Abstract
α-Phenyl-N-tert-butylnitrone (PBN), a free radical spin trap, has been shown previously to protect retinas against light-induced neurodegeneration, but the mechanism of protection is not known. Here we report that PBN-mediated retinal protection probably occurs by slowing down the rate of rhodopsin regeneration by inhibiting RPE65 activity. PBN (50 mg/kg) protected albino Sprague-Dawley rat retinas when injected 0.5–12 h before exposure to damaging light at 2,700 lux intensity for 6 h but had no effect when administered after the exposure. PBN injection significantly inhibited in vivo recovery of rod photoresponses and the rate of recovery of functional rhodopsin photopigment. Assays for visual cycle enzyme activities indicated that PBN inhibited one of the key enzymes of the visual cycle, RPE65, with an IC50 = 0.1 mm. The inhibition type for RPE65 was found to be uncompetitive with Ki = 53 μm. PBN had no effect on the activity of other visual cycle enzymes, lecithin retinol acyltransferase and retinol dehydrogenases. Interestingly, a more soluble form of PBN, N-tert-butyl-α-(2-sulfophenyl) nitrone, which has similar free radical trapping activity, did not protect the retina or inhibit RPE65 activity, providing some insight into the mechanism of PBN specificity and action. Slowing down the visual cycle is considered a treatment strategy for retinal diseases, such as Stargardt disease and dry age-related macular degeneration, in which toxic byproducts of the visual cycle accumulate in retinal cells. Thus, PBN inhibition of RPE65 catalytic action may provide therapeutic benefit for such retinal diseases.
Keywords: Enzyme Inhibitors, Radicals, Retina, Rhodopsin, Signal Transduction, Vision, alpha-Phenyl-N-tert-butylnitrone, Light Damage, RPE65, Visual Cycle
Introduction
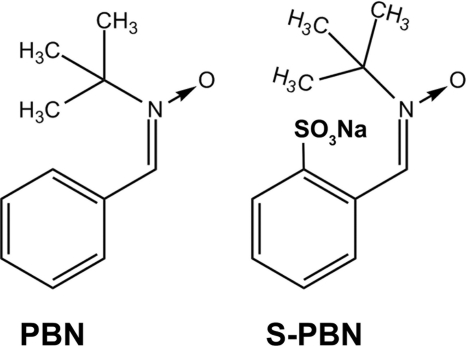
In several previous publications, we have shown that α-phenyl-N-tert-butylnitrone (PBN)4 (see Fig. 1), a commonly used free radical spin trap, provides remarkable protection of photoreceptor and RPE cells from light-induced damage (1–5). Because of its anti-oxidant properties and based on several reports demonstrating beneficial pharmacological effects, including reduction in mortality associated with endotoxin shock (6–8), neuroprotection in ischemia-reperfusion and aging models (9–10), and prevention of streptozotocin-induced diabetes in mice (11), we speculated that PBN could be a useful therapeutic intervention against retinal degenerative diseases, such as age-related macular degeneration. Because the retina has a high oxygen demand, is chronically exposed to light, and contains several photosensitizers, oxidative stress is presently considered to be a cause of disease progression in age-related macular degeneration (12). Thus, we speculated that PBN, which is already known to be effective against age-related and accumulative oxidative stress (13, 14), might also be effective against age-related macular degeneration.
FIGURE 1.
Structures of PBN and S-PBN.
The mechanism(s) underlying the PBN-mediated protection of photoreceptor cells are not well understood. Given the role of oxidative stress in retinal light damage (15–18) and the free radical scavenging properties of PBN, it seemed logical to propose that PBN exerts antioxidant function in the retina by quenching reactive oxygen species generated in the early stages of light stress. However, besides free radical scavenging, PBN has a multitude of pharmacological effects in neuroprotection, such as modulating the expression of various cytokine genes, Cox-2 (cyclooxygenase 2), inducible nitric-oxide synthase, and the transcription factors NF-κB and AP-1 (activator protein 1) (19, 20). Because AP-1 is involved in light-induced photoreceptor cell death (21, 22), inhibition of c-fos activation has been proposed as one of the mechanisms of PBN-mediated protection of photoreceptors (5). However, the mechanism of c-fos gene down-regulation by PBN is not clear. Because a functional rhodopsin visual pigment is necessary to elicit the damaging effects of light (23), we speculated that PBN might influence the rate of visual pigment regeneration by slowing the retinoid visual cycle.
The retinoid visual cycle is a multistep process for the recycling of 11-cis-retinal (the chromophore of both rod and cone visual pigments) and is essential for regeneration of visual pigment and maintenance of normal vision. The key step of the visual cycle is the hydrolysis-isomerization of all-trans-retinyl ester to 11-cis-retinol. Previously, we and others have established that the enzyme that catalyzes the isomerization step is RPE65 (retinal pigment epithelium-specific protein of 65 kDa) isomerohydrolase (24–26). Intact RPE65 function is essential for vision because mutations in the RPE65 gene have been reported to cause several forms of inherited retinal dystrophies (27–31). Moreover, no 11-cis-retinoids were detected in the Rpe65−/− mice, suggesting that RPE65 is the only enzyme that can produce 11-cis-retinoids in the eye (32, 33). Rpe65−/− mice were found to be completely protected against light-induced apoptosis (34). In the current study, we established that RPE65 isomerohydrolase activity is efficiently inhibited by PBN slowing down the visual pigment regeneration rate.
EXPERIMENTAL PROCEDURES
Animal Care
All procedures were performed according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the University of Oklahoma Health Sciences Center Guidelines for Animals in Research. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees of the University of Oklahoma Health Sciences Center and the Dean A. McGee Eye Institute. Albino Sprague-Dawley rats and BALB/c mice were born and raised in the Dean A. McGee Eye Institute vivarium and maintained from birth under dim cyclic light (5 lux, 12 h on/off, 7 a.m. to 7 p.m. central time).
PBN Treatment and Light Exposure Regimens
PBN, purchased from Sigma-Aldrich, is a lipophilic compound that is sparsely soluble in aqueous solvents (solubility is up to 15 mg/ml in phosphate-buffered saline (PBS)). We also purchased N-tert-butyl-α-(2-sulfophenyl) nitrone sodium salt (S-PBN) from the same source, which is much more soluble in water (Fig. 1) and also possesses similar free radical trapping properties (35). Consistent with previous publications (1–2, 5), a dose of 50 mg/kg PBN and S-PBN dissolved in saline was chosen for intraperitoneal injection in the rats in the treatment group. An equal volume of saline was injected into control rats.
PBN injection in dark-adapted rats 0.5 h before damaging light exposure provides almost complete protection of the retina from light-induced damage (1, 2, 5). We used a light stress paradigm of 2,700 lux for 6 h to test the PBN effect at different time points before (0.5, 3, 6, 12, 16, and 24 h) or after (0.5 and 3 h) light exposure. The effect of S-PBN was tested using the same dose and protocol of PBN at 0.5 h before light exposure.
