Abstract
Na+/K+-ATPase, a plasma membrane protein abundantly expressed in epithelial tissues, has been identified and linked to numerous biological events, including ion transport and reabsorption. In Na+/K+-ATPase, the β-subunit plays a fundamental role in the structural integrity and functional maturation of holoenzyme. Estrogens are important circulating hormones that can regulate Na+/K+-ATPase abundance and activity; however, the specific molecules participating in this process are largely unknown. Here, we characterize that N-myc downstream-regulated gene 2 (NDRG2) is an estrogen up-regulated gene. 17β-Estradiol binds with estrogen receptor β but not estrogen receptor α to up-regulate NDRG2 expression via transcriptional activation. We also find that NDRG2 interacts with the β1-subunit of Na+/K+-ATPase and stabilizes the β1-subunit by inhibiting its ubiquitination and degradation. NDRG2-induced prolongation of the β1-subunit protein half-life is accompanied by a similar increase in Na+/K+-ATPase-mediated Na+ transport and Na+ current in epithelial cells. In addition, NDRG2 silencing largely attenuates the accumulation of β1-subunit regulated by 17β-estradiol. Our results demonstrate that estrogen/NDRG2/Na+/K+-ATPase β1 pathway is important in promoting Na+/K+-ATPase activity and suggest this novel pathway might have substantial roles in ion transport, fluid balance, and homeostasis.
Keywords: Estrogen; Gene Regulation; Na,K-ATPase; Protein Degradation; Ubiquitination; Ionic Transportation; NDRG2; Na+/K+-ATPase
Introduction
Na+/K+-ATPase, a plasma membrane ion pump, has numerous physiological functions. Of note, it is consisted of three subunits (1), α, β, and γ, and the holoenzyme activity required by the participation of the three subunits. α is the catalytic subunit of the enzyme that utilizes ATP hydrolysis to pump K+ into the cell in exchange for Na+, which is essential for maintaining normal resting membrane potentials and facilitating the exchange of other materials needed for cellular homeostasis and activity (2–4). β-Subunit is responsible for the formation and integrity of the holoenzyme. In vertebrate cells, β-subunit may stabilize the correct folding of the α-subunit to facilitate its delivery to the plasma membrane (5–7). In addition, evidence showed that β-subunit is related to the cell motility and invasion (8, 9). At present, four α-isoforms known as α1, α2, α3, α4 as well as three different β-polypeptides termed as β1, β2, and β3 (3) have been identified. Among these isoforms, α1β1 distributes in nearly every tissue whereas other isoforms exhibit a tissue-specific pattern of expression. γ-Subunit, a small hydrophobic polypeptide that has only one isoform, is involved in the modulation of Na+/K+-ATPase function (10, 11). The ionic homeostasis maintained by the Na+/K+-ATPase is also critical for cell survival, differentiation, and cell apoptosis (12, 13).
As an important molecule in charge of so many biological events, Na+/K+-ATPase is regulated by a number of hormones, including aldosterone, thyroid hormone, glucocorticoid, catecholamines, insulin, carbachol, and androgen. These circulating hormones can exert either short term or long term regulation on Na+/K+-ATPase via changing the gene expression level, reversible phosphorylation of its subunits, or modulating its trafficking (14, 15). Recently, accumulated evidence indicates that estrogen has a great effect on the activity and expression of Na+/K+-ATPase. It has been demonstrated that 17β-estradiol (E2)4 can enhance the activity of Na+/K+-ATPase in H9C2 cardiac myocytes and rat hearts (16, 17). Meanwhile, ovariectomized rats showed a decreased Na+/K+-ATPase activity (18). In erythrocytes in women, the activity of Na+/K+-ATPase was increased when estrogen levels reached a peak during the menstrual cycle (19, 20). Subsequent studies showed that estrogen also associates with Na+/K+-ATPase-induced Na+ transport in other cells such as alveolar epithelial cells and osteoblasts (21, 22). Together, these studies suggest that estrogen may influence Na+/K+-ATPase activation as well as ion transport. The regulation of estrogen on Na+/K+-ATPase is likely to be responsible for the cardioprotective effects, fluid balance and homeostasis. However, the precise molecular mechanisms by which estrogen alter pump activity and expression are still poorly understood.
N-myc downstream-regulated gene 2 (NDRG2) is a member of the NDRG family and was first cloned in our laboratory from a normal human brain cDNA library (23). NDRG2 shares roughly 60% residue identity with the other members NDRG1, NDRG3, and NDRG4 (24). The currently available bioinformatic analysis does not indicate any known motif or domain in NDRG2 (25). NDRG2 is involved in cell growth, differentiation, stress, and hormonal responses (26). Previous studies have shown that the expression of NDRG2 is regulated by many hormones, including dexamethasone, insulin, androgens, and aldosterone (27–29). Analysis of the promoter region flanking 5′ of the NDRG2 gene revealed a putative estrogen-response element (ERE) in the region (5′-nnAGTCAnnnTGACCnn-3′) (supplemental Fig. S1), which suggests that estrogen, may also play a regulative role in NDRG2 expression.
To gain further insights into the function of NDRG2, we screened for NDRG2-binding partners by using a yeast two-hybridization assay and identified Na+/K+-ATPase β1 as a protein that interacts with NDRG2. Our previous studies have shown that as a cytoplasmic protein NDRG2 is expressed in many human normal tissues and is expressed highly in the salivary glands, brain, heart, skeletal muscle, and kidney where Na+/K+-ATPases were enriched (30, 31). Importantly, the interaction of NDRG2 with Na+/K+-ATPase β1 also occurred in human HEK293 cells as determined by using a mammalian two-hybrid system (supplemental Fig. S2B).
Based on these previous studies, we wondered about the following: first, whether estrogen exerts its regulation on NDRG2; second, what would be the significance of the interaction between NDRG2 and Na+/K+-ATPase β1; and third, whether there are any possibilities that NDRG2 is involved in the estrogen-mediated regulation of Na+/K+-ATPase. In this study, we tested the hypothesis that a possible estrogen/NDRG2/Na+/K+-ATPase β1 pathway participates in the regulation of Na+/K+-ATPase.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
All cells were cultured in a humidified incubator under 5% CO2 in a Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum, 50 units/ml penicillin, 50 μg/ml streptomycin, and 2 mm l-glutamine. DNA and RNA transfection of cells was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. All cell-based assays were performed 48 h after transfection.
Construction of Plasmids
The plasmid (pGL3) for the luciferase reporter of the human NDRG2 promoter has been described previously (32). The mutants of the human NDRG2 promoter were generated by PCR using the cloned promoter as template. The plasmids for the mammalian expression of ERα and ERβ were generated by PCR amplification of the ER gene from pRST7-ERα and PSG5-ERβ (generously provided by Drs. Donald P. McDonnell (33) and Jan-Åke Gustafsson (34)), and the PCR products were subcloned into pcDNA3.1, respectively. The authenticity of newly constructed plasmids was confirmed by sequencing.
Real Time PCR
Total RNA was extracted from individual samples using TRIzol (Invitrogen) and reversely transcribed into cDNA using the RevertAidTM First-Strand cDNA synthesis kit (Fermentas, Lithuania), according to the manufacturer's instruction. The levels of mRNA transcripts were determined by quantitative real time PCR using the cDNA as the template, specific primers, and the standard SYBR® Green RT-PCR kit (Takara, Japan), according to the manufacturer's instructions. The PCRs (25 μl/tube, in triplicate) were performed first at 95 °C for 10 s for denaturation and subjected to 35 cycles of 95 °C for 5 s and 60 °C for 34 s using the specific primers (supplemental Table S1). The relative levels of target gene mRNA transcripts were normalized to the control β-actin.
Luciferase Assays
Cells (2 × 104 cells/well) were cultured in 10% FBS DMEM in 96-well plates overnight and transfected with one of the reporter vectors (0.1 μg) containing WT, truncated mutants, or point mutants (M1, M2, or M3) of NDRG2 promoter along with 5 ng of pHRL using Lipofectamine 2000 (Invitrogen) for 48 h and then treated with 10 nm E2, PPT, DPN, or ethanol for 12 h. The luciferase activities of individual samples were determined using the Dual-Luciferase reporter assay system.
