Abstract
Toxic shock syndrome (TSS) is a clinical consequence of the profound amplification of host pro-inflammatory cytokine signaling that results from staphylococcal enterotoxin (SE) exposure. We recently reported that MyD88−/− mice were resistant to SEA or SEB toxic shock and displayed reduced levels of pro-inflammatory cytokines in their serum. Here we report that SEB stimulation of total mononuclear cells up-regulated MyD88 in monocytes and T cells. Further, MyD88 gene silencing in primary human cells using siRNA prevented SEB or SEB plus lipopolysaccharide (LPS) induction of interleukin-1β (IL-1β) transcriptional activation, suggesting that MyD88-mediated signaling is an essential component of SEB toxicity. We synthesized small molecules that mimic the conserved BB-loop in the Toll/IL-1 receptor (TIR) domain of MyD88. In primary human cells, these mimetics attenuated SEB-induced pro-inflammatory cytokine production. SEB stimulation of primary cells with mimetic affected newly synthesized MyD88 and downstream signaling components. Furthermore, LPS-induced MyD88 signaling was likewise inhibited in a cell-based reporter assay. More importantly, administration of mimetic reduced cytokine responses and increased survivability in a murine SEB challenge model. Collectively, these results suggest that MyD88 BB-loop mimetics interfere with SEB-induced pro-inflammatory signaling and toxicity, thus offering a potential approach in the therapy of toxic shock.
Keywords: Bacterial Toxins, Cytokines Induction, MyD88, Signal Transduction, Toll-like Receptors (TLR), BB-loop, Inflammation, Staphylococcal Enterotoxin B, Synthetic Mimetics, Toxic Shock
Introduction
Toxic shock syndrome (TSS)4 is caused by the inflammatory cytokine response to toxins produced by toxigenic strains of the Gram-positive bacteria Staphylococcus aureus and Streptococcus pyogenes, and often progresses to sepsis, multi-organ dysfunction, and death (1–4). Staphylococcal enterotoxin B (SEB) belongs to this family of related enterotoxins (5), which also includes SEA and Toxic Shock Syndrome Toxin 1 (TSST-1). TSS results from the ability of such enterotoxins to act as superantigens. Superantigens stimulate immune cell expansion and rampant pro-inflammatory cytokine production in a manner that bypasses normal MHC-restricted antigen processing. These bacterial toxins can be isolated from patients with sepsis (6–7). SEB is listed by the Center for Disease Control and Prevention (CDC) as a select agent due to its potential use as an aerosolized biological weapon, which adds further impetus to the development of medical countermeasures. Studies show that mice are more resistant to the pathological effects of SEs than humans. However, in the presence of lipopolysaccharide (LPS, endotoxin) they respond strongly (8–9). SEB/LPS-injected mice show distinct patterns of cytokine release in their serum: elevated amounts of tumor necrosis factor-α (TNF-α), interferon γ (IFN-γ), interleukin-1β (IL-1β), IL-1α, and interleukin-6 (IL-6) as well as prolonged secretion of IL-6, IL-2, and IFN-γ (8). In patients, severe sepsis often occurs after exposure to both SE and LPS. Exposure to SE or SE plus LPS triggers vigorous intracellular signaling events in T cells and monocyte/macrophages (APCs), which leads to hyper-inflammation and release of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-1α, IL-2, and IL-6. Innate immune response is an essential component of host defense but it is a delicate balancing act. Dys-regulation of the innate immune reactions, by either default or excess, has dramatic consequences for the infected host, as seen in severe sepsis and septic shock. It is generally known that MyD88 is predominantly responsible for directing intracellular signaling critical for innate immune regulation (10). It is also known that IL-1β and IFN-γ induces signaling via MyD88-dependent pathways (11). Recent results from our laboratory demonstrated that MyD88−/− mice were protected from a lethal SEA or SEB challenge and showed a significantly reduced level of pro-inflammatory cytokines in their serum. In contrast, the potent cytokine response of wild type mice was significant and lethal (12–13). These results suggest that MyD88 plays an essential role in pro-inflammatory cytokine signaling in eliciting SE toxicity. However, to date there has not been any attempt made to evaluate MyD88 as a potential target for therapeutic intervention of SEB-induced toxicity.
MyD88 is an anchor adaptor protein that integrates and transduces intracellular signals generated by TLRs and IL-1 receptor (TLR/IL-1R) super-family (10–14). All TLR/IL-1 members possess cytoplasmic Toll/IL-1 receptor (TIR) domains (15). The IL-1 (α/β) signaling begins with activation of IL-1R1, which forms a heterodimer with IL-1 receptor accessory protein (IL-1RAcP) via their TIR domains. This is followed by recruitment of MyD88 and subsequent activation of the Interleukin Receptor-associated kinases 1–4 (IRAK1–4). Phosphorylated IRAK-1 dissociates from MyD88 and then associates with TRAF-6, which in turn causes activation of TGF-β-activated kinase 1. This complex signaling pathway eventually induces the downstream activation of the IKβ kinase complex and the release of the transcription factor NF-kB and MAPKs p38 and JNK (16) as well as inflammatory cytokines such as TNF-α, IL-6, and IL-1β and chemokines such as IFN-γ-inducible protein 10 (IP-10). A conserved sequence, (F/Y)-(V/L/I)-(P/G), called the BB-loop appears in the TIR domain of most members of the TLR/IL-1R family. The BB-loop is reported to be involved in TIR-TIR interaction and is critical in MyD88-mediated signaling (17). Previous work showed that a synthetic molecule, hydrocinnamoyl-l-valyl-pyrrolidine, (Compd1), mimicking the BB-loop of the TIR domain, disrupted TLR/IL-1R signaling (18). The mimetic blocked IL-1 signaling by disrupting MyD88 and IL-1R association, and reduced fever associated with inflammation in mice. In this study, we have used synthetic BB-loop mimetics such as Compd1 to assess whether MyD88 is an effective target for therapeutic intervention of SEB toxicity. We evaluated the biological effect of the mimetics in primary human cells and tested them in a mouse model of lethal SEB challenge. The BB-loop mimetics Compd1, Compd2, and Compd3 attenuated the production of pro-inflammatory cytokines in response to SEB, TSST-1, and SEB/LPS intoxication in primary cells and increased survival of mice in a lethal SEB challenge.
EXPERIMENTAL PROCEDURES
Reagents
Staphylococcal enterotoxin B (SEB) was purchased from Porton Down, Inc. (Salisbury, UK) and stored at −50 °C. SEB was endotoxin free and prepared under GMP conditions. TSST-1 was purchased from Toxin Technology (Sarasota, FL). Escherichia coli LPS (055:B5) was purchased from Difco laboratories (Detriot, MI). Pooled human AB sera were obtained from Pel-Freez (Brown Deer, WI). A cytometric bead array (CBA) kit was purchased from Pharmingen (San Diego, CA). Meso Scale Discovery (MSD) multi spot array ultra sensitive cytokine assay kit was purchased from MSD (Gaithersburg, MD). Tri-Reagent was obtained from Molecular Research Center Inc, (Cincinnati, OH). MML reverse transcriptase was purchased from Perkin Elmer (Waltham, MA). Magnetic bead-conjugated anti-CD14, anti-CD3, anti-CD11c mAbs were obtained from Miltenyi Biotech Inc., (Auburn, CA). Ficoll-Hypaque was purchased from GE Healthcare Biosciences (Piscataway, NJ). Trans- NF-kB p50 and p65 kits were purchased from Active Motif (Carlsbad, CA). Human MyD88 siRNA and siRNA transfecting reagents were purchased from Dharmacon (Lafayette, CO). Power Sybr Green PCR master mix and 7900HT Fast Real Time PCR System were obtained from Applied Biosystems (Foster City, CA). Primary anti-MyD88 antibody was obtained from AnaSpec, Inc. (San Jose, CA). Anti-β-actin antibody was purchased from Cell Signaling Technology (Danvers, MA). HEK 293(TLR4-MD2-NF-kB-SEAPorter transfected) stable cell line was purchased from Imgenex (San Diego, CA). Poly IC (low molecular weight, endotoxin free) was purchased from InvivoGen (San Diego, CA).
