Abstract
Alzheimer’s disease (AD), the leading cause of dementia worldwide, is characterized by the accumulation of the β-amyloid peptide (Aβ) within the brain along with hyperphosphorylated and cleaved forms of the microtubule-associated protein tau. Genetic, biochemical, and behavioral research suggest that physiologic generation of the neurotoxic Aβ peptide from sequential amyloid precursor protein (APP) proteolysis is the crucial step in the development of AD. APP is a single-pass transmembrane protein expressed at high levels in the brain and metabolized in a rapid and highly complex fashion by a series of sequential proteases, including the intramembranous γ-secretase complex, which also process other key regulatory molecules. Why Aβ accumulates in the brains of elderly individuals is unclear but could relate to changes in APP metabolism or Aβ elimination. Lessons learned from biochemical and genetic studies of APP processing will be crucial to the development of therapeutic targets to treat AD.
Keywords: Neurodegeneration, dementia, BACE1, α-secretase, γ-secretase, aging
HISTORY OF ALZHEIMER’S DISEASE
In 1907, Alois Alzheimer reported the results of an autopsy on a 55-year-old woman named Auguste Deter, who had died from a progressive behavioral and cognitive disorder. Alzheimer noted the presence of two distinctive pathologies in Deter’s brain: neurofibrillary tangles, which he correctly surmised were abnormal intracellular aggregates (and which were later shown to be composed of hyperphosphorylated and cleaved forms of the microtubule-associated protein tau), and neuritic plaques (which he called miliary foci), which were dystrophic neuronal processes surrounding a “special substance in the cortex” (Alzheimer et al. 1995). This “special substance” was isolated and purified in 1984 by Glenner & Wong (1984), who showed that it was a 4.2-kDa peptide, primarily 40 or 42 amino acids in length, which they speculated was cleaved from a larger precursor. Their prediction was verified in short order when the amyloid precursor protein (APP) was cloned in 1987 (Kang et al. 1987). The peptide isolated by Glenner & Wong has come to be known as the Aβ peptide, short for amyloid-β peptide.
Alzheimer was convinced that the case of Auguste Deter represented an unusual cause of dementia. It was not until the seminal work of Blessed, Tomlinson, and Roth (Blessed et al. 1968) that a relationship between the amount of neuritic Aβ plaques in the brains of elderly subjects and the risk of dementia was established. Alzheimer’s disease (AD) is now recognized as a common dementing disorder of the elderly (see sidebar, Alzheimer’s Disease: What’s in a Name) with characteristic pathological findings (Figure 1). Young onset, frequently genetic, forms of the disease are a rare but important subset. AD currently afflicts 26 million people worldwide with projections of a fourfold increase in that number by 2050 (Brookmeyer et al. 2007).
Figure 1.

Pathology of Alzheimer’s disease. (a, b) Brain sections from a patient with dementia are stained with silver, revealing neuritic plaques in panel a and a neurofibrillary tangle in panel b. The plaques in panel a consist of an amorphous reddish protein (Aβ) with dystrophic neurites (yellow arrows, dark black material ). (c) An Aβ plaque stained with an anti-Aβ antibody (red ) shows infiltrating microglia stained with an IBA1 antibody ( green). Each line is 40 microns.
RELATIONSHIP OF BRAIN Aβ ACCUMULATION TO DEMENTIA IN HUMAN PATHOLOGIC SPECIMENS
Cohorts of subjects who are followed with serial neuropsychological testing during life and then donate their brains to scientific research after death (Dolan et al. 2010a) have become a crucial tool for understanding the determinants of cognitive decline in older subjects. Most studies agree that the classical pathological criteria for AD, neuritic plaques and neurofibrillary tangles, can account for 40%–70% of the variance in cognition seen in elderly subjects, with additional pathologies such as cerebrovascular disease (Dolan et al. 2010b) and Lewy body pathology (Schneider et al. 2007) working together with AD pathology to account for an additional 20%–30% of dementia cases.
It was historically unclear whether the accumulating Aβ plaques in the brains of patients with dementia caused the dementia or simply indicated the presence of dying neurons. Indeed, studies of head trauma and brain ischemia in humans and animals demonstrate transient increases in brain Aβ deposition (Gentleman et al. 1997, Qi et al. 2007). However, studies of chronic brain injury and ischemia in humans do not suggest that Aβ is a nonspecific marker of neuronal injury (Dolan et al. 2010b, McKee et al. 2009), and there is no increase in the age-expected prevalence of Aβ pathology in patients with other neurodegenerative disorders such as Parkinson’s disease and frontotemporal dementia.
STRUCTURE AND FUNCTION OF APP
The amyloid precursor protein (APP) is one member of a family of related proteins that includes the amyloid precursor-like proteins (APLP1 and APLP2) in mammals and the amyloid precursor protein-like (APPL) in Drosophila. All are single-pass transmembrane proteins with large extracellular domains (Figure 2), and all are processed in a manner similar to APP. Only APP generates an amyloidogenic fragment owing to sequence divergence at the internal Aβ site. Alternate splicing of the APP transcript generates 8 isoforms, of which 3 are most common: the 695 amino acid form, which is expressed predominantly in the CNS, and the 751 and 770 amino acid forms, which are more ubiquitously expressed (Bayer et al. 1999).
Figure 2.
Sequential cleavage of the amyloid precursor protein (APP) occurs by two pathways. (a) The APP family of proteins has large, biologically active, N-terminal ectodomains as well as a shorter C-terminus that contains a crucial Tyrosine–Glutamic Acid-Asparagine-Proline-Threonine-Tyrosine (YENPTY) protein-sorting domain to which the adaptor proteins X11 and Fe65 bind. The Aβ peptide starts within the ectodomain and continues into the transmembrane region (red ). (b) Nonamyloidogenic processing of APP involving α-secretase followed by γ-secretase is shown. (c) Amyloidogenic processing of APP involving BACE1 followed by γ-secretase is shown. Both processes generate soluble ectodomains (sAPPα and sAPPβ) and identical intracellular C-terminal fragments (AICD). Figure 2 was adapted from Thinakaran G, Koo EH. 2008 Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283:29615–19
ALZHEIMER’S DISEASE: WHAT’S IN A NAME.
