Abstract
Objective
To investigate the clinical correlates of central nervous system (CNS) alterations among women with vulvodynia. Altered central sensitization has been linked to dysfunction in CNS inhibitory pathways (e.g. GABAergic), and metrics of sensory adaptation, a centrally mediated process that is sensitive to this dysfunction, could potentially be used to identify women at risk of treatment failure using conventional approaches.
Methods
Twelve women with vulvodynia and twenty age-matched controls participated in this study, which was conducted by sensory testing of the right hand’s index and middle fingers. The following sensory precepts were assessed: 1) vibrotactile detection threshold; 2) amplitude discrimination capacity (defined as the ability to detect differences in intensity of simultaneously delivered stimuli to two fingers); and 3) a metric of adaptation (determined by the impact that applying conditioning stimuli have on amplitude discriminative capacity).
Results
Participants did not differ on key demographic variables, vibrotactile detection threshold, and amplitude discrimination capacity. However, we found significant differences from controls in adaptation metrics in one subgroup of vulvodynia patients. Compared to healthy controls and women with a shorter history of pain (n=5; duration (yr) = 3.4 ± 1.3), those with a longer history (n=7; duration (yr) = 9.3 ±1.4)) were found to be less likely to have adaptation metrics similar to control values.
Discussion
Chronic pain is thought to lead to altered central sensitization, and adaptation is a centrally mediated process that is sensitive to this condition. This report suggests that similar alterations exist in a subgroup of vulvodynia patients.
Keywords: Vulvodynia, Central sensitization, Adaptation
Introduction
Vulvodynia is a heterogeneous family of idiopathic pain disorders affecting upward of 16% of reproductive age women in the US1. It is characterized by both provoked and unprovoked pain in and surrounding vulvar skin, mucosa and underlying musculature. Clinically, vulvodynia is classified into subgroups based on anatomical location (vulvar mucosa vs. hairy/non-hairy epithelium) and temporal characteristics as provoked vs. unprovoked. While a given patient may experience both provoked and unprovoked pain, the most common complaint is that of provoked pain on contact, precipitated by tampon use or intercourse. Unlike unprovoked pain -where the clinical examination is non-specific- the majority of women with provoked pain have localized tenderness in vulvar mucosa (a.k.a. vestibule) 2. Additionally, women with provoked vulvodynia tend to be younger, and in most instances unaware of their condition until coital debut or the first attempt at using a tampon.
While both peripheral and central abnormalities have been implicated in vulvodynia, the extent to which peripheral vs. central factors contribute to the pain state in an individual patient remains unknown. A substantial portion of women with vulvodynia show hypersensitivity at extra-genital sites (e.g. arms and feet); this non-specific hypersensitivity has conventionally been attributed to changes in ‘central sensitization’ caused by the chronic pain state. To date, clinical signs and symptoms associated with central dysregulation in subgroups of women with vulvodynia remains unknown. Thus, understanding of the mechanistic (central vs. peripheral) implication of clinical signs and symptoms in vulvodynia is a necessary first step towards individualized, symptom based treatment approach.
Current literature 1,3,4 suggests that symptoms of vulvodynia are likely to be triggered by peripheral factors in the skin and/or underlying musculature. With time (and chronicity), varying degrees of central dysregulation may develop. In this setting, patients may experience superimposed unprovoked (spontaneous) pain in otherwise unaffected tissue. Thus, investigating clinical correlates of central involvement in vulvodynia (e.g., how sensory information processing is altered) may provide us with a unique opportunity to investigate the mechanisms of clinically similar disorders (e.g. localized pain at the vulvar vestibule vs. generalized vulvar pain). Once the fundamental mechanisms of the centrally vs. peripherally mediated vulvar pain is understood, this knowledge will enable the development of robust research and clinical tools that could improve diagnosis and lead to informed therapeutic options.
In this study, we investigated sensory information processing in subgroups of patients with vulvodynia and healthy controls. The quantitative sensory testing methodology utilized in this study has been demonstrated to be sensitive to systemic cortical alteration 5-7, and in pilot studies, has been shown to return to normative values with treatment (Tommerdahl; personal communication, 2010). In this study, we hypothesized that women who had experienced a longer time course with pain and/or had unprovoked symptoms are more likely to have measures consistent with altered central sensitization when compared to healthy control subjects or those subjects who had experienced a shorter duration of provoked pain.
Materials and Methods
In this study, a convenience sample of twelve women with vulvodynia and twenty healthy controls without gynecological pain were recruited from the University of North Carolina, Pelvic Pain Clinic and the surrounding community, respectively. The groups did not differ in basic demographic characteristics. All the participants were naïve both to the study design and issue under investigation. The study was performed in accordance with the Declaration of Helsinki, all subjects gave their written informed consent, and the experimental procedures were reviewed and approved in advance by an institutional review board.
Experimental sessions were conducted with the subjects seated comfortably in a chair with the right arm resting on an arm rest attached to the head unit of a portable four-site vibrotactile stimulator (Fig. 1; CM4; Cortical Metrics, LLC). Vibrotactile stimulation was conducted via 5mm probes that come in contact with subject’s digit 2 (index finger) and digit 3 (middle finger). Glabrous pads of digit 2 (D2) and digit 3 (D3) were chosen as the test sites for two reasons: (1) to allow the convenience of access and comfort of the subject, and (2) because of the wealth of neurophysiological information that exists for the corresponding somatotopic regions of cortex in primates. The independent probe tips are computer controlled and capable of delivery of a wide range of vibrotactile stimulation of varying frequencies (measured in Hertz) and amplitudes (measured in micrometers, μm). Stimulus parameters are specified by test algorithms that are based on specific protocols and subjects’ responses during those protocols.
Fig. 1.