Synthesis of MyD88 Mimetics
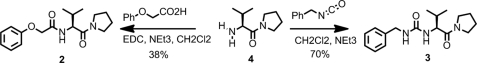
(Structure 1) (S)-N-(3-methyl-1-oxo-1-(pyrrolidin-1-yl) butan-2-yl)-2-phenoxyacetamide (2). To a solution of amine 4 (1.277 g, 4.4 mmol) and NEt3 (1.251 ml, 8.9 mmol) in CH2Cl2 (50 ml) at 0 °C was added phenoxyacetic acid (717 mg, 4.7 mmol) and EDC (945 mg, 4.9 mmol). After 18 h stirring under nitrogen, the organic phase was washed with 20 ml of 1 m HCl, dried over Na2SO4 and concentrated. The crude residue was purified by column chromatography on silica gel (Hexane/AcOEt : 1/0 to 1/1) to give compound 2 (583 mg, 1.9 mmol, 38%) as a colorless oil. 1H NMR: (250 MHz, CDCl3) δ 7.33–7.20 (m, 3H), 7.00 (m, 0.4H), 6.97 (m, 0.6H), 6.94 (m, 1.2H), 6.90 (m, 0.8H), 4.64 (m, 1H), 4.48 (m, 2H), 3.70 (m, 1H), 3.45 (m, 3H), 2.12–1.76 (m, 5H), 0.93 (d, J = 6.7 Hz, 3H), 0.85 (d, J = 6.7 Hz, 3H); 13C NMR: (75 MHz, CDCl3) δ 169.5, 167.8, 157.0, 129.5, 121.8, 114.6, 67.0, 55.1, 46.6, 45.7, 31.3, 25.9, 24.0, 19.4, 17.6; HRMS: (ESI-TOF) C17H24N2O3H+ expected: 305.1860. found: 305.1860.
STRUCTURE 1.
Reaction scheme synthesis of MyD88 mimetics.
(S)-1-benzyl-3-(3-methyl-1-oxo-1-(pyrrolidin-1-yl) butan-2-yl) urea (3). To a solution of amine 4 (1 g, 3.5 mmol) and NEt3 (1.719 ml, 12.3 mmol) in CH2Cl2 (50 ml) at 0 °C was added benzylisocyanate (0.508 ml, 4.1 mmol) dropwise. After 18 h stirring under nitrogen, 20 ml of HCl was added carefully. The organic phase was collected and concentrated as above. The crude residue was purified by column chromatography on silica gel (Hexane/AcOEt : 1/0 to 1/1) to give urea 3 (742 mg, 2.4 mmol, 70%) as a colorless oil. 1H NMR: (600 MHz, CDCl3) δ 7.32 (m, 4H), 7.20 (m, 1H), 6.70 (m, 1H), 6.45 (m, 1H), 4.48 (m, 1H), 4.37 (m, 2H), 3.78 (m, 1H), 3.41 (m, 1H), 2.92 (m, 1H), 2.76 (m, 1H), 1.91 (m, 1H), 1.83 (m, 2H), 1.60 (m, 2H), 0.98 (d, J = 6.7 Hz, 3H), 0.94 (d, J = 6.7 Hz, 3H); 13C NMR: (150 MHz, CDCl3) δ 172.3, 158.5, 139.6, 128.2, 127.8, 126.7, 56.3, 46.8, 45.2, 44.2, 31.5, 25.6, 24.0, 19.3, 18.0; HRMS: (ESI-TOF) C17H25N3O2H+ expected: 304.2019. found: 304.2021.
Mice
Pathogen-free, 6–8-week-old BALB/c mice were obtained from Charles River (NCI-Frederick, Frederick, MD).5
Cell Isolation and Purification
Peripheral blood mononuclear cells (MNC), obtained from consenting healthy donors in accordance with Institutional Review Board-approved research donor protocol, were isolated by standard density gradient centrifugation with Ficoll-Hypaque, harvested from the interface, washed, and resuspended in RPMI 1640 medium. CD14+ monocytes and CD3+ T-cells were isolated as previously described (19). Isolated cell populations had >98% purity. Purified CD14+ and CD3+ cells were cultured to evaluate MyD88 gene silencing by siRNA transfection. All experiments were performed at least three times.
Cytokine Analysis
Cell cultures were incubated (37 °C, 5% CO2) for 16 h. Cytokines in culture supernatants were measured by a CBA kit using captured beads coated with antibodies specific for cytokines and flow cytometry analysis as described elsewhere (20). Cytokine measurements were confirmed by dilution of culture supernatant using Human Inflammation and Th1/Th2 CBA kits and acquiring 1800 beads. We also used Meso Scale Discovery (MSD) multi spot array ultra sensitive cytokine assay kit for measuring cytokines in culture supernatants (according to the manufacturer's protocol). Briefly, the 96 well cytokine assay plate was blocked with diluent 2 (according to manufacturer's protocol) for 30 min at room temperature with constant shaking at 400 rpm. The calibrators were processed according to manufactures protocol. 25 μl of calibrator and samples were added to the plate in triplicates for 2 h at room temperature with constant shaking at 400 rpm. After the 2 h incubation, the plates were washed three times with 1× PBS+0.05% Tween-20, and 25 μl of the detection antibody solution was added to each well of the plate. This was incubated for 2 h at room temperature with constant shaking at 400 rpm. Following the 2 h incubation the plates were washed 3 times with 1× PBS + 0.05% Tween-20. According to the manufacturer's protocol, 150 μl of 2× Read Buffer T was added to each well of the plate and analyzed on the SECTOR Imager. The assay results were read using an MSD SECTOR Image 2400 incorporating a CCD. Sample cytokine concentrations were determined with Softmax Pro Version 4.6 software, using curve fit models (log-log or 4-PL) as suggested by the manufacturer for the specific cytokine.
Proliferation Assay
Total mononuclear cells (2 × 106) were cultured in a 96-well plate with SEB (200 ng/ml) in the presence or absence of Compd1 (10 and 40 mm) for 3 days at 37 °C. After 3 days, cultures were pulsed with 1 μCi of [3H]thymidine/well for 10 h and proliferation was measured by harvesting the cells onto microplate unifilters and measuring radioactivity in a liquid scintillation counter (Packard, Meriden, CT).
NF-κB Assay
Activation of NF-κB was measured in nuclear extracts of cells as described earlier (12–13). To prepare nuclear extract, cells (5 × 106) were washed and resuspended with 1 ml of cold PBS and placed on ice for 15 min to allow the cells to swell. 50 μl of 10% Nonidet P-40 was added to the cells and briefly centrifuged. The nuclear pellet was resuspended in lysis buffer (Active Motif) containing DTT and protease inhibitor for 30 min on ice, centrifuged for 10 min at 14,000 × g at 4 °C and collected extract for NF-κB assay. The TransAm Chemi kit (Active Motif, Carlsbad, CA) had NF-κB binding oligonucleotides immobilized to a 96-well plate. To the 96 -well plate, nuclear extracts of spleen cells were placed in duplicates for an hour. The plates were then washed several times and diluted NF-κB antibody was added to the plates for 1 h at room temperature. The plates were washed and a diluted horseradish peroxidase (HRP)-conjugated secondary antibody was added to the plate for an hour at room temperature. After incubation, the plates were washed with wash buffer and a chemiluminescent working solution was added to detect NF-κ B recognition of an epitope on p50 subunit.
