Abstract
It is well known that insulin can activate both PI3K/Akt pathway, which is responsible for glucose uptake, and MAPK pathway, which is crucial for insulin resistance formation. But, it is unclear exactly how the two pathways coordinate to regulate insulin sensitivity upon hyperinsulinism stress of type 2 diabetes mellitus (T2DM). Here, we show that an early response transcription factor Egr-1 could tilt the signalling balance by blocking PI3K/Akt signalling through PTEN and augmenting Erk/MAPK signalling through GGPPS, resulting in insulin resistance in adipocytes. Egr-1, PTEN and GGPPS are upregulated in the fat tissue of T2DM patients and db/db mice. Egr-1 overexpression in epididymal fat induced systematic insulin resistance in wild-type mice, and loss of Egr-1 function improved whole-body insulin sensitivity in diabetic mice, which is mediated by Egr-1 controlled PI3K/Akt and Erk/MAPK signalling balance. Therefore, we have revealed, for the first time, the mechanism by which Egr-1 induces insulin resistance under hyperinsulinism stress, which provides an ideal pharmacological target since inhibiting Egr-1 can simultaneously block MAPK and augment PI3K/Akt activation during insulin stimulation.
Keywords: AKT, Egr-1, insulin resistance, MAPK
Introduction
It is well known that insulin resistance is a central defect in metabolic syndromes such as type 2 diabetes mellitus (T2DM). These disorders have been associated with a chronic low-grade inflammatory state (Wellen and Hotamisligil, 2005; Hotamisligil, 2006; Shoelson et al, 2006) or an activation of cellular stress responses caused by high levels of triglycerides or an elevation of glucose or insulin (Nishikawa et al, 2000; Evans et al, 2003; Frayn, 2007; Kammoun et al, 2009). Upon insulin stimulation, two signalling pathways are activated. The insulin receptor is able to recruit and phosphorylate insulin receptor substrate (IRS) proteins after ligand binding, leading to the activation of the PI3K/Akt pathway, which is responsible for glucose metabolism, including glucose uptake and glycogenesis (Sun et al, 1991; Burgering and Coffer, 1995; Franke et al, 1995; Biddinger and Kahn, 2006). Additionally, insulin stimulation activates the MAP kinases (Erk 1 and 2) and SAP kinases (JNK and p38MAPK), which results in decreased insulin signalling and is crucial for insulin resistance (Aguirre et al, 2000; Hirosumi et al, 2002; Fujishiro et al, 2003). Thus, the balance of these two signalling pathways, PI3K/Akt and MAPK, is regarded as the key controller of insulin sensitivity (Guilherme et al, 2008; Isenovic et al, 2009). Therefore, identifying the key regulator for the coordination between the two signalling pathways may not only shed light on the mechanisms of insulin resistance but also provide a new strategy for diabetes intervention.
During the development of T2DM, patients are normally subjected to hyperinsulinism because excess insulin is secreted from β-cells to compensate for the reduced insulin sensitivity even before the patients are clinically diagnosed as T2DM (Polonsky et al, 1996; Cavaghan et al, 2000). Hyperinsulinaemia might impair the balance of PI3K/Akt and MAPK signalling, resulting in desensitization to PI3K/Akt activation and excess MAPK activation, thus impairing glucose metabolism and enhancing MAPK signalling-regulated insulin resistance-related gene expression (Samuelsson et al, 2006; Esma et al, 2008). Blocking MAPK signalling and augmenting PI3K/Akt signalling have the potential to prevent the development of insulin resistance and increase insulin sensitivity during stress exposure (Manning and Davis, 2003; Kaneto et al, 2004; Emanuelli et al, 2008; Luan et al, 2009). Benz et al (2010) have reported that fibroblast growth factor-1 (FGF-1) could induce downstream gene expression via both increase of MAPK and inhibition of AKT/PKB simultaneously in hippocampal neurons. They found when phosphorylated MAPK reached maximal activation; phosphorylated AKT was at its lowest levels, suggesting an interaction between MEK-1/2 and PI3K during gene expression induction. However, it is still not clear what causes the imbalance between PI3K/AKT signalling and the MAPK pathway during the development of T2DM.
It has been reported that insulin-sensitive tissues are able to respond to insulin stimulation even under an insulin-resistant status (Elchebly et al, 1999). Although the expression of some genes is not responsive to high level of insulin, other genes like early growth response 1 (Egr-1) have been reported to remain insulin sensitive in insulin-resistant 3T3-L1 adipocytes (Sartipy and Loskutoff, 2003a). As a stress response gene, Egr-1 is often rapidly and transiently activated by a variety of signals, including serum, growth factors, cytokines and hormones (Gashler and Sukhatme, 1995). According to our previous study, Egr-1 exerts an important function in chronic obstructive pulmonary disease (Ning et al, 2004) and has a crucial role in response to cigarette smoke extract or other stresses (Li et al, 2007). Also, Egr-1 can exert its inhibitory effect on adipocyte differentiation in 3T3-L1 cells (Boyle et al, 2009). Because T2DM patients exhibit hyperinsulinism status, we then investigated whether Egr-1 participates in insulin sensitivity under chronic high insulin-induced stress.
Additionally, as an important zinc finger transcription factor, Egr-1 can recognize highly conserved GC-rich consensus nucleotide sequences (GCG G/TGG GCG) (Chavrier et al, 1988; Cao et al, 1992; Gashler and Sukhatme, 1995; Yan et al, 2000) and activate the transcription of many genes, such as phosphatase and tensin homologue deleted on chromosome 10 (PTEN) (Virolle et al, 2001), an inhibitor of PI3K/Akt signalling. Our previous results also suggested that Egr-1 is able to augment Erk/MAPK signalling through one of its target genes, geranylgeranyl diphosphate synthase (GGPPS) (Shen et al, 2011a). Therefore, we hypothesize that Egr-1 may be an important regulator of insulin sensitivity by controlling the balance of PI3K/Akt and MAPK signalling via PTEN and GGPPS, respectively.
In this study, we provided in vitro and in vivo evidence that Egr-1 is able to mediate the insulin sensitivity by altering the balance of PI3K/Akt and MAPK signalling pathways and promoting the release of inflammatory cytokines from fat tissue. Loss of functional Egr-1 in the epididymal fat tissue of BKs db/db (C57BL/KsJ.Cg-m+/+Lepr db) mice increased insulin sensitivity. More importantly, we also found that blocking Egr-1 function in fat tissue relieved the insulin resistance of other tissues, such as the liver, and thus improved whole-body insulin resistance disorders. Our results suggested that Egr-1 might be an ideal pharmacological target for the development of drugs that prevent insulin resistance and increase insulin sensitivity.
