Abstract
The research reported herein was designed to assess whether the bacterium, Dietzia subspecies C79793-74, used as a probiotic, could prevent development of parameters indicative of bovine paratuberculosis after potential in utero, birthing and neonatal (colostrum) exposure to Mycobacterium avium subspecies paratuberculosis (MAP). Such exposure avenues are especially relevant for dairy farms practicing good management procedures since calves on these farms could be infected via dams that have yet to be identified as MAP-positive. Indeed, of 18 calves in the present study that became paratuberculosis parameter-positive, five had dams that were negative for all parameters pre-calving. Parameters used herein to define paratuberculosis status were serum ELISA, serum agar gel immunodiffusion, cultureable fecal MAP, histopathology at necropsy and clinical disease. Thirty-four newborn calves whose dams were paratuberculosis-positive were assigned to four different treatment groups. Ten were treated daily for 60 days with viable Dietzia added to their antibiotic-free milk feedings; none became positive for any parameter with age. In contrast, seven of eight calves that were not treated became positive for one or more paratuberculosis-associated parameter. Sixteen calves were treated with viable Dietzia for the first two days of life; eight were then not treated further, whereas the other eight were treated an additional 58 days with Dietzia added to tetracycline-fortified milk (Dietzia is sensitive to tetracycline). In these two groups, positivity developed in five of eight and six of eight, respectively. These results indicated that (a) a daily, 60-day treatment with viable Dietzia effectively prevented development of parameters indicative of paratuberculosis and (b) this treatment, in combination with good management practices, has the potential to eradicate MAP from animals/herds, which should curtail the spread of MAP. Such results should significantly reduce human exposure to MAP, which in turn, could have relevance for the controversial role of MAP in Crohn's disease, type-1 diabetes mellitus, sarcoidosis, Blau syndrome, ulcerative colitis, irritable bowel syndrome and multiple sclerosis.
Key words: bovine Johne's disease, Dietzia probiotic, Mycobacterium avium subsp. paratuberculosis (MAP), idiopathic inflammatory bowel diseases (IIBD), ulcerative colitis, Crohn's, diarrhea, preventive therapy, diabetes, multiple sclerosis
Introduction
Johne's disease, caused by Mycobacterium avium, subspecies paratuberculosis (MAP), is a worldwide economic problem on dairy,1,2 sheep and goat farms due to animal movement from one farm to another, herd expansion, farm intensification and confinement, and most importantly, the absence of an effective preventive vaccine or therapeutic drug treatment. Routes by which MAP infection of ruminants may occur are: in utero, colostrum or milk, ingestion of fecal MAP during birthing and ingestion of environmental MAP-laced manure after calving.3–12 Animals in well managed operations may be infected by any of the first three routes, whereas all are potential sources in poorly managed operations. Outside a probiotic therapeutic protocol,13–15 there are no curative treatments for Johne's disease. Presently, whole herd testing for risk-based management is considered by many as the best means of curtailing the spread of MAP from animal to animal, as well as farm to farm.11,16–23 Unfortunately, even though these management practices have successfully reduced the incidence of Johne's disease, they have yet to, and most likely will not, eliminate MAP from farms or animals; thus, other preventive vaccines and/or curative measures are needed. Furthermore, as Johne's disease continues to become more prevalent, and as more human diseases are found to be associated with MAP, what role, if any, food animals play in the transmission of MAP to humans via dairy products,25–27,65 meat24,29,31,33 and ground water28,30,32 is becoming more relevant.
As one means to curtail the spread of MAP, Dietzia subspecies C79793-74, used as a probiotic, has been tested in adult paratuberculosis cattle for the past several years; the impetus coming from a report that MAP growth in vitro was inhibited by Dietzia under specific culture conditions34 in a similar way to that achieved in vitro by antimicrobial drugs.35,36 The results of these previous investigations13–15 indicated that (a) longevity of late-stage, diseased cows was significantly extended by an extensive Dietzia treatment (high daily dose of >5 × 1011 cfu for months) and (b) 40% of cows in the early stages of Johne's disease were “cured” by a similar extensive treatment; i.e., those cured became negative for all parameters used to define paratuberculosis,37 lived a normal uneventful life and succumbed to nonparatuberculosis insults. More importantly, the Dietzia cures were long-lasting post-treatment and were therefore unlike the palliative benefits of anti-mycobacterials described for paratuberculosis goats and cattle.35,36,38 Although instructive for designing human clinical trials,15 the adult Dietzia protocols were unfortunately (a) very lengthy, (b) less effective for more advanced stages of disease and (c) costly ($5–10/day for 12–18 months), making them financially prohibitory for farmers. Therefore, the experiments reported herein were undertaken to assess whether a cost-effective (estimated <$1/day) Dietzia treatment of MAP-exposed fetuses or neonatal calves (via colostrum or during birthing) would prevent subsequent adulthood paratuberculosis-associated parameters indicative of Johne's disease.
Results
Calves from paratuberculosis dams.
Although MAP is not easily detected in colostrum obtained from paratuberculosis cows, colostrum is one of the sources by which calves are infected.8–10 Therefore, as one means to increase the probability for infection, all calves in the present study received colostrum from their dams only. Unfortunately, of 12 paratuberculosis colostrums tested for the presence of MAP, only one, from a Stage III positive dam (see Materials and Methods for definition of disease stages), was found positive by culture, none by PCR. Such negativity was not informative and testing for MAP in colostrum was discontinued. However, feeding paratuberculosis colostrum was continued and its impact on outcomes of Dietzia treatments will be addressed.
The results in Table 2 are those obtained for eight calves never exposed to Dietzia. Seven became ELISA-positive, four fecal-positive and none AGID-positive—those testing positive are highlighted in bold print. Calves 1-1 and 1-6 succumbed with clinical Johne's disease, all others from unrelated conditions. All dams of these untreated calves were or became ELISA-positive (only pre-calving and maximum values are shown), including the five that were negative prior to calving. Of these dams, all but two were AGID- and/or fecal-positive at some point in their lives and six were euthanized with clinical disease. The mean ELISA OD value of 1.34 for these dams at pre-calving was lower than those of the other three groups; maximum mean values, however, were similar for all four groups (Table 1).
Table 2.
Parameters for calves not fed Dietzia and for their paratuberculosis dams
| Group # calf ID # | Breed1 | Sex | calf E/A/F2 | Age (months) at testing3 | Dam E/A/F | Pregnant/fresh clinical stage of dam5 | |
| Pre-calving | maximum | ||||||
| 1-1* | J | F | 2.7/−/6 | 20 | 0.65/−/0 | 3.5/−/>300 | I* |
| 1-2 | J | F | 2.2/−/4 | 17.5 | 0.71/−/0 | 1.5/−/0 | I |
| 1-3 | H | F | 0.81/−/0 | 36 | 0.80/−/0 | 2.8/−/nd | I |
| 1-4 | J | F | 2.0/−/0 | 18.5 | 0.84/−/0 | 3.7/+/30 | I* |
| 1-5 | H | F | 1.7/−/0 | 46 | 1.1/−/0 | 3.1/+/13 | I* |
| 1-6* | J | F | 3.6/−/60 | 41 | nd4 | 3.1/+/70 | I/II* |
| 1-7 | H | F | 3.0/−/2 | 46 | 2.0/−/0 | 3.7/+/20 | II* |
| 1-86 | J | M | 2.6/−/nd4 | 5 | 3.3/+/2 | 4.2/+/2 | II* |
Note: Bold indicates calves with paratuberculosis-positive test parameters.
indicates animal died with clinical Johne's disease.
J, Jersey; H, Holstein.
Result for each calf at first positive or last negative E, ELISA OD405 nm (≤1.4 considered negative), A, AGID (positive/negative); F, Fecal culture (cfu/2 g).
Age at which calf first tested positive for either E, A, F or if E, A, F were negative, age at final test.
nd, not tested.
Clinical Stage (Whitlock and Buergelt, 1996).
One male was tested at five months of age (no evidence at the time for clinical Johne's disease) because of a non-paratuberculosis, presumed eminent, lethal condition.
Table 1.
