Abstract
A Kinase Anchoring Proteins, AKAPs, bind multiple signaling proteins and have subcellular targeting domains that allow them to greatly impact cellular signaling. AKAPs localize, specify, amplify and accelerate signal transduction within the cell by bringing signaling proteins together in space and time. AKAPs also organize higher-order network motifs such as feedforward and feedback loops that may create complex network responses including adaptation, oscillation, and ultrasensitivity. Computational models have begun to provide insight into how AKAPs regulate signaling dynamics and cardiovascular pathophysiology. Models of MAPK and EGFR scaffolds have revealed additional design principles and new methods for representing signaling scaffolds mathematically. Coupling computational modeling with quantitative experimental approaches will be increasingly necessary for dissecting the diverse information processing functions performed by AKAP signaling complexes.
Keywords: mathematical modeling, cellular signaling, scaffold, kinase
A-kinase anchoring proteins (AKAPs) are a family of proteins that share the ability to bind the regulatory subunit of protein kinase A (PKA). AKAPs regulate a wide range of signaling molecules and cellular processes, binding both PKA and other partners simultaneously. But AKAPs generally do not directly mediate signal propagation. Instead, AKAPs act as scaffolds that modulate the signaling between constituent binding partners. AKAPs affect the location and dynamics of signal transduction by tethering specific signaling molecules to a particular location in the cell. Over the past several years, a wealth of molecular mechanisms underlying AKAP signaling has been revealed, much of which is described in the other reviews of this series on AKAPs [1] or reviewed previously elsewhere [2–4].
In parallel with increasing molecular characterization, the role of AKAPs in physiology and disease has also been increasingly established. Thirteen different AKAPs have been identified in cardiac myocytes [5]. Several of these AKAPs have been shown to regulate of the inotropic, chronotropic and lusitropic state of the heart [6]. AKAPs have been shown to have important roles in pathological states such as cardiac arrhythmia [7,8] cardiac hypertrophy [9] and familial breast cancer [10]. AKAPs are of particular interest as potential drug targets due to their specific, modulating role in systems governed by effectors that also have many other functions. While inhibiting a highly conserved kinase such as PKA would have widespread consequences in many tissues, AKAP-targeted therapies may allow tissue or even subcellular compartment-specific action. But the indirect signaling role that makes AKAPs attractive therapeutic targets also makes it difficult to assess AKAP function directly using traditional experimental approaches.
Computational modeling can aid understanding of complex networks where intuition alone is insufficient or misleading. Complementing experiments with computational models can allow one to identify key mechanisms underlying a biological response, predict the multiscale physiological consequences of these mechanisms, and perform in silico experiments that are not otherwise feasible [11]. Recent efforts have begun to include AKAPs and other scaffolds into models of signaling networks, helping to clarify the functional role of these scaffolds in cellular information processing. This review provides an overview of how computational models are being used to reveal fundamental principles by which AKAPs shape cellular signals.
Design Principles for AKAP Function
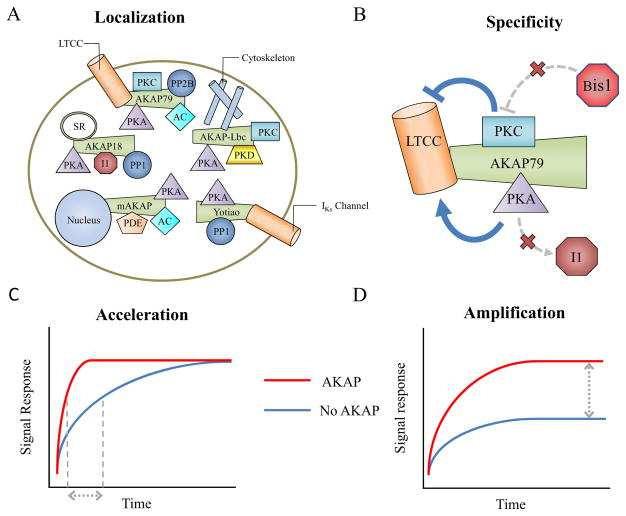
Given that AKAPs act as modifiers rather than direct transducers of cellular signaling, what are the design principles that govern their function? To answer this question, we must examine the fundamental biophysical mechanisms that enable AKAPs to modify signal flow through a pathway. Here, we outline four main design principles by which AKAPs have been shown to influence signaling: localization, specificity, amplification, and acceleration (Figure 1).
Figure 1. Design principles of AKAP signaling.
A) AKAPs localize many signaling proteins to specific locations within the cell [12–15]. B) AKAPs can create preferential interactions on the scaffold. C) AKAPs can increase the rate at which signal transduction occurs. D) AKAPs can increase the magnitude of the signal response.
AKAPs have the ability to localize their binding partners to specific physical domains within the cell. Many AKAPs have targeting domains that anchor them to various subcellular compartments such as the plasma membrane, mitochondria, endoplasmic reticulum, centrosome, and nuclear membrane (Figure 1A) [12–15]. It is not a requirement that AKAPs have a targeting domain, but most AKAPs have been shown to contain localization capabilities [12,16]. Targeting to specific subcellular compartments helps mediate signaling that is specific to that compartment. For example, AKAP-Lbc localizes its protein complex to the cytoskeleton, allowing it to direct cell migration, influence PKA activity gradients at the plasma membrane, and regulate cardiac myocyte hypertrophy [9,17]. AKAP localization can also be dynamic, with post-translational modifications causing AKAP translocation in response to a cellular signal. This is evident through reversible palmitoylation that targets AKAP18 to the plasma membrane and the dynamic recruitment of PKA to mitochondria during oocyte maturation [13,18].