Real-time PCR
For measuring transcriptional activation of MyD88, total RNA was extracted from cells using Tri-Reagent and reverse transcribed into cDNA with Maloney murine leukemia virus reverse transcriptase according to the manufacturer's instructions (Perkin Elmer). Quantitative real time PCR was performed using cDNA collected as described elsewhere (21). Amplification was performed using 4 μl of a 1 μm final concentration of gene specific primers, MyD88 (Forward 5′-CACTCGCAGTT TGTTGGATG-3′; Reverse5′-CGCAGGA TACTGGGAAAGTC-3′), and β-actin (Forward 5′- TCCTGTGGCATCC ACGAAACT-3′; Reverse 5′-GAAGCATT TGCGGTGGACGAT-3′), 10 μl of Power Syber Green PCR master mix, and 6 μl cDNA according to the manufacturer's instructions. Finally, the 20 μl PCR reaction was amplified using a 7900HT Fast Real Time PCR System. The final normalized results were calculated by dividing the relative transcript levels of the test genes by the relative amount of the β-actin RNA.
RNA Interference Technique
CD14+ and CD3+ cells were plated in 6 well plates (107 per well) for 16 h. Non-adherent cells were harvested. The adherent cells were washed with Optimem media and 2 ml of the fresh media containing the non-adherent T cells were added. A 100 nm final concentration of the 21-nucleotide siRNA duplex of MyD88 gene (accession number NM-0002468.30 at nucleotide (nt) 749–767 (sense:5′-CGACUGAAGUUGUGUGU GUUU-3′;antisense:5′-P.ACACACACAACUUCAGUCGUU-3′) or control siRNA was added to DharmaFECT4 and transfected monocytes plus T cells for 48 h. After 48h incubation, transfected cells were stimulated with SEB (200 ng/ml) or LPS (1 μg/ml) for 24 h. The cells were then collected for RNA. Culture supernatant was used to measure cytokine activity.
Flow Cytometry Analysis
To examine up-regulation of MyD88 in T cells, MNCs were activated with SEB or kept untreated (4 h, at 37 °C). Intracellular expression of MyD88 protein in CD3+ T cells was examined using a modified method similar to intracellular staining of phosphorylated proteins in lymphocytes (BD Biosciences) as described elsewhere (21). Briefly, the cells were pelleted, washed once with PBS, and immediately fixed by mixing one volume of cells to 20 volumes of 1× Phosphoflow Lyse/Fix buffer (BD Pharmingen, San Diego, CA). Cells were incubated (15 min, 37 °C), pelleted, and permeabilized with PhosFlow Perm Buffer (III) (BD Pharmingen) for 30 min on ice, washed twice with BD Pharmingen TM stain buffer and pelleted by centrifugation (300 × g) for 5 min. Cells were suspended (107/ml) in BD Pharmingen stain buffer. BD FcR-block antibody (0.06 μg) was added for each 107 cells, which were incubated on ice for 15 min. Cells were labeled with APC conjugated anti-CD3 and primary anti-MyD88 antibody (rabbit polyclonal) followed by secondary goat anti-rabbit IgG (H+L) conjugated to Alexa Flour® 488 (Molecular Probe) or appropriate isotype-matched control antibody. The labeled cells were washed, suspended in stain buffer, and MyD88 expression was measured by flow cytometry.
Cytotoxicity Assay
T cells, MNCs, and CD14+ selected cells (5 × 105) were cultured in a 96 well plate with SEB and/or LPS in the presence of Compd1. After 24 h, cultures were lysed with 100 μl of nucleotide-releasing agent (Lumitech), followed by the addition of an ATP-monitoring agent (20 μl). Light emission was measured in a luminometer (Wallac 1420 Victor3V; Perkin Elmer), and specific cytotoxic effect of compd1 was calculated by subtracting experimental from maximum target-cell lysis and determined as percentage change from control.
Western Blot Analysis
Human MNC (5 × 106/ml) were stimulated with SEB (200 ng/ml) or SEB plus different concentration of Compd1 (10 mm to 0.01 mm), or left untreated for 2 h. Cells were collected into fresh 1.5 ml centrifuge tubes and chilled on ice for 5 min before centrifuging. Membrane and cytoplasm were separated by resuspending the pellets in 50 μl of lysis buffer (Active Motif) in the presence of DTT, protease inhibitors and phosphatase inhibitors on ice for 30–60 min. The membrane fraction was collected by centrifuging lysates at 14,000 × g for 20 min. Supernatant contained the cytoplasmic fraction and pellet contained membrane fraction. Samples containing 10 μg of total cytoplasmic proteins separated by SDS-PAGE and transferred to nitrocellulose membranes. Nitrocellulose membranes were blocked overnight in Tris-buffered saline containing 0.1% Tween 20 and 3% BSA at 4 °C. Blots were extensively washed and probed with anti-MyD88 polyclonal antibody followed by HRP-conjugated secondary Ab. Blots were washed extensively and developed with chemiluminescent substrate in the presence of hydrogen peroxide using Immun-Star WesternC Chemiluminescent kit (Bio-Rad). An imaging system VersaDoc Model 4000 (Bio-Rad) was used to capture the image.
Intracellular Staining and Confocal Microscopy
Human monocytes (0. 8 × 106/chamber) were cultured in 8 chambered slides with SEB (200 ng/ml) in the presence of Compd1 (0.1 mm). After 2 h, cells were fixed with a 1% paraformaldehyde containing 0.1% glutaraldehyde for 5 min at room temperature. The cells were washed several times with 1× PBS and 0.5% BSA. The fixed cells were then permeabilized using Perm Buffer III (BD Biosciences) for 30 min at room temperature. The cells were washed a few times with 1× PBS and 0.5% BSA. The cells were blocked with PBS + 5% BSA for 15 min at room temperature. The cells were washed with 1× PBS+0.5% BSA several times, incubated with anti-MyD88 antibody for 1 h at room temperature. The cells were washed with 1× PBS + 0.5% BSA. An Alexa 488 goat anti-rabbit antibody was added to the cells for 1 h at room temperature. The cells were washed several times with 1× PBS+0.5% BSA, mounted with Hoecht Stain (Invitrogen) and observed under a confocal microscope.
Secreted Alkaline Phosphatase (SEAP) Assay
TLR4/MD-2/NF-kB/SEAPorter HEK 293 cells (5 × 105 cells/ml/well) were cultured with LPS (1 μg/ml), poly IC (1 μg/ml), poly IC or LPS with varying concentration of Compd1 in 24-well plate and incubated at 37 °C for 16 h. The culture supernatant was collected and centrifuged to remove any cell debris. The Great EscAPe SEAP Assay from Clontech was used to determine the amount of alkaline phosphatase that is secreted into the supernatant. A 1× dilution buffer is prepared from a 5× stock solution and 75 μl of the 1× dilution buffer is mixed with 25 μl of the supernatant, incubated for 30 min at 65 °C to inactivate endogenous alkaline phosphatase. The samples were then placed on ice for 3 min and then equilibrated at room temperature. SEAP substrate solution (100 μl) was added to each sample and read at 10 min intervals using a chemiluminescent reader.