Summary of MAP parameters for paratuberculosis dams of calves in treatment Groups 1–4
| Parameter | at | Treatment group | |||
| None | 2 day viable | 60 day viable | 2 day viable + 58 nonv | ||
| ELISA | pre-calving | 1.34 ± 0.371 | 2.50 ± 0.51 | 2.51 ± 0.40 | 2.91 ± 0.51 |
| maximum | 3.20 | 3.81 | 3.48 | 3.86 | |
| MAP+ | pre-calving | 1/72 | 4/8 | 6/10 | 6/8 |
| maximum | 6/7 | 8/8 | 9/10 | 7/8 | |
| AGID+ | pre-calving | 1/7 | 4/8 | 4/10 | 2/8 |
| maximum | 5/8 | 6/8 | 5/10 | 5/8 | |
| Dam's clinical stage (I:II:III:IV) at calving3 | |||||
| 5:2/34:0:0 | 2:5:1:0 | 2:6:2:0 | 0:7:1:0 | ||
Mean OD405 nm ± Standard Error Mean.
# animals+/# total animals tested.
Whitlock and Buergelt, 1996.
One cow was not ELISA- or fecal-tested precalving, as she was clinically asymptomatic.
Five of eight calves treated with Dietzia for only the first two days after birth (Group 2, Table 3) had positive parameters for Johne's disease (calves 2-1 and 2-8 succumbed with clinical disease), whereas all ten that were treated daily for the first 60 days of life tested negative (Group 3, Table 4). All dams in both groups were ELISA-positive multiple times (3–4 tests/year) and six in Group 2 and five in Group 3 were AGID-positive at some point in their life. Only one dam in Group 3 and none in Group 2 tested MAP negative. Six dams in Group 2 and six in Group 3 were euthanized because of clinical disease.
Table 3.
Parameters for calves fed Dietzia for first two days of life and for their paratuberculosis dams
| Group # calf ID # | Breed1 | Sex | calf E/A/F2 | Age (months) at testing3 | Dam E/A/F | Pregnant/fresh clinical stage of dam4 | |
| Pre-calving | maximum | ||||||
| 2-1* | J | F | 1.7/+/33 | 49 | 0.62/−/0 | 2.2/−/4 | I |
| 2-2 | J | F | 0.86/−/0 | 60.5 | 1.0/−/0 | 3.8/−/60 | I* |
| 2-3 | H | F | 0.85/−/0 | 50 | 1.5/−/0 | 4.1/+/05 | II |
| 2-4 | X | F | 2.1/−/58 | 17.5 | 1.6/−/0 | 3.5/+/39 | II* |
| 2-5 | H | F | 0.50/−/0 | 22.5 | 3.5/+/50 | 3.6/+/50 | II* |
| 2-6 | X | M | 4.2/+/0 | 16.5 | 3.9/+/>300 | 4.3/+/>300 | III* |
| 2-7 | X | F | 3.5/−/0 | 17.5 | 3.9/+/>300 | 4.4/+/>300 | II* |
| 2-8* | J | F | 3.4/+/>300 | 49.5 | 4.0/+/2 | 4.6/+/>300 | II* |
Note: Bold indicates calves with paratuberculosis-positive test parameters.
indicates animal died with clinical Johne's disease.
J, Jersey; H, Holstein; X, Cross.
Result for each calf at first positive or last negative E, ELISA OD405 nm (≤1.4 considered negative); A, AGID (positive/negative); F, Fecal culture (cfu/2 g).
Age at which calf first tested positive for either E, A, F or if E, A, F were negative, age at final test.
Clinical Stage (Whitlock and Buergelt, 1996).
Culture-positive intestinal lymph node at autopsy.
Table 4.
Parameters for calves fed Dietzia for 60 days and for their paratuberculosis dams
| Group # calf ID # | Breed1 | Sex | calf E/A/F2 | Age (months) at testing3 | Dam E/A/F | Pregnant/fresh clinical stage of dam3 | |
| Pre-calving | maximum | ||||||
| 3-1 | X | F | 0.74/−/0 | 53.5 | 0.95/−/0 | 2.7/−/8 | I* |
| 3-2 | J | M | 0.39/−/0 | 20.5 | 1.3/−/0 | 2.1/−/58 | I |
| 3-3 | X | F | 1.1/−/0 | 23 | 1.5/−/0 | 2.3/−/0 | II |
| 3-4 | X | M | 0.62/−/0 | 15.5 | 1.6/−/0 | 3.0/−/17 | II |
| 3-5 | X | F | 0.48/−/0 | 51 | 1.7/−/17 | 3.0/−/17 | II |
| 3-6 | X | M | 0.52/−/0 | 23 | 2.7/−/65 | 4.2/+/>300 | III* |
| 3-7 | J | M | 0.81 /−/0 | 51.3 | 3.2/+/>300 | 3.7/+/>300 | II* |
| 3-8 | J | M | 0.57/−/0 | 25 | 3.8/+/>300 | 4.6/+/>300 | II* |
| 3-9 | H | F (twin) | 0.63/−/0 | 23.5 | 3.9/+/>300 | 4.3/+/>300 | III* |
| 3-10 | X | F | 0.80/−/0 | 60.5 | 4.4/+/>300 | 4.9/+/>300 | II* |
J, Jersey; H, Holstein; X, Cross.
Result for each calf at last test E, ELISA OD405 nm (≤1.4 considered negative), A, AGID (positive/negative); F, Fecal culture (cfu/2 gm).
Age at final test for E, A and F.
Clinical Stage (Whitlock and Buergelt, 1996).
indicates animal died with clinical Johne's disease.
To test whether some component of Dietzia (protein, nucleic acid, other) would be as effective as viable Dietzia, calves (Group 4, Table 5) were treated with viable Dietzia for only the first 2 days of life followed by 58 days of Dietzia added to milk replacer that contained tetracycline/neomycin. Dietzia is tetracyclinesensitive. As shown, six of eight calves in this group tested positive and three succumbed with clinical Johne's disease. Again all dams were ELISA-positive multiple times, five AGID-positive, seven fecal-positive and six succumbed with clinical disease.
Table 5.
Parameters for calves fed viable Dietzia for first two days of life followed by Dietzia (non-viable) in antibiotic-fortified milk replacer for 58 days and for their paratuberculosis dams
| Group # calf ID # | Breed1 | Sex | calf E/A/F2 | Age (months) at testing3 | Dam E/A/F | Pregnant/fresh clinical stage of dam4 | |
| Pre-calving | maximum | ||||||
| 4-1* | J | F | 4.0/+/22 | 31 | 1.7/−/0 | 2.2/−/0 | II |
| 4-2* | X | F | 2.7/−/8 | 36 | 1.8/−/2 | 3.7/+/30 | II* |
| 4-3* | H | F | 4.9/+/>300 | 23 | 2.2/−/0 | 3.8/+/>225 | II* |
| 4-4 | A | M | 4.0/−/39 | 22.5 | 3.3/−/>300 | 3.6/+/>300 | III* |
| 4-5 | H | M | 3.8/+/44 | 22.5 | 3.4/−/16 | 4.3/−/16 | II* |
| 4-6 | X | M | 1.5/−/0 | 23 | 3.7/+/220 | 4.6/+/>300 | II* |
| 4-7 | H | F | 1.0/−/0 | 58.5 | 4.3/−/>300 | 4.3/−/>300 | II |
| 4-8 | X | M | 0.69/−/0 | 21 | 4.4/+/>300 | 4.4/+/>300 | II* |
Note: Bold indicates calves with paratuberculosis-positive test parameters.
indicates animal died with clinical Johne's disease.
J, Jersey; H, Holstein; X, Cross; A, Ayrshire.
Result for each calf at first positive or last negative E, ELISA OD405 nm (≤1.4 considered negative); A, AGID (positive/negative); F, Fecal culture (cfu/2 g).
Age at which calf first tested positive for either E, A, F or if E, A, F were negative, age at final test.
Clinical Stage (Whitlock and Buergelt, 1996).
The median times at which calves became sero- and/or fecal-positive in Groups 1, 2 and 4 were 20, 17.5 and 23 months, respectively (Table 6), which is in sharp contrast to no calves testing positive in Group 3. However, the median test-time for animals in Group 3 of 24.5 months was similar to the test-positive medians of the other three Groups. The mean age of calves at first positive for Groups 1, 2 and 4 was 28.8, 35.4 and 29.7 months and at the final negative-test-time for Group 3 was 34.8; none being statistically different. Since animals that continually tested negative skew these values higher, perhaps a more informative way to calculate means is to exclude negative animals. These somewhat lower values are 27.7, 30.0 and 26.3 months for Groups 1, 2 and 4, respectively; a Group 3 value is not calculable.
Table 6.
Summary of data for calves of paratuberculosis dams in treatment groups 1–4
| Dietzia treatment1 | Mean test-age of2 | Median age3 for positivity | #clinical/total4 | |
| Only positives | All | |||
| None | 27.7 | 28.8 | 20 | 2/6 |
| 2v days | 30.0 | 35.4 | 17.5 | 2/5 |
| 60v days5 | N | 34.8 | NA | 0/4 |
| 2v + 58nv days | 26.3 | 29.7 | 23 | 3/4 |
Not treated or treated for 2 or 60 days with viable Dietzia or for 2 days with viable Dietzia followed by 58 days Dietzia in antibiotic-fortified milk replacer.
