Abstract
Morphine and other opiates mediate their effects through activation of the μ-opioid receptor (MOR), and regulation of the MOR has been shown to critically affect receptor responsiveness. Activation of the MOR results in receptor phosphorylation, β-arrestin recruitment, and internalization. This classical regulatory process can differ, depending on the ligand occupying the receptor. There are two forms of β-arrestin, β-arrestin1 and β-arrestin2 (also known as arrestin2 and arrestin3, respectively); however, most studies have focused on the consequences of recruiting β-arrestin2 specifically. In this study, we examine the different contributions of β-arrestin1- and β-arrestin2-mediated regulation of the MOR by comparing MOR agonists in cells that lack expression of individual or both β-arrestins. Here we show that morphine only recruits β-arrestin2, whereas the MOR-selective enkephalin [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin (DAMGO), recruits either β-arrestin. We show that β-arrestins are required for receptor internalization and that only β-arrestin2 can rescue morphine-induced MOR internalization, whereas either β-arrestin can rescue DAMGO-induced MOR internalization. DAMGO activation of the receptor promotes MOR ubiquitination over time. Interestingly, β-arrestin1 proves to be critical for MOR ubiquitination as modification does not occur in the absence of β-arrestin1 nor when morphine occupies the receptor. Moreover, the selective interactions between the MOR and β-arrestin1 facilitate receptor dephosphorylation, which may play a role in the resensitization of the MOR and thereby contribute to overall development of opioid tolerance.
Keywords: G Protein-coupled Receptors (GPCR), Receptor Desensitization, Receptor Endocytosis, Receptor Regulation, Ubiquitination, Morphine, Receptor Resensitization, Tolerance, Arrestin, Phosphorylation
Introduction
Morphine and other opiates are among the most clinically useful analgesics, and their actions are mediated largely through activation of μ-opioid receptors (MORs).3 As a G protein-coupled receptor (GPCR), the MOR is subject to regulation paradigms that include phosphorylation by GPCR kinases (GRKs) and subsequent interactions with β-arrestins (β-arrestin1, also known as arrestin2, and β-arrestin2, also known as arrestin3). β-Arrestins can then initiate receptor internalization, which in turn can promote both receptor down-regulation and resensitization (1–3). β-Arrestins can also facilitate these regulatory events by scaffolding ubiquitination machinery, such as E3 ligases, to GPCRs, as has been shown for the β2 adrenergic receptor (β2AR), V2 vasopressin receptor, and the chemokine receptor (CXCR4) (4–6), however this has not been demonstrated for the MOR.
MOR regulation has been shown to be contingent upon the particular agonist acting at the receptor as morphine promotes different regulatory events than other opioid ligands, including the d-enkephalin analog, [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin (DAMGO), fentanyl, methadone, and etorphine, although all of these ligands are full agonists at the MOR with respect to G protein coupling (7). The difference between agonists was first recognized when Arden et al. (8) observed that although DAMGO promotes robust internalization of the MOR in HEK-293 cells, morphine treatment fails to do so. Subsequent studies have demonstrated that morphine's attenuated ability to promote MOR internalization is a consequence of morphine being less effective at promoting MOR phosphorylation and β-arrestin recruitment compared with other opioids. In fact, overexpression of GRK2 in cells can augment morphine-induced MOR phosphorylation, β-arrestin2 recruitment, and internalization (3, 9, 10). These data also suggest that in addition to the ligand, MOR regulation and trafficking are also dependent on the complement of intracellular interacting proteins expressed in residence with the receptor.
In contrast to morphine, DAMGO induces robust MOR phosphorylation and β-arrestin recruitment and subsequent MOR trafficking. These agonist-directed differences demonstrate functional selectivity at the MOR, wherein both agonists promote MOR activation but differ in their abilities to promote β-arrestin interactions (1–3, 9, 11–17).
Opioid-induced MOR regulation also differs, at the level of inducing selective interactions with β-arrestin1 and/or β-arrestin2. Using confocal microscopy, it was shown that the MOR may have a higher affinity for β-arrestin2 than β-arrestin1 when activated with the highly potent opioid, etorphine (18). Further microscopy studies have shown that whereas etorphine induces translocation of both β-arrestins to the receptor, morphine appears to only lead to β-arrestin2 recruitment (1). However, these findings are based on the assumption that the two β-arrestin-GFP constructs have an equal opportunity to interact with the activated MOR in the transfected cells therefore, the comparisons are largely qualitative.
Interestingly, agonist- and β-arrestin-dependent differences are also revealed in vivo, wherein mice lacking β-arrestin2 (βarr2-KO) display a myriad of behavioral differences in response to morphine compared with their WT littermates, including enhanced thermal antinociception, reduced antinociceptive tolerance, reduced constipation, reduced signs of withdrawal, enhanced dopamine release, and enhanced reward profiles (1, 19–23). In contrast, whereas morphine's effects are dramatically altered in the βarr2-KO mice, several other agonists, such as etorphine, fentanyl, and methadone, promote similar antinociceptive profiles in both the WT and βarr2-KO mice (1, 19–23). Moreover, both genotypes develop equivalent degrees of tolerance to methadone and fentanyl, whereas morphine tolerance is greatly attenuated in the βarr2-KO mice (23). The lack of differences between the WT and βarr2-KO mice in response to fentanyl and methadone may be a consequence of their ability to promote interactions with β-arrestin1, which may be able to compensate for the absence of β-arrestin2. Supported by the qualitative microscopy studies, it has been suggested that the morphine-bound MOR cannot recruit β-arrestin1, and thus this compensatory regulation cannot occur (1).
In this study, we have asked whether there are consequences to receptor regulation based upon the specific β-arrestin interactions that occur in response to distinct agonists. Here we utilize mouse embryonic fibroblasts (MEFs) that do not express either both β-arrestins (βarr1/2-KO) or individual β-arrestins (βarr1-KO and βarr2-KO MEFs) to assess the ability of morphine and DAMGO, to promote MOR regulation as a function of β-arrestin expression. We show that DAMGO induces recruitment of both β-arrestin1 and β-arrestin2 to MOR and that either β-arrestin is sufficient to promote DAMGO-induced internalization. DAMGO promotes MOR ubiquitination that does not occur in the absence of β-arrestin1. Morphine, however, only promotes interactions between the MOR and β-arrestin2. This interaction is sufficient to promote MOR internalization but not MOR ubiquitination, further implicating β-arrestin1 in facilitating this regulatory event. Moreover, β-arrestin1 significantly contributes to the rate of receptor dephosphorylation following agonist stimulation.
EXPERIMENTAL PROCEDURES
Drugs
DAMGO (Tocris, Ellisville, MO) and morphine (morphine sulfate pentahydrate; Sigma) were dissolved in distilled water to 10 mm. 10 mm stock solutions were diluted in PBS to working concentrations.
cDNA Constructs
The mouse μ-opioid receptor (gi1055230) was tagged on the N terminus with hemagglutinin (HA-MOR); the HA-MOR was tagged on the C terminus with Renilla luciferase (HA-MOR-Rluc) for BRET studies. Rat β-arrestin1 (gi949985) and β-arrestin2 (gi949986) were tagged on the C terminus with enhanced green fluorescent protein (βarr2-GFP and βarr1-GFP) for microscopy studies or with GFP2 for BRET studies (βarr1-GFP2 and βarr2-GFP2). Rat β-arrestin1 and β-arrestin2 were Myc-tagged on the N terminus (Myc-βarr1 and Myc-βarr2) for internalization rescue studies.