Results
Egr-1 levels correspond to the diabetic state in the abdominal fat of humans and the epididymal (epi) fat of mice
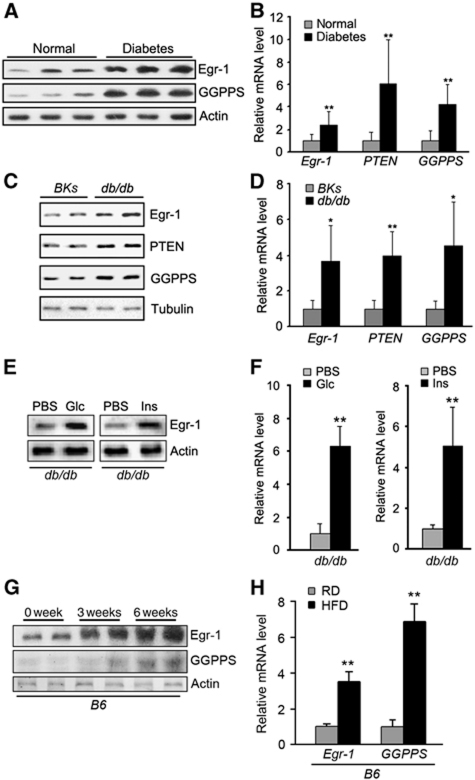
Before the patients are clinically diagnosed as T2DM, they are already exposed to chronic hyperinsulinism stress. Insulin-sensitive tissues are subjected to a constant or intermittent overstimulation of above normal level of insulin. Although Egr-1 is normally transiently activated within hours of insulin stimulation, we found both the protein (Figure 1A; Supplementary Figure S1A) and mRNA (Figure 1B) levels of Egr-1 were elevated in the adipose tissues of clinical samples of type 2 diabetes patients (clinical characteristics are shown in Supplementary Table 1). The expression of two Egr-1 target genes, PTEN and GGPPS, was also significantly higher in T2DM patients than in control groups (Figure 1A and B). In diabetic mice (db/db), both the protein and the mRNA levels of Egr-1, PTEN and GGPPS are also elevated in epi fat tissues (Figure 1C and D; Supplementary Figure S1B). Egr-1 was still responsive to glucose and insulin challenge in diabetic mice. When db/db mice were challenged with glucose or insulin, both the protein (Figure 1E; Supplementary Figure S1C) and mRNA (Figure 1F) levels of Egr-1 in epi fat were significantly increased, which suggested that Egr-1 still can be regulated by insulin in epi fat even in insulin-resistant mice. Still, in a more physiological insulin-resistant or prediabetic model that wild-type B6 mice were fed with high fat diet for 3 and 6 weeks, Egr-1 expression was also increased in epi fat tissue (Figure 1G and H; Supplementary Figure S1D), which further confirmed that Egr-1 in epi fat might involve in diabetes development.
Figure 1.
Egr-1, PTEN and GGPPS levels are elevated in diabetic patients’ abdominal fat and BKs db/db mice epi fat tissue. (A) Immunoblot of Egr-1 and GGPPS expression in adipose tissue of diabetic patients (n=10) and non-diabetic patients (n=10). Egr-1 and GGPPS protein level of diabetic patients was significantly higher than that of non-diabetic patients. (B) Quantitative RT–PCR of Egr-1, PTEN and GGPPS expression in adipose tissue of diabetic patients. Egr-1, PTEN and GGPPS mRNA of diabetic patients were significantly higher than that of non-diabetic patients. (C) Immunoblot of Egr-1, PTEN and GGPPS expression in adipose tissue of BKs wild-type and BKs db/db mice. Egr-1, PTEN and GGPPS expression levels were higher in the epi fat of db/db mice. (D) Quantitative RT–PCR of Egr-1, PTEN and GGPPS expression in adipose tissue of BKs wild-type and BKs db/db mice. Egr-1, PTEN and GGPPS mRNA of db/db mice were significantly higher than that of BKs wild-type mice. (E) Immunoblot of Egr-1 expression in epi fat of BKs db/db mice after challenged with glucose (1.8 g/kg of body weight) or insulin (2 units/kg of body weight) for 30 min. (F) Quantitative RT–PCR of Egr-1 expression in epi fat of BKs db/db mice after challenged with glucose or insulin. Egr-1 was increased in epi fat. (G) Immunoblot of Egr-1 and GGPPS expression in adipose tissue of B6 wild-type mice fed a high fat diet for 3 and 6 weeks. (H) Quantitative RT–PCR of Egr-1 and GGPPS expression in adipose tissue of B6 wild-type mice fed a regular or high fat diet for 6 weeks. All groups of animal experiments contained at least five mice and all the immunoblots were representative of the replicates. Data are presented as the mean and s.e.m. *P<0.05, versus control; **P<0.005, versus control.
Egr-1 in epi fat tissue modulates systematic glucose tolerance and insulin sensitivity in mice
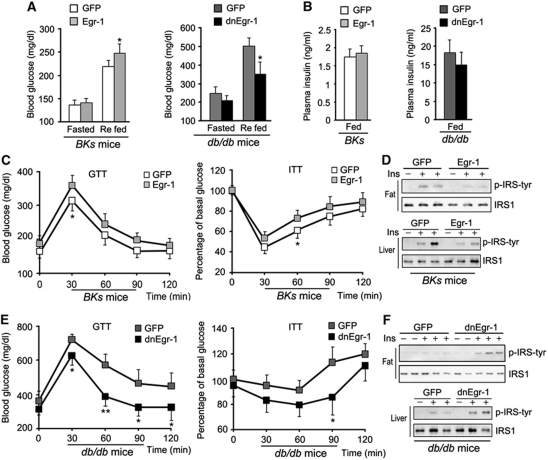
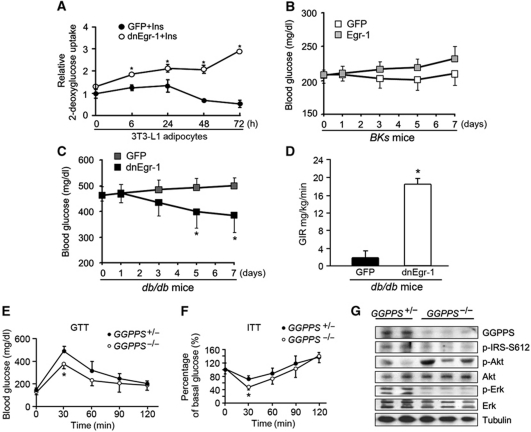
As the body's largest endocrine organ and one of the most important insulin targeting tissues, adipose tissue has a critical role in insulin sensitivity and glucose homeostasis (Steppan and Lazar, 2002; Kershaw and Flier, 2004). To determine the potential function of Egr-1 in adipose tissue, we overexpressed Egr-1 in wild-type mice and inhibited Egr-1 transcriptional activity in diabetic db/db mice by injecting adenovirus carrying either Egr-1 or its dominant-negative form into epi fat pads, which is the predominant fat tissue in mice intra-abdominal cavity. Western blotting and Q–PCR against GFP showed that the adenovirus only infected epi fat and not other tissues (Supplementary Figure S2A and B). Immunocytochemistry results showed that the adenovirus mainly infected into the adipocytes (Supplementary Figure S2C). Q–PCR indicated that Egr-1 was highly expressed in epi fat tissue after Egr-1 adenovirus injection (Supplementary Figure S2D). The injection did not affect the body weight (Supplementary Figure S2E) or food intake (Supplementary Figure S2F) of BKs db/db mice or wild-type controls. After fasting and refeeding, the blood glucose of Egr-1 BKs mice kept higher than GFP control group after Egr-1 infection (Figure 2A), whereas the blood glucose levels of dnEgr-1-infected diabetic mice were effectively decreased (Figure 2A). However, there was no significant difference of plasma insulin in the fed state (while the insulin level was significantly high in diabetic mice; Figure 2B). Moreover, glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) revealed that Egr-1 overexpression slightly impaired glucose tolerance and insulin sensitivity (Figure 2C) in wild-type BKs mice, whereas expression of dnEgr-1 was able to significantly improve glucose tolerance and increase insulin sensitivity (Figure 2E) in BKs db/db mice. Examination of IRS-1 phosphorylation in the epi fat and liver tissue of mice further confirmed that Egr-1 overexpression blocked IRS-1 phosphorylation of not only epi fat but also liver in wild-type mice (Figure 2D; Supplementary Figure S2G), while dnEgr-1 enhanced IRS-1 phosphorylation of not only epi fat but also liver response to insulin in db/db mice (Figure 2F; Supplementary Figure S2H). These results suggested that the Egr-1 might relate to insulin sensitivity not only in epi fat but also in other tissues of the body like liver.
Figure 2.