Electroretinography
After light exposure, rats were returned to their dim cyclic light (5–10 lux) environment for 7 days before ERG recordings were performed. Flash ERGs were recorded with the Diagnosys Espion E2 ERG system (Diagnosys, LLC, Lowell, MA). Rats were maintained in total darkness overnight and prepared for ERG recording under dim red light. They were anesthetized with ketamine (120 mg/kg body weight) and xylazine (6 mg/kg body weight) intramuscularly. One drop of 10% (v/v) phenylephrine was applied to the cornea to dilate the pupil, and one drop of 0.5% (v/v) proparacaine HCl was applied for local anesthesia. Rats were kept on a heating pad at 37 °C during recordings. A gold electrode was placed on the cornea, a reference electrode was positioned in the mouth, a ground electrode was placed on the foot, and rats were placed inside a Ganzfeld illuminating sphere. Responses were differentially amplified, averaged, and stored. For the assessment of rod photoreceptor function (scotopic ERG), five strobe flash stimuli were presented at flash intensities at −2.3, −1.3, 0.7, and 2.7 log cd·s/m2. The amplitude of the a-wave was measured from the prestimulus base line to the a-wave trough. The amplitude of the b-wave was measured from the trough of the a-wave to the peak of the b-wave. For the evaluation of cone function (photopic ERG), a strobe flash stimulus (3.3 log cd·s/m2) was presented to dilated, light-adapted (5 min at 1.7 log cd·s/m2) rats. The amplitude of the cone b-wave was measured from the trough of the a-wave to the peak of the b-wave.
Possible defects in the visual cycle were analyzed by measuring the time course of dark adaptation (recovery of rod photoreceptor sensitivity) following a bleaching light exposure. Rats were fully dark-adapted and injected intraperitoneally with PBN (50 mg/kg) or saline. Full-field scotopic ERGs for both eyes were recorded after 3 h of PBN injection. A single test flash of 2.3 log cd·s/m2 was presented to elicit the rod a-wave response under fully dark-adapted conditions. Rats were then exposed to a steady field of log 2.3 cd·s/m2 for 2 min in the Ganzfeld dome to bleach rod photoreceptors. Immediately following the bleaching period (time = 0 min) and every 10 min thereafter (time = 10, 20, 30, 40, 50, and 60 min), the same test flash of 2.3 log cd·s/m2 was presented. The a-wave responses at the indicated times after bleaching were normalized to the initial dark-adapted response for each rat.
To evaluate cone function under conditions in which rod recovery is suppressed, rats were injected with PBN (50 mg/kg) or saline 60 min prior to recording single-flash photopic and flicker-flash ERG recordings. Rats were placed under a steady adapting field of 1.7 log cd·s/m2 for at least 7 min. A single flash of 3.3 log cd·s/m2 was presented under the same adapting field to elicit a maximal cone response. Cone responses were further evaluated by presenting 1.2 log cd·s/m2 stimuli flickering at frequencies of 3, 10, 20, and 30 Hz under the same adapting field.
Histology
After ERG recordings, rats were killed by carbon dioxide asphyxiation for light microscopic evaluation of retinal structure. Immediately after death, eyes were excised, placed in fixative (4% (w/v) paraformaldehyde, 2% (v/v) trichloroacetic acid, 20% (v/v) isopropyl alcohol, 2% (v/v) zinc chloride, and 72% (v/v) distilled water), and embedded in paraffin. Sections of 5 μm were cut along the vertical meridian through the optic nerve and stained with hematoxylin and eosin (H&E). The thickness of the outer nuclear layer (ONL) was measured at 0.5-mm distances from the optic nerve to the inferior and superior ora serrata and plotted as a spider diagram.
Measurement of Rhodopsin Regeneration
To measure the effect of PBN on the rate of rhodopsin regeneration, dark-adapted rats were injected intraperitoneally with PBN (50 mg/kg) or PBS 0.5 h prior to a 2-h bleach in room light (∼400 lux). Immediately following the bleach (time = 0 min), a group of PBN- and vehicle-treated rats were killed, and retinas were removed and snap frozen. The remaining rats were dark-adapted, and retinas were collected under dim red light at 45, 90, and 180 min after the bleach. An additional group of rats was dark-adapted overnight, injected with PBN or vehicle, and maintained in darkness for 2 h to serve as dark-adapted controls and to determine if PBN had a direct effect on the binding of chromophore to rhodopsin.
Rhodopsin measurements were performed as described previously (2, 36) with slight modification. Briefly, under dim red light, each retina was homogenized in 450 μl of buffer containing 10 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 2% (w/v) octyl glucoside, and 50 mm hydroxylamine. Homogenates were centrifuged at 16,000 × g, and soluble lysates were scanned from 270 to 800 nm in a spectrophotometer (Ultrospec 3000 UV-visible spectrophotometer, GE Healthcare). Samples were then bleached under room light for 10 min and scanned again. The difference spectra at 500 nm between pre- and postbleached samples were used to determine rhodopsin content using a molar extinction coefficient of 42,000 m−1 (37). The values were normalized to the total lysate volume, and data are presented as rhodopsin content/retina.
Assay for Retinal RDH Activity
The effect of PBN on retinal dehydrogenase (RDH) enzymes was determined in vitro using mouse retinal microsomes. To prepare retinal microsomes, dissected mouse retinas were homogenized in sucrose buffer (25 mm sucrose, 10 mm Tris-Cl (pH 7.2), and 1 mm EDTA), containing protease inhibitors (2 μg/ml aprotinin, 5 μg/ml pepstatin A, 10 μg/ml leupeptin, and 0.5 mm phenylmethylsulfonyl fluoride, final concentrations), using a Polytron PT-1200 CL homogenizer (Kinematica Inc., Bohemia, NY). Homogenates were centrifuged at 10,000 × g for 10 min at 4 °C. The resulting supernatants were then centrifuged at 100,000 × g for 1 h at 4 °C, and microsomal pellets were resuspended in storage buffer (50 mm Tris-Cl (pH 7.2), 1 mm EDTA, 20% glycerol, 1 mm dithiothreitol, and the protease inhibitors described above). Protein concentrations were measured, and microsomal fractions were stored at −80 °C after snap freezing in liquid nitrogen.