Chromatin Immunoprecipitation
HSG and HeLa cells (2 × 106 cells/dish) were transfected with 10 μg of WT ERβ or control vector for 48 h in 10-cm culture dishes, respectively, and treated with 10 nm E2 for 12 h. The cells were harvested, cross-linked with 1% formaldehyde, and then lysed, as described previously (35). The chromatin DNA was sonicated to yield an average size of 300–500 bp and incubated with rabbit anti-ERβ polyclonal antibodies (Santa Cruz Biotechnology) or rabbit IgG (negative control) in a ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 2 mm EDTA, 167 mm NaCl, 16.7 mm Tris-HCl, pH 8.1, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A) at 4 °C overnight with gentle rotation. The immunocomplex was precipitated by the salmon sperm DNA/protein A-agarose 50% beads. After washing, the precipitated DNA was amplified by PCR using the primers flanking the ERE of the NDRG2 promoter (−1378/−1240) or the control region (−75/+50). The plasmid containing the NDRG2 promoter and the original portions of lysates containing the sonicated input chromatin were used as positive controls. The PCR products were resolved by agarose gel electrophoresis, and their identities were determined by DNA sequencing.
Electrophoretic Mobility Shift Assay
HSG cells were transfected with human WT ERβ or control vector and treated with 10 nm E2 for 12 h. EMSA was performed using the EMSA kit, according to the manufacturer's protocol (Pierce). Endogenous or exogenous ERβ protein was prepared using the NE-PER nuclear and cytoplasmic extraction reagents. Briefly, the candidate WT ERE in the NDRG2 promoter and the corresponding complementary sequence were synthesized and labeled with, or without, biotin at the 5′ terminus. A mutant ERE sequence (supplemental Table S1) in the NDRG2 promoter was synthesized and used as a control. The biotinylated sequence was used as the hot probe, and the unbiotinylated sequence and the mutant were used as the cold competitors. HSG cells (1 × 107) were incubated with 200 μl of cytoplasmic extraction reagent I containing protease inhibitors on ice for 10 min and treated with 11 μl of cytoplasmic extraction reagent II for 1 min, followed by centrifuging at 12,000 × g for 10 min. The cell pellets were resuspended in 50 μl of nuclear extraction reagent containing protease inhibitors and rotated at 4 °C for 1 h, followed by centrifuging at 12,000 × g for 15 min for the recovery of nuclear proteins in the supernatants. The collected nuclear proteins (3 μg) from individual samples were incubated with 10 fmol of the biotinylated hot probe (WT NDRG2 ERE) in the presence or absence of 1 μg of anti-ERβ or control rabbit IgG at 4 °C for 1 h. Subsequently, the DNA-protein complexes were separated on a 6% nondenaturing polyacrylamide gel in 2.5% glycerol, 0.5×TBE and transferred to nylon membranes. After UV-cross-linking and blocking, the membranes were probed with peroxidase-conjugated streptavidin and visualized using the enhanced chemiluminescence (Pierce) and exposing to x-ray film. In addition, the nuclear proteins were preincubated with 50–200-fold excess of the cold probes (WT or its mutant) for 1 h at room temperature and then interacted with the hot probe for competition.
Immunoprecipitation
Transfected or untransfected cells were incubated with 1 ml of lysis buffer containing 150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1% Lubrol, and 5 mm EDTA and protease inhibitors for 30 min at 4 °C. The insoluble fraction was eliminated through centrifugation at 10,000 × g for 30 min at 4 °C. After the centrifugation, the lysates were incubated with the antibody of interest, and protein A or G was conjugated to Sepharose (Pierce) for 8 h at 4 °C. To quantify the total amount of protein loaded, 20 μl of the lysates was saved. Beads were washed four times with lysis buffer. Proteins were eluted in SDS-PAGE sample buffer and separated by SDS-PAGE and analyzed by immunoblotting. Blots were then probed with peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies and visualized by enhanced chemiluminescence reagent (Pierce).
Immunofluorescent Assay
HSG cells were fixed in a freshly prepared solution of 4% paraformaldehyde, rinsed, and permeabilized with 0.1% Triton X-100 in PBS. Permeabilized cells were then incubated with horse serum in PBS to block nonspecific binding. After washing with PBS, cells were incubated overnight at 4 °C with mouse anti-NDRG2 antibody (diluted 1:150), rabbit anti-Na+/K+-ATPase β1 (diluted 1:150) and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody (diluted 1:400; Sigma) or Cy3-conjugated anti-rabbit antibody (diluted 1:400; Sigma). The isotype mouse and rabbit IgGs were used as negative controls. Dual-color detection was performed by a laser confocal microscope after treatment with 4′,6-diamidino-2-phenylindole (DAPI) to label nuclear DNA.
Transepithelial Electrical Resistance
HSG cells (5 × 105 cells/well) were cultured in 10% FBS DMEM on polyester filters of 6-well Transwell-coll culture chambers (Costar, Cambridge, MA), and the resistance of the HSG cell monolayer was measured using a Millicell-ERS volt-ohmmeter (Millipore Corp., Billerica, MA), according to the manufacturers' instruction. Individual values were normalized by subtracting the background resistance of a filter and medium alone and recorded as ohms/cm2.
Ionic Transport Measurements
HSG cells (5 × 105 cells/well) were cultured in 10% FBS DMEM on the collagen I-coated filters in the apical chamber of 6-well Transwell-col culture chambers. When confluent, the cells were exposed to 1.6 and 2.5 ml of fresh minimal medium in the apical and basolateral chambers for down-regulating or up-regulating the expression of NDRG2. The medium in both the apical and basolateral chambers was harvested separately. The ionic concentrations in the medium were determined with a Hitachi 7600 chemical auto-analyzer (Hitachi, Co., Ltd., Tokyo, Japan).
Whole-cell Patch Clamp Recordings
Na+-K+-ATPase
HSG cells were voltage clamped using the whole-cell patch technique. Patch electrodes were made from borosilicate glass capillaries (Clark Electromedical Instruments, Reading, UK) and were fire-polished (DMG Universal Puller, Zeitz-Instrumente VentreibsGmbH, Germany). The electrodes had a resistance of 3–5 megohms when filled with the standard pipette solution. Current signals were recorded using an Axopatch 1-C single electrode voltage clamp amplifier (Axon Instruments) controlled by a microcomputer running pClamp software (version 6, Axon Instruments). A gigaohm seal was rapidly established between the electrode and the cell surface, and the resistance was monitored by a repetitive +5-mV pulse (five is duration) from a holding potential of 0 mV. Access resistance on gaining access to the inside of the cell was <6 megohms. The pipette and extracellular solutions were designed to inhibit all voltage-gated channels and the Na+/Ca2+ exchanger. The standard pipette solution was made of deionized water and contained the following (Sigma) in mm: NaCl 15, MgCl2 1, CsCl 8, HEPES 10, EGTA 5, MgATP 5, creatine phosphate 5, CsCH3O3S 90, NaCH3O3S 35, pH 7.2. The standard extracellular solution contained the following (in mm): NaCl 140, KCl 5, MgCl2 1, NiCl2 2, BaCl2 1, glucose 10, HEPES 10, pH 7.4, or without KCl and correction for osmolarity for the potassium-free extracellular solution. Under these conditions, the Na+/K+-ATPase current can be defined as that inhibited by the removal of extracellular potassium.
ENaC
Whole-cell macroscopic current recordings of ENaC reconstituted in HSG cells were made under voltage clamp conditions using standard methods. Current through ENaC was the inward amiloride-sensitive Na+ current with a bath solution of (in mm) NaCl 124, KCl 2.5, CaCl2 2, MgSO4 1, NaHCO3 25, NaH2PO4 1, glucose 10, pH 7.2, and the pipette solution of (in mm) CsCl 140, NaCl 5, MgCl2 2, EGTA 1, HEPES 10, MgATP 2, Na3GTP 0.1, pH 7.4. All currents were filtered at 1 kHz. The cells were clamped to a −60 mV holding potential voltage ramp (500 ms) step protocols with potentials ranging from −100 mV to 40 mV in 20-mV increments to elicit current. Series resistances, on average 3–5 megohms, were also compensated.