Mean age (months) for only those calves that tested positive or mean age for all calves in the group.
Median age at which calves tested seroand/or fecal-positive. (This is not a survival age).
Number calves developing clinical disease out of the number monitored over their natural lifetime. Outside one male in group 3, all males were excluded.
None of the calves in this group tested sero- or fecal-positive and none developed clinical disease.
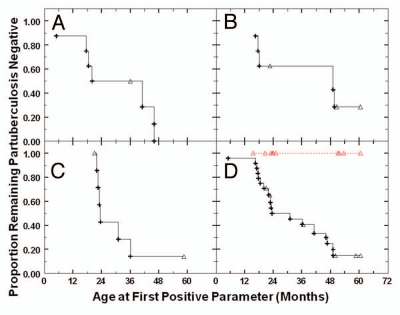
Kaplan-Meier analysis of each treatment-group is shown in Figure 1. The percentage of infected calves in Group 1, not treated (Fig. 1A) and treated Groups 2 and 4 (Fig. 1B and C) were not significantly different from each other (p values ranged from 0.28–0.50). Zero positivity in the 60-day group (Fig. 1D) was not only significantly less, but infinitely less, than that of each of the other three groups, with p values of 0.002, 0.0065 and 0.0015 vs. not treated, 2-day-treated and 2 day + 58 day antibiotic-treated, respectively. Interestingly, eleven of the 18 animals (61.1%) that developed positive parameters indicative of paratuberculosis did so by 23.5 months of age; in this same time period, none of the 10 in the 60-day treatment group were positive. Comparison of the combined results of Groups 1, 2 and 4 to the 60-day-treated group is also shown in (Fig. 1D).
Figure 1.
Kaplan-Meier plot of paratuberculosis parameters of calves undergoing different treatments. (A) Calves (Group 1) were not treated (n = 8). (B) Calves (Group 2) were treated with viable Dietzia for only the 1st 2 days of life (n = 8). (C) Calves (Group 4) were treated with viable Dietzia for the 1st 2 days of life, followed by 58 days of Dietzia in the presence of antibiotics (n = 8). (D) Calves (Group 3, n = 10) were treated with viable Dietzia for 60 days  . Also shown is a compilation of the data for Groups 1, 2 and 4 (----) shown in (A–C) (n = 18). (+) represent the age at which each calf first tested sero- and/or fecal-paratuberculosis-positive and (Δ) the age at which an animal left the herd testing negative.
. Also shown is a compilation of the data for Groups 1, 2 and 4 (----) shown in (A–C) (n = 18). (+) represent the age at which each calf first tested sero- and/or fecal-paratuberculosis-positive and (Δ) the age at which an animal left the herd testing negative.
Calves from paratuberculosis-negative dams.
Fifty-one calves, whose dams were paratuberculosis-negative (test-negative up to five years of age), were treated as described for calves from paratuberculosis-positive dams (Groups 1–4 in Tables 2–5). Based on SCVF herd management practices and on the primary objective of the study, no effort was made to have equal numbers of these 51 calves in each group; instead most were assigned to the 60-day treatment group since this treatment was anticipated to have the most efficacious outcome. Of these 51 calves, ten were not treated, one was treated with viable Dietzia for two days only, 36 were treated with viable Dietzia for 60 days and four were treated with viable Dietzia for two days followed by tetracycline- “killed” Dietzia for 58 days. All 51 tested negative (data not shown) over their lifetime (3–4 times per year) for sero-, fecal- and clinical-parameters used to define paratuberculosis.
Discussion
The validity of the therapeutic benefit of the 60-day probiotic treatment described herein is dependent upon: (a) reliability and predictability of assays used to define Johne's disease; (b) evidence that each calf 's dam was paratuberculosis-positive; and (c) sufficient exposure to MAP to result in infection. Fulfillment of each is discussed below.
Assays.
ELISA, AGID, MAP present in feces or necropsied tissues, postmortem pathology and end-stage clinical disease were previously examined longitudinally37 for reliability to identify Johne's disease in 75 individual adult animals over their natural lifetime. The most relevant finding for the present investigation is that, of these 75, all were ante-mortem, ELISA-positive, and all but seven either (a) became/were positive for a second parameter indicative of paratuberculosis (fecal shedding or positive serum AGID), (b) developed end-stage clinical disease, and/or (c) possessed necropsied tissue consistent with paratuberculosis histopathology; i.e., 90.7% were definitively positive. Of the seven that only tested ELISA-positive, five tested positive multiple times (maximum values ranged from 2.2 to 4.0), one, whose dam succumbed with clinical disease, was terminated because of other insults within three months of first testing positive (had a single test time) and one, whose maternal sister and dam died with Stage III and Stage IV clinical disease respectively, was part of an earlydisease, adult-group that was cured by a lengthy Dietzia treatment.13–15 Thus, these seven were considered highly likely to also be paratuberculosis-positive and it was concluded that an Allied Monitor ELISA OD value >1.4 accurately identifies paratuberculosis cattle.
In the present investigation, the >1.4 OD cut-off value also defined paratuberculosis of dams, since (a) all (except dam of paratuberculosis-positive calf 1-2) had positive ELISA values multiple times over their lifetime, including eight of nine that were ELISA negative at pre-calving, (b) 21 became AGID-positive, (c) 22 succumbed with severe Stage IV clinical disease and (d) 30 of the 33 tested, were MAP culture-positive (fecal or post-mortem tissue). Evidence supporting positivity of the three ELISA-only-positive dams (dams of calves 1-2, 3-3 and 4-1) is that 2 (calves 1-2 and 4-1) of these 3 calves tested both ELISA- and fecal-positive—both calves were born prior to initiating a Dietzia treatment that cured the dams—prevented development of fecal shedding.13–15 Therefore, based on the predictive accuracy of the Allied Monitor ELISA found with the previous 75 adults,37 plus similar accuracy found for the dams in the present study, the probability that all animals (dams and calves) that were only ELISA testpositive as adults, irrespective of their age, are not paratuberculosis-positive is extremely remote.
Status of dams.
Except for Group 1, calves were stratified among the four treatment groups based on ELISA values of the dams to achieve a presumed, similar degree of infection, with at least eight calves per group. The mean pre-calving ELISA ± SEM OD values of dams in Groups 2, 3 and 4 of 2.50 ± 0.51, 2.51 ± 0.40 and 2.91 ± 0.51 respectively, not being significantly different, supports this attempt.
Group 1 (the exception group) had more calves from dams that tested paratuberculosis-negative at calving than the other groups, resulting in a lower mean, pre-calving ELISA OD of 1.34 ± 0.37. In contrast the maximum value means for all groups were similar. Surprisingly, although not significantly, calves in Group I had the highest percentage of positives and the youngest mean age at which positivity was detected. Thus, the potential for calves in this non-treated group to be under-infected was not substantiated. Further, each group had dams that were negative for all MAP parameters pre-calving, (except for Group 4) as well as dams that were or were not AGID and/or fecal-positive. None of the dams had clinical Stage IV disease prior to calving. Based on all tests performed every three to four months, plus clinical disease status, all 34 dams of calves potentially infected with MAP were paratuberculosis-positive.
Exposure to MAP.
To partially mimic on-farm, good management practices, calves, post-calving, were not deliberately exposed to environmental fecal material possessing MAP. Although it may be of interest to test how effective Dietzia would be in curtailing this type exposure, such a group was not included because it can and should be controlled by management practices as defined by others.11,16–23
The only calves found positive as adults for parameters indicative of MAP infection were those whose dams were positive for Johne's disease. Of 51 calves from paratuberculosis-negative dams (10 not treated, 36 treated with Dietzia for 60 days and five in either treatment Groups 2 or 4), none tested positive for any Johne's disease-associated parameter. Based on the results obtained with the 60-day treatment of calves from paratuberculosis dams, it was expected that there would not be any positives in the 36 treated for 60 days. The absence of positives in the other 15 (51 minus 36) suggests that our management practices at SCVF were sufficient to prevent cross-contamination by inadvertent exposure to fecal MAP of positive cows or positive calves.39,40 Moreover, based on the similar percent positivity in Groups 1, 2 and 4 vs. its absence in Group 3, no Dietzia calf-to-calf crossprotection was indicated.
