Abstract
RACK1 (receptor for activated protein kinase C 1) is an intracellular scaffolding protein known to interact with the inositol-1,4,5-trisphosphate receptor and thereby enhance calcium release from the sarcoplasmic reticulum. Because calcium signaling may affect vascular smooth muscle cell proliferation, we investigated whether RACK1 regulates proliferation of rat preglomerular microvascular smooth muscle cells. Western blot analysis indicated that preglomerular microvascular smooth muscle cells robustly express RACK1 protein, and co-immunoprecipitation experiments demonstrated that RACK1 binds the inositol-1,4,5-trisphosphate receptor. RACK1 siRNA decreased RACK1 mRNA and protein expression, significantly (p=0.0225) reduced steady-state basal levels of intracellular calcium (6712 ± 156 versus 7408 ± 248, arbitrary florescence units in RACK1 siRNA-treated versus control cells, respectively) and significantly (p<0.0001) decreased cell proliferation by approximately 50%. Xestospongin C and 2-aminoethoxydiphenyl borate (antagonists of inositol-1,4,5-trisphosphate receptors), cyclopiazonic acid (sarcoplasmic reticulum Ca2+-ATPase inhibitor) and calmidazolium (calmodulin inhibitor) mimicked the effects of RACK1 siRNA on proliferation, and RACK1 siRNA had no additional effects on proliferation in the presence of these agents. RACK1 siRNA did not affect the expression of cyclin D1/2 or phosphorylation of retinoblastoma protein (pro-growth cell cycle regulators), yet caused compensatory decreases in the expression of p21Cip1/Waf1 and p27Kip1 (anti-growth cell cycle regulators). Like preglomerular microvascular smooth muscle cells, glomemular mesangial cells also expressed high levels of RACK1, and RACK1 siRNA inhibited their proliferation.
Conclusion
RACK1 modulates proliferation of preglomerular microvascular smooth muscle cells and glomemular mesangial cells, likely via the inositol-1,4,5-trisphosphate receptor/calcium/calmodulin pathway. RACK1 may represent a novel druggable target for treating renal diseases such as glomerulosclerosis.
Keywords: Receptor for Activated Protein Kinase C 1, RACK1, Cell Proliferation, Microvascular Smooth Muscle Cells, Glomerular Mesangial Cells, Calcium, Calmodulin
INTRODUCTION
Healthy kidneys require a structurally appropriate renal microcirculation, which in turn depends on the proper regulation of proliferation of renal microvascular cellular elements including renal microvascular smooth muscle cells, such as preglomerular vascular smooth muscle cells (PGVSMCs). Dysregulation of PGVSMCs and their phenotypically similar counterparts in the glomerulus (i.e., glomerular mesangial cells; GMCs) contributes to nephropathies such as hypertensive and diabetic renal disease1–3. Thus, the elucidation of novel, druggable molecular targets that regulate growth of PGVSMCs and GMCs is a logical first step in the development pathway for generating new pharmacological therapies to treat renal diseases that involve the renal microcirculation and glomeruli.
Receptor for activated protein kinase C 1 (RACK1) is a seven-sided propeller protein with seven WD40 repeats, and RACK1 participates in cell signaling by functioning as a scaffold protein4, 5. Although RACK1’s name reflects its role in localizing activated C kinases, more recent studies confirm that RACK1 serves as a highly diverse scaffold protein that binds a large array of proteins and regulates multiple signaling pathways by interacting with WD40, SH2, C2, PH and NUF domains in binding partner proteins 5. Importantly, recent studies show that in some cell types RACK1 binds to the inositol-1,4,5-trisphosphate receptor (IP3R) and increases its binding affinity for inositol-1,4,5-trisphosphate (IP3)6 and thereby increases intracellular calcium levels by triggering release of calcium from the sacrcoplamic reticulum (SR) calcium-releasing system. RACK1 also binds to BKCa channels in rat pulmonary artery smooth muscle cells and in rat basilar artery smooth muscle cells, a process that is thought to impair BKCa channel function and increase localized compartments of intracellular calcium7. Because increases in intracellular calcium stimulate proliferation of vascular smooth muscle cells8–13, we hypothesize that RACK1 may regulate growth of PGVSMCs and GMCs. The main goal of this study was to evaluate this hypothesis.