AKAPs have the ability to bring not only PKA but multiple interacting partners together to create a separate signaling nanodomain termed a “signalosome” [4,19]. Indeed, a large number of proteins have been shown to bind to AKAPs [3]. By defining which proteins are in a particular signalosome, the AKAP may able to enhance signaling pathways within the scaffold while minimizing the spread of signaling outside the scaffold, creating response specificity (Figure 1B). This may be especially important for AKAPs due to the fact that PKA may have >250 protein substrates [20]. It has been hypothesized that scaffolds were evolutionarily selected because they allow the cell to maintain fewer signaling proteins while still ensuring specificity [22]. Indeed, protein scaffolds appear to be early adopters of the mantra “reduce, reuse, recycle” as they reconfigure existing components for new uses. One example of bringing interacting proteins together is mAKAP, which binds PKA, phosphodiesterase PDE4D3, adenylyl cyclase-5 (AC5) and others [23,24]. This increased proximity helps PKA activate the PDE and negatively feedback on AC5, downregulating cAMP. These particular interactions have important physiological consequences for cardiovascular physiology, as disrupting the binding of mAKAP to AC5 caused an increase in global cAMP levels and myocyte hypertrophy [24]. In perhaps the clearest example of AKAP specificity to date, Hoshi et al. found that AKAP79 not only enhanced interactions between PKC and its substrates but also prevented interactions with certain molecules not on the AKAP [25]. While a noncompetitive PKC inhibitor was unaffected by AKAP-PKC interactions, AKAP-bound PKC was insensitive to the competitive PKC inhibitor BIS-1 [25]. This raises an interesting challenge for drug target selection as AKAP-induced specificity may alter the pharmacology of its binding partners.
In addition to arranging specific signaling proteins in new configurations, AKAPs may accelerate signal transduction by positioning the enzyme and substrate in close proximity (Figure 1C). The acceleration of downstream activation was clearly shown by Zhang et al. through the development of a fluorescence resonance energy transfer (FRET)-based A-kinase activity reporter (AKAR) [26]. They used targeting sequences derived from AKAPs to localize PKA to the AKAR reporter and showed that with rapid cAMP stimulation, the PKA phosphorylated its substrate ~10-fold faster than without tethering [26]. Similarly, Hoshi et al. found that fusing AKAP79 to a C Kinase Activity Reporter, CKAR, increased the rate of response to muscarinic stimulation to equal the response time of the endogenous KCNQ2 channel [25].
AKAPs also have the ability to amplify the signal response (Figure 1D). For example, AKAP75 amplifies PKA activity in the nucleus, which can affect transcription factors such as cyclin-dependent kinase-2 inhibitor p27kip1 [27,28]. This amplification is presumed to be due to localization of AKAP75 to the nuclear membrane to increase the rate of nuclear transport. Another way that AKAPs may amplify a signal is through dynamic regulation of substrate binding affinity. For example, AKAP-Lbc amplifies PKD activation by PKC and is involved in PKA regulation to further amplify PKD signaling [29]. It is hypothesized that the amplification by PKA is due to phosphorylation of AKAP-Lbc by PKA, which reduces the binding affinity of PKD and thus allows greater turnover of PKD and amplification of PKD signaling [29].
The ability of scaffolds to accelerate and amplify cell signals appears closely linked. Indeed, in a classic example of the drosophila phototransduction cascade, mutants of the scaffolding protein InaD decreased both response magnitudes and response times to light [30]. The signal acceleration and amplification by AKAPs has been hypothesized to be a result of the increase of the local concentrations of the enzyme and its intended target [31]. In a rough order-of-magnitude approximation Zeke et al. calculated that the “effective concentration” of a protein on a scaffold could be up to 3000 times greater than in solution [22]. Indeed, for a single molecule tethered within a 10 nm radius sphere, its effective concentration would be ~400 μM. Experimental evidence that AKAPs increase the effective concentration of an effector was shown by Tavalin et al. where introduction of AKAP79 caused a 4-fold acceleration and a 20-fold shift in the concentration of PKC needed to regulate GluR1 receptor currents [32]. These responses are similar to the 10-fold acceleration of PKA-mediated phosphorylation shown by Zhang et al. [26], but the quantitative extent to which “effective concentration” is enhanced may be different for each AKAP. Work done by Persechini et al. on calmodulin provides additional direct experimental evidence of the effective concentration hypothesis [33]. Calmodulin mimics a scaffold because it contains two distinct Ca-binding lobes that both interact with target proteins, thus acting like two separate regulators tethered together. By titrating calmodulin fragments containing just one lobe or the other, they were able to show that once one calmodulin lobe binds to a target protein, the effective concentration of the second lobe is ~1 mM [33].
The ability of AKAPs to localize, specify, accelerate and amplify biochemical signals are important design principles for understanding the information processing provided by cell signaling networks. Characterizing these principles experimentally requires quantitative experimental approaches such as the fluorescent reporters and electrophysiology described above. These experiments will provide the necessary ingredients for computational models that enable a quantitative understanding of the relationships between biophysical mechanisms and physiological consequences of AKAP signaling.