Egr-1 modulates systematic glucose tolerance and insulin sensitivity. Egr-1 was overexpressed in wild-type mice or its transcriptional activity was inhibited in diabetic db/db mice by injecting adenovirus carrying either Egr-1 or its dominant-negative form into epididymal (Epi) fat pads for 7 days. (A) Seven days after adenovirus administration, the blood glucose level was measured after fasting and refeeding. The blood glucose level of BKs wild-type mice gained an increase after Egr-1 administration, whereas dnEgr-1 administered diabetic mice were effectively suppressed. (B) Plasma insulin showed no significant change after adenovirus administration after refeeding. (C) GTT and ITT 7 days after adenovirus administration in BKs wild type. Egr-1 overexpression slightly impaired glucose tolerance and insulin tolerance in wild-type BKs mice. (D) Immunoblot analysis of IRS-1 activation in the epi fat and liver by Egr-1 overexpression in the epi fat of BKs wild-type mice after insulin challenge (2 units/kg of body weight). Overexpression of Egr-1 in the epi fat of normal BKs mice impaired IRS-1 phosphorylation of not only epi fat but also liver. (E) GTT and ITT 7 days after adenovirus administration in epi fat and liver of BKs db/db mice. dnEgr-1 was able to significantly improve glucose tolerance and increase insulin sensitivity in BKs db/db mice. (F) Immunoblot analysis of dnEgr-1-overexpressing in the epi fat of BKs db/db mice after insulin challenge (2 units/kg of body weight). dnEgr-1 overexpression was able to augment insulin-stimulated IRS-1 tyrosine phosphorylation of not only fat but also liver. All groups of animal experiments contained at least five mice and all the immunoblots were representative of the replicates. Data are presented as the mean and s.e.m. *P<0.05, **P<0.005, versus control.
Egr-1 regulates the balance of PI3K/Akt and Erk/MAPK pathways
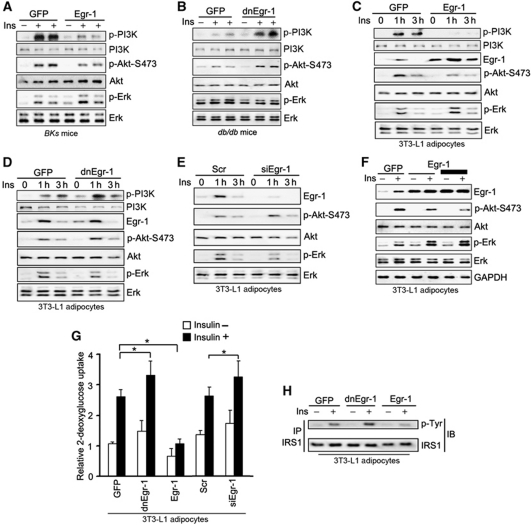
MAPK and PI3K/Akt are two critical pathways that mediate insulin's intracellular signal transduction which are crucial for insulin function (Sun et al, 1991; Burgering and Coffer, 1995; Franke et al, 1995; Aguirre et al, 2000; Hirosumi et al, 2002; Fujishiro et al, 2003; Biddinger and Kahn, 2006). But, it is unclear exactly how the two pathways coordinate to regulate insulin sensitivity in mice and insulin responsive cells. Signalling studies in the epi fat tissue of Egr-1 overexpressed wild-type mice and dnEgr-1 overexpressed diabetic db/db mice indicated that insulin-induced PI3K/Akt phosphorylation was inhibited by Egr-1 overexpression, at the same time, insulin-induced Erk1/2 phosphorylation was enhanced (Figure 3A; Supplementary Figure S3A). In contrast, inhibition of the transcriptional activity of Egr-1 with dnEgr-1 enhanced insulin-stimulated PI3K/Akt phosphorylation and blocked Erk1/2 phosphorylation (Figure 3B; Supplementary Figure S3B). Signalling pathway examination in 3T3-L1 adipocytes also indicated that insulin-induced PI3K/Akt phosphorylation was inhibited by Egr-1 overexpression, at the same time, insulin-induced Erk1/2 phosphorylation was enhanced (Figure 3C; Supplementary Figure S3C). In contrast, inhibition of the transcriptional activity of Egr-1 with dnEgr-1 (Figure 3D; Supplementary Figure S3D) or knockdown Egr-1 with siRNA (Figure 3E; Supplementary Figure S3E) enhanced insulin-stimulated Akt phosphorylation and blocked Erk1/2 phosphorylation (siRNAs efficiency of Egr-1, PTEN and GGPPS was shown in Supplementary Figure S3F). Overexpression of Egr-1 or blocking Egr-1's activity with dnEgr-1 or siEgr-1 had no effect on the differentiation of 3T3-L1 adipocytes (Supplementary Figure S3G). Further study showed that Egr-1 overexpression could inhibit Akt phosphorylation and enhance Erk1/2 phosphorylation in a dose-dependent manner (Figure 3F; Supplementary Figure S3H). When we overexpressed Egr-1 in 3T3-L1 adipocytes, the cells failed to respond to insulin stimulation, and the glucose uptake was suppressed (Figure 3G). Both the inhibition of the transcriptional function of Egr-1 with dnEgr-1 and knocking down of Egr-1 with siRNA were able to increase insulin sensitivity in 3T3-L1 adipocytes (Figure 3G). Analysis of IRS-1 tyrosine phosphorylation in 3T3-L1 adipocytes showed that overexpression of Egr-1 decreased IRS-1 phosphorylation when 3T3-L1 adipocytes were stimulated with insulin; whereas inhibition of Egr-1 transcriptional activity significantly increased IRS-1 phosphorylation (Figure 3H; Supplementary Figure S3I). Our results suggested Egr-1 could tilt the balance of PI3K/Akt and Erk/MAPK signalling to the direction of decreasing insulin sensitivity, and thus might involve T2DM development of epi fat tissue.
Figure 3.
Egr-1 regulates the balance of PI3K/Akt and Erk/MAPK pathways. (A, B) Immunoblot analysis of epididymal fat pad extracts from Egr-1 or dnEgr-1-overexpressing BKs wild-type or BKs db/db mice after insulin challenge (2 units/kg of body weight). Overexpression of Egr-1 in normal BKs mice impaired PI3K/Akt activation while augmenting Erk1/2 phosphorylation (A). However, dnEgr-1 overexpression was able to augment insulin-stimulated PI3K/Akt phosphorylation while reduce Erk1/2 phosphorylation in BKs db/db mice (B). (C–E) Immunoblot analysis of phosphorylated PI3K/Akt and Erk1/2 in 3T3-L1 adipocytes. Cells were treated with Egr-1 adenovirus (C), dnEGR-1 adenovirus (D) and Egr-1 siRNA (E) followed by high insulin (1000 nM) treatment for indicated times. Densitometric analysis (Supplementary Figure S3) suggested that Egr-1 was a negative regulator of PI3K/Akt and positive regulator of Erk1/2. (F) Egr-1 overexpression could inhibit Akt phosphorylation, whereas enhance Erk1/2 phosphorylation in a dose-dependent manner. (G) Glucose uptake of 3T3-L1 adipocytes responded to high insulin stimulation. Cells were pretreated with 1.5 μl of [3H]D-glucose (200 μCi/mmol/l) and then treated with insulin (1000 nM) for 3 h. Overexpression of Egr-1 suppressed glucose uptake, whereas inhibiting Egr-1's transcriptional function with dnEgr-1 or knockdown of Egr-1 with siRNA (siEgr-1) was able to largely increase their insulin sensitivity. (H) Immunoblot analysis of IRS-1 tyrosine phosphorylation in 3T3-L1 adipocytes after insulin stimulation for 1 h. Overexpression of Egr-1 decreased IRS-1 phosphorylation, whereas inhibition of Egr-1 transcriptional activity significantly increased IRS-1 tyrosine phosphorylation. All groups of animal experiments contained at least five mice and all the immunoblots were representative of the replicates. Data are presented as the mean and s.e.m. *P<0.05, versus control.