RESULTS
MyD88 Mediates Up-regulation of IL-1β in Monocytes and T Cells after SEB Stimulation
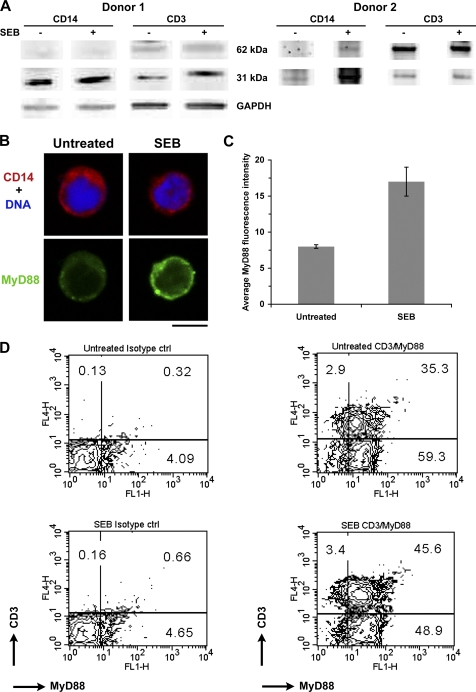
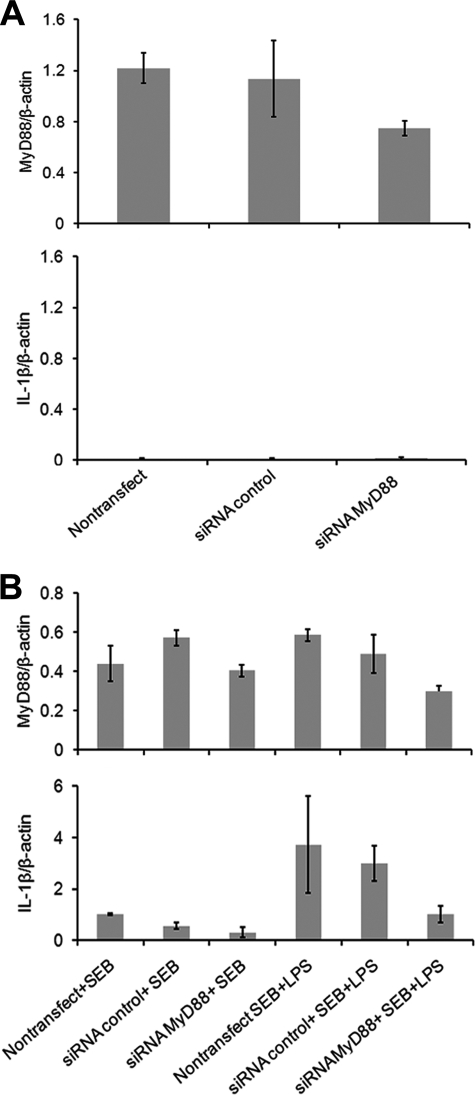
To examine whether SEB stimulation activated monocytes and T cells through up-regulation of MyD88, we isolated MHC class II+, CD14+ monocytes, and CD3+ T cells after SEB stimulation of total MNC or from untreated MNC. Cell extracts were prepared from monocytes and T cells and examined for MyD88 up-regulation by Western blot analysis. Results shown in Fig. 1A demonstrate an up-regulation of 62 and 31 kDa protein in monocytes and T cells compared with untreated cells. The up-regulation of MyD88 in monocytes and T cells was further confirmed by immunofluorescence. Total MNC were activated with SEB, stained for CD14 and MyD88. Confocal microscopy results shown in Fig. 1, B and C indicate up-regulation of MyD88 in CD14+ monocytes. Results from dual color flow cytometry analysis, shown in Fig. 1D, likewise indicated that SEB stimulation of MNCs increased MyD88 in CD3+ T cells (CD3+MyD88+) compared with untreated MNCs. These results suggest that up-regulation of MyD88 was induced by SEB stimulation in both monocytes and T cells. In addition to MyD88, our earlier report indicated other downstream signaling components such as IRAK-4 and TRAF-6 were also up-regulated after SEB stimulation (21). We therefore examined the involvement of MyD88 in pro-inflammatory cytokine signaling, such as expression of IL-1β, in human primary cells after SEB or SEB/LPS stimulation. We employed siRNA-mediated gene silencing to knock down MyD88 gene function using primers from its gene sequence (siRNA-MyD88). Freshly isolated purified human monocytes (CD14+) and T cells in in vitro cultures were transfected with siRNA-MyD88 or control primer. siRNA targeting of the MyD88 gene knocked down MyD88 at the mRNA level compared with non-transfected or control transfected cells (Fig. 2A). After 48 h, non-transfected cells, control transfected cells and siRNA-MyD88 transfected cells were stimulated with SEB or SEB/LPS and harvested after 24 h. Consequently, transcriptional activation of IL-1β was not up-regulated (Fig. 2B) and the release of IL-1β was diminished after SEB or SEB/LPS stimulation in MyD88-deficient cells (siRNA-MyD88-transfected cells) compared with non-transfected or control transfected cells (data not shown). These results suggest that MyD88 gene silencing prevented induction of IL-1β after SEB or SEB/LPS stimulation. These results are consistent with our earlier observation that MyD88 deficiency in primary spleen cells impaired SEA- or SEB-induced pro-inflammatory cytokine responses (12–13).
FIGURE 1.
Up-regulation of MyD88 in monocytes and T cells after SEB stimulation of total MNC. A, SEB induced up-regulation of MyD88 synthesis in cytoplasmic fractions of monocytes and T cells. B, confocal images showing up-regulation of MyD88 in CD14+ monocytes treated with SEB or left untreated for 2 h. Scale bar, 5 μm. C, the attached graph shows the mean MyD88 fluorescence intensity (error bars are S.E.) for untreated, and SEB. For each condition, N is greater than or equal to 24 cells. A Mann Whitney rank sum test showed SEB-treated to be significantly higher than untreated (p < 0.001). D, up-regulation of MyD88 in CD3+ T cells. Increase in CD3+MyD88+ T cells after SEB stimulation of MNCs. Dual color FACS analysis was performed using appropriate isotype controls with untreated MNCs and MNCs treated with SEB.
FIGURE 2.
Reduced expression of MyD88 by siRNA gene silencing prevents up-regulation of IL-1β after stimulation of primary cells with SEB or SEB plus LPS. A, siRNA MyD88 transfection knocked down MyD88 mRNA. B, siRNA knocked down of MyD88 prevented up-regulation of IL-1β. MyD88 is required for IL-1β induction following SEB or SEB plus LPS stimulation. Cells were kept untreated (nontransfect) or transfected with siRNA control or siRNA MyD88 for 48 h. Then cells were stimulated with SEB or SEB and LPS. MyD88 and IL-1β expression levels were measured by quantitative real time PCR 24 h after stimulation. MyD88 and IL-1β expression were normalized to the expression of β-actin. Expression levels are expressed as means ± S.D.
Designing a Synthetic Mimetic Structurally Similar to the BB-loop of MyD88
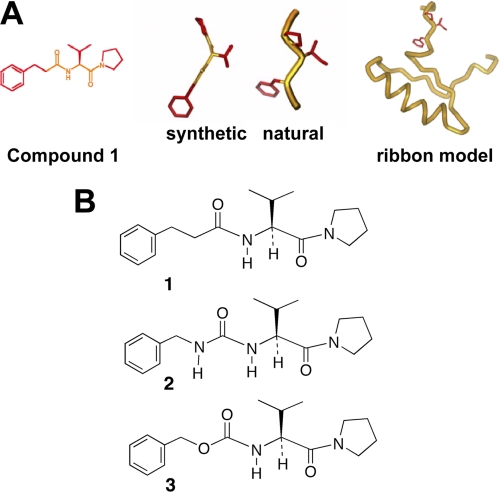
SEB stimulation of primary cells induced up-regulation of MyD88 and MyD88 gene silencing was able to reduce SEB-induced IL-1β production in human primary culture. Taken together with our earlier results that MyD88−/− mice were resistant to SEA or SEB-induced lethality (12–13), this suggests that MyD88 is a critical component of SEB induced pro-inflammatory signaling. We therefore hypothesized that targeting of MyD88 would interfere with SEB-induced pro-inflammatory cytokine signaling. Synthetic mimetics of the conserved BB-loop region (17) in the TIR domain of MyD88, as shown in Fig. 3A, were thus designed. We synthesized Compd1 and structural analogs based on the tripeptide sequence of the BB-loop ((F/Y)-(V/L/I)-(P/G)) of the Toll/IL-1 receptor (TIR) domain binding surface of MyD88 (Fig. 3B). Similar to the BB-loop structure, the basic structure of the synthetic mimetics contained an aromatic ring, an amino acid and a pyrrolidine group. It was anticipated that because of the structural homology, synthetic mimetic binding to this region would interfere with recruitment of MyD88 and subsequent downstream signaling.