Network Motifs on AKAP Signaling Complexes
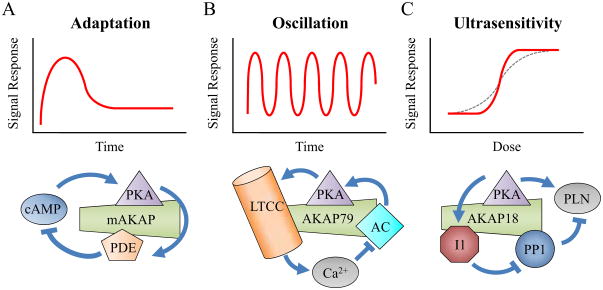
While the four design principles discussed above focus on how AKAPs modulate the interaction between two proteins, additional functional properties are revealed when we begin to examine higher-order interactions occurring on a scaffold. Several of the systems concepts useful for understanding AKAP signaling are defined in Table 1. Recurring patterns of interactions called network motifs form the building blocks of complex networks [34]. Common network motifs such as feedback, feedforward, and bifan structures have been shown to be statistically enriched across many types of networks, from internet structure and social networks to microbial transcription [34]. By recruiting specific binding partners to the scaffold, there is increasing evidence that AKAPs form network motifs that may have important functional consequences for their cellular signaling. Network motifs found on AKAPs have been shown to cause characteristic signal response behaviors such as adaptation [23], oscillation [35] and ultrasensitivity [36].
Table 1.
Key systems concepts relevant to understanding AKAP signaling.
| Term | Definition | References |
|---|---|---|
| Design Principles | fundamental ways in which AKAPs shape cellular signals | |
| localization | tethering of a protein to a physical subcellular location | [12–16,47] |
| specificity | preferential interaction of proteins bound to an AKAP | [23–25] |
| acceleration | increase in the speed of response to a stimulus | [25,26,30] |
| amplification | increase in the magnitude of response to a stimulus | [27–30,32] |
| Network Motifs | topological patterns in signaling cascades | [34] |
| feedback | “downstream” protein acting on an “upstream” protein | [23,34,37,40] |
| feedforward | “upstream” protein affecting a “downstream” protein through two separate pathways | [34,37,46] |
| Network Responses | system behaviors not attributable to any individual component | |
| adaptation | return of a cellular signal to its pre-stimulated state while under continued stimulation | [37] |
| oscillation | repeated fluctuations of a cellular signal | [35,39,40] |
| ultrasensitivity | switch-like response to increasing input signal, exhibiting a dose response curve with Hill coefficient >1 | [36,43,44] |
One common signaling response behavior is adaptation, defined as the ability of a network to respond to a sustained input but then return to the pre-stimulated state (Figure 2A) [37]. This is important to be able to sense the change as opposed to the magnitude of a stimulus. Ma et al. computationally tested all possible interactions between three signaling proteins and found just two basic motifs allowing adaptation: negative feedback and incoherent feed forward loops [37]. Negative feedback occurs when a “downstream” protein inhibits an “upstream” protein, while incoherent feedforward loops are formed when a particular protein activates and then later inhibits the same protein. For example, mAKAP organizes a negative feedback motif where PKA phosphorylates PDE4, which degrades cAMP and results in decreased PKA activity, see Figure 2A [23]. Dodge et al. built a synthetic AKAP complex incorporating the PKA reporter AKAR, PKA and PDE, which exhibited adaptive PKA activity in response to stimuli, whereas complexes without PDE binding did not [23]. Adaptive responses exhibit either a single overshoot (shown in Figure 2A) or damped oscillations, depending on the nonlinearity and time constants involved as shown clearly for the NFκB system [38]. While feedback and feedfoward motifs can drive adaptation, their existence in a network does not guarantee that adaptation will occur.
Figure 2. Examples of complex signaling responses and corresponding network motifs that are possible on AKAPs.
A) The negative feedback loop through PDE can create signal adaptation [23]. B) Calcium negative feedback with significant delay can create oscillation in PKA activity [40]. C) The dual actions of PKA on Phospholamban (PLN) and the phosphatase inhibitor I1 can both increase the Hill coefficient of the signal response [46].
Oscillation is another physiologically important signaling response which has been shown to be caused primarily through negative feedback loops (Figure 2B) [39]. One special requirement that separates this response from adaptation is that the feedback must be sufficiently delayed [39]. AKAPs have been shown to affect the frequency and amplitude of estradiol-induced Ca2+ oscillations in neurons [35]. This was hypothesized to be due to the AKAP’s regulation of the L-type Calcium Channel (LTCC) and possibly other downstream effectors of scaffold-bound PKC such as MAPK and PI3K [35]. Another important physiological example of oscillation is pulsatile insulin secretion in the pancreas, which involves oscillatory Ca2+-triggered exocytosis [40]. Ni et al. showed MIN6 cells exhibited Ca2+ oscillations whose amplitude and magnitude are regulated by PKA activity [40]. This oscillatory circuit is hypothesized to be formed by PKA potentiation of the LTCC, resulting in Ca2+ dependent negative feedback on cAMP, either through PDE or AC (see Figure 2B) [40]. This finding of direct PKA regulation of Ca2+ oscillation may implicate a coordination role of AKAPs in this process because PKA, LTCC and AC5/AC6 associate with AKAP79 in these cells [31,41,42]. It is important to mention that even though AKAPs can enhance oscillations, it may also be possible for AKAPs to diminish oscillations by reducing the delay in the negative feedback of the network through acceleration.