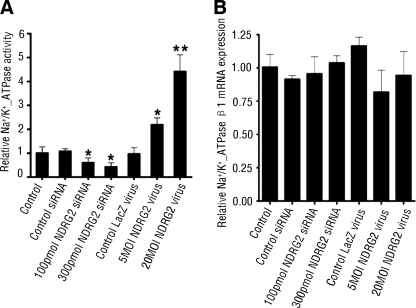
Plasma Membrane Na+/K+-ATPase Activity Assays
HSG cells (5 × 105cells/well) were infected with NDRG2 or control LacZ adenovirus (5 and 20 multiplicity of infection) or transfected with the NDRG2-specific siRNA or control siRNA (100 and 300 pmol, Invitrogen). The cells were harvested, and their Na+/K+-ATPase activities were measured essentially as described (36). Briefly, the cell suspension (50 μl) was first cooled on ice, and one portion of it was transferred to the Na+/K+-ATPase assay medium. The reactions were shocked at −20 °C and then at 37 °C for 15 min, and the contained proteins were precipitated by trichloroacetic acid/charcoal, followed by centrifugation. The 32P liberated in the supernatant was counted. The Na+/K+-ATPase activity was calculated as the difference between testing samples (total ATPase activity) and samples assayed in a medium devoid of Na+ and K+ and in the presence of 2 mm ouabain (ouabain-insensitive ATPase activity). Protein determination was performed according to the BCA protein assay kit (Pierce).
Cellular Fractionation
Cells were washed twice with ice-cold phosphate buffered saline, scraped, and homogenized in 1 ml of lysis buffer (in 250 mm sucrose, 10 mm HEPES, pH 7.4, 2.5 mm MgCl2, 0.5 mm EDTA, 100 μm Na3VO4, 100 μm PMSF, and complete protease inhibitor (Roche Applied Science)). After two centrifugations at 1,000 × g for 10 min at 4 °C, the anucleated supernatant were sonicated (power of 3 watts, using a Microson 2425 from Misonix, Inc.) for 10 s on ice and centrifuged at 28,000 × g for 15 min at 4 °C. The membrane and cytosolic pellets were resuspended in 150 μl of lysis buffer, and protein concentration was measured using the BCA method.
Statistical Analyses
All values were expressed as mean ± S.D. The difference among the different groups was analyzed by analysis of variance and post hoc analysis, and the difference between two groups was analyzed by Student's t test using the GraphPad Prism software version 4.0 (GraphPad software, San Diego). A value of p < 0.05 was considered statistically significant.
RESULTS
Estrogen Up-regulates Expression of NDRG2
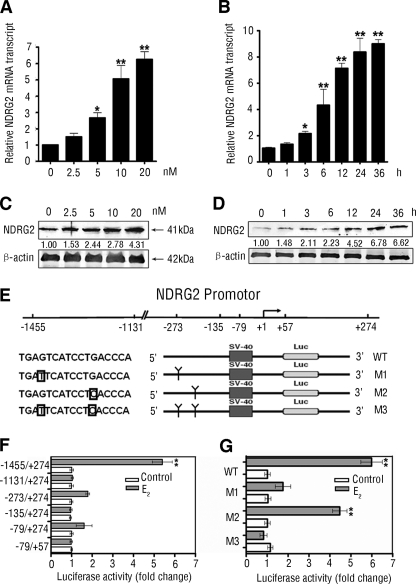
Given that the promoter region of NDRG2 had a putative estrogen-response element, we tested the hypothesis that estrogen may directly modulate the NDRG2 expression. We found that treatment with E2 significantly increased the levels of NDRG2 mRNA transcripts in a dose- and time-dependent manner in HeLa cells (Fig. 1, A and B). A similar pattern of NDRG2 protein was detected following treatment with different doses of E2 for varying periods (Fig. 1, C and D).
FIGURE 1.
Estrogen up-regulates expression of NDRG2 by binding to the putative ERE in NDRG2 promoter. A and C, dose-dependent up-regulation of NDRG2 expression. HeLa cells were treated with 0–20 nm E2 for 24 h, and the NDRG2 expression was determined by real time PCR (A) and immunoblotting (C). B and D, time-dependent up-regulation of NDRG2 expression. HeLa cells were treated with 10 nm E2 for 0–36 h and the NDRG2 expression was determined by real time PCR (B) and immunoblotting (D). Data are expressed as means ± S.D. of the relative levels of NDRG2 mRNA transcripts to control β-actin (A and B) and representative images (C and D) of each group of cells from three separate experiments. E, scheme of NDRG2 promoter region and the deletion and point mutants. The framed letter indicates the point mutation. F and G, HeLa cells were transfected with different deletion and point mutants of the NDRG2 promoter for 48 h and treated with E2, or control ethanol for 12 h, and the relative promoter reporter luciferase activity (firefly luciferase/Renilla luciferase) of individual groups of cells was analyzed. Luciferase activity experiments were performed in triplicate. Data are expressed as the means ± S.D. of relative changes of individual groups to the control. *, p < 0.05; **, p < 0.01 versus control.
To explore the mechanism by which E2 up-regulated NDRG2 expression, we cloned the promoter region flanking 5′ of the NDRG2 gene (−1455/+274) (32), and we generated a series of the truncated promoters for testing their responses to E2 stimulation by luciferase reporter assays. We found that E2, but not control ethanol, dramatically increased the luciferase activity in HeLa cells that had been transfected with the wild type (WT) NDRG2 promoter but not in the cells transfected with any of the truncated promoters (Fig. 1F). These data suggest E2 response region located within −1455 to −1131 bp of the NDRG2 promoter. Accordingly, we generated three mutants of the NDRG2 promoter by point mutation of one or two nucleotides in the putative ERE in HeLa cells (Fig. 1E). We found that M1 and M3, not M2, significantly attenuated the luciferase activity induced by E2 (Fig. 1G). These data strongly suggest that the putative ERE in the NDRG2 promoter is critical for the responsiveness to E2.
ERβ Participates in the Transcriptional Regulation of Estrogen on NDRG2 Promoter
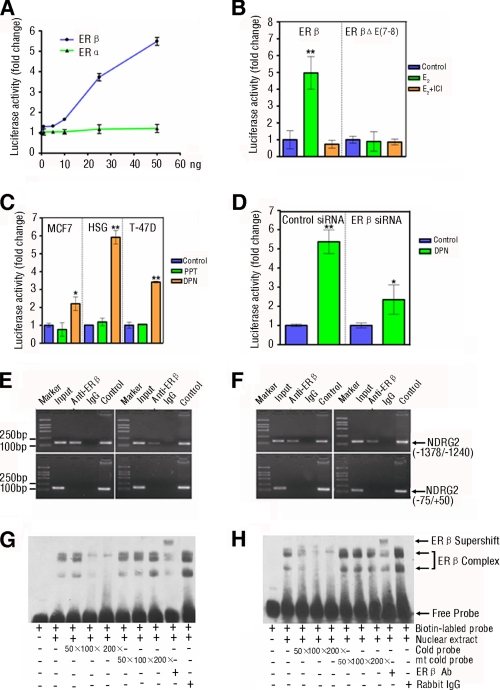
Estrogens usually bind to their nuclear receptors, ERα or ERβ, which act as transcription factors, regulating the expression of downstream genes (37), and the ERα and ERβ are encoded by distinct genes and expressed in many tissues with different patterns (38). To determine which type of the ER is responsible for transcriptional regulation on NDRG2, we analyzed the expression of ERα and ERβ in human salivary tissues and HSG (human submandibular epithelial duct) cells where high levels of NDRG2 mRNA transcripts were detected (30). Both ERα and ERβ appeared to express predominantly in the salivary duct cells and HSG cells, which were co-localized with NDRG2, but the intensity of anti-ERβ staining was much stronger than that of anti-ERα (supplemental Fig. S3). To further illustrate the role of ERα or ERβ in the estrogen-mediated up-regulation of NDRG2 expression, HEK293 cells were transfected with the NDRG2 promoter-controlled luciferase reporter and the plasmid of ERα or ERβ and treated with different doses of E2. High levels of luciferase activity were detected in the cells transfected with ERβ, but not ERα, and the E2-induced activation of the NDRG2 promoter appeared to be dose-dependent (Fig. 2A). This activity was not mediated by the ERβ mutant and was completely abrogated by treatment with ER antagonist ICI 182,780 (Fig. 2B). Next, we examined whether the endogenous ERβ was a necessary component for transactivation of NDRG2 promoter. We transfected MCF7 (human breast cancer), HSG, and T-47D (human breast cancer) cells that express both endogenous ERα and ERβ with the NDRG2 reporter and treated with the ERα- or ERβ-specific agonist, PPT or DPN, respectively. We found that DPN, the ERβ-specific agonist, activated the NDRG2 promoter and elevated endogenous NDRG2 protein expression in these cells (Fig. 2C and supplemental Fig. S4A). Moreover, we examined whether ERβ silencing could attenuate the responsiveness of the NDRG2 promoter to DPN. We found that there were significantly reduced levels of NDRG2 promoter luciferase activity and NDRG2 protein expression in HSG cells that had been transfected with ERβ-specific siRNA, as compared with that in cells transfected with control siRNA (Fig. 2D and supplemental Fig. S4B). Therefore, ERβ, but not the ERα, mediated the regulation of estrogen on the activation of the NDRG2 promoter.