As a means to potentially increase the number of infected neonates over that achieved in utero (or during birthing), each calf was fed colostrum from its paratuberculosis dam only.8–10 These types of exposure were deemed relevant since MAP infection could easily occur on any dairy farm practicing good management via in utero/colostrum/birthing of cows yet to be identified as paratuberculosis-positive. Indeed, the five positive calves derived from the seven, initially sero- and fecal-negative asymptomatic dams in Groups 1, 2 and 4 supports this presumption. However, of colostrums from 12 paratuberculosis cows tested for the presence of MAP by either DNA technology and by culture, only one was found positive by culture, none by PCR. Was MAP absent or merely undectable? This absence is in agreement with that of Streeter et al. in which cultureable MAP was present almost exclusively in colostrums of cows that were heavy fecal shedders. Since 16 of 33 (one not tested) paratuberculosis dams were negative prior to calving for fecal shedding and seven more were light shedders, undetectable MAP in colostrum was anticipated. These findings are, however, in disagreement with that reported by Stabel for colostrum of subclinical animals,10 perhaps because of differences in preparation protocols. In addition, she found that colostrum from paratuberculosis dams (vs. pasteurized colostrum from negative dams) increased severity of, but not percentage of, MAP infection in calves whose dams were positive.10 Using a different approach,41 calves (all from dams with undefined Johne's disease status) in only one of 12 herds fed plasma-derived commercial colostrum replacer developed significantly less adulthood paratuberculosis parameters compared to calves that received non-pasteurized colostrum from their dams. In the other eleven herds, no significant differences were found, even though in two herds, plasma replacer was actually less effective. Unfortunately, neither this plasma study, Stabel's study, nor the present study clarifies the relative contribution of in utero vs. colostrum-derived MAP for infection.
A second aspect of feeding colostrum from paratuberculosis dams that will need to be further explored in order to fully understand its role in MAP infection is, the presence of and concentration of antibodies specific for MAP—levels that, like MAP fecal levels, are dependent upon the stage of disease. Moreover, colostrum MAP antibodies are not reliably quantifiable by milk ELISA (see AntelBio's Technical Report on Milk ELISA). The significance of these antibodies stems from the findings that MAP-specific antibodies cross-react with antigenic epitopes shared with Dietzia (Richards, personal communication and Click, unpublished). Thus, colostrum antibodies specific for MAP could enhance or impair Dietzia's alteration of MAP levels15 by either antibody-mediated elimination of Dietzia (not a desirable event), or by a preferably enhanced opsonization of Dietzia/MAP by phagocytes possessing MAP/Dietzia.15 One consequence of close proximity of Dietzia and MAP is that Dietzia could either inhibit MAP growth via competition for a nutrient(s), or could directly kill it. The most intriguing aspect of this postulate is that for Dietzia to effectively reduce and eliminate MAP in adult cattle, immune reactivity (with an emphasis on humoral) to MAP was found essential, even though such activity in the absence of Dietzia is not beneficial/curative (discussed in more detail in ref. 15, where elimination/reduction of MAP by Dietzia in adults depended upon a dexamethasone-sensitive immune event). This immune dependence emphasizes an additional concern relative to the use of MAP-free colostrum (which presumably would also be MAP antibody-free), which is a critical component of good management practices; namely, would such antibody-free colostrum result in a less effective 60-day Dietzia treatment for calves? Based on the pre-calving serum ELISA values of dams in Group 3, the answer appears to be no. Why? Because five dams had low serum ELISA values at calving (which would suggest low antibody levels in their colostrum) and five dams had high serum ELISA values at calving (suggesting a significant concentration of antibody in their colostrum)—and yet, none of the 10 calves from these dams developed paratuberculosis-positive parameters! Obviously more research will be necessary to address the impact of colostrum on Dietzia's efficacy.
In utero infection reported previously in references 5 and 6 was based on culture-detected MAP in fetal tissue. MAP that was detected was primarily in fetuses of cows that were heavily infected as defined by fecal shedding and MAP culture-positive lymph nodes. The percentage of positive fetuses from cows with low infection was negligible or absent; a result not unexpected when looking for miniscule numbers of MAP in a 100# fetus. In contrast to these necrosis findings, the present results suggest that there was no relationship of, when, and if, a calf became positive as an adult and any ante-mortem parameter used to define the status of the dam pre-calving. Thus, in contrast to that proposed by others,42 a high percentage of calves in the present investigation, derived from ELISA “negative” (5 of 7 = 71%), “suspect” (3 of 4 = 75%) and “positive” (10 of 13 = 77%) paratuberculosis dams, monitored into adulthood, became positive for parameters indicative of paratuberculosis. Based on these findings, the 60-day therapeutic treatment most likely eliminated MAP from calves rather than simply reducing the load of MAP sufficiently to slow development of positive parameters. The high infection percentage reported herein confirms (a) that of Stabel,10 in which all calves from subclinical and clinical dams, fed pasteurized non-paratuberculosis colostrum, tested positive (presumably via in utero/birthing infection), (b) the conclusions of a critical review and meta-analysis,78 and (c) that found for farmed red deer in which 9 of 10 fetuses were culture-positive for MAP.43 Thus, the efficacy found for the 60-day Dietzia treatment would likely not be altered by clarification of the importance of in utero exposure verse colostrum exposure, even though in utero/birthing seems the most likely non-environmental source.
Dietzia treatments.
Based on studies in which viable Dietzia effectively controlled/reversed disease in adult paratuberculosis cows,13–15 whereas gamma-radiated, inactivated Dietzia was ineffective,15 it was anticipated that if any preventive intervention was found for calves, it would be with viable Dietzia. In contrast to varying Dietzia dosages based on clinical disease status of adult cows (a parameter unavailable for monitoring in calves), a single, fixed dose (adjusted for cow/calf weight differences) based on an extremely efficacious dose for adult cattle (>1012 cfu) was chosen for treating calves. The duration of treatment to achieve therapeutic results, however, remained to be established. As shown, a two-day treatment, although very cost-effective, did not prevent development of positive parameters; instead, the percent positivity was not significantly different from that found for untreated calves. Likewise, the number of calves with positive parameters that were fed Dietzia in tetracycline-fortified milk replacer for 58 days was comparable to that for calves not treated and to those fed viable Dietzia for only two days. Thus, feeding “killed” Dietzia had no obvious adverse or beneficial effect, suggesting a role for an active process, just as found for adult cows, rather than specific inert molecules, emphasizing the importance of proper storage and handling of Dietzia to maintain viability.
Based on test ages of <25 months for six of the 10 calves in the 60-day therapeutic group, raises a question as to the significance of (1) differences found for this group and those of Groups 1, 2 and 4 and (2) degree of prevention over a lifetime. Evidence to counter these are:
Cox Proportional Hazard Survival Analysis of Kaplan-Meier estimates of different treatments indicated that only the 60-day treatment resulted in a lower, statistical significant, percentage positive animals vs. Group 1, Group 2, Group 4 (p = 0.0003, 0.0065, 0.0015 respectively). Moreover, the percentage of positive calves in Groups 1, 2 and 4 were not significantly different from one another and appeared to be independent of breed or sex; although because of limited numbers, it is not possible to draw conclusions regarding the latter variables;
Six of 10 calves from dams with ELISA OD values >2.5 in Groups 1, 2 and 4 tested positive at, or before, 25 months of age; thus, it would be expected that of the five calves in Group 3, whose dams also had ELISA values >2.5 at calving, three would likewise have tested positive by 25 months of age and yet none did;
Of the 18 calves (out of 24) in groups 1, 2 and 4 that tested positive, 11 were positive at <25 months of age, irrespective of any pre-calving parameter of the dams. Based on this 45.8% positivity, a similar percentage would be expected for Group 3 at <25 months; i.e., 4.58 calves would be expected to be positive and again all ten tested negative at <25 months; and five of eight (62.5%) calves in Groups 1, 2 and 4 tested positive at >40 months of age whereas none of four in the 60-day treated group were positive at >40 months.
Reduction of MAP in the food chain—relevance for human diseases.