Ultrasensitivity is a switch-like response to increasing input, characterized by an increased apparent Hill coefficient (Figure 2C) [43]. Ultrasensitivity is important because it allows the system to be activated quickly or allow the network to filter out weak signal noise [44]. Ultrasensitivity has been shown to have four underlying mechanisms: cooperativity, multistep, zero-order, and inhibitor [43,44]. The classic example of ultrasensitivity is seen in the cooperative oxygen binding to hemoglobin. Multistep ultrasensitivity arises when a signaling effector has multiple feed-forward interactions [43]. For example, MAPK proteins require two distinct phosphorylations by a single upstream kinase for activation which creates multistep ultrasensitivity [44]. Zero-order ultrasensitivity occurs when enzymes are operating at near saturation levels, which occurs when the substrate concentration is relatively large compared to the Michaelis constant [43]. It is possible that AKAPs may create zero-order sensitivity by increasing the effective local concentration of the substrate to saturating levels. Finally, it has been shown that the presence of a stoichiometric inhibitor can cause ultrasensitivity because at low stimulation levels the enzyme is blocked by the inhibitor [44]. For example, PKI, a PKA inhibitor, was predicted to enhance the ultrasensitivity of cAMP-dependent PKA activation [45].
Ultrasensitivity may result from a combination of the above mechanisms and other network motifs on AKAPs. For example, Mutalik and Venkatesh used a computational model to show that incorporation of Inhibitor-1 (I-1) in the signaling cascade may greatly increase the sensitivity of many downstream effectors [36]. The system studied by Mutalik and Venkatesh had already been shown to exhibit zero-order ultrasensitivity but the increased sensitivity was hypothesized as being due to feedforward actions of PKA and the PP1 inhibition by I-1 (see Figure 2C). While that study was not focused on AKAPs, others have shown that that AKAP18 binds PKA, I-1 and PP1 and that I-1 phosphorylation by PKA is increased by I-1 binding to the scaffold [46]. By bringing these three components together on an AKAP, it may be possible to create an ultrasensitive signaling response.
AKAPs may enable network responses such as adaptation, oscillation and ultrasensitivity, by organizing the required network motifs efficiently. This may allow them to localize these signal responses to a specific area and provide a compartment specific response to a signal.
Computational Models of AKAP Signaling
Computational modeling of signaling networks with scaffolds has proved useful in understanding how protein scaffolds integrate the effects seen at the protein interaction and signaling network levels. To do this one must consider the above design principles and determine how to represent these mathematically. Building a computational model of a signaling network requires a specific list of the biochemical reactions with stoichiometry, rate constants for each reaction (generally determined from prior biochemical experiments) and protein expression levels. When rate constants or expression levels have not been measured directly, these parameters may in some cases be inferred by fitting the model to quantitative timecourse data. A detailed introduction to these modeling approaches has been described previously [48]. Here we review computational models that have incorporated AKAPs into signaling networks as well as several models of other relevant protein scaffolds.
The first computational model to incorporate an AKAP into a signaling network was developed by Saucerman et al. [45]. This kinetic model examined how the β-adrenergic signaling pathway regulates excitation-contraction coupling in cardiac myocytes. While there are many components in this large kinetic model, one subtlety is the inclusion of an AKAP that localizes PKA, PP1, and PP2A to the L-type calcium channel (LTCC). The inclusion of this AKAP was necessary to be able to achieve the dynamic range of LTCC phosphorylation in response to β-adrenergic agonists seen experimentally. It was hypothesized that the AKAP accelerates PKA-mediated phosphorylation by increasing the effective concentration of the AKAP-bound LTCC [45]. This was achieved mathematically by multiplying scaffold-bound proteins by a 10-fold increase in effective local concentration, based on the acceleration of PKA-mediated phosphorylation seen experimentally when PKA was tethered to a fluorescent reporter [26,45].
The necessity of the AKAP in this model emphasized the importance of AKAP scaffolding in signaling dynamics. But while an “effective concentration” AKAP model was sufficient to explain the experimental data in this case, there are several limitations that should be considered. First, the use of an effective concentration is somewhat phenomenological, because it does not mechanistically represent the interactions within the scaffold. Further, the effective concentration used was based on a recombinant fluorescent reporter rather than the endogenous LTCC, because such data was not available. It is likely that the degree of acceleration will vary both from AKAP to AKAP and between proteins on a given AKAP. Therefore it will be important to obtain more specific experimental data on a case-by-case basis and better characterize the biophysical determinants of signal acceleration.
This computational model of β-adrenergic signaling was subsequently extended to examine how a mutation in the IKs channel causes Long QT (LQT) syndrome and arrhythmias during sympathetic stimulation [49]. This study was motivated by a clinically-observed G589D mutation in the KCNQ1 gene that was shown to disrupt the binding of an AKAP, yotiao, to the IKs channel [8]. Yotiao anchors PKA and PP1 to the IKs channel; by disrupting this interaction the mutation prevents PKA-mediated phosphorylation of IKs [8]. Despite these advances in molecular biology, the sequence of physiological mechanisms linking molecular interactions to clinical phenotype could not be addressed directly using genetic perturbations due the minimal role for IKs in mouse electrophysiology. Saucerman et al. incorporated these signaling mechanisms into the β-adrenergic signaling model discussed above, again using an “effective concentration” model to simulate reactions within the yotiao/IKs channel complex [49]. But in this case, increasing mechanistic detail was used to represent the reversible binding of yotiao to KCNQ1 and the reversible binding of PKA and PP1 to yotiao. By including this detail, they were able to further model the effect of the KCNQ1 mutation as a decreased affinity of yotiao for KCNQ1. The model showed that the disruption of the yotiao/KCNQ1 interface prevented β-adrenergic regulation of IKs seen experimentally, and was able to further predict increased action potential duration and early after depolarizations in myocytes along with LQT and increased transmural dispersion of repolarization in the ventricular wall. Thus, the computational model was able to provide a mechanistic and multi-scale explanation of how the KCNQ1-G589D gene mutation leads to a clinically relevant phenotype. Indeed, a subsequent clinical case study of a LQT1 patient demonstrated remarkably similar “Himalayan” T-waves to those predicted by the model [50].