Stable Expression of HA-MOR in MEFs
MEFs were transduced with HA-MOR using murine stem cell virus. Transfected cells were selected in the presence of puromycin (1 μg/ml; Calbiochem). A FACS Aria flow cytometer was used to select for high expressing cells (top ∼25%) using an anti-HA AlexaFluor 488 conjugate antibody (1:200; Invitrogen). WT and βarr1/2-KO MEFs were subcloned three times. Saturation binding was used to isolate cell lines of each genotype that express similar amounts of [3H]naloxone binding sites: HA-MOR-WT MEFs (KD = 2.39 ± 0.499 nm; 1302 ± 64.65 fmol/mg membrane protein), HA-MOR βarr1/2-KO MEFs (KD = 2.158 ± 0.448 nm; 1356 ± 103.5 fmol/mg membrane protein), HA-MOR βarr1-KO MEFs (KD = 3.629 ± 1.136 nm; 1203 ± 67.58 fmol/mg membrane protein), and HA-MOR βarr2-KO MEFs (KD = 2.639 ± 0.588 nm; 1595 ± 317.6 fmol/mg membrane protein). Uniform cell surface expression of the HA-MOR under basal conditions was confirmed by confocal microscopy (Fig. 2).
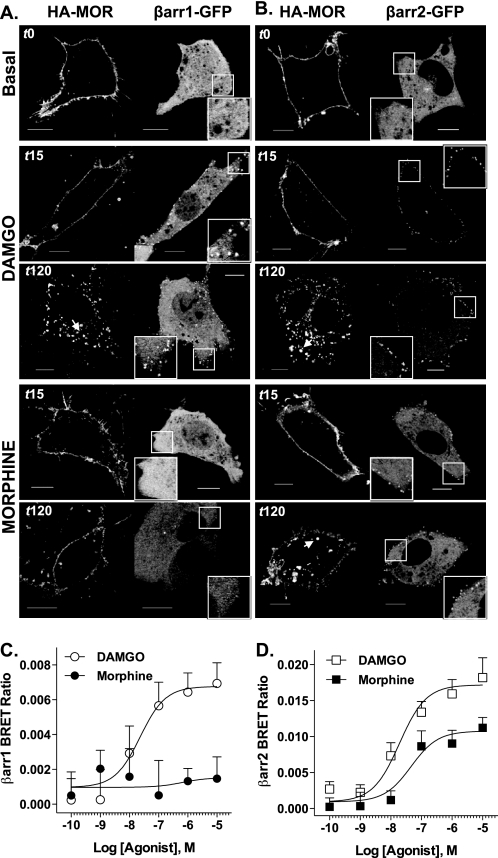
FIGURE 2.
Agonist-induced β-arrestin recruitment. A and B, confocal microscopy. HA-MOR (left side of each panel) and either βarr1-GFP (A) or βarr2-GFP (B) (right side of each panel, puncta; inset, ×2 magnification) expressed in βarr1/2-KO MEFs were treated with DAMGO (1 μm) or morphine (10 μm). Representative confocal images of live cell β-arrestin-GFP translocation observed 15 and 120 min after drug addition are shown from at least three experiments (scale bars, 10 μm). C and D, BRET. HEK-293 cells expressing MOR-Rluc, GRK2, and either βarr1-GFP2 (C) or βarr2-GFP2 (D) were stimulated with opioid agonists for 5 min prior to substrate addition. Graphs show mean ± S.E. (n = 5–8); data were fit to a non-linear regression curve using GraphPad Prism software. C, DAMGO induces a concentration-dependent increase in βarr1-GFP2 BRET with MOR, but morphine does not (nonconvergence). D, both DAMGO and morphine induce concentration-dependent increase of βarr2-GFP2 interactions with the MOR, but morphine is less efficacious (extra sum of squares F test, p = 0.0008).
Radioligand Binding
Membranes of MEFs were prepared using Teflon on glass Dounce homogenization and centrifugation at 20,000 × g at 4 °C for 30 min. Membranes were resuspended in 50 mm Tris, pH 7.4, using Dounce homogenization, and protein content was quantified. Total binding was assessed in 200 μl reactions containing 20 μg of membrane protein and increasing concentrations (0.1–3 nm) of [3H]naloxone (PerkinElmer Life Sciences). Nonspecific binding was determined in the presence of 40 μm 6β-naltrexol. Reactions were incubated at room temperature for 1 h and were terminated by rapid filtration through GF/B filters and washes with ice-cold water. Radioactivity was determined using liquid scintillation counting. Affinity (KD) and receptor number (Bmax) were determined by fitting data to a saturation binding curve using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). Data are provided above.
MOR Phosphorylation
[32P]Phosphate Incorporation
HA-MOR WT and βarr1/2-KO MEFs were incubated in serum- and phosphate-free media containing [32P]phosphate (100 mCi/ml; PerkinElmer) for 1 h, followed by a 10 min treatment with vehicle (PBS), DAMGO (1 μm), or morphine (10 μm). The HA-MOR was immunoprecipitated from cell lysates as described previously (9) and resolved by electrophoresis. Gels were dried and exposed to film (Kodak Biomax, Carestream Health, Rochester, NY) for 3 days at −80 °C. Film was scanned, and bands were quantified using ImageJ (National Institutes of Health).
Western Blotting
MOR phosphorylation was performed as described previously with slight modification (9). Briefly, HA-MOR WT and βarr1/2 KO MEFs were serum-starved for 15 min, followed by a 10-min treatment with vehicle (PBS), DAMGO (1 μm), or morphine (10 μm). The HA-MOR was immunoprecipitated from cell lysates and analyzed by Western blot using antibodies against the MOR phosphorylated at Ser-375 (1:500; Cell Signaling, Danvers, MA) and the C terminus of the MOR (1:1000; Neuromics, Edina, MN). Densitometry was assessed on the prominent ∼75 kDa band using Kodak imaging software, and the level of phosphorylated MOR detected was normalized to the amount of total MOR immunoprecipitated in each sample.
β-Arrestin Translocation
Microscopy
βarr1/2 KO MEFs were transiently transfected with HA-MOR (5 μg) and either βarr1-GFP or βarr2-GFP (2.5 μg) using electroporation and plated on collagen-coated glass coverslips. After incubation at 37 °C for 36 h, cells were incubated with an anti-HA AlexaFluor 594 conjugate antibody in serum-free media for 30 min at 37 °C. Cells were washed with serum-free media, and basal images were obtained. HA-MOR-expressing cells were monitored for βarr-GFP translocation up to 30 min post-drug treatment. Fluorescence was visualized with a ×100 objective on an Olympus Fluoview 300 confocal microscope and Olympus Fluoview imaging software version 4.3.
Bioluminescence Resonance Energy Transfer (BRET)
HEK-293 cells stably overexpressing GRK2 were virally transduced with HA-MOR-Rluc. Cells expressing high levels of HA-MOR-Rluc (top 30% of positive cells) were selected by flow cytometry. Cells were then transiently transfected with either βarr1-GFP2 or βarr2-GFP2 by electroporation. Two days post-transfection, cells were collected in BRET buffer (PBS supplemented with 0.1 g/liter CaCl2, 0.1 g/liter MgCl2, and 1 g/liter glucose), and 100,000 cells (in 40 μl of buffer) were added to each well of a white 96-well OptiPlate (PerkinElmer Life Sciences). Cells were treated with 5 μl of varying concentrations of drug and allowed to incubate at 37 °C for 5 min, followed by the addition of 5 μl of coelenterazine 400A (Biotium, Hayward, CA; final concentration of 5 μm) (also known as DeepBlueC from Packard Bioscience). Luminescence readings were taken at 515 and 395 nm using an Envision plate reader. The BRET ratio equals 510 nm/395 nm. Background signal (cells not expressing βarr1/2-GFP2) was subtracted from all ratios. Ratios were then normalized to vehicle responses. Agonist-induced interactions between MOR and β-arrestins were detected as an increase in normalized BRET ratio. Data were fit to a dose-response curve using GraphPad Prism.
MOR Internalization
Immunofluorescence
Internalization was assessed in HA- MOR WT and βarr1/2-KO MEFs. Cells were serum-starved for 30 min, followed by drug treatment for 2 h. Cells were fixed (1:1 methanol/acetone, 20 min, −20 °C) and permeabilized in PBS+ (PBS with 5% goat serum, 0.3% Triton X-100, and 0.02% sodium azide, 30 min, room temperature). HA-MOR was labeled using an anti-HA AlexaFluor 488 conjugate antibody (1:200). Immunostaining was visualized and imaged as described above.