Dementia is a clinical term that refers to the development of progressive cognitive deterioration associated with an inability to perform normal activities of daily living. When dementia afflicts the young, there is usually a single pathologic process present on autopsy such as the neuritic plaques and neurofibrillary tangles, which are indicative of Alzheimer’s disease (AD). In the elderly, however, in whom dementia affects 1 out of 3 individuals, the brain pathology is usually mixed, with most demented individuals having a combination of AD pathology, atherosclerosis, and Lewy body pathology. Moreover, recent work with autopsy cohorts and neuroimaging studies has shown that many cognitively normal elderly subjects carry moderate amounts of AD or cerebrovascular pathology without symptoms as long as they do not have a second comorbid process. Thus, in the elderly, it seems more appropriate to use the term dementia to describe the clinical process and denote “Alzheimer-type pathology” as a risk factor for dementia. In the future, the relative amount of each of these pathologies in a symptomatic or asymptomatic individual will be quantifiable using neuroimaging and cerebrospinal fluid analysis, allowing the design of pathologically relevant clinical trials.
The precise physiological function of APP is not known and remains one of the vexing issues in the field. In most studies, APP overexpression shows a positive effect on cell health and growth. This effect is epitomized in transgenic mice that overexpress wild-type APP and have enlarged neurons (Oh et al. 2009). In transiently transfected cell lines, APP modulates cell growth, motility, neurite outgrowth, and cell survival, functions that can be reproduced by the soluble ectodomain, which is released by cleavage of APP (Figure 2). These observations were extended in vivo by a recent study (Young-Pearse et al. 2007), which found neuronal migration abnormalities in embryonic rodents injected with APP RNAi. In adult animals, intracerebral injections of the APP ectodomain can improve cognitive function and synaptic density (Meziane et al. 1998, Roch et al. 1994). The sites most responsible for the bioactivity of the APP ectodomain appear to be its two heparin-binding domains (Mok et al. 1997). The second heparin-binding domain is also the site of binding F-spondin, the only potential ligand identified for APP (Ho & Sudhof 2004). Identifying a ligand-binding partner for APP is of some importance because APP has been compared with the developmental signaling molecule Notch, which is structurally similar to APP. Notch proteolysis is triggered by binding to a member of the Delta, Serrate, Lag2 (DSL) family of ligands (Louvi & Artavanis-Tsakonas 2006), which causes Notch to be cleaved by many of the same secretases as APP, releasing a soluble ectodomain and an intracellular domain (NICD) that regulates nuclear target genes. Although F-spondin plays a role in neuronal development and repair, evidence demonstrating that APP is crucial to this process is lacking. Other potential N-terminal binding partners for APP include collagen, netrin-1, laminin, the Aβ peptide, and molecules that interact with APP when coexpressed in the same cell, including other members of the APP family and Notch itself (Chen et al. 2006).
Nikolaev et al. (2009) recently reported that the secreted APP ectodomain acts as a ligand for Death Receptor 6 (DR6). In this formulation, growth factor deprivation triggers cleavage of APP by the secretase BACE1, releasing the ectodomain, which then binds to DR6 and activates caspase 6 and caspase 3, causing axonal and cell body apoptotic degeneration, respectively. The growth factor deprivation, which triggers cleavage of APP, could be part of normal axonal pruning or could be a primary factor in neuronal degeneration. This work has raised the intriguing possibility that the APP ectodomain released by BACE1 cleavage (sAPPβ) has different properties than does the APP ectodomain released by α-cleavage (sAPPα), which has an extra 16 amino acids in its C-terminus.
In addition to a physiological role for APP, the Aβ peptide itself plays an important role in synaptic physiology, regulating synaptic scaling (Kamenetz et al. 2003) and synaptic vesicle release (Abramov et al. 2009).
It is disappointing, however, that deletion of APP in mice (and thus Aβ production) produces very little phenotype and does not suggest that a loss of APP or Aβ function is deleterious to the adult animal in any way. Triple knockouts involving APP, APLP1, and APLP2 (Herms et al. 2004) show scattered cortical migration abnormalities. Double knockout mice lacking APP and APLP2 exhibit a mismatch between presynaptic and postsynaptic markers at the neuromuscular junction along with excessive nerve terminal sprouting (Wang et al. 2005). This same phenotype is seen in DR6 knockout mice and in fly APPL deletions (Ashley et al. 2005), suggesting a role for APP and its family members in neuritic outgrowth and synaptic pruning. None of these, however, suggests a role for APP in the mature CNS, in which APP production is known to continue at a very high rate.
The intracellular C-terminus of APP is also important for its function and has been proposed to serve two roles, one as a transcriptional regulator and the other, related to its YENPTY amino acid domain (Figure 2), as a regulator of its own intracellular sorting. The YENPTY domain regulates clathrin-coated pit internalization (Chen et al. 1990) through a series of binding partners (see below). It is 100% conserved in all forms of APP from the fly to the human. Mutation at this site alters endocytosis of APP (Perez et al. 1999) and diminishes Aβ production (Ring et al. 2007). Phosphorylation of Thr 668, 14 amino acids away from the YENPTY domain, by cyclin-dependent kinase 5 interferes with at least some of the protein-protein interactions of the YENPTY domain (Ando et al. 2001), although mutation of this site did not alter brain Aβ accumulation in mice (Sano et al. 2006).
Interaction with the YENPTY domain of APP requires the presence of a phosphotyrosine binding domain on the interacting protein. The two best characterized APP binding partners are X11 and Fe65, which were isolated using the yeast-two-hybrid screen. X11 contains one polypyrimidine-tract binding (PTB) domain as well as two postsynaptic density-DlgA-ZO1 (PDZ) domains (Feng & Zhang 2009), whereas Fe65 has two PTB domains, each with different binding specificities, and a tryptophan repeat domain that interacts with the actin cytoskeleton-associated proteins Mena and Evl (Borg et al. 1996, Lambrechts et al. 2000). Both X11 and Fe65 are highly expressed in brain and interact with all APLPs. Tissue culture studies have suggested that both binding partners couple APP to SorLA/LR11 in the trans-Golgi network (TGN), preventing APP from interacting with BACE1 (Pietrzik et al. 2004, Saito et al. 2008). When heterozygous X11 or Fe65 knockout mice are crossed with APP overexpressing mice, brain Aβ accumulation increases significantly (Saluja et al. 2009); moreover, mice that overexpress X11 or Fe65 have diminished brain Aβ accumulation (Lee et al. 2003, McLoughlin & Miller 2008).