Others have recently explored alternative mathematical approaches to modeling AKAP scaffolds. Andrei and Calder developed a stochastic reward-based analysis method for modeling interactions occurring on and off a scaffold [51]. They used this approach to develop a semi-quantitative model with interactions between cAMP, PKA, PDE and Raf on an AKAP, predicting “pulsations” of signaling. While such behaviors have not been reported experimentally, FRET reporters incorporating a cAMP-PKA-PDE negative feedback loop have shown adaptation in PKA activity [23].
Computational Models of Other Signaling Scaffolds
There have been a number of modeling studies of other scaffolds that are relevant to our understanding of AKAPs. Many of these examine MAPK scaffolds and GAB scaffolds that are associated with EGFR signaling. The focus here is to highlight the assumptions and methods that have been applied to model these scaffolds and the design principles of scaffold signaling elucidated by computational modeling.
Levchenko et al. developed a computational model to examine how scaffold concentration affects the acceleration and amplification of MAPK signaling [52]. To do this, they examined a two-member scaffold that binds both a MAPKK and its substrate MAPK. This model allowed for MAPK phosphorylation both in solution and on the scaffold, but kinases in solution were assumed not to phosphorylate MAPK on the scaffold. In contrast to the “effective concentration” AKAP model described above, Levchenko assumed that phosphorylation on the scaffold occurred at a maximal catalytic rate independent of the Michaelis constant Km. MAPK activation generally requires phosphorylation at two distinct sites and require two distinct enzyme interactions (dissociative), which as discussed above can create ultrasensitivity. But in this model it was assumed that both phosphorylation reactions on the scaffold occur simultaneously.
With these assumptions, Levchenko et al. showed that moderate concentrations of scaffold can both accelerate and amplify the signal response [52]. However, at high scaffold concentrations there was little or no amplification, forming a biphasic dependence on scaffold. The surprising prediction of decreased signaling at high scaffold concentrations was due to increasing the number of partially-filled scaffolds where MAPKK or MAPK proteins were isolated and inactive [52]. This suggested that cells may be able to modulate signaling pathways through changes in scaffold expression. This model also suggested that by rewiring sequential phosphorylation events to be simultaneous, the MAPK scaffold may diminish ultrasensitivity [43]. However, later experiments showed that MAPK scaffold signaling was still ultrasensitive [53].
O’Shaughnessy et al. investigated the properties of MAPK scaffold signaling further by combining synthetic biology and computational modeling [53]. They expressed the Raf-MEK-Erk cascade in yeast, along with varying expression levels of the MAPK scaffold Pax. Like the model predictions of Levchenko et al., they found that high scaffold expression levels diminish signaling by increasing the number of partially-filled scaffolds. But in contrast to the predictions of Levchenko et al., moderate concentrations of scaffold did not enhance signaling [53]. O’Shaughnessy developed a computational model of this system and showed that the lack of amplification by the scaffold may be because this particular MAPK cascade is inherently catalytically efficient when in solution [53]. These experimental and model results indicate that the effects of scaffolding may depend on the kinetic rate constants and concentrations for the particular system of interest.
Another model examining MAPK scaffold dynamics was developed by Locasale et al., which used stochastic spatial simulations to analyze how scaffolds may either amplify or attenuate signal propagation [54]. Their simulations indicated a key balance between the diffusion rate of the kinase and the activity of the phosphatase that dictates whether a scaffold causes amplification or attenuation. While in general one would expect a scaffold to amplify a signal (as discussed above), their model predicted that in fact scaffolds may limit amplification by reducing the number of substrates accessible to a mobile kinase. On the other hand, if the opposing phosphatase activity is high, the kinase may require scaffolding in order to effectively phosphorylate its substrates, resulting in amplification. Like the results of Levchenko and O’Shaugnessy et al., these simulation results highlight that while the intuitions and design principles outlined above may be good rules of thumb, the functional impact of scaffolds will depend on a number of factors and will need to be studied quantitatively on a case-by-case basis. While many of the lessons from MAPK scaffolding may be helpful to understanding AKAPs, there are some key differences. One difference is that MAPK scaffolds process information in a linear manner (MAPKKK to MAPKK to MAPK), whereas AKAPs can bring together many different signals and contain much more complex network motifs [55].