Cell Surface Biotinylation
Internalization was quantified by a cell surface biotinylation assay, which was performed as described previously, with slight modification (9). HA-MOR WT and βarr1/2 KO MEFs were serum-starved for 30 min prior to biotinylation and then treated with vehicle, DAMGO (1 μm), or morphine (10 μm) for 2 h at 37 °C. Protected biotinylated proteins were precipitated from cell lysates (1000 μg) with Avidin beads (40 μl; Pierce) and analyzed by Western blot using an antibody against the C terminus of the MOR (1:1000; Neuromics).
β-Arrestin Rescue of MOR Internalization
Immunofluorescence
HA-MOR βarr1/2-KO MEFs were transiently transfected with Myc-βarr1 (1 μg) or Myc-βarr2 (10 μg) using electroporation. After incubation at 37 °C for 36 h, cells were serum-starved for 30 min at 37 °C followed by treatment with vehicle (PBS), DAMGO (1 μm), or morphine (10 μm) for 2 h at 37 °C. Cells were fixed and permeabilized as above. Cells were incubated with three antibodies in PBS+ in the following order, with several PBS+ washes after each antibody: anti-Myc (1:100, 4 °C, overnight; Clontech, Mountain View, CA), goat anti-mouse AlexaFluor 568 conjugate (1:2000, room temperature, 2 h; Invitrogen), and anti-HA AlexaFluor 488 conjugate (1:100, 4 °C, overnight; Invitrogen). Immunostaining was visualized and imaged as described above. HA-MOR internalization was assessed only in cells expressing Myc-β-arrestin.
Biotinylation
HA-MOR βarr1/2-KO MEFs were transiently transfected with Myc-βarr1 (1 μg) or Myc-βarr2 (10 μg) using electroporation because these amounts yield similar protein expression levels. Biotinylation experiments were performed 2 days post-transfection as described above. Immunostaining of parallel samples was used to verify ≥20% transfection efficiency was obtained, and Myc expression in total lysates was determined by Western blot using antibodies against Myc (1:500, 4 °C, overnight; Clontech).
MOR Ubiquitination
MEFs were treated with drug for the times indicated. Cells were washed twice with PBS on ice and collected in lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 0.1% SDS, 10% glycerol, 1% Nonidet P-40, 0.5% deoxycholate, 10 mm sodium orthovanadate, 10 mm NaF, protease inhibitor mixture (Roche Applied Science), and 10 mm N-ethylmaleimide. Lysates were homogenized on a rotator for at least 20 min at 4 °C and cleared at 12,000 × g for 30 min at 4 °C. Equal amounts of protein (1000 μg) were added to 25 μl of anti-HA-agarose conjugate and incubated overnight on a rotator at 4 °C. Precipitate was washed four times in PBS, and proteins were eluted in 35 μl of 1× XT sample buffer (Bio-Rad) with 1.25% β-mercaptoethanol at 55 °C for 30 min. 30 μl of supernatant was removed and boiled at 100 °C for 3 min. 25 μl of precipitated proteins were resolved on 4–12% BisTris NuPAGE gels (Invitrogen) and transferred to PVDF membranes (Immobilon-P; Millipore, Billerica, MA). Blots were blocked with 5% milk in TBST and incubated simultaneously with an antibody against ubiquitin (P4D1, 1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and an antibody against the C terminus of the MOR (1:4500; Neuromics) overnight at 4 °C. Blots were washed in TBST and incubated simultaneously with a goat anti-rabbit IRDye 680 conjugate (1:15000; LI-COR, Lincoln, NE) and goat anti-mouse IRDye 800 conjugate (1:15000; LI-COR) in 5% milk for 1 h at room temperature protected from light. Signal was detected using the LI-COR Odyssey imager. Band intensity was determined using Odyssey software version 1.2. Ubiquitin signal was normalized to the total receptor per lane.
MOR Dephosphorylation
MEFs were serum-starved for 15 min and treated with DAMGO (1 μm) or morphine (10 μm) for 30 min at 37 °C. Cells were washed three times with PBS and incubated in the absence of drug for the indicated times (washout). MOR phosphorylation was determined as described above. The extent of dephosphorylation was determined by first normalizing the phospho-MOR to the total receptor immunoprecipitated, and then each time point post-washout was normalized to the phospho-MOR levels 30 min post-agonist addition without washout. Representative Western blots are shown, and any alterations to enhance brightness or contrast were applied to the entire gel image.
Graphical Modifications to Images
All immunoblots presented have been digitally enhanced to optimize contrast, and the optimization has been uniformly applied to the entire gel image, wherein control samples have been included. Microscopy images have also been optimized to reveal contrast, and all modifications have been uniformly applied within a figure such that all red images received the same adjustments, and all green images received the same adjustments within a particular figure. The magnified image insets have been subject to the same modifications as the parent image.
Statistical Analysis
Statistical tests are indicated in the figure legends and were performed using GraphPad Prism 5.0 software.
RESULTS
Generation of MOR-expressing MEF Lines
To determine the role that β-arrestins play in the agonist-directed regulation of the MOR, we stably transfected WT, βarr1/2-KO, βarr1-KO, and βarr2-KO MEFs with the HA-MOR. MEF lines expressing similar levels of the HA-MOR were identified by [3H]naloxone binding (data are presented under “Experimental Procedures”).
Agonist-induced HA-MOR Phosphorylation in WT and βarr1/2-KO MEFs
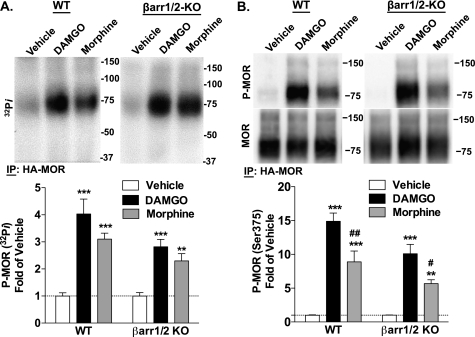
Because MOR phosphorylation can determine β-arrestin interactions, we first assessed whether the absence of β-arrestins impacts upon agonist-induced MOR phosphorylation. DAMGO is generally more potent than morphine in most assays, and therefore a higher concentration of morphine was used for comparison (10 μm morphine versus 1 μm DAMGO). In the HA-MOR WT and βarr1/2-KO MEFs, both DAMGO and morphine induced phosphorylation of MOR (Fig. 1A). Although a trend for more robust phosphorylation with DAMGO than morphine is observed, these results did not reach statistical significance. This whole cell phosphorylation assay may represent the phosphorylation of at least 12 serine and threonine residues in the C-terminal tail and intracellular loops of the MOR (24–27). Several studies have shown that the specific pattern of phosphorylation of these residues depends on the agonist used (10, 24–26, 28, 29). Importantly, Ser-375 has been correlated with regulatory events in which β-arrestins have been implicated, including receptor internalization and desensitization (10, 26). Therefore, we assessed MOR phosphorylation at Ser-375 using a phospho-specific antibody. Both the phospho-MOR and total MOR are detected as a primary band at >75 kDa and a less intense band at ∼150 kDa, which may represent the glycosylated monomer and dimer, respectively (30–32). In the HA-MOR WT and βarr1/2-KO MEFs, both DAMGO and morphine induced phosphorylation of MOR at Ser-375 (Fig. 1B). Consistent with previous observations in other heterologous expression systems (3, 10), morphine induced significantly less robust phosphorylation of this residue than DAMGO regardless of genotype, thus demonstrating that the agonist-dependent differences in MOR phosphorylation at Ser-375 are preserved in the absence of β-arrestins.
FIGURE 1.