Egr-1 decreases insulin sensitivity through regulating PI3K/Akt and Erk/MAPK signalling balance by promoting PTEN and GGPPS transcription
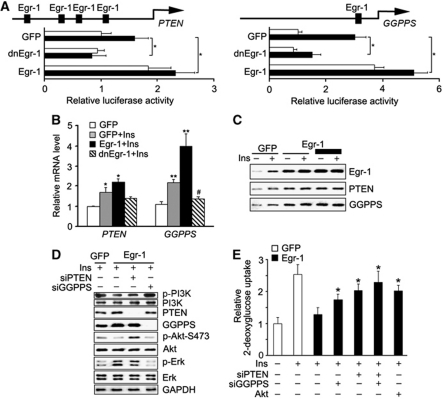
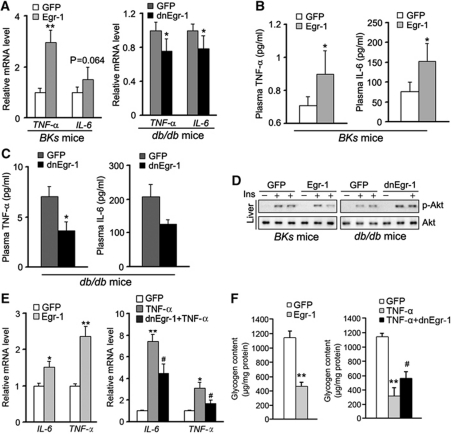
It has been reported that Egr-1 directly activates PTEN transcription during irradiation-induced signalling (Virolle et al, 2001). The previous study in our laboratory also revealed that Egr-1 was able to enhance Erk/MAPK signalling through directly activating GGPPS transcription during cigarette smoke stress in pulmonary cells (Shen et al, 2011a). Both the protein and the mRNA level of PTEN and GGPPS were also upregulated in the abdominal fat tissue of T2DM patients (Figure 1A and B) and diabetic mice (Figure 1C and D). Use of a PTEN or GGPPS promoter-driven luciferase assay confirmed the ability of Egr-1 to regulate the expression of these genes in 3T3-L1 adipocytes (Figure 4A). Q–PCR indicated that insulin was able to increase PTEN and GGPPS transcription in an Egr-1-dependent manner (Figure 4B). When Egr-1 was overexpressed in 3T3-L1 adipocytes, the protein level of PTEN and GGPPS was increased in a dose-dependent manner with the stimulation of insulin (Figure 4C; Supplementary Figure S4A). Further examination suggested that the effect of Egr-1 on PI3K/Akt and Erk1/2 was presumably mediated by the ability of PTEN to block the activity of AKT and GGPPS to stimulate the activation of Erk1/2 because their effects were reversed in the presence of siRNA of GGPPS or PTEN (Figure 4D; Supplementary Figure S4B). The glucose uptake assessment showed that the suppression of glucose uptake induced by Egr-1 could be rescued by GGPPS siRNA, PTEN siRNA or constitutively activated Akt (Figure 4E). All of data suggested Egr-1 induced PTEN and GGPPS expression tilted the balance of PI3K/Akt and Erk/MAPK signalling to the direction of decreasing insulin sensitivity in fat tissue.
Figure 4.
Egr-1 regulates PI3K/Akt and Erk/MAPK signalling balance by promoting PTEN and GGPPS transcription and then modulates insulin resistance development. (A) PTEN or GGPPS promoter-driven luciferase assay in 3T3-L1 adipocytes. The cells were infected with dnEgr-1 adenovirus and Egr-1 adenovirus followed by high insulin (1000 nM) treatment for 6 h. Insulin increased PTEN and GGPPS transcription activity in an Egr-1-dependent manner. (B) Quantitative RT–PCR of PTEN and GGPPS mRNA after Egr-1 overexpression in 3T3-L1 adipocytes. PTEN and GGPPS mRNA were increased by Egr-1 overexpression. (C) Immunoblotting of PTEN and GGPPS after Egr-1 overexpression in 3T3-L1 adipocytes. PTEN and GGPPS levels increased in an Egr-1 dose-dependent manner. (D) Signalling pathway analysis of 3T3-L1 adipocytes that transfected with Egr-1, siPTEN or siGGPPS, followed by high insulin (1000 nM) treatment for 1 h. The result suggested that the regulation of Egr-1 on PI3K/Akt and Erk1/2 was dependent on Egr-1-induced PTEN and GGPPS transcription, respectively. (E) Glucose uptake of 3T3-L1 adipocytes transfected with Egr-1, siGGPPS, siPTEN or constitutively active Akt as indicated. Cells were pretreated with 1.5 μl of [3H]D-glucose (200 μCi/mmol/l) followed by high insulin (1000 nM) treatment for 3 h. The results suggested that Egr-1 decreased glucose uptake in a GGPPS- and PTEN-dependent manner. Data are presented as the mean and s.e.m. *P<0.05, versus control; **P<0.005, versus control; #P<0.05, versus GFP plus insulin group.
Blocking Egr-1 transcriptional activity in epi fat tissue increases systematic insulin sensitivity
Patients with T2DM are normally subjected to hyperinsulinism-induced stress that impairs the balance of insulin signalling in insulin-sensitive tissues. To mimic the high insulin situation, 3T3-L1 adipocytes were exposed to a high level of insulin (1000 nM) for as long as 72 h, during which the fresh medium containing insulin was changed every 12 h. Long-term hyperinsulinism treatment resulted in a decreased glucose uptake in 3T3-L1 adipocytes (Figure 5A). However, this effect of insulin on glucose uptake was reversed by blocking the Egr-1 function with the dnEgr-1 adenovirus (Figure 5A). This suggested that long-term hyperinsulinism exposure of 3T3-L1 adipocytes could enhance insulin resistance in an Egr-1-dependent manner in vitro. It has been reported that hyperinsulinism could downregulate the insulin receptor (Gavin et al, 1974) which could also be observed in our study (Supplementary Figure S5A). In vivo assessment was further conducted by overexpressing Egr-1 in wild-type mice (Figure 5B) and inhibiting Egr-1 transcriptional activity in diabetic db/db mice (Figure 5C) in their epididymal fat pads. The results showed that the blood glucose levels in normal wild-type mice were gradually increased by overexpression of Egr-1 compared with the GFP control group (Figure 5B), whereas overexpression of dnEgr-1 effectively decreased blood glucose levels in diabetic db/db mice (Figure 5C). To define the role for Egr-1 in modulating whole-body insulin sensitivity further, we performed hyperinsulinaemic–euglycaemic clamp studies. We found that overexpression of dnEgr-1 in epi fat tissue of db/db mice led to better whole-body insulin sensitivity compared with the control group (Figure 5D). This indicated that disruption of Egr-1 in the epi fat tissue could contribute to the improvement of whole-body insulin resistance. To confirm whether GGPPS, which is the Egr-1 target gene, was crucial for the maintenance of insulin sensitivity in vivo, we knocked out the adipose-specific GGPPS gene in the mice and evaluated their insulin sensitivity. Indeed, the results indicated that GGPPS−/− male mice were more tolerant to glucose challenge (Figure 5E) and more sensitive to insulin challenge (Figure 5F), the mice uptook plasma glucose more quickly than control group did, a phenotype that had also been observed in PTEN knockout mice in adipose tissues (Kurlawalla-Martinez et al, 2005). However, the insulin sensitivity of female mice with GGPPS adipose-specific deletion was not improved compared with their littermate controls (data not shown). The reason of gender difference is that the isoprenoids like FPP and GGPP are the precursors of the cholesterol and terpenoids which are highly related with the steroid hormone synthesis (Olivier et al, 2000; Miller, 2002). GGPPS deletion might alter steroid hormone situation in different gender mice, which changes insulin sensitivity or affects intracellular signalling pathway. Signalling pathway examination in GGPPS−/− epi fat tissue indicated that Akt phosphorylation was enhanced while Erk1/2 phosphorylation was inhibited in a basal level (Figure 5G; Supplementary Figure S5B). The inhibitory serine phosphorylation of IRS-1 was also decreased in epi fat tissue after GGPPS deletion (Figure 5G). These in vitro and in vivo data suggested that blocking Egr-1/GGPPS pathway could increases systematic insulin sensitivity through regulating both PI3K/Akt and Erk/MAPK signalling, which might serve as a potential pharmacological target for the development of drugs that prevent insulin resistance and increase insulin sensitivity.