Another system that has benefitted significantly from computational modeling of scaffolds is endothelial growth factor (EGF) receptor signaling. Kiyatkin et al. combined experimental and computational approaches to investigate how the scaffold protein Grb2-associated binder (GAB1) regulates the balance of MAPK and PI3K pathways in response to EGF [56]. They developed a comprehensive computational model of this system and experimentally validated its predictions under multiple EGF doses, GAB1 suppression, and pharmacological inhibitors. Their models and experiments helped them identify how GAB1 acts to amplify the PI3K pathway and extend the duration of transient MAPK activity. In addition to the feedback and feedforward motifs involved, this network was particularly challenging to model due to the numerous simultaneous docking interactions. Their model included six binding sites on GAB1, 3 binding domains on Grb2 and both cytoplasmic or membrane targeted scaffolds, resulting in vast number of possible combinations of multi-protein complexes [56]. For example, when considering just EGF receptor dimerization and possible phosphorylation states and binding partners, without GAB there can be as many as 1128 different states. This combinatorial state explosion has also been observed in models of FcεRI signaling, where 354 distinct states and 3680 chemical reactions are possible [57,58].
The large number of protein complex combinations would generally make these models both computationally expensive and prohibitively time consuming to implement correctly. But several groups have identified new model reduction approaches or numerical algorithms to manage this complexity. The model reduction approach in the Kiyatkin model was developed by Borisov et al., which by assuming independent binding collapses many of the states into groups called macrostates [59,60]. A strength of this approach is that often the macrostates correspond with experimental observables, such as the overall Gab1 phosphorylation level rather combinations of modifications that are not accessible experimentally. Another method to reduce the burden of combinatorial complexity was developed by Faeder et al., which automatically generates the equations for each state using a reduced number of variables [58]. This takes advantage of the fact that while there may be a large number of possible states, often the reactions that occur on the multi-protein complexes only depend on a few of the states of the complex and are the same across many different combinations. This allows the definition of reaction classes which are all defined by the same kinetic equation and rate constants. For example, a model of FcεRI signaling was developed to have 15 reaction classes with 21 rate constants to describe the 354 states in contrast to implementing each of the 3680 chemical reactions individually [58]. Finally, Sneddon et al. developed stochastic modeling software, NFsim, which keeps track of every individual molecular complex and uses rules to describe state changes [61]. This approach becomes beneficial when the number of potential states is much larger than the number of individual molecular species in the simulation.
The modeling studies discussed above provide a number of insights into the design principles of signaling on scaffolds. Studies of MAPK signaling have shown how scaffolds can accelerate or amplify signaling by increasing the effective concentration within the complex. On the other hand, certain combinations of rate constants can cause the scaffold to attenuate signaling. Models of the Gab1 scaffold have examined the impact of localization and how scaffolds may recruit entire protein complexes to the plasma membrane. The Gab1 model also nicely illustrates how scaffolds may help establish network motifs such as feedback loops that shape signaling dynamics. While specificity has not been directly studied in these studies, the effects of specificity are often included by assuming no enzymatic activity between the scaffold and cytosol. While the described models have been deterministic, there is increasing recognition of the functional importance of biological noise [62]. Bhalla examined the differences between stochastic and deterministic modeling for four different signaling cascades, finding that the effects of noise begin to dominate at volumes approaching 10−15 L [63]. Localization by AKAPs creates compartments of this size and commonly stoichiometries of just 1 protein per complex, warranting further examination of how randomness affects AKAP signaling.
Conclusions
AKAPs play an important role in shaping cellular signals and regulating physiology and disease. Computational modeling is beginning to provide insights into the design principles fundamental to AKAP signaling function including localization, specificity, acceleration and amplification. AKAPs also help form a variety of feedback and feedforward network motifs, considerably increasing the diversity of signaling dynamics. Recent modeling efforts of MAPK and EGFR scaffolds provide additional examples of how scaffolds may regulate signaling networks. Mathematical models aid formulation of quantitative hypotheses that best explain experimental data and prediction of responses to perturbations that are not currently feasible experimentally. In the future, models of AKAP signaling are also likely to be helpful in identifying novel drug targets for cardiovascular disorders including hypertrophy, arrhythmia, and heart failure.
Acknowledgments
Sources of support: This work was supported by National Institutes of Health (grant HL094476 to J.S.) and the American Heart Association (grant 0830470N to J.S.).