Interestingly, Johne's disease has many manifestations in common with Crohn's disease,44–46 including both debilitating diarrhea and absence of a preventive vaccine/drug or curative treatment. Because a large percentage of Crohn's patients are systemically infected with MAP,47–57 it is not surprising that MAP is at the center of controversy as to its role in this disease. Although association of Crohn's disease with MAP appears specific, the question of whether it is causative, perpetuitive, or merely an opportunistic bystander still remains to be resolved.15,47–52 Moreover, a similar controversy will likely develop regarding the significance of MAP found in patients with sarcoidosis,58 type-1 diabetes mellitus,59–61 Blau syndrome,62 ulcerative colitis,51 irritable bowel syndrome51 and multiple sclerosis.63 Perhaps even more intriguing: What is the significance of MAP found in control, non-diseased subjects by numerous laboratories? In a recent report, an astonishingly 33% of control subjects were found MAP-infected!64 The presence of MAP in control subjects raises an interesting question as to what percentage of the population is exposed to (infected with) MAP at some point in their lifetime? An answer will clearly depend upon (a) the frequency at which normal subjects get tested in their lifetime, (b) the difficulty in documenting infection in humans75,76 and (c) consequences of appropriate susceptibility genes77 (and other factors) that severely curtail infected individuals from contracting disease. Thus, the number of individuals in the general population that are exposed/infected with MAP is probably grossly underestimated and indeed, could be at an epidemic level.
A second related question is: What is the source and transmission route by which patients are infected with MAP?32,44–49,52–57 Similar to MAP-infected subjects, there are no curative treatments for ruminants with Johne's disease (outside the Dietzia treatment). As a consequence, both asymptomatic and clinically diseased cattle are in general sent to slaughter where the meat is processed into ground beef.31 This meat, when not cooked well,24 plus viable MAP present in retail cheeses65 and in pasteurized milk on grocery shelves25–27 puts humans at risk for consuming MAP.29,66 Furthermore, MAP-contaminated water reservoirs may also be an important source for human exposure due to animal feces present in pastures and streams.28,30,32 Thus, it is imperative that MAP be eliminated from the food chain as one means to reduce potential exposure of humans to viable MAP. The present results suggest that this can be most easily and cost-effectively achieved by treating neonatal rather than treating adult cattle.
Conclusions
The results presented herein, plus the fact that no adverse side effects have been observed in Dietzia treated calves or cows for over sixteen years of experimentation, suggest that a 60-day Dietzia treatment of calves, under good management practices, would be cost-effective (estimated <$1/day), safe and sufficient to prevent development of Johne's disease from in utero/colostrum/vaginal birthing MAP modes of infection. How effective it would be after exposure to environmental fecal material on poorly managed farms is an open question. In addition, whether a treatment less than 60 days but greater than 2 days would be beneficial, also remains to be determined. To more firmly document the efficacy of Dietzia reported herein, to extensively examine long-term benefits, and to define how Dietzia mechanistically15 intervenes paratuberculosis processes, further investigations will need to be undertaken.
Materials and Methods
Adult cows.
The goal of the present research was to define a protocol which could potentially ameliorate Johne's disease on any well managed dairy farm; it is most likely not applicable to poorly managed farms. To mimic the situation on a “typical” small USA dairy farm, a roughly 4:1 ratio of paratuberculosis-negative to paratuberculosis-positive animals (see below for definition of each), under the ownership and management of St. Croix Valley Farm (SCVF), were housed together in a tie-stall facility. At any given time, the herd was comprised of 50–60 females. Cows from the SCVF herd that tested both sero- and fecal-negative every four to six months over their entire life were considered paratuberculosis-free. Presumed paratuberculosis-positive, pregnant cows were purchased over a two year period from seven moderately high prevalence herds based on the following criteria: (a) they were ELISA-positive when tested by the owner (ELISA test performed by Wisconsin state laboratory); or (b) they tested negative but were offspring of paratuberculosis dams (paratuberculosis being defined by either positive ELISA values, fecal MAP shedding and/or clinical disease). At the time of purchase, these cows, approaching their second or third lactation, were clinically evaluated and retested for sero- and fecal-parameters by Allied Monitor (see Table 1); they were then classified as at Stage I (n = 9), II (n = 20), III (n = 4), and one, not retested, was at Stage I or II—none were at stage IV. Stages of disease severity67 are defined as follows; animals that were asymptomatic and negative for all test-parameters were classified as at Stage I, those asymptomatic, but either ELISA- and/or fecal-positive were classified as being at Stage II. Stage III animals had signs of early clinical disease (change in fecal consistency, loss of appetite and reduced milk production, all noted by the author) and were either ELISA- and/or fecal-positive, whereas, Stage IV animals had severe, end-stage clinical disease, irrespective of whether they were ELISA- and/or fecal-negative/positive (almost all were fecal shedding). The magnitude of ELISA values and fecal shedding cfu were not used to define these stages, since previous longitudinal assessment37 showed that there was no correlation. All dams were tested three to four times per year over their lifetime and maintained in the herd until local veterinarians humanely euthanized recumbent, emaciated and/or cachectic animals that could not rise and stand on their own by intravenous injection of a sodium pentobarbital solution (Fatal Plus, 6 g/ml) at a dose of 1 ml per 4.5 kg body weight. All nine cows at Stage 1 (all parameters were negative pre-calving), subsequently over their lifetime of 2–3 years, tested ELISA-positive, seven became fecal shedders and four were euthanized with Stage IV clinical disease.
Cows, irrespective of whether they were paratuberculosispositive or negative, were calved on pasture in good weather and in maternity pens during inclement weather. To mimic on-farm “good management practice,” a conscientious effort was made to minimize calf exposure to environmental fecal material possessing MAP. To this end, each cow was fitted with a bra at least five days prior to the expected calving date to prevent newborns from nursing. Calves were removed from their dams and fed colostrum (with or without Dietzia—see below), most within 2–3 h—none longer than 10 h—of calving and housed in a tie-stall facility upwind from the cows in individual, impervious stalls to prevent potential calf-to-calf transmission of Dietzia and/or MAP.39,40 An additional procedure implemented as the standard milking protocol was use of a 5% glycerin-3% sodium hypochlorite pre-dip solution to clean teats prior to milking because bleach (at a final concentration of 3% sodium hypochlorite) reduced MAP (Click RE, unpublished) in feces from >300 to 3 colony forming units (cfu) and reduced MAP in biofilms on materials commonly used to construct livestock watering troughs.68
Calf treatments.
There were two basic groups of calves in the present study—34 were from paratuberculosis-positive dams and 51 were from paratuberculosis-negative dams; results with the former, which represent the primary focus and goal of the study, should always be considered the most important. Calves in both groups were treated similarly and once weaned were raised together. To increase the number of calves projected to be MAP-infected in utero/vaginal birthing, each received (naturally or tubed) four feedings of 2–3 quarts/feeding colostrum from their own dam only. Likewise calves of nonparatuberculosis dams were fed colostrum from only their dams. Thereafter, all were fed one of two milk replacers obtained from a local commercial source for 58 days. Both milk replacers were nutritionally identical—however, one contained 200 g/ton tetracycline plus 400 g/ton neomycin, whereas the other was devoid of antibiotics. All calves were given free choice antibiotic-free, texturized calf starter during this time. After weaning, they were group-housed based solely on size—not on treatment protocol—fed 2–3 lbs of grain per day plus an unlimited amount of hay and raised outdoors, isolated from adult cattle, with access to a south-sided open shelter. All calves were dehorned and males castrated at four weeks of age or younger. Females were eventually reintroduced into the herd after they became pregnant and calved.
The thirty-four calves derived from paratuberculosis dams were assigned to one of four treatment-groups, with at least 8 per group. In an effort to attain, on average, presumed equivalent exposure to natural non-environmental infection (Group 1 was an exception) they were stratified based on ELISA values of their dams. Group 1, not treated, had more calves derived from dams that tested paratuberculosis-negative at calving (Stage I), but subsequently tested positive at multiple test-dates (tested every three to four months per year). These calves were not treated primarily because their dams were presumed negative. This misclassification had the potential to underestimate the extent of infection, which as discussed earlier was not found.
The dose of Dietzia chosen for calf treatments was weightadjusted based on a dose that was extremely efficacious for paratuberculosis cows (>1012 cfu/650 kg).14 In contrast to varying Dietzia dosages based on clinical disease status of adult cows (a parameter unavailable for monitoring in calves), a single, fixed dose of 1–2 × 1011 cfu was chosen for treating calves. Calves in Group 1 were never exposed to Dietzia and served as untreated controls. Those in Groups 2 and 3 were fed viable Dietzia in each of the four colostrum feedings (2×/day) and then those in Group 3 only were fed viable Dietzia once per day for an additional 58 d; both groups were fed non-antibiotic-fortified milk replacer post-colostrum feedings. To assess whether a product produced by Dietzia (such as a protein, nucleic acid or other moiety) could replace viable Dietzia, calves in Group 4 were fed viable Dietzia twice per day for two days (colostrum feedings) and then once per day in milk replacer fortified with tetracycline, to which Dietzia is sensitive, for an additional 58 d.