Reactions were carried out in 1 ml of reaction buffer (100 mm Tris-HCl (pH 7.2), 200 mm NaCl, 1 mm dithiothreitol, 2 mg/ml bovine serum albumin, and 1% (v/v) glycerol) with 5 μg of microsomes, 0 or 75 μm NADPH, 0 or 2 mm PBN, and increasing concentrations of the substrate all-trans-retinal. After a 1-h incubation at room temperature, reactions were terminated by the addition of 1 volume of cold methanol. Retinoids were extracted with 2 volumes of hexane and analyzed by HPLC. Samples were dissolved in HPLC mobile phase (11.2% (v/v) ethyl acetate, 2.0% (v/v) dioxane, 1.4% (v/v) octanol in hexane), and retinoids were separated by using a Lichrosphere Sil 60A (Phenomenex, Torrance, CA) 5-μm column. The peaks of all-trans-retinal (substrate) and all-trans-retinol (product of the reaction) were identified and quantified by comparison with pure retinoid isomeric standards. All procedures were performed under dim red light. For each condition, the non-enzymatic conversion of all-trans-retinal to all-trans-retinol measured in the absence of NADPH was subtracted from the conversion obtained with NADPH to determine the NADPH-dependent activity of retinal RDHs.
Isomerohydrolase Activity Assay and PBN Inhibition
Rats were sacrificed, and eyes were enucleated and dissected to remove the anterior part, including lens, vitreous, and retina. The remaining eyecups containing RPE were sonicated three times for 20 s in a cold 0.32 m sucrose, 0.1 m sodium phosphate buffer (pH 7.4). The homogenate was centrifuged (20 min, 20,000 × g) to sediment sclera, unbroken cells, nuclei, and mitochondria. The obtained supernatant was recentrifuged (1 h, 100,000 × g), and the microsomal pellet was resuspended in 10 mm 1,3-bis[tris(hydroxymethyl)-methylamino]propane (pH 8.0) and 100 mm NaCl and stored at −80 °C. All-trans-[11,12-3H]retinol (1 mCi/ml, 45.5 Ci/mmol, American Radiolabeled Chemicals, Inc., St. Louis, MO) in N,N-dimethyl formamide was used as the substrate for the isomerohydrolase assay. For each reaction, 50 μg of microsomal proteins from the rat RPE were added into 200 μl of reaction buffer (10 mm 1,3-bis[tris(hydroxymethyl)-methylamino]propane (pH 8.0), and 100 mm NaCl) containing 0.2 μm all-trans-retinol, 1% (w/v) BSA, and 25 μm cellular retinaldehyde-binding protein. For the inhibition studies, PBN or S-PBN dissolved in the PBS (pH 7.4) was added to the reaction prior to the addition of all-trans-retinol. The reaction was stopped, and retinoids were extracted with 300 μl of cold methanol and 300 μl of hexane and centrifuged at 10,000 × g for 5 min. The upper layer was collected, and the generated retinoids were analyzed by normal phase HPLC as described (38). The peak of each retinoid isomer was identified based on its characteristic retention time of retinoid standards. The isomerohydrolase activity was calculated from the area of the 11-cis-retinol peak using Radiomatic 610TR software (PerkinElmer Life Sciences) with synthetic 11-cis-[3H]-retinol as a standard. Alternatively, liposomes composed of 3.3 μm all-trans-retinyl palmitate and a 250 μm concentration of a mixture of 1,2-dioleoyl-sn-glycero-3-phosphocholine/1,2-dilauroyl-sn-glycero-3-phosphocholine (85:15) were incubated with 125 μg of total protein lysates from 293A cells that had been infected with chicken RPE65 adenovirus. Liposomes and chicken RPE65 were prepared as described previously (39). Non-linear regression analysis of v versus [S] data was used to calculate Vm(app) and Km(app) in the absence and in the presence of PBN. The inhibition constant for PBN was calculated from the equation, Ki = [I]/(Vmi/Vm − 1), where [I] represents the concentration of PBN, Vmi is maximal velocity in the presence of PBN, and Vm is maximal velocity in the absence of PBN.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). The quantitative data are expressed as mean ± S.D. or S.E. for each group. Student's and paired t tests were performed to assess differences between means.
RESULTS
PBN Protection of Retina from Light Stress
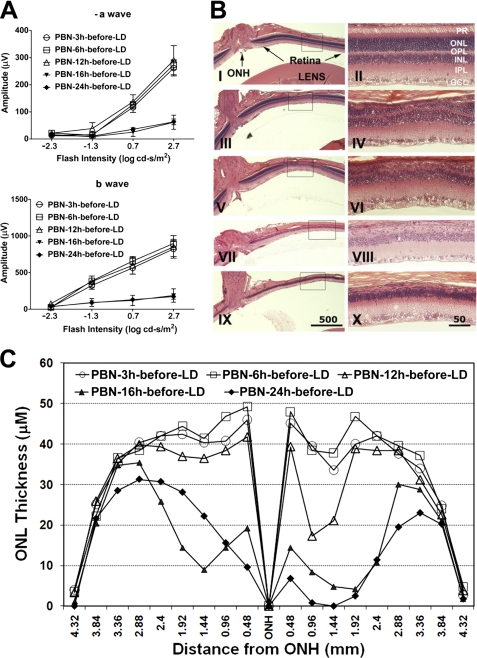
In previous studies, we have shown that 50 mg/kg PBN administered 0.5 h before light stress (2,700 lux for 24 h) provided significant (80–90%) protection of retinal structure and function (1). Here, we have used the same dose of light but for a shorter duration (6 h), which also causes significant loss of photoreceptor cell function (Fig. 2). Electroretinography was performed 7 days after light exposure; thus, the reductions in ERG responses (Fig. 2A) reflect the loss of photoreceptor cells (Fig. 2B). Representative H&E-stained sections of retina 7 days after light damage are shown in Fig. 2B. By 7 days, the dead photoreceptors were removed, and the remaining nuclei in the ONL represent the viable photoreceptors. The findings obtained by functional ERG analysis were confirmed by quantitative morphometry of the thickness of the ONL of the retina, which provided a direct indicator of photoreceptor cell number (Fig. 2C). PBN injection 0.5 h before light exposure almost completely protected against light-induced loss of retinal function (rod a- and b-wave responses; Fig. 2A) and structure (Fig. 2, B and C) (compare no light damage (NLD) versus saline-injected versus PBN-injected). Interestingly, the same dose of PBN administered 0.5 h after light exposure did not provide any protection (Fig. 2, A–C). Water-soluble S-PBN also failed to protect against light-induced loss of photoreceptor structure and function (Fig. 2, A–C).
FIGURE 2.
Effect of PBN on protection against light-induced retinal degeneration. A, retinal rod photoreceptor function was measured by ERG 7 days after light exposure. Full-field dark-adapted ERGs were recorded for flashes of increasing intensities (−2.3–2.7 log cd·s/m2). Scotopic a-wave and b-wave responses (mean ± S.E. (error bars), n = 6–10 rats) are presented. NLD, no light damage; LD, light-damaged. B, after ERG recordings, eyes were harvested for histology, marked for orientation, and fixed. Five-μm sections were cut along the vertical meridian through the optic nerve and stained with H&E. Representative sections from each treatment were imaged from the superior-central retina, which is specifically affected by exposure to damaging light. Panels on the right represent higher magnifications of the superior-central retina. I and II, no light damage; III and IV, saline before light damage; V and VI, PBN before light damage; VII and VIII, PBN after light damage; IX and X, S-PBN before light damage. C, quantitative morphometric measurement of ONL thickness was measured from H&E-stained slides along the vertical meridian from superior to inferior retina (n = 8–10). ONH, optic nerve head.