Agonist-induced HA-MOR phosphorylation in WT and βarr1/2-KO MEFs. HA-MOR WT and βarr1/2-KO MEFs were treated with vehicle, DAMGO (1 μm), or morphine (10 μm) for 10 min prior to immunoprecipitation (IP) of the HA-MOR. A, whole cell phosphorylation was determined by [32P]phosphate incorporation. Top, representative autoradiographs. Bottom, densitometric analysis of four experiments shows mean ± S.E. (error bars). DAMGO and morphine induced phosphorylation in both genotypes (one-way ANOVA, p < 0.0001; Bonferroni's post-test, p < 0.001 (***) and p < 0.01 (**) for agonist treatment versus vehicle of the same genotype; n = 8). B, phosphorylation at Ser-375 was detected by Western blotting. Top, representative immunoblots. Bottom, densitometric analysis of seven experiments shows mean ± S.E. of phospho-MOR (P-MOR) normalized to total MOR. DAMGO and morphine induce phosphorylation at Ser-375 in both genotypes, and morphine induces less robust phosphorylation than DAMGO in both genotypes (one-way ANOVA, p < 0.0001; Bonferroni's post-test, p < 0.001 (***) and p < 0.01 (**) for agonist treatment versus vehicle of the same genotype, p < 0.05 (#) and p < 0.01 (##) versus DAMGO of the same genotype; n = 8–9).
Agonist-induced β-Arrestin Recruitment
Receptor phosphorylation has been shown to enhance GPCR-β-arrestin interaction affinities (1, 3, 9, 10, 33). To ascertain whether different agonists impart preference for recruiting individual β-arrestins to the receptor, βarr1/2-KO MEFs were transiently transfected with HA-MOR and either βarr1-GFP or βarr2-GFP, and β-arrestin translocation was determined by confocal microscopy (Fig. 2, A and B). In the absence of agonist, βarr1-GFP expression was localized in the cytosol and nucleus, whereas βarr2-GFP expression was primarily confined to the cytosol; HA-MOR was expressed primarily on the plasma membrane, as assessed by immunolabeling. DAMGO induced visible translocation of both βarr2-GFP and βarr1-GFP, as visualized by green puncta localized at the plasma membrane. Although morphine promotes detectable translocation of βarr2-GFP, βarr1-GFP translocation was not observed up to 120 min post-drug treatment. Importantly, the images in Fig. 2 were taken 15 min after drug addition, a time at which the MOR remains primarily localized to the plasma membrane. At later time points (up to 120 min), we observe internalization of the MOR, but the translocated β-arrestin (puncta) remains at the membrane and does not internalize with the MOR, consistent with its classification as a Class A GPCR (18). The transient interaction between β-arrestins and the MOR may be involved in determining early protein complex associations with the MOR that ultimately determine receptor fate.
To obtain a quantitative assessment of DAMGO and morphine-induced interactions between the MOR and β-arrestins, a BRET assay was employed (Fig. 2, C and D). BRET assays were performed in the presence of overexpressed GRK2 because this kinase has been shown to facilitate opioid-induced β-arrestin recruitment (1, 3, 9). HEK-293 cells stably expressing GRK2 and HA-MOR-Rluc were transiently transfected with either βarr1-GFP2 or βarr2-GFP2, and agonist-induced β-arrestin-MOR interactions are reported as an increase in the BRET ratio. DAMGO and morphine promoted robust interactions between MOR and β-arrestin2 with similar potencies (EC50 (log EC50 ± S.E.)): DAMGO, 19.2 nm (−7.72 ± 0.26); morphine, 46.1 nm (−7.34 ± 0.42) (p > 0.05, F(1–72) = 0.743, extra sum of squares F test, n = 5–8). Morphine, however, was significantly less efficacious at promoting β-arrestin2 interactions when compared with DAMGO (Emax ± S.E.): DAMGO, 0.0172 ± 0.00102; morphine, 0.0108 ± 0.00116 (p = 0.0008, F(1,72) = 12.4, extra sum of squares F test, n = 5–8; Fig. 2D). Interestingly, whereas DAMGO promoted β-arrestin1 interactions with the MOR (EC50 (logEC50 ± S.E.): DAMGO, 23.2 nm (−7.63 ± 0.274), n = 5–8), morphine was unable to induce this interaction, even in the presence of GRK2 overexpression (morphine: not converged, R2 = −0.2; Fig. 2C). Collectively, the microscopy and BRET studies demonstrate that whereas DAMGO recruits both β-arrestin1 and β-arrestin2 to the MOR, morphine selectively induces interactions between the receptor and β-arrestin2 (1) .
Agonist-induced MOR Trafficking in HA-MOR WT and βarr1/2-KO MEFs
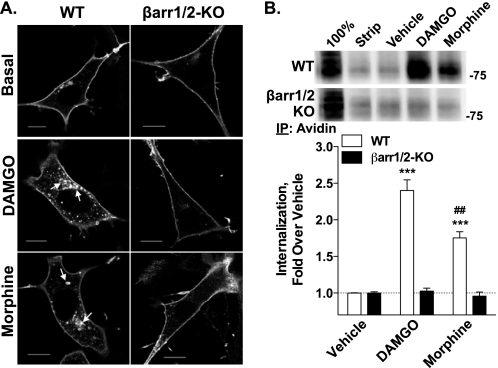
β-Arrestins can promote GPCR internalization by scaffolding the receptor to internalization machinery (33–38). Therefore, we asked whether MORs could be internalized in the absence of β-arrestins. DAMGO and, to a lesser extent, morphine, induced internalization of MORs in WT MEFs, but neither drug induced receptor internalization in the βarr1/2-KO MEFs, as shown by confocal microscopy (Fig. 3A). These observations were confirmed and quantified using a cell surface biotinylation assay, wherein both agonists caused receptor internalization in WT MEFs, although DAMGO internalizes the MOR to a greater degree than morphine. Again, MOR internalization was not observed with either agonist in the β-arrestin null MEFs (Fig. 3B).
FIGURE 3.
Agonist-induced MOR trafficking in HA-MOR WT and βarr1/2-KO MEFs. A, internalization of HA-MOR was determined with an anti-HA antibody following agonist incubation (2 h). DAMGO (1 μm) and morphine (10 μm) lead to an increase in intracellular receptor staining only in the WT MEFs (arrows). In βarr1/2-KO MEFs, the HA-MOR remains on the cell surface after treatment with either drug. Representative images are shown from at least three experiments (scale bars, 10 μm). B, agonist-induced HA-MOR internalization was quantified in HA-MOR WT and βarr1/2-KO MEFs using a cell surface biotinylation assay. Representative immunoblots are shown. Controls for protein biotinylation (100%; cells were not incubated in stripping buffer) and stripping (Strip; cells were biotinylated, stripped, and lysed without drug treatment) are included. Densitometric analysis of seven biotinylation experiments done in duplicate shows the mean ± S.E. (error bars) of internalized HA-MOR. DAMGO and morphine induce internalization in WT MEFs (***, p < 0.001 versus vehicle; Student's t test; n = 8–9), but not in the βarr1/2 KO MEFs (p > 0.05; Student's t test; n = 10–14). Morphine induces significantly less MOR internalization than DAMGO in the WT MEFs (##, p < 0.01; Student's t test; n = 7–8). IP, immunoprecipitation.
β-Arrestin Rescue of HA-MOR Internalization in βarr1/2-KO MEFs
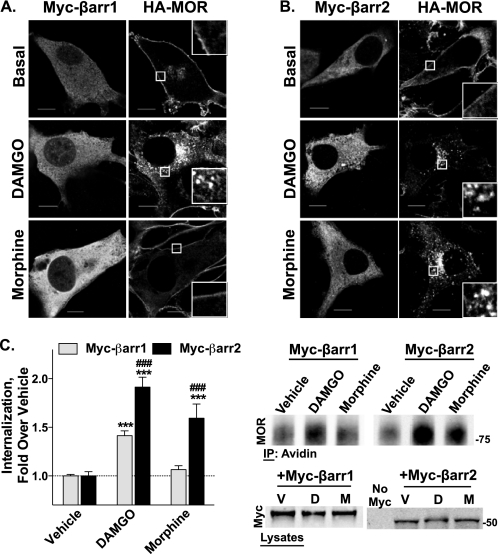
To determine the role of each β-arrestin in agonist-induced MOR internalization, we assessed the ability of individual Myc-tagged β-arrestins to rescue MOR internalization in the βarr1/2-KO MEFs. Transfection of Myc-βarr2 rescued the abilities of both DAMGO and morphine to induce MOR internalization in β-arrestin null MEFs, as shown by confocal microscopy. In contrast, transfection of Myc-βarr1 only restored DAMGO-induced internalization (Fig. 4, A and B). These effects were quantitated using the cell surface biotinylation assay, which recapitulates the effects observed with microscopy, wherein DAMGO is able to internalize the MOR when either β-arrestin is transfected, but only transfection of Myc-βarr2 rescues morphine-induced internalization (Fig. 4C).