FIGURE 2.
ERβ mediates the estrogen-induced NDRG2 transcriptional activation by binding to the ERE of NDRG2 promoter in vivo and in vitro. A, ERβ, but not ERα, mediates the estrogen-induced NDRG2 transcriptional activation. HEK293 cells were co-transfected with NDRG2 promoter and increasing amounts of the expression vectors for human WT ERα or ERβ for 48 h, and then the cells were treated with 10 nm E2 for 12 h. The luciferase activity was measured. B, mutation in the ligand binding domain of ERβ loses the function. HEK293 cells were co-transfected with NDRG2 promoter and WT ERβ or the mutant with a deletion in ligand binding domain (ERβΔE(7–8)) for 48 h and treated with 10 nm E2 for 12 h in the presence or absence of 10 nm ICI 182,780 (ER antagonist). The luciferase activity was measured. C, DPN, but not PPT, enhances the NDRG2 transcriptional activation. MCF7, HSG, and T-47D cells that express endogenous ERα and ERβ were transfected with the NDRG2 promoter for 48 h and treated with ERα- or ERβ-selective agonist, 10 nm PPT or 10 nm DPN, or control ethanol for 12 h. The luciferase activity of individual groups of cells was measured. D, silence of ERβ expression reduces their responses to DPN. HSG cells were co-transfected with the NDRG2 promoter and 100 pmol of ERβ-specific siRNA or control siRNA for 48 h and treated with DPN for 12 h. The luciferase activity of individual groups of cells was measured. B–D, above experiments were performed in triplicate. Data are expressed as the means ± S.D. of relative changes of individual groups of cells to the control cells. *, p < 0.05; **, p < 0.01 versus control. E and F, binding of exogenous (left panel) or endogenous (right panel) ERβ to the ERE in the NDRG2 promoter in vivo was measured by ChIP assay. HSG (E) and HeLa (F) cells were transfected with expression vector for human ERβ or control vector for 48 h and treated with 10 nm E2 for 12 h. Subsequently, cell lysates were immunoprecipitated with anti-ERβ-specific antibody and analyzed by PCR amplification using the primers flanking the ERE in the NDRG2 promoter (−1378/−1240) or the control region (−75/+50), as indicated in supplemental Table S1. NDRG2 promoter plasmid and input chromatin (the remaining portion of cell lysates) were used as positive controls. ERβ antibody isotype rabbit IgG was used as negative control. G and H, binding of exogenous (G) or endogenous ERβ (H) to the ERE in NDRG2 promoter in vitro was measured by EMSA. HSG cells were transfected with the expression vector for human ERβ or control vector for 48 h and treated with 10 nm E2 for 12 h. Their nuclei were extracted and incubated with biotinylated WT probe for NDRG2-ERE in the presence or absence of increasing amounts of competitive unlabeled cold probes for NDRG2-ERE (WT or mutant) or the ERβ-specific antibody, respectively. The DNA-protein complexes were separated on a 6% TBE gel, transferred to nylon membranes, and visualized using peroxidase-conjugated streptavidin and substrate. Data shown are representative images from two repeated experiments.
ERs can act as transcription factors, activating the downstream genes. To explore whether ERβ could directly bind to the ERE of NDRG2 promoter, HSG and HeLa cells were transfected with ERβ plasmid, and the potential complex of the ERβ/NDRG2 promoter was characterized by chromatin immunoprecipitation (ChIP) using anti-ERβ and amplifying the ERE-containing fragment of the NDRG2 promoter (−1378/−1240 bp). Interestingly, the ERE-containing fragment of NDRG2 promoter (−1378/−1240 bp), but not the control sequence (NDRG2 promoter, −75/+50 bp), was precipitated by anti-ERβ in both types of cells with or without ERβ transfection (Fig. 2, E and F), indicating that inducible and endogenous ERβ bound to this region of NDRG2 promoter. In addition, the direct binding of ERβ to the ERE region of NDRG2 promoter was further demonstrated by electrophoretic mobility shift assay (EMSA). Incubation of the biotinylated WT ERE-NDRG2 DNA with the nuclear extracts from the ERβ-transfected (Fig. 2G) or nontransfected HSG cells (Fig. 2H) changed the mobilization behaviors of the probe, which was further shifted by the addition of anti-ERβ, indicating the formation of ERβ-DNA complex. The binding of ERβ to the ERE region of NDRG2 promoter was competitively inhibited by preincubation of nuclear proteins with an excessive amount of WT cold probe but not with the same amount of mutant cold probe. Together, the data clearly demonstrate that estrogen up-regulates the expression of NDRG2 through direct binding ERβ to the ERE region (position of −1455 to −1131 bp) of NDRG2 promoter.
NDRG2 Interacts with the β1-subunit of Na+/K+-ATPase
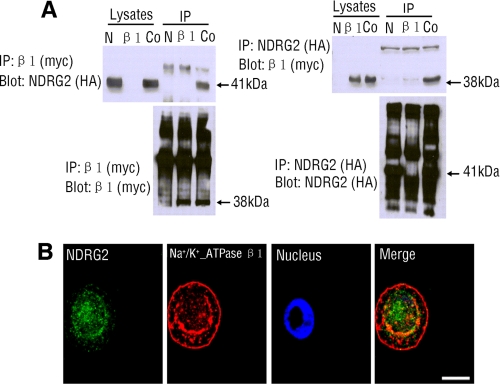
Data obtained from yeast and mammalian two-hybrid assays suggest that NDRG2 might interact with Na+/K+-ATPase β1 (supplemental Fig. S2). To further confirm that NDRG2 interacts with β1-subunit of Na+/K+-ATPase, co-immunoprecipitation was performed using lysates prepared from HEK293 cells that were transfected with pCMV-HA-NDRG2 and pCMV-Myc-Na+/K+-ATPase β1. A 41-kDa protein, which corresponded to NDRG2, was precipitated by anti-Myc mAb and probed by anti-HA antibody, whereas a 38-kDa protein corresponding to Na+/K+-ATPase β1 was precipitated by the anti-HA antibody and probed by anti-Myc mAb (Fig. 3A). We also demonstrated that endogenous Na+/K+-ATPase β1 co-immunoprecipitated with endogenous NDRG2 in HSG cells, and vice versa (supplemental Fig. S5). Collectively, these experiments revealed that NDRG2 bound to Na+/K+-ATPase β1.
FIGURE 3.
NDRG2 interacts with Na+/K+-ATPase β1. A, HA-tagged full-length NDRG2 and/or Myc-tagged Na+/K+-ATPase β1-subunit were/was transfected into HEK293 cells. β1-subunit can be detected in an NDRG2 immunoprecipitate (IP), and conversely, NDRG2 can be detected in a β1-subunit immunoprecipitate, when the β1-subunit and NDRG2 are transfected together. Vector transfection alone showed no bands in either direction. Immunoprecipitation antibodies were either anti-Myc (β1-subunit) or anti-HA (NDRG2), with detection antibodies either anti-Myc or anti-HA. The location of various proteins is indicated with an arrowhead. N, pCMV-HA-NDRG2 transfection alone; β1, pCMV-Myc-Na+/K+-ATPase β1 transfection alone; Co, co-transfection with both plasmids; Blot, immunoblotting; IP, immunoprecipitation. B, co-localization of NDRG2 and Na+/K+-ATPase β1 in HSG cells. The distribution of endogenous NDRG2 and endogenous Na+/K+-ATPase β1 was characterized by immunofluorescent assays using FITC and Cy3 antibodies. Data shown are representative images from three independent experiments. Scale bars, 10 μm.