METHODS
Animals
Studies utilized male adult (16-weeks-of-age) normotensive Wistar-Kyoto rats from Taconic Farms (Germantown, NY). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Culture of PGVSMCs and GMCs
PGVSMCs and GMCs were cultured by explant from freshly isolated rat renal microvessels and glomeruli as described in detail by us previously14, 15.
Effects of RACK1 siRNA on Cell Proliferation
Cells (3rd to 5th passage) were cultured in DMEM/F12 medium containing 10% fetal calf serum (FCS), 20 units/ml penicillin, 20 μg/ml streptomycin and 0.05 μg/ml amphotericin at 37°C with 5% CO2. One day before transfection, cells were plated in 500 μl of DMEM/F12 medium containing 10% FCS (for [3H]-thymidine incorporation studies) or 2.5% FCS (for cell number experiments) without antibiotics in a 24-well plate. On the day of tranfection, 40 pmoles of RACK1 siRNA pool or non-targeting siRNA pool (Dharmacon; Lafayette, CO) and 1.5 μl of DharmaFECT 1 (Dharmacon) were diluted to 50 μl in Opti-MEM I medium and incubated for 5 minutes at room temperature. Then the diluted siRNA and DharmaFECT 1 were combined and incubated for 20 minutes at room temperature to allow transfection complexes to form. DMEM/F12 medium containing 0.1% FCS (low serum) or 2.5% FCS (high serum) without antibiotics were added to the complexes (transfection mixture). In some experiments, the transfection mixture included either xestospongin C [5 μM; antagonist of IP3Rs16], 2-aminoethoxydiphenyl borate [2-APB, 100 μM; alternative antagonist of IP3Rs17, 18], cyclopiazonic acid [5 μM; blocks calcium pump in the SR19] or calmidazolium [1 μM; inhibits calmodulin20]. Xestospongin C, 2-APB, cyclopiazonic acid and calmidazolium were obtained from Sigma-Aldrich (St. Louis, MO). The growth medium was removed from the cells and replaced with the tranfection mixture, and the cells were incubated with the transfection mixture at 37°C with 5% CO2. For thymidine incorporation studies (DNA synthesis), at 68 hours, the medium was changed to DMEM/F12 containing both 0.1% FCS and [3H]-thymidine (1 μCi/mL). Four hours later, the experiments were terminated by washing the cells twice with Dulbecco’s PBS and twice with ice-cold trichloroacetic acid (10%). The precipitate was solubilized in 500 μL of 0.3N NaOH and 0.1% sodium dodecylsulfate after incubation at 50°C for 2 hours. Samples were mixed with 10 mL scintillation fluid and counted in a liquid scintillation counter. For cell number experiments, at 72 hours, the transfection mixture was replaced with fresh DMEM/F12 medium containing either 0.1% or 2.5% FCS with or without xestospongin C, 2-APB, cyclopiazonic acid or calmidazolium and at 96 hours, cells were dislodged and counted on a Coulter counter.
Assessment of siRNA Knockdown of RACK1 mRNA
RNA was isolated (TRIZOL Reagent; Life Technologies; Carlsbad, CA), and cDNA was synthesized using iScript™ cDNA synthesis kit (Bio-Rad; Herucles, CA). The RACK1 primers were: forward, 5′-gtgctcttcgaggtcactcc-3′; reverse, 5′-cggttgtcagaggagaaagc-3′; 184 bp amplification product. β-actin primers were: forward, 5′-actcttccagccttccttc-3′; reverse, 5′-atctccttctgcatcctgtc-3′; 171 bp amplification product. Real-time polymerase chain reaction (PCR) analysis was performed using SYBR Green PCR Master Mix (Applied Biosystems; Foster City, CA) in the AB 7300 Real-Time PCR System (Applied Biosystems). Threshold cycle (Ct) for target was subtracted from Ct for β-actin to calculate 2ΔCt.