We then performed an experiment to test the effect of PBN administration at several time points before light exposure. PBN administration up to 12 h prior to light exposure protected against the light-induced loss of photoreceptor cells but was not protective when administered 16 or 24 h before light exposure (Fig. 3, A–C). Although some ONL thinning was observed in a small region of the superior central retina when PBN was administered 6 or 12 h before damaging light exposure (Fig. 3B), the loss of photoreceptors in this small area was not reflected in the full field ERG responses, which showed complete functional protection (Fig. 3A). The preservation of cone function as measured by single flash photopic b-wave amplitude 7 days after damaging light exposure paralleled the results shown for rods. Cone function was preserved in retinas in which PBN was administered between 0.5 and 12 h prior to light exposure. However, cone function was not preserved if PBN was administered 16 or 24 h before light exposure (supplemental Fig. 1). As seen for rods, PBN did not protect cone function when administered after light damage, and S-PBN failed to protect cone function (supplemental Fig. 1). In summary, the results indicate that PBN must be administered within a specific time window to protect effectively and that the protective effect is only effective with the more hydrophobic version of this compound.
FIGURE 3.
Effect of the time of PBN administration on the ability to protect against light-induced retinal degeneration. A, rod photoreceptor function was measured by ERG 7 days after light exposure. B, representative H&E sections as described in the legend to Fig. 2B. I and II, PBN 3 h before LD; III and IV, PBN 6 h before LD; V and VI, PBN 12 h before LD; VII and VIII, PBN 16 h before LD; IX and X, PBN 24 h before LD. C, quantitative morphometry of ONL thickness of the samples described in A and B (n = 6–8). Error bars, S.E. ONH, optic nerve head.
Effect of PBN on the Rate of Recovery of Rod Photoresponses; Dark Adaptation ERG
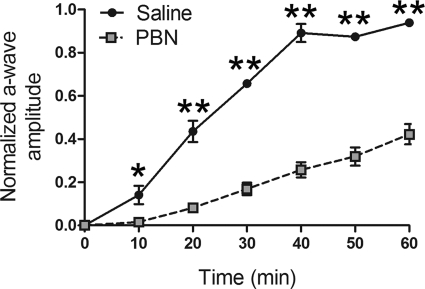
The damaging effect of bright light requires a functional rhodopsin photopigment (23). It is well established that PBN protects against light damage (1), and we previously speculated that PBN, as part of its protective mechanism, might down-regulate the rhodopsin regeneration rate (2). We tested this hypothesis by measuring the in vivo recovery of rod photoresponses following a bleaching illumination as described previously (40, 41). After overnight dark adaptation, rats were injected with PBN or saline 30 min prior to exposure to a 2.3 log cd·s/m2 test flash to elicit a saturated rod a-wave response. These fully dark-adapted a-wave responses from PBN-treated and control rats were indistinguishable (PBN, −441.5 ± 70.66 μV; saline, −430.9 ± 27.81 μV; n = 4), indicating that PBN did not directly affect the rod photocascade when the test flash was presented to fully dark-adapted retinas. However, as shown in Fig. 4, PBN treatment dramatically reduced the rate of recovery of rod photoresponses such that 40 min after the bleaching illumination, PBN-treated rats had only recovered 25% of the initial fully dark-adapted response, whereas vehicle-treated rats had recovered completely. These results demonstrate that PBN reduces the dark adaptation rate of rods.
FIGURE 4.
PBN suppresses recovery of rod responses after bleaching. Recovery of rod photoresponses after bleaching (2.3 log cd/m2 for 2 min) in PBN-treated (gray square; n = 4) and saline-treated rats (solid circle; n = 4). a-wave responses before and after bleaching were elicited by 2.3 log cd×s/m2 flashes and were normalized to the amplitude of the initial, dark-adapted response. PBN administration significantly reduced the recovery of rod responses at the indicated time points (*, p ≤ 0.05; **, p ≤ 0.005; unpaired t test, n = 4). Error bars, S.E.
To assess the effect of PBN administration on cone function under conditions when rod recovery was suppressed, we performed single- and flicker-flash ERG recordings under a steady, rod-suppressing background illumination 60 min after PBN administration. As shown in Fig. 5, cone function was not affected by PBN under conditions when rod recovery was completely suppressed.
FIGURE 5.
PBN administration does not inhibit cone function. PBN or saline were administered 60 min prior to recording single-flash photopic and flicker-flash ERGs under a steady adapting field of 1.7 log cd/m2. Representative single-flash photopic (A) and flicker-flash (B) responses are indicated. C, quantitative analysis of single-flash and flicker-flash ERG responses (n = 4) indicated that PBN did not suppress cone function under conditions when rod recovery is inhibited. No significant differences were found between PBN and saline for any cone responses (unpaired t test, n = 4). Error bars, S.E.
Effect of PBN on Rhodopsin Regeneration
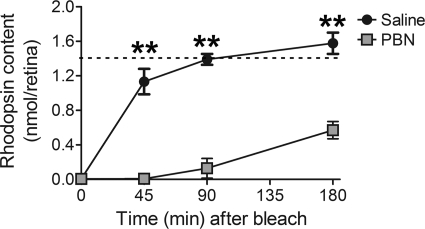
To test whether the dramatic PBN-induced delay of dark adaptation was mediated by direct inhibition of the rate of regeneration of functional rhodopsin, we measured rhodopsin content, spectrophotometrically, at several time points before, during, and after a complete photobleach. As indicated in Fig. 6, rhodopsin recovery was significantly inhibited by administration of PBN. PBN was administered to fully (overnight) dark-adapted rats that were subsequently exposed to room light for 2 h, resulting in a complete bleach of rhodopsin in both saline- and PBN-injected rats (Fig. 6, time = 0 min). When returned to darkness for 45 min, virtually no rhodopsin was regenerated in PBN-treated rats, whereas rhodopsin recovery in controls was already 75% of fully dark-adapted values (Fig. 6, dotted line). Even after dark adaptation for 3 h, only ∼30% of the bleachable rhodopsin had recovered in PBN-treated rats, whereas saline-injected control levels had fully recovered. These results demonstrate profound inhibition of rhodopsin recovery by PBN administration in vivo. Administration of PBN to rats maintained in darkness did not reduce rhodopsin content (PBN, 1.38 ± 0.14 nmol/eye; saline, 1.41 ± 0.07 nmol/eye) (mean ± S.E.), indicating that PBN did not directly displace chromophore from the visual pigment.
FIGURE 6.