Fe65 has also assumed a unique place in Alzheimer’s research because it is known to bind the transcription factor complex CP2-LSF-LBP-1 and the histone deacetylase Tip60 via the non-APP-binding PTB domain (Cao & Sudhof 2001, Zambrano et al. 1998) and regulate transcription in cultured cell lines. Investigators initially felt that Fe65 acted in concert with the APP C-terminus to regulate transcription. More recent work has emphasized the independent role of Fe65 (Yang et al. 2006), suggesting that full-length APP may serve as a docking station to keep Fe65 out of the nucleus.
AMYLOID PRECURSOR PROTEIN PROCESSING
APP is produced in large quantities in neurons and is metabolized very rapidly (Lee et al. 2008). Multiple alternate pathways exist for APP proteolysis, some of which lead to generation of the Aβ peptide and some of which do not (Figures 2 and 3). After sorting in the endoplasmic reticulum and Golgi, APP is delivered to the axon, where it is transported by fast axonal transport to synaptic terminals (Koo et al. 1990). APP had been reported to function as a receptor for kinesin-1-mediated axonal transport (Kamal et al. 2000), but subsequent work has not confirmed the association of APP and kinesin-1 (Lazarov et al. 2005).
Figure 3.
APP trafficking in neurons. Newly synthesized APP ( purple) is transported from the Golgi down the axon (1) or into a cell body endosomal compartment (2). After insertion into the cell surface, some APP is cleaved by α-secretase (6) generating the sAPPα fragment, which diffuses away ( green), and some is reinternalized into endosomes (3), where Aβ is generated (blue). Following proteolysis, the endosome recycles to the cell surface (4), releasing Aβ(blue) and sAPPβ. Transport from the endosomes to the Golgi prior to APP cleavage can also occur, mediated by retromers (5).
Crucial steps in APP processing occur at the cell surface and in the TGN (Figure 3). From the TGN, APP can be transported to the cell surface or directly to an endosomal compartment. Clathrin-associated vesicles mediate both these steps. On the cell surface, APP can be proteolyzed directly by α-secretase and then γ-secretase, a process that does not generate Aβ, or reinternalized in clathrin-coated pits into another endosomal compartment containing the proteases BACE1 and γ-secretase. The latter results in the production of Aβ, which is then dumped into the extracellular space following vesicle recycling or degraded in lysosomes. Although most APP must pass through the cell surface as part of its processing, this step is very rapid, as little APP is on the surface at any point in time. Why some surface APP is internalized into endosomes and some proteolyzed directly by α-secretase is unclear, although segregation of APP and BACE1 into lipid rafts may be a crucial element (Ehehalt et al. 2003). Finally, to complete the APP cycling loop, retrograde communication occurs between endosomal compartments and the TGN, mediated by a complex of molecules called retromers.
Experiments that have examined this model directly have found that 80% of Aβ release is blocked by preventing surface endocytosis (Koo & Squazzo 1994). Moreover, all the enzymes appear to be in the correct places. FRET analysis indicates that BACE1 interacts with APP predominantly in endosomes under native conditions (Kinoshita et al. 2003), whereas γ-secretase activity is present on the cell surface, where it complements α-secretase activity, and in endosomal compartments, where it complements BACE1 activity (Fukumori et al. 2006, Parvathy et al. 1999).
The enzymes that cleave APP have been extensively characterized. BACE1, a transmembrane aspartic protease, is directly involved in the cleavage of APP at the +1 (prior to amino acid 1) and +11 sites of Aβ. Neurons from BACE1−/− mice do not produce Aβ, confirming that BACE1 is the neuronal β-secretase (Cai et al. 2001). Following BACE1 cleavage and release of the sAPPβ ectodomain, the APP C-terminal fragment is cleaved by the γ-secretase complex at one of several sites varying from +40 to +44 to generate Aβ peptides (1–40 and 1–42 being most common) and the APP intracellular domain (Figure 2).
γ-secretase is a multiprotein complex composed of presenilin 1 (PS1) or presenilin 2 (PS2); nicastrin (Nct), a type I transmembrane glycoprotein; and Aph-1 and Pen-2, two multipass transmembrane proteins (Bergmans & De Strooper 2010). This complex is essential for the sequential intramembranous proteolysis of a variety of transmembrane proteins. PS1 and PS2 contain two aspartyl residues that play crucial roles in intramembranous cleavage; substitutions of these residues (D257 in TM 6 and at D385 in TM 7) reduces cleavage of APP and Notch1 (De Strooper et al. 1999, Wolfe et al. 1999). The functions of the various γ-secretase proteins and their interactions in the complex are not yet fully defined, but it has been suggested that the ectodomain of nicastrin recognizes and binds to the aminoterminal stubs of previously cleaved transmembrane proteins. Aph-1 aids the formation of a precomplex, which interacts with PS1 or PS2 while Pen-2 enters the complex to initiate the cleavage of PS1 or PS2 to form an N-terminal 28-kDa fragment and a C-terminal 18-kDa fragment, both of which are critical to the γ-secretase complex (Takasugi et al. 2003).
Several aspects of the standard model deserve comment. α-cleavage of APP (+17) is attributed to the ADAM (a disintegrin and metalloproteinase) family of proteases (Asai et al. 2003; Jorissen et al. 2010) and occurs, to a large extent, on the cell surface. However, there is some α-secretase activity in the trans-Golgi. This is of some significance because activation of protein kinase C (Mills & Reiner 1999) causes a significant increase in α-cleavage of APP by increasing transport of APP to the cell surface (Hung et al. 1993), by blocking access of cell surface APP to endosomes, and by stimulating α-cleavage in the TGN (Skovronsky et al. 2000). Because α-cleavage occurs within the Aβ sequence, it prevents Aβ generation. Indeed, increased expression of ADAM 10 or SIRT1, a regulator of ADAM 10 gene expression, in a mouse model of AD significantly attenuated Aβ deposition and cognitive deficits (Donmez et al. 2010; Postina et al. 2004).