The interaction between NDRG2 and Na+/K+-ATPase β1 prompted us to examine their subcellular distribution. In accordance with previous studies, NDRG2 was predominantly localized in the cytoplasm (31), whereas Na+/K+-ATPase β1 was in plasma membrane and perinuclear region of the cytoplasm (7). Indeed, we found that a portion of endogenous NDRG2 and endogenous Na+/K+-ATPase β1 were co-located in the peri-nuclear cytoplasm region in HSG cells (Fig. 3B).
NDRG2 Modulates the Ionic Transportation via Regulating the Na+/K+-ATPase Activity
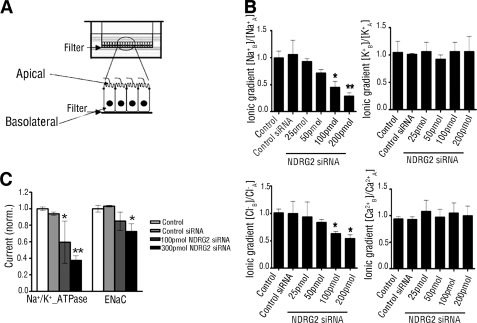
Na+/K+-ATPase is responsible for maintaining membrane potential and ionic gradients by transporting sodium and potassium ions against their concentration gradients (3). To investigate the potential impact of NDRG2 on Na+/K+-ATPase, we detected the transepithelial ions transport by establishing a polarized monolayer of salivary duct cells cultured on Transwell filters (39), as shown in Fig. 4A. HSG monolayer cells on the filters of the apical chamber exhibited a high trans-epithelial electrical resistance (3.71 ± 0.12 kilo-ohms/cm2), which meant that our model can mimic the in vivo environment. In salivary glands, Na+/K+-ATPase localizes mainly at the basolateral plasma membrane of the striated duct and excretory ducts (40). Under basal conditions, cells generated basolateral to apical Na+ and Cl− gradients, indicating a Na+/Cl− reabsorption from the apical to the basolateral side. A significant decrease in Na+ reabsorption and comparable decrease in Cl− reabsorption were induced in NDRG2-silenced HSG cells; however, it did not alter K+ and Ca2+ gradients (Fig. 4B). Consistent with above result, induction of NDRG2 overexpression by infecting HSG cells with NDRG2 adenovirus significantly elevated the Na+ and Cl− gradients but did not affect the K+ and Ca2+ gradients in HSG cells (supplemental Fig. S6C). We also checked the effect of NDRG2 on Na+-activated currents in HSG cells using whole-cell patch clamp. NDRG2 silencing significantly reduced Na+/K+-ATPase-mediated Na+ current (Fig. 4C). Consistent a with previous study in oocytes (41), NDRG2 silencing could also reduce ENaC-mediated Na+ current.
FIGURE 4.
NDRG2 stimulates ionic transport and Na+/K+-ATPase-mediated Na+ current. A, schematic diagram of HSG cells grown on a filter of the top well. HSG cells were cultured on collagen I-coated Transwell Col filters for monolayer. B, HSG cells on the filter were transfected with different doses of NDRG2-specific or control siRNA for 48 h. The concentrations of Na+, K+, Cl−, and Ca2+ in the supernatants from the apical and basolateral compartments were measured, and the ratio of individual electrolyte concentrations in the basolateral to that in the apical well was calculated for the ionic gradients. The value of control cells was designated as 1. Data are expressed as means ± S.D. of the relative values of individual electrolytes in individual groups of cells from three independent determinations. A, apical; B, basolateral. C, HSG cells were transfected with different doses of NDRG2-specific siRNA or control siRNA for 48 h. Ouabain-sensitive current (Na+/K+-ATPase-mediated Na+ current) and amiloride-sensitive current (ENaC-mediated Na+ current) were measured under whole-cell patch clamp conditions. Na+/K+-ATPase-mediated Na+ current was the outward current at a cell potential of 0 mV, which was blocked by 1 mm ouabain. ENaC-mediated Na+ current was the inward current at a cell potential of −60 mV, which was blocked by 10 μm amiloride. Data are expressed as means ± S.D. of the relative values of each group of cells from 12 independent experiments, and the value of control cells was designed as 1. *, p < 0.05; **, p < 0.01 versus control.
Given that NDRG2 interacted with Na+/K+-ATPase β1, and it affected the Na+ transport and Na+ current, we next measured the Na+/K+-ATPase activity, the motor of sodium reabsorption. As shown in Fig. 5A, NDRG2 silencing reduced Na+/K+-ATPase activity, and accordingly, NDRG2 overexpression increased Na+/K+-ATPase activity in HSG cells. Taken together, these results suggest that NDRG2 is a positive regulator of Na+/K+-ATPase activity.
FIGURE 5.
NDRG2 promotes Na+/K+-ATPase activity but not affects Na+/K+-ATPase β1 mRNA transcripts. A, HSG cells were infected with NDRG2 adenovirus or transfected with NDRG2-specific siRNA for 48 h. The Na+/K+-ATPase activity in different groups of cells was determined. Results are expressed as means ± S.D. of the relative values of each group to control from three independent determinations, and the value of control cells was designated as 1. B, HSG cells were infected with NDRG2 adenovirus or transfected with NDRG2-specific siRNA for 48 h. The levels of Na+/K+-ATPase β1 mRNA transcripts were determined by real time PCR. Data are expressed as the mean ± S.D. of the relative levels of Na+/K+-ATPase β1 mRNA transcripts to control β-actin of individual groups of cells from three independent experiments and the value of control cells was designated as 1. *, p < 0.05; **, p < 0.01 versus control.
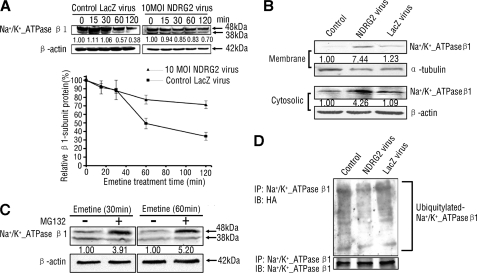
NDRG2 Inhibits the Ubiquitination and Degradation of β1-subunit
To investigate the mechanism of increased Na+/K+-ATPase activity regulated by NDRG2, we examined whether NDRG2 regulates the abundance of Na+/K+-ATPase β1. Notably, NDRG2 did not change the levels of Na+/K+-ATPase β1 mRNA transcripts in HSG cells (Fig. 5B), and because of that, the amount of Na+/K+-ATPase at the plasma membrane could be modified by changes in the rate of synthesis or degradation (14, 42). We hypothesized that NDRG2 may exert a regulation on the degradation of Na+/K+-ATPase β1. To test this hypothesis, HSG cells that had been infected with NDRG2 adenovirus were then treated with emetine, a protein synthesis inhibitor. We detected significantly high levels of Na+/K+-ATPase β1 protein in NDRG2 overexpression cells 60 min post-emetine, as compared with that in controls (Fig. 6A). The amounts of Na+/K+-ATPase β1 in the membrane and cytosolic fractions were substantially increased in NDRG2-overexpressed HSG cells (Fig. 6B). Thus, NDRG2 could inhibit the natural degradation and prolong the half-life of Na+/K+-ATPase β1.
FIGURE 6.