Figure 5.
Egr-1 and systematic insulin sensitivity. (A) 3T3-L1 adipocytes were transfected with dnEgr-1 and then given high insulin (1000 nM) treatment as indicated times, with a fresh medium (plus insulin) provided every 12 h. Cells were then pretreated with 1.5 μl of [3H]D-glucose (200 μCi/mmol/l) followed by insulin treatment for 3 h. Long-tern exposure to high-level insulin gradually decreased insulin sensitivity, which could be recovered by blocking Egr-1 transcriptional activity with dnEgr-1. (B, C) Glucose assessment after overexpressing Egr-1 in wild-type mice (B) or dnEgr-1 in db/db mice (C) by injecting adenovirus into epididymal fat pads. Egr-1 overexpression could increase blood glucose levels in normal wild-type mice (B); whereas dnEgr-1 overexpression could effectively decrease blood glucose levels in diabetic db/db mice (C). (D) Hyperinsulinaemic–euglycaemic clamp studies performed in db/db mice (6 weeks old) which were subjected to overexpression of dnEgr-1 (n=3) or control adenovirus (n=3) in the epi fat tissue. The glucose infusion rate (GIR) was shown. (E, F) GTT and ITT in GGPPS+/− or GGPPS−/− male mice. The GGPPS−/− male mice showed improved glucose tolerance (E) and more sensitive to insulin challenge (F). (G) Immunoblot analysis of epididymal fat pad extracts from GGPPS+/− or GGPPS−/− male mice. GGPPS deletion augmented Akt phosphorylation while reduced Erk1/2 phosphorylation. GGPPS deletion also decreased IRS-1 inhibitory serine 612 site phosphorylation. All groups of animal experiments contained at least five mice and all the immunoblots were representative of the replicates. Data are presented as the mean and s.e.m. *P<0.05, versus control.
Egr-1 mediates adipokines release from adipocytes that account for systematic insulin sensitivity
Our data showed that Egr-1 in epi fat also altered IRS-1 phosphorylation in liver tissue (Figure 2D and F). As fat is a metabolically active secretory tissue that has an important role in the development of systemic insulin resistance through the synthesis and release of adipokines, such as TNF-α, IL-6, or IL-1β, we next explored whether expression of Egr-1 in epi fat tissue regulated insulin resistance through these adipokines. When we overexpressed Egr-1 in the epi fat tissue of wild-type BKs mice, TNF-α and IL-6 expression levels in epi fat tissue were elevated (Figure 6A) and plasma levels of TNF-α and IL-6 were also increased (Figure 6B). When we inhibited the transcriptional activity of Egr-1 in epi fat tissue of BKs db/db mice, TNF-α and IL-6 levels in plasma were reduced (Figure 6C). Accordingly, mRNA levels of TNF-α and IL-6 in epi fat tissue were decreased (Figure 6A). Other inflammatory cytokines had no change, like IL-13, IL-10, resistin and IL-4 although resistin and IL-4 level in db/db mice are much higher than that in wild-type BKs mice (Supplementary Figure S6A and B) which suggest that the viruses injection did not effect immune cells function. Since interruption of Egr-1 could affect IRS-1 phosphorylation of not only epi fat but also liver (Figure 2D and F), we further examined the situation of insulin signalling pathway in liver. Egr-1 overexpression in epi fat blocked liver Akt activation in wild-type mice, while dnEgr-1 in epi fat enhanced Akt phosphorylation response to insulin in db/db mice (Figure 6D; Supplementary Figure S6C). To determine whether the liver's response is a direct result of adipokines released from epi fat tissue, we co-cultured 3T3-L1 adipocytes and hepatocytes in different layers of a transwell system when either Egr-1 or dnEgr-1 was overexpressed in the 3T3-L1 adipocytes. As shown in Figure 6E, both TNF-α and IL-6 expression levels were increased by Egr-1 and decreased by dnEgr-1 in 3T3-L1 adipocytes. When stimulated with insulin, the glycogen synthesis of hepatocytes co-cultured with Egr-1-expressing 3T3-L1 adipocytes was markedly reduced (Figure 6F). However, when hepatocytes co-cultured with TNF-α-induced insulin-resistant 3T3-L1 adipocytes, dnEgr-1 expression could restore insulin-stimulated glycogen synthesis (Figure 6F). These data indicated that Egr-1 was able to affect insulin sensitivity not only in fat tissue but also in other tissue like liver, and thus efficiently regulate glucose levels in the body.
Figure 6.
Egr-1 mediates adipokine expression in epi fat tissue and affects glycogen synthesis of liver responding to insulin. The adenovirus of Egr-1 or its dominant-negative form was injected into epididymal fat pads to overexpress Egr-1 in wild-type BKs mice or inhibit its transcription activity in BKs db/db mice. Expression of TNF-α and IL-6 in the epi fat tissue was determined by quantitative RT–PCR. The mice were fasted overnight, and the plasma levels of TNF-α and IL-6 were determined with ELISA kits. Egr-1 overexpression in wild-type mice was able to increase TNF-α and IL-6 expression in epi fat (A) and plasma (B), whereas dnEgr-1 overexpression in db/db mice had the opposite effect on TNF-α and IL-6 in epi fat (A) and plasma (C). (D) Immunoblot analysis of liver from Egr-1 or dnEgr-1 overexpressing in the epi fat pad of BKs db/db and BKs wild-type mice after insulin challenge (2 units/kg of body weight). Overexpression of Egr-1 in normal BKs mice epi fat pads impaired the Akt activation in liver. However, dnEgr-1 overexpression in BKs db/db mice epi fat pads was able to augment insulin-stimulated Akt phosphorylation in liver. (E, F) 3T3-L1 adipocytes and hepatocytes were co-cultured in different layers of a transwell. Egr-1 or dnEgr-1 was overexpressed in 3T3-L1 adipocytes. 3T3-L1 adipocytes were treated with TNF-α 5 ng/ml for 24 h to induce insulin resistance and then incubated in fresh medium without TNF-α for 12 h. Both TNF-α and IL-6 expression levels were increased by Egr-1 and decreased by dnEgr-1 in 3T3-L1 adipocytes (E). After co-incubation with 3T3-L1 adipocytes for 12 h, hepatocytes were stimulated with insulin (100 nM) for 30 min and determined the glycogen content. When stimulated with insulin, the glycogenesis of hepatocytes co-cultured with Egr-1-expressing 3T3-L1 adipocytes was markedly reduced. However, when hepatocytes were co-cultured with dnEgr-1-expressing 3T3-L1 adipocytes, insulin-stimulated glycogen synthesis was restored (F). All groups of animal experiments contained at least five mice and all the immunoblots were representative of the replicates. Data are presented as the mean and s.e.m. *P<0.05, **P<0.005, versus control; #P<0.05, versus TNF-α group.
