Abstract
Genome evolution of bacteria is usually influenced by ecology, such that bacteria with a free-living stage have large genomes and high rates of horizontal gene transfer, while obligate intracellular bacteria have small genomes with typically low amounts of gene exchange. However, recent studies indicate that obligate intracellular species that host-switch frequently harbor agents of horizontal transfer such as mobile elements. For example, the temperate double-stranded DNA bacteriophage WO in Wolbachia persistently transfers between bacterial coinfections in the same host. Here we show that despite the phage's rampant mobility between coinfections, the prophage's genome displays features of constraint related to its intracellular niche. First, there is always at least one intact prophage WO and usually several degenerate, independently-acquired WO prophages in each Wolbachia genome. Second, while the prophage genomes are modular in composition with genes of similar function grouping together, the modules are generally not interchangeable with other unrelated phages and thus do not evolve by the Modular Theory. Third, there is an unusual core genome that strictly consists of head and baseplate genes; other gene modules are frequently deleted. Fourth, the prophage recombinases are diverse and there is no conserved integration sequence. Finally, the molecular evolutionary forces acting on prophage WO are point mutation, intragenic recombination, deletion, and purifying selection. Taken together, these analyses indicate that while lateral transfer of phage WO is pervasive between Wolbachia with occasional new gene uptake, constraints of the intracellular niche obstruct extensive mixture between WO and the global phage population. Although the Modular Theory has long been considered the paradigm of temperate bacteriophage evolution in free-living bacteria, it appears irrelevant in phages of obligate intracellular bacteria.
Introduction
Bacteriophages, viruses that infect bacteria, play a major role in bacterial genome evolution and ecology through their global abundance [1] and their ability to laterally transfer their genomes between bacteria [2], [3], [4]. The most common bacterial viruses, the double-stranded (ds)DNA tailed phages, outnumber prokaryotic cells by 10-fold in environmental samples [5] and are responsible for the majority of intraspecific genome diversification in bacteria [6], [7], [8]. This diversification is due in part to bacteriophages triggering genomic rearrangement in their host bacteria and transmitting new genetic material both within and sometimes between different bacterial species. A striking example of bacteriophage diversity is found in Mycobacterium smegmatis, where eighty different dsDNA tailed phages from 21 different viral clusters are present [9], [10], [11], [12]. Bacteriophages are also known to alter bacterial cell biology by facilitating the transfer of virulence factors such as superantigens, extracellular toxins, effector proteins that modulate host-cell invasion, and host-cell adhesion factors [13].
The Modular Theory of dsDNA phages, as originally proposed by Botstein (1980), asserts that phage genomes consist of conserved clusters of functionally-related genes (i.e. modules) that can be interchanged by horizontal transfer among a large common phage gene pool [14], [15]. These phage modules are composed of contiguous sets of genes involved in a similar function, such as head assembly, tail formation, or regulation of the lysis and lysogeny cycles. While the genome of the phage is the total composite of the phage's DNA, the Modular Theory asserts that phage evolution primarily acts at the level of gene modules due to promiscuous module exchange between unrelated phages, where essentially one module is replaced with another that has the same general biological function. Comparative approaches suggest modularity and mosaicism are major evolutionary hallmarks of dsDNA phages. However, generalizing the principles to all dsDNA phages will require an expanded analysis of phage genomics in diverse ecological ranges [16]. In this regard, obligate intracellular bacteria, which live and replicate within the cytosol of host cells, are an ideal test of the Modular Theory since the intracellular niche may pose ecological restraints on exposure to novel phage gene pools.
The genome sequences of obligate intracellular bacteria have brought a renewed interest in mobile element evolution in bacteria prone to genome reduction [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. Comparative analyses of multiple genomes of obligate intracellular species demonstrate that although these bacteria have tiny genomes, their ecological range correlates with mobile element abundance. Specifically, species that host-switch tend to harbor significantly more mobile elements than those species that transmit vertically through the maternal line [31], [32]. Of the mobile elements studied in obligate intracellular bacteria, temperate bacteriophages are noteworthy for their ability to spread intercellularly [28], [33], [34], [35] and diversify the host bacterial genome [36].
At least three arguments suggest phages from host-restricted bacteria may not evolve by the Modular Theory. First, point mutations can be the major cause of phage diversification [37] across a core genome that is recalcitrant to lateral gene transfer. Second, some phage types, such as the T4-like phages, show a mixed genomic structure involving both modular exchanges and a conserved core genome [38]. Third, and perhaps most importantly, constraints on phage evolution in a restricted intracellular niche could suppress recombination with novel gene pools and lead to a preponderance of single nucleotide mutations and deletions.
Here we test for the first time whether the Modular Theory governs the genome evolution of double-stranded DNA phages in an obligate intracellular genus (Wolbachia). The WO bacteriophages are an ideal system to discern the evolutionary forces that are shaping phage genome and protein evolution in obligate intracellular bacteria. Wolbachia are predicted to infect two out of three arthropod species globally [39], [40], [41] in addition to 90% of filarial nematode species [42]. Extrapolation of the infection frequency using estimates of the number of arthropod species makes this bacterium one of the most infectious microbes on the planet. Wolbachia are transmitted both vertically within species and horizontally between species, which promotes a higher exposure rate to novel gene pools. For this study, we selected sequences from three complete Wolbachia genomes (wMel, wPip, and wRi) containing WO prophages [29], [30], [43] and complete prophage sequences from two additional Wolbachia (wCauB and wVitA) [28], [44]. These five Wolbachia induce cytoplasmic incompatibility, a reproductive modification that typically results in embryonic lethality between crosses of infected males and uninfected females. Each fully sequenced Wolbachia genome contains two to five prophage WO haplotypes, demonstrating that phage diversity is common within Wolbachia genomes. Molecular surveys have placed bacteriophage WO in 89% of Wolbachia from the A and B phylogenetic supergroups that infect arthropods [45], [46]. Thus, the abundance of phage WO and its ability to rampantly transfer between Wolbachia coinfections [28], [33], [35], [45] in one of the most prevalent bacterial infections in animals demonstrates its potential impact on bacterial symbiont diversity and host-symbiont interactions.
To determine the molecular evolutionary forces shaping prophage WO genomes, we addressed four interconnected questions. (i) First, does the Modular Theory explain the genetic changes in WO genomes or do point mutations provide most of the genetic diversity? (ii) Second, does the obligate intracellular niche constrain the acquisition of new genes and/or modules in WO prophages? (iii) Third, is the WO integration site and mechanism conserved in Wolbachia? We explore WO integration by comparing the recombinases encoded in each WO type and the areas of the host Wolbachia genome surrounding the integrated prophages. (iv) Finally, what is the relative strength of selection and recombination on prophage WO protein evolution across the functional modules of the genome?
Results
I. Does the Modular Theory explain the evolution of prophage WO genomes?
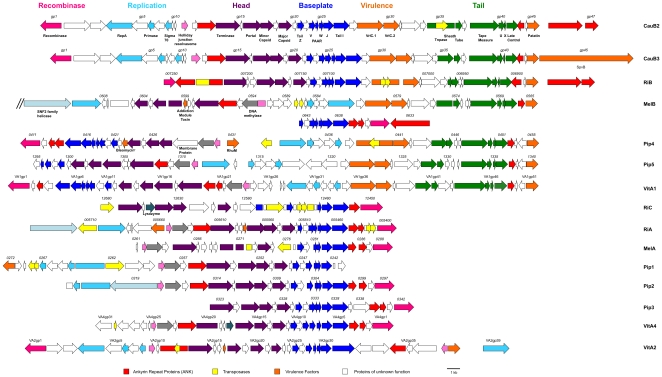
Comparative sequence analyses of 16 prophage WO genomes from Wolbachia strains that induce cytoplasmic incompatibility (Table 1) specify the largest WO prophages, WOCauB2 and WOCauB3, as 43.2 kb (46 genes) and 45.2 kb (47 genes), respectively. We define each prophage as a contiguous set of phage-related genes and each haplotype as a genetic variant of the prophage WO family. There are six haplotypes that are capable of forming virions, including WOCauB2 and WOCauB3 [47], WOVitA1 [28], [48], and at least one haplotype each from Wolbachia infecting Culex pipiens [49], Drosophila simulans, and D. melanogaster [50]. For the analyses below, the prophage region cutoffs for each haplotype are estimated according to the terminal genes of the WOCauB2 and WOCauB3 reference genomes. The first and main observation from these comparisons (Figure 1) is that the WO haplotypes do not exemplify one of the two patterns consistent with the Modular Theory [14]. While the genomes are modular in nature, there is no evidence of promiscuous exchange of functional modules between unrelated phages. The closest sequence relatives of all gene modules in WO are from other WO haplotypes based on nucleotide and protein BLAST searches. Thus, the recent, genetic changes of prophage WO within the niche of Wolbachia principally arose from descent with modification. However, the ancestral gene modules of WO were previously annotated to be from diverse phages [44],
Table 1. Prophage strains used in this study.