Although the standard model suggests that little Aβ is generated outside of endosomal pathways, which (a) dump it outside the cell and (b) have a compulsory cell-surface transition prior to internalization, such is not necessarily the case. Shunting directly from the TGN to the endosome and back (Figure 3) is a potentially important pathway in APP processing. Indeed, early work in transfected cell lines had suggested that significant amounts of APP were processed to Aβ intracellularly (Greenfield et al. 1999), and evidence provides support for BACE1 in the TGN (Huse et al. 2002) and for intracellular Aβ accumulation in neurons from patients with early AD (Gouras et al 2000; LaFerla et al 2007). A key to intracellular generation of Aβ is the concept of retromer transport of APP and BACE1. Retromers are intracellular complexes that shuttle cargo predominantly but not exclusively from the endosome to the TGN. Adaptor proteins affix cargo to the retromer complex. SorLA, a member of the low density lipoprotein receptor superfamily, is one such adaptor protein and binds APP (Andersen et al. 2005) via its N-terminal complement-like domain and binds to the retromer complex via its vacuolar protein-sorting domain ( Jacobsen et al. 2001). C-terminal interactions also exist between APP, BACE1, and SorLA (Spoelgen et al. 2006) mediated through adaptor proteins such as Fe65 and X11 (Schmidt et al. 2007). Most current data suggest that SorLA keeps APP from interacting with BACE1 and promotes transport of APP to the Golgi and away from endosomes, reducing Aβ production. SorLA expression is diminished in neurons from patients with AD (Scherzer et al. 2004), whereas a reduction of SorLA in transgenic animals leads to Aβ accumulation (Andersen et al. 2005).
THE GENETICS OF ALZHEIMER’S DISEASE
The most important lessons to be learned from genetic cases of AD is that the pathology of autosomal dominant AD is very similar to sporadic AD (Shepherd et al. 2009), including the development of neurofibrillary tangles and microglial infiltration. This single observation is central to the concept that Aβ deposition is also the primary event in sporadic AD.
Autosomal Dominant Mutations Associated with AD
There are 32 APP, 179 PSEN1 (presenilin 1 gene locus), and 14 PSEN2 gene mutations that result in early-onset, autosomal dominant, fully penetrant AD. The mutations can be examined in detail at the Alzheimer Disease and Frontotemporal Dementia Mutation Database Web site online (http://www.molgen.ua.ac.be/ADmutations/). In APP, mutations cluster around the γ-secretase cleavage site, although the most famous APP mutation (APP-swe) causes a change in amino acids adjacent to the BACE1 cleavage site. PSEN gene mutations (which give rise to proteins called presenilins, PS1 and PS2) predominantly alter the amino acids in their nine transmembrane domains. The common thread to all these mutations is that they increase production of the less soluble and more toxic Aβ42 relative to Aβ40 (Shen & Kelleher 2007).
In Down’s syndrome, overexpression of APP results in brain Aβ deposition when individuals are in their late 20s. Neurofibrillary tangles develop later and correlate with the onset of the mid-life cognitive decline that is common in these individuals (Hof et al. 1995).
APOE
Late-onset sporadic AD also has a significant genetic component, estimated at 50%–70%. Almost half that risk is conferred by the apolipoprotein E (APOE) allele (Avramopoulos 2009), although some recent data has challenged that association (Roses 2010). The APOE gene comes in three variants that encode proteins (designated ApoE2, ApoE3, and ApoE4) that differ at two amino acids. The APOE E4 allele, which is present in 10%–20% of various populations (Singh et al. 2006), increases the risk for AD threefold in individuals carrying one copy and 15-fold for homozygous individuals. ApoE is the predominant apolipoprotein of the HDL complex in the brain. Although ApoE has many roles in brain physiology (Kim et al. 2009), the most compelling data regarding its role in the development of AD relates to its ability to bind Aβ.
Pathologically, the APOE E4 allele is strongly associated with increased brain Aβ deposition (Tiraboschi et al. 2004), and ApoE2 and -E3 but not -E4 bind Aβ tightly (Tokuda et al. 2000). Human APOE genes expressed in mice diminish Aβ deposition except for APOE E4, which increases Aβ deposition (Holtzman et al. 1999). Thus, the effect of ApoE on AD risk may be entirely explained by its effect on Aβ deposition. It is appealing to think ApoE increases clearance or cellular uptake of Aβ via receptor-mediated binding, but the data are not yet clear on this issue (Kim et al. 2009).
MECHANISMS OF Aβ TOXICITY
The primacy of APP in the development of Alzheimer’s disease depends on the toxicity of the Aβ peptide (Figure 4) because evidence does not show that loss of APP function is deleterious. In addition, Aβ toxicity must also explain other pathological aspects of AD including neurofibrillary tangles, inflammation, and oxidative damage.
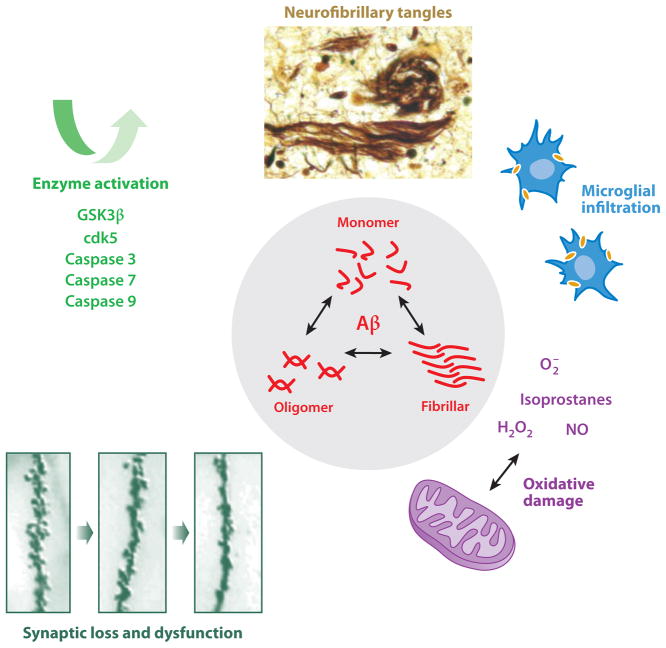
Figure 4.
Aβ toxicity. An equilibrium between several species of extracellular and intracellular Aβ, including monomeric, oligomeric, and fibrillar forms, causes toxicity through several mechanisms including microglial infiltration, the generation of reactive oxygen species, and synaptic damage. Neurofibrillary tangles are generated by Aβ-induced tau phosphorylation and cleavage. Enzymes activated directly by extracellular Aβ include GSK3β, Cdk5, and multiple caspases, which activate tau cleavage and phosphorylation among their many deleterious effects.