Assessment of siRNA Knockdown of RACK1 Protein
Protein was extracted (Mammalian Protein Extraction Reagent; Pierce Biotechnology Inc.; Rockford, IL), measured (BCA assay; Pierce) and boiled (5 minutes in Laemmli buffer). SDS-polyacrylamide-gel electrophoresis was performed on polyacrylamide gels (8–16%) with 40 μg of protein per lane. Proteins were transferred to PDVF membranes. Membranes were blocked in tris-buffered saline tween-20 containing 5% milk and probed with an anti-RACK1 mouse monoclonal primary antibody (1:400; Santa Cruz Biotechnology; Santa Cruz, CA). Membranes were exposed to horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (1:4000; Pierce) and visualized with the Bio-Rad VersaDoc Imaging System using luminal-based enhanced chemiluminescence substrate (Supersignal West Dura Extended Duration Substrate; Pierce).
Analysis of Cell Cycle Regulators
Cellular levels of cyclin D1/2, phosphorylated and hyperphosphorylated retinoblastoma protein (pRB and ppRb, respectively), p21Cip1/Waf1 and p27Kip1 were measured by Western blotting as previously described by us in detail21.
Co-Immunoprecipitation of RACK1 With IP3R and Calmodulin
Each batch of PGVSMCs was cultured in a 75-cm2 flask and suspended in trypsin in a separate 15-mL tube. The sample was centrifuged at 4°C for 10 minutes (2000 rpm), and the pellet was resuspended in 10 ml of PBS and then again centrifuged for 10 minutes. Each pellet was resuspended in 1 mL of ice-cold RIPA lysis buffer (Santa Cruz, catalogue # sc-24948), and after 30 minutes, the sample was sonicated, incubated for another 30 minutes, centrifuged at 4°C for 10 minutes (10,000 × g) and the supernatant transferred to a 1.5-ml microcentrifuge tube. Preclearing B-Matrix-Rabbit solution (45 μL; Santa Cruz, catalogue # sc-45059) was added to the sample, and the sample was incubated at 4°C for 10 minutes while rotating. Then each sample was centrifuged in a microcentrifuge at maximum speed for 30 seconds at 4°C and the supernatant was transferred to a microfuge tube and placed on ice.
To prepare the precipitating complex, 45 μL of IP-Matrix (ExactaCruz B; Santa Cruz, catalogue # sc-45039), 500 μL of PBS and 20 μL of IP3R or calmodulin 1° rabbit polyclonal antibody (200 μg/mL; Santa Cruz) were incubataed overnight at 4 °C with rotation in a microcentrifuge tube. The precipitating complex was pelleted by microcentrifugation at maximum speed for 30 seconds at 4°C. The pellet was resuspended in 500 μL of PBS and washed twice, and the precleared cell lysate was added to the pellet (IP-antibody + IP-matrix complex) and incubated at 4°C for 1 hour while rotating. The sample was pelleted by microcentrifugation at maximum speed for 30 seconds at 4°C and washed 3 times in PBS. The pellet was resuspended in sample buffer with reducing agent.
Sixteen μg of protein from each sample was placed in 16 μL of sample buffer, boiled for 10 minutes, incubated on ice for 5 minutes, flash centrifuged and loaded onto an Invitrogen NuPAGE Novex Bis-Tris 12% gel. Gels were run in NuPAGE MOPS SDS running buffer at 120V for 2 hours, and proteins were transferred onto a PVDF membrane in NuPAGE transfer buffer at 0.21 amp overnight. PVDF membranes were washed briefly with methanol and blocked in PBS (5% dry non-fat milk, 0.05% tween-20) at room temperature for 2 hours. Blots were incubated with 1° RACK-1 mouse monoclonal antibody in PBS (5% dry non-fat milk, 0.05% tween-20) at 1:350 ratio at 4°C overnight, washed 5 times in PBS, incubated with anti-mouse HRP-conjugated ExactaCruz detection reagent in PBS (5% dry non-fat milk, 0.05% tween-20) at 1:8500 ratio for 1 hour at room temperature, washed three times in PBS, covered with SuperSignal West Femto reagent (Pierce) and imaged with a Fotodyne (Hartland, WI) gel imaging system for an 8 minute exposure.