| Prophage | Host insect | Common Name | Wp group | Wp strain | Rp | ORFs | Head/Bp | Vir./Tail | Rec. | Rep. | Unchar. | Ref |
| WOCauB2 | Cadra cautella | moth | B | wCauB | CI | B2gp1-gp47 | gp11-gp27 | gp28-gp47 | gp1-gp3 | gp4-gp10 | NA | [44] |
| WOCauB3 | B2gp1-gp46 | gp12-gp28 | gp29-gp46 | gp1-gp3 | gp4-gp11 | NA | ||||||
| WOPip1 | Culex pipiens | mosquito | B | wPip | CI | WPa_0242-0272 | WPa_0242-0261 | NA | NA | WPa_0262-0270 | WPa_0271-0272 | [29] |
| WOPip2 | WPa_0297-0322 | WPa_0301-0318 | NA | NA | WPa_0319-0322 | NA | ||||||
| WOPip3 | WPa_0323-0342 | WPa_0323-0336 | NA | WPa_0337-0342 | NA | NA | ||||||
| WOPip4 | WPa_0411-0455 | WPa_0415-0430 | WPa_0438-0455 | WPa_0411-0414 | WPa_0433-0437 | WPa_0431-0432 | ||||||
| WOPip5 | WPa_1294-1340 | WPa_1294-1311 | WPa_1321-1340 | NA | WPa_1312-1318 | WPa_1319-1320 | ||||||
| WOMelA | Drosophila melanogaster | fruit fly | A | wMel | CI | WD_0261-0288 | WD_0261-0284 | NA | WD0285-0288 | NA | NA | [30] |
| WOMelB1 | WD_0565-0610 | WD_0593-0605 | WD_0581-0565 | NA | WD_0582-0592 | WD_0606-0610 | ||||||
| WOMelB2 | WD_0634-0644 | WD_0638-0644 | NA | WD_0634-0637 | NA | NA | ||||||
| WORiA-1 | Drosophila simulans Ri. | fruit fly | A | wRi | CI | wRi_005400-005720 | wRi_005460-005650 | NA | wRi_005400-005450 | NA | wRi_005660-005720 | [43] |
| WORiA-2 | wRi_010060-010380 | wRi_010120-010310 | NA | wRi_010060-010110 | NA | wRi_010320-010380 | ||||||
| WORiB | wRi_006880-007250 | wRi_p07230-007070 | wRi_007060-006880 | wRi_p07240-007250 | NA | NA | ||||||
| WORiC | wRi_012450-012670 | wRi_012670-012470 | NA | wRi_012450-012460 | NA | NA | ||||||
| WOVitA1 | Nasonia vitripennis | jewel wasp | A | wVitA | CI | VA1gp1-gp51 | VA1gp5-gp23 | VA1gp35-gp51 | VA1gp1-gp4 | VA1gp27-gp34 | VA1gp24-gp26 | [28] |
| WOVitA2 | VA2gp1-gp39 | VA2gp9-gp31 | NA | VA2gp1-gp3 | VA2gp4-gp8 | VA2gp32-gp39 | ||||||
| WOVitA4 | VA4gp1-gp31 | VA4gp5-gp27 | NA | VA4gp1-gp4 | NA | VA4gp28-gp31 |
Wp-Wolbachia pipientis; Rp- reproductive parasitism; CI – cytoplasmic incompatibility; NA – not applicable; Bp – Baseplate; Vir. – Virulence; Rec. – Recombinase; Rep. – Replication; Unchar. – Uncharacterized. WOVitA3 was not analyzed because the initial identification via PCR product published in [45] was found to be a chimera. For the purposes of this study, WOMelB1 and WOMelB2 are combined into one haplotype, WOMelB; see text for justification. Similarly, WORiA-1 and WOiRA-2 are analyzed as one haplotype, WORiA, since they are identical copies.
Figure 1. Prophage WO genomes are modular.
A schematic of gene synteny across the prophage WO genomes is depicted. Complete WO prophage sequences are available with the exception of wPip2 and wPip3, as the wPip genome sequence was artificially connected between genes within these two prophages [29]. These two prophages are treated as separate and complete. The two prophages from wCauB have been shown to be excisable by the mapping of their att sites [44] in conjunction with visualizing phage particles [44], [47]. Inverse PCR and sequencing analysis showed that WOCauB2 is conjoined between the integrase B2gp1 and the ankyrin repeat protein B2gp47, and WOCauB3 is conjoined between the integrase B3gp1 and the putative SpvB family toxin B3gp45 and hypothetical protein encoding B3gp46 genes [44]. WO haplotypes from wMel [46], wPip [49], and wVitA [48] are presumed excisable due to observations of lytic phage particles in each system. Genes are colored based on functional type and homology. Bright pink: integrase/recombinase; Red: Ankyrin-repeat protein; Turquoise: Replication module; Purple: Head module; Blue: Baseplate module; Orange: Putative virulence factors; Green: Tail module; Yellow: Transposases; Light pink: Holliday junction resolvasome/endonuclease; Grey: DNA methylase, Light teal: SNF2 helicase, Dark teal: lysozyme. The numbers above genes refer to the locus tag of that gene in the published genome.
Second, each Wolbachia strain has at least one complete prophage with head, baseplate, virulence, and tail modules in addition to one or more partial prophages (Figure 1). We classify partial prophages as genomes that lack one of these modules; they are unlikely to be active by themselves as they are generally missing tail genes that are required for adsorption and infection. However, an intact copy of each known structural gene in a Wolbachia genome could allow for bacteriophage protein “commandeering” where the prophages that lack the tail module could use proteins encoded by the other functional haplotype within the genome to complete their assembly and movement. Alternatively, these partial prophages may form virions that are tailless or they do not form virions at all.
The presence of partial prophage sequences can be explained by three possible scenarios: (i) recurrent infections by new WO types followed by degeneration, (ii) duplications of the resident WO haplotype(s) by errors in replication or recombination, followed by degeneration of one of the copies, or (iii) a combination of the two scenarios. To distinguish these alternatives, we compared the average nucleotide identity of seven WO prophage genes within each Wolbachia to that between different Wolbachia (Table S1). If the haplotypes arose by duplication within a Wolbachia genome, then we expect to observe higher prophage sequence homology within a Wolbachia genome than between them. Six of the genes selected for this analysis are homologs of WOCauB2 genes gp17, gp18, gp19, gp21, gp22, and gp23; they occur in all of the prophages. The seventh gene is a homolog of WOCauB2 gp15 that is absent only in WOPip4. The average nucleotide identity of these prophage genes within a Wolbachia genome ranged from 53.4% in wCauB (gp23) to 99.1% in wPip (gp15) (Table S1). The genes analyzed from most WO phages never had the highest level of nucleotide identity with another prophage in the same Wolbachia genome. For example, WOMelB and WORiA-1 and WORiA-2 (identical copies, hereafter referred to as WORiA) are more closely related to each other (99.9% identical) than to the other prophages within their Wolbachia genomes (75.6% in wMel and 80% in wRi). One exception is strain wPip from Culex pipiens, where prophage WO genes are more likely to have their closest homolog in the same genome. The genes in WOPip1, WOPip2, and WOPip3 are most closely related to each other, with an average of 93.5% similarity, when compared to other WO prophages (MWU, two-tailed, p<0.001). For five of the seven genes and four out of six genes, respectively, WOPip5 and WOPip4 are most closely related to another wPip prophage. Therefore, with the exception of wPip that appears prone to within-genome WO duplications, independent acquisition of new WO haplotypes explains the variation within a single Wolbachia genome.
A third observation of prophage WO genomes is that while the genes are syntenous within modules, the position of the modules within the prophages is highly variable. For instance, Figure 1 shows that in WOCauB2, WOCauB3, and WORiB, the head, baseplate, and tail genes are oriented in the same direction. However, in WOPip4, WOPip5, and WOVitA1, the head and baseplate modules are inverted compared to the tail module. In WOMelB, the head and tail module (denoted WOMelB1) are contiguous but inverted from each other, while the presumed baseplate module and the recombinase (WOMelB2) that belong to this prophage are located 34.7 kb downstream. These two fragments of the prophage were putatively conjoined at one point because they are proximate to each other. The insertion between them is derived from a lateral transfer event with a Rickettsia plasmid [36], and the two prophage fragments complement each other exactly to make an intact prophage. Interestingly, despite the orientation of the other modules in the genome, the recombinase gene is always positioned so that the 3′ end is adjacent to the flanking region of the prophage (Figure 1), which typically contains an ankyrin repeat family protein.
