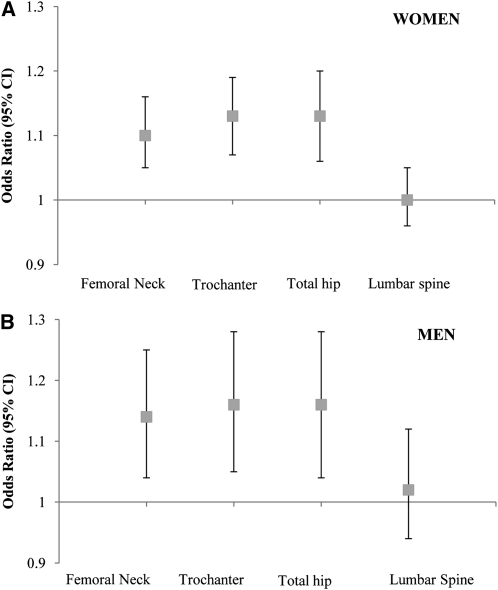
In women, the multiple adjusted ORs of osteoporosis or osteopenia at the femoral neck, trochanter, total femur, and lumbar spine for every 100-g greater body weight–adjusted AFM were 1.10 (95% CI: 1.05, 1.16), 1.13 (95% CI: 1.07, 1.19), 1.13 (95% CI: 1.06, 1.20), and 1.00 (95% CI: 0.96, 1.05), respectively (Figure 2A). In men, higher body weight–adjusted AFM was associated with a higher likelihood of osteoporosis or osteopenia at all 3 hip sites, but not at the lumbar spine. The ORs for osteoporosis or osteopenia at the femoral neck, trochanter, total femur, and lumbar spine for every 100-g greater AFM were 1.14 (95% CI: 1.04, 1.25), 1.16 (95% CI: 1.05, 1.28), 1.16 (95% CI: 1.04, 1.28), and 1.02 (95% CI: 0.94, 1.12), respectively (Figure 2B). The P value for the Hosmer-Lemeshow test statistic was >0.50, which indicated that the logistic regression model was a good fit.
FIGURE 2.
ORs (and 95% CIs) of osteoporosis or osteopenia for every 100-g increase in body weight–adjusted abdominal fat mass in women (n = 465; A) and in men (n = 164; B). Adjusted for age (y), current smoking status (yes or no), education (<9th grade, 9th–12th grade, General Education Development, some college, college, or graduate school), alcohol intake (g/d), energy intake (kcal/d), season of bone mineral density measurement (spring, summer, fall, or winter), osteoporosis prescription medication use (yes or no), physical activity score (%), calcium intake (mg/d), plasma 25-hydroxyvitamin D status (ng/mL), body weight (kg), and height (m) by using logistic regression (PROC LOGISTIC; SAS Institute).
DISCUSSION
In this cross-sectional study in Puerto Rican older men and women, both body weight–adjusted AFM and TFM were inversely associated with BMD. Yet, effect sizes were much smaller for TFM than for AFM. In women, higher body weight–adjusted AFM was associated with lower BMD at all 4 bone sites. In men, this association was restricted to the femoral neck. In both sexes, the strongest associations were seen at the femoral neck. In both men and women, the likelihood of osteoporosis or osteopenia at all 3 hip sites increased with every 100-g increase in body weight–adjusted AFM. Thus, AFM appears to have a strong inverse association with bone mass beyond the mechanical loading effect of body weight and differences in height. To our knowledge, the current study is the first to show the inverse association between AFM, measured by using DXA, and bone mass specifically in a Hispanic population.
The inverse associations between body weight–adjusted AFM and BMD are particularly noteworthy. Fat mass is a major component of body weight. Obesity, a condition characterized by excessive fat mass, has been traditionally thought to be protective for bone mass. In fact, low body weight, especially in older adults, is an established risk factor for osteoporosis. Moreover, in the WHO fracture risk assessment tool (23), a higher body weight is associated with a lower 10-y risk of fracture. The primary mechanism for the positive relation between fat and bone is due to the load on the skeleton by body weight. However, a few studies (24, 25) have shown that, when the mechanical loading effect of body weight is statistically removed, fat mass is negatively associated with bone. Most recently, Reid (26) contested that fat mass should not be adjusted for body weight because of the potential collinearity between the 2 variables. However, our hypothesis was that central fat mass is negatively associated with BMD after adjustment for the mechanical loading effect of body weight. To avoid collinearity between AFM and body weight, we included AFM residuals, as opposed to AFM, as our main exposure variable.