FIGURE 3.
A schematic representation of BB-loop in the TIR domain of MyD88. A, schematic representation of the ribbon model represents the drawn amino acid residues contributing to the conserved BB-loop as shown. Natural and synthetic structural drawings are the representation of the BB-loop part as shown in the ribbon model. Hydrocinnamoyl-l-valyl-pyrrolidine (Compd1) is a synthetic mimetic of BB-loop. The basic structure of the Compd1 contains an aromatic ring, an amino acid and a pyrrolidine group. B, compounds 2 and 3 are structural analogs of Compd1.
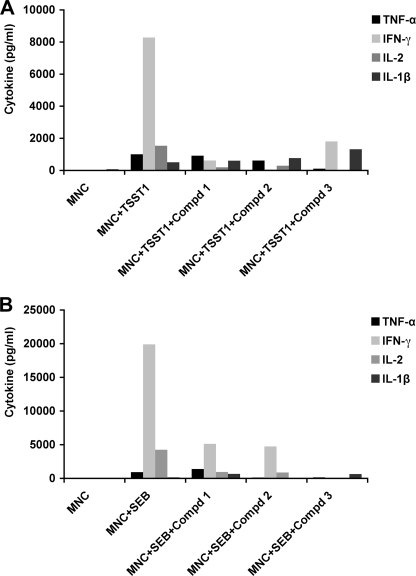
BB-loop Mimetics Attenuated Pro-inflammatory Cytokine Production in Response to SEB or TSST-11 in Primary Culture
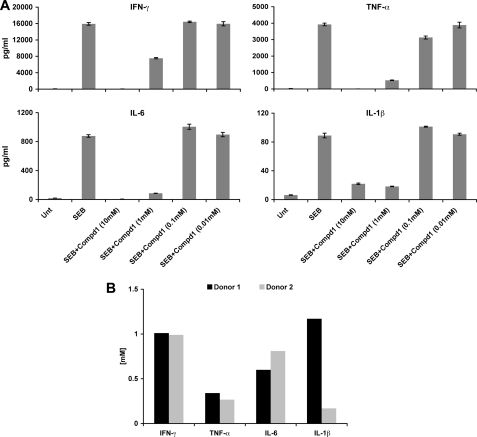
To assess the effects of synthetic BB-loop mimetics on cytokine inhibition, MNCs were first treated with Compds 1, 2, and 3 for 30 min, and cultured with SEB or TSST-1. The cultures were incubated for 16 h, and culture supernatants were collected to measure cytokines. As shown in Fig. 4, Compds 1, 2, and 3 (10 mm) lead to inhibition of cytokine responses elicited with TSST-1 (Fig. 4A), or SEB (Fig. 4B). While Compd 3 appears to be a potent inhibitor of SEB stimulation, it had no inhibitory effect on TSST-1-induction of IL-1β (Fig. 4A), whereas Compd1 inhibited cytokine production in response to both SEB as well as TSST-1. Despite differences among the mimetics used, these preliminary results suggest that the synthetic mimetics overall were capable of inhibiting pro-inflammatory cytokine (IL-1β, IL-2, IFN-γ, TNF-α) production in response to SEB or TSST-1. To examine a more detailed analysis of the effects of the mimetics on cytokine production, we selected Compd1 for further testing in primary cells from two different donors. We used varying concentrations of Compd1 (10, 1, 0.1, and 0.01 mm) and cultured it with MNCs in the presence of SEB. Compd1 inhibited SEB induced IL-1β, TNF-α, IFN-γ, and IL-6 production in a dose-dependent manner (Fig. 5A). The average inhibitory concentration (IC50) of Compd1 ranged from 200 μm to 1.2 mm for most of the cytokines except IL-1β (Fig. 5B). There was a major difference in IC50 value of IL-1β between the two donors. We therefore repeated measurements of IL-1β expression with two additional donors. MNCs were isolated from donor 3 and donor 4 and treated with SEB in the presence and absence of Compd1 and IL-1β response was measured. Our results indicated that while IL-1β response varied from donor to donor, SEB-induced IL-1β response was inhibited by Compd1 (supplemental Fig. S1).
FIGURE 4.
MyD88 mimetics attenuate pro-inflammatory cytokine production in response to SEB or TSST-1. Human MNC (106/well) were cultured with SEB or TSST1 (200 ng/ml) and Compd1 (10 mm) for 16 h. Culture supernatants were tested for IL-1β, IL-2, IFN-γ, and TNF-α using CBA assay. A, effects of compounds 1, 2, and 3 on TSST-1- induced cytokine production. B, effects of compounds 1, 2, and 3 on SEB- induced cytokine production. Data presented are representative of two independent experiments.
FIGURE 5.
Compd1 inhibits cytokine production in a dose-dependent manner in response to SEB in primary cells. Human MNC (5 × 105/well) were cultured with SEB (200 ng/ml) with varying concentration of compd1 (10, 1, 0.1, and 0.01 mm) for 16 h. Culture supernatants were tested for cytokine release using a MSD assay. A, compd1 induced dose-dependent inhibition of IL-1β, IL-6, IFN-γ, and TNF-α production. B, IC50 of Compd1. Total MNC from two different donors were tested to determine IC50. The IC50 value was calculated as the concentration required for inhibition of cytokine production by 50% relative to the control.