FIGURE 4.
β-Arrestin rescue of HA-MOR internalization in βarr1/2-KO MEFs. HA-MOR βarr1/2-KO MEFs were transiently transfected with either Myc-βarr1 or Myc-βarr2 and treated with DAMGO (1 μm) or morphine (10 μm) for 2 h. A and B, confocal microscopy. Receptor internalization was detected by anti-HA immunocytochemistry following drug treatment. Myc-β-arrestins are labeled with an anti-Myc antibody, followed by an anti-mouse secondary AlexaFluor 568 conjugate (fluorescence on the left side of each panel). HA-MOR is labeled using anti-HA AlexaFluor 488 conjugate (right side of each panel; inset, ×4 magnification). A, expression of Myc-βarr1 rescues HA-MOR internalization only by DAMGO and not morphine. B, expression of Myc-βarr2 rescues both DAMGO- and morphine-induced HA-MOR internalization. Representative images are shown from at least three experiments (scale bars, 10 μm). C, biotinylation. Agonist-induced HA-MOR internalization was quantified using cell surface biotinylation assays. Densitometric analysis of three experiments done in duplicate or triplicate shows the mean ± S.E. (error bars) of internalized HA-MOR. Expression of Myc-βarr2 rescues both DAMGO- and morphine-induced internalization, whereas Myc-βarr1 transfection rescues only DAMGO-induced HA-MOR internalization (one-way ANOVA, p < 0.0001; Bonferroni's multiple comparison test, p < 0.001 (***) versus vehicle of the same transfection and p < 0.001 (###) versus Myc-βarr1 with the same treatment; n = 4–8). Representative immunoblots of biotin pull-downs and Myc transfections are shown.
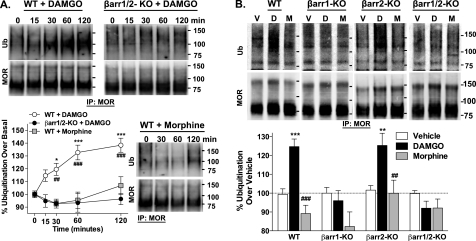
Agonist-induced MOR Ubiquitination
Trafficking of GPCRs can serve as a means to compartmentalize signaling scaffolds, and the association of β-arrestins can determine the inclusion of certain proteins within the receptor complex (39, 40). By acting to scaffold E3 ubiquitin ligases, β-arrestins have been shown to play a critical role in agonist-induced ubiquitination of several GPCRs (41). Therefore, we asked whether the MOR was ubiquitinated in response to agonist treatment and whether this ubiquitination was dependent upon the presence of a particular β-arrestin. We found that DAMGO promoted MOR ubiquitination in a time-dependent manner in WT MEFs that peaked 1 h after treatment and persisted to at least 2 h; however, no ubiquitination was observed in the absence of β-arrestins, indicating that this event is dependent on β-arrestins (Fig. 5A). Moreover, morphine did not promote MOR ubiquitination in the WT MEFs.
FIGURE 5.
Agonist-induced MOR ubiquitination. The HA-MOR was immunoprecipitated from cell lysates of WT and β-arrestin KO MEFs treated with DAMGO (1 μm) or morphine (10 μm) for the times indicated. Ubiquitination was detected by Western blotting. Representative immunoblots, as well as densitometric analysis of ubiquitinated MOR normalized to total MOR and then expressed as changes in regard to basal levels, are shown (mean ± S.E., from eight separate experiments, n = 3–29 for each data point). A, comparison of agonists in WT and βarr1/2 KO MEFs. In WT MEFs, DAMGO induces a time-dependent increase in MOR ubiquitination, whereas morphine does not (one-way ANOVA; DAMGO, p < 0.0001; morphine, p > 0.05), and the time courses differ significantly (two-way ANOVA; WT DAMGO versus WT morphine; p < 0.01 for time, p < 0.001 for drug, and p < 0.01 for the interaction; Bonferroni's post-test, p < 0.05 (*) and p < 0.001 (***)). DAMGO is unable to promote MOR ubiquitination in the βarr1/2-KO MEFs (one-way ANOVA: p > 0.05), and the DAMGO time courses differ significantly between genotypes (two-way ANOVA, WT DAMGO versus KO DAMGO, p < 0.01 for time, p < 0.001 for genotype, and p < 0.001 for the interaction; Bonferroni's post-test, p < 0.01 (##) and p < 0.001 (###)). B, comparison of DAMGO (1 μm) and morphine (10 μm) effects in WT, βarr1-KO, βarr2-KO, and βarr1/2-KO MEFs (60-min treatment). Representative immunoblots are shown, and densitometric analysis was performed as above (mean ± S.E. (error bars) from at least three separate experiments performed in triplicate, n = 9–23 per condition). In WT and βarr2-KO MEFs, only DAMGO induces MOR ubiquitination (one-way ANOVA, p < 0.0001 for WT and p < 0.01 for βarr2-KO; Bonferroni's multiple comparisons test, p < 0.001 (***) and p < 0.01 (**) versus vehicle of the same genotype; p < 0.001 (###) and p < 0.01 (##) versus DAMGO of the same genotype). For the βarr1-KO and βarr1/2-KO MEFs, agonist-induced MOR ubiquitination is not observed (one-way ANOVA, p > 0.05). IP, immunoprecipitation.
Using MEFs that lack expression of each individual β-arrestin (βarr1-KO or βarr2-KO), we assessed the role of each β-arrestin in agonist-induced MOR ubiquitination. The deletion of β-arrestin2 did not disrupt DAMGO-induced ubiquitination of MOR. However, DAMGO did not promote MOR ubiquitination in the absence of β-arrestin1. Interestingly, morphine did not induce MOR ubiquitination in any of the cell lines (Fig. 5B). Because morphine does not promote β-arrestin1 recruitment, these findings suggest that β-arrestin1, and not β-arrestin2, plays a critical role in agonist-induced ubiquitination of the MOR.
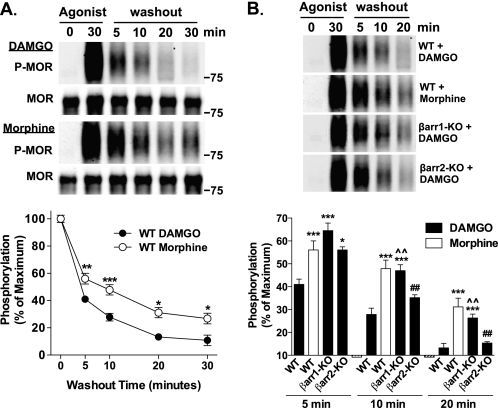
Dephosphorylation of the HA-MOR at Ser-375 following Removal of Agonist
Internalization of a GPCR can represent a point at which the receptor is destined for recycling or degradation, and ubiquitination may play a role in this divergence (42). Because β-arrestin1 is essential for MOR ubiquitination, we asked whether it impacts receptor dephosphorylation, an early indicator of receptor recycling. WT and β-arrestin null MEFs stably expressing the HA-MOR were treated for 30 min with DAMGO or morphine to induce peak MOR phosphorylation (as determined by time course studies; data not shown). Cells were then incubated in the absence of agonist for the indicated times to allow for the dephosphorylation of the receptor. MOR phosphorylation at Ser-375 remaining after this “washout” period was measured. In the WT MEFs treated with DAMGO, MOR was almost fully dephosphorylated 20 min after the removal of drug. In contrast, morphine-induced MOR phosphorylation was more evident at all time points compared with DAMGO, suggesting that the rate of dephosphorylation of the morphine-stimulated MOR is delayed compared with DAMGO (Fig. 6A).
FIGURE 6.