The differences in the effect sizes of body weight–adjusted AFM and TFM with BMD are particularly striking. AFM is known to be more metabolically and biologically active and produces a variety of autocrine and paracrine hormones, chemokines, and cytokines that affect bone metabolism. The flux of free fatty acids to the liver via the portal vein is greater in individuals with excess visceral fat. An increase in the delivery of free fatty acids to the liver signals a greater production of glucose output by the liver, which eventually leads to an insulin-resistant state (27). Insulin resistance, an essential feature of type 2 diabetes, has been shown to increase the risk of fracture (28, 29). A second potential mechanism for the negative association between body weight adjusted AFM and BMD may have to do with the production of proinflammatory molecules such as IL-6 and TNF-α. Recent research has established that the release of many inflammatory adipokines by adipose tissue is enhanced in obese individuals, although these cytokines are primarily released by the nonfat cells of human adipose tissue (30). Visceral adipose tissue is known to release greater amounts of cytokines than is abdominal subcutaneous tissue (31). Concentrations of high-sensitivity C-reactive protein, a marker of systemic inflammation, are also elevated in individuals with abdominal obesity (32, 33), independent of BMI. Both prospective and cross-sectional analyses have indicated that higher circulating concentrations of proinflammatory cytokines—including C-reactive protein (34–36), IL-6 (37), and TNF-α (34)—are associated with lower BMD and greater fracture risk (38). In addition to a greater production of proinflammatory cytokines by abdominal adipose tissue, it is also known that production of adiponectin is reduced in obese individuals. Adiponectin, an adipose-derived hormone, is inversely associated with visceral fat (39, 40) and other measures of central obesity, such as waist circumference (41). Elegant in vitro and animal studies have elucidated the role of adiponectin on the skeleton. Adiponectin exerts an activity to increase bone mass by suppressing osteoclastogenesis and by activating osteoblastogenesis (42). Furthermore, the adiponectin receptors AdipoR1 and AdipoR2 are expressed in bone-forming cells (43). However, most recently, adiponectin knockout mice were shown to have increased bone mass, which suggests that adiponectin may have other indirect effects on bone (44). Finally, serum osteocalcin, a bone-derived protein that regulates bone formation, was recently found to be inversely associated with visceral adiposity (38). The modest effect sizes noted for associations of TFM and BMD may indicate that TFM may have small or negligible effects on BMD beyond its weight-bearing effect.
Our results are consistent with those from other studies that used computed tomography, magnetic resonance imaging, or anthropometric measure to determine abdominal obesity. Gilsanz et al (45) noted that visceral, but not subcutaneous, fat was negatively associated with the structure and strength of the femur in young women. Similarly, in a group of obese adolescent girls, visceral adipose tissue was a negative predictor of both hip and spine BMD (46). Likewise, Huang et al (47) showed that lumbar spine BMD is reduced in association with greater visceral fat in HIV-infected men with lipodystrophy. Using waist-to-hip ratio as a marker for visceral fat, 2 independent studies in Korean men (48) and postmenopausal women (49) found that BMD of the calcaneus (48) and the lumbar spine (49) were negatively correlated with waist-to-hip ratio, after adjustment for BMI or body weight. Unlike the study populations of Huang et al (47), Russell et al (46), and Kim et al (49), we found no associations at the lumbar spine in men, possibly because of the presence of osteophytes, disc space narrowing, and end-plate sclerosis and the presence of other structural artifacts such as extraskeletal calcifications. Lumbar spine BMD measurements can be confounded by these structural artifacts that can artificially increase the BMD measurement (50, 51). Nevertheless, our finding of a strong association of AFM with femoral neck BMD is of public health importance because the death rate within 1 y of a fractured neck of femur is between 20% and 35% (52).
The results of the current study should be interpreted in the context of a few limitations. First, because we used DXA to measure AFM, we were unable to differentiate between visceral and subcutaneous fat. In addition, we had no data to validate AFM against measures of visceral fat from computed tomography or magnetic resonance imaging. However, a recent study in adolescent girls showed that percentage trunk fat from DXA was more significantly associated with visceral (r = 0.83, P < 0.0001) than with subcutaneous (r = 0.77, P < 0.0001) fat (53). Furthermore, whereas visceral fat is thought to be more strongly associated with disease risk, a recent study showed that measures of central obesity were better associated with coronary artery calcium than with direct measures of visceral adiposity (54). These data suggest that the total amount of central obesity is more important than the relative distribution of visceral compared with subcutaneous fat. Still, future studies should evaluate the independent roles of visceral compared with subcutaneous fat depots on bone. Second, as with any observational study, residual confounding is still a possibility. However, covariates included in our models were carefully selected on the basis of underlying biological mechanisms. Finally, our study was cross-sectional in nature; hence, we were unable to make inferences of causality.