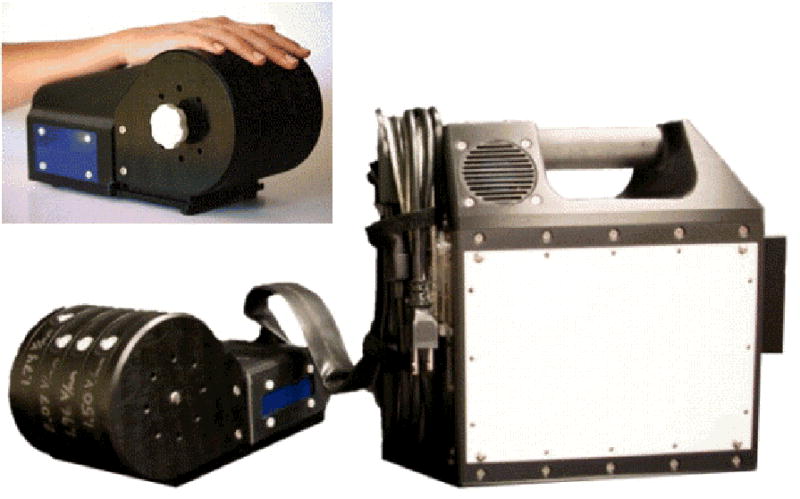
Images of the multi-site vibrotactile stimulator. Stimulators are positioned by rotating each of the 4 independently positioned drums to maximize contact between fingers and the stimulator tips. During an experimental session, the subject was seated comfortably in a chair with the right arm resting on the arm rest attached to the head unit of the stimulator. Index and middle finger were positioned for D2 and D3 stimulation.
Participants viewed a computer monitor which provided continuous visual cueing during the experimental session. Specifically, an on-screen light panel indicated to the subject when the stimulus was on and when the subject was to respond. Practice trials were performed before each test which allowed the subject to become familiar with the tests, and correct responses on 5 consecutive training trials were required before commencing with each test. The subject was not given performance feedback or knowledge of the results during data acquisition.
The sensory testing session was conducted by application of low frequency (25 Hz) vibration to right hand’s index and middle finger(s). The protocols –from start to finish- lasted approximately 30 minutes and consisted of the following 5 modules: (1) static detection threshold; (2) dynamic detection threshold; (3) amplitude discrimination between two concurrent and stationary stimuli; (4) the impact of single-site adaptation on amplitude discrimination capacity; and (5) dynamic amplitude discrimination. Exemplary use, technical description and neurobiological basis of individual modules have previously been described in detail 5-10. An overview of the procedures and the previously published normative findings is provided below.
Static detection threshold
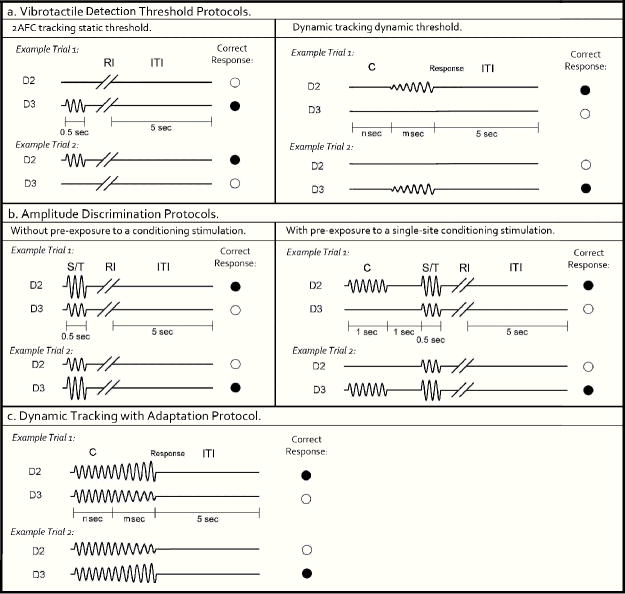
Each participant’s vibrotactile detection threshold was measured using a 20-trial Two Alternative Forced Choice (2AFC) tracking protocol (for recent description with this experiment setup, see previous studies 9-13). The left panel of Fig. 2a shows the schematic of the protocol. During each trial a 25 Hz vibrotactile test stimulus was delivered to either D2 or D3; the stimulus location was randomly selected on a trial-by-trial basis in order to minimize subject’s inattention and distraction. Following each vibrotactile stimulus, the subject was prompted to select the skin site (index (D2) vs. middle (D3) finger) that was perceptually larger. After a 5 sec delay –based on subject response- the stimulation was repeated until the completion of the 20 trials. The stimulus amplitude was started at 15 μm and was modified based on the subject’s response in the preceding trial. A 1-up/1-down algorithm was used for the purposes of amplitude modification in the first 10 trials. For example, the stimulus amplitude was decreased by 1 μm if the subject’s response in the preceding trial was correct. However, it was increased by the same amount if the response was incorrect. After the initial 10 trials, the amplitude was varied using a 2-up/1-down algorithm (two correct/one incorrect subject response(s) resulted in a decrement/increment, respectively, in the amplitude of the stimulus). The rationale for using 1up/1down algorithm in the first 10 trials was to expedite determination of subject’s vibrotactile discriminative range without affecting the results, and this approach has been previously reported 6-10,14,15.
Fig. 2.
- Vibrotactile detection threshold protocols. Left panel: 2AFC tracking protocol: In each trial, a 25 Hz vibrotactle test stimulus was delivered to either D2 or D3 for 0.5 sec, followed by a subject response interval (RI). Subject was prompted to select the skin site that perceived the stimulus. A 5 sec inter-trial interval (ITI) intervened between stimulus response and onset of the next trial. Right panel: Dynamic tracking protocol: A delay period (n sec = 0, 1.5, 2 or 3 sec) without any stimulation was applied. After the initial delay, a 25 Hz vibrotactile stimulus was delivered to either D2 or D3. The amplitude of the stimulus was initiated from zero and increased in steps of 2 μm/sec. The stimulation was terminated with subject response to the perceived stimulus.
- Amplitude discrimination protocols. Left panel: Amplitude discrimination at baseline: Two 25 Hz vibrotactile stimuli, the standard (S) and test (T), were delivered simultaneously for 0.5 sec. Subject was asked to choose the stimulus that was perceptually larger. Right panel: Amplitude discrimination task with pre-exposure to conditioning stimulation. A 25 Hz conditioning stimulus was delivered 1 sec prior to the presentation of the test and standard stimuli.
- Dynamic tracking with adaptation protocol: Two identical 25 Hz vibrotactile stimuli were delivered simultaneously for a fixed interval (n sec = 0, 1.5, 2, or 3 sec). After the initial constant stimulus period, the amplitude of the two stimuli were dynamically increased/decreased, in steps of 25 μm/sec. Stimulation was terminated with subject response to the location at which the most intense stimulus was delivered.
