Abstract
Background
Age-related macular degeneration remains the leading cause of irreversible blindness in the United States and the developed world. Intravitreal injections of anti–vascular endothelial growth factor (VEGF) medications have become standard of care for the treatment of the wet form of the disease. Recent reports have demonstrated an association with various immune factors. We aimed to investigate the effect of immunosuppressive therapy in the clinical course of the wet form of the disease. We compared anti-VEGF therapy plus one of three systemic immunosuppressive therapies versus anti-VEGF therapy alone for recurrent choroidal neovascularization associated with age-related macular degeneration.
Methods
This was a pilot, Phase I/II, prospective, randomized, unmasked, single-center trial. Patients with subretinal exudation secondary to recurrent choroidal neovascularization associated with age-related macular degeneration were included in the study. Patients were randomized to 1 of 3 systemic arms immunosuppressive agents (daclizumab, rapamycin, or infliximab) for 6 months plus intraocular anti-VEGF therapy if indicated, compared with a group who received only anti-VEGF therapy if indicated.
Results
The number of anti-VEGF injections per group, visual acuity, retinal thickness, and safety measures were assessed in all groups. Thirteen patients were randomized; comparing anti-VEGF injections before and during the study, a decrease in the number of injections from 0.73 injections per month to 0.42 for daclizumab and from 0.67 to 0.34 for sirolimus was seen, while no apparent decrease was seen for either infliximab or observation. Visual acuities were maintained in all groups.
Conclusion
These preliminary data suggest that some immunosuppressive agents given systemically can alter the clinical course of the wet form of the disease and support the notion that more definitive clinical trials of immune mediation of age-related macular degeneration are indicated.
Keywords: age-related macular degeneration, immunosuppression, T cell, choroidal neovascularization
Age-related macular degeneration (AMD) remains the leading cause of irreversible blindness in the United States and the developed world.1 It has been calculated that, with the aging population, the prevalence of the disease will reach epidemic proportions, with an estimated 3 million citizens of the United States having the advanced form of the disease by 2020.1 Genetic association studies have suggested a relationship with several factors controlling immune responses, such as complement factor H and HTRA1, as well as C3 and C5.2 Direct immune dysregulation has also been reported.3,4We report here a randomized pilot study comparing three immunosuppressive agents and standard of care in the treatment of advanced AMD with choroidal neovascularization (CNV).
Methods and Materials
The study was conducted at the Clinical Center, National Institutes of Health under an investigational new drug application (IND#6705) and a clinical research protocol (05-EI-0208). The study protocol was reviewed and approved by the Institutional Review Board of the National Institutes of Health, and all procedures conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. While not falling directly under its regulations, the National Institutes of Health intramural program’s Clinical Center is Health Insurance Portability and Accountability Act compatible. The study was registered at http://www.Clinicaltrials.gov. The study was a pilot, Phase I/II, prospective, randomized, unmasked, single-center trial that consisted of 3 systemic adjunctive immunologic treatments and a control group with 6-month follow-up: rapamycin, daclizumab, infliximab, and observation. Randomization was performed by the National Institutes of Health pharmacy. All treatment arms (including observation) received standard care, which included intravitreal anti–vascular endothelial growth factor (VEGF) injections at the treating physicians’ discretion.
Participants who were 55 years or older with CNV associated with AMD were eligible. Eligibility further required that they have recurrent exudation from neovascular AMD requiring intravitreal injections of an anti-VEGF agent. If both eyes had CNV requiring treatment, the referring retinal physician chose the eye requiring the anti-VEGF intravitreal injection as the study eye.
Injections of either bevacizumab (Avastin 1.25 mg/0.05 mL or 2.5 mg/0.1 mL) or ranibizumab (Lucentis 0.5 mg) were given if there was recurrence of intraretinal or subretinal fluid as seen on Stratus ocular coherence tomography (OCT) (Carl Zeiss Meditec, Dublin, CA). Patients were required to have received intravitreal antiangiogenic therapy because of exudative changes secondary to the wet type of AMD within 7 days of entry into this study. All patients demonstrated the presence of drusen larger than 125 µm; had vision in the study eye between 20/20 and 20/400, had either classic or occult CNV seen on fluorescein angiography, did not have an inflammatory or other diseases to explain the presence of the CNV, did not have a history of cancer in the past 5 years, and had no evidence of active tuberculosis or cardiac insufficiency. All potential participants of the study were evaluated by an Internal Medicine team with a physical examination, chest X-ray, tuberculosis testing, and review of systems.