WO is temperate and should therefore have identifiable endolysin genes. Surprisingly, the prophages do not contain a conserved endolysin, despite electron micrograph evidence that phage WO can lyse Wolbachia [35], [48]. No holins and only two lysozymes (in WOVitA4 and WORiC) were identified in the WO prophages. Therefore, the proteins encoding the lysin by which WO exits the bacterial cell may be novel. The patatin-like phospholipase encoded on the terminal portion of the tail module could be involved in cell exit or entry of the bacteriophage. Likewise, other than the integrase, proteins that comprise a typical lysogeny module, such as transcriptional regulators, are unidentified.
A RepA-family helicase, a primase, and a sigma-70 subunit that may direct the bacterial RNA polymerase to these genes for initiation of DNA strand synthesis are present in the predicted replication module. At least one of the genes is encoded in ten of the sixteen WO prophages, but only six WO genomes encode all three genes. Two other genes with a predicted function, a Holliday junction resolvasome/endonuclease and a DNA methylase, are present that could be involved in DNA replication and packaging. These two genes are adjacent to each other and lie between the replication and head modules in the ten prophages for which they are both present. The endonuclease could assist DNA packaging of mature phage heads by cleaving branched DNA structures of replicated phage DNA. The methylases may modify the packaged phage DNA such that it becomes resistant to bacterial restriction systems. The endonuclease is present in three additional prophages in which the methylase is absent. Interestingly, the endonuclease and methylase genes are oriented so that they appear to be part of the head/baseplate modules and not the replication module. The endonuclease is likely to degrade either bacterial DNA to inhibit the host during WO's lytic cycle or superinfecting phage DNA. In three prophages (WOMelB, WORiA-1, and WORiA-2), a second repA gene is present and adjacent to a SNF2-family helicase. In WOPip2, the SNF2 helicase is part of the replication module that also contains a single repA and the sigma-70 subunit.
II. Does the obligate intracellular niche constrain the acquisition of new genes in WO phages?
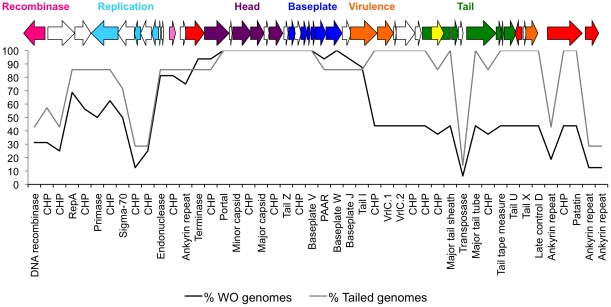
Figures 1 and 2 show that WO is comprised of core genes that are present in nearly all WO types and dispensable genes that are only present in some prophage WO types. When comparing gene content between the prophages with and without tail genes, there is a demarcation in whether certain functional modules are preserved. Across all genomes, the baseplate and head modules span 15 genes and may comprise a single module based on gene orientation and the close proximity of reading frames. These modules are also present in nearly all WO genomes (Figure 2). Furthermore, an integrase gene is present in 14/16 WO genomes, but this gene is highly variable and groups into three major phylogenetic clusters (Figure S1). For example, the family of integrase found in WOCauB2 is only present in four other WO genomes. In contrast, the dispensable gene clusters chiefly include the replication and tail/virulence gene modules. However, when just considering the prophages with tail genes, the tail genes and putative virulence genes VrlA, VrlC, and patatin are present in 100% of these WO prophages (Figure 2), suggesting that these genes play a functional role in tailed WO or Wolbachia biology. Indeed, patatins were originally annotated as storage proteins in potatoes, but they also have the lipolytic activity of phospholipase, catalyzing the cleavage of fatty acids from membrane lipids. Such enzymatic activity would be especially useful for phage WO when entering or exiting a membrane-bound intracellular bacteria.
Figure 2. The core genome of prophage WO consists only of head and baseplate genes.
The percentage of prophage WO genomes (N = 16) containing genes present in the active phage genome, WOCauB2, is depicted. Percentages were calculated for both all WO genomes (black) and only WO genomes with tail genes (grey). A gene map of WOCauB2 is shown above the plot with colors corresponding to the labels in Figure 1.
Notably, there are a few WO prophages that contain genes that are not present in the reference genome of WOCauB2. These ‘unique genes’ are summarized in Table S2 and encode conserved hypothetical proteins, an M1 lysozyme, an addiction module toxin, an RNA-directed DNA polymerase, a helicase of the SNF2 family, and a DNA methylase. The presence of an addition module toxin but not an antitoxin gene to rescue it is unexpected. Toxin-antitoxin loci are common in mobile elements of free-living bacteria and employed as post-segregational killers to spread the mobile genetic elements they are associated with. The observation of a toxin gene in prophage WO without an antitoxin complement may indicate that this toxin has evolved a new function in the intracellular niche, such as killing the host Wolbachia cell during lysis. The presence of a DNA methylase in WO is also interesting, as it is present in a high fraction of WO haplotypes (9/16). Methylases are common on bacteriophages and may modify the DNA such that it becomes resistant to bacterial restriction systems.
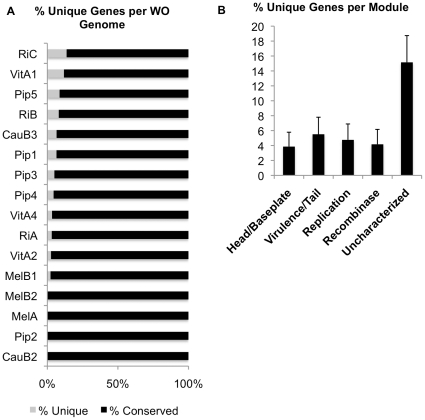
Additionally, a few genes only occur once within the 16 WO genomes, making them unique to that particular prophage haplotype (Figure 3A, Table S3). These genes can comprise up to 13% of a prophage genome and are distributed broadly across 12/16 prophages with the exception of WOMelA, WOPip2, WOMelB2, and WOCauB2. Unique prophage genes can be classified into two groups – those that differentiate WO genomes but that also occur in other locations in the Wolbachia genome, including conserved hypothetical genes and a prophage uncharacterized gene (Table S3), and those genes that are found solely within the WO genome, including insecticidal toxin gene SpvB of WOCauB3, a bleomycin resistance gene found in WOPip4, ankyrin repeat protein-encoding genes, and conserved hypothetical protein-encoding genes.
Figure 3. Unique genes in WO genomes are rare and scattered across functional modules.
(A) The percentage of genes specific to a single prophage WO haplotype was calculated. (B) The percentage of unique genes present in each functional region across all WO prophages and standard error was identified by calculating the number of unique genes divided by the number of genes in the module.
As the prophage functional modules are comprised of operons that could be disrupted or enhanced by the acquisition of new gene content, we assessed if unique genes to a WO genome occurred in specific modules of the genome or randomly across the prophage genome. Novel genes are distributed in all prophage modules, with the highest percentage of novel genes found in the head/baseplate region (39.3%) and the virulence/tail region (25%). The remaining 10.7%, 7.1%, and 17.9% of unique genes are found in the replication, recombinase, and uncharacterized modules, respectively. The uncharacterized areas are found either between the head/baseplate module and the tail module (WOVitA1 and WOPip5) or the terminal region of the prophage upstream from the head genes (WOVitA4, WORiA, WOPip1, WOPip2, WOPip4, WOMelA, and WOMelB1). However, after normalizing the data to the gene number of these different regions (Figure 3B), as larger regions with more genes could contain more unique DNA, the fractions of unique genes per module were 3.9% (11 unique genes/285 total genes) for the head/baseplate module, 4.2% (2/48) for the recombinase region, 5.5% (7/127) for the virulence/tail module, 4.8% (3/63) for the replication module, and 15.2% (5/33) for the regions not assigned to a specific module. In summary, prophage WO is capable of acquiring a limited number of novel genes throughout the prophage WO genome, especially in the uncharacterized regions that may be under relaxed selection relative to the structural or lifecycle modules.
WO genomes are also clearly prone to degradation due to transposon insertions from multiple different families and gene mutations that lead to non-functional proteins (Figure S2A). One genome lacking a tail module, WORiC, and one genome for which the core modules are separated by a large genomic segment, WOMelB1, contain the highest fractions of pseudogenes (13.6% and 8.9%, respectively), including mutations to genes required to generate an active phage particle. The other five prophage genomes for which pseudogenes are present contain a lower percentage (2.1–.7.1%) of pseudogenes. After the data is normalized to account for the number of total genes in each module, transposons are also most frequent in the uncharacterized modular region (2/33 or 6.06%). Interestingly, there is little difference between degradation in prophages with and without tail genes, as 44% of pseudogenes are found in prophages with tail genes and 56% are found in prophages without tail genes (Fisher's exact test, two tailed, p = 0.7395).