Fifty-one calves whose dams always tested negative were assigned to one of the same four treatment groups without regard to having equal numbers in each. This was done because our management was such that no/few calves were anticipated to be infected with environmental MAP-laced manure. These calves were included merely to assess both our management practices and the efficacy of different Dietzia protocols on infection that might have occurred via inadvertent environmental fecal exposure; these calves were not the focus of the study.
It should be emphasized that paratuberculosis positivity in the calves was based on positive sero- and/or fecal parameters and NOT on clinical disease, primarily because of longitudinal associations reported previously for adult cows.37 The age at which calves were generally first tested for both serological and fecal parameters was 16–18 months based on other's findings that ELISA-positivity occurred as early as 134 d post-MAP infection69,70 and because of on-farm convenience for sampling. All animals, irrespective of whether they tested negative or positive, were retested every three to four months until they succumbed to either Johne's disease, to other medical conditions, or they were terminated. The final test of steers (except #3-7, who was kept to increase the number of animals tested long-term) and free-martin females was at the time they reached slaughter-weight; they were never reintroduced into the herd. The months shown in the Tables as “Age (months) at testing” are those at which an animal first tested positive or, for those that tested negative, at their final test-age. These ages are not the age at which an animal succumbed with/without Johne's disease.
Serum and MAP detection protocols.
All assays were performed at independent laboratories in an effort to attain unbiased measurements. Since the studies on Dietzia were initiated 16 years ago, all assays performed at that time by Allied Monitor, Inc. (Fayette, MO) were continued without variation for the duration of the study—this was done in an effort to ensure that data collected at any time would be reasonably comparable. Thus, many improvements of the assays were not adapted for our investigations. Fecal material collected directly from the rectum using individual disposable gloves and blood obtained aseptically from the tail vein, were transferred to sterile containers, coded and sent chilled on the day of collection to Allied Monitor. Colostrum was also obtained aseptically and sent to Allied. Some duplicate colostrum and fecal samples were sent to Antel BioSystems, Inc. (Lansing, MI). Both Allied Monitor and Antel BioSystems are USDA- and NVSL-approved laboratories that specialize in assays for Johne's disease. Serum ELISA and AGID assays and fecal MAP cultures were performed upon receipt. Validity, sensitivity and specificity of these assays when used longitudinally for individual cows were previously assessed.37
ELISA.
ELISA was performed using a crude, soluble, MAP protoplasmic antigen prepared by Allied. Test sera were preabsorbed with Mycobacterium phlei. The content of each well was read at a wavelength of 405 nm. ELISA values were calculated by dividing the test-sample OD by a value equivalent to ¼ the OD of a standard reference positive serum (range 0.13–0.14). Allied's classification of Negative (≤1.4 OD), Suspect (1.5 to 2.0 OD) and Positive (>2.0 OD) was modified slightly based on the finding that 10 of 13 adult animals with initial “suspect” serum ELISA values eventually became fecal shedders (n = 9) and/or succumbed with end-stage clinical disease (n = 5).37 Of the three that were only ELISA-positive, two had multiple values >2.0. The dam with only a single positive ELISA value gave birth to a calf that became both ELISA- and fecal-positive (calf 1-2, Table 2 herein)—this calf was born prior to the dam beginning an adult Dietzia treatment protocol that led to her negative paratuberculosis parameters. Therefore, the “suspect” category was not used and all animals with serum ELISA OD values >1.4 were considered paratuberculosis-positive.
AGID.
The AGID test71,72 was performed by Allied with the same crude MAP antigen used in the ELISA. The six peripheral wells were inoculated with 50 µl of test sera or a reference serum. The central well was loaded with 30 µl of antigen. Final readings were performed after 48 h. The reference paratuberculosis-positive sera used by Allied in both the ELISA and AGID assays was obtained from naturally MAP infected adult paratuberculosis cows.
MAP detection.
Fecal samples of 2 g were weighed into 50 ml sterile conical centrifugation tubes containing 35 ml of sterile 0.75% hexadecylpyridinium chloride (HPC) (Sigma Aldrich, St. Louis, MO). Tubes were shaken for 30 min by a horizontal shaker, after which the contents allowed to settle for 2 h. Approximately 2 ml of supernatant, just above the sedimentation layer, was removed using a sterile transfer pipette. The supernatant-loaded pipette was placed upright into a sterile culture tube (20 × 75 mm) at room temperature for overnight decontamination. Triplicate (occasionally quadruple) Herrold's egg yolk (HEY) agar slants containing 50 mg each Naladixic acid, Vancomycin and Amphotericin B (Becton Dickinson and Company, Sparks, MD) with mycobactin-J, plus one slant without (to evaluate mycobactin-J dependency), were inoculated with 4 drops (∼0.1 ml) of the decontaminated supernatant. Slants were maintained at 37°C in a horizontal position for one week, after which they were placed in the upright position and incubated for a total of 13 weeks. Enumeration of slightly raised white-yellow colonies evaluated for typical acid fastness and morphological appearance of MAP was recorded and summed together. Slants with so many colonies that made it impossible to accurately count were estimated and expressed as >100 cfu and are shown in the Tables as >300 cfu if all three slants were >100 cfu. For triplicates in which all slants were not >100, then a number less than 300 was calculated and expressed with a > sign. Any fecally shed MAP was considered as pass-through only if all ELISA values, prior to, at and post-MAP detection, were negative and all other test parameters were/remained negative (pass-through was found four times out of >1,500 individual tests).
The presence of MAP in randomly chosen colostrum samples was determined using both PCR (IS900) by Antel BioSystems and culture by Allied Monitor. Samples were processed using a centrifugation method;73 50 ml was dispensed into a sterile conical centrifugation tube and then centrifuged at 900x g for 30 min. The fat layer was removed from the surface and the liquid decanted. The pellet was then suspended in 25 ml of sterile 0.75% HPC, vortexed and incubated overnight at room temperature. Tubes were centrifuged at 900x g for 30 minutes, supernatant discarded and the pellet processed as fecal samples.
Necropsy.
The ileo-cecum lymph node plus other lymph nodes along the small intestine were obtained at sacrifice from all male calves in Group 4 and tested for the presence of MAP via PCR (IS900) and culture at the University of Minnesota Veterinarian Diagnostic Laboratory (St. Paul, MN). This test was discontinued because (a) positive/negative status was the same as that defined by their fecal cultures, (b) costs and inconvenience and (c) as reported previously, longitudinal analysis of ELISA values of individual animals was the best predictor of changes in disease status.37
Dietzia.
Dietzia was isolated from feces of a sero- and fecal-MAP-positive cow.34 It was reclassified as Dietzia, based on its 16S rRNA sequence—the gold standard for bacterial identification74 by MIDI Labs, Inc. (Newark, DE). Dietzia was grown under contract in 75-liter fermenters at the University of Minnesota Biotechnology Institute (St. Paul, MN) for 4–5 d at 29°C in fructose supplemented tryptic soy broth. Samples were centrifuged, washed and concentrated 20-fold prior to storage in 45 ml aliquots at −80°C (long term) or −20°C (short term). New lots were prepared as needed, approximately every 2–3 mo. The cfu were determined prior to use. Once thawed, excess material was stored at 4°C for only 7 d, at which time a new aliquot was thawed.
Statistical methods.
Differences in positivity for the four groups in which negative animals were lost to follow-up were analyzed using the Kaplan-Meier method to estimate probabilities. Cox Proportional Hazard Survival Analysis was used to assess differences in these estimates. The Student's t-test was used to assess differences in mean ELISA values. For all comparisons, p values <0.05 were considered statistically significant.
Acknowledgments
This research was funded, in part, by NIH Grant R01AI027331 (prior to retirement from Univ. WI-RF) and by Altick Associates, River Falls, WI. I wish to thank William D. Richards for the initial ICON 6 isolate (Dietzia) and Dr. Craig Van Kampen for statistical analysis and editorial assistance.