The primary objective of this pilot study was to detect evidence of activity in one or several of the treatment arms, and therefore, sample size calculations were not performed. The primary outcome was the number of antiangiogenic injections given during the 6-month period of the study. During the study period, patients were seen at least monthly by their treating retinal consultant and received antiangiogenic therapy deemed necessary to treat their AMD regardless of the treatment arm they were assigned. Criterion for reinjection was detection of any intraretinal or subretinal fluid on OCT. Thus, all patients were evaluated on identical therapeutic intervention criteria. The number of injections in the study eye throughout the study was compared among the study arms. In addition, the number of injections in the study eye during the study was also compared with “prestudy” injections in the same eye. Prestudy injection rates were calculated by total injection number divided by months over which repeated injections were given for each participant. The in-study injection rate was calculated by injection number divided by the study duration (6 months). Only study eye injections were calculated (both for prestudy and in-study).
Study Medications Used
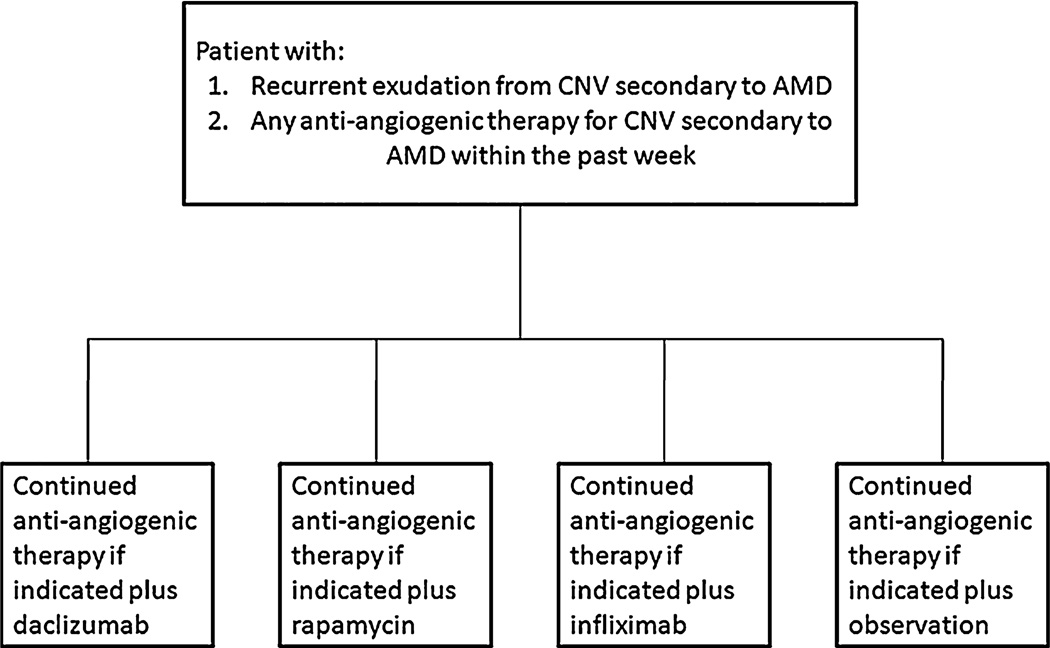
Patients were randomized (1:1:1:1) to 1 of the 3 systemic immunosuppressive agents (intravenous daclizumab, intravenous infliximab, or oral rapamycin tablets) or observation (Figure 1). Patients randomly assigned to daclizumab received 8 mg/kg of daclizumab intravenously at time zero, then 4 mg/kg at Week 2, and then 2 mg/kg (intravenously) monthly for the rest of the 6-month study. Patients randomly assigned to infliximab received 3 mg/kg of intravenous infliximab monthly for 6 months, and patients randomly assigned to rapamycin received 2 mg in capsule form every other day for 6 months.