III. Is the WO integration site and mechanism conserved in Wolbachia?
To determine if the WO recombinases are homologous in Wolbachia, and thus mediate integration in a similar fashion, a protein alignment of the 14 site-specific recombinases was constructed. Three major families of integrases are represented in the WO prophages (Figure S1). First, the integrases encoded on a Nasonia vitripennis wVitA non-phage genome segment and WORiB are members of a family of phage-related tyrosine recombinases (93.9% amino acid identity) with the closest homolog found in Ehrlichia canis. Second, the integrases of WOPip2, WOPip3, WOPip4, WOVitA4, WOMelA and WOMelB are not closely related to the above integrases and belong to the serine recombinase family, and thus function using a different mechanism than the tyrosine recombinases [51]. These WO integrases share 84.4% amino acid identity. Finally, there are two more subgroups of recombinases including those in WOCauB2, WOCauB3, WOVitA2 and a WO remnant from wRi (96.4% amino acid identity) and those in WOVitA1 and WORiC (83.3% amino acid identity). These two groups of integrases also belong to the serine recombinase family. The high level of genetic and functional diversity in the recombinase genes supports the lack of a common integration site for all WO haplotypes and could be an indication of mosaic evolution that appears to not extend to other prophage WO modules.
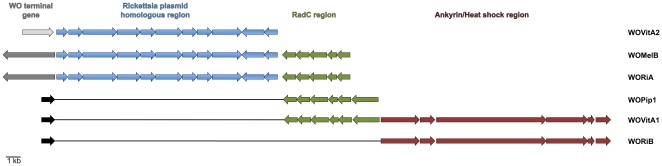
In order to confirm that members of prophage WO do not target conserved gene sequences for integration, the genes flanking prophage WO were compared. While there is no conserved gene set in all WO flanking regions, there are similarities between some WO types (Figure 4). Four prophages spanning three haplotypes (WOMelB, WORiA1, WORiA2, WOVitA2) have termini that are adjacent to a group of eleven genes also found in a plasmid from a Rickettsia symbiont of Ixodes scapularis ticks [52]. The average pairwise nucleotide identity between these four prophages in this region is 85.3%. In all but WOVitA2, the gene preceding this cluster is an SNF2-family helicase that, in eukaryotes, can regulate transcription, maintain chromosome stability during mitosis, and process DNA damage [53]. The presence of these genes within a prophage region was first reported by Ishmael et al. [36], who also demonstrated by microarray analysis that three closely-related Wolbachia infections from fruit flies (wAu, wSim, and wSan) contained the same genetic region. The more divergent Wolbachia infections of wPip and wBm do not have this region. A BLASTx search determined that this region is found in the genomic shotgun sequences of wWil and wAna of Drosophila.
Figure 4. WO flanking regions contain conserved gene sets.
The prophages WOVitA2, WOMelB, and WORiA are flanked on one end by a segment of genes that is conserved on a Rickettsial plasmid [36] (blue). In WOMelB and WORiA, a second conserved gene set (green), comprised of transcriptional regulators and the DNA repair gene radC, is found downstream of the Rickettsia gene homologs. This region is the prophage-flanking region in WOPip1 and WOVitA1. A third conserved gene segment (red) of ankyrin repeat proteins, a heat shock protein, and a conserved hypothetical protein, is found in WOVitA1 and flanking the prophage terminal gene in WORiB. Light gray: RepA; Dark gray: SNF2-family helicase; Black: patatin.
Immediately downstream from this conserved gene cluster in WOMelB and WORiA is a second set of conserved genes. These genes are also found adjacent to the phage terminal patatin gene in the phages WOPip1 and WOVitA1 (Figure 4), indicating a possible deletion of the Rickettsia homologs after prophage integration. These genes are oriented in the same direction, indicating that they may be cotranscribed, and include transcriptional regulators, the DNA repair protein RadC, and a conserved hypothetical protein. In WOVitA1 and WOPip1, this region extends to the DNA mismatch repair gene mutL. Interestingly, the region downstream of these genes in WOVitA1 (consisting of ankyrin repeat genes, the heat shock protein hspC2, and a hypothetical protein) is adjacent to the patatin in WORiB. This gene group is oriented in the same direction relative to each other, but opposite to the RadC gene cluster.
Eleven of the 16 prophages have the DNA repair protein RadC in the host Wolbachia regions that flank prophage WO, but never in the genome segment adjacent to the recombinase. The remaining WO phages have unknown flanking regions on the non-recombinase end (WOCauB2, WOPip2, WOPip3, and WOVitA4) or are not flanked by conserved gene segments (WORiC). While the majority of the prophages containing a radC homolog in the Wolbachia flanking regions do not have a large syntenous region, WOMelA, WOCauB3, WOPip4, and WOPip5 all contain a set of genes of similar function, including radC, transcriptional regulators, and hypothetical genes.
IV. What is the relative strength of selection and recombination on phage WO protein evolution across the functional modules of the genome?
A complete view of prophage evolution in obligate intracellular bacteria involves a balance among the forces of genetic drift, adaptive evolution, functional constraints, and recombination. Analyses of molecular evolution, when applied to loci across the prophage modules, provide insight on how the modules may be differentially evolving. One important caution, however, is that temperate bacteriophages in general are highly recombinogenic [15]. There is abundant evidence of recombination within the minor capsid protein of WO [33], [45] and this raises a concern that different regions of the locus or genome may have experienced different evolutionary histories due to recombination; therefore the inference of selection using maximum likelihood phylogenetic approaches (i.e., PAML) is inappropriate. We analyzed variation in selection (ω = dN/dS) across the WO modules using the omegaMap software package [54]. This method employs a Bayesian approach to parameter estimation that is independent of phylogeny, and therefore, is less likely to falsely identify sites subject to diversifying selection in sequences that display clear evidence of recombination [55], [56].
To address how selection is affecting phage protein evolution, we applied the program omegaMap to test for variation in the nature and strength of selection (ω) across specific phage loci and whether this variation corresponds to specific phage functions. Strikingly, we found throughout the entire phage genome that the average ω value per gene was <1, indicating that WO prophage genes are overall under strong, purifying selection (Table 2). Individual residues rarely experience significant, positive selection. The only exception in the dataset is gp45, which is predicted to encode phospholipases of the patatin family that may facilitate phage entry or exit into or out of the Wolbachia cell by digesting lipids. Its four 3′ terminal nucleotides are under significant, positive selection (mean ω = 6.61–6.69; posterior probability of positive selection >0.95).
Table 2. Recombination and Selection in WO genes.
| Gene | Length | n | θw | r2, d | |D′|, d | Plk | Pr2 | P|D′| | 2Ner/Locus (-InL) | 2Ner/site/θw | Mean ω |
| Untailed | |||||||||||
| gp15 | 222 | 6 | 0.105 | −0.170 | na | 0.86 | 0.001 | 0.002 | 0(−13173.81) | 0.000 | 0.111 |
| gp17 | 957 | 6 | 0.119 | −0.052 | na | 0 | 0.001 | 0 | 0(−304995.29) | 0.000 | 0.179 |
| gp18 | 366 | 6 | 0.111 | 0.177 | na | 0.004 | 0 | 0.051 | 0(−40621.8) | 0.000 | 0.303 |
| gp19 | 1002 | 6 | 0.069 | −0.033 | −0.028 | 0.372 | 0.014 | 0.012 | 0(−118253.22) | 0.000 | 0.052 |
| gp21 | 387 | 6 | 0.145 | −0.129 | −0.069 | 0 | 0 | 0 | 1.0(−70346.48) | 0.018 | 0.277 |
| gp22 | 462 | 6 | 0.154 | −0.031 | 0.015 | 0.001 | 0.015 | 0.843 | 1.0(−95555.08) | 0.014 | 0.206 |
| gp23 | 438 | 6 | 0.119 | −0.115 | −0.085 | 0 | 0 | 0 | 1.0(−68581.6) | 0.019 | 0.199 |
| Tailed | |||||||||||
| gp15 | 222 | 6 | 0.142 | −0.077 | −0.044 | 0 | 0.001 | 0.11 | 2.0(−22892.43) | 0.063 | 0.090 |
| gp17 | 957 | 6 | 0.131 | −0.063 | −0.073 | 0 | 0 | 0 | 2.0(−309686.81) | 0.016 | 0.190 |
| gp18 | 366 | 6 | 0.134 | −0.148 | −0.037 | 0 | 0 | 0.004 | 2.0(−50448.29) | 0.041 | 0.286 |
| gp19 | 1002 | 6 | 0.083 | −0.036 | −0.028 | 0 | 0 | 0 | 14.0(−154609.48) | 0.167 | 0.056 |
| gp21 | 387 | 6 | 0.165 | −0.026 | −0.004 | 0 | 0.029 | 0.414 | 1.0(−84186.15) | 0.016 | 0.217 |
| gp22 | 462 | 6 | 0.183 | −0.081 | −0.006 | 0 | 0 | 0.251 | 5.0(−117532.59) | 0.059 | 0.173 |
| gp23 | 438 | 6 | 0.214 | −0.012 | −0.015 | 0.002 | 0.091 | 0.028 | 3.0(−136640.64) | 0.032 | 0.204 |
| gp28 | 681 | 6 | 0.150 | −0.143 | −0.051 | 0 | 0 | 0 | 4.0(−210583.82) | 0.039 | 0.155 |
| gp30 | 1173 | 6 | 0.096 | −0.076 | 0.015 | 0 | 0 | 0.978 | 11.0(−275129.17) | 0.097 | 0.094 |
| gp31 | 189 | 6 | 0.160 | −0.198 | −0.108 | 0 | 0 | 0 | 4.0(−17092.57) | 0.132 | 0.171 |
| gp32 | 1230 | 6 | 0.120 | −0.228 | −0.070 | 0 | 0 | 0 | 1.0(−534906.31) | 0.007 | 0.274 |
| gp37 | 495 | 6 | 0.143 | −0.037 | −0.041 | 0 | 0.006 | 0 | 2.0(−104015.99) | 0.028 | 0.044 |
| gp40 | 336 | 6 | 0.220 | −0.007 | 0.003 | 0.636 | 0.254 | 0.645 | 0.0(−110168.19) | 0.000 | 0.241 |
| gp41 | 171 | 6 | 0.207 | −0.065 | −0.043 | 0.532 | 0.017 | 0.033 | 0.0(−18167.72) | 0.000 | 0.201 |
| gp42 | 867 | 6 | 0.196 | −0.003 | −0.005 | 0.999 | 0.157 | 0.089 | 0.0(−516916.65) | 0.000 | 0.226 |
| gp45 | 906 | 6 | 0.155 | −0.155 | −0.086 | 0 | 0 | 0 | 1.0(−393658.44) | 0.007 | 0.236 |
θw – the population mutation rate per site; r – correlation coefficient between pairs of loci; r2, d – correlation of r2 with distance; D′|, d - correlation of |D′| with distance; Plk , Pr2, P|D′| - probabilities resulting form testing the null hypothesis of no recombination with a likelihood permutation test; 2Ner/Locus (-InL) – the population recombination rate per locus under a coalescent framework; 2Ner/site/θw – the population recombination rate per locus per site; Mean ω-the mean ratio of dn/ds.