NDRG2 stabilizes Na+/K+-ATPase β1 through inhibiting its ubiquitination. A, upper panel, HSG cells were infected with NDRG2 or LacZ adenovirus for 48 h and then treated with 100 μm emetine for the indicated periods. Bottom panel, relative levels of β1-subunit to β-actin were quantified by densitometry. Data shown are representative images from three independent experiments. B, cell fractions were prepared from HSG cells infected with NDRG2 or LacZ adenovirus for 48 h. Membrane and cytosolic fractions of endogenous β1-subunit were detected. α-Tubulin and β-actin served as loading controls. C, HSG cells were treated with DMSO or MG132 for 4 h and emetine for 30 or 60 min; β1-subunit levels were analyzed by immunoblotting. D, HSG cells were transfected with HA-ubiquitin for 6 h and then infected with NDRG2 or LacZ adenovirus for 48 h, and cell lysates were collected and subjected to immunoprecipitation and immunoblotting with β1-subunit and HA antibodies. MOI, multiplicity of infection; IP, immunoprecipitation; IB, immunoblotting; HA, hemagglutinin.
To investigate the mechanism that NDRG2 inhibits Na+/K+-ATPase β1 protein degradation, we first detected the protein degradation pathway of the β1-subunit. MG132, a proteasome inhibitor, led to greater accumulations of Na+/K+-ATPase β1 in HSG cells (Fig. 6C), indicating that the β1-subunit degradation was proteasome-mediated, consistent with a previous study that ubiquitylation, as well as endocytosis, was involved in the fast degradation of the β1-subunit (42). Furthermore, ectopic NDRG2 expression led to a decrease in Na+/K+-ATPase β1 ubiquitination in HSG cells (Fig. 6D). These results indicated that overexpressed NDRG2 bound to Na+/K+-ATPase β1 and inhibited the proteasome-mediated Na+/K+-ATPase β1 ubiquitination and degradation, leading to increased Na+/K+-ATPase β1 protein level and Na+/K+-ATPase activity.
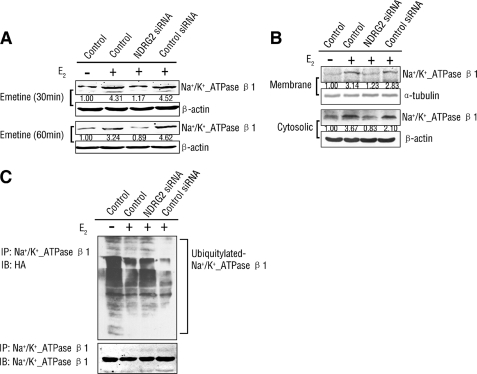
NDRG2 Is Involved in E2-mediated Regulation of Na+/K+-ATPase β1
Previous studies showed that estrogen up-regulates and activates Na+/K+-ATPase (16, 18–20). As our results demonstrated that E2 can enhance the expression of NDRG2 and NDRG2 inhibits the degradation of Na+/K+-ATPase β1, we postulated that NDRG2 may be involved in the E2-mediated regulation of Na+/K+-ATPase. We found that E2 can elevate the protein level of Na+/K+-ATPase β1. In addition, a significantly lower level of Na+/K+-ATPase β1 protein was detected in NDRG2-silenced HSG cells compared with control groups with E2 treatment (Fig. 7A). The increased quantity of endogenous Na+/K+-ATPase β1 protein in the membrane and cytosolic fractions with E2 treatment was also attenuated by NDRG2 silencing (Fig. 7B). Next, we found E2 can inhibit the ubiquitination of Na+/K+-ATPase β1, whereas NDRG2 silencing partly counteracted E2 mediated above inhibition (Fig. 7C). These assays indicated that the regulation of E2 on Na+/K+-ATPase β1 was at least partly mediated by NDRG2.
FIGURE 7.
NDRG2 participates in the regulation of estrogen on Na+/K+-ATPase β1 protein. A–C, HSG cells were transfected with NDRG2 siRNA or control siRNA for 48 h and treated with E2 or ethanol for 12 h. A, cell lysates of HSG cells pretreated with 100 μm emetine for 30 or 60 min were analyzed by immunoblotting with β1-subunit antibody. B, cell fractions were prepared, and membrane and cytosolic fractions of endogenous β1-subunit were detected. C, cells were transfected with HA-ubiquitin plasmid for 6 h, and cell lysates were collected and subjected to immunoprecipitation and immunoblotting with β1-subunit and HA antibodies. IP, immunoprecipitation; IB, immunoblotting; HA, hemagglutinin.
DISCUSSION
Previous studies have shown that the expression of NDRG2 is regulated by Myc (32), TNFα, IGF-1 (28), hypoxia (43), DNA damage (44), and many hormones, including dexamethasone, insulin, androgens, and aldosterone (27–29). Nevertheless, regulation of NDRG2 is still not fully understood and merits further investigation. In our study, we analyzed the promoter region flanking 5′ of NDRG2 and found a potential ERE. Moreover, we revealed that E2 up-regulated the expression of NDRG2 in dose- and time-dependent manner. In addition, we demonstrated that ERβ, but not ERα, bound specifically to the ERE at position of −1455 to −1131 bp of the NDRG2 promoter and transactivated the NDRG2 promoter. These data mean that estrogen, as an important circulating hormone, also plays a regulatory role in NDRG2 expression. myc is a downstream target of estrogen and ERs by transcriptional regulation, which has profound mitogenic effects on cancer cells (45). In our previous study, we found Myc represses NDRG2 gene expression via Miz-1-dependent interaction with NDRG2 core promoter region (32). In addition, estrogen also regulates the PI3K/Akt pathways (46). Interesting, NDRG2 is one of the phosphorylated substrates of Akt (47). Thus, we infer that estrogen not only regulates NDRG2 through ERβ-mediated transcriptional regulation but also through many other patterns, such as Myc or Akt-mediated signaling. These complicated yet accurate cross-talk pathways need to be further studied.
NDRG2 seems to be broadly involved in stress responses, cell proliferation, and differentiation (26). To identify NDRG2, interacted proteins may provide important information for understanding its precise molecular and cellular functions. Our previous study characterized a cell cycle-dependent transcription factor MSP58 (58-kDa microspherule protein)/MCRS1 (microspherule protein 1) was a binding partner of NDRG2 (25). NDRG2 could translocate from cytosol to the nucleus to form a complex with MSP58 upon NiCl2 treatment. Data obtained from yeast and mammalian two-hybrid assays suggest that Na+/K+-ATPase β1 might also interact with NDRG2 (supplemental Fig. S2). The β-subunit of Na+/K+-ATPase serves as a chaperone that promotes proper membrane insertion of the α-subunit. After formation of the holoenzyme, the β-subunit stabilizes the α-subunit in the membrane and modulates cation sensitivity of the pump (48, 49). In this study, we confirmed that NDRG2 directly interacted with Na+/K+-ATPase β1 using co-immunoprecipitation. The binding of NDRG2 and Na+/K+-ATPase β1 prompted us to analyze whether NDRG2 and Na+/K+-ATPase β1 localize in the same subcellular compartment. NDRG2 was predominantly localized in the cytoplasm (31), whereas Na+/K+-ATPase β1 was in the plasma membrane and perinuclear region of the cytoplasm (7). Our data showed that NDRG2 and Na+/K+-ATPase β1 were co-located in the peri-nuclear cytoplasm region.
NDRG2 is expressed in many human tissues, highly in salivary glands, brain, heart, skeletal muscle, and kidney (30), where Na+/K+-ATPases are enriched. Based on the salivary duct cell monolayer model, we demonstrated that NDRG2 induces the transport of Na+ in ductal epithelial cells. Down-regulation of endogenous NDRG2 by siRNA was associated with a dramatic decrease of Na+ and Cl− gradients and the Na+/K+-ATPase-mediated Na+ current. Consistent with the study of Wielputz et al. (41) in oocytes and fisher rat thyroid cells, our data showed that NDRG2 silencing could also reduce ENaC-mediated Na+ current. It is well known that reabsorption of ion, a vital mechanism for humoral regulation, is an important function in tight epithelium mainly through Na+/K+-ATPase, ENaC, and Cl− channels. Previous studies have indicated that Na+/K+-ATPase and ENaC are expressed in tight epithelial cells and are critical components for the control of Na+ reabsorption (50–52). Our data suggest that NDRG2 may regulate Na+ reabsorption in epithelial cells by directly interacting with Na+/K+-ATPase β1 and affect Na+/K+-ATPase holoenzyme, promoting the formation of an electrochemical gradient of Na+ across the basolateral plasma membrane, leading to low cytosolic concentrations of Na+, which stimulates the sensors of cytosolic Na+ and activates ENaC in apical plasma membrane, facilitating the reabsorption of Na+.