Fig. 1.
Randomization scheme.
Optical Coherence Tomography
The Stratus OCT (software Version 5.0), a time-domain OCT instrument, was used (Carl Zeiss Meditec). Information on scanning modes and image analyses were obtained from the manufacturer. Scanning with the Stratus OCT was performed using the fast macular thickness map (FastMac, Carl Zeiss Meditec, Dublin, CA) protocol, which acquires 6 evenly distributed 6-mm radial lines, consisting of 128 A-scans per line, intersecting at the fovea (total of 768 sampled points) within a scan time of 1.9 seconds.
Cell Surface Marker Staining
Whole blood samples from patients were collected immediately before starting the protocol and at different time points after the beginning of the study. A 4-color whole-blood flow cytometry staining protocol was used to analyze surface expression levels of CD3, CD4, or CD8 on T cells, CD22 on B cells, and CD56 on natural killer (NK) cells, as well as CD11b and CD11c on monocytes. Surface expression of chemokine/chemokine receptors (CX3CR1, CXCR3, CCR4, CCR5, and CCR7) and interleukin (IL)-2Rα (CD25) and IL-2Rβ (CD122) were also examined. Data were analyzed using the FlowJo software (TriStar, San Jose, CA). The lymphocytes and monocytes were gated based on forward scatter plot versus side scatter plot. The expression levels of chemokine/chemokine receptors and IL-2 receptor subunits on T cells, B cells, NK, and monocytes and subsets of T and NK cells were analyzed based on gating on positive staining for anti-CD3, anti-CD8, and anti-CD56. All antibodies were obtained from BD Biosciences. The data obtained from a FACSCaliber (BD Biosciences, San Jose, CA) were analyzed using FlowJo software (TreeStar). Natural killer “bright” cells (CD56brightCD3−) were noted in a previous publication5 to be elevated in patients with uveitis receiving daclizumab, and thus, NK cells were gated based on CD56 and CD3 staining. CD56 bright cells were gated on CD56bright CX3CR1 negative/CD3 negative or CD56bright CXCR3positive CD3 negative. The results are represented as the percentage of CD56bright subset in the CD56-positive CD3-negative NK subpopulation. The percentages of the positive cells from all patients at different time points during the course of the treatment were compared for changes of expression levels.
Results
A total of 13 patients met the criteria for the study and were enrolled from September 2006 to May 2008 (Table 1). There were 12 women and 1 man. The mean age of the participants was 80 years (60–92 years). The mean time to begin anti-VEGF injections before entering the study was similar in all groups: 16 months for daclizumab, 14 months for rapamycin, 13 months for infliximab, and 16 months for the observation group. Additional medications are listed in Table 1. Of the 13 patients enrolled, 12 completed the 6-month follow-up. One patient withdrew from the study before 6 months (Patient 7 completed 3.5 months). This patient withdrew for nonstudy reasons; the patient fell during the follow-up period resulting in several fractures and the inability to continue study treatment and follow-up.
Table 1.