To statistically detect recombination within WO loci, we used the program LDhat [57], which analyzes sequence alignments and estimates the significance of intragenic recombination and the population rate of recombination (2Ner). It has been widely applied in several systems [57], including Helicobacter pylori, HIV, human mtDNA, and Wolbachia [45], [58]. Four estimates were calculated (Table 2) across genes that occurred in all prophages, or prophages that are separated into those with and without tail genes: (i) the population mutation rate (θw), (ii) the correlation coefficients of linkage disequilibrium (LD) with distance, (iii) the significance of the correlation using three different permutation tests, and (iv) the population recombination rate (2Ner) per locus under a coalescent framework. Genes used in the analysis are listed in Table S4 and Table S5 and were chosen based on the criteria that these genes lacked stop codon mutations. Sampling of the prophage taxa was restricted to the fully coding prophages with tail genes, WOCauB2, WOCauB3, WORiB, WOPip5, WOVitA1, and WOMelB, and the prophages without tail genes, WOPip1, WOPip2, WOPip3, WORiA, and, WOVitA4.
To determine if specific genes/modules are more likely to recombine than others, it is helpful to control for variation in population sizes (Ne) that can affect the estimates of recombination rate. The ratio 2Ner/θW (per site) reduces to 2Ner/2Neμ and then to r/μ, yielding the likelihood of a base pair experiencing a recombination event relative to mutation in a given gene. The r/μ ratio for head and baseplate genes is notably different between the prophages with and without tail genes, where there is a significant eight-fold difference between the recombination rates (0.056, tailed vs 0.007, nontailed; MWU, two tailed p = 0.007) (Table 2). All seven genes analyzed from the head/baseplate modules for both the prophages with and without tail genes show evidence of recombination (at least 2/3 permutation tests with a p<0.05, Table 2). Seven of the nine genes in the virulence/tail module are positive for recombination; only the tailU (gp40) and late control D (gp42) genes do not show recombination (Table 2).
Among the prophages with tail genes, three genes in particular account for the higher rates of recombination relative to mutation in comparison to the prophages without tail genes: major capsid gene gp19 (0.167), putative virulence VrlC homolog gp30 (0.097), and conserved hypothetical gp31 (0.132) (Table 2). When just comparing the rate of recombination between modules within the prophages with tail genes, the tail module had the lowest rate of recombination (0.016), which is 3.6-fold lower than the head/baseplate module (0.056; MWU, two tailed, p = 0.01) and 3.4-fold lower than the virulence module (0.058; MWU, two tailed, p = 0.031).
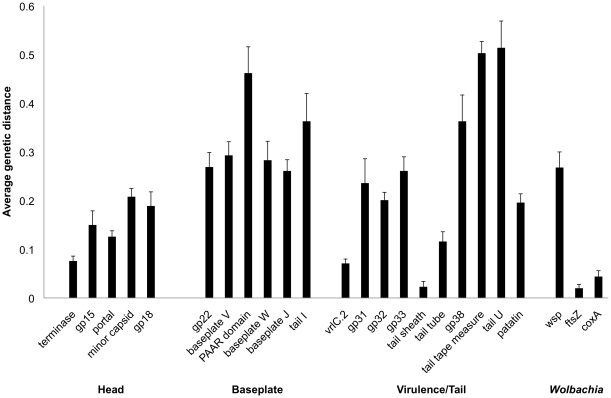
We also determined the average genetic distance, which is the average proportion of amino acid substitutions between a pair of proteins within a gene family, between homologs of genes across prophage WO (Figure 5). Proteins from the head region are the most evolutionarily conserved (average 0.1498) and have a significantly reduced genetic distance relative to the baseplate region (average 0.3218, MWU, two-tailed, p = 0.004) but not to the tail region (average 0.2484, MWU, two-tailed, p = 0.31). Elevated rates of change in the baseplate and some tail protein sequences is further evident by their similar genetic distances to the hypervariable Wolbachia surface protein wsp.
Figure 5. Mean genetic distance of WO and Wolbachia genes.
The average genetic distance and standard error was calculated for genes across Wolbachia prophage WO haplotypes. The values for the hypervariable Wolbachia surface protein gene wsp and the highly conserved Wolbachia housekeeping genes coxA and ftsZ are provided for comparison. Black bars represent genes having a predicted function while gray bars represent genes for which no function can be predicted.
Discussion
A null hypothesis for the evolution of dsDNA phages, known as the Modular Theory, is that phage genomes consist of clusters of functionally-related genes that can be interchanged by horizontal exchange within a large common gene pool [14], [15]. This theory of modular and mosaic evolution is well established in phage from free-living bacteria. However, access should not be uniform in the niche of an intracellular bacterium because the host cell is presumably a significant barrier to exchange with the global population of phages. In this regard, obligate intracellular bacteria are an ideal test of the Modular Theory as the intracellular niche may pose ecological restraints on exposure to novel phage gene pools.
In order to determine if Modular Theory applies to dsDNA phages in the obligate intracellular niche, we analyzed the prophage gene pool from Wolbachia. We show that WO prophages do not have a recent history of modular exchange and instead evolve through point mutations, deletion, recombination, and purifying selection. This non-mosaic evolution is also partly seen in the structural genes of the P2 family of phages that infect E. coli [59]. However, unlike P2 where evolution coincides with that of its host, phage WO evolution is incongruent from its host Wolbachia [45], a feature likely due to the rampant phage transfer between Wolbachia coinfections [28], [33], [34], [45].
While phage WO lacks mosaicism, it is modular in structure. The head and baseplate gene modules are found in every copy of WO, and the replication, virulence and tail modules are present in at least one WO type per Wolbachia genome. Given this pattern of one complete and at least one partial phage per Wolbachia genome, it is unsurprising that WO degradation is tolerated and pervasive. The presence of at least one intact copy of each known structural gene in a Wolbachia genome could allow for bacteriophage protein “commandeering” where the prophages that have mutations or lack the tail module could use proteins encoded on other WO prophages within the genome to complete their assembly and movement. A similar mechanism of transfer by defective phages has been shown for the Sp family from E. coli O157 [60].
Despite a reduced exposure to novel gene pools, WO is not completely immune to acquiring new genes. Transposons are frequently found within WO genomes and are known to play important roles in shaping the genomic diversity of Wolbachia [61], [62]. Additionally, the majority of WO prophages contain a few, unique genes with no defined role in phage functionality. Brussow and Hendrix [5] postulate that novel genes located within bacteriophages are frequently under their own transcriptional control and are maintained because they are advantageous to the bacterial host. Although a majority of novel WO genes are located in uncharacterized regions, some could be important to bacterial biology. Several of the novel genes are also found in non-phage regions of the Wolbachia genome, which indicates that WO could be exchanging new genes through recombination with the host genome.