Measurement of Intracellular Calcium
One day before transfection, 20,000 PGVSMCs were suspended in 100 μl of DMEM/F12 medium containing 2.5% FCS without antibiotics, and cells were placed in wells of a 96-well plate so that cells would be less than 30% confluent at the time of transfection. Eight pmol of RACK1 siRNA pool or non-targeting siRNA pool (Dharmacon) and 0.3 μl of DharmaFECT 1 (Dharmacon) were diluted to 10 μl in Opti-MEM I medium and incubated for 5 min at room temperature. Then the diluted siRNA and DharmaFECT 1 were combined and incubated for 20 min at room temperature to allow transfection complexes to form. Eighty μl of DMEM/F12 medium containing 2.5% FCS without antibiotics were added to the complexes (transfection mixture). The growth medium was removed from the cells and replaced with the tranfection mixture, and the cells were incubated with the transfection mixture for 72 hrs at 37°C with 5 % CO2. At 72 hours, the transfection mixture was removed, and 100 μl of the dye loading solution (Fluo-4 NW Calcium Assay Kit, Molecular Probes; Eugene, OR) was added to each well. Cells were incubated at 37oC for 30 minutes, then at room temperature for an additional 30 minutes. Fluorescence was measured using a Victor2 plate reader (PerkinElmer; Waltham, MA) with instrument settings for excitation at 485 nm and emission at 535 nm.
Statistical Analysis
Variables were compared with either a Student’s t-test or an independent-sampling 2-factor analysis of variance. Statistical analysis was performed using the Number Cruncher Statistical System (Kaysville, UT, U.S.A.), and all values in the text and figures refer to means ± SEM.
RESULTS
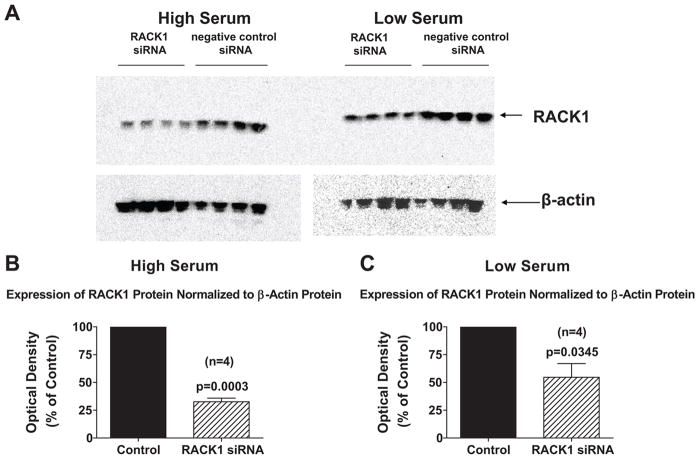
Because the level of growth factors in the medium could alter the cellular expression of RACK1 binding partners, which could potentially alter the role of RACK1 in regulating growth, we conducted our initial studies in cells cultured under both high (2.5%) and low (0.1%) serum conditions. Accordingly, our first objective was to determine the feasibility of using RACK1 siRNA to reduce the expression of RACK1 under the cell culture conditions employed in this study. Importantly, in PGVSMCs, RACK1 siRNA decreased RACK1 mRNA expression (as assessed by real time PCR) by 70% (p=0.0014) and 87% (p=0.0001) in cells cultured under high and low serum conditions, respectively. Also, RACK1 siRNA reduced RACK1 protein expression by 67% (p=0.0003) and 45% (p=0.0345) in cells cultured under high (Figure 1, panels A and B) and low (Figure 1, panels A and C) serum conditions, respectively.
Figure 1.
Panel A shows Western blots demonstrating the effects of RACK1 siRNA versus negative control siRNA on RACK1 protein expression in preglomerular vascular smooth muscle cells when cells were grown in the presence of either high (2.5%) or low (0.1%) serum in the medium. For both negative control siRNA-treated cells (Control) and RACK1 siRNA-treated cells (RACK1 siRNA), the RACK1 protein expression was normalized to β-actin protein expression by calculating the optical density ratio (optical density of RACK1 band divided by optical density of β-actin band). The optical density ratio for RACK1 siRNA cells was expressed as a % of the average optical density for the Control cells. Panels B and C show results for cells grown in presence of high or low serum in the medium. Values represent means ± SEM, and p-values are from unpaired Student’s t-test.