Discussion
Currently, the most common drugs used to treat T2DM are those in the sulphonylurea group, including glibenclamide and gliclazide. These drugs can increase glucose-stimulated insulin secretion by the pancreas, even in cases of insulin resistance (Gregorio et al, 1996). Thus, the patients might be exposed to more severe hyperinsulinism status after drug administration. The sustained or repetitious hyperinsulinism will impair the balance of the PI3K/Akt and MAPK signalling pathways evoked by insulin and enhance insulin resistance. So any molecules that can tilt the balance can be used to ameliorate hyperinsulinism-induced insulin resistance. Our results suggest that Egr-1 is such a molecule to control the balance of these two signalling pathways by inducing the expression of downstream genes involved in PI3K/Akt and MAPK signalling. Sustained expression of Egr-1 during hyperinsulinaemia can stimulate PTEN and GGPPS transcription. Increased PTEN blocks PI3K/Akt signalling and thus impairs insulin-induced glucose metabolism. Meanwhile, increased GGPPS causes sustained MAPK/Erk1/2 activation that is responsible for the development of insulin resistance. When we overexpressed Egr-1 in mouse epididymal fat tissue, some characteristics of insulin resistance were observed, such as an increase in blood glucose levels and a decrease in insulin sensitivity.
Our results suggest that Egr-1 is able to serve as a target for pharmacological development of drugs for T2DM because inhibiting Egr-1 is able to simultaneously block MAPK signalling as well as augment PI3K-Akt signalling. For example, when we injected dominant-negative Egr-1 adenovirus into epididymal fat tissue of BKs db/db mice to inhibit its transcriptional activity, symptoms of insulin resistance in the mice were largely improved. Loss of Egr-1 function was also able to partially restore insulin-stimulated PI3K/Akt activation and IRS-1 tyrosine phosphorylation in both 3T3-L1 adipocytes and epi fat tissue.
There are three possibilities that Egr-1 can decrease insulin sensitivity. The first possibility is that Egr-1 can directly interrupt insulin receptor-mediated signalling in epi fat tissue. Besides stimulating PTEN transcription, Egr-1-induced MAPK/Erk1/2 sustained activation can also directly block insulin signalling through phosphorylating IRS-1 at its inhibitory serine site. We have found that the phosphorylation of Erk1/2 and IRS-1 serine 612 decreased in GGPPS-deleted epi fat tissue (Figure 5G). Our previous report also showed that Egr-1 could regulate hyperinsulinism-induced IRS-1 612 serine phosphorylation in a GGPPS/Erk1/2-dependent manner (Shen et al, 2011b). Although it has been reported that the hyperinsulinism could downregulate the insulin receptor itself (Gavin et al, 1974) which had nothing to do with Egr-1, we think that the IRS-1 inhibition by serine phosphorylation resulted from Egr-1 would be another cause for insulin signalling interruption and disruption of Egr-1 could restore the insulin sensitivity by increasing the IRS-1 tyrosine phosphorylation (Figures 2F and 3H) which could compensate the downregulation of insulin receptor. The second possibility is that Egr-1 affects the function of adipocyte. Boyle et al (2009) showed that adipogenesis is carefully regulated by Egr-1 and Egr-2, which exert opposing influences on adipocyte differentiation. We also found that dnEgr-1 administration in epididymal fat tissue could affect the size of adipocyte (Supplementary Figure S2C). So the elevation of Egr-1 in 3T3-L1 adipocytes under hyperinsulinism stress can regulate insulin sensitivity through changing the situation of lipid metabolism of the cells. The third possibility that Egr-1 can regulate insulin sensitivity is that Egr-1 increases the synthesis and release of adipokines from 3T3-L1 adipocytes. TNF-α, a target gene of Egr-1 (Son et al, 2008), and IL-6 are elevated in obese, diabetic subjects (Hotamisligil et al, 1993; Sabio et al, 2008), and regulate glucose metabolism in multiple cell types (Emanuelli et al, 2008). The TNF-α-deficient obese mice have lower levels of circulating free fatty acids and are protected from the obesity-related reduction in the insulin receptor signalling of fat tissues (Uysal et al, 1997). We found that both the mRNA of IL-6 and TNF-α in epi fat tissue and the protein levels of IL-6 and TNF-α in the plasma of BKs db/db mice are significantly decreased by injection of dominant-negative Egr-1 adenovirus into epididymal fat tissue, which suggests that Egr-1 can regulate insulin sensitivity through affecting the synthesis and release of adipokines.
Adipose tissue is the body's largest endocrine organ and is a highly active metabolic site of insulin action. Adipose tissue has a critical role in insulin metabolism and whole-body homeostasis (Kershaw and Flier, 2004; Pou et al, 2007). Visceral fat that is predominant in the intra-abdominal cavity is involved in the development of metabolic syndrome, and this type of fat is more frequently accompanied by disorders of glucose and lipid metabolism than subcutaneous fat tissue (Matsuzawa et al, 1994; Fukuhara et al, 2005). Others have reported that augmented UCP1 expression in epi fat improves glucose tolerance in both diet-induced and genetically obese mouse models (Yamada et al, 2006). Intra-abdominal fat has been shown to have high levels of cytokine release, lipogenesis and lipolysis, and its accumulation induces high levels of inflammatory cytokines and free fatty acids, which travel to the liver directly through portal circulation (Hotamisligil et al, 1993; Hug and Lodish, 2005). Excess inflammatory cytokines cause enhanced gluconeogenesis as well as insulin resistance, resulting in hyperinsulinism, glucose intolerance and other metabolic disorders (Hofmann et al, 1994). Our results suggest that Egr-1 expression in epi fat tissue may also involve in whole-body insulin resistance. Blocking Egr-1 in epi fat tissue can improve whole-body insulin sensitivity.
Together, these results demonstrate that Egr-1 is an important mediator of insulin resistance. Egr-1 is able to regulate the balance of PI3K-Akt and Erk/MAPK signalling and regulate TNF-α and IL-6 expression in adipose tissue. Thus, Egr-1 may be a perfect pharmacological target to develop drugs that prevent insulin resistance and increase insulin sensitivity.
Materials and methods
Subjects
The study sample comprised 20 individuals (12 men and 8 women) from the Jiangsu Traditional Chinese Medicine Hospital in China, a population-based study among non-institutionalized Chinese subjects aged 46–77 years in Jiangsu. All participants attended a clinical examination that included standard anthropometric measurements and fasting plasma samples collection. Height and weight were measured with participants dressed in lightweight clothing without shoes. Glucose was measured enzymatically on an automatic analyzer (Hitachi 7080) with reagents purchased from Wako Pure Chemical Industries (Osaka, Japan). Diabetes was defined either by 1999 World Health Organization criteria (Gabir et al, 2000) or self-report of being previously diagnosed as diabetic or treated with medication for diabetes confirmed by medical record review. Informed consent was obtained from all participants, and study protocols were approved by the institute review board of the Institute for Nutritional Sciences. Descriptive characteristics of the population are given in Supplementary Table 1. This clinical study was conducted according to the Declaration of Helsinki principles.
Materials
Antibodies for immunoblotting were Erk1/2, Akt (Cell Signaling Technology, MA) and their phosphorylated forms (Cell Signaling Technology), Egr-1 (Abcam, Cambridge, UK), GFP, IRS-1 and phosphorylated form (Santa Cruz Biotechnology, CA), PI3K and its phosphorylation form (Bioworld Technology, Minneapolis, MN). Secondary antibodies were alkaline phosphatase conjugated (Santa Cruz Biotechnology). Control GFP, dnEgr-1 and Egr-1-expressing adenoviruses were constructed using AdEasy adenoviral vector system (Stratagene, CA), purified by CsCl gradient centrifugation and dialysed in PBS containing 10% glycerol. Egr-1, GGPPS and PTEN siRNAs were purchased from Invitrogen (Carlsbad, CA). TNF-α was purchased from Sigma. Murine TNF-α and IL-6 ELISA Kits were purchased from USCN Life Science. Murine insulin Elisa kit was purchased from Millipore (MA).