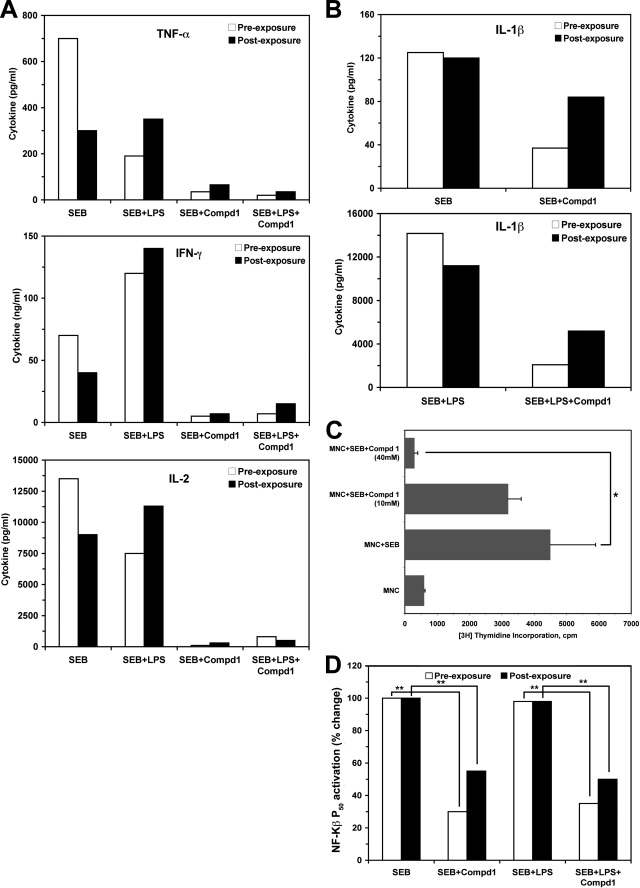
We extended this observation to determine the complete inhibitory effect of Compd1 in specific cell types that are primarily responsible for SEB-induced cytokine production. For this, we purified human MHC class II+ monocytes and T cells, combined them in culture and treated them with Compd1 at varying concentrations for 30 min prior to SEB or SEB plus LPS stimulation (pre-exposure). Alternatively, cells were stimulated with SEB or SEB plus LPS for 30 min followed by treatment with Compd1 (post-exposure). Supernatants from each culture were tested for cytokine release. Consistent with literature reports, our results showed that, compared with SEB, stimulation with SEB plus LPS induced higher levels of IL-1β, IFN-γ, TNF-α, and IL-2 production, particularly in the post exposure group (Fig. 6, A and B). In both pre- and post-exposure groups, Compd1 blocked the release of cytokines in a dose-dependent manner (10 mm and 1 mm, data not shown) and, at 40 mm, almost complete inhibition was observed (Fig. 6, A and B). However, inhibition of cytokine production was less with Compd1 treatment after SEB or SEB+LPS stimulation (post-exposure) compared with pre-exposure (Fig. 6, A and B). It is important to note that IL-1 signaling initiates with the recruitment of MyD88 to IL-1R1 and results in secretion of many pro-inflammatory cytokines such as IL-2, TNF-α, and INF-γ (22). Our results showed that Compd1 treatment inhibited not only IL-1β but also IL-2, TNF-α, and IFN-γ in cultures of MHC class II+ monocytes and T cells when stimulated with SEB or SEB plus LPS (Fig. 6, A and B). Consistent with attenuation of cytokine production, Compd1 inhibited T lymphocyte proliferation (Fig. 6C) and lowered NF-κB p50 activation in both pre- and post-exposure stimulations (Fig. 6D). Given that NF-κB can still be activated via MyD88-independent pathways, it is not surprising that complete inhibition was not achieved. The Compd1 had no cytotoxic effects on purified monocytes, T cells, CD14+/T cells, or unseparated mononuclear cells (MNC) when treated with Compd1 alone or with SEB (supplemental Fig. S2). Compd1 showed a low level of toxicity (average 18%) when cells were stimulated with SEB plus LPS (compare SEB+LPS versus SEB+LPS+Compd1), which was not unusual. These results suggest that Compd1 attenuates pro-inflammatory cytokine responses while enabling immune cell survival.
FIGURE 6.
Pre or postexposure treatment of Compd1 attenuates TNF-α, IFN-γ, IL-2, and IL-1β production. A and B, monocytes and T cells were purified from human blood and cultured (107/well) with compd1 (40 mm) for 30 min followed by SEB (200 ng/ml), or SEB plus LPS (1 μg/ml) stimulation (pre-exposure) or first stimulated with SEB and/or LPS for 30 min followed by Compd1 treatment (post-exposure) and incubated for 16 h. Culture supernatants were collected and cytokine release was measured by CBA. C, compd1 inhibited lymphocyte proliferation after stimulation of SEB (p ≤ 0.01, *). D, inhibition of NF-κB p50 activation in the presence of Compd1 in cells stimulated with SEB or SEB+LPS. Significant differences (p ≤ 0.01) are indicated for untreated versus cells treated with Compd1 (**).
Compd1 Targets MyD88 Signaling
MyD88 plays an important role in IL-1 signaling and the MyD88-death domain is critical for MyD88-induced activation of downstream NF-κB and JNK, which are involved in pro-inflammatory responses (23). Our earlier results demonstrated that SEB stimulation up-regulates MyD88 and other downstream components of MyD88 signaling in primary monocytes (21). To determine whether Compd1 inhibits SEB-induced pro-inflammatory cytokine production by targeting MyD88 signaling, we examined intracellular levels of MyD88 in CD3+ T cells after stimulation of MNCs with SEB. Compd1 inhibited MyD88 up-regulation in a dose-dependent manner in CD3+ T cells, as determined by dual color flow cytometry (supplemental Fig. S3). Like MyD88 protein, up-regulation of downstream components of MyD88 signaling such as IRAK-1, IRAK-4, and TRAF-6 after LPS (supplemental Fig. S4) or SEB exposure (data not shown) was diminished in MNCs and monocytes. These results indicate that Compd1 interferes with up-regulation of MyD88 and its downstream components after SEB stimulation in monocytes and T cells.
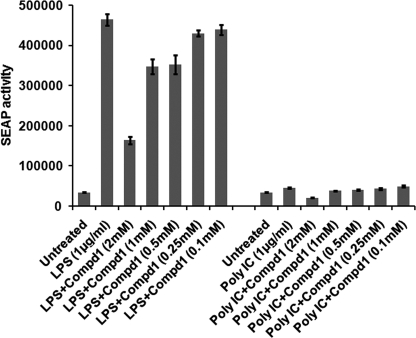
To further confirm that Compd1 specifically targets MyD88, we examined a cell-based reporter assay for detecting MyD88-mediated response using a stably co-transfected(TLR4-MD2-NF-κB/SEAPorterTM) HEK 293 cell line. LPS induced NF-κB-driven SEAP reporter gene expression was evaluated. Results shown in Fig. 7 indicated that Compd1 inhibited LPS induced TLR4- MD2-MyD88-NF-kB-SEAP response in a dose-dependent manner. In contrast, TLR3 ligand poly IC stimulation, which does not utilize MyD88, had no effect on SEAP response (Fig. 7). These results suggest that Compd1 specifically targeted MyD88-mediated signaling. It is important to note that Compd1 inhibited SEAP activity almost equivalent to inhibitory concentration for most of the cytokines (IC50 of Compd1 ranged from 200 μm to 1.2 mm).
FIGURE 7.
Compd1 targets MyD88 signaling. Compd1 inhibits LPS-induced SEAP activity via MyD88-mediated NF-κB-driven signaling pathway. HEK 293 stable transfected cell line (TLR4-MD2-NF-κB-SEAP) were activated with LPS (TLR-4 ligand) or poly IC (TLR3-ligand, control) with varying concentrations of Compd1 (2 mm to 100 μm) or without Compd1 and culture supernatants were tested for SEAP activity. Results are representative of four experiments.