PBN administration inhibits rhodopsin regeneration. Rats were dark-adapted overnight and injected with PBN or PBS (vehicle) immediately prior to a complete bleach for 2 h in room light (∼400 lux). Rats were returned to darkness for the indicated times, and retinas were collected for spectrophotometric determination of rhodopsin content. Values represent the mean nmol of rhodopsin/eye, and the error bars represent S.E. PBN significantly inhibited rhodopsin regeneration at the indicated time points (**, p ≤ 0.005; unpaired t test, n ≥ 4). No significant differences were observed between fully dark-adapted rats injected with either PBN (1.38 ± 0.14 nmol/eye) or PBS (1.41 ± 0.07 nmol/eye). The dotted line represents the mean rhodopsin content of fully dark-adapted rats injected with or without PBN.
Effect of PBN on Retinol Dehydrogenase Activity
The dark-adapted ERG and the rhodopsin regeneration kinetics presented in Figs. 4 and 6, respectively, indicated that PBN delayed the regeneration of functional visual pigment without directly affecting the association of 11-cis-retinal with rhodopsin. These results suggested that PBN might exert its effects on some enzymes of the visual cycle. To test this, we measured the effect of PBN on the activities of major RDHs and RPE enzymes (LRAT and RPE65) responsible for maintaining the visual cycle. Several RDHs are expressed in the retina and participate in the regeneration of the 11-cis-retinal chromophore of rhodopsin by catalyzing the first step of the visual cycle, which is the reduction of all-trans-retinal to all-trans-retinol, using NADPH as coenzyme. To determine if PBN could directly inhibit retinal RDH activity, we used an in vitro assay with mouse retinal microsome preparation as a source of RDH enzymes. NADPH-dependent reduction of all-trans-retinal was measured in the presence and absence of PBN. A representative saturation curve is shown in Fig. 7A. The addition of PBN did not inhibit retinal RDH activity. Results from the average of three experiments presented in Fig. 7B show that PBN does not significantly inhibit RDH activity.
FIGURE 7.
PBN does not inhibit retinol dehydrogenase activity. Retinal RDH activity was measured in the presence and absence of PBN with increasing concentrations of substrate. The representative experiment shown in A was repeated two times with similar results. Histograms of the relative RDH activities at three different substrate concentrations and 2 mm PBN are shown in B. In this graph, the RDH activity without PBN represents 100%. No statistically significant differences were found between RDH activities in the absence and in presence of PBN. Error bars, S.D.
Effect of PBN on RPE65 Isomerohydrolase Activity
It has been previously shown that conversion of all-trans-retinyl ester to 11-cis-retinol catalyzed by RPE65 is a rate-limiting step in the visual cycle (42). To determine the direct inhibition of PBN on isomerohydrolase activity of RPE65, we used an in vitro isomerohydrolase assay. Although all-trans-retinyl ester is the endogenous substrate for the RPE65 isomerohydrolase (38), the insolubility of retinyl ester in water prevented its direct use in the reaction. Therefore, all-trans-[3H]retinol was used to produce retinyl esters by LRAT in rat RPE microsomes; these esters were then converted directly to 11-cis-[3H]retinol by RPE65. Incubation of the rat RPE microsomes with all-trans-[3H]retinol resulted in formation of all-trans-retinyl esters and significant amounts of 11-cis-[3H]retinol, as shown by the HPLC elution profile (Fig. 8A). The addition of 1 mm PBN to the reaction resulted in almost complete inhibition of 11-cis-retinol generation (Fig. 8B), whereas the production of retinyl ester did not decrease, suggesting that LRAT was not inhibited by PBN. The PBN inhibition of isomerohydrolase activity was concentration-dependent (Fig. 8D) with an apparent IC50 of 0.1 mm. Interestingly, S-PBN in the same enzymatic assay did not inhibit RPE65 generation of 11-cis-retinol at a concentration as high as 5 mm (Fig. 8C).
FIGURE 8.
PBN inhibits retinol isomerohydrolase activity in vitro. RPE microsomal protein (50 μg) was incubated with 0.2 μm all-trans-[3H]retinol with and without PBN for 1 h at 37 °C. The generated retinoids were analyzed by normal phase HPLC. Shown are HPLC elution profiles without PBN (A), with 1 mm PBN (B), and with 5 mm S-PBN (C). Peak 1, retinyl esters; peak 2, 11-cis-retinol; peak 3, all-trans-retinol. D, PBN concentration-dependent inhibition of 11-cis-retinol generation (mean ± S.E. (error bars), n = 3).
To completely exclude the possibility that diminished 11-cis-retinol production could result from PBN-mediated inhibition of LRAT activity and to analyze the model of inhibition, we employed a recently developed liposome-based isomerohydrolase assay (39). In this assay, a hydrophobic substrate of RPE65, all-trans-retinyl palmitate, was incorporated into 1,2-dioleoyl-sn-glycero-3-phosphocholine/1,2-dilauroyl-sn-glycero-3-phosphocholine liposomes and could be directly converted to 11-cis-retinol in the isomerohydrolase reaction, allowing the determination of the type of inhibition. In this experiment, we used recombinant chicken RPE65, which has a more efficient isomerohydrolase activity compared with that from rod-dominant species (43). To determine the type of inhibition, we measured the isomerohydrolase activity at various substrate concentrations in the presence and absence of PBN. As shown in the Lineweaver-Burk graph (Fig. 9), both Vm and Km decreased in the presence of PBN, resulting in the plot consisting of two parallel lines characteristic of uncompetitive inhibition. The inhibition constant was calculated according to the formula for uncompetitive inhibition (44), which yields Ki = 53 ± 7 μm. These results agree well with the Ki recently reported for PBN (45).
FIGURE 9.
Uncompetitive inhibition of RPE65 isomerohydrolase by PBN in the liposome-based isomerohydrolase assay. Lineweaver-Burk plots of 11-cis-retinol generated by RPE65. Liposomes with increasing concentrations of all-trans-retinyl palmitate (S) were incubated with equal amounts (125 μg) of chicken RPE65 expressed in 293A cells, with (■) and without (○) PBN (0.1 mm).
DISCUSSION
Light-induced retinal degeneration is a useful model for retinal research because photoreceptor cell death occurs by apoptosis, as is the case in hereditary retinal degenerations, such as retinitis pigmentosa and age-related macular degeneration (46). Because of its advantage of causing synchronized and regulated cell death, this model has been used extensively to screen putative neuroprotective compounds (16, 47–48). Since its discovery in 1966 by Noell et al. (49), a number of studies have identified that photobleaching of rhodopsin is the essential trigger for retinal light damage (34, 50). The availability of retinol is also crucial because vitamin A-deficient rats are protected against light damage (51, 52). Rhodopsin knock-out (KO) mice, which lack the opsin apoprotein, and RPE-65 KO mice, which have opsin apoprotein but lack the ability to generate 11-cis-retinal, are both protected against light damage (33–34, 50).