Demographic, Randomization, Therapeutic, and Cholesterol Information
| Patient Number |
Age, Years |
Ethnicity | Gender | Randomization | Cholesterol | Additional Medications | |
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | ||||||
| 1 | 86 | White | Female | Sirolimus | 117 | 144 | Plavix, omega 3 fatty acids, MVI, lutein glucosamine-chondroitin complex, atenolol, Zocor, calcium carbonate, triamterene with HCTZ |
| 2 | 85 | White | Female | Infliximab | 267 | 275 | Synthroid, Prilosec, lutein, Glycolax, vitamin B12, acetaminophen 2 |
| 3 | 82 | Hispanic | Female | Daclizumab | 130 | 193 | Levothyroxine, metformin, Byetta, Norvasc, folic acid, Lipitor, lidocaine 5% patch |
| 4 | 85 | White | Female | Observation | 227 | 203 | Actonel, Allegra, aspirin, Ocuvite, MVI, calcium with vitamin D, Patanol ophthalmic solution |
| 5 | 72 | White | Female | Observation | 211 | 238 | Lovastatin, levothyroxine, Lopressor, colchicine, Ocuflox |
| 6 | 78 | White | Female | Infliximab | 244 | 242 | Calcium, vitamin D, cranberry extract, vitamin C, vitamin E, omega 3 fatty acid, glucosamine-chondroitin complex, Ocuvite, Preservision, L-tyrosine |
| 7 | 92 | White | Female | Sirolimus | 215 | N/A | Fosamax, Norvasc, Klor-Con, Ocuvite, chlorthalidone, lisinopril |
| 8 | 60 | White | Female | Daclizumab | 211 | N/A | Prozac, Cartia XT, Voltaren, Diovan, Toprol XL |
| 9 | 85 | White | Female | Daclizumab | 168 | 117 | Evista, Celebrex, Centrum Silver, Norvasc, lisinopril, lovastatin, atenolol, Microzide, Calcium |
| 10 | 69 | White | Male | Infliximab | 116 | 120 | Neurontin, verapamil, HCTZ, Cozaar, Glucotrol, Glucophage, Vytorin, Ocuvite, vitamin B12, calcium, MVI |
| 11 | 77 | White | Female | Observation | 266 | 247 | Nexium, Synthroid, glucosamine-chondroitin sulfate, metronidazole 1% gel, lutein, calcium, retinavites, MVI |
| 12 | 83 | White | Female | Sirolimus | 186 | 261 | Synthroid, Prilosec, aspirin, vitamin D, omega 3 fatty acids, Citracal Plus, lidocaine 5% patch, vitamin B12, Claritin, AREDS soft gel gaps |
| 13 | 90 | White | Female | Daclizumab | 180 | 164 | Levothyroxine, aspirin, Didronel, calcium Centrum Silver, retinavites |
HCTZ, hydrochlorothiazide; MVI, multivitamins.
Table 2 shows the anti-VEGF injection history per patient. Patients are grouped by the study arm to which they were assigned. The prestudy and in-study injection rates are provided for each patient with the mean and median for each group. The lesion types varied among groups, but occult lesions slightly predominated (Table 2). All patients in the daclizumab group were classified as having occult CNV; in the rapamycin group, one third were classified as occult and two thirds as classic; and in the infliximab group, there were a mixed group of membranes. Monthly rates of anti-VEGF injections were 0.42 and 0.34 for daclizumab and rapamycin, respectively, compared with 0.83 for both the control group and the infliximab group during the 6-month study period. Injection rates during the study compared with prestudy rates showed a decrease in rapamycin and daclizumab arms from a median of 0.67 injections per month (prestudy) to a median of 0.34 and from 0.73 to 0.42, respectively; no decrease was seen for either infliximab or observation (Table 2).
Table 2.
Anti-VEGF Injection History Per Patient During the Study
| Patient | Rx Arm | Study Eye |
Prestudy | In-Study | Prestudy Median/Mean |
Study Median/Mean |
Lesion Type |
|---|---|---|---|---|---|---|---|
| 3 | Daclizumab | OS | 0.6 | 0.34 | 0.725 | 0.420 | Occult |
| 9 | Daclizumab | OD | 0.74 | 0.34 | 0.805 | 0.503 | Occult |
| 13 | Daclizumab | OS | 1.17 | 0.83 | — | — | Occult |
| 8 | Daclizumab | OD | 0.71 | 0.5 | — | — | Occult |
| 2 | Infliximab | OD | 0.67 | 0.5 | 0.700 | 0.830 | Min classic |
| 6 | Infliximab | OS | 0.93 | 0.83 | 0.767 | 0.777 | Occult |
| 10 | Infliximab | OS | 0.7 | 1 | — | — | Classic |
| 4 | Observation | OS | 0.5 | 0.83 | 0.670 | 0.830 | Occult |
| 5 | Observation | OD | 0.75 | 0.5 | 0.640 | 0.777 | Min classic |
| 11 | Observation | OD | 0.67 | 1 | — | — | Classic |
| 1 | Rapamycin | OS | 1 | 0.5 | 0.67 | 0.34 | Occult |
| 7 | Rapamycin | OD | 0.44 | 0.29 | 0.703 | 0.377 | Classic |
| 12 | Rapamycin | OD | 0.67 | 0.34 | — | — | Classic |
Rx, therapeutic arm; OD, right eye; OS, left eye; Min, minimally.