Dynamic detection threshold
At the beginning of each trial (as shown in Fig. 2a, right panel), a delay period which includes no stimulation was applied. Four conditions of delay (n sec) were employed, in separate trials: 0, 1.5, 2, and 3 sec. After the initial delay, a 25 Hz vibrotactile stimulus was delivered to either D2 or D3 (the stimulus location was randomly selected on a trial-by-trial basis). The amplitude of the stimulus was initiated from zero and increased in steps of 2 μm/sec. The subject was instructed to indicate the skin site that received the stimulus as soon as the vibration was detected. The subject’s detection threshold was calculated as the average of the stimulus amplitude at the time of subject response (msec).
Amplitude discrimination at baseline
Each subject’s amplitude discrimination capacity was assessed using a 2AFC tracking protocol that has been described and implemented in a number of previous studies 6-10,14,15. As shown in Fig. 2b left panel, during the 20-trial experimental run, a vibrotactile test stimulus (25 Hz, amplitude between 105 and 200 μm) was delivered to one digit pad at the same time that a standard stimulus (25 Hz, amplitude fixed at 100 μm) was applied to the other digit pad. The loci of the test and standard stimuli were randomly selected on a trial-by-trial basis. At the beginning of the experimental run, the test amplitude was 200 μm and the standard amplitude was 100 μm. The difference between the amplitudes of the test and standard stimuli was adjusted on the basis of the subject’s response in the preceding trial, such that the difference was decreased/increased after a correct/incorrect response, respectively. The same tracking algorithm as that described for the tactile detection threshold protocol (2AFC tracking protocol) was employed to track the subject’s ability to determine the most intense stimulus between the test and standard stimuli (i.e., the subject’s difference limen (DL) was determined). The step size was held constant at 10 μm throughout the experimental run.
Amplitude discrimination with single-site adaptation
In order to measure the effects that conditioning stimuli have on subsequent test stimuli, the previously described amplitude discrimination protocol was modified. As shown in Fig. 2b right panel, a 25 Hz 200 μm conditioning stimulus was delivered 1 sec prior to the presentation of the test and standard stimuli. When the conditioning stimulus is delivered to the same site as the test stimulus, the gain effect of adaptation (reducing the perceived intensity) can be quantified by comparison of the amplitude discrimination DL obtained in the adapted vs. non-adapted conditions 6-8,10. The amplitude discrimination tracking algorithm used in the previously described protocol was employed.
Dynamic amplitude discrimination
To further characterize the effects of adaptation on amplitude discrimination, a dynamic tracking protocol was implemented (for recent description with this experimental setup, see previous study 10). At the start of each run (shown in Fig. 2c), two vibrotactile stimuli (25 Hz; initially identical in amplitude at 300 μm) were delivered simultaneously to D2 and D3. Four conditions of initial constant stimulus duration (n sec) were employed in separate experimental trials: 0, 1.5, 2, and 3 sec. After the initial constant or stationary stimulus period, the amplitudes of both stimuli were dynamically altered such that the amplitude of one stimulus was increased and the amplitude of the other stimulus was decreased at the rate of 25 μm/sec. The subject was instructed to indicate the location at which the most intense stimulus was delivered as soon as the two stimuli felt distinctly different in intensity. For each trial, the DL was recorded as the actual difference between the two test amplitudes at the time of subject response (m sec). Averaged DLs were obtained for the four different durations of conditioning stimuli that preceded each trial.
Analysis
Repeated-measures analysis of variance (ANOVA) was used to evaluate the difference of the subject’s performance under different conditions. Data are presented as means and standard errors (SE). A probability of less than 0.05 was considered statistically significant.
Results
The present study compared women with vulvodynia and matched healthy controls in a series of sensory perceptual measures that assessed: (1) vibrotactile detection threshold on the fingertip; (2) amplitude discrimination capacity; and (3) the impact of conditioning stimuli on amplitude discrimination capacity. The results show that patients with vulvodynia deviated very little from that of healthy controls in most of the sensory measures obtained in the absence of conditioning stimuli – such as threshold detection and amplitude discriminative capacity, although the patients with vulvodynia demonstrated a tendency to have lower tactile thresholds on the fingertips than controls. Most importantly, the measures of the effects of conditioning stimuli on amplitude discrimination revealed that the patients’ data clustered into two distinct sub-groups (which will be referred to as Group A and Group B). Group B data was very similar to that obtained from healthy control subjects, and Group A demonstrated a significantly reduced impact of adaptation on the sensory percept. While the average ages and demographics of the two sub-groups were not significantly different, there was a significant difference in the duration that the two sub-groups of patients had pain: Group A (n=7) subjects had suffered from vulvodynia for a long duration (average duration: 9.3 ± 1.4 years; average age: 35.7 ± 3.2 years); and Group B (n=5) subjects had suffered from vulvodynia for a relatively shorter duration (average duration: 3.4 ±1.3 years; average age: 34.6 ± 4.3 years).
Patients with vulvodynia exhibit slightly lower tactile detection thresholds
Fig. 3 summarizes the group-averaged detection thresholds. As shown in the left panel of Fig. 3, the group-averaged static thresholds observed were 12.37±1.34 μm for controls, 9.77±2.23 μm for patients in Group A, and 10.32±1.85 μm for patients in Group B. The data suggest an elevated sensitivity for patients with vulvodynia compared to controls, although this difference was not statistically significant (Group A vs. controls: p=0.35; Group B vs. controls: p=0.51). This finding is consistent with data reported by Pukall 16 which showed that women suffering from vulvodynia had a lower tactile threshold than controls at sites distant to the genitalia area.
Fig. 3.
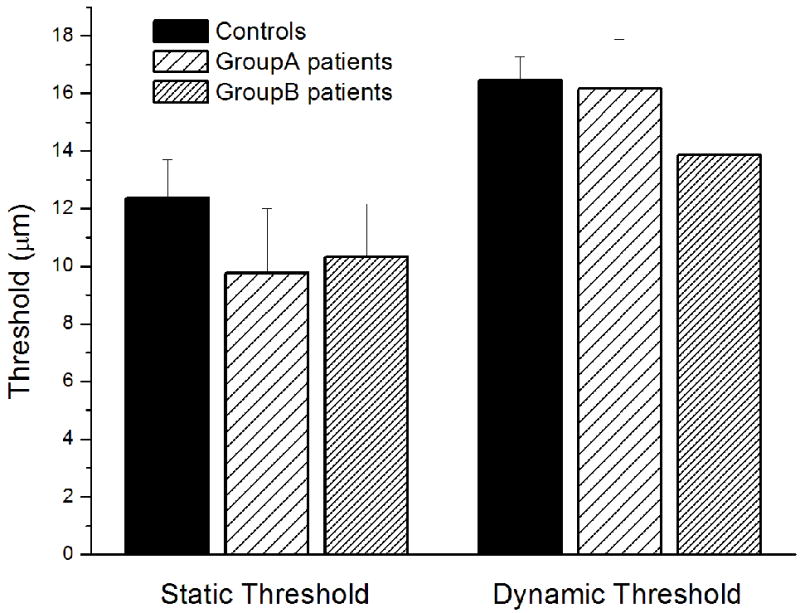
Summary of group-averaged vibrotactile detection thresholds obtained with two different methods on two sub-groups of patients with vulvodynia and controls. Static threshold: No significant difference were observed on the static thresholds between any patients group and controls (Group A vs. controls: p = 0.35; Group B vs. controls: p = 0.51). Dynamic threshold: The group-averaged dynamic thresholds of patients with vulvodynia did not significantly differ from that of controls, while data from patients in Group B show a trend for lower dynamic threshold than controls.