A steady state rhodopsin level is achieved by the balance between its bleaching and regeneration, which involves both the RPE and photoreceptors. Therefore, the rate of rhodopsin regeneration is an important factor in light damage susceptibility. Fast regeneration of functional rhodopsin after bleaching increases retinal sensitivity to light damage, whereas slowing the flux of retinoids through the visual cycle increases the resistance of photoreceptors to light-induced insult. For example, slowing rhodopsin regeneration and inhibiting the visual cycle with 13-cis-retinoic acid prevents light damage in albino rats (53). 13-cis-Retinoic acid was shown to be a competitive inhibitor of RPE65, the rate-limiting enzyme of the visual cycle (54). More recently, it has been shown that the RPE65 inhibitor retinyl amine provides efficient protection from light damage (55). In mice, RPE65 enzymatic activity and the rate of rhodopsin regeneration are related, and slowing regeneration results in resistance to light damage (56). It has been shown that the extent of light damage is significantly lower in a mouse strain with L450M mutation in RPE65 (56). This mutation decreased the isomerohydrolase activity in RPE by ∼5-fold (38), making the retina of Met-450 mice significantly resistant to light damage (57).
Most of the small molecules inhibiting the visual cycle are structurally similar to retinoids. Although PBN does not have structural similarity to RPE65 substrates (Fig. 1), we nevertheless decided to analyze its effect on the visual cycle enzymes, such as RDH and RPE65. Given the profound protective effect of PBN against light-induced retinal degeneration, we hypothesized that PBN may interfere with rhodopsin regeneration during the continuous illumination in our light damage animal model. This would be predicted to desensitize the retina to the damaging light. When we observed that PBN significantly slowed the rate of dark adaptation after bleaching (Figs. 4 and 6), we speculated that the mechanism could be one of the following: 1) PBN may interfere directly with the binding of 11-cis-retinal chromophore to opsin, or 2) it may inhibit one or more of the enzymes of the visual cycle involved in the generation of 11-cis-retinal chromophore. Our results support the latter mechanism. We found that in the dark, PBN did not have any effect on opsin-chromophore interaction or on phototransduction (Figs. 4 and 6). We therefore tested the direct effect of PBN on the enzymatic activities of visual cycle RDHs, LRAT, and RPE65. We observed that PBN did not affect the activity of RDHs or LRAT (Fig. 7) but significantly inhibited RPE65 activity (Figs. 8 and 9). RPE65 is the only isomerohydrolase in RPE cells, and it catalyzes the rate-limiting step of the visual cycle converting all-trans-retinyl esters to 11-cis-retinol. PBN efficiently inhibited this activity with an IC50 of 0.1 mm (Fig. 8) and significantly decreased the supply of 11-cis retinal to the photoreceptors in the retina. Thus, by inhibiting RPE65, PBN reduces the rate of visual pigment regeneration and substantially decreases the quantity of bleachable rhodopsin (Fig. 6). Therefore, it is reasonable to propose that because PBN reduces the quantity of the primary and necessary trigger for light damage (i.e. bleachable rhodopsin), this is the mechanism by which it provides significant protection from light damage. This also explains why PBN is not effective when administered after light exposure (Figs. 2 and 3), because light-damaging rhodopsin activation was already induced. We observed that the effect of PBN on rhodopsin regeneration lasted for several h (Fig. 6) and could prevent light damage when administered up to 12 h before the exposure. We cannot completely rule out the additional potentially beneficial effect of PBN on scavenging the oxidants generated by intense light exposure. However, given the profound effect on RPE65 activity and on rhodopsin regeneration, we favor the hypothesis that this is the primary protective mechanism in light damage.
Although PBN clearly and potently inhibited rhodopsin regeneration and recovery of rod photoreceptor responsiveness after bleaching, cone function was maintained in the presence of PBN. These results support a growing body of evidence indicating a non-canonical visual cycle in the retina that rapidly provides 11-cis-retinaldehyde to cones (reviewed recently (58)). Although RPE65 is expressed in mammalian cones (59), it is unclear whether it acts as the isomerohydrolase for the cone-specific visual cycle. Assuming that RPE65 expressed in cones can be inhibited by PBN, the results of the present study suggest that either RPE65 is not necessary for the retina-specific cone visual cycle or PBN is preferentially taken up by the RPE and does not reach a high enough concentration in the retina to inhibit the RPE65 expressed in cones. Our results demonstrate substantial retention of visual function (i.e. normal cone function) under conditions where PBN completely inhibits the canonical, RPE visual cycle. These findings provide support for the use of PBN as a therapeutic agent to slow the accumulation of toxic retinoid intermediates that accumulate in the RPE in diseases such as Stargardt maculopathies without affecting diurnal vision provided by cone photoreceptors.
The mechanisms of RPE65 inhibition by PBN as well as the mechanism of RPE65 reaction are currently unclear. Recently, it has been proposed that the isomerohydrolase reaction may proceed through the formation of an intermediate retinoid radical cation, which facilitates the retinoid polyene chain isomerization by lowering the energy of activation barrier (60). It is likely that PBN can trap this intermediate radical and interrupt the reaction. Although the spin trapping of retinoid radicals has not been reported in the literature, it has been shown that PBN can produce stable adducts with carotenoid radicals (61). Alternatively, PBN can bind at the active site of RPE65, competing with the binding of retinyl ester substrate. To distinguish between these two alternatives, we studied PBN inhibition using chicken recombinant RPE65 in the liposome-based isomerohydrolase assay. PBN inhibits RPE65 uncompetitively (Fig. 9), suggesting that it does not bind to the free RPE65 enzyme but rather binds to the enzyme-substrate complex (44). This supports the idea that PBN may act as a spin trap at the active site of RPE65, binding the retinoid radical intermediate that forms a complex with RPE65.
Retinoid radicals are unstable and most likely can exist only as intermediates at the active site of RPE65. Therefore, to be efficient, the spin trap should have easy access to the active site of RPE65. The RPE65 active site with catalytic iron is located deep inside the hydrophobic tunnel that serves to bind the highly hydrophobic retinyl ester substrate of RPE65 (62). Because of its relatively high hydrophobicity, PBN can penetrate into the active site and interact with the retinoid radical, interrupting the formation of the 11-cis-retinol product. S-PBN is significantly more hydrophilic and probably cannot reach the RPE65 active site due to unfavorable interaction with hydrophobic aromatic residues at the substrate binding tunnel. This may explain why S-PBN does not interfere with RPE65 isomerohydrolase activity and consequently does not protect the retina from light stress. Similar results obtained from an independent study showed that aromatic and more lipophilic nitrone spin traps inhibit RPE65 activity effectively (45).