In conclusion, our finding of a negative association between AFM and bone mass in a Hispanic population provides compelling evidence that AFM is a significant risk factor for osteoporosis. Although our results should be replicated in other populations, our findings support the urgent need for development of public health programs tailored to specific ethnic groups that focus on the prevention and treatment of abdominal obesity.
Acknowledgments
The authors’ responsibilities were as follows—SNB and KLT: study design; SNB: data analysis, data interpretation, and manuscript writing; KLT: study oversight; and BD-H, MTH, and AHL: data interpretation and critical revision of the manuscript. The authors had no conflicts of interest.
Footnotes
AFM, abdominal fat mass; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; GED, General Education Development; TFM, total fat mass.
REFERENCES
- 1.National Osteoporosis Foundation Prevalence report. Washington, DC. Available from: http://www.nof.org/advocacy/resources/prevalencereport (cited 27 September 2010) [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75 [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999-2008. Int J Obes (Lond) 2010 [DOI] [PubMed] [Google Scholar]
- 4.Looker AC, Flegal KM, Melton LJ., III Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporos Int 2007;18:307–13 [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Melton LJ, III, Harris TB, Borrud LG, Shepherd JA. Prevalence and trends in low femur bone density among older US adults: NHANES 2005-2006 compared with NHANES III. J Bone Miner Res 2010;25:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, Thun MJ, Gapstur SM. Waist circumference and all cause mortality in a large US cohort. Arch Intern Med 2010;170(15):1293–301 [DOI] [PubMed] [Google Scholar]
- 7.Perry CD, Alekel DL, Ritland LM, Bhupathiraju SN, Stewart JW, Hanson LN, Matvienko OA, Kohut ML, Reddy MB, Van Loan MD, et al. Centrally located body fat is related to inflammatory markers in healthy postmenopausal women. Menopause 2008;15:619–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis 2005;183(2):308–15 [DOI] [PubMed] [Google Scholar]
- 9.dos Santos RE, Aldrighi JM, Lanz JR, Ferezin PC, Marone MM. Relationship of body fat distribution by waist circumference, dual-energy X-ray absorptiometry and ultrasonography to insulin resistance by homeostasis model assessment and lipid profile in obese and non-obese postmenopausal women. Gynecol Endocrinol 2005;21(5):295–301 [DOI] [PubMed] [Google Scholar]
- 10.Katzel LI, Busby-Whitehead MJ, Goldberg AP. Adverse effects of abdominal obesity on lipoprotein lipids in healthy older men. Exp Gerontol 1993;28:411–20 [DOI] [PubMed] [Google Scholar]
- 11.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med 2010;70(12):1988–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnishi H, Saitoh S, Akasaka H, Mitsumata K, Chiba M, Furugen M, Furukawa T, Mori M, Shimamoto K. Incidence of hypertension in individuals with abdominal obesity in a rural Japanese population: the Tanno and Sobetsu study. Hypertens Res 2008;31(7):1385–90 [DOI] [PubMed] [Google Scholar]
- 13.Tucker KL, Falcon LM, Bianchi LA, Cacho E, Bermudez OI. Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and non-Hispanic white neighborhood comparison group. J Gerontol A Biol Sci Med Sci 2000;55:M90–7 [DOI] [PubMed] [Google Scholar]
- 14.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Society for Clinical Densitometry Official positions of the International Society for Clinical Densitometry. West Hartford, CT: 2007:4–5 [Google Scholar]
- 16.White J, Harris SS, Dallal GE, Dawson-Hughes B. Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom 2003;6(2):159–62 [DOI] [PubMed] [Google Scholar]
- 17.Paffenbarger RS, Wing A. L, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol 1978;108:161–75 [DOI] [PubMed] [Google Scholar]
- 18.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993;328:538–45 [DOI] [PubMed] [Google Scholar]
- 19.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol 1998;148:507–18 [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med 1991;115:505–12 [DOI] [PubMed] [Google Scholar]
- 21.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med 1989;321:1777–83 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 suppl):1220S–8S; discussion 9S–31S [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Available from: http://www.sheffield.ac.uk/FRAX/tool.jsp (cited 27 September 2010)
- 24.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr 2006;83(1):146–54 [DOI] [PubMed] [Google Scholar]