References
- 1.Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med. 1999;40:179–192. doi: 10.1016/s0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 2.Chi J, VanLeeuwen J, Weersink A, Keefe GP. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leucosis virus, Mycobacterium avium subspecies paratuberculosis and Neorsporta caninum. Prev Vet Med. 2002;55:137–153. doi: 10.1016/s0167-5877(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TK, Wilks CR, McQueen DS. Isolation of Mycobacterium paratuberculosis from the milk of a cow with Johne's disease. Vet Res. 1981;109:532–533. [PubMed] [Google Scholar]
- 4.Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 5.Seitz SE, Heider LE, Heuston WD, Bech-Nielsen S, Rings DM, Spangler L. Bovine fetal infection with Mycobacterium paratuberculosis. J Am Vet Med Assoc. 1989;194:1423–1426. [PubMed] [Google Scholar]
- 6.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis isolated from fetuses of infected cows not manifesting signs of the disease. Am J Vet Res. 1992;53:477–480. [PubMed] [Google Scholar]
- 7.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol. 1992;30:166–171. doi: 10.1128/jcm.30.1.166-171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streeter RN, Hoffsis GF, Cech-Nielsen S, Shulaw WP, Rings DM. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res. 1995;56:1322–1324. [PubMed] [Google Scholar]
- 9.Sweeney RW. Transmission of paratuberculosis. Vet Clin North Am Food Anim Pract. 1996;12:305–312. doi: 10.1016/s0749-0720(15)30408-4. [DOI] [PubMed] [Google Scholar]
- 10.Stabel JR. Pasteurization of colostrum reduces the incidence of paratuberculosis in neonatal dairy calves. J Dairy Sci. 2008;91:3600–3606. doi: 10.3168/jds.2008-1107. [DOI] [PubMed] [Google Scholar]
- 11.Benedictus A, Mitchell RM, Linde-Widmann M, Sweeney R, Fyock T, Schukken WH, et al. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev Vet Med. 2008;83:215–227. doi: 10.1016/j.prevetmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Whittington RJ, Windsor PA. In utero infection of cattle with Mycobacterium avium subspecies paratuberculosis: A critical review and meta-analysis. Vet J. 2009;179:60–69. doi: 10.1016/j.tvjl.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Click RE, Van Kampen CL. Progression of Johne's disease curtailed by a probiotic. J Dairy Sci. 2009;92:4846–4851. doi: 10.3168/jds.2009-2129. [DOI] [PubMed] [Google Scholar]
- 14.Click RE, Van Kampen CL. Assessment of Dietzia subsp. C79793-74 for treatment of cattle with evidence of paratuberculosis. Virulence. 2010;1:145–155. doi: 10.4161/viru.1.3.10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Click RE. Successful treatment of asymptomatic or clinically terminal bovine Mycobacterium avium subspecies paratuberculosis infection (Johne's disease) with the bacterium Dietzia used as a probiotic alone or in combination with dexamethasone—Adaption to chronic human diarrheal diseases. Virulence. 2011;2:131–143. doi: 10.4161/viru.2.2.15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells SJ, Wagner BA. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J Am Vet Med Assoc. 2000;216:1450–1457. doi: 10.2460/javma.2000.216.1450. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DJ, Benedictus G. Control of Mycobacterium avium subspecies paratuberculosis infection in agricultural species. Rev Sci Tech. 2001;20:151–179. doi: 10.20506/rst.20.1.1274. [DOI] [PubMed] [Google Scholar]
- 18.McKenna SL, Keefe GP, Tiwari A, Van Leeuwen J, Barkema HW. Johne's disease in Canada, part II: disease impacts, risk factors and control programs for dairy producers. Can Vet J. 2006;47:1089–1099. [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, Mitchell RM, Smith RL, Van Kessel JS, Chapagain PP, Schukken YH, et al. The importance of culling in Johne's disease control. J Theor Biol. 2008;254:135–146. doi: 10.1016/j.jtbi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Tavornpanich S, Johnson WO, Anderson RJ, Gardner IA. Herd characteristics and management practices associated with seroprevalence of Mycobacterium avium subspecies paratuberculosis infection in dairy herds. Am J Vet Res. 2008;69:904–911. doi: 10.2460/ajvr.69.7.904. [DOI] [PubMed] [Google Scholar]
- 21.Ferrouillet C, Wells SJ, Hartmann WL, Godden SM, Carrier J. Decrease of Johne's disease: prevalence and incidence in six Minnesota USA, dairy cattle herds on a long-term management program. Prev Vet Med. 2009;88:128–137. doi: 10.1016/j.prevetmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Collins MT, Eggleston V, Manning EJB. Successful control of Johne's disease in nine dairy herds: Results of a six-year field trial. J Dairy Sci. 2010;93:1638–1643. doi: 10.3168/jds.2009-2664. [DOI] [PubMed] [Google Scholar]
- 23.Ridge SE, Heuer C, Cogger N, Heck A, Moor S, Baker IM, et al. Herd management practices and the transmission of Johne's disease with infected dairy herds in Victoria, Australia. Pre Vet Med. 2010;95:186–197. doi: 10.1016/j.prevetmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 24.McDowell RM, McElvaine MD. Long-term sequelae to foodborne disease. Rev Sci Tech. 1997;16:337–341. [PubMed] [Google Scholar]
- 25.Grant IR, Ball HJ, Rowe MT. Incidence of Mycobacterium avium subspecies paratuberculosis in bulk raw and commercially pasteurized cow's milk from approved dairy processing establishments in the United Kingdom. Appl Environ Microbiol. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayele WY, Svastova P, Roubal P, Bartos M, Pavlik I. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cows milk in the Czech Republic. Appl Environ Microbiol. 2005;71:1210–1214. doi: 10.1128/AEM.71.3.1210-1214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellingson JLE, Anderson JL, Koziczkowski JJ, Radcliff RP, Sloan SJ, Allen SE, et al. Detection of viable Mycobacterium avium subs. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J Food Protection. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. [DOI] [PubMed] [Google Scholar]
- 28.Pickup RW, Rhodes G, Bull TL, Arnott S, Sidi-Moumedine K, Hurley M, et al. Mycobacterium avium subspecies paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: diverse opportunities for environmental cycling and human exposure. Appl Environ Microbiol. 2006;72:4067–4077. doi: 10.1128/AEM.02490-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirone K, Morsella C, Romano M, Paolicchi F. Mycobacterium avium subspecies paratuberculosis in food and its relationship with Crohn's disease. Review Argentina Microbiol. 2007;39:57–68. [PubMed] [Google Scholar]
- 30.Abubakar I, Myhill DJ, Hart AR, Lake IR, Harvey I, Rhodes JM, et al. A case-control study of drinking water and dairy products in Crohn's disease—further investigation of the possible role of Mycobacterium avium paratuberculosis. Am J Epidemiol. 2007;165:776–783. doi: 10.1093/aje/kwk067. [DOI] [PubMed] [Google Scholar]
- 31.Antognoli MC, Garry FB, Hirst HL, Lombard JE, Dennis MM, Gould DH, et al. Characterization of Mycobacterium avium subspecies paratuberculosis disseminated infection in dairy cattle and its association with antemortem test results. Vet Micro. 2008;127:300–308. doi: 10.1016/j.vetmic.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Pierce ES. Possible transmission of Mycobacterium avium subspecies paratuberculosis through potable water: lessons from an urban cluster of Crohn's disease. Gut Pathog. 2009;1:17–21. doi: 10.1186/1757-4749-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutharia LM, Klassen MD, Faires J, Barbut S, Gill CO. Mycobacterium avium subspecies paratuberculosis in muscle, lymphatic and organ tissues from cows with advanced Johne's disease. J Food Microbiol. 2010;136:340–344. doi: 10.1016/j.ijfoodmicro.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Richards WD. In vitro and in vivo inhibition of Mycobacterium paratuberculosis by iron deprivation: A hypothesis. Proc Conf Johne's Disease, Australia. 1988:87–94. [Google Scholar]
- 35.St. Jean G. Treatment of clinical paratuberculosis in cattle. Vet Clin North Am Food Anim Pract. 1996;12:417–430. doi: 10.1016/s0749-0720(15)30414-x. [DOI] [PubMed] [Google Scholar]
- 36.St. Jean G, Jernigan AD. Treatment of Mycobacterium paratuberculosis infection in ruminants. Vet Clin North Am Food Anim Pract. 1991;7:793–804. doi: 10.1016/s0749-0720(15)31085-9. [DOI] [PubMed] [Google Scholar]
- 37.Click RE, Van Kampen CL. Comparison of antemortem assays to assess progression-regression of paratuberculosis in individual dairy animals. Virulence. 2010;1:134–144. doi: 10.4161/viru.1.3.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slocombe RF. Combined streptomycin-isoniazid-rifampin therapy in the treatment of Johne's disease in a goat. Can Vet J. 1982;23:160–163. [PMC free article] [PubMed] [Google Scholar]