Surprisingly, homologs of several genes required for complete function in dsDNA phages (holins, lysozymes, transcription factors) could not be identified in all prophage WO. It is possible that the large number of conserved hypothetical genes provide those missing functions. There is precedent for such genes that lack sequence homology to known functional phage proteins but perform identical roles [5], [63]. Further, the modular structure of phages is conserved among phages that infect Bacteria and Archaea, but there is little to no sequence homology between genes that provide an equivalent function.
Recombinases are one example of WO genes that are diverse in nucleotide identity but are functionally comparable. Prophage WO haplotypes encode a range of recombinases. Since recombinase genes are frequently interspersed throughout the Wolbachia genome, it is easy to extrapolate that recombination between WO and the host genome facilitates the switching of prophage recombinases. The diversity of recombinases in WO correlates with the apparent lack of a consensus in their integration site. Unlike prophages that recombine into a specific gene, such as a tRNA gene, the integration point of prophage WO cannot be predicted and seems to vary based on the prophage haplotype.
Evidence from ssDNA phages that do not evolve modularly has shown that structural genes (“self” genes) are more evolutionarily conserved, while genes that interact with the host (“nonself” genes) evolve more rapidly [64]. In this system, the viral coat proteins have fewer than 1% amino acid changes, while the assembly proteins have 1–10% amino acid differences, and the genes required for phage entry and release are more than 10% divergent. This theory offers one explanation for how phages isolated from diverse areas and at different times have a high degree of genetic similarity. The WO prophages follow this trend. The head module, which does not interact with the host, is the most conserved while the baseplate module, which is involved in phage-host recognition, is the most genetically divergent.
Temperate bacteriophages, such as WO, tend to be highly recombinogenic. Within WO, rates of intragenic recombination are 8-fold greater in genes from prophages with tail genes than in genes from prophages without tail genes. Since prophages with complete tail modules have a greater chance of forming virions and being transferred into new genomes, the increase in recombination could be due to a wider exposure over time to other WO phages. The question still remains if WO prophages that occur in the same genome can recombine with each other.
The nature of selection within prophage genes results in a complex dichotomy between what is advantageous for the bacteria versus what is best for the phage. Prophage WO genes are under strong purifying selection, where deleterious mutations are selected against and removed from the population. One major hypothesis of phage evolution is through illegitimate recombination, which often occurs within open reading frames [65]. If the recombination leads to knockdown of a functional module and lack of a viable phage, the deleterious event will be discarded [66], [67], [68]. In this case, the phage genes under strong purifying selection are akin to ‘housekeeping’ genes that are conserved to maintain function.
While phage WO is, to date, unique in the obligate intracellular bacteria, a modular dsDNA tailed phage, known as APSE, is present in the facultative symbiont of pea aphids, Hamiltonella defensa [69], [70], [71], [72]. Diversity in APSE is driven by recombination and it has >90% nucleotide identity with other APSE genomes [72], indicating that, like WO, it may not evolve by modular evolution. Other similarities between APSE and WO include the ability to gain novel genes and its variable copy number in host genomes [70]. If the Modular Theory does not apply to both WO and APSE, then it must be considered that phages in bacterial endosymbionts have a reduced ability to exchange DNA with other phages owing to their restricted niche and limited exposure to other phage gene pools.
Materials and Methods
Prophages used in this study
Prophages analyzed in this study were i) from whole genome Wolbachia sequences from the infections of Culex pipiens Pel (wPip, NC_010981) [29], Drosophila melanogaster yw (wMel, NC_002978) [30], Drosophila simulans Riverside (wRi, NC_012416) [43], and ii) from shotgun or partial genome Wolbachia sequences from the infections of Cadra cautella (wCauB, AB161975.2, AB478515.1, AB478516.1) [44] and Nasonia vitripennis (wVitA, HQ906662, HQ906663, and HQ906664) [28]. Prophages were divided into head, baseplate, recombinase, replication, virulence, and tail regions based on the predicted function of groups of genes oriented in the same direction (Table 1). Functionality was inferred based on i) the current gene annotation found in NCBI, ii) the annotation of non-Wolbachia homologs identified in a tblastx search of the nr database, and/or iii) the presence of conserved protein domains.
Identification of phage gene homologs and unique genes
A tblastx search using each annotated gene from the sixteen prophage WO genomes as the query was performed against the whole genome sequences of wPip [29], wMel [30], wRi [43], and wVitA (unpublished data) and the prophage and flanking sequences of WOCauB2 and WOCauB3 [44]. Genes were considered homologs if there was greater than 50% amino acid homology over 30% of the coding length. The bacterial species from which the closest relatives were identified was noted. Genes that did not have a homolog in Wolbachia, and thus considered unique to their phage haplotype, were used as the query in a tblastx search against the NCBI nr database to identify potential homologs in other bacteria.
Gene content and synteny
Prophage gene homologs of the WOCauB2 genes gp17, gp18, gp19, gp21, gp22, and gp23 (identified in the tBLASTx search described above) were aligned using the MUSCLE plugin [73] in Geneious version 5.0.4 [74]. Each representative from each haplotype was aligned with every other prophage WO homolog, and the percent nucleotide identity was compared between prophages integrated within the same Wolbachia genome and between prophages integrated in different Wolbachia genomes.
Comparison of recombinases
The amino acid sequences of the annotated recombinases found in the WO prophages were aligned using MUSCLE. A neighbor-joining consensus phylogenetic tree using the Jukes-Cantor general distance model, no outgroup, and 100 bootstrap replicates was constructed using the Geneious Tree Builder. A blastx search of the nr database was performed to identify the closest homologs and recombinase protein families for each phage WO representative.
Comparison of WO flanking regions
The Wolbachia genomic sequences flanking each prophage were compared using the Mauve [75], [76] plug-in in Geneious to identify homologous genes. For wCauB phages WOCauB2 and WOCauB3, the entire known flanking sequence was compared. For the phages for which the whole Wolbachia genome is sequenced, a minimum of 13.4 kb of flanking sequence was used for comparison.
Recombination and selection
The prophage WO homologs of seven genes from the head/baseplate region (Table S4) and nine genes from the virulence/tail region (Table S5) were aligned using MUSCLE. Criteria for the taxa analyzed were (i) they must have coding genes and (ii) the taxa were consistent among all of the alignments. Analysis of the head/baseplate genes was restricted to WOCauB2, WOCauB3, WOPip1, WOPip2, WOPip3, WOPip5, WOMelB1, WOMelB2, WORiA, WORiB, WOVitA1 and WOVitA4. Analysis of the tail region was restricted to WOCauB2, WOCauB3, WOPip5, WOMelB1, WORiB, and WOVitA1. Indels were removed from the alignment using MacClade version 4.08 [77].
To investigate the influence of recombination, the program LDhat [57] was used. LDhat estimates the population recombination rate by composite-likelihood method and employs a parametric approach, based on natural coalescence, to estimate the scaled parameter 2N e r where Ne is the effective population size, and r is the rate of recombination. The estimate of the population recombination rate is normalized by the mutation rate (θ), which is estimated using a finite-series version of the Watterson estimator. All the data sets were run through both crossing-over model L and Gene conversion model C with respective gene length and average tract length of 50-bp and 100-bp for the analysis of biallelic sites. Because recombination tract lengths are unknown for Wolbachia and the estimates of 2N e r can be highly dependent on the recombination tract lengths, recombination rates from the genetic exchange model producing the best-likelihood score are presented and should be interpreted with some caution. Nonetheless, all data sets produced the best-likelihood score with a gene conversion, 50-bp tract model.
In order to address the strength of adaptive evolution on point mutations, we used the method implemented in OmegaMap [54] that employs a Bayesian approach to parameter estimation that is independent of phylogeny, and therefore, less likely to falsely identify sites subject to diversifying selection in sequences that display clear evidence of recombination [55], [56]. The program estimates both variation in selection (ω = dn/ds) and the population recombination rate (ρ). The following prior distributions were used for the analyses: μ, κ and Öindel: improper inverse, ù: inverse with range 0.01–10, ρ: inverse with range 0.01–10. The variable block model was chosen for both ω and ρ, with block sizes of 30 and 30, respectively. Analyses were performed with 500,000 iterations and 10 reorderings as suggested in omegaMap documentation (Wilson, 2006). To summarize the data, the Summarize module of omegaMap program, which summarizes the results from every 100th generation of the run, was used. The first 50,000 sequences were discarded as a burn-in.