Cell culture, transfection, adenovirus infection and luciferase assay
3T3-L1 cells were cultured in DMEM medium with 10% FBS. 3T3-L1 adipocytes were induced to differentiate from 3T3-L1 cells following the protocol as previously described (Furukawa et al, 2005). Mouse hepatoma Hepa1c1c7 cells were cultured in MEM supplemented with 10% FBS and 1% penicillin/streptomycin. Insulin-resistant cells were obtained by incubating the differentiated 3T3-L1 adipocytes for 3 days in the presence of 3 ng/ml TNF-α in fresh medium and changed each day as described (Hotamisligil et al, 1994; Samad et al, 2000; Sartipy and Loskutoff, 2003b). For adenovirus infection, cells were exposed to adenovirus at a multiplicity of infection of 200 in media overnight. GGPPS and PTEN promoter constructs were transfected into 3T3-L1 adipocytes with Lipo2000 (Invitrogen). To control for transfection efficiency, cells were co-transfected with the pCMVh-galactosidase plasmid. For knockdown studies, cells were transfected with control or Egr-1, GGPPS, PTEN siRNA (in low-serum Opti-MEM using Lipo2000 reagent from Invitrogen), according to the manufacturer's recommendations. Twenty-four hours after transfection, cells were treated with insulin for 3 h, then collected, lysed in 1 × reporter lysis buffer, and luciferase or β-galactosidase activity was measured.
3T3-L1 adipocytes/hepatocytes co-culture
For co-culture experiments, 3T3-L1 adipocytes were treated with TNF-α for 24 h, and then fresh medium without TNF-α was added. 3T3-L1 adipocytes were then co-incubated with hepatocytes for 12 h in the upper layer of a transwell system. Next, the hepatocytes were stimulated with insulin (100 nM) for 30 min and were processed for glucose uptake.
Metabolite determinations
Glycogen content was measured by scraping cells into 30% KOH, boiling the extract for 15 min, and centrifuging at 5000 g for 15 min. Glycogen in the cleared supernatants was measured as described (Chan and Exton, 1976).
Glucose uptake
Glucose uptake measurement of cells was performed as described with modification (Moyers et al, 1996). Briefly, cells were stimulated with 1000 nM insulin in KRH containing 0.1% BSA buffer for 3 h, and then incubated with 1 μCi of [3H]2-deoxyglucose for 3 h. After incubation, the cells were washed three times with ice-cold PBS and then dissolved in 1 N NaOH. The sample solution was neutralized with 1 N HCl and counted by liquid scintillation.
Cell lysates, immunoprecipitation and immunoblotting
Cells were washed in ice-cold PBS and harvested with RIPA buffer containing vanadate and protease inhibitors. Lysates (400 mg) were immunoprecipitated with anti-IRS1 antibodies (Upstate, NY) and then protein A coupled to agarose beads (GE Healthcare Biosciences, Piscataway, NJ). For immunoblotting, cell and tissue protein extracts (250 mg total protein) were boiled in loading buffer, separated by 10% SDS–PAGE and then transferred onto polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA). The membranes were incubated overnight with appropriate primary antibodies. Bound antibodies were then visualized using alkaline phosphatase-conjugated secondary antibodies. The immunoblots were visualized with Strom 840 (Amersham, Buckinghamshire, UK). The intensities of bands were quantified with the NIH Image 1.62 program.
RNA extraction and gene expression
RNA extraction from cells and tissues was extracted using Trizol reagent (Invitrogen) according to the manufacturer's protocol. Complementary DNA was synthesized from total RNA with Superscript II enzyme and random hexamer primers (TaKaRa, Tokyo, Japan). cDNAs were amplified with SYBRGreen Master Mix in a Mastercycler (Eppendorf AG 22331, Hamburg, Germany). All data were normalized to the expression of 18S rRNA. Primer sequences are available from the authors on request.
Animal studies
Male BKs db/db mice (C57BL/KsJ.Cg-m+/+Lepr. db) and control littermates were purchased from the Model Animal Research Center of Nanjing University. All of the mice were maintained on a 12-h light/dark cycle. All protocols for animal use were reviewed and approved by the Animal Care Committee of Nanjing University in accordance with Institutional Animal Care and Use Committee guidelines. The in vivo adenovirus administration experiment was performed as described previously (Furukawa et al, 2005). Briefly, BKs wild-type and BKs db/db mice (6 weeks old) were anesthetized before abdomen exposure. The adenoviral preparation (1 × 108 plaque-forming units in a volume of 20 μl) was injected at four points on each side of the epididymal fat tissue in db/db and in wild-type mice. The mice were maintained on a standard diet (65% carbohydrate, 4% fat and 24% protein). Blood was obtained from the vena cava 7 days after injection, and the plasma was stored at −80°C until analysis. Plasma TNF-α and IL-6 levels were measured with commercial kits (Uscnlife, Missouri city, USA) according to the manufacturer's protocols. Plasma insulin level was measured with sensitive insulin Elisa kit (Millipore). The body weight of the db/db and wild-type mice was also measured when the mice were killed.
Generation of adipose-specific GGPPS deletion mice
The GGPPS genomic DNA was isolated from a 129SVJ mouse genomic library and used to construct the GGPPS targeting vector by standard techniques. Briefly, the GGPPS gene was cloned into a targeting vector that contained a neomycin selection cassette flanked by Flp recombinase target sites. Two LoxP sites were inserted into the flank of exons 3–4 of the GGPPS gene. GGPPS-LoxP-targeted mice were subsequently crossed with Fabp4-Cre transgenic mice to generate adipose-specific GGPPS knockout mice. The transgenic lines (129) were back crossed for at least six generations into the C57BL/6 background, which is the background of the GGPPS-LoxP mice. All the offsprings were genotyped by PCR analysis using mouse-tail DNA and specific primers: Cre: forward: 5′-GCGGTCTGGCAGTAAAAACTATC-3′, reverse: 5′-GTGAAACAGCATTGCTGTCACTT-3′; LoxP: forward: 5′-AATTGTGTGTGGTAGGGGTA-3′, reverse: 5′-AACTTGCTTCAG AACTGAGC-3′. All the mice used in this study were male ones.
Glucose and insulin tolerance and insulin sensitivity assessment in vivo
To test glucose tolerance, male mice were given an intraperitoneal injection of glucose (1.8 mg/g body weight) after a 16-h fast. To determine insulin sensitivity, mice were given an intraperitoneal injection of insulin (2 units/kg of body weight) at feeding after a 16-h fast. Blood glucose was determined at the indicated times from tail blood using the OneTouch Ultra glucometer (Lifescan, Burnaby, Canada).
Hyperinsulinaemic–euglycaemic clamp study
Four days before the hyperinsulinaemic–euglycaemic clamp studies, indwelling catheters were placed into the right internal jugular vein extending to the right atrium. Meanwhile, the adenoviral preparation (1 × 108 plaque-forming units in a volume of 20 μl) was injected at four points on each side of the epididymal fat tissue in db/db mice. After being fasted for overnight, mice were studied in a restrainer. The protocol consisted of a 120-min experimental period (t=0–120 min). The insulin clamp was begun at t=0 min with continuous infusion of human insulin (18 mU/kg min). Euglycaemia (120–130 mg/dl) was maintained during clamps by measuring blood glucose every 5 min starting at t=0 min and infusing 20% dextrose from t=10 min as necessary. The glucose infusion rate adjusted for body weight was used as one measure of insulin sensitivity.
Data analysis
All data are presented as the mean±s.e.m. Data were analysed using an one-way ANOVA followed by Fisher's LSD post hoc test. Calculations were performed using SPSS/Windows version 12.5S statistical package (SPSS, Chicago, USA). In all cases, statistical significance is displayed as P<0.05 (one asterisk) or P<0.005 (two asterisks).