Since several studies have reported that psychophysical measurement methods had a significant influence on vibrotactile thresholds 17,18, in current study, the subject’s vibrotactile threshold was also measured by a dynamic tracking protocol. The group-averaged dynamic thresholds are shown in the right panel of Fig. 3. There was no significant difference between the controls and two vulvodynia patients groups, although data from patients in Group B showed a lower (though not statistically significant) dynamic threshold than controls.
While amplitude discrimination capacity was not significantly different between the controls and patients with vulvodynia, the impact of conditioning stimuli on performance during this task revealed that the vulvodynia subjects were clustered into two distinct sub-groups
Fig. 4 summarizes the group-averaged performance during amplitude discrimination tests for the controls and two sub-groups of patients with vulvodynia. Weber’s fractions (WF) were determined by normalizing each subject’s DL to the amplitude of standard stimulus (100 μm). As shown in the left panel of Fig. 4, during which amplitude discrimination was measured in the absence of conditioning stimulus, there was no significant difference in performance between the controls and groups of vulvodynia patients. Specifically, control subjects were able to discriminate the difference between the test and standard stimuli that is 24.4% of the standard amplitude (WF = 0.244), and the patients in Group A and Group B were able to discriminate respectively 33.5% (WF = 0.335) and 31.6% (WF = 0.316) of the standard amplitude. However, pre-exposure to a single-site conditioning stimulus dramatically changed the subjects’ performance (shown in Fig. 4, right panel). While the WF of controls and patients in Group B is significantly elevated in the adapted condition compared to the un-adapted condition, patients in Group A performed equally well under both adapted and un-adapted conditions. Previous reports have demonstrated that single-site adaptation impairs control subject’s amplitude discrimination capacity 6-8, 10. One interpretation of the impairment observed in current study is that a 1 sec conditioning stimulus reduces the perceived intensity of the subsequent test stimulus to the extent that a test stimulus with amplitude of approximately 162% (controls)/ 171% (Group B) of the standard amplitude was perceived nearly the same in intensity as the standard stimulus. Comparing to the significant degradation of performance of the controls (p < 0.01) and the patients in Group B (p = 0.017) due to adaptation, no change was observed in the patients in Group A (p = 0.52). Moreover, under the adapted condition the group-averaged performance is significantly different between controls and patients in Group A (p = 0.036). Therefore, conditioning stimulation significantly impaired the performance of the controls and the patients in Group B, but has no effects on the patients in Group A.
Fig. 4.
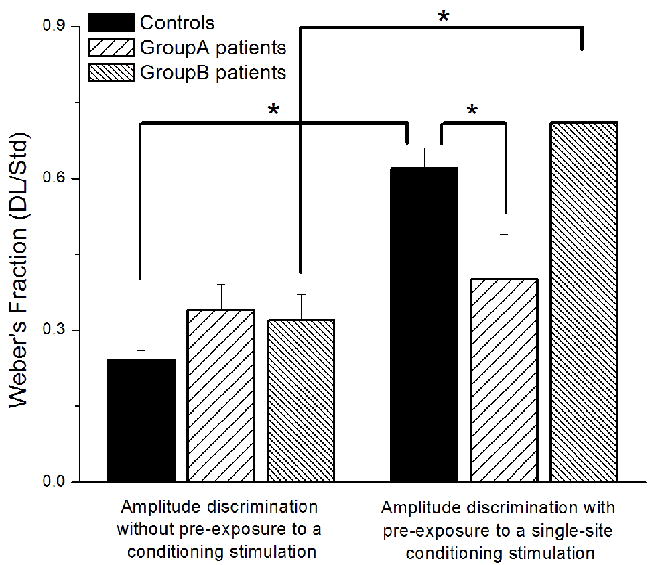
Comparison of weber’s fraction obtained with amplitude discrimination protocols (without/with pre-exposure to a single-site conditioning stimulus). In the absence of conditioning stimulus, no significant difference was observed between the performance of controls and sub-groups of vulvodynia patients. Pre-exposure to a single-site conditioning stimulation (1 sec in duration) caused a significant degradation of performance in the controls (p < 0.01) and the patients in Group B (p = 0.017). Contrary to controls and Group B, patients in Group A performed equally well under both adapted and un-adapted conditions. Under the condition with adaptation, the group-averaged performance is significantly different between controls and Group A (p = 0.036).
In order to determine whether the differential effects of adaptation observed between groups were consistent within subjects, each subject’s WF obtained under the adapted condition was normalized to the un-adapted condition. As shown in Fig. 5, The 1 sec conditioning stimulus significantly impaired amplitude discrimination capacity by nearly 30% for both the controls and the patients in Group B, while there was much less of an effect (3%) of adaptation observed in the patients in Group A (p < 0.01).
Fig. 5.