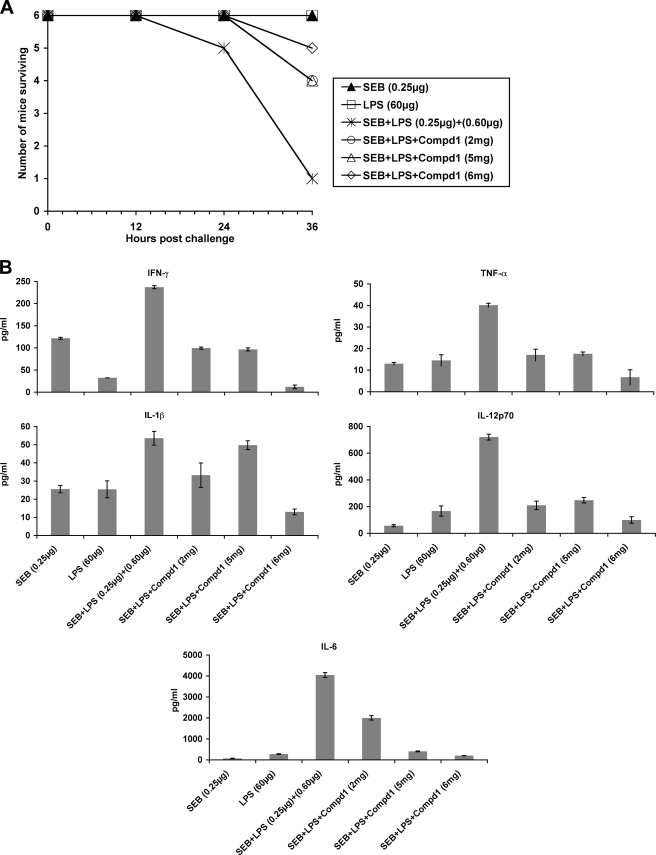
Administration of Compd1 in Mice Increased Survival to Lethal SEB Challenge
To determine whether Compd1 attenuates SEB toxicity in vivo, we used the LPS potentiation model of SEB toxicity in mice (8). BALB/c mice (n = 6) were injected with different amounts of Compd1 (2 to 6 mg/mouse), 30 min later they were treated with SEB (0.25 μg/mouse) followed by LPS 2 h later. Untreated animals showed only a 10% survival by 36 h. In contrast, 66–83% mice treated with Compd1 (3–6 mg/mouse) were alive (p = 0.0937) (Fig. 8A). Similar to experimental settings as in Fig. 8A, we determined pro-inflammatory serum cytokine profile at 24 h time point. The results shown in Fig. 8B indicated that cytokine response was reduced in Compd1-treated mice, with maximum reduction at 6 mg/mouse. These results suggest that cytokine responses correlated to survival of mice. Thus, Compd1 targeting of MyD88 interfered with pro-inflammatory cytokine signaling and protected mice from SEB intoxication. The results point to the potential of MyD88 mimetics for use as therapeutics in vivo to limit SEB-induced toxicity.
FIGURE 8.
Compd1 attenuates pro-inflammatory cytokines and SEB induced lethality in mice. A, administration of Compd1 increased survival of mice. BALB/c mice (n = 6) were injected with different amounts of Compd1 (2, 5, or 6 mg, 100 μl volume/mouse), 30 min later injected with SEB (0.25 μg/mouse) followed by LPS 2 h later. Mice were observed for survival. Control mice injected with 60 μg of LPS or 1 μg of SEB survived. Data are representative of two separate experiments. B, administration of Compd1 in mice inhibited pro-inflammatory cytokine response. Similar to experimental settings as in Fig. 8A, mice (n = 6) were bled at 24 h, serum were pooled from each group (SEB, LPS, SEB+LPS, Compd1 (2 mg/mouse) +SEB+LPS, Compd1 (5 mg/mouse) +SEB+LPS, and Compd1 (6 mg/mouse) +SEB+LPS) and measured serum cytokine profile in triplicates using 7-plex MSD assay.
DISCUSSION
In this study, our data provide evidence that synthetic low molecular weight compounds structurally similar to the BB-loop in the TIR domain of MyD88 attenuated SEB induced pro-inflammatory cytokine production in primary human cells. Further, administration of Compd1 inhibited pro-inflammatory cytokine response in a dose-dependent manner and increased survival of mice from toxic shock-induced death after lethal SEB challenge. These results are consistent with our earlier report, which demonstrated that MyD88−/− mice are resistant to SEA or SEB challenge due to impaired pro-inflammatory cytokine secretion in their serum (12–13). MyD88 was believed to be solely associated with TLR/IL-1R signaling. However, it has recently been reported that its involvement goes beyond the signaling of TLR/IL-1R family members. MyD88 deficiency reportedly results in diminished production of TNF-α and IFN-γ inducible protein IP-10 in macrophages treated with IFN-γ (11) even though Interferon γ receptor1 (IFNGR1) lacks a TIR domain. Additional evidence against an absolute dependence of MyD88 on a TIR domain comes from recent identification of a TIR-less variant of TRIF that has been found to be important for TLR3-mediated signaling (24). In this study, our results demonstrated that MyD88 is up-regulated in monocytes and T cells after stimulation with SEB, which cross-links MHC class II molecules and T cell receptors. Similar to IFNGR1, both MHC class II molecules and T cell receptors lack the TIR domain. Nevertheless, suppression of MyD88 activation by MyD88 gene silencing diminished IL-1 response in human primary cells, suggesting a role for MyD88 as a signaling component in SEB induced pro-inflammatory cytokine responses. In addition to pro-inflammatory innate immune response, MyD88 signaling has also been reported to be involved in antigen specific response. It was reported that MHC class II-peptide-specific T cells responded to ova peptide-specific Th1 cytokine response in a MyD88-dependent manner (25). MyD88−/− mice failed to elicit Th1 cytokine response to ova peptide. A recent report also indicates that, in B cells, MyD88 is required for the production of natural antibodies. MyD88B−/− chimeric mice exhibited an impairment in development of IFN-γ effector T cells and a lack of Ig2c response due to lack of B cell signaling via MyD88 (26). Similar interactions have been detected between MyD88 and other proteins lacking TIR and death domains such as Bruton's tyrosine kinase and interferon regulatory factor 7 (27–28). More recently, it has been reported that MyD88 controls a B cell-intrinsic, TIR-independent, TACI receptor-dependent pathway for immunoglobulin diversification through class switching (29). Thus, in addition to inflammatory responses, a linkage of MHC class II in B cells and MyD88 is beginning to emerge. Although MHC class II molecules are known to serve as signal-transducing receptors (30–32), MHC class II-based recognition of ligands that activate MyD88-mediated pro-inflammatory signaling has not been characterized before. Results from our laboratory demonstrated that MHC class II-SEA or SEB interaction activate MyD88-dependent pro-inflammatory cytokine responses in human primary monocytes and B cells (21). Recently, MHC class II molecules were shown to promote TLR signaling (33–34), thus it is clear now that TLR and MHC-mediated responses both engage MyD88. Earlier reports indicated that, along with an IL-1 response, IFN-γ and TNF-α significantly contribute to toxic shock-induced death (8). In the present study, we observed that MyD88 mimetics are capable of attenuating pro-inflammatory responses, not only by affecting IL-1, but by inhibiting T-cell-derived cytokines such as TNF-α, IFN-γ, and IL-2 and NF-κB activation. This inhibition of cytokine responses by the MyD88-mimetics after SEB stimulation is likely a consequence of MyD88-mediated signal interruption. Our data in SEB-stimulated primary monocytes and transfected HEK 293 cells indicated that Compd1 targets MyD88. Ligand (LPS) induced MyD88-mediated NF-κB-driven cell-based inhibitory SEAP reporter activity data further suggest that Compd1 genuinely targets MyD88. Based on these results, it is likely that, while SEB stimulation up-regulates MyD88-mediated signaling for pro-inflammatory responses in human primary cells and mice, small molecules such as Compd1 that target MyD88, interfere with MyD88-mediated pro-inflammatory signaling. It is important to note that the inhibition of cytokine responses and SEAP reporter activity was observed at similar concentrations. Interestingly, it was also observed that MyD88 downstream signaling components such as IRAK-1, IRAK-4, and TRAF-6 were also affected by Compd1 in a similar manner. Although these results suggest that Compd1 targets MyD88, the precise mode of binding is yet to be determined. It is likely that binding of Compd1 to MyD88 limits the recruitment of MyD88 to receptor complex and affects the downstream signaling for cytokine responses. Taken together, our results suggest that MyD88 represents an intracellular therapeutic target where MyD88 mimetic binding limits the recruitment of MyD88, thereby inhibiting downstream signaling cascades and attenuating pro-inflammatory cytokine responses to SEB exposure. These results are in agreement with our previous observation in mice which demonstrated that MyD88−/− mice were resistant to lethal SEB-induced toxicity due to reduced level of pro-inflammatory cytokine responses.