- 39.van Roermund HJ, Bakker D, Willemsen PT, de Jong MC. Horizontal transmission of Mycobacterium avium subspecies paratuberculosis in cattle in an experimental setting: calves can transmit the infection to other calves. Vet Microbiol. 2007;122:270–279. doi: 10.1016/j.vetmic.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell RM, Whitlock RH, Stehman SM, Benedictus A, Chapagain PP, Grohn YT, et al. Simulation modeling to evaluate the persistence of Mycobacterium avium subspecies paratuberculosis (MAP) on commercial dairy farms in the United States. Prev Vet Med. 2008;83:360–380. doi: 10.1016/j.prevetmed.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Pithua P, Godden SM, Wells SJ, Oakes MJ. Effect of feeding plasma-derived commercial colostrum replacer for the prevention of transmission in Holstein calves. J Am Vet Med Assoc. 2009;234:1167–1176. doi: 10.2460/javma.234.9.1167. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen H, Nielsen SS, Berg P. Genetic variation and heritability of the antibody response to Mycobacterium avium subspecies paratuberculosis in Danish Holstein cows. J Dairy Sci. 2004;87:2108–2113. doi: 10.3168/jds.S0022-0302(04)70029-6. [DOI] [PubMed] [Google Scholar]
- 43.van Kooten HC, Mackintosh CG, Koets AP. Intrauterine transmission of paratuberculosis (Johne's disease) in farmed red deer. N Z Vet J. 2006;54:16–20. doi: 10.1080/00480169.2006.36598. [DOI] [PubMed] [Google Scholar]
- 44.Chiodini RJ. Crohn's disease and the mycobacterioses: A review and comparison of two disease entities. Clin Micro Rev. 1989;2:90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Path. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 46.Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, et al. Mycobacterium avium subsp. paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn's disease and Johne's disease: common neural and immune pathogenicities. J Clin Micro. 2007;45:3883–3890. doi: 10.1128/JCM.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chamberlin WM, Naser SA. Integrating theories of the etiology of Crohn's disease. On the etiology of Crohn's disease; questioning the hypotheses. Med Sci Monit. 2006;12:27–33. [PubMed] [Google Scholar]
- 48.Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, et al. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- 49.Behr MA, Kapur V. The evidence for Mycobacterium paratuberculosis in Crohn's disease. Curr Opin Gastro. 2008;24:17–21. doi: 10.1097/MOG.0b013e3282f1dcc4. [DOI] [PubMed] [Google Scholar]
- 50.Pierce ES. Where are all the Mycobacterium avium subspecies paratuberculosis in patients with Crohn's disease? PLoS Pathog. 2009;5:1–11. doi: 10.1371/journal.ppat.1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pierce ES. Ulcerative colitis and Crohn's disease: is Mycobacterium avium subspecies paratuberculosis the common villain? Gut Path. 2010;2:21–32. doi: 10.1186/1757-4749-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Patho. 2009;1:1–15. doi: 10.1186/1757-4749-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng J, Stellakis ML, Sumar N. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastrol. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlin W, Graham DY, Hulten K, El-Zimaity HM, Schwartz MR, Naser S, et al. Review article: Mycobacterium avium subspecies paratuberculosis as one cause of Crohn's disease. Aliment Pharmacol Ther. 2001;15:337–346. doi: 10.1046/j.1365-2036.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- 55.Harris JE, Lammerding AM. Crohn's disease and Mycobacterium avium subspecies paratuberculosis: current issues. J Food Prot. 2001;64:2103–2110. doi: 10.4315/0362-028x-64.12.2103. [DOI] [PubMed] [Google Scholar]
- 56.Hermon-Taylor J, Bull T. Crohn's disease caused by Mycobacterium avium subspecies paratuberculosis: a public health tragedy whose resolution is long overdue. J Med Microbiol. 2002;51:3–6. doi: 10.1099/0022-1317-51-1-3. [DOI] [PubMed] [Google Scholar]
- 57.Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401–410. doi: 10.1002/ibd.20276. [DOI] [PubMed] [Google Scholar]
- 58.El-Zaatari FA, Naser SA, Markesich DC, Kalter DC, Engstand L, Graham DY. Identification of Mycobacterium avium complex in sarcoidosis. J Clin Microbiol. 1996;34:2240–2245. doi: 10.1128/jcm.34.9.2240-2245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sechi LA, Rosu V, Pacifico A, Fadda G, Ahmed N, Zanetti S. Humoral immune responses of Type 1 Diabetes patients to Mycobacterium avium subspecies paratuberculosis lend support to the infectious trigger hypothesis. Clin Vaccine Immunol. 2008;15:320–326. doi: 10.1128/CVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paccagnini D, Sieswerda L, Rosu V, Masala S, Pacifico A, Gazouli M, et al. Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PloS One. 2009;4:7109. doi: 10.1371/journal.pone.0007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rani PS, Sechi LA, Ahmed N. Mycobacterium avium subspecies paratuberculosis as a trigger of type-1 diabetes: destination Sardinia or beyond? Gut Pathog. 2010;2:1–6. doi: 10.1186/1757-4749-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dow CT, Ellingson JLE. Detection of Mycobacterium avium ss. paratuberculosis in Blau Syndrome tissues. Autoimmune Dis. 2010;127:692–697. doi: 10.4061/2010/127692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cossu D, Cocco E, Paccagnini D, Masala S, Ahmed N, Frau J, et al. Association of Mycobacterium avium subsp. paratuberculosis with Multiple Sclerosis in Sardinian Patients. PLoS ONE. 2011;6:18482. doi: 10.1371/journal.pone.0018482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rath T, Roderfeld M, Blocher S, Rhode A, Basler T, Akineden O, et al. Presence of intestinal Mycobacterium avium subspecies paratuberculosis (MAP) DNA is not associated with altered MMP expression in ulcerative colitis. BMC Gastroentero. 2011;11:34–53. doi: 10.1186/1471-230X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikonomopoulos J, Pavlik I, Bartos M, Svastova P, Ayele WY, Roubal P, et al. Detection of Mycobacterium avium subsp. paratuberculosis in retail cheeses from Greece and the Czech Republic. App Environ Micro. 2005;71:8934–8936. doi: 10.1128/AEM.71.12.8934-8936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stabel J. Johne's disease and milk: do consumers need to worry? J Dairy Sci. 2000;83:1659–1663. doi: 10.3168/jds.S0022-0302(00)75034-X. [DOI] [PubMed] [Google Scholar]
- 67.Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology) Vet Clin North Am Food Anim Pract. 1996;12:345–356. doi: 10.1016/s0749-0720(15)30410-2. [DOI] [PubMed] [Google Scholar]
- 68.Cook KL, Britt JS, Bolster CH. Survival of Mycobacterium avium subs. paratuberculosis films on livestock watering trough materials. Vet Microbiol. 2010;141:103–109. doi: 10.1016/j.vetmic.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Koets AP, Adugna G, Janss LL, van Weering HJ, Kalis CH, Wentink GH, et al. Genetic variation of susceptibility to Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J Dairy Sci. 2000;83:2702–2708. doi: 10.3168/jds.S0022-0302(00)75164-2. [DOI] [PubMed] [Google Scholar]
- 70.Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, et al. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subspecies paratuberculosis infection of calves. Infect Immun. 2003;71:5130–5138. doi: 10.1128/IAI.71.9.5130-5138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherman DM, Markham RJ, Bates F. Agar gel immunodiffusion test for diagnosis of clinical paratuberculosis in cattle. J Am Vet Med Assoc. 1984;185:179–182. [PubMed] [Google Scholar]
- 72.Sherman DM, Bray B, Gay JM, Bates F. Agar gel immunodiffusion test for diagnosis of subclinical paratuberculosis in cattle. Am J Vet Res. 1984;50:525–530. [PubMed] [Google Scholar]
- 73.Whitlock RH. Protocol for culture of Mycobacterium paratuberculosis from feces, milk and tissue. New Bolton Center University of Pennsylvania. 1988;6 [Google Scholar]
- 74.Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 76.Juste RA, Elguezabal N, Pavon A, Garrido JM, Geijo M, Sevilla I, et al. Association between Mycobacterium avium subspecies paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Intl J Infect Dis. 2009;13:247–254. doi: 10.1016/j.ijid.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 77.Cukovic-Cavka S, Vermeirem S, Hrstic I, Claessens G, Kolacek S, Jakic-Cazumovic J, et al. NOD2/CARD15 mutations in Croatian patients with Crohn's disease: prevalence and genotype-phenotype relationship. Eur J Gastro Hep. 2006;18:895–899. doi: 10.1097/00042737-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Whittington RJ, Windsor PA. In utero infection of cattle with Mycobacterium avium subsp. paratuberculosis: A critical review and meta-analysis. Vet J. 2009;179:60–69. doi: 10.1016/j.tvjl.2007.08.023. [DOI] [PubMed] [Google Scholar]