Dephosphorylation of the HA-MOR at Ser-375 following the removal of agonist. WT and β-arrestin KO MEFs were treated with DAMGO (1 μm) or morphine (10 μm) for 30 min. Following drug washout at the indicated time points, HA-MOR was immunoprecipitated, and the extent of MOR phosphorylation was determined by Western blotting. Representative immunoblots are shown; densitometric analysis includes normalization of phosphorylated MOR (P-MOR) over total MOR (MOR), expressed as the percentage of maximal phosphorylated MOR levels observed after 30 min of agonist treatment. (In B, total MOR blots are not shown for brevity). A, comparison of agonists between WT and βarr1/2 KO MEFs. In the WT MEFs, washout after DAMGO treatment results in a time-dependent loss of P-MOR detection, with levels approaching basal detection at 20 min. Although washout after morphine treatment also induces a time-dependent loss in phosphorylated MOR detection, it is not as robust as compared with that seen with DAMGO (two-way ANOVA, DAMGO versus morphine, p < 0.0001 for drug, p < 0.0001 for time, and p < 0.01 for the interaction; Bonferroni's post-test, p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***), n = 9–18). B, comparison of the contribution of individual β-arrestins to the extent of dephosphorylation over time. βarr1-KO MEFs treated with DAMGO resemble WT MEFs treated with morphine because both treatments result in significantly more phosphorylated MOR remaining after washout compared with WT MEFs treated with DAMGO. Further, DAMGO washout results in equivalent amounts of phosphorylated MOR remaining between WT and βarr2-KO MEFs at 10 and 20 min, yet phosphorylated MOR levels were higher in βarr2 KO MEFs at 5 min (one-way ANOVA for each time point, p < 0.0001; Tukey's multiple comparison test within each time point, p < 0.05 (*) and p < 0.001 (***) versus WT DAMGO; p < 0.01 (##) versus WT morphine; and p < 0.01 (^^) versus βarr2-KO DAMGO). Error bars, S.E.
To determine the contributions of individual β-arrestins to MOR dephosphorylation, the HA-MOR-expressing βarr1-KO and βarr2-KO MEFs were evaluated in response to DAMGO. MOR dephosphorylation in WT MEFs following removal of either DAMGO or morphine was run in parallel for comparison. In the absence of β-arrestin2, DAMGO-induced MOR phosphorylation approached base-line levels at 20 min after agonist washout, which is similar to what was observed in WT MEFs treated with DAMGO. In the absence of β-arrestin1, however, DAMGO-induced MOR phosphorylation remained higher compared with DAMGO-treated WT MEFs at all time points (Fig. 6B). Interestingly, in the absence of β-arrestin1, the response profile following DAMGO washout resembles that observed for the morphine-treated WT MEFs. These studies suggest that interactions with β-arrestin1 more significantly impact the dephosphorylation state of MOR that occurs following the removal of agonist.
DISCUSSION
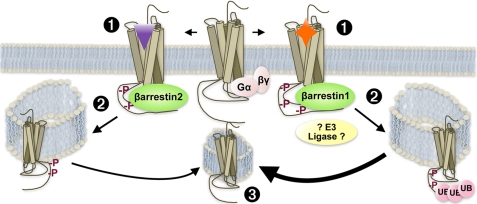
In this report, we show for the first time quantitative evidence that morphine, even in the presence of excess GRK2, exclusively leads to MOR recruitment of β-arrestin2 and not β-arrestin1, whereas DAMGO promotes recruitment of both β-arrestins (Fig. 2). Further, we show that β-arrestins are required for MOR internalization (Fig. 3) and that either β-arrestin isoform can facilitate this event if the agonist is capable of promoting receptor interactions with that particular β-arrestin (Fig. 4). We also demonstrate for the first time that β-arrestins are necessary for promoting MOR ubiquitination and, whereas either β-arrestin can restore MOR internalization, β-arrestin1 selectively mediates MOR ubiquitination. As such, morphine, which does not recruit β-arrestin1, does not promote MOR ubiquitination (Fig. 5). Finally, we provide evidence that β-arrestin1 promotes the dephosphorylation of the MOR because this event is delayed when β-arrestin1 is absent. Further, the MOR is dephosphorylated at a slower rate after morphine treatment, which does not induce β-arrestin1 recruitment, than after DAMGO treatment, which does promote β-arrestin1 interactions with the MOR (Fig. 6). These studies suggest that the ubiquitination of the MOR may be important for promoting receptor dephosphorylation because β-arrestin1, which selectively facilitates MOR ubiquitination, is also involved in promoting MOR dephosphorylation following activation. A summary of results is provided in Fig. 7.
FIGURE 7.
The ability of a ligand to recruit different β-arrestins to the MOR impacts regulation of the receptor. 1, the nature of the agonist determines the interaction with a particular β-arrestin. In this study, we have shown that morphine preferentially induces interactions with β-arrestin2, whereas DAMGO promotes recruitment of both β-arrestins. 2, the particular β-arrestin determines receptor fate. In this study, we show that the morphine occupied MOR can functionally utilize β-arrestin2 but not β-arrestin1 to induce receptor internalization, whereas DAMGO can promote MOR internalization via either β-arrestin. Further, recruitment of β-arrestin1 is necessary for receptor ubiquitination (UB), which may involve β-arrestin1-mediated recruitment of an E3 ligase (although this has not been demonstrated in this study). 3, while the receptor can be dephosphorylated following agonist washout independent of the agonist, the nature of the β-arrestin appears to influence the rate of dephosphorylation whereby β-arrestin1 interactions positively influence the rate of MOR dephosphorylation (thicker arrow), presumably via stabilizing recruitment of phosphatases.
Agonists that promote robust MOR phosphorylation, such as DAMGO, have previously been shown to promote robust β-arrestin2 recruitment (1, 3, 9, 18, 43). Morphine-induced β-arrestin2 recruitment has been much more difficult to assess and can only be visualized with GRK2 overexpression in HEK-293 cells or in the absence of competing endogenous β-arrestins, as shown in β-arrestin null MEFs (1) (Fig. 2). On the other hand, β-arrestin1 interactions with MOR have received much less attention. Previously, βarr1-GFP was shown to interact with MOR upon etorphine but not morphine stimulation in β-arrestin null MEFs, as assessed by confocal microscopy (1). The current study shows that, like etorphine, DAMGO stimulation leads to βarr1-GFP recruitment, but morphine does not; importantly, these findings have been quantitated using BRET in HEK-293 cells overexpressing GRK2 (Fig. 2, C and D). GRK2 overexpression has been shown to enhance MOR phosphorylation and to enhance β-arrestin2 associations with the receptor (1, 3). Therefore, our data demonstrate that morphine does not induce β-arrestin1 interactions with the MOR, even in a cellular environment that has been biased to facilitate such interactions. Taken together, our data suggest that the morphine-bound MOR is a poor substrate for β-arrestin1 interactions, even in the presence of GRK2.
Morphine-induced MOR regulation seems to be highly dependent on the intracellular environment in which the MOR is expressed. Many cell culture studies have manipulated kinase or β-arrestin expression to alter morphine-induced MOR trafficking. In neurons, Haberstock-Debic et al. (44, 45) showed that morphine-induced MOR trafficking differs between neurons and even within different regions of the same neuron. MOR trafficking was observed in the dendrites of nucleus accumbens neurons but not in the soma of the same neurons (45). It is attractive to postulate that the expression of accessory proteins (perhaps GRKs) may differ between these regions and may result in differential MOR regulation. Indeed, the most robust example of the sensitivity of the morphine-bound MOR to expression of regulatory proteins is the dramatically altered morphine-induced behavioral profile in the βarr2-KO mice (19–22). Other opioids, which promote robust MOR phosphorylation, such as DAMGO and methadone, may promote a receptor conformation that has high affinity for GRKs and both β-arrestins. Therefore, such opioids may not be as sensitive to the specific complement of intracellular proteins.