Mean evolutionary distance
To estimate the level of conservation for individual proteins within the WO phage family, homologous amino acid sequences for each protein were first aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2). The alignment was then imported into the program MEGA4 [78], and the overall mean distance was calculated under an equal input model. This model assumes that each amino acid site has the same substitution rate but adjusts for differing amino acid frequencies in the protein. Gaps within the alignment were ignored so that only sites that were present in all sequences were considered in the analysis. The overall mean distance represents the average proportion of amino acids that differ among the sequences aligned. For example, an overall mean distance of 0.2 indicates that, on average, the homologous proteins differ from one another in 20% of their amino acid residues. For comparison, the overall mean distances of two Wolbachia housekeeping genes, ftsZ and coxA, and one Wolbachia gene with a high level of variability among strains, wsp, were calculated using protein sequences from the same strains of Wolbachia in which the WO prophages are located. Standard error for each overall mean distance estimate was also calculated by MEGA4 using bootstrap analysis with 500 replications.
Supporting Information
WO Recombinases are Diverse. A neighbor-joining phylogenetic tree based on nucleotide sequences demonstrates that prophage WO recombinases cluster into three major groups. Groups A belongs to the tyrosine-recombinase family. Groups B and C belong to the serine-recombinase family.
(TIF)
Degradation of WO Genomes. The number of transposon insertions and pseudogenes were tallied in order to measure the degradation and gene loss in WO prophage genomes. A) The fraction of transposase genes and pseudogenes out of the total number of genes in each prophage WO genomes are denoted along with the standard error of proportion. B) The fraction of transposons and pseudogenes per functional module, normalized to account for difference in module size, is shown.
(TIF)
Synteny analysis of the WOCauB2 family of phages. Alignments were performed between the prophages and flanking regions of WORiB, WOCauB2, and WOVitA2. Dotplot analysis shows that these prophage genomes are syntenous and contain few breakpoints between the genomes.
(TIF)
Average percent nucleotide identity for each prophage gene within a Wolbachia genome. Parentheses indicate the number of phage genes/haplotypes per Wolbachia .
(DOC)
Genes present in WO haplotypes that are not present in WOCauB2.
(DOC)
Genes found in a single WO haplotype.
(DOC)
Genes used in selection and recombination analysis from the head and baseplate modules of both tailed and untailed phages.
(DOC)
Genes used in selection and recombination analysis from the virulence and tail modules.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Foundation grant number IOS-0852344 and the National Institutes of Health grant number R01 GM085163-01 to S.R.B. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiology and molecular biology reviews : MMBR. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brussow H. Phage as agents of lateral gene transfer. Curr Opin Microbiol. 2003;6:417–424. doi: 10.1016/s1369-5274(03)00086-9. [DOI] [PubMed] [Google Scholar]
- 3.Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Valera F, Martin-Cuadrado AB, Rodriguez-Brito B, Pasic L, Thingstad TF, et al. Explaining microbial population genomics through phage predation. Nature Reviews Microbiology. 2009;7:828–836. doi: 10.1038/nrmicro2235. [DOI] [PubMed] [Google Scholar]
- 5.Brussow H, Hendrix RW. Phage genomics: small is beautiful. Cell. 2002;108:13–16. doi: 10.1016/s0092-8674(01)00637-7. [DOI] [PubMed] [Google Scholar]
- 6.Banks DJ, Beres SB, Musser JM. The fundamental contribution of phages to GAS evolution, genome diversification and strain emergence. Trends Microbiol. 2002;10:515–521. doi: 10.1016/s0966-842x(02)02461-7. [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi M, Kurokawa K, Hayashi T. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends Microbiol. 2001;9:481–485. doi: 10.1016/s0966-842x(01)02173-4. [DOI] [PubMed] [Google Scholar]
- 8.Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, et al. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol. 2003;185:1018–1026. doi: 10.1128/JB.185.3.1018-1026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatfull GF, Cresawn SG, Hendrix RW. Comparative genomics of the mycobacteriophages: insights into bacteriophage evolution. Res Microbiol. 2008;159:332–339. doi: 10.1016/j.resmic.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry M, O'Sullivan O, Sleator RD, Coffey A, Ross RP, et al. In silico analysis of Ardmore, a novel mycobacteriophage isolated from soil. Gene. 2010;453:9–23. doi: 10.1016/j.gene.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Pope WH, Jacobs-Sera D, Russell DA, Peebles CL, Al-Atrache Z, et al. Expanding the diversity of mycobacteriophages: insights into genome architecture and evolution. PloS One. 2011;6:e16329. doi: 10.1371/journal.pone.0016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson T, Broussard GW, Marinelli LJ, Jacobs-Sera D, Ray M, et al. Mycobacteriophages BPs, Angel and Halo: Comparative genomics reveals a novel class of ultra-small mobile genetic elements. Microbiology. 2009;155:2962–2977. doi: 10.1099/mic.0.030486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd EF, Brussow H. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends in Microbiology. 2002;10:521–529. doi: 10.1016/s0966-842x(02)02459-9. [DOI] [PubMed] [Google Scholar]
- 14.Botstein D. A theory of modular evolution for bacteriophages. Ann N Y Acad Sci. 1980;354:484–490. doi: 10.1111/j.1749-6632.1980.tb27987.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendrix RW, Smith MC, Burns RN, Ford ME, Hatfull GF. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proceedings of the National Academy of Sciences. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix RW, Hatfull GF, Smith MC. Bacteriophages with tails: chasing their origins and evolution. Res Microbiol. 2003;154:253–257. doi: 10.1016/S0923-2508(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 17.Tran-Nguyen LT, Kube M, Schneider B, Reinhardt R, Gibb KS. Comparative genome analysis of “Candidatus Phytoplasma australiense” (subgroup tuf-Australia I; rp-A) and “Ca. Phytoplasma asteris” Strains OY-M and AY-WB. J Bacteriol. 2008;190:3979–3991. doi: 10.1128/JB.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshima K, Kakizawa S, Nishigawa H, Jung HY, Wei W, et al. Reductive evolution suggested from the complete genome sequence of a plant-pathogenic Phytoplasma. Nature Genetics. 2004;36:27–29. doi: 10.1038/ng1277. [DOI] [PubMed] [Google Scholar]
- 19.Andersson SG, Eriksson AS, Naslund AK, Andersen MS, Kurland CG. The Rickettsia prowazekii genome: a random sequence analysis. Microbial & Comparative Genomics. 1996;1:293–315. [PubMed] [Google Scholar]
- 20.Andersson SG, Zomorodipour A, Andersson JO, Sicheritz-Ponten T, Alsmark UC, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 21.Blanc G, Ogata H, Robert C, Audic S, Claverie JM, et al. Lateral gene transfer between obligate intracellular bacteria: evidence from the Rickettsia massiliae genome. Genome Res. 2007;17:1657–1664. doi: 10.1101/gr.6742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felsheim RF, Kurtti TJ, Munderloh UG. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PloS One. 2009;4:e8361. doi: 10.1371/journal.pone.0008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier PE, El Karkouri K, Leroy Q, Robert C, Giumelli B, et al. Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genomics. 2009;10:166. doi: 10.1186/1471-2164-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLeod MP, Qin X, Karpathy SE, Gioia J, Highlander SK, et al. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. Journal of Bacteriology. 2004;186:5842–5855. doi: 10.1128/JB.186.17.5842-5855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata H, La Scola B, Audic S, Renesto P, Blanc G, et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genetics. 2006;2:e76. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison DW, Clark TR, Sturdevant DE, Virtaneva K, Porcella SF, et al. Genomic comparison of virulent Rickettsia rickettsii Sheila Smith and avirulent Rickettsia rickettsii Iowa. Infection and Immunity. 2008;76:542–550. doi: 10.1128/IAI.00952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent BN, Salichos L, Gibbons JG, Rokas A, Newton IL, et al. Complete bacteriophage transfer in a bacterial endosymbiont (Wolbachia) determined by targeted genome capture. Genome Biology and Evolution. 2011;3:209–218. doi: 10.1093/gbe/evr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol. 2008;25:1877–1887. doi: 10.1093/molbev/msn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004;2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bordenstein SR, Reznikoff WS. Mobile DNA in obligate intracellular bacteria. Nat Rev Microbiol. 2005;3:688–699. doi: 10.1038/nrmicro1233. [DOI] [PubMed] [Google Scholar]
- 32.Newton IL, Bordenstein SR. Correlations Between Bacterial Ecology and Mobile DNA. Curr Microbiol. 62(1):198–208. doi: 10.1007/s00284-010-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chafee ME, Funk DJ, Harrison RG, Bordenstein SR. Lateral phage transfer in obligate intracellular bacteria (Wolbachia): verification from natural populations. Mol Biol Evol. 2010;27:501–505. doi: 10.1093/molbev/msp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masui S, Kamoda S, Sasaki T, Ishikawa H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J Mol Evol. 2000;51:491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- 35.Masui S, Kuroiwa H, Sasaki T, Inui M, Kuroiwa T, et al. Bacteriophage WO and virus-like particles in Wolbachia, an endosymbiont of arthropods. Biochem Biophys Res Commun. 2001;283:1099–1104. doi: 10.1006/bbrc.2001.4906. [DOI] [PubMed] [Google Scholar]