Initial work in tissue culture showed that Aβ fibrils are acutely toxic to neurons (Yankner et al. 1989), resulting in complete death of all cells within 24 h of exposure. The mechanism of death in these cultured cells is likely to be apoptosis (Deshpande et al. 2006), triggered by oxidative effects of Aβ. Mutation of a single amino acid (methionine 35) of the Aβ peptide eliminates its ability to generate reactive oxidative species (Kanski et al. 2002). The form of the Aβ peptide that is toxic to neurons is controversial. Evidence exists for picomolar toxicity of an oligomeric assembly of Aβ, possibly a dimer (Shankar et al. 2008), as well as for a multimeric pore-like complex of Aβ monomers. However, there is still compelling evidence for toxicity caused by Aβ monomers, especially Aβ42 (Butterfield 2002), and by truncated, oxidized, and insoluble species of Aβ (Yankner & Lu 2009). There is also controversy about the relative toxicities of intracellular versus extracellular Aβ because intracellular injection of Aβ42 but not Aβ40 also kills neurons and intracellular Aβ is seen early in AD (LaFerla et al. 2007).
Work in vivo also supports the notion that Aβ is toxic to neurons. Mice that overexpress mutant human APPs develop Aβ deposition by 4 to 6 months and show evidence of subsequent neuronal injury. Studies show loss of synaptic terminals (Irizarry et al. 1997, Spires et al. 2005), synaptic dysfunction (Kamenetz et al. 2003, Shankar et al. 2008), abnormalities on spatial memory tests (Chen et al. 2000), and inflammation (El Khoury et al. 2007). These animals, along with human AD brains, show activation of multiple caspases, including caspases 3, 6, 7, 8, and 9, along with evidence of caspase cleavage of actin, fodrin, and the proteasome subunit p97 (Halawani et al. 2010, Rissman et al. 2004, Rohn & Head 2009). Moreover, caspase inhibitors (Rohn et al. 2009) and overexpression of the antiapoptotic protein Bcl-2 (Rohn et al. 2008) ameliorate Aβ toxicity in transgenic mice. One of the more interesting aspects of caspase-induced cell damage is whether APP itself is a target for caspase cleavage, releasing a unique, potentially toxic, C-terminal intracellular fragment (Galvan et al. 2006, Gervais et al. 1999). Caspase-induced damage in AD may occur independent of apoptosis (Hyman 2011), to which mature neurons are resistant.
Soluble Aβ can also control cleavage and phosphorylation of tau, both of which are crucial for neurofibrillary tangle (NFT) generation. Phosphorylation of tau is regulated by several kinases, including GSK3β and cdk5, both of which are activated by extracellular Aβ (Hernandez & Avila 2008, Lee et al. 2000). Pathways leading to tau cleavage including GSK3β, caspase 3, caspase 9, and calpain are also activated by soluble Aβ species (Cho & Johnson 2004, Chung et al. 2001). Moreover, it appears that tau is an important downstream mediator of Aβ toxicity. Triple transgenic mice with mutant APP, PS1, and tau proteins develop Aβ deposition prior to the appearance of NFT pathology (Oddo et al. 2003). Reducing levels of Aβ by immunotherapy prevents tau pathology from developing and abrogates spatial memory problems (Billings et al. 2005, Oddo et al. 2006). Moreover, when transgenic mice that overexpress APP are crossed with mice lacking tau, no detioration in spatial memory function is seen even though Aβ deposition is exuberant (Roberson et al. 2007).
WHY IS THERE AGE-RELATED BRAIN Aβ ACCUMULATION
Large amounts of APP are continuously metabolized to Aβ in the brain (Bateman et al. 2006). Early in the development of Alzheimer’s disease, the concentration of Aβ42 in the cerebrospinal fluid (CSF) starts to fall (Shaw et al. 2009), while the concentration of Aβ42 in the brain is rising (Steinerman et al. 2008), suggesting a diminution in Aβ transport from the brain, a result strongly supported by a recent metabolic analysis of brain Aβ clearance in humans (Mawuenyega et al. 2010). Alternatively, a change in the ratio of Aβ42 to Aβ40 within the brain or any change in the production of CSF or the molecules that buffer Aβ in CSF, such as ApoE, may result in more Aβ aggregation and less CSF clearance.
One mechanism to eliminate extracellular Aβ in the brain includes the proteases neprilysin and insulin degrading enzyme (Selkoe 2001), the polyfunctional endothelial transport proteins P-glycoprotein, receptor for advanced glycation endproducts (RAGE), and low-density lipoprotein-like receptor (LRP1). Animal work has shown that each of these proteins can enhance either Aβ degradation (Leissring et al. 2003) or Aβ transport (Cirrito et al. 2005, Deane et al. 2004). However, the relevance of any one of these proteins to Aβ accumulation in the earliest stages of Alzheimer’s disease is unclear.
One alternate explanation for Aβ accumulation in the elderly would be an alteration in the cleavage of APP. Excessive age-associated acetylation of the α-secretase gene may diminish nonamyloidogenic processing of APP (Donmez et al. 2010), whereas an increase in BACE1 activity, reported in early AD brain tissue, would increase amyloidogenic processing (Holsinger et al. 2002, Yang et al. 2003). Underlying the increase in brain BACE1 activity seen early in AD could be HIF1a, induced by oxidative stress (Guglielmotto et al. 2009). Several micro RNAs, altered early in AD, also regulate BACE1 RNA. Among these are miR-107, miR-298, miR-328, and the miR-29a/b-1 cluster (Hebert et al. 2008).
One very important emerging area of research is the role of environmental enrichment and exercise on brain APP metabolism and Aβ elimination. Enriched environments, including exercise, reduce brain Aβ in transgenic mice (Yuede et al. 2009) in part by increasing brain peptidases but also by stimulating synaptic processes and growth factors. This work is consistent with human research that shows intellectual stimulation and exercise are also protective against dementia (Rovio et al. 2005, Verghese et al. 2006). Combined with data showing that synaptic activity regulates Aβ production in neurons (Cirrito et al. 2008), there is powerful incentive for future work in this area.