β-Arrestins have been shown to act as scaffolds for both internalization and ubiquitination machinery (41, 46). Here, we observed that in some cases, either β-arrestin can substitute for certain regulatory events, such as internalization, given that the agonist can promote recruitment of either β-arrestin. However, agonist-induced ubiquitination of MOR is dependent on β-arrestin1. The preference for a particular β-arrestin in mediating agonist-induced GPCR regulation has been observed previously. The β2AR and the V2 vasopressin receptors have been shown to utilize β-arrestin2 to promote receptor ubiquitination (5, 6, 47), whereas the chemokine receptor CXCR4 and insulin-like growth factor-1 receptor (IGF-1R) have been shown to preferentially utilize β-arrestin1 to promote receptor ubiquitination (4, 48). An attractive hypothesis is that β-arrestins promote receptor ubiquitination by serving as adaptors between the receptors and E3 ligases, which are enzymes responsible for conjugating ubiquitin moieties to proteins, thus facilitating receptor ubiquitination. The identity of the E3 ligase(s) utilized for the MOR, however, remains undetermined. In the case of the well studied β2AR, somewhat contradictory observations have been reported. In reconstitution assays, Mdm2 was shown to ubiquitinate the β2AR, and β-arrestin2 was required for this event (5). Additional studies have also implicated NEDD4 as an E3 ligase for the β2AR, and NEDD4 has been shown to interact with the receptor in a β-arrestin-dependent (47) and β-arrestin-independent manner (49). Although these varying results may be due to differences in the assay design, they may also suggest that multiple E3 ligases may be able to ubiquitinate the same receptor. Therefore, studies conducted in cell culture systems (including this one) must be interpreted with caution. The identification of the particular E3 ligase involved in MOR regulation may best be concluded in vivo.
Ubiquitination is thought to mediate trafficking of receptors to the lysosome for degradation (41, 50). Our studies showing that DAMGO, but not morphine, induces ubiquitination of the MOR could indicate that the DAMGO-bound receptor is then degraded, whereas the morphine-bound receptor is not. This would be in agreement with several studies that have observed that chronic treatment with morphine does not cause MOR down-regulation in whole mouse brain or brainstem (19, 51–53), which may be due to morphine's inability to promote MOR ubiquitination.
Although endocytic trafficking can lead to receptor degradation, it can also facilitate receptor recycling or resensitization. MOR dephosphorylation was measured as an early indicator of MOR resensitization; however, functional resensitization of the receptor was not tested, and therefore the results must be interpreted with caution. Further, MOR phosphorylation at Ser-375 was used to assess the state of dephosphorylation in these studies for several reasons. First, this residue has been shown to be important for MOR internalization and desensitization, and both of these events are known to also involve β-arrestins (10, 26). Second, morphine-promoted MOR phosphorylation requires this residue (10). Finally, the whole cell phosphorylation results closely mirrored the Ser-375 phosphorylation results (Fig. 1). By assessing the phosphorylation state of Ser-375 of the MOR following removal of agonist over time, our data suggest that β-arrestin1 may play a significant role in MOR dephosphorylation following DAMGO stimulation. Dephosphorylation following morphine treatment was delayed, which is in agreement with an earlier report (9). Interestingly, the dephosphorylation profile obtained following DAMGO in the absence of β-arrestin1 highly resembled that obtained with morphine, an agonist that does not induce MOR-β-arrestin1 interactions. This suggests that the differences in MOR dephosphorylation rate are due to the agonist's ability to recruit β-arrestin1 to the receptor. Importantly, the possible continued phosphorylation of other residues cannot be discounted, including threonines 180 and 394, because both of these residues have been shown to be important for DAMGO-induced MOR desensitization (24, 25). It is possible that the collective pattern of MOR phosphorylation will be critical in the overall functional state of the MOR.
It remains to be determined whether the β-arrestin1-mediated ubiquitination of the receptor facilitates its dephosphorylation and whether dephosphorylation correlates with MOR resensitization. Further, although β-arrestin2 does not contribute to MOR ubiquitination, its role in dephosphorylation cannot be eliminated because we find that its absence delays the dephosphorylation of MOR at the early time point (5 min following washout of DAMGO) in comparison with WT MEFs (Fig. 6B). Previous reports have also demonstrated that β-arrestin2 is critical for the desensitization of the MOR because DAMGO-induced G protein coupling in brainstem and PAG is enhanced in the absence of β-arrestin2 (19, 21). Because dephosphorylation may be an initial step in MOR resensitization, and the rate of receptor resensitization will contribute to the overall state of desensitization, the two components, both influenced by β-arrestins, may play a role in determining opioid responsiveness in vivo.
Recently, different clinically used opioids were evaluated for their propensity to induce antinociceptive tolerance in mice lacking β-arrestin2 (23). Although morphine does not induce tolerance in the hot plate test following chronic pump administration in βarr2-KO mice (48 mg/kg/day EC50 shift, ∼1.3-fold over basal) compared with WT mice (same dose, EC50 shift ∼3-fold), methadone and fentanyl produce equivalent degrees of tolerance in both genotypes (∼2-fold shift in EC50). Interestingly, in contrast to morphine, these latter two agonists lead to MOR phosphorylation to the same extent as etorphine or DAMGO (3, 54). It is attractive to speculate that in the absence of β-arrestin2, β-arrestin1 can substitute for agonists that promote robust phosphorylation of the MOR, such as fentanyl and methadone, to lead to desensitization and antinociceptive tolerance (19, 21). Further, the fact that relatively less tolerance is observed in the methadone- and fentanyl-treated mice compared with the morphine-treated WT mice (∼2-fold versus ∼3-fold) may be due to the ability of the DAMGO-like agonists to recruit β-arrestin1 and promote dephosphorylation of MOR. The morphine-bound MOR, due to its inability to recruit β-arrestin1, would be predicted to have impaired capacity for dephosphorylation and perhaps resensitization. Therefore, β-arrestins may dually impact MOR desensitization and resensitization, the balance of which may ultimately determine the extent of opioid antinociceptive tolerance.
Overall, our results demonstrate that MOR regulation is highly dependent on the agonist, such that an agonist's ability to promote MOR/β-arrestin interactions dictates MOR internalization, ubiquitination, and dephosphorylation. Collectively, these data support the hypothesis that morphine solely recruits β-arrestin2 to the MOR, and therefore in the βarr2-KO mice, β-arrestin1 may not compensate for the absence of β-arrestin2 in regulating the MOR. Hence, dramatic morphine-induced behavioral differences are observed between the WT and βarr2-KO mice. In contrast, agonists that promote robust MOR phosphorylation, such as fentanyl and methadone, can promote β-arrestin1-mediated MOR regulation in the βarr2-KO mice, which may account for the lack of difference in antinociceptive profiles from WT mice and the lesser degree of tolerance that develops to these drugs compared with morphine. It remains to be determined if such regulation and β-arrestin selectivity occurs in vivo. Studies evaluating the endogenous state of MOR phosphorylation, signaling, and ubiquitination are ongoing.
Acknowledgment
We thank Dr. Robert Lefkowitz (Duke University) for providing the WT and β-arrestin MEF cell lines.
This work was supported, in whole or in part, by National Institutes of Health, National Institute on Drug Abuse, Grants DA14600 and DA18860 (to L. M. B.).