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
References
- 1.Kapiloff MS. AKAPs: Temporal and Spatial Regulation of Intracellular Signal Transduction in the Cardiovascular System. Journal of Cardiovascular Pharmacology. 2011 doi: 10.1097/FJC.0B013E31822D5C08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michel JJ, Scott JD. AKAP mediated signal transduction. Annual review of pharmacology and toxicology. 2002 Jan;42:235–57. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- 3.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nature reviews Molecular cell biology. 2004 Dec;5:959–70. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 4.Dodge-Kafka KL, Bauman A, Kapiloff MS. A-kinase anchoring proteins as the basis for cAMP signaling. Handbook of experimental pharmacology. 2008 Jan;:3–14. doi: 10.1007/978-3-540-72843-6_1. [DOI] [PubMed] [Google Scholar]
- 5.Ruehr ML, Russell Ma, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. Journal of molecular and cellular cardiology. 2004 Sep;37:653–65. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, Rebolledo B, Pennypacker J, Thurston J, Rodriguez-Pinto N, Self C, Olson G, Insel Pa, Giles WR, Taylor SS, Roth DM. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. The Journal of biological chemistry. 2010 Sep;285:27632–40. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, Young SG, Vranizan K, Kwok PY, Whooley MA, Conklin BR. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007 May;104:8461–6. doi: 10.1073/pnas.0610393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science (New York, NY) 2002 Jan;295:496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 9.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Molecular cell. 2008 Oct;32:169–79. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirtenberger M, Schmutzhard J, Hemminki K, Meindl A, Sutter C, Schmutzler RK, Wappenschmidt B, Kiechle M, Arnold N, Weber BHF, Niederacher D, Bartram CR, Burwinkel B. The functional genetic variant Ile646Val located in the kinase binding domain of the A-kinase anchoring protein 10 is associated with familial breast cancer. Carcinogenesis. 2007 Feb;28:423–6. doi: 10.1093/carcin/bgl164. [DOI] [PubMed] [Google Scholar]
- 11.Yang JH, Saucerman JJ. Computational models reduce complexity and accelerate insight into cardiac signaling networks. Circulation research. 2011 Jan;108:85–97. doi: 10.1161/CIRCRESAHA.110.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. The Journal of cell biology. 1999 Dec;147:1481–92. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LJ, Wang L, Ma Y, Durick K, Perkins G, Deerinck TJ, Ellisman MH, Taylor SS. NH2-Terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. The Journal of cell biology. 1999 May;145:951–9. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO reports. 2000 Dec;1:524–9. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. Journal of cell science. 1999 Aug;112( Pt 1):2725–36. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 16.Carr D, DeManno D, Atwood A, Hunzicker-Dunn M, Scott J. Follicle-stimulating hormone regulation of A-kinase anchoring proteins in granulosa cells. J Biol Chem. 1993 Oct;268:20729–20732. [PubMed] [Google Scholar]
- 17.Paulucci-Holthauzen AA, Vergara LA, Bellot LJ, Canton D, Scott JD, O’Connor KL. Spatial distribution of protein kinase A activity during cell migration is mediated by A-kinase anchoring protein AKAP Lbc. The Journal of biological chemistry. 2009 Feb;284:5956–67. doi: 10.1074/jbc.M805606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Current biology: CB. 2006 Feb;16:321–7. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burack WR, Shaw aS. Signal transduction: hanging on a scaffold. Current opinion in cell biology. 2000 Apr;12:211–6. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 20.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J. Signaling diversity of PKA achieved via a Ca(2+)-cAMP-PKA oscillatory circuit. Nature chemical biology. 2011 Jan;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraeutler MJ, Soltis AR, Saucerman JJ. Modeling cardiac beta-adrenergic signaling with normalized-Hill differential equations: comparison with a biochemical model. BMC systems biology. 2010 Nov;4:157. doi: 10.1186/1752-0509-4-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeke A, Lukacs M, Lim Wa, Remenyi A. Scaffolds: interaction platforms for cellular signalling circuits. Trends in cell biology. 2009 Aug;19:364–74. doi: 10.1016/j.tcb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005 Sep;437:574–8. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. The Journal of biological chemistry. 2009 Aug;284:23540–6. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Molecular cell. 2010 Feb;37:541–50. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proceedings of the National Academy of Sciences of the United States of America. 2001 Dec;98:14997–5002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indolfi C, Stabile E, Coppola C, Gallo A, Perrino C, Allevato G, Cavuto L, Torella D, Di Lorenzo E, Troncone G, Feliciello A, Avvedimento EV, Chiariello M. Membrane-Bound Protein Kinase A Inhibits Smooth Muscle Cell Proliferation In Vitro and In Vivo by Amplifying cAMP-Protein Kinase A Signals. Circ Res. 2001;88:319–324. doi: 10.1161/01.res.88.3.319. [DOI] [PubMed] [Google Scholar]
- 28.Feliciello A, Li Y, Avvedimento EV, Gottesman ME, Rubin CS. A-kinase anchor protein 75 increases the rate and magnitude of cAMP signaling to the nucleus. Current Biology. 1997 Dec;7:1011–1014. doi: 10.1016/s0960-9822(06)00424-6. [DOI] [PubMed] [Google Scholar]
- 29.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Molecular cell. 2004 Sep;15:889–99. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998 Oct;395:805–8. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 31.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green Sa, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997 Jul;19:185–96. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 32.Tavalin SJ. AKAP79 Selectively Enhances Protein Kinase C Regulation of GluR1 at a Ca2+-Calmodulin-dependent Protein Kinase II/Protein Kinase C Site. The Journal of biological chemistry. 2008;283:11445–11452. doi: 10.1074/jbc.M709253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persechini A, McMillan K, Leakey P. Activation of myosin light chain kinase and nitric oxide synthase activities by calmodulin fragments. J Biol Chem. 1994;269:16148–16154. [PubMed] [Google Scholar]