Table 3 shows the visual acuities and OCT measurements for both the study eyes and the fellow eyes. Study eyes in all groups maintained approximately stable visual acuities over the 6-month follow-up period; OCTs performed in all the treatment groups at the end of the study were also similar to those obtained at baseline.
Table 3.
Visual Acuities and OCT Measurements for the Study Eyes
| Patient Number |
Baseline Vision |
Sixth-Month Vision |
Baseline Vision |
Sixth Month Vision |
Baseline OCT |
Sixth-Month OCT |
Baseline OCT |
Sixth-Month OCT |
|---|---|---|---|---|---|---|---|---|
| Study Eye | Study Eye | Fellow Eye | Fellow Eye | Study Eye | Study Eye | Fellow Eye | Fellow Eye | |
| Rapamycin | ||||||||
| 1 | 20/40 | 20/25 | 20/400 | 20/640 | 234* | 230 | 211 | 203 |
| 7† | 20/63 | 20/50 | 20/640 | 20/640 | 361 | 239 | 242 | 186 |
| 12 | 20/32 | 20/32 | 20/32 | 20/32 | 327 | 264 | 226 | 205 |
| Daclizumab | ||||||||
| 3 | 20/40 | 20/40 | 20/40 | 20/32 | 216 | 189 | 352 | 206 |
| 8 | 20/32 | 20/32 | 20/32 | 20/32 | 185 | 208 | 218 | 149 |
| 9 | 20/250 | 20/250 | 20/63 | 20/40 | 297 | 196 | 215 | 210 |
| 13 | 20/50 | 20/40 | 20/25 | 20/20 | 329 | 344 | 305 | 317 |
| Infliximab | ||||||||
| 2 | 20/40 | 20/32 | 20/63 | 20/125 | 205 | 177 | 190 | 181 |
| 6 | 20/250 | 20/800 | 20/63 | 20/80 | 290 | 243 | 262 | 252 |
| 10 | 20/50 | 20/32 | 20/125 | 20/125 | 291 | 288 | 453 | 347 |
| Observation | ||||||||
| 4 | 20/63 | 20/63 | 20/40 | 20/25 | 229 | 196 | 189 | 171 |
| 5 | 20/50 | 20/25 | CF | CF | 426 | 225 | 270 | 213 |
| 11 | 20/32 | 20/50 | 20/200 | 20/63 | 318 | 305 | 158 | 169 |
Microns;
measurements performed at 3.5 months.
CF, counting fingers.
Table 4 shows the Grade 2 or 3 adverse events that occurred during the study. None of the adverse events required cessation of the study medication. Rapamycin has been reported to be associated with an increase in serum cholesterol levels. However, no clinically important changes in serum cholesterol levels between baseline and 6 months were seen, and no patient required any change in cholesterol-lowering medication during the study period. Participant 7 fell during the follow-up period resulting in several fractures and the inability to continue study follow.
Table 4.
List of Grade 2 or 3 Adverse Events that Occurred During the Study
| Participant Number |
Category | Description | Intervention Required |
Inpatient Care |
Outcome | Effect on Therapy |
Related to Agent |
Severity |
|---|---|---|---|---|---|---|---|---|
| 3 | Neurology | Episode of right-sided numbness | Other | No | Recovered, no residual effects | Held | Possibly | Severe/Grade 3 |
| 7 | Cardiovascular | Irregular heart rate | Other | Yes | Recovered, with sequelae | Discontinued | Possibly | Severe/Grade 3 |
| Metabolic | Electrolyte imbalance | Other | Yes | Recovered, no residual effects | Discontinued | Possibly | Severe/Grade 3 | |
| Trauma | Fall | Monitoring | Yes | Recovered, with sequelae | Discontinued | Possibly | Severe/Grade 3 | |
| 8 | Neurology | Sciatica to left leg and hip/back | Rx med | No | Persistent condition not expected to resolve | None | Not related | Moderate/Grade 2 |
| GI | S/P colonoscopy diagnosed with colitis | Rx med | No | Persistent condition | Held | Remotely | Moderate/Grade 2 | |
| 10 | Dermatology | Rash arms bilateral | Rx med | No | Recovered, no residual effects | None | Not related | Moderate/Grade 2 |
| 12 | Pulmonary | Bronchitis | Rx med | No | Recovered, no residual effects | None | Possibly | Moderate/Grade 2 |
| Other | Elevated lipids | Rx med | No | Recovered, no residual effects | None | Possibly | Moderate/Grade 2 | |
| Musculoskeletal | Pain in back between shoulder blades | Rx med | No | Recovered, no residual effects | Held | Possibly | Moderate/Grade 2 | |
| Musculoskeletal | Polymyalgia rheumatica | Rx med | No | Recovered, no residual effects | None | Not related | Moderate/Grade 2 | |
| Pulmonary | Dry persistent cough | Rx med | No | Recovered, no residual effects | None | Not related | Moderate/Grade 2 |
Rx med, treated with medication.