The β-subunit of Na+/K+-ATPase facilitates the transport of the α-subunit to plasma membrane, and the α-subunit would execute the catalytic performance (6). We found NDRG2 is a positive regulator of Na+/K+-ATPase activity, the motor of sodium reabsorption. To investigate the mechanism of increased Na+/K+-ATPase activity regulated by NDRG2, we examined whether NDRG2 regulates the abundance of Na+/K+-ATPase β1 and found that Na+/K+-ATPase β1 protein, but not RNA, was dramatically affected by NDRG2. Although NDRG2 did not affect the transcript of Na+/K+-ATPase β1, it inhibited the ubiquitination and degradation of the β1-subunit. Based on the observation that NDRG2 and Na+/K+-ATPase β1 were co-located in the cytoplasm region, we may infer that NDRG2 serves as a chaperone for the β1-subunit that protects it from being degraded during its translocation to the plasma membrane. Therefore, NDRG2 is a physiological regulator of the reabsorption of Na+ by binding and stabilizing Na+/K+-ATPase β1 in salivary duct epithelial cells. Whether this mechanism could apply to brain, heart, skeletal muscle, and kidney where Na+/K+-ATPase and NDRG2 were both enriched needs further experiments.
Intricate regulations have developed to adjust the expression and activity of Na+/K+-ATPase. Most of the regulators of this pump have been identified to be hormones. Previous studies reported that estrogens can regulate Na+/K+-ATPase activity, which is governed by several ways, including an allosteric manner (20) and transcriptional regulation (16). However, the molecular mechanisms by which estrogen alters Na+/K+-ATPase expression and activity have not been fully understood. Here, we demonstrated that human NDRG2 is up-regulated by E2 and under the control of E2-mediated transcriptional regulation. We also found NDRG2 can up-regulate Na+/K+-ATPase activity and Na+/K+-ATPase-mediated Na+ transport by binding and stabilizing the Na+/K+-ATPase β1 protein. Based on above data, we postulate that estrogen may affect Na+/K+-ATPase via NDRG2-mediated Na+/K+-ATPase β1 regulation. Our results show that E2 promotes the accumulation of Na+/K+-ATPase β1 protein by inhibiting its ubiquitination and degradation. In addition, knockdown of NDRG2 could attenuate the effects of E2 on Na+/K+-ATPase β1, which indicated that the regulation of estrogen on Na+/K+-ATPase was at least partly mediated by NDRG2. Interestingly, recent evidence showed that Na+/K+-ATPase β1 also plays an important role in cell-cell tight junctions (9), whereas ERβ-deficient mice failed to maintain the polarized phenotype of epithelial cells (53). This research further implies the possibility of a regulation pathway for E2/ERβ-induced NDRG2 expression and enhanced β1-subunit stability. NDRG2 is able to inhibit proliferation and enhance apoptosis of breast cancer as well as many other malignant tumors (26). In this study, we found that NDRG2 can reduce the natural degradation of Na+/K+-ATPase β1. Given that Na+/K+-ATPase β1 is involved in cell motility and invasion, this may partly explain the reason that NDRG2 can serve as a tumor suppressor gene candidate in various highly invasive and migratory cancers. However, estrogens exert both genomic and nongenomic effects by complicated signaling pathways. Whether this system is related to breast cancer treatment needs to be directly determined in future studies.
On the basis of our data and previous observations, we propose the following mechanistic model for NDRG2 contributing to estrogen-induced Na+/K+-ATPase regulation. The supplemental Fig. S7A shows NDRG2 transcripts at low levels without estrogen treatment or stimulation. Na+/K+-ATPase β1 is ubiquitinated and targeted for rapid destruction by the proteasome in cytosol. Na+/K+-ATPase and ENaC mediate Na+ transport on basolateral and apical sides of plasma membrane, respectively, at low levels in epithelial cells. The supplemental Fig. S7B shows in the presence of estrogen that estrogen binds to ERβ, forming a transcription factor, which activates NDRG2 transcription. NDRG2 protein binds and stabilizes the β1-subunit by inhibiting the ubiquitin-proteasome pathway-mediated degradation. The holoenzyme of Na+/K+-ATPase accumulates and translocates into the basolateral side of cell plasma membrane, where Na+ is pumped out and an electrochemical gradient of Na+ is established across the plasma membrane. ENaC localized in the apical membranes senses this Na+ electrochemical gradient and facilitates the reabsorption of Na+ in epithelial cells.
Taken together, our data combined with previous studies indicated that estrogen can regulate Na+/K+-ATPase via multiple mechanisms. The diversity of the regulatory mechanisms may be required by cells under various physiological and pathological conditions. Therefore, characterization of the novel estrogen/NDRG2/Na+/K+-ATPase β1 regulation pathway broadened the understanding of the regulatory role of estrogen on Na+/K+-ATPase, and modulation of this pathway may potentially provide a basis for the intervention of ion transport, fluid balance and internal environment homeostasis.
Supplementary Material
Acknowledgment
We thank Dr. Minggao Zhao for technical help with the patch clamp assays.
This work was supported by Chinese National Key Basic Research and Development Program Grant 2010CB529705 and National Natural Science Foundation of China Grants 31070681, 30830054, 30801121, 81100764, 81072973, and 81000563.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7 and Table S1.
- E2
- 17β-estradiol
- ENaC
- epithelial sodium channel
- ER
- estrogen receptor
- ERE
- estrogen response element
- NDRG
- N-myc downstream-regulated gene
- PPT
- 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol)
- DPN
- 2,3-bis(4-hydroxyphenyl)-propionitrile)
- HSG
- human salivary gland.