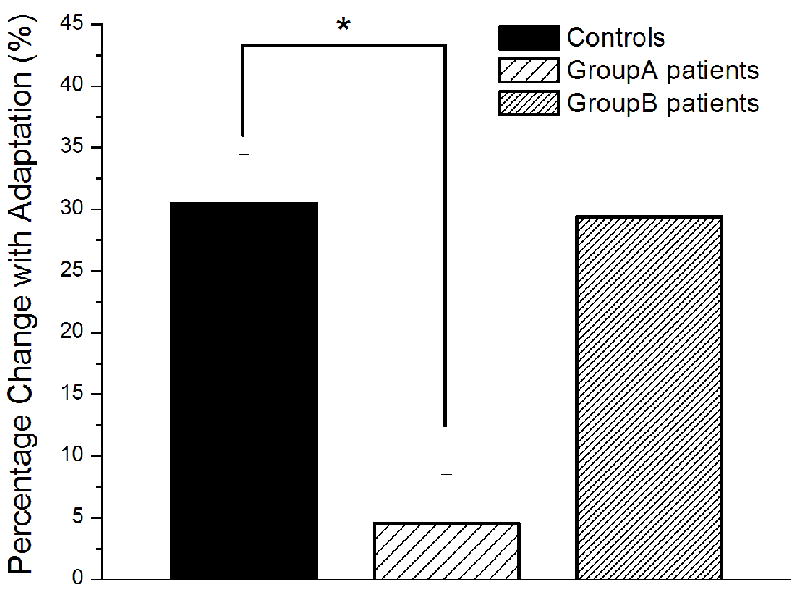
Weber’s fraction obtained under the condition with adaptation was normalized on a subject-by-subject basis to the un-adapted condition. The 1 sec conditioning stimulus significantly impaired the subjects’ amplitude discrimination capacity by nearly 30% for both the controls and the patients in Group B, while there were much lesser effects (3%) of adaptation observed in the patients in Group A.
Dynamic amplitude discrimination
A dynamic amplitude discrimination protocol was employed which is able to effectively compare the degree to which a subject adapts to simultaneously delivered dual-site vibrotactile stimuli at different durations of conditioning stimulation. Fig. 6 summarizes the group-averaged performance with dual-site adaptation at the four different durations of conditioning stimulation (0, 1.5, 2, and 3 sec) for the controls and two sub-groups of patients with vulvodynia. The results show that increasing the duration of the conditioning stimuli delivered to both sites of skin led to an improvement of a subject’s capacity to detect the difference in amplitude between the two stimuli. For example, after pre-exposure to 1.5 sec, 2 sec, or 3 sec conditioning stimulus, control subjects were, on average, able to attain a DL (156 μm, 141 μm, 94 μm) that was ~73%, ~66%, or ~42% of the DL (208 μm) obtained without adaptation. Compared to controls, two sub-groups of patients with vulvodynia have distinct performance differences. Specifically, the DLs were significantly higher in patients of Group A compared to controls (0 sec adaptation: p < 0.01; 1.5 sec adaptation: p = 0.01; 2 sec adaptation: p < 0.01; 3 sec adaptation: p = 0.06), but there was no significant difference between patients of Group B and controls in the DLs obtained under all the conditions. In summary, data obtained from patients in Group A showed little effect with conditioning stimulation while the data obtained from patients in Group B deviated very little from that of controls.
Fig. 6.
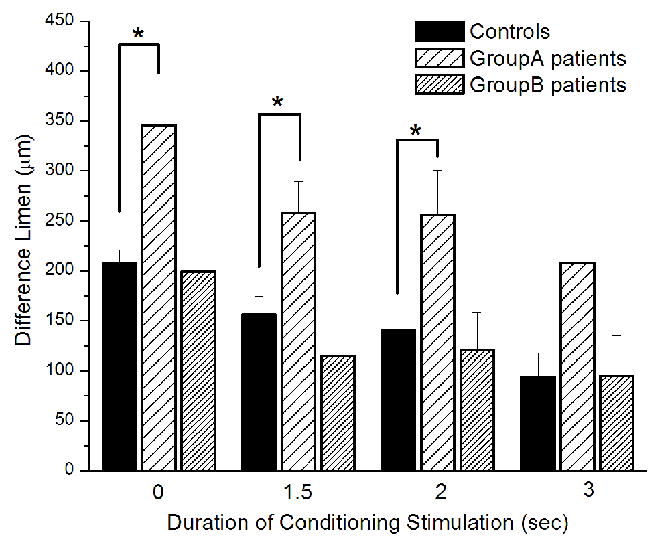
Comparison of the group-averaged performance with dual-site adaptation at the four different durations of dual-site conditioning stimulation (0, 1.5, 2 and 3 sec) for the controls and two sub-groups of patients with vulvodynia. Increasing the duration of the conditioning stimuli led to an improvement of performance (i.e., reduced DL). As the data obtained from patients in Group B deviated very little from that of controls, DLs obtained from patients in Group A were significantly higher compared to controls and showed only little effect with adaptation.
Discussion
In this study, sensory perceptual measures were obtained on 12 patients diagnosed with vulvodynia and 20 healthy control subjects. Five tests were performed to assess: (1) detection threshold on the fingertips; (2) amplitude discrimination capacity; (3) the effects of adaptation on tactile discrimination capacity. The results suggest that women with vulvodynia have – although not statistically significantly - lower tactile thresholds on the fingertips than do control subjects. Furthermore, as amplitude discrimination capacity was not significantly different between the controls and patients with vulvodynia, the impact of single site conditioning (or adaptation) on performance of the dual-site task demonstrated a remarkable difference. Specifically, the observations of the conditioned sensory measures revealed that the patients with vulvodynia were clustered into two distinct sub-groups. Group B had data that was very similar to that obtained from healthy control subjects, while Group A demonstrated a significantly reduced impact of adaptation on the sensory percept. The primary difference between the compositions of the two sub-groups is the duration or longevity of pain of the patients in each sub-group. Group B was composed of patients that reported pain for an average of 3.4 ±1.3 years, while Group A was composed of patients who reported pain for an average duration of 9.3 ± 1.4 years.