In summary, our results indicate that several synthetic mimetics structurally similar to the BB-loop in the TIR domain of MyD88 inhibited pro-inflammatory cytokine production in human primary cells. In addition, administration of BB-loop mimetic, such as Compd1, to mice inhibited pro-inflammatory cytokine response and increased survival from toxic shock induced death following lethal SEB challenge. Taken together, these results provide the first proof-of-concept that synthetic BB-loop targeting the TIR-domain of MyD88 limits hyper-inflammation. Thus, use of synthetic mimetics to attenuate MyD88 signaling may provide a potential strategy for treating toxic shock syndrome. An ongoing effort is underway to refine Compd1 by chemical modification to increase its potency and drug-like properties for potential use against toxic shock.
Supplementary Material
Acknowledgments
We thank Thomas Plummer for his help in animal experiments and Lorraine Farinick for figure preparation. Views expressed in this manuscript are those of the authors and do not purport to reflect official policy of the U.S. Government USAMRIID administrators.
This work was supported by the Defense Threat Reduction Agency (to K. U. S.) and the Skaggs Institute.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Peripheral blood mononuclear cells used in this study were obtained from healthy donors with written consents, in accordance with guidelines of the human use committee (HUC) and institutional (USAMRIID) review board-approved research donor protocol FY 05-05.
- TSS
- toxic shock syndrome
- SEB
- Staphylococcal enterotoxin B
- MyD88
- Myeloid differentiation protein 88
- TIR
- Toll-interleukin receptor domain
- LPS
- lipopolysaccharide
- SEAP
- secreted alkaline phosphatase.
REFERENCES
- 1. Scholl P., Diez A., Mourad W., Parsonnet J., Geha R. S., Chatila T. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 4210–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ulrich R. G., Bavari S., Olson M. A. (1995) Trends Microbiol 3, 463–468 [DOI] [PubMed] [Google Scholar]
- 3. Blank C., Luz A., Bendigs S., Erdmann A., Wagner H., Heeg K. (1997) Eur. J. Immunol. 27, 825–833 [DOI] [PubMed] [Google Scholar]
- 4. Miethke T., Duschek K., Wahl C., Heeg K., Wagner H. (1993) Eur. J. Immunol. 23, 1494–1500 [DOI] [PubMed] [Google Scholar]
- 5. Marrack P., Kappler J. (1990) Science 248, 705–711 [DOI] [PubMed] [Google Scholar]
- 6. Vincent J. L., Bihari D. J., Suter P. M., Bruining H. A., White J., Nicolas-Chanoin M. H., Wolff M., Spencer R. C., Hemmer M. (1995) JAMA 274, 639–644 [PubMed] [Google Scholar]
- 7. Richards M. J., Edwards J. R., Culver D. H., Gaynes R. P. (1999) Crit Care Med. 27, 887–892 [DOI] [PubMed] [Google Scholar]
- 8. Stiles B. G., Bavari S., Krakauer T., Ulrich R. G. (1993) Infect Immunity 61, 5333–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beno D. W., Uhing M. R., Masakatsu G., Chen J. V. A., Kimura R. E. (2001) Am. J. Physiol. Gastrointest Liver Physiol. 280, G866-G872 [DOI] [PubMed] [Google Scholar]
- 10. Akira S., Takeda K. (2004) Nat. Rev. Immunol. 4, 499–511 [DOI] [PubMed] [Google Scholar]
- 11. Sun D., Ding A. (2006) Nature Immunol. 7, 375–381 [DOI] [PubMed] [Google Scholar]
- 12. Kissner T. L., Cisney E. D., Ulrich R. G., Fernandez S., Saikh K. U. (2010) Immunology 130, 516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kissner T. L., Ruthel G., Cisney E. D., Ulrich R. G., Fernandez S., Saikh K. U. (2010) Innate Immunity published online 10 August 2010; DOI: 10.1177/1753425910374092 [DOI] [PubMed] [Google Scholar]
- 14. McGettrick A. F., O'Neill L. A. J. (2004) Mol. Immunol. 41, 577–582 [DOI] [PubMed] [Google Scholar]
- 15. Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr (1998) Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 16. Dunne A., O'Neill L. (2003) A Sci. STKE 3, 171. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y., Tao X., Shen B., Horng T., Medzhitov R., Manley J. L., Tong L. (2000) Nature 408, 111–115 [DOI] [PubMed] [Google Scholar]
- 18. Bartfai T., Behrens M. M., Gaidarova S., Pemberton J., Shivanyuk A., Rebek J., Jr. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7971–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saikh K. U., Khan A. S., Kissner T., Ulrich R. G. (2001) Clin. Exp. Immmunol. 126, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saikh K. U., Kissner T. L., Sultana A., Ruthel G., Ulrich R. G. (2004) J. Immunol. 173, 7426–7434 [DOI] [PubMed] [Google Scholar]
- 21. Kissner T. L., Ruthel G., Alam S., Ulrich R. G., Fernandez S., Saikh K. U. (2011) PLoS ONE 6, e15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mills K. H., Dunne A. (2009) Nat. Med. 15, 1363–1364 [DOI] [PubMed] [Google Scholar]
- 23. Burns K., Martinon F., Esslinger C., Pahl H., Schneider P., Bodmer J. L., Di Marco F., French L., Tschopp J. (1998) J. Biol. Chem. 27, 12203–12209 [DOI] [PubMed] [Google Scholar]
- 24. Han K. J, Yang Y., Xu L. G., Shu H. B. (2010) J. Biol. Chem. 285, 12543–12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barr T. A., Brown S., Mastroeni P., Gray D. (2009) J. Immunol. 183, 1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buatois V., Baillet M., Bécart S., Mooney N., Leserman L., Machy P. (2003) J. Immunol. 171, 5812–5819 [DOI] [PubMed] [Google Scholar]
- 27. Jefferies C.A., Doyle S., Brunner C., Dunne A., Brint E., Wietek C., Walch E., Wirth T., O'Neill L. A. (2003) J. Biol. Chem. 278, 26258–26264 [DOI] [PubMed] [Google Scholar]
- 28. Honda K., Yanai H., Mizutani T., Negishi H., Shimada N., Suzuki N., Ohba Y., Takaoka A., Yeh W. C., Taniguchi T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He B., Santamaria R., Xu W., Cols M., Chen K., Puga I., Shan M., Xiong H., Bussel J. B., Chiu A., Puel A., Reichenbach J., Marodi L., Döffinger R., Vasconcelos J., Issekutz A., Krause J., Davies G., Li X., Grimbacher B., Plebani A., Meffre E., Picard C., Cunningham-Rundles C., Casanova J. L., Cerutti A. (2010) Nat. Immunol. 11, 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hauschildt S., Bessler W. G., Scheipers P. (1993) Eur. J. Immunol. 23, 2988–2992 [DOI] [PubMed] [Google Scholar]
- 31. Spertini F., Chatlia T., Geha R. S. (1992) J. Immunol. 149, 65–70 [PubMed] [Google Scholar]
- 32. Faassen A. E., Pierce S. K. (1995) J. Immunol. 155, 1737–1745 [PubMed] [Google Scholar]
- 33. Liu X., Zhan Z., Li D., Xu L., Ma F., Zhang P., Yao H., Cao X. (2011) Nat Immunol. 12, 416–424 [DOI] [PubMed] [Google Scholar]
- 34. Hassan G. S., Mourad W. (2011) Nat. Immunol. 12, 375–376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.