- 36.Ishmael N, Dunning Hotopp JC, Ioannidis P, Biber S, Sakamoto J, et al. Extensive Genomic Diversity of Closely Related Wolbachia Strains. Microbiology. 2009;155(7):2211–2222. doi: 10.1099/mic.0.027581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chibani-Chennoufi S, Canchaya C, Bruttin A, Brussow H. Comparative genomics of the T4-Like Escherichia coli phage JS98: implications for the evolution of T4 phages. Journal of bacteriology. 2004;186:8276–8286. doi: 10.1128/JB.186.24.8276-8286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comeau AM, Bertrand C, Letarov A, Tetart F, Krisch HM. Modular architecture of the T4 phage superfamily: a conserved core genome and a plastic periphery. Virology. 2007;362:384–396. doi: 10.1016/j.virol.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 40.Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc Biol Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?–A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitol Today. 1999;15:437–442. doi: 10.1016/s0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- 43.Klasson L, Westberg J, Sapountzis P, Naslund K, Lutnaes Y, et al. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci U S A. 2009;106:5725–5730. doi: 10.1073/pnas.0810753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka K, Furukawa S, Nikoh N, Sasaki T, Fukatsu T. Complete WO phage sequences revealed their dynamic evolutionary trajectories and putative functional elements required for integration into Wolbachia genome. Appl Environ Microbiol. 2009;75(17):5676–5686. doi: 10.1128/AEM.01172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordenstein SR, Wernegreen JJ. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol. 2004;21:1981–1991. doi: 10.1093/molbev/msh211. [DOI] [PubMed] [Google Scholar]
- 46.Gavotte L, Vavre F, Henri H, Ravallec M, Stouthamer R, et al. Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol Biol. 2004;13:147–153. doi: 10.1111/j.0962-1075.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 47.Fujii Y, Kubo T, Ishikawa H, Sasaki T. Isolation and characterization of the bacteriophage WO from Wolbachia, an arthropod endosymbiont. Biochem Biophys Res Commun. 2004;317:1183–1188. doi: 10.1016/j.bbrc.2004.03.164. [DOI] [PubMed] [Google Scholar]
- 48.Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog. 2006;2:e43. doi: 10.1371/journal.ppat.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanogo YO, Dobson SL. WO bacteriophage transcription in Wolbachia-infected Culex pipiens. Insect Biochem Mol Biol. 2006;36:80–85. doi: 10.1016/j.ibmb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, et al. A Survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Mol Biol Evol. 2007;24:427–435. doi: 10.1093/molbev/msl171. [DOI] [PubMed] [Google Scholar]
- 51.Groth AC, Calos MP. Phage integrases: biology and applications. Journal of molecular biology. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 52.Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisen JA, Sweder KS, Hanawalt PC. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res. 1995;23:2715–2723. doi: 10.1093/nar/23.14.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson DJ, McVean G. Estimating diversifying selection and functional constraint in the presence of recombination. Genetics. 2006;172:1411–1425. doi: 10.1534/genetics.105.044917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shriner D, Nickle DC, Jensen MA, Mullins JI. Potential impact of recombination on sitewise approaches for detecting positive natural selection. Genetical research. 2003;81:115–121. doi: 10.1017/s0016672303006128. [DOI] [PubMed] [Google Scholar]
- 56.Anisimova M, Nielsen R, Yang Z. Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics. 2003;164:1229–1236. doi: 10.1093/genetics/164.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McVean G, Awadalla P, Fearnhead P. A coalescent-based method for detecting and estimating recombination from gene sequences. Genetics. 2002;160:1231–1241. doi: 10.1093/genetics/160.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiggins FM. The rate of recombination in Wolbachia bacteria. Mol Biol Evol. 2002;19:1640–1643. doi: 10.1093/oxfordjournals.molbev.a004228. [DOI] [PubMed] [Google Scholar]
- 59.Nilsson AS, Haggard-Ljungquist E. Evolution of P2-like phages and their impact on bacterial evolution. Research in Microbiology. 2007;158:311–317. doi: 10.1016/j.resmic.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Asadulghani M, Ogura Y, Ooka T, Itoh T, Sawaguchi A, et al. The defective prophage pool of Escherichia coli O157: prophage-prophage interactions potentiate horizontal transfer of virulence determinants. PLoS Pathogens. 2009;5:e1000408. doi: 10.1371/journal.ppat.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordaux R. ISWpi1 from Wolbachia pipientis defines a novel group of insertion sequences within the IS5 family. Gene. 2008;409:20–27. doi: 10.1016/j.gene.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 62.Cordaux R, Pichon S, Ling A, Perez P, Delaunay C, et al. Intense transpositional activity of insertion sequences in an ancient obligate endosymbiont. Mol Biol Evol. 2008;25:1889–1896. doi: 10.1093/molbev/msn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brussow H, Desiere F. Comparative phage genomics and the evolution of Siphoviridae: insights from dairy phages. Molecular Microbiology. 2001;39:213–222. doi: 10.1046/j.1365-2958.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 64.Saren AM, Ravantti JJ, Benson SD, Burnett RM, Paulin L, et al. A snapshot of viral evolution from genome analysis of the tectiviridae family. Journal of Molecular Biology. 2005;350:427–440. doi: 10.1016/j.jmb.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 65.Brussow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiology and Molecular Biology Reviews. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hendrix RW. Bacteriophages: evolution of the majority. Theoretical Population Biology. 2002;61:471–480. doi: 10.1006/tpbi.2002.1590. [DOI] [PubMed] [Google Scholar]
- 67.Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, et al. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. Journal of Molecular Biology. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- 68.Pedulla ML, Ford ME, Houtz JM, Karthikeyan T, Wadsworth C, et al. Origins of highly mosaic mycobacteriophage genomes. Cell. 2003;113:171–182. doi: 10.1016/s0092-8674(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 69.van der Wilk F, Dullemans AM, Verbeek M, van den Heuvel JF. Isolation and characterization of APSE-1, a bacteriophage infecting the secondary endosymbiont of Acyrthosiphon pisum. Virology. 1999;262:104–113. doi: 10.1006/viro.1999.9902. [DOI] [PubMed] [Google Scholar]
- 70.Degnan PH, Moran NA. Diverse phage-encoded toxins in a protective insect endosymbiont. Appl Environ Microbiol. 2008;74:6782–6791. doi: 10.1128/AEM.01285-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Degnan PH, Moran NA. Evolutionary genetics of a defensive facultative symbiont of insects: exchange of toxin-encoding bacteriophage. Mol Ecol. 2008;17:916–929. doi: 10.1111/j.1365-294X.2007.03616.x. [DOI] [PubMed] [Google Scholar]
- 72.Moran NA, Degnan PH, Santos SR, Dunbar HE, Ochman H. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc Natl Acad Sci. 2005;102:16919–16926. doi: 10.1073/pnas.0507029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drummond AJ, Ashton B, Cheung M, Heled J, Kearse M, et al. Geneious v. 5.0. 2010. Available from http://www.geneious.com.
- 75.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Research. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PloS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maddison WP, Maddison DR. Interactive analysis of phylogeny and character evolution using the computer program MacClade. Folia Primatologica; International Journal of Primatology. 1989;53:190–202. doi: 10.1159/000156416. [DOI] [PubMed] [Google Scholar]
- 78.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WO Recombinases are Diverse. A neighbor-joining phylogenetic tree based on nucleotide sequences demonstrates that prophage WO recombinases cluster into three major groups. Groups A belongs to the tyrosine-recombinase family. Groups B and C belong to the serine-recombinase family.
(TIF)
Degradation of WO Genomes. The number of transposon insertions and pseudogenes were tallied in order to measure the degradation and gene loss in WO prophage genomes. A) The fraction of transposase genes and pseudogenes out of the total number of genes in each prophage WO genomes are denoted along with the standard error of proportion. B) The fraction of transposons and pseudogenes per functional module, normalized to account for difference in module size, is shown.
(TIF)
Synteny analysis of the WOCauB2 family of phages. Alignments were performed between the prophages and flanking regions of WORiB, WOCauB2, and WOVitA2. Dotplot analysis shows that these prophage genomes are syntenous and contain few breakpoints between the genomes.
(TIF)
Average percent nucleotide identity for each prophage gene within a Wolbachia genome. Parentheses indicate the number of phage genes/haplotypes per Wolbachia .
(DOC)
Genes present in WO haplotypes that are not present in WOCauB2.
(DOC)
Genes found in a single WO haplotype.
(DOC)
Genes used in selection and recombination analysis from the head and baseplate modules of both tailed and untailed phages.
(DOC)
Genes used in selection and recombination analysis from the virulence and tail modules.
(DOC)