The reduction of the adaptation metric in patients with vulvodynia studied in this paper has not been previously reported. There have been few studies to date that have assessed the changes in perception that normally result from repetitive vibrotactile stimulation on the population of chronic pain patients, though Hollins and colleagues did report decreased effects of adaptation in subjects with tempormandibular disorders 19. Neurophysiological studies have demonstrated that repetitive stimulation results in temporal changes of cortical activity, the most prominent of which is a reduction in cortical response with extended stimulus duration. At the single cell level, both visual and somatosensory cortical pyramidal neurons undergo prominent use-dependent modifications of their receptive fields and response properties with repetitive stimulation. These modifications can attain full development within a few tens of milliseconds of stimulus onset, and can disappear within seconds after the stimulus ends (visual cortical neurons 20-30; alternatively, for review of short-term cortical neuron dynamics in visual cortex 31; for review of short-term primary somatosensory cortical neuron dynamics 32-36). Optical imaging studies have also characterized the short-term dynamics of the population-level response of squirrel monkey contralateral primary somatosensory (SI) cortex using different amplitudes and durations of vibrotactile stimulation 37-39. Guided by the scientific work mentioned above, our research group has designed a series of tactile sensory diagnostics which effectively assess the impact that adaptation has on perception 5,7-10,15. For example, the protocols employed in the current study directly measure the change in amplitude discrimination capacity that occurs with prior conditioning stimuli. Previous studies using this measure demonstrated that a subject’s ability to discriminate between two simultaneously delivered vibrotactile stimuli – differing only in amplitude and location – was very robust and repeatable across a large number of healthy subjects, but it was also very sensitive to varying conditions of conditioning stimuli. For instance, changing the duration of the conditioning stimulus delivered to one of the two sites before the amplitude discrimination task significantly altered a subject’s ability to determine the actual difference between the two stimuli in a predictive and quantifiable fashion. As a result, these methods could be viewed as a reliable indicator of the influence of adapting stimuli on central nervous system response, as changes in the peripheral response are not significantly changed at these short stimulus durations (for discussion, see 5,8,9,40,41). Centrally mediated adaptation is dependent on several factors (e.g., GABAergic and NMDA receptor mediated neurotransmission, neuron-glial interactions) which play significant roles in the way in which cortical information processing capacities of a number of clinically identified subject populations are impacted by their respective disorder. For example, conditioning stimuli do not have as pronounced an impact on the amplitude discriminative capacity of subjects with autism as it does with typically developing subjects (for discussion of GABA-deficiencies in autism, see 5,6,41). Additionally, subjects administered a relatively small dose of an NMDA receptor antagonist (60 mg of dextromethorphan) also demonstrated a degraded adaptation metric 7.
Two aspects of the adaptation process were measured in this study. The first, the gain effects of adaptation, was derived from the amplitude discrimination task in which a conditioning stimulus was delivered on one of the two test sites. The effect of that conditioning stimulus was on the gain of the conditioned site – that site was now perceived to be much smaller and thus, a reduction in gain was manifested, and subsequently, subjects (normally) become worse at the task. The second facet of adaptation that was measured was a contrast effect, in which contrast between two stimuli improve after conditioning stimuli have been delivered to both of the test sites, and the subjects (normally) perform better after conditioning than they do without. In this study, the data obtained from the vulvodynia subjects clustered into two distinct sub-groups consistently with both of these aspects of adaptation. The patients in Group B performed very similar as healthy controls did, and the performance of the patients in Group A showed a significantly reduced impact of conditioning stimulation on the sensory percept. However, other sensory measures obtained in the absence of conditioning stimuli – such as threshold detection and amplitude discriminative capacity – demonstrated no statistically significant difference between the two sub-groups. The primary difference between the compositions of the two sub-groups of note is the duration that patients of the sub-groups have had pain, while average age of the two sub-groups was not significantly different. Considering the metrics of adaptation (measuring the effects of conditioning stimulation on sensory perception) could be a reliable indicator of systemic alterations on central nervous function, it is speculated that the performance difference between the two sub-groups of patients with vulvodynia observed in the current study might reflect the level of dysregulation of their central nervous system due to chronic vulvar pain.
The involvement of both peripheral and central mechanisms in the development and maintenance of vulvodynia has been supported by a series of studies 3, 16, 42-47. For example, it has been found that patients with vulvodynia have increased sensitivity to sensory stimulation at both genital regions and sites distant to it 3, 16, 44. This suggests that not only peripheral sensitization but also a generalized central abnormality is involved in vulvodynia and could be similar to that observed in patients with other pain syndromes, implying a widespread disturbance in the CNS 45.The observation of increased tactile sensitivity of the skin area distant to the vulvar region – including the static thresholds of all vulvodynia subjects in this report - is consistent with altered central sensitization that develops with chronic pain.
All subjects, including controls, demonstrated a dynamic threshold that was higher than their static threshold. This noticeable difference in the threshold between the two tasks is consistent with previous reports 10,18. Although this could possibly be explained by the influence that psychophysical measurement methods have on tactile detection 17,18, we believe an alternate explanation is much more plausible. Mechanistically, this phenomenon could be the result of feed-forward inhibition that is generated by the initial subthreshold stimulus that occurs when the threshold test is ramped from zero to the detectable level 48. The significance of this is that this type of feed-forward inhibition takes place in somatosensory cortical input layer 4 49, in which local layer 4 inhibitory cells receive direct thalamocortical input and in turn suppress responses of neighboring layer 4 excitatory cells to their thalamocortical drive, thereby sharpening their RF properties 50-55. These inhibitory cells are more responsive to weak (near-threshold) afferent drive than are the excitatory layer 4 cells, and thus, sub-threshold or weak stimulus inputs will have the effect of raising the threshold at which excitatory layer 4 cells begin to respond to peripheral stimuli. Thus, though not statistically significant, the observation of the difference between the Group A and B patients in their dynamic thresholds is that the difference between the ratio of the respective dynamic and static thresholds are clearly evident, and suggestive of below normal feed-forward inhibition. If this alteration is, as we believe, sensitive to the time dependency of the GABAb receptor, then the measure itself might be an indicator that GABAb efficiency has been compromised in some individuals.
Our data on vulvodynia patients is consistent with existing constructs in the pain literature and supports the notion that the relative contribution of peripheral and central factors differ in subgroups of women with vulvodynia, and that clinical signs and symptoms alone are insufficient in identifying the underlying mechanism of pain as peripheral, central or a combination of both. A wide range of therapies for vulvodynia have been proposed that include topical therapies, pharmacologic regimens, physical therapy, surgery, and cognitive behavioral therapy 56. However, outcomes with these therapies vary widely. For example, as a commonly reported therapy for localized vestivular dysesthesia, vestibulectomy is most effective for a specific subset of patients, specifically women under 30 years old who have localized vulvar pain and provoked pain 57,58. These findings suggest that it’s possible that this type of pain represents a localized nociceptor mechanism, while unprovoked and generalized pain could have a different mechanism. Our data suggest that women suffering vulvar pain for long duration or with unprovoked pain have more CNS involvement or dysregulation. The CNS involvement occur de novo (e.g. genetic polymorphism) or secondary to an intractable pain state; the latter is the likely mechanism by which women with provoked vulvodynia transition into unprovoked and/or chronic pain state. It is well documented that an intractable peripheral process can lead to neuroplastic changes (via central sensitization) at all levels of the CNS and “generalization of pain” 59.