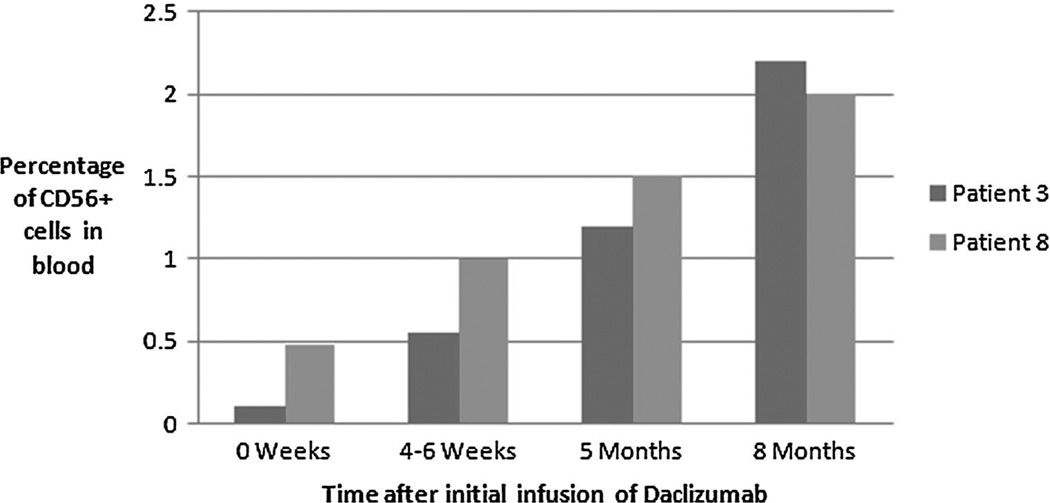
We monitored for possible immune alterations in the peripheral blood of study patients. When compared with age-matched controls, no differences in T-cell and monocyte subgroups were noted in any of the patients tested. Natural killer populations were monitored for in most patients as well, including three of the four patients of the daclizumab group. In those 3 patients, an increase in the CD56bright NK cells was noted in the 2 tested at 8 months after the initiation of daclizumab therapy (Figure 2). This NK cell subgroup increase has been seen in patients with uveitis receiving this medication.5
Fig. 2.
Time after initial infusion of Daclizumab.
Discussion
This randomized pilot study evaluated the use of 3 systemic immunosuppressive agents, which predominantly target T cells in the treatment of recurrent CNV secondary to AMD. We noted that over the 6-month period of the study, 2 medications, daclizumab and rapamycin, appeared to decrease the need for anti-VEGF intravitreal injections by approximately half, compared with the observation or the infliximab group. These observations suggest that treating a major component of the acquired immune system may result in a positive clinical outcome for patients with the exudative type of AMD.