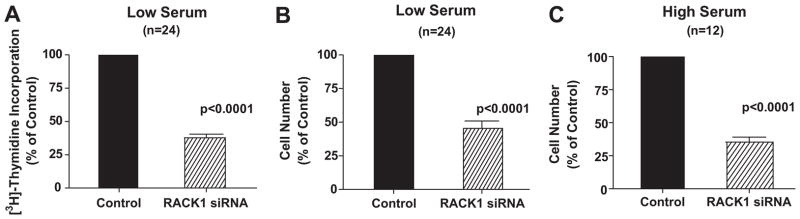
Having validated the ability of RACK1 siRNA to reduce the expression of RACK1, we next applied this approach to determine the role of RACK1 in modulating growth of PGVSMCs. In this regard, we first examined the effects of RACK1 siRNA on [3H]-thymidine incorporation because this index of DNA synthesis is a highly sensitive technique for assessing changes in cell growth. Importantly, in PGVSMCs cultured under low serum conditions (Figure 2, panel A), RACK1 siRNA significantly reduced [3H]-thymidine incorporation by 62% (p<0.0001).
Figure 2.
Bar graphs depict effects in preglomerular vascular smooth muscle cells of RACK1 siRNA versus negative control siRNA (Control) on [3H]-thymidine incorporation under low serum (0.1%) conditions (panel A), on cell number under low serum conditions (panel B) and on cell number under high serum (2.5%) conditions (panel C). [3H]-thymidine incorporation or cell number for RACK1 siRNA cells was expressed as a % of the average [3H]-thymidine incorporation or cell number for the Control cells. [3H]-thymidine incorporation in low serum Control cells was 32,477 dpm, and the cell counts in low and high serum Control cells were 73,142 and 474,500, respectively. Values represent means ± SEM, and p-values are from unpaired Student’s t-test.
This information provided confidence that cell counting experiments would be able to detect siRNA-induced changes of the magnitude observed in the [3H]-thymidine incorporation experiments, and from this point forward we used direct cell counting to assess cell proliferation because this method is not subject to error introduced by aneuploidy. RACK1 siRNA significantly reduced cell counts in PGVSMCs by 55% (p<0.0001) and 65% (p<0.0001) when cells were cultured under low (Figure 2, panel B) and high (Figure 2, panel C) serum conditions, respectively. Because the results were similar when cells were cultured under high versus low serum conditions, in all subsequent proliferation studies we employed high (2.5%) serum conditions to increase cell number and facilitate the detection of changes.
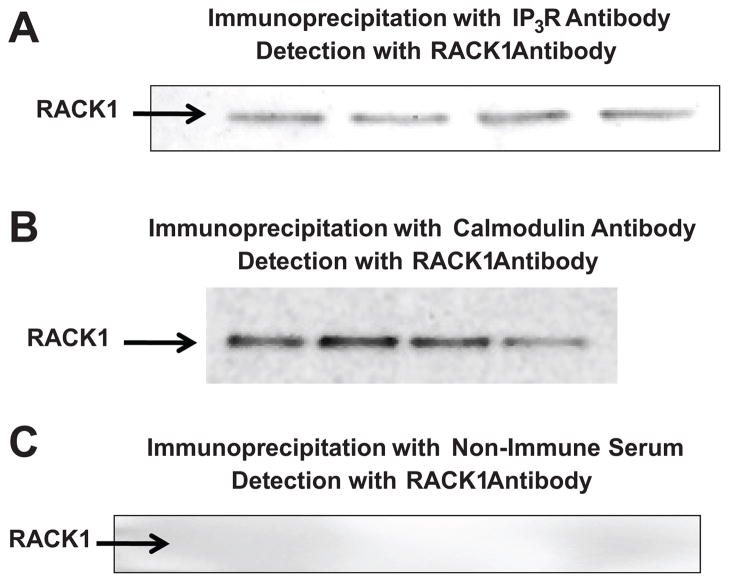
Because in some cell types RACK1 binds IP3Rs in the SR leading to increased IP3-induced release of SR calcium6, we tested the hypothesis that RACK1 physically interacts with IP3Rs in PGVSMCs. In this regard, we performed co-immunoprecipitation experiments using an antibody to IP3Rs to immunoprecipitate proteins in PGVSMCs, followed by Western blotting and probing with a RACK1 antibody. As shown in Figure 3 (panel A) in PGVSMCs RACK1 strongly co-immunoprecipitated with IP3Rs. Moreover, using the Fluo-4 NW Calcium Assay Kit (Molecular Probes) we examined the effects of chronic RACK1 siRNA treatment on basal (steady state) levels of intracellular calcium. These experiments revealed a significant (p=0.0225) reduction in intracellular calcium in RACK1-treated (n=25) versus control (n=26) PGVSMCs (6712 ± 156 versus 7408 ± 248, arbitrary florescence units in RACK1 siRNA versus control cells, respectively).