The findings in this study are consistent with the idea that chronic pain, caused by vulvodynia, alters central sensitization that leads to changes in sensory information processing. These changes are manifested in lower sensory thresholds (or higher sensitivity) in sites without provoked pain – because of a change in the balance between excitation and inhibition (or glutamatergic and gabaergic neurotransmission). Lower thresholds are consistent with this imbalance; decreasing inhibition will result in less suppression of cortical activity. In other words, a simple stimulus on the skin will generate more cortical activity if altered central sensitization has resulted in decreased inhibition or increased excitation. However, threshold testing has not been considered as an efficient method in measuring altered central sensitization due to large inter-individual variability. And in order to show these small differences, group differences of repeated measurements are normally necessary. Alternatively, using a measure – such as an adaptation metric – in which the patient provides their own individual baseline (i.e., the adaptation metric is derived on how amplitude discriminative capacity is impacted by conditioning) – could prove to be a more effective indicator of altered central sensitization that can be obtained reliably and efficiently (protocols employed in the current study can be obtained within 2-3 minutes). Sensory based measures of altered central sensitization appear to differentiate chronicity within subgroups of vulvodynia, and future studies will continue to investigate the changes in sensitization that appear to occur with the time course of the history of vulvodynia.
Acknowledgments
This work was supported, in part, by the Department of Defense CDMRP (W81XWH-07-1-0287), NIH/NINDS (PO1 NS045685-061A), and NIH/NICH (K23 HD 053631).
References
- 1.Danby CS, Margesson LJ. Approach to the diagnosis and treatment of vulvar pain. Dermatol Ther. 2010;23:485–504. doi: 10.1111/j.1529-8019.2010.01352.x. [DOI] [PubMed] [Google Scholar]
- 2.Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. Am J Obstet Gynecol. 2001;185:545–550. doi: 10.1067/mob.2001.116748. [DOI] [PubMed] [Google Scholar]
- 3.Giesecke J, Reed BD, Haefner HK, et al. Quantitative sensory testing in vulvodyniapatients and increased peripheral pressure pain sensitivity. Obstet Gynecol. 2004;104:126–133. doi: 10.1097/01.AOG.0000129238.49397.4e. [DOI] [PubMed] [Google Scholar]
- 4.Gunter J. Vulvodynia: New Thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62:812–819. doi: 10.1097/01.ogx.0000290350.14036.d6. [DOI] [PubMed] [Google Scholar]
- 5.Tommerdahl M, Tannan V, Cascio CJ, et al. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res. 2007;1154:116–123. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannan V, Holden J, Zhang Z, et al. Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res. 2008;1:223–230. doi: 10.1002/aur.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folger SE, Tannan V, Zhang, et al. Effects of the N-methyl-D-Aspartate receptor antagonist dextromethorphan on vibrotactile adaptation. BMC Neurosci. 2008:9–87. doi: 10.1186/1471-2202-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannan V, Simons S, Dennis RG, et al. Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res. 2007;1186:164–170. doi: 10.1016/j.brainres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francisco E, Tannan V, Zhang Z, et al. Vibrotactile amplitude discrimination capacity parallels magnitude changes in somatosensory cortex and follows Weber’s Law. Exp Brain Res. 2008;191:49–56. doi: 10.1007/s00221-008-1494-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Francisco E, Holden JK, et al. The impact of non-noxious heat on tactile information processing. Rrain Res. 2009;1302:97–105. doi: 10.1016/j.brainres.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Tannan V, Dennis R, Tommerdahl M. A novel device for delivering two-site vibrotactile stimuli to the skin. J of Neurosci Methods. 2005;147:75–81. doi: 10.1016/j.jneumeth.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Tannan V, Dennis GR, Tommerdahl M. Stimulus-dependent effects on tactile spatial acuity. Behav Brain Funct. 2005;1:18. doi: 10.1186/1744-9081-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannan V, Whitsel BL, Tommerdahl MA. Vibrotactile adaptation enhances spatial localization. Brain Res. 2006;1102:109–116. doi: 10.1016/j.brainres.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Tannan V, Dennis RG, Zhang Z. A portable tactile sensory diagnostic device. J of Neurosci Methods. 2007;164:131–138. doi: 10.1016/j.jneumeth.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Tannan V, Holden JK. A quantitative method for determining spatial discriminative capacity. BioMed Eng Online. 2008;7:12. doi: 10.1186/1475-925X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pukall CF, Binik YM, Khalife S. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96:163–175. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, Grinffin MJ. A comparison of vibrotactile thresholds on the finger obtained with different measuring algorithms. Stockholm workshop proceedings of hand-arm vibration syndrome: diagnostics and quantitative relationships to exposure. 1995;94:85–95. [Google Scholar]
- 18.Morioka M, Griffin MJ. Dependence of vibrotactile thresholds on the psychophysical measurement method. Int Arch Occup Environ Health. 2002;75:78–84. doi: 10.1007/s004200100280. [DOI] [PubMed] [Google Scholar]
- 19.Hollins M, Sigurdsson A, Fillingim L, et al. Vibrotactile threshold is elevated in temporomandibular disorders. Pain. 1996;67:89–96. doi: 10.1016/0304-3959(96)03083-7. [DOI] [PubMed] [Google Scholar]
- 20.Bredfeldt CE, Ringach DL. Dynamics of spatial frequency tuning in macaque V1. J Neurosci. 2002;22:1976–1984. doi: 10.1523/JNEUROSCI.22-05-01976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celebrini S, Thorpe S, Trotter Y, et al. Dynamics of orientation coding in area V1 of the awake primate. Vis Neurosci. 1993;10:811–825. doi: 10.1017/s0952523800006052. [DOI] [PubMed] [Google Scholar]
- 22.Das A, Gilbert CD. Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J Neurophysiol. 1995;74:779–792. doi: 10.1152/jn.1995.74.2.779. [DOI] [PubMed] [Google Scholar]