REFERENCES
- 1. Dempski R. E., Friedrich T., Bamberg E. (2009) Biochim. Biophys. Acta 1787, 714–720 [DOI] [PubMed] [Google Scholar]
- 2. Lingrel J. B., Kuntzweiler T. (1994) J. Biol. Chem. 269, 19659–19662 [PubMed] [Google Scholar]
- 3. Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 4. Skou J. C., Esmann M. (1992) J. Bioenerg. Biomembr. 24, 249–261 [DOI] [PubMed] [Google Scholar]
- 5. Chow D. C., Forte J. G. (1995) J. Exp. Biol. 198, 1–17 [DOI] [PubMed] [Google Scholar]
- 6. McDonough A. A., Geering K., Farley R. A. (1990) FASEB J. 4, 1598–1605 [DOI] [PubMed] [Google Scholar]
- 7. Vagin O., Sachs G., Tokhtaeva E. (2007) J. Bioenerg. Biomembr. 39, 367–372 [DOI] [PubMed] [Google Scholar]
- 8. Rajasekaran S. A., Palmer L. G., Quan K., Harper J. F., Ball W. J., Jr., Bander N. H., Peralta Soler A., Rajasekaran A. K. (2001) Mol. Biol. Cell 12, 279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajasekaran S. A., Huynh T. P., Wolle D. G., Espineda C. E., Inge L. J., Skay A., Lassman C., Nicholas S. B., Harper J. F., Reeves A. E., Ahmed M. M., Leatherman J. M., Mullin J. M., Rajasekaran A. K. (2010) Mol. Cancer Ther. 9, 1515–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivard C. J., Almeida N. E., Berl T., Capasso J. M. (2005) Front. Biosci. 10, 2604–2610 [DOI] [PubMed] [Google Scholar]
- 11. Dempski R. E., Lustig J., Friedrich T., Bamberg E. (2008) Biochemistry 47, 257–266 [DOI] [PubMed] [Google Scholar]
- 12. Yu S. P. (2003) Biochem. Pharmacol. 66, 1601–1609 [DOI] [PubMed] [Google Scholar]
- 13. Lefranc F., Kiss R. (2008) Neoplasia 10, 198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ewart H. S., Klip A. (1995) Am. J. Physiol. 269, C295–C311 [DOI] [PubMed] [Google Scholar]
- 15. Efendiev R., Das-Panja K., Cinelli A. R., Bertorello A. M., Pedemonte C. H. (2007) Br. J. Pharmacol. 151, 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C. G., Xu K. Q., Xu X., Huang J. J., Xiao J. C., Zhang J. P., Song H. P. (2007) Clin. Exp. Pharmacol. Physiol. 34, 998–1004 [DOI] [PubMed] [Google Scholar]
- 17. Dzurba A., Ziegelhöffer A., Vrbjar N., Styk J., Slezák J. (1997) Mol. Cell. Biochem. 176, 113–118 [PubMed] [Google Scholar]
- 18. Kaur G., Sharma P., Bhardwaj S., Kaur G. (1997) Mol. Cell. Biochem. 167, 107–111 [DOI] [PubMed] [Google Scholar]
- 19. Melis M. G., Troffa C., Manunta P., Pinna Parpaglia P., Soro A., Pala F., Madeddu P., Pazzola A., Tonolo G., Patteri G. (1990) Boll. Soc. Ital. Biol. Sper. 66, 679–684 [PubMed] [Google Scholar]
- 20. Ziegelhöffer A., Dzurba A., Vrbjar N., Styk J., Slezák J. (1990) Bratisl. Lek. Listy 91, 902–910 [PubMed] [Google Scholar]
- 21. Laube M., Küppers E., Thome U. H. (2011) Pediatr. Res. 69, 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G. Z., Nie H. G., Lu L., Chen J., Lu X. Y., Ji H. L., Li Q. N. (2011) Cell. Mol. Biol. 57, (suppl.) OL1480–OL1486 [PMC free article] [PubMed] [Google Scholar]
- 23. Deng Y., Yao L., Liu X., Nie X., Wang J., Zhang X., Su C. (2001) Shengwu. Huaxue. Yu. Shengwu. Wuli. Jinzhan. 28, 72–76 [Google Scholar]
- 24. Qu X., Zhai Y., Wei H., Zhang C., Xing G., Yu Y., He F. (2002) Mol. Cell. Biochem. 229, 35–44 [DOI] [PubMed] [Google Scholar]
- 25. Zhang J., Liu J., Li X., Li F., Wang L., Zhang J., Liu X., Shen L., Liu N., Deng Y., Yang A., Han H., Zhao M., Yao L. (2007) Biochem. Biophys. Res. Commun. 352, 6–11 [DOI] [PubMed] [Google Scholar]
- 26. Yao L., Zhang J., Liu X. (2008) Acta Biochim. Biophys. Sin. 40, 625–635 [DOI] [PubMed] [Google Scholar]
- 27. Boulkroun S., Fay M., Zennaro M. C., Escoubet B., Jaisser F., Blot-Chabaud M., Farman N., Courtois-Coutry N. (2002) J. Biol. Chem. 277, 31506–31515 [DOI] [PubMed] [Google Scholar]
- 28. Foletta V. C., Prior M. J., Stupka N., Carey K., Segal D. H., Jones S., Swinton C., Martin S., Cameron-Smith D., Walder K. R. (2009) J. Physiol. 587, 1619–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boulkroun S., Le Moellic C., Blot-Chabaud M., Farman N., Courtois-Coutry N. (2005) Pflugers Arch. 451, 388–394 [DOI] [PubMed] [Google Scholar]
- 30. Deng Y., Yao L., Chau L., Ng S. S., Peng Y., Liu X., Au W. S., Wang J., Li F., Ji S., Han H., Nie X., Li Q., Kung H. F., Leung S. Y., Lin M. C. (2003) Int. J. Cancer 106, 342–347 [DOI] [PubMed] [Google Scholar]
- 31. Hu X. L., Liu X. P., Deng Y. C., Lin S. X., Wu L., Zhang J., Wang L. F., Wang X. B., Li X., Shen L., Zhang Y. Q., Yao L. B. (2006) Cell Tissue Res. 325, 67–76 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J., Li F., Liu X., Shen L., Liu J., Su J., Zhang W., Deng Y., Wang L., Liu N., Han W., Zhang J., Ji S., Yang A., Han H., Yao L. (2006) J. Biol. Chem. 281, 39159–39168 [DOI] [PubMed] [Google Scholar]
- 33. Hall J. M., McDonnell D. P. (1999) Endocrinology 140, 5566–5578 [DOI] [PubMed] [Google Scholar]
- 34. Ogawa S., Inoue S., Watanabe T., Hiroi H., Orimo A., Hosoi T., Ouchi Y., Muramatsu M. (1998) Biochem. Biophys. Res. Commun. 243, 122–126 [DOI] [PubMed] [Google Scholar]
- 35. Chen L., Peng Z., Bateman E. (2004) Nucleic Acids Res. 32, 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. (1990) Nature 347, 386–388 [DOI] [PubMed] [Google Scholar]
- 37. Stossi F., Barnett D. H., Frasor J., Komm B., Lyttle C. R., Katzenellenbogen B. S. (2004) Endocrinology 145, 3473–3486 [DOI] [PubMed] [Google Scholar]
- 38. Nilsson S., Mäkelä S., Treuter E., Tujague M., Thomsen J., Andersson G., Enmark E., Pettersson K., Warner M., Gustafsson J. A. (2001) Physiol. Rev. 81, 1535–1565 [DOI] [PubMed] [Google Scholar]
- 39. Wakabayashi Y., Chua J., Larkin J. M., Lippincott-Schwartz J., Arias I. M. (2007) Histochem. Cell Biol. 127, 463–472 [DOI] [PubMed] [Google Scholar]
- 40. Sims-Sampson G., Gresik E. W., Barka T. (1984) Anat. Rec. 210, 53–60 [DOI] [PubMed] [Google Scholar]
- 41. Wielpütz M. O., Lee I. H., Dinudom A., Boulkroun S., Farman N., Cook D. I., Korbmacher C., Rauh R. (2007) J. Biol. Chem. 282, 28264–28273 [DOI] [PubMed] [Google Scholar]
- 42. Yoshimura S. H., Iwasaka S., Schwarz W., Takeyasu K. (2008) J. Cell Sci. 121, 2159–2168 [DOI] [PubMed] [Google Scholar]
- 43. Wang L., Liu N., Yao L., Li F., Zhang J., Deng Y., Liu J., Ji S., Yang A., Han H., Zhang Y., Zhang J., Han W., Liu X. (2008) Cell. Physiol. Biochem. 21, 239–250 [DOI] [PubMed] [Google Scholar]
- 44. Liu N., Wang L., Li X., Yang Q., Liu X., Zhang J., Zhang J., Wu Y., Ji S., Zhang Y., Yang A., Han H., Yao L. (2008) Nucleic Acids Res. 36, 5335–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Butt A. J., McNeil C. M., Musgrove E. A., Sutherland R. L. (2005) Endocr. Relat. Cancer 12, S47–S59 [DOI] [PubMed] [Google Scholar]
- 46. Haynes M. P., Li L., Sinha D., Russell K. S., Hisamoto K., Baron R., Collinge M., Sessa W. C., Bender J. R. (2003) J. Biol. Chem. 278, 2118–2123 [DOI] [PubMed] [Google Scholar]
- 47. Burchfield J. G., Lennard A. J., Narasimhan S., Hughes W. E., Wasinger V. C., Corthals G. L., Okuda T., Kondoh H., Biden T. J., Schmitz-Peiffer C. (2004) J. Biol. Chem. 279, 18623–18632 [DOI] [PubMed] [Google Scholar]
- 48. Béguin P., Hasler U., Staub O., Geering K. (2000) Mol. Biol. Cell 11, 1657–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hasler U., Wang X., Crambert G., Béguin P., Jaisser F., Horisberger J. D., Geering K. (1998) J. Biol. Chem. 273, 30826–30835 [DOI] [PubMed] [Google Scholar]
- 50. Verrey F. (1995) J. Membr. Biol. 144, 93–110 [DOI] [PubMed] [Google Scholar]
- 51. Garty H., Palmer L. G. (1997) Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 52. Tang C. H., Wu W. Y., Tsai S. C., Yoshinaga T., Lee T. H. (2010) J. Comp. Physiol. B 180, 813–824 [DOI] [PubMed] [Google Scholar]
- 53. Zhao C., Dahlman-Wright K., Gustafsson J. A. (2008) Nucl. Recept. Signal. 6, e003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.