- 25.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92(5):1640–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid IR. Fat and bone. Arch Biochem Biophys 2010;503(1):20–7 [DOI] [PubMed] [Google Scholar]
- 27.Girard J, Lafontan M. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: Visceral adipose tissue production and liver metabolism. Diabetes Metab 2008;34(5):439–45 [DOI] [PubMed] [Google Scholar]
- 28.Faulhaber GA, Premaor MO, Moser Filho HL, Silla LM, Furlanetto TW. Low bone mineral density is associated with insulin resistance in bone marrow transplant subjects. Bone Marrow Transplant 2009;43(12):953–7 [DOI] [PubMed] [Google Scholar]
- 29.Melton LJ, III, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 2008;23:1334–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm 2010;2010:513948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004;145(5):2273–82 [DOI] [PubMed] [Google Scholar]
- 32.Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, Vaccaro O. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care 2009;32(9):1734–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol 2003;13(10):674–82 [DOI] [PubMed] [Google Scholar]
- 34.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab 2008;93(5):1952–8 [DOI] [PubMed] [Google Scholar]
- 35.Kim BJ, Yu YM, Kim EN, Chung YE, Koh JM, Kim GS. Relationship between serum hsCRP concentration and biochemical bone turnover markers in healthy pre- and postmenopausal women. Clin Endocrinol (Oxf) 2007;67(1):152–8 [DOI] [PubMed] [Google Scholar]
- 36.Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int 2005;16:1263–71 [DOI] [PubMed] [Google Scholar]
- 37.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 2001;86:2032–42 [DOI] [PubMed] [Google Scholar]
- 38.Cauley JA, Danielson ME, Bounderau RM, Forrest KY, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res 2007;22:1088–95 [DOI] [PubMed] [Google Scholar]
- 39.Nomura K, Eto M, Kojima T, Ogawa S, Iijima K, Nakamura T, Araki A, Akishita M, Ouchi Y. Visceral fat accumulation and metabolic risk factor clustering in older adults. J Am Geriatr Soc 2010;58:1658–63 [DOI] [PubMed] [Google Scholar]
- 40.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18(5):884–9 [DOI] [PubMed] [Google Scholar]
- 41.Zhuo Q, Wang ZQ, Fu P, Piao JH, Tian Y, Xu J, Yang XG. Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomed Environ Sci 2010;23(1):53–61 [DOI] [PubMed] [Google Scholar]
- 42.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 2005;331(2):520–6 [DOI] [PubMed] [Google Scholar]
- 43.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004;35(4):842–9 [DOI] [PubMed] [Google Scholar]
- 44.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150(8):3603–10 [DOI] [PubMed] [Google Scholar]
- 45.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94(9):3387–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 2010;95(3):1247–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang JS, Rietschel P, Hadigan CM, Rosenthal DI, Grinspoon S. Increased abdominal visceral fat is associated with reduced bone density in HIV-infected men with lipodystrophy. AIDS 2001;15:975–82 [DOI] [PubMed] [Google Scholar]
- 48.Seo HJ, Kim SG, Kim CS. Risk factors for bone mineral density at the calcaneus in 40-59 year-old male workers: a cross-sectional study in Korea. BMC Public Health 2008;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CJ, Oh KW, Rhee EJ, Kim KH, Jo SK, Jung CH, Won JC, Park CY, Lee WY, Park SW, et al. Relationship between body composition and bone mineral density (BMD) in perimenopausal Korean women. Clin Endocrinol (Oxf) 2009;71(1):18–26 [DOI] [PubMed] [Google Scholar]
- 50.Muraki S, Yamamoto S, Ishibashi H, Horiuchi T, Hosoi T, Orimo H, Nakamura K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int 2004;15:724–8 [DOI] [PubMed] [Google Scholar]
- 51.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA. A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J Rheumatol 1995;22:932–6 [PubMed] [Google Scholar]
- 52.Goldacre MJ, Roberts SE, Yeates D. Mortality after admission to hospital with fractured neck of femur: database study. BMJ 2002;325:868–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savgan-Gurol E, Bredella M, Russell M, Mendes N, Klibanski A, Misra M. Waist to hip ratio and trunk to extremity fat (DXA) are better surrogates for IMCL and for visceral fat respectively than for subcutaneous fat in adolescent girls. Nutr Metab (Lond) 2010;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho JS, Cannaday JJ, Barlow CE, Willis B, Haskell WL, FitzGerald SJ. Comparative relation of general, central, and visceral adiposity measures for coronary artery calcium in subjects without previous coronary events. Am J Cardiol 2009;104(7):943–6 [DOI] [PubMed] [Google Scholar]