PBN is a commonly used free radical spin trap. Several derivatives of PBN are in preclinical trials for protection of tissues from oxidative damage, such as occurs in stroke and ischemic injuries. Our previous findings already indicated that PBN might be a good therapeutic candidate for human retinal degenerations. Interference with the normal visual cycle is one of the strategies for treating Stargardt diseases. This approach could preserve vision by decreasing the accumulation of toxic retinoid metabolites, such as the retinal fluorophore A2E, a major component of lipofuscin and a side product of the visual cycle (42). Following a similar strategy, there are at least two other compounds entered in human clinical trials, fenretinide-N-(4-hydroxyphenyl) retinamide (Sirion Therapeutics, Tampa, FL) and ACU-4429 (Acucela, Bothell, WA), demonstrating the potential of this therapeutic strategy (63, 64). Our results show that PBN did not inhibit cone visual cycle and thus is not anticipated to affect diurnal vision if taken as a drug. However, we note that its effect on rod visual function may result in reduced night vision, as has been observed for other inhibitors of the visual cycle (53, 64, 65). PBN is already a proven compound for photoreceptor preservation, and this determination of its mechanism of action makes it a potential agent for targeted therapies for Stargardt disease and dry age-related macular degeneration in which accumulation of A2E leads to pathogenesis.
Supplementary Material
Acknowledgments
We are grateful to Mark Dittmar and Alicia Avila (Dean A. McGee Eye Institute, Oklahoma City, OK) for assistance with animal breeding and feeding and to Louisa J. Williams and Linda S. Boone (Dean A. McGee Eye Institute, Oklahoma City, OK) for assistance in histology.
This work was supported, in whole or in part, by National Institutes of Health (NIH), NEI Grants EY 012190, EY 04149, EY 019494, EY 012231, and EY 00871 and NIH, NCRR, Grant RR 17703. This work was also supported by the Foundation Fighting Blindness, USA, Research to Prevent Blindness, Inc., USA, and the University of Oklahoma College of Medicine Alumni Association.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- PBN
- α-phenyl-N-tert-butylnitrone
- LRAT
- lecithin retinol acyltransferase
- RDH
- retinol dehydrogenase
- NF-κB
- nuclear factor κB
- Cox-2
- cyclooxygenase 2
- AP-1
- activator protein 1
- RPE65
- retinal pigment epithelium-specific protein of 65 kDa
- S-PBN
- N-tert-butyl-α-(2-sulfophenyl) nitrone sodium salt
- ERG
- electroretinography
- ONL
- outer nuclear layer
- RPE
- retinal pigment epithelium cells
- A2E
- pyridinium bis-retinoid
- cd
- candela.
REFERENCES
- 1. Ranchon I., Chen S., Alvarez K., Anderson R. E. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1375–1379 [PubMed] [Google Scholar]
- 2. Ranchon I., LaVail M. M., Kotake Y., Anderson R. E. (2003) J. Neurosci. 23, 6050–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanito M., Elliott M. H., Kotake Y., Anderson R. E. (2005) Invest. Ophthalmol. Vis. Sci. 46, 3859–3868 [DOI] [PubMed] [Google Scholar]
- 4. Tanito M., Kaidzu S., Anderson R. E. (2007) Invest. Ophthalmol. Vis. Sci. 48, 1864–1872 [DOI] [PubMed] [Google Scholar]
- 5. Tomita H., Kotake Y., Anderson R. E. (2005) Invest. Ophthalmol. Vis. Sci. 46, 427–434 [DOI] [PubMed] [Google Scholar]
- 6. Miyajima T., Kotake Y. (1995) Biochem. Biophys. Res. Commun. 215, 114–121 [DOI] [PubMed] [Google Scholar]
- 7. Novelli G. P., Angiolini P., Tani R., Consales G., Bordi L. (1986) Free. Radic. Res. Commun. 1, 321–327 [DOI] [PubMed] [Google Scholar]
- 8. Hamburger S. A., McCay P. B. (1989) Circ. Shock 29, 329–334 [PubMed] [Google Scholar]
- 9. Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 3633–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillis J. W., Clough-Helfman C. (1990) Neurosci. Lett. 116, 315–319 [DOI] [PubMed] [Google Scholar]
- 11. Tabatabaie T., Kotake Y., Wallis G., Jacob J. M., Floyd R. A. (1997) FEBS Lett. 407, 148–152 [DOI] [PubMed] [Google Scholar]
- 12. Zarbin M. A. (2004) Arch. Ophthalmol. 122, 598–614 [DOI] [PubMed] [Google Scholar]
- 13. Saito K., Yoshioka H., Cutler R. G. (1998) Biosci. Biotechnol. Biochem. 62, 792–794 [DOI] [PubMed] [Google Scholar]
- 14. Sack C. A., Socci D. J., Crandall B. M., Arendash G. W. (1996) Neurosci. Lett. 205, 181–184 [DOI] [PubMed] [Google Scholar]
- 15. De La Paz M. A., Anderson R. E. (1992) Invest. Ophthalmol. Vis. Sci. 33, 2091–2096 [PubMed] [Google Scholar]
- 16. Organisciak D. T., Darrow R. M., Jiang Y. I., Marak G. E., Blanks J. C. (1992) Invest. Ophthalmol. Vis. Sci. 33, 1599–1609 [PubMed] [Google Scholar]
- 17. Organisciak D. T., Wang H. M., Li Z. Y., Tso M. O. (1985) Invest. Ophthalmol. Vis. Sci. 26, 1580–1588 [PubMed] [Google Scholar]
- 18. Wiegand R. D., Giusto N. M., Rapp L. M., Anderson R. E. (1983) Invest. Ophthalmol. Vis. Sci. 24, 1433–1435 [PubMed] [Google Scholar]
- 19. Kotake Y., Sang H., Miyajima T., Wallis G. L. (1998) Biochim. Biophys. Acta 1448, 77–84 [DOI] [PubMed] [Google Scholar]
- 20. Sang H., Wallis G. L., Stewart C. A., Kotake Y. (1999) Arch. Biochem. Biophys. 363, 341–348 [DOI] [PubMed] [Google Scholar]
- 21. Hafezi F., Steinbach J. P., Marti A., Munz K., Wang Z. Q., Wagner E. F., Aguzzi A., Remé C. E. (1997) Nat. Med. 3, 346–349 [DOI] [PubMed] [Google Scholar]
- 22. Hao W., Wenzel A., Obin M. S., Chen C. K., Brill E., Krasnoperova N. V., Eversole-Cire P., Kleyner Y., Taylor A., Simon M. I., Grimm C., Remé C. E., Lem J. (2002) Nat. Genet. 32, 254–260 [DOI] [PubMed] [Google Scholar]
- 23. Organisciak D. T., Vaughan D. K. (2010) Prog. Retin. Eye Res. 29, 113–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. (2005) Cell 122, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gu S. M., Thompson D. A., Srikumari C. R., Lorenz B., Finckh U., Nicoletti A., Murthy K. R., Rathmann M., Kumaramanickavel G., Denton M. J., Gal A. (1997) Nat. Genet. 17, 194–197 [DOI] [PubMed] [Google Scholar]