POTENTIAL EVIDENCE AGAINST THE PRIMACY OF APP IN ALZHEIMER’S DISEASE
Aβ Immunization
Fortunately, we have now entered an era in which disease-modifying therapies for Alzheimer’s disease are in human clinical trials (http://www.alz.org/trialmatch). These therapies target Aβ production, tau aggregation, oxidation, and inflammation. None has created the interest of Elan’s initial AN1792 trial, which was an active immunization protocol against the full-length Aβ42 peptide (Schenk et al. 2005). Although the trial was stopped because encephalitis developed in some participants, eight subjects who received active immunization have undergone autopsies. Remarkably, Aβ pathology was virtually absent in three of the eight brains (Holmes et al. 2008). Unfortunately, all three of these subjects continued to have a relentlessly progressive disease course and neurofibrillary pathology was advanced at the time of death. Many explanations have been offered for these results, especially the failure to immunize patients early enough in the disease. However, other possible explanations for the results, such as the importance of intracellular Aβ or a bystander role for extracellular Aβ, need also be considered.
Abnormal Notch Processing as the Real Cause of AD
Besides cleaving APP, γ-secretase and BACE1 cleave other single-pass transmembrane proteins, including Notch (De Strooper et al. 1999), neuregulin, and E-cadherin (Parks & Curtis 2007). This raises the question of whether PS1 mutations or APP processing cause cognitive problems due to interference with the processing of other, more essential, substrates. Because almost every PS1 mutation that causes dementia is associated with significant Aβ deposition, this explanation seems unlikely. Moreover, although conditional PS1 deletions in mice result in impairments in memory and synaptic plasticity (Saura et al. 2004), more relevant reductions in PS1 activity have not had a similar effect.
Growth Factor and Hormone Deprivation as the Cause of Alzheimer’s Disease
Several different growth factors and signaling molecules are linked to Alzheimer’s disease. First, the levels of brain-derived neurotrophic factor (BDNF) in the brains of patients with AD decrease (Lee et al. 2005, Peng et al. 2005), and BDNF infusion can improve cognitive function in aged primates (Nagahara et al. 2009). However, BDNF polymorphisms, which have been associated with other cognitive syndromes, have not been consistently associated with AD (Zuccato & Cattaneo 2009).
Second, levels of the nerve growth factor (NGF) prohormone proNGF are significantly increased in early AD (Peng et al. 2004), potentially caused by a reduction in the expression of the NGF receptor TrkA (Counts et al. 2004). Cross talk occurs between APP processing and NGF signaling pathways such that Aβ generation may be altered by NGF signaling (Calissano et al. 2010). The most remarkable animal model of Alzheimer’s disease is a mouse line engineered to express anti-NGF antibodies. At 15 months of age, these animals develop amyloid plaques and neurofibrillary tangles along with abnormalities in spatial learning (Capsoni et al. 2000). Combined with work suggesting growth factor deprivation can trigger APP cleavage by BACE1 and activate DR6 (Nikolaev et al. 2009), this line of investigation is a very interesting twist on the primacy of APP.
Third, a prospective study from the Framingham cohort (Lieb et al. 2009) showed that lower plasma levels of the hormone leptin were correlated with a significantly higher risk of developing Alzheimer’s disease. Moreover, leptin supplementation in APP transgenic mice reduced Aβ accumulation and improved cognition (Greco et al. 2010). Pathways that may mediate this effect include leptin’s ability to affect Aβ production and clearance (Fewlass et al. 2004, Greco et al. 2009b) and its ability to alter dendritic morphology and synaptic density (O’Malley et al. 2007). However it still remains to be shown that low leptin levels cause AD pathology and are not simply the result of those changes.
Systems Degeneration
Interesting recent research has suggested that very early AD may involve the degeneration of cortical areas coordinately active at rest called the “default mode network” (Seeley et al. 2009; Sorg et al. 2007). These are also the areas (posterior cingulate and parietal cortex) with the earliest Aβ accumulation (Sperling et al. 2009). This work suggests that there is a metabolic or synaptic component to the disease and that modulating neuronal activity as well as APP metabolism might be a useful approach. Alternative theories that AD pathology spreads as a synaptic contagion due to toxins or misfolded (prion-like) proteins have also been proposed.
CONCLUSIONS AND FUTURE DIRECTIONS
On the basis of the data presented in this review, there is much to suggest that abnormal processing of APP and the toxicity of the Aβ peptide are central to the development of dementia in the elderly. However, genetic data has suggested that targeting the components of APP processing as a pharmacologic strategy will not be without consequences. BACE1 null mice show altered performance on tests of cognition and emotion (Laird et al. 2005, Savonenko et al. 2008) and have abnormalities of myelination, reflecting alterations in the biology of neuregulin (Hu et al. 2006, Willem et al. 2006). Conditional PS1 deletions result in impairments in memory and in hippocampal synaptic plasticity (Saura et al. 2004), whereas nicastrin heterozygote knockout mice develop skin tumors (Li et al. 2007b). Other roadblocks also exist. The BACE1 catalytic site is quite large, and we do not know yet whether investigators can achieve adequate brain penetration of a compound of sufficient size to inhibit its activity.
γ-secretase activity is also an attractive target. Both genetic and pharmaceutical lowering of γ-secretase activity decrease production of Aβ (Li et al. 2007a,b). However, γ-secretase activity is also essential for processing Notch and a variety of other transmembrane proteins (Louvi & Artavanis-Tsakonas 2006). The γ-secretase inhibitor LY–411, for instance, reduces brain Aβ production but also has profound effects on T- and B- cell maturation (Barten et al. 2005).
Although challenges exist, significant progress has been made over the past 30 years. The future is likely to include multidrug regimens targeting several steps in Aβ production and clearance (Chow et al. 2010) given to individuals with asymptomatic Aβ accumulation detected by positron-emission topography (PET) scans or spinal fluid analysis. In this version of the future, our parents will be able to live out their lives with dignity and grace.