- 34.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science (New York, NY) 2002 Oct;298:824–7. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Blackman BE, Schonemann MD, Zogovic-Kapsalis T, Pan X, Tagliaferri M, Harris HA, Cohen I, Pera RAR, Mellon SH, Weiner RI, Leitman DC. Estrogen receptor beta-selective agonists stimulate calcium oscillations in human and mouse embryonic stem cell-derived neurons. PloS one. 2010 Jan;5:e11791. doi: 10.1371/journal.pone.0011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutalik VK, Venkatesh KV. Theoretical Biology and Medical Quantification of the glycogen cascade system: the ultrasensitive responses of liver glycogen synthase and muscle phosphorylase are due to distinctive regulatory designs. Theoretical Biology and Medical Modelling. 2005;18:1–18. doi: 10.1186/1742-4682-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma W, Trusina A, El-Samad H, Lim Wa, Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009 Aug;138:760–73. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science (New York, NY) 2002 Nov;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 39.Novak B, Tyson JJ. Design principles of biochemical oscillators. Nature reviews Molecular cell biology. 2008 Dec;9:981–91. doi: 10.1038/nrm2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J. Signaling diversity of PKA achieved via a Ca(2+)-cAMP-PKA oscillatory circuit. Nature chemical biology. 2011 Jan;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lester LB, Faux MC, Nauert JB, Scott JD. Targeted Protein Kinase A and PP-2B Regulate Insulin Secretion through Reversible Phosphorylation. Endocrinology. 2001 Mar;142:1218–1227. doi: 10.1210/endo.142.3.8023. [DOI] [PubMed] [Google Scholar]
- 42.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DMF, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Molecular cell. 2006 Sep;23:925–31. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koshland DE, Goldbeter A, Stock JB. Amplification and adaptation in regulatory and sensory systems. Science (New York, NY) 1982 Jul;217:220–5. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- 44.Ferrell J. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends in Biochemical Sciences. 1996 Dec;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 45.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. The Journal of biological chemistry. 2003 Nov;278:47997–8003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 46.Singh A, Redden JM, Kapiloff M, Dodge-Kafka KL. The large isoforms of AKAP18 mediate the phosphorylation of Inhibitor-1 by PKA and the inhibition of PP1 activity. Molecular pharmacology. 2011 Dec;79:533–540. doi: 10.1124/mol.110.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langeberg LK, Scott JD. A-kinase-anchoring proteins. Journal of cell science. 2005 Aug;118:3217–20. doi: 10.1242/jcs.02416. [DOI] [PubMed] [Google Scholar]
- 48.Saucerman JJ, McCulloch AD. Mechanistic systems models of cell signaling networks: a case study of myocyte adrenergic regulation. Progress in biophysics and molecular biology. 2004;85:261–78. doi: 10.1016/j.pbiomolbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch AD. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circulation research. 2004 Dec;95:1216–24. doi: 10.1161/01.RES.0000150055.06226.4e. [DOI] [PubMed] [Google Scholar]
- 50.Darbar D, Roden DM, Ali MF, Yang T, Wathen MS. Images in cardiovascular medicine. Himalayan T waves in the congenital long-QT syndrome. Circulation. 2005 Mar;111:e161. doi: 10.1161/01.CIR.0000159092.92366.CD. [DOI] [PubMed] [Google Scholar]
- 51.Andrei O, Calder M. A Model and Analysis of the AKAP Scaffold. Electronic Notes in Theoretical Computer Science. 2010:1–14. [Google Scholar]
- 52.Levchenko A, Bruck J, Sternberg PW. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proceedings of the National Academy of Sciences of the United States of America. 2000 May;97:5818–23. doi: 10.1073/pnas.97.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Shaughnessy EC, Palani S, Collins JJ, Sarkar Ca. Tunable Signal Processing in Synthetic MAP Kinase Cascades. Cell. 2011 Jan;144:119–131. doi: 10.1016/j.cell.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locasale JW, Shaw AS, Chakraborty AK. Scaffold proteins confer diverse regulatory properties to protein kinase cascades. Proceedings of the National Academy of Sciences of the United States of America. 2007 Aug;104:13307–12. doi: 10.1073/pnas.0706311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science (New York, NY) 2009 Nov;326:1220–4. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiyatkin A, Aksamitiene E, Markevich NI, Borisov NM, Hoek JB, Kholodenko BN. Scaffolding protein Grb2-associated binder 1 sustains epidermal growth factor-induced mitogenic and survival signaling by multiple positive feedback loops. The Journal of biological chemistry. 2006 Jul;281:19925–38. doi: 10.1074/jbc.M600482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hlavacek WS, Faeder JR, Blinov ML, Perelson AS, Goldstein B. The complexity of complexes in signal transduction. Biotechnology and bioengineering. 2003 Dec;84:783–94. doi: 10.1002/bit.10842. [DOI] [PubMed] [Google Scholar]
- 58.Faeder JR, Hlavacek WS, Reischl I, Blinov ML, Metzger H, Redondo A, Wofsy C, Goldstein B. Investigation of early events in Fc epsilon RI-mediated signaling using a detailed mathematical model. Journal of immunology (Baltimore, Md: 1950) 2003 Apr;170:3769–81. doi: 10.4049/jimmunol.170.7.3769. [DOI] [PubMed] [Google Scholar]
- 59.Borisov NM, Markevich NI, Hoek JB, Kholodenko BN. Signaling through receptors and scaffolds: independent interactions reduce combinatorial complexity. Biophysical journal. 2005 Aug;89:951–66. doi: 10.1529/biophysj.105.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borisov NM, Markevich NI, Hoek JB, Kholodenko BN. Trading the micro-world of combinatorial complexity for the macro-world of protein interaction domains. Bio Systems. 2006;83:152–66. doi: 10.1016/j.biosystems.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sneddon MW, Faeder JR, Emonet T. Efficient modeling, simulation and coarse-graining of biological complexity with NFsim. Nature Methods. 2011 Feb;8:177–183. doi: 10.1038/nmeth.1546. [DOI] [PubMed] [Google Scholar]
- 62.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010 Sep;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhalla US. Signaling in small subcellular volumes. I. Stochastic and diffusion effects on individual pathways. Biophysical journal. 2004 Aug;87:733–44. doi: 10.1529/biophysj.104.040469. [DOI] [PMC free article] [PubMed] [Google Scholar]