Choroidal neovascularization can be immunologically driven and treated with immunosuppressive therapy.6,7 It is further known that proinflammatory cytokines are angiogenic8 and are seen in other disorders manifesting as retinal neovascularization or CNV, such as diabetes and uveitis. Age-related macular degeneration has been often described as a degenerative disorder. However, the notion that AMD is significantly mediated by immune mechanisms has been suggested for more than 20 years.9 Autoantibodies to retinal elements4,10,11 and histopathologic reports of monocyte invasion into the posterior segment in early stages of the disease have been reported in AMD.9,12,13 Anderson et al,14 in reviewing AMD eyes, noted the deposition of parts of the complement cascade in and around drusen, further supporting the concept of immune activation as part of the pathogenesis of the disease. Recently, several single-nucleotide polymorphisms have been associated with AMD, including complement factor H,2 HTRA1,15 age-related maculopathy susceptibility protein 2 (ARMS2),16 IL-817, and pleckstrin homology domain-containing family A (phosphoinositide binding specific) member 1 (PLEKHA1)18 Some of these products have been localized to drusen and the surrounding tissues, but the functional importance of these variants is yet to be elucidated. However, the multiple variant associations would suggest an ocular immune environment dysregulation and not an alteration limited to a single disease.19
Animal models have been reported to mimic certain parts of the human clinical presentation of AMD.20 Most models rely on some aspect of immune dysregulation as a result of knockout technology. However, one model, reported by Hollyfield et al21 showed that immunization with adducted carboxyethylpyrrole, which is found in drusen, will induce an AMD-like disease in older mice. Further, AMD patients have been noted to have antibodies directed against this protein.
Several anecdotal reports have reported a positive therapeutic response with infliximab, both given systemically and intraocularly in the treatment of AMD.22,23We have used rapamycin to effectively treat CNV secondary to punctate inner choroidopathy with sustained clinical control of the CNV.7 Rapamycin has been shown to be effective as well in preventing neovascularization in two models of posterior segment new vessel growth.6 We have used daclizumab extensively in the treatment of uveitis.24
The mechanisms of two medications used in this study predominantly affect T-cell function (particularly activation), while infliximab’s action may be broader, possibly affecting innate and acquired immunity and effector function. Daclizumab, a humanized antibody directed against the alpha portion of the IL-2 (CD25) receptor, is found highly expressed in activated T cells.25 It is a recombinant monoclonal immunoglobulin of the human IgG1 isotype, which is a composite of human (90%) and murine (10%) antibody sequences. The IL-2 receptor system is a well-characterized lymphokine receptor system that plays a central role in the induction of immune responses.
Rapamycin is a macrocyclic lactone produced by Streptomyces hygroscopicus that inhibits T-lymphocyte activation and proliferation in response to both antigenic and cytokine (IL-1 IL-2, IL-4, and IL-15) stimulation by a mechanism that is distinct from that of other immunosuppressants.26 Rapamycin also inhibits antibody production. In cells, rapamycin binds to the immunophilin, FK binding protein-12, to generate an immunosuppressive complex that binds to and inhibits the activation of the mammalian target of rapamycin, a key regulatory kinase. This inhibition suppresses cytokine-driven T-cell proliferation. In the ocular system, sirolimus has been shown to inhibit experimental autoimmune uveoretinitis in the rat.27 Rapamycin, in addition to its potent immunosuppressive effects, has been noted to have antitumor and antiangiogenic properties. Dejneka et al6 evaluated systemically administered rapamycin in a laser-induced CNV model in mice and in a second model inducing retinal neovascularization by hyperoxia/hypoxia.
Infliximab is a chimeric human/murine monoclonal antibody of IgG1 isotype with specificity for human tumor necrosis factor (TNF). It neutralizes the biologic activity of TNF-alpha by binding to the soluble and transmembrane forms of TNF-alpha and inhibits binding of TNF-alpha to its receptors.28 Infliximab specifically inhibits the activity of TNF-alpha through neutralization of the cytokine. Interestingly, our observations over a 6-month period did not support the notion that infliximab may be useful as an immunosuppressive agent.
It may be that AMD belongs to the group of disorders, such as atherosclerosis and Alzheimer’s disease, that are now believed to be immune mediated; therefore, immunosuppressive therapy would be a reasonable approach to this disorder. It may be that these disorders will share common underlying mechanisms. The multiple genetic variant associations may be reflective of the complicated nature of the downregulatory immune environment of the eye rather than AMD itself. We have hypothesized that any alteration in this downregulatory environment puts one at risk for the development of disorders with the choriocapillaris/retinal pigment epithelium/outer retinal interface.19,29 If this concept can be affirmed, immunosuppressive therapy would then be indicated for both forms of advanced AMD and perhaps even for eyes at particularly high risk for developing advanced AMD. This study is a proof of concept and cannot be considered as a long-term solution to immunotherapy of AMD patients. The challenge will be drug delivery, whether local or systemic, that could be administered for extended periods without important side effects in an elderly population, to prevent the alterations that are the result of chronic inflammatory disease.