- 23.DeAngelis GC, Anzai A, Ohzawa I, et al. Receptive field structure in the visual cortex: does selective stimulation induce plasticity? Proc Natl Acad Sci U S A. 1995;92:9682–9686. doi: 10.1073/pnas.92.21.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinse HR, Kruger K. Contribution of area 19 to the foreground-background-interaction of the cat: an analysis based on single cell recordings and behavioural experiments. Exp Brain Res. 1990;82:107–122. doi: 10.1007/BF00230843. [DOI] [PubMed] [Google Scholar]
- 25.Pack CC, Born RT. Temporal dynamics of a neural solution to the aperture problem in visual area MT of macaque brain. Nature. 2001;409:1040–1042. doi: 10.1038/35059085. [DOI] [PubMed] [Google Scholar]
- 26.Pettet MW, Gilbert CD. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992;89:8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringach DL, Hawken MJ, Shapley R. Dynamics of orientation tuning in macaque primary visual cortex. Nature. 1997;387:281–284. doi: 10.1038/387281a0. [DOI] [PubMed] [Google Scholar]
- 28.Shevelev IA, Eysel UT, Lazareva NA, et al. The contribution of intracortical inhibition to dynamics of orientation tuning in cat striate cortex neurons. Neuroscience. 1998;84:11–23. doi: 10.1016/s0306-4522(97)00363-1. [DOI] [PubMed] [Google Scholar]
- 29.Shevelev IA, Volgushev MA, Sharaev GA. Dynamics of responses of V1 neurons evoked by stimulation of different zones of receptive field. Neuroscience. 1992;51:445–450. doi: 10.1016/0306-4522(92)90328-y. [DOI] [PubMed] [Google Scholar]
- 30.Sugase Y, Yamane S, Ueno S, et al. Global and fine information coded by single neurons in the temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- 31.Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 32.Kohn A, Whitsel B. Sensory cortical dynamics. Behav Brain Res. 2002;135:119–126. doi: 10.1016/s0166-4328(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 33.Tommerdahl M, Delemos KA, Favorov OV, et al. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80:3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- 34.Tommerdahl M, Favorov OV, Whitsel BL. Effects of high-frequency skin stimulation on SI cortex: mechanisms and functional implications. Somatosens Mot Res. 2005;22:151–169. doi: 10.1080/08990220500084461. [DOI] [PubMed] [Google Scholar]
- 35.Tommerdahl M, Simons SB, Chiu JS, et al. Response of SII cortex to ipsilateral, contralateral and bilateral flutter stimulation in the cat. BMC neurosci. 2005;6:11. doi: 10.1186/1471-2202-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tommerdahl M, Whitsel BL, Vierck CJ, Jr, et al. Effects of spinal dorsal column transection on the response of monkey anterior parietal cortex to repetitive skin stimulation. Cereb Cortex. 1996;6:131–155. doi: 10.1093/cercor/6.2.131. [DOI] [PubMed] [Google Scholar]
- 37.Chiu JS, Tommerdahl M, Whitsel BL, et al. Stimulus-dependent spatial patterns of response in SI cortex. BMC Neurosci. 2005;6:47. doi: 10.1186/1471-2202-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons SB, Chiu J, Favorov OV, et al. Duration-dependent response of SI to vibrotactile stimulation in squirrel monkey. J Neurophysiol. 2007;97:2121–2129. doi: 10.1152/jn.00513.2006. [DOI] [PubMed] [Google Scholar]
- 39.Simons SB, Tannan V, Chiu J, et al. Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci. 2005;6:43. doi: 10.1186/1471-2202-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tommerdahl M, Tannan V, Zachek M, et al. Effects of stimulus-driven synchronization on sensory perception. Behav Brain Funct. 2007;3:61. doi: 10.1186/1744-9081-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tommerdahl M, Tannan V, Holden JK, et al. Absence of stimulus-driven synchronization effects on sensory perception in autism: Evidence for local underconnectivity? Behav Brain funct. 2008;4:19. doi: 10.1186/1744-9081-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive--behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297–306. doi: 10.1016/S0304-3959(00)00449-8. [DOI] [PubMed] [Google Scholar]
- 43.Marinoff SC, Turner ML. Vulvar vestibulitis syndrome: an overview. Am J Obstet Gynecol. 1991;165:1228–1233. doi: 10.1016/s0002-9378(12)90732-2. [DOI] [PubMed] [Google Scholar]
- 44.Bohm-Starke N, Hilliges M, Brodda-Jansen G, et al. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94:177–183. doi: 10.1016/S0304-3959(01)00352-9. [DOI] [PubMed] [Google Scholar]
- 45.Pukall CF, Strigo IA, Binik YM, et al. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Gordon AS, Panahian-Jand M, Mccomb F, et al. Characteristics of women with vulvar pain disorders: responses to a Web-based survey. J Sex Marital Ther. 2003;29:45–58. doi: 10.1080/713847126. [DOI] [PubMed] [Google Scholar]
- 47.Zolnoun D, Hartmann K, Lamvu G, et al. A conceptual model for the pathophysiology of vulvar vestibulitis syndrome. Obstet Gynecol Surv. 2006;61:395–401. doi: 10.1097/01.ogx.0000219814.40759.38. [DOI] [PubMed] [Google Scholar]
- 48.Tommerdahl M, Favorov OV, Whitsel BL. Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev. 2010;34:160–170. doi: 10.1016/j.neubiorev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Favorov OV, Kursun O. Neocortical layer 4 as a pluripotent function linearizer. J Neurophysiol. 2011 Jan 19; doi: 10.1152/jn.00708.2010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Douglas RJ, Koch C, Mahowald M, et al. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 51.Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11:488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 52.Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alonso JM, Swadlow H. Thalamocortical specificity and the synthesis of sensory cortical receptive fields. J Neurophysiol. 2005;94:26–32. doi: 10.1152/jn.01281.2004. [DOI] [PubMed] [Google Scholar]
- 54.Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein AT, Marinoff SC, Haefner HK. Vulvodynia: strategies for treatment. Clin Obstet Gynecol. 2005;48:769–785. doi: 10.1097/01.grf.0000182213.21599.49. [DOI] [PubMed] [Google Scholar]
- 57.Traas MA, Bekkers RL, Dony JM, et al. Surgical treatment for the vulvar vestibulitis syndrome. Obstet Gynecol. 2006;107:256–262. doi: 10.1097/01.AOG.0000195058.91506.ae. [DOI] [PubMed] [Google Scholar]
- 58.Bornstein J, Goldik Z, Stolar Z, et al. Predicting the outcome of surgical treatment of vulvar vestibulitis. Obstet Gynecol. 1997;89:695–698. doi: 10.1016/s0029-7844(97)00102-6. [DOI] [PubMed] [Google Scholar]
- 59.Woolf CJ, Doubell TP. The pathophysiology of chronic pain - increased sensitivity to low threshold Ab-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]