Supplementary Material
Acknowledgments
This work was supported by National Basic Research Program of China (2009CB918703 and 2006CB943500) and National Natural Science Foundation of China (30671086) awarded to Professor Chao-Jun Li. This work was also supported by National Natural Science Foundation of China (30700394) and the Natural Science Foundation of Jiangsu Province of China (07KJB180053) awarded to Dr Bin Xue.
Author contributions: BX and CJL conceived and designed the experiments. XY, NS, MLZ and FYP performed the experiments. CW, WPJ, CL, QG, XG, BX and CJL analysed the data. CJL, XY and NS wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF (2000) The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem 275: 9047. [DOI] [PubMed] [Google Scholar]
- Benz AH, Shajari M, Peruzki N, Dehghani F, Maronde E (2010) Early growth response-1 induction by fibroblast growth factor-1 via increase of mitogen-activated protein kinase and inhibition of protein kinase B in hippocampal neurons. Br J Pharmacol 160: 1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR (2006) From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68: 123–158 [DOI] [PubMed] [Google Scholar]
- Boyle KB, Hadaschik D, Virtue S, Cawthorn WP, Ridley SH, O'Rahilly S, Siddle K (2009) The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death Differ 16: 782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BMT, Coffer PJ (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376: 599–602 [DOI] [PubMed] [Google Scholar]
- Cao XM, Guy GR, Sukhatme VP, Tan YH (1992) Regulation of the Egr-1 gene by tumor necrosis factor and interferons in primary human fibroblasts. J Biol Chem 267: 1345–1349 [PubMed] [Google Scholar]
- Cavaghan MK, Ehrmann DA, Polonsky KS (2000) Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest 106: 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TM, Exton JH (1976) A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes* 1. Anal Biochem 71: 96–105 [DOI] [PubMed] [Google Scholar]
- Chavrier P, Zerial M, Lemaire P, Almendral J, Bravo R, Charnay P (1988) A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J 7: 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544. [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Eberl D, Suzuki R, Kahn CR (2008) Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc Natl Acad Sci U S A 105: 3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esma I, Maxenence F, Goran K, Emina S, Jelena V, Branislava D, Pierre M (2008) Insulin regulation of proliferation involves activation of AKT and ERK 1/2 signaling pathways in vascular smooth muscle cells. Exp Clin Endocrinol Diabetes 16: P652. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52: 1–8 [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736 [DOI] [PubMed] [Google Scholar]
- Frayn KN (2007) Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc 60: 375–380 [DOI] [PubMed] [Google Scholar]
- Fujishiro M, Gotoh Y, Katagiri H, Sakoda H, Ogihara T, Anai M, Onishi Y, Ono H, Abe M, Shojima N (2003) Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3-L1 adipocytes. Mol Endocrinol 17: 487. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307: 426. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H (2005) Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2: 119–129 [DOI] [PubMed] [Google Scholar]
- Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC (2000) The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care 23: 1108. [DOI] [PubMed] [Google Scholar]
- Gashler A, Sukhatme VP (1995) Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50: 191–224 [DOI] [PubMed] [Google Scholar]
- Gavin JR, Roth J, Neville DM, De Meyts P, Buell DN (1974) Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A 71: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio F, Ambrosi F, Cristallini S, Filipponi P, Santeusanio F (1996) Effects of glimepiride on insulin and glucagon release from isolated rat pancreas at different glucose concentrations. Acta Diabetol 33: 25–29 [DOI] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin K, Hotamisligil GS (2002) A central role for JNK in obesity and insulin resistance. Nature 420: 333–336 [DOI] [PubMed] [Google Scholar]
- Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, Spiegelman BM (1994) Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology 134: 264. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860–867 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM (1994) Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A 91: 4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87. [DOI] [PubMed] [Google Scholar]
- Hug C, Lodish HF (2005) Visfatin: a new adipokine. Science 307: 366–367 [DOI] [PubMed] [Google Scholar]
- Isenovic ER, Kedees MH, Tepavcevic S, Milosavljevic T, Koricanac G, Trpkovic A, Marche P (2009) Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovasc Haematol Disord Drug Targets 9: 172–180 [DOI] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferr P, Foufelle F (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119: 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka T, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M (2004) Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 10: 1128–1132 [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548. [DOI] [PubMed] [Google Scholar]
- Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H (2005) Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol 25: 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Ning W, Matthay MA, Feghali-Bostwick CA, Choi AMK (2007) MAPK pathway mediates EGR-1-HSP70-dependent cigarette smoke-induced chemokine production. Am J Physiol Lung Cell Mol Physiol 292: L1297. [DOI] [PubMed] [Google Scholar]
- Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G (2009) Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature 457: 1146–1149 [DOI] [PubMed] [Google Scholar]
- Manning AM, Davis RJ (2003) Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2: 554–565 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K (1994) Pathophysiology and pathogenesis of visceral fat obesity. Diabetes Res Clin Pract 24: S111–S116 [DOI] [PubMed] [Google Scholar]
- Miller WL (2002) Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol 198: 7–14 [DOI] [PubMed] [Google Scholar]
- Moyers JS, Bilan PJ, Reynet C, Kahn CR (1996) Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem 271: 23111. [DOI] [PubMed] [Google Scholar]
- Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z (2004) Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A 101: 14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790 [DOI] [PubMed] [Google Scholar]
- Olivier LM, Kovacs W, Masuda K, Keller GA, Krisans SK (2000) Identification of peroxisomal targeting signals in cholesterol biosynthetic enzymes: AA-CoA thiolase, HMG-CoA synthase, MPPD, and FPP synthase. J Lipid Res 41: 1921. [PubMed] [Google Scholar]
- Polonsky KS, Sturis J, Bell GI (1996) Non-insulin-dependent diabetes mellitus—a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med 334: 777. [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr, Meigs JB, Lipinska I, Kathiresan S (2007) Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ (2008) A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322: 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad F, Pandey M, Bell PA, Loskutoff DJ (2000) Insulin continues to induce plasminogen activator inhibitor 1 gene expression in insulin-resistant mice and adipocytes. Mol Med 6: 680. [PMC free article] [PubMed] [Google Scholar]
- Samuelsson AM, Bollano E, Mobini R, Larsson BM, Omerovic E, Fu M, Waagstein F, Holmang A (2006) Hyperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am J Physiol Heart Circ Physiol 291: H787. [DOI] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ (2003a) Expression profiling identifies genes that continue to respond to insulin in adipocytes made insulin-resistant by treatment with tumor necrosis factor-a*. J Biol Chem 278: 52298–52306 [DOI] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ (2003b) Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100: 7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Gong T, Wang JD, Meng FL, Qiao L, Yang RL, Xue B, Pan FY, Zhou XJ, Chen HQ, Ning W, Li CJ (2011a) Cigarette smoke-induced pulmonary inflammatory responses are mediated by Egr-1/GGPPS/MAPK signaling. Am J Pathol 178: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Yu X, Pan FY, Gao X, Xue B, Li CJ (2011b) An early response transcription factor, Egr-1, enhances insulin resistance in type 2 diabetes with chronic hyperinsulinism. J Biol Chem 286: 14508–14515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SW, Min BW, Lim Y, Lee YH, Shin SY (2008) Regulatory mechanism of TNF [alpha] autoregulation in HaCaT cells: the role of the transcription factor EGR-1. Biochem Biophys Res Commun 374: 777–782 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Lazar MA (2002) Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 13: 18–23 [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF (1991) Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352: 73–77 [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-∣Á function. Nature 389: 610–614 [DOI] [PubMed] [Google Scholar]
- Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, de Belle I (2001) The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol 3: 1124–1128 [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A (2006) Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab 3: 223–229 [DOI] [PubMed] [Google Scholar]
- Yan SF, Pinsky DJ, Mackman N, Stern DM (2000) Egr-1: is it always immediate and early? J Clin Invest 105: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.