This preliminary study has not demonstrated a definitive beneficial effect of immunosuppressants for AMD. While the 95% confidence intervals can give an estimate of the variability of the prestudy injection rate (not shown), none of individuals or groups had a statistically significant difference between the prestudy rate and the study rate, although for the daclizumab and rapamycin groups, the decrease in rate is notable. Using a sign test to see whether the observed number of decreases (i.e., any decrease) in injection rate is greater than expected if only chance were operating (i.e., the probability of a decrease is 0.5), the only group that approaches statistical significance is the daclizumab group, where the probability of observing improvement in 4 of 4 patients, given that the true probability for a single patient is 0.5, is 0.062, borderline statistically significant. For the rapamycin group, the probability is 0.125, and for the other 2 groups, the P value is even farther from statistical significance. While still very small numbers, the results do support further assessment of possible immunologic intervention in patients at risk for vision loss from AMD.
Acknowledgment
We thank Dr Susan Vitale of the NEI for her exceptional help with the statistical evaluations.
Supported by intramural funds of the National Eye Institute, National Institutes of Health, Bethesda, Maryland.
Footnotes
No authors have any financial/conflicting interests to disclose.
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 4.Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–274. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 6.Dejneka N, Kuroki A, Fosnot J, Tang W, Tolentino M, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;10:964–972. [PubMed] [Google Scholar]
- 7.Nussenblatt RB, Coleman H, Jirawuthiworavong G, et al. The treatment of multifocal choroiditis associated choroidal neovascularization with sirolimus (rapamycin) Acta Ophthalmol Scand. 2007;85:230–231. doi: 10.1111/j.1600-0420.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 8.Roh MI, Kim HS, Song JH, Lim JB, Koh HJ, Kwon OW. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with AMD after bevacizumab treatment. Retina. 2009;29:523–529. doi: 10.1097/IAE.0b013e318195cb15. [DOI] [PubMed] [Google Scholar]
- 9.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu J, Pauer GJ, Yue X, et al. Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol Cell Proteomics. 2009;8:1338–1349. doi: 10.1074/mcp.M800453-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4:613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 13.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 16.Kanda A, Chen W, Othman M, et al. Avariant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goverdhan SV, Ennis S, Hannan SR, et al. Interleukin-8 promoter polymorphism −251A/T is a risk factor for age-related macular degeneration. Br J Ophthalmol. 2008;92:537–540. doi: 10.1136/bjo.2007.123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leveziel N, Souied EH, Richard F, et al. PLEKHA1-LOC387715-HTRA1 polymorphisms and exudative age-related macular degeneration in the French population. Mol Vis. 2007;13:2153–2159. [PubMed] [Google Scholar]
- 19.Nussenblatt RB, Ferris F., III Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–540. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 23.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol. 2009;147:825–830. doi: 10.1016/j.ajo.2008.12.004. 30 e1. [DOI] [PubMed] [Google Scholar]
- 24.Nussenblatt RB, Thompson DJ, Li Z, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21:283–293. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 25.Mottershead M, Neuberger J. Daclizumab. Expert Opin Biol Ther. 2007;7:1583–1596. doi: 10.1517/14712598.7.10.1583. [DOI] [PubMed] [Google Scholar]
- 26.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:S7–S14. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 27.Martin DF, DeBarge LR, Nussenblatt RB, Chan CC, Roberge FG. Synergistic effect of rapamycin and cyclosporin A in the treatment of experimental autoimmune uveoretinitis. J Immunol. 1995;154:922–927. [PubMed] [Google Scholar]
- 28.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine. 1995;7:251–259. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 29.Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: an immunologically driven disease. Curr Opin Investig Drugs. 2009;10:434–442. [PubMed] [Google Scholar]