- 28. Marlhens F., Bareil C., Griffoin J. M., Zrenner E., Amalric P., Eliaou C., Liu S. Y., Harris E., Redmond T. M., Arnaud B., Claustres M., Hamel C. P. (1997) Nat. Genet. 17, 139–141 [DOI] [PubMed] [Google Scholar]
- 29. Morimura H., Fishman G. A., Grover S. A., Fulton A. B., Berson E. L., Dryja T. P. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3088–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorenz B., Gyürüs P., Preising M., Bremser D., Gu S., Andrassi M., Gerth C., Gal A. (2000) Invest. Ophthalmol. Vis. Sci. 41, 2735–2742 [PubMed] [Google Scholar]
- 31. Thompson D. A., Gyürüs P., Fleischer L. L., Bingham E. L., McHenry C. L., Apfelstedt-Sylla E., Zrenner E., Lorenz B., Richards J. E., Jacobson S. G., Sieving P. A., Gal A. (2000) Invest. Ophthalmol. Vis. Sci. 41, 4293–4299 [PubMed] [Google Scholar]
- 32. Fan J., Rohrer B., Moiseyev G., Ma J. X., Crouch R. K. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13662–13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redmond T. M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J. X., Crouch R. K., Pfeifer K. (1998) Nat. Genet. 20, 344–351 [DOI] [PubMed] [Google Scholar]
- 34. Grimm C., Wenzel A., Hafezi F., Yu S., Redmond T. M., Remé C. E. (2000) Nat. Genet. 25, 63–66 [DOI] [PubMed] [Google Scholar]
- 35. Williams H. E., Claybourn M., Green A. R. (2007) Free Radic. Res. 41, 1047–1052 [DOI] [PubMed] [Google Scholar]
- 36. Tanito M., Brush R. S., Elliott M. H., Wicker L. D., Henry K. R., Anderson R. E. (2009) J. Lipid Res. 50, 807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irreverre F., Stone A. L., Shichi H., Lewis M. S. (1969) J. Biol. Chem. 244, 529–536 [PubMed] [Google Scholar]
- 38. Moiseyev G., Crouch R. K., Goletz P., Oatis J., Jr., Redmond T. M., Ma J. X. (2003) Biochemistry 42, 2229–2238 [DOI] [PubMed] [Google Scholar]
- 39. Nikolaeva O., Takahashi Y., Moiseyev G., Ma J. X. (2009) FEBS J. 276, 3020–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. (1999) Cell 98, 13–23 [DOI] [PubMed] [Google Scholar]
- 41. Kasus-Jacobi A., Ou J., Birch D. G., Locke K. G., Shelton J. M., Richardson J. A., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Edwards A. O. (2005) J. Biol. Chem. 280, 20413–20420 [DOI] [PubMed] [Google Scholar]
- 42. Maiti P., Kong J., Kim S. R., Sparrow J. R., Allikmets R., Rando R. R. (2006) Biochemistry 45, 852–860 [DOI] [PubMed] [Google Scholar]
- 43. Moiseyev G., Takahashi Y., Chen Y., Kim S., Ma J. X. (2008) J. Biol. Chem. 283, 8110–8117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fersht A. (1985) Enzyme Structure and Mechanism, pp. 108–109, W.H. Freeman and Co., New York [Google Scholar]
- 45. Poliakov E., Parikh T., Ayele M., Kuo S., Chander P., Gentleman S., Redmond T. M. (2011) Biochemistry 50, 6739–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Remé C. E., Grimm C., Hafezi F., Marti A., Wenzel A. (1998) Prog. Retin. Eye Res. 17, 443–464 [DOI] [PubMed] [Google Scholar]
- 47. Wenzel A., Grimm C., Seeliger M. W., Jaissle G., Hafezi F., Kretschmer R., Zrenner E., Remé C. E. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1653–1659 [PubMed] [Google Scholar]
- 48. Sugawara T., Sieving P. A., Iuvone P. M., Bush R. A. (1998) Invest. Ophthalmol. Vis. Sci. 39, 2458–2465 [PubMed] [Google Scholar]
- 49. Noell W. K., Walker V. S., Kang B. S., Berman S. (1966) Invest. Ophthalmol. 5, 450–473 [PubMed] [Google Scholar]
- 50. Humphries M. M., Rancourt D., Farrar G. J., Kenna P., Hazel M., Bush R. A., Sieving P. A., Sheils D. M., McNally N., Creighton P., Erven A., Boros A., Gulya K., Capecchi M. R., Humphries P. (1997) Nat. Genet. 15, 216–219 [DOI] [PubMed] [Google Scholar]
- 51. Noell W. K., Albrecht R. (1971) Science 172, 76–79 [DOI] [PubMed] [Google Scholar]
- 52. Noell W. K., Delmelle M. C., Albrecht R. (1971) Science 172, 72–75 [DOI] [PubMed] [Google Scholar]
- 53. Sieving P. A., Chaudhry P., Kondo M., Provenzano M., Wu D., Carlson T. J., Bush R. A., Thompson D. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gollapalli D. R., Rando R. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10030–10035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maeda A., Maeda T., Golczak M., Imanishi Y., Leahy P., Kubota R., Palczewski K. (2006) Mol. Pharmacol. 70, 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wenzel A., Reme C. E., Williams T. P., Hafezi F., Grimm C. (2001) J. Neurosci. 21, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grimm C., Wenzel A., Williams T., Rol P., Hafezi F., Remé C. (2001) Invest. Ophthalmol. Vis. Sci. 42, 497–505 [PubMed] [Google Scholar]
- 58. Wang J. S., Kefalov V. J. (2011) Prog. Retin. Eye Res. 30, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Znoiko S. L., Crouch R. K., Moiseyev G., Ma J. X. (2002) Invest. Ophthalmol. Vis. Sci. 43, 1604–1609 [PubMed] [Google Scholar]
- 60. Redmond T. M., Poliakov E., Kuo S., Chander P., Gentleman S. (2010) J. Biol. Chem. 285, 1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Konovalova T. A., Kispert L. D., Polyakov N. E., Leshina T. V. (2000) Free Radic. Biol. Med. 28, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 62. Kiser P. D., Golczak M., Lodowski D. T., Chance M. R., Palczewski K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17325–17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mata N. L., Vogel R. (2010) Curr. Opin. Ophthalmol. 21, 190–196 [DOI] [PubMed] [Google Scholar]
- 64. Kubota R., Boman N. L., David R., Mallikaarjun S., Patil S., Birch D. (2011) Retina, in press [DOI] [PubMed] [Google Scholar]
- 65. Radu R. A., Mata N. L., Nusinowitz S., Liu X., Sieving P. A., Travis G. H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4742–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.