Glossary
- Neuritic plaques
Large extracellular aggregates of the amyloid Aβ peptide surrounded by dystrophic neurites (dendrites) containing aggregated tau
- APP
amyloid precursor protein
- Aβ
amyloid β peptide
- Dementia
a progressive decline in cognition associated with an inability to perform normal activities owing to the cognitive deterioration
- Alzheimer’s disease (AD)
The most common underlying cause of dementia. Pathology shows frequent Aβ amyloid deposition and neurofibrillary tangles
- Notch
Historically and developmentally important signaling protein with similar structure and processing as APP. The Notch intracellular domain regulates transcription
- Secretase
protease designed to cleave transmembrane proteins to release bioactive forms or metabolize proteins prior to degradation
- SorLA
sorting protein–related receptor, also called LR11
- Trans-Golgi network (TGN)
final sorting stack of the Golgi system from which vesicles bud on their way to the cell surface, endosome, and lysosome
- Endosome
an acidic transitional compartment in equilibrium with the cell surface, TGN, and lysosome, which compartmentalizes proteolytic function
- PS1
presenilin 1 protein
- PS2
presenilin 2 protein
- ApoE4
apolipoprotein E4 protein
- APOE E4
apolipoprotein E4 gene locus
- Neurofibrillary tangles (NFT)
Insoluble intracellular inclusions that stain darkly with silver and are composed primarily of hyperphosphorylated and cleaved forms of the microtubule-associated protein tau
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–76. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–66. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001;276:40353–61. doi: 10.1074/jbc.M104059200. [DOI] [PubMed] [Google Scholar]
- Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, et al. Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun. 2003;301:231–35. doi: 10.1016/s0006-291x(02)02999-6. [DOI] [PubMed] [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–55. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramopoulos D. Genetics of Alzheimer’s disease: recent advances. Genome Med. 2009;1:34.1–34.7. doi: 10.1186/gm34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten DM, Guss VL, Corsa JA, Loo A, Hansel SB, et al. Dynamics of {beta}-amyloid reductions in brain, cerebrospinal fluid, and plasma of {beta}-amyloid precursor protein transgenic mice treated with a (Lilly)-secretase inhibitor. J Pharmacol Exp Ther. 2005;312:635–43. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–61. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Cappai R, Masters CL, Beyreuther K, Multhaup G. It all sticks together—the APP-related family of proteins and Alzheimer’s disease. Mol Psychiatry. 1999;4:524–28. doi: 10.1038/sj.mp.4000552. [DOI] [PubMed] [Google Scholar]
- Benn SC, Woolf CJ. Adult neuron survival strategies—slamming on the brakes. Nat Rev Neurosci. 2004;5:686–700. doi: 10.1038/nrn1477. [DOI] [PubMed] [Google Scholar]
- Bergmans BA, De Strooper B. Gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol. 2010;9:215–26. doi: 10.1016/S1474-4422(09)70332-1. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol. 1996;16:6229–41. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–13. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–34. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Calissano P, Matrone C, Amadoro G. Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. Dev Neurobiol. 2010;70:372–83. doi: 10.1002/dneu.20759. [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–20. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. Alzheimer-like neurodegener-ation in aged antinerve growth factor transgenic mice. Proc Natl Acad Sci USA. 2000;97:6826–31. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Oh SY, Hinman JD, Abraham CR. Visualization of APP dimerization and APP-Notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J Neurochem. 2006;97:30–43. doi: 10.1111/j.1471-4159.2006.03705.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–79. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–23. [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem. 2004;279:54716–23. doi: 10.1074/jbc.M403364200. [DOI] [PubMed] [Google Scholar]
- Chow VW, Savonenko AV, Melnikova T, Kim H, Price DL, et al. Modeling an anti-amyloid combination therapy for Alzheimer’s disease. Sci Transl Med. 2010;2:13ra1. doi: 10.1126/scitranslmed.3000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–72. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–90. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann Neurol. 2004;56:520–31. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–4. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotox-icity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–18. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dolan D, Troncoso J, Crain B, Resnick S, Zonderman A, O’Brien R. Age, dementia and Alzheimer’s disease in the BLSA. Brain. 2010a;133:2225–231. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan H, Troncoso J, Crain B, Resnick S, Zonderman A, O’Brien R. Atherosclerosis and Alzheimer’s disease in the Baltimore Longitudinal Study of Aging. Ann Neurol. 2010b;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–23. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–38. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsy-naptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–78. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Fukumori A, Okochi M, Tagami S, Jiang J, Itoh N, et al. Presenilin-dependent gamma-secretase on plasma membrane and endosomes is functionally distinct. Biochemistry. 2006;45:4907–14. doi: 10.1021/bi052412w. [DOI] [PubMed] [Google Scholar]
- Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, et al. Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci USA. 2006;103:7130–35. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Greenberg BD, Savage MJ, Noori M, Newman SJ, et al. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport. 1997;8:1519–22. doi: 10.1097/00001756-199704140-00039. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, et al. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, et al. Chronic leptin supplementation ameliorates pathology and improves cognitive performance in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2009a doi: 10.3233/JAD-2009-1308. Abstract. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1155–67. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009b;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, et al. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci USA. 1999;96:742–47. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–56. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- Halawani D, Tessier S, Anzellotti D, Bennett DA, Latterich M, LeBlanc AC. Identification of Caspase-6-mediated processing of the valosin containing protein (p97) in Alzheimer’s disease: a novel link to dysfunction in ubiquitin proteasome system-mediated protein degradation. J Neurosci. 2010;30:6132–42. doi: 10.1523/JNEUROSCI.5874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA. 2008;105:6415–20. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J, Anliker B, Heber S, Ring S, Fuhrmann M, et al. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–15. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Avila J. The role of glycogen synthase kinase 3 in the early stages of Alzheimers’ disease. FEBS Lett. 2008;582:3848–54. doi: 10.1016/j.febslet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Ho A, Sudhof TC. Binding of F-spondin to amyloid-beta precursor protein: a candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc Natl Acad Sci USA. 2004;101:2548–53. doi: 10.1073/pnas.0308655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. Age-related distribution of neu-ropathologic changes in the cerebral cortex of patients with Down’s syndrome. Quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol. 1995;52:379–91. doi: 10.1001/archneur.1995.00540280065020. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–86. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–25. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- Hung AY, Haass C, Nitsch RM, Qiu WQ, Citron M, et al. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993;268:22959–62. [PubMed] [Google Scholar]
- Huse JT, Liu K, Pijak DS, Carlin D, Lee VM, Doms RW. Beta-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer’s disease brain. J Biol Chem. 2002;277:16278–84. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- Hyman BT. Caspase activation without apoptosis: insight into Abeta initiation of neurodegeneration. Nat Neurosci. 2011;14:5–6. doi: 10.1038/nn0111-5. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, et al. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–59. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001;276:22788–96. doi: 10.1074/jbc.M100857200. [DOI] [PubMed] [Google Scholar]
- Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, et al. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J Neurosci. 2010;30:4833–44. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–59. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–36. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kanski J, Aksenova M, Butterfield DA. The hydrophobic environment of Met35 of Alzheimer’s Abeta(1–42) is important for the neurotoxic and oxidative properties of the peptide. Neurotox Res. 2002;4:219–23. doi: 10.1080/10298420290023945. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci. 2003;116:3339–46. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA. 1990;87:1561–65. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–89. [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, et al. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275:36143–51. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J Neurosci. 2005;25:2386–95. doi: 10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fukumoto H, Orne J, Klucken J, Raju S, et al. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, et al. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283:11501–8. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lau KF, Perkinton MS, Standen CL, Shemilt SJ, et al. The neuronal adaptor protein X11alpha reduces Abeta levels in the brains of Alzheimer’s APPswe Tg2576 transgenic mice. J Biol Chem. 2003;278:47025–29. doi: 10.1074/jbc.M300503200. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–64. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–93. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Das P, Smithson LA, et al. Epidermal growth factor receptor and notch pathways participate in the tumor suppressor function of gamma-secretase. J Biol Chem. 2007a;282:32264–73. doi: 10.1074/jbc.M703649200. [DOI] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Laird FM, Ma G, et al. Moderate reduction of gamma-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci. 2007b;27:10849–59. doi: 10.1523/JNEUROSCI.2152-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–72. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, et al. Chronic traumatic en-cephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin DM, Miller CC. The FE65 proteins and Alzheimer’s disease. J Neurosci Res. 2008;86:744–54. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, et al. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA. 1998;95:12683–88. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J, Reiner PB. Mitogen-activated protein kinase is involved in N-methyl-D-aspartate receptor regulation of amyloid precursor protein cleavage. Neuroscience. 1999;94:1333–38. doi: 10.1016/s0306-4522(99)00381-4. [DOI] [PubMed] [Google Scholar]
- Mok SS, Sberna G, Heffernan D, Cappai R, Galatis D, et al. Expression and analysis of heparin-binding regions of the amyloid precursor protein of Alzheimer’s disease. FEBS Lett. 1997;415:303–7. doi: 10.1016/s0014-5793(97)01146-0. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–37. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–89. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–70. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–23. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Oh ES, Savonenko AV, King JF, Fangmark Tucker SM, Rudow GL, et al. Amyloid precursor protein increases cortical neuron size in transgenic mice. Neurobiol Aging. 2009;30:1238–44. doi: 10.1016/j.neurobiolaging.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–72. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–50. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Cleavage of Alzheimer’s amyloid precursor protein by alpha-secretase occurs at the surface of neuronal cells. Biochemistry. 1999;38:9728–34. doi: 10.1021/bi9906827. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–49. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93:1412–21. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, et al. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–56. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–65. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–64. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JP, Wu H, Yang Y, Wang DD, Chen YX, et al. Cerebral ischemia and Alzheimer’s disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis. 2007;12:335–41. doi: 10.3233/jad-2007-12406. [DOI] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, et al. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–26. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. An inherited variable poly-T repeat genotype in TOMM40 in Alzheimer disease. Arch Neurol. 2010;67:536–41. doi: 10.1001/archneurol.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–30. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–54. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roch JM, Masliah E, Roch-Levecq AC, Sundsmo MP, Otero DA, et al. Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proc Natl Acad Sci USA. 1994;91:7450–54. doi: 10.1073/pnas.91.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer’s disease: Is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2:108–18. [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Kokoulina P, Eaton CR, Poon WW. Caspase activation in transgenic mice with Alzheimer-like pathology: results from a pilot study utilizing the caspase inhibitor, Q-VD-OPh. Int J Clin Exp Med. 2009;2:300–8. [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008;28:3051–59. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sano Y, Vassar R, Gandy S, Nakaya T, et al. X11 proteins regulate the translocation of amyloid beta-protein precursor (APP) into detergent-resistant membrane and suppress the amyloidogenic cleavage of APP by beta-site-cleaving enzyme in brain. J Biol Chem. 2008;283:35763–71. doi: 10.1074/jbc.M801353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja I, Paulson H, Gupta A, Turner RS. X11alpha haploinsufficiency enhances Abeta amyloid deposition in Alzheimer’s disease transgenic mice. Neurobiol Dis. 2009;36:162–68. doi: 10.1016/j.nbd.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Nakaya T, Pedrini S, Takeda S, Iijima-Ando K, et al. Physiological mouse brain Abeta levels are not related to the phosphorylation state of threonine-668 of Alzheimer’s APP. PLoS One. 2006;1:e51. doi: 10.1371/journal.pone.0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA. 2008;105:5585–90. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk DB, Seubert P, Grundman M, Black R. A beta immunotherapy: lessons learned for potential treatment of Alzheimer’s disease. Neurodegener Dis. 2005;2:255–60. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–5. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- Schmidt V, Sporbert A, Rohe M, Reimer T, Rehm A, et al. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J Biol Chem. 2007;282:32956–64. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–80. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA. 2007;104:403–9. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol. 2009;118:37–52. doi: 10.1007/s00401-009-0521-4. [DOI] [PubMed] [Google Scholar]
- Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-Golgi network. J Biol Chem. 2000;275:2568–75. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104:18760–65. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–87. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, et al. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26:418–28. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinerman JR, Irizarry M, Scarmeas N, Raju S, Brandt J, et al. Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol. 2008;65:906–12. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–41. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–83. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J. 2000;348(Pt. 2):359–65. [PMC free article] [PubMed] [Google Scholar]
- Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–27. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yang G, Mosier DR, Chang P, Zaidi T, et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–25. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–66. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–17. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Yang Z, Cool BH, Martin GM, Hu Q. A dominant role for FE65 (APBB1) in nuclear signaling. J Biol Chem. 2006;281:4207–14. doi: 10.1074/jbc.M508445200. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245:417–20. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Lu T. Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:4755–59. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007;27:14459–69. doi: 10.1523/JNEUROSCI.4701-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–32. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano N, Minopoli G, de Candia P, Russo T. The Fe65 adaptor protein interacts through its PID1 domain with the transcription factor CP2/LSF/LBP1. J Biol Chem. 1998;273:20128–33. doi: 10.1074/jbc.273.32.20128. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–22. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]