- MOR
- μ-opioid receptor
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]enkephalin
- βarr
- β-arrestin
- MEF
- mouse embryonic fibroblast
- BRET
- bioluminescence resonance energy transfer
- β2AR
- β2 adrenergic receptor
- ANOVA
- analysis of variance
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Bohn L. M., Dykstra L. A., Lefkowitz R. J., Caron M. G., Barak L. S. (2004) Mol. Pharmacol. 66, 106–112 [DOI] [PubMed] [Google Scholar]
- 2. Whistler J. L., von Zastrow M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang J., Ferguson S. S., Barak L. S., Bodduluri S. R., Laporte S. A., Law P. Y., Caron M. G. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Girnita L., Shenoy S. K., Sehat B., Vasilcanu R., Girnita A., Lefkowitz R. J., Larsson O. (2005) J. Biol. Chem. 280, 24412–24419 [DOI] [PubMed] [Google Scholar]
- 5. Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001) Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 6. Martin N. P., Lefkowitz R. J., Shenoy S. K. (2003) J. Biol. Chem. 278, 45954–45959 [DOI] [PubMed] [Google Scholar]
- 7. McPherson J., Rivero G., Baptist M., Llorente J., Al-Sabah S., Krasel C., Dewey W. L., Bailey C. P., Rosethorne E. M., Charlton S. J., Henderson G., Kelly E. (2010) Mol. Pharmacol. 78, 756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arden J. R., Segredo V., Wang Z., Lameh J., Sadée W. (1995) J. Neurochem. 65, 1636–1645 [DOI] [PubMed] [Google Scholar]
- 9. Groer C. E., Tidgewell K., Moyer R. A., Harding W. W., Rothman R. B., Prisinzano T. E., Bohn L. M. (2007) Mol. Pharmacol. 71, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulz S., Mayer D., Pfeiffer M., Stumm R., Koch T., Höllt V. (2004) EMBO J. 23, 3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alvarez V. A., Arttamangkul S., Dang V., Salem A., Whistler J. L., Von Zastrow M., Grandy D. K., Williams J. T. (2002) J. Neurosci. 22, 5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borgland S. L., Connor M., Osborne P. B., Furness J. B., Christie M. J. (2003) J. Biol. Chem. 278, 18776–18784 [DOI] [PubMed] [Google Scholar]
- 13. Celver J., Xu M., Jin W., Lowe J., Chavkin C. (2004) Mol. Pharmacol. 65, 528–537 [DOI] [PubMed] [Google Scholar]
- 14. Finn A. K., Whistler J. L. (2001) Neuron 32, 829–839 [DOI] [PubMed] [Google Scholar]
- 15. Keith D. E., Murray S. R., Zaki P. A., Chu P. C., Lissin D. V., Kang L., Evans C. J., von Zastrow M. (1996) J. Biol. Chem. 271, 19021–19024 [DOI] [PubMed] [Google Scholar]
- 16. Koch T., Widera A., Bartzsch K., Schulz S., Brandenburg L. O., Wundrack N., Beyer A., Grecksch G., Höllt V. (2005) Mol. Pharmacol. 67, 280–287 [DOI] [PubMed] [Google Scholar]
- 17. Trapaidze N., Gomes I., Cvejic S., Bansinath M., Devi L. A. (2000) Brain Res. Mol. Brain Res. 76, 220–228 [DOI] [PubMed] [Google Scholar]
- 18. Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. (2000) J. Biol. Chem. 275, 17201–17210 [DOI] [PubMed] [Google Scholar]
- 19. Bohn L. M., Gainetdinov R. R., Lin F. T., Lefkowitz R. J., Caron M. G. (2000) Nature 408, 720–723 [DOI] [PubMed] [Google Scholar]
- 20. Bohn L. M., Lefkowitz R. J., Caron M. G. (2002) J. Neurosci. 22, 10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohn L. M., Lefkowitz R. J., Gainetdinov R. R., Peppel K., Caron M. G., Lin F. T. (1999) Science 286, 2495–2498 [DOI] [PubMed] [Google Scholar]
- 22. Raehal K. M., Walker J. K., Bohn L. M. (2005) J. Pharmacol. Exp. Ther. 314, 1195–1201 [DOI] [PubMed] [Google Scholar]
- 23. Raehal K. M., Bohn L. M. (2011) Neuropharmacology 60, 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Celver J. P., Lowe J., Kovoor A., Gurevich V. V., Chavkin C. (2001) J. Biol. Chem. 276, 4894–4900 [DOI] [PubMed] [Google Scholar]
- 25. Deng H. B., Yu Y., Pak Y., O'Dowd B. F., George S. R., Surratt C. K., Uhl G. R., Wang J. B. (2000) Biochemistry 39, 5492–5499 [DOI] [PubMed] [Google Scholar]
- 26. El Kouhen R., Burd A. L., Erickson-Herbrandson L. J., Chang C. Y., Law P. Y., Loh H. H. (2001) J. Biol. Chem. 276, 12774–12780 [DOI] [PubMed] [Google Scholar]
- 27. Pak Y., O'Dowd B. F., George S. R. (1997) J. Biol. Chem. 272, 24961–24965 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J., Barak L. S., Winkler K. E., Caron M. G., Ferguson S. S. (1997) J. Biol. Chem. 272, 27005–27014 [DOI] [PubMed] [Google Scholar]
- 29. Johnson E. A., Oldfield S., Braksator E., Gonzalez-Cuello A., Couch D., Hall K. J., Mundell S. J., Bailey C. P., Kelly E., Henderson G. (2006) Mol. Pharmacol. 70, 676–685 [DOI] [PubMed] [Google Scholar]
- 30. Rozenfeld R., Devi L. A. (2007) FASEB J. 21, 2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaturvedi K., Bandari P., Chinen N., Howells R. D. (2001) J. Biol. Chem. 276, 12345–12355 [DOI] [PubMed] [Google Scholar]
- 32. Wang D., Sun X., Bohn L. M., Sadée W. (2005) Mol. Pharmacol. 67, 2173–2184 [DOI] [PubMed] [Google Scholar]
- 33. Ferguson S. S., Zhang J., Barak L. S., Caron M. G. (1998) Life Sci. 62, 1561–1565 [DOI] [PubMed] [Google Scholar]
- 34. Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996) Science 271, 363–366 [DOI] [PubMed] [Google Scholar]
- 35. Laporte S. A., Miller W. E., Kim K. M., Caron M. G. (2002) J. Biol. Chem. 277, 9247–9254 [DOI] [PubMed] [Google Scholar]
- 36. Laporte S. A., Oakley R. H., Holt J. A., Barak L. S., Caron M. G. (2000) J. Biol. Chem. 275, 23120–23126 [DOI] [PubMed] [Google Scholar]
- 37. Laporte S. A., Oakley R. H., Zhang J., Holt J. A., Ferguson S. S., Caron M. G., Barak L. S. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3712–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reiter E., Lefkowitz R. J. (2006) Trends Endocrinol. Metab. 17, 159–165 [DOI] [PubMed] [Google Scholar]
- 39. Hall R. A., Lefkowitz R. J. (2002) Circ. Res. 91, 672–680 [DOI] [PubMed] [Google Scholar]
- 40. Lefkowitz R. J., Shenoy S. K. (2005) Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 41. Shenoy S. K. (2007) Circ. Res. 100, 1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hicke L. (2001) Cell 106, 527–530 [DOI] [PubMed] [Google Scholar]
- 43. Molinari P., Vezzi V., Sbraccia M., Grò C., Riitano D., Ambrosio C., Casella I., Costa T. (2010) J. Biol. Chem. 285, 12522–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haberstock-Debic H., Kim K. A., Yu Y. J., von Zastrow M. (2005) J. Neurosci. 25, 7847–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haberstock-Debic H., Wein M., Barrot M., Colago E. E., Rahman Z., Neve R. L., Pickel V. M., Nestler E. J., von Zastrow M., Svingos A. L. (2003) J. Neurosci. 23, 4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luttrell L. M., Lefkowitz R. J. (2002) J. Cell Sci. 115, 455–465 [DOI] [PubMed] [Google Scholar]
- 47. Shenoy S. K., Xiao K., Venkataramanan V., Snyder P. M., Freedman N. J., Weissman A. M. (2008) J. Biol. Chem. 283, 22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhandari D., Trejo J., Benovic J. L., Marchese A. (2007) J. Biol. Chem. 282, 36971–36979 [DOI] [PubMed] [Google Scholar]
- 49. Nabhan J. F., Pan H., Lu Q. (2010) EMBO Rep. 11, 605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen J., Benedict Gomes A., Gallagher A., Stafford K., Yoburn B. C. (2000) Synapse 38, 322–327 [DOI] [PubMed] [Google Scholar]
- 52. Stafford K., Gomes A. B., Shen J., Yoburn B. C. (2001) Pharmacol. Biochem. Behav 69, 233–237 [DOI] [PubMed] [Google Scholar]
- 53. Yoburn B. C., Billings B., Duttaroy A. (1993) J. Pharmacol. Exp. Ther. 265, 314–320 [PubMed] [Google Scholar]
- 54. Raehal K. M., Schmid C. L., Groer C. E., Bohn L. M. (2011) Pharmacol. Rev. 63, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]