Figure 3.
Western blots show the results of co-immunoprecipitation experiments in four separate batches of preglomerular vascular smooth muscle cells using the Santa Cruz BT: ExactaCruz IP kit. The immunoprecipitation step was conducted with an antibody against either the inositol 1,4,5-trisphosphate receptor (IP3R; panel A) or calmodulin (panel B) or with non-immune serum (panel C). Immunoprecipitates were analyzed by Western blotting using an anti-RACK1 antibody for detection.
Because calmodulin mediates many of the signaling functions of intracellular calcium, we also investigated whether RACK1 scaffolds calmodulin. As shown in Figure 3 (panel B) co-immunoprecipitation experiments using an antibody to calmodulin to immunoprecipitate proteins in PGVSMCs, followed by Western blotting and probing with a RACK1 antibody revealed that in PGVSMCs RACK1 strongly co-immunoprecipitated with calmodulin. Importantly, RACK1 was not detected in blots in which immunoprecipitation was performed with non-immune serum (Figure 3, panel C).
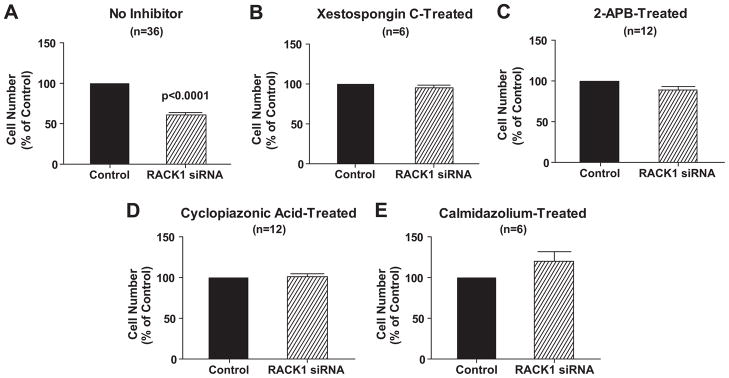
To explore further the hypothesis that RACK1 modulates proliferation of PGVSMCs via the IP3R/calcium/calmodulin pathway, we examined the effects of inhibition of IP3Rs with xestospongin C or 2-APB, depletion of SR calcium stores with cyclopiazonic or inhibition of the calcium signaling transducer calmodulin with calmidazlolium in control versus RACK1 siRNA-treated PGVSMCs. Xestospongin C, 2-APB, cyclopiazonic acid and calmidazolium significantly (p<0.05) reduced cell proliferation (176,042 ± 11,458, 118,229 ± 7,556, 135,417 ± 4237 and 145,833 ± 11,932 cells/well, repectively, versus 209,375 ± 6,790 in control cells). Moreover, in the presence of these inhibitors, RACK1 siRNA had no additional effect on proliferation of PGVSMCs (Figure 4), suggesting a common mechanism of action of RACK1 siRNA and these inhibitors. In contrast to their effects on RACK1 siRNA, the inhibitors of calcium signaling did not affect the anti-proliferative response to 2-chloroadenosine (data not shown), a metabolically stable analogue of adenosine that inhibits vascular smooth muscle cell proliferation via the adenosine A2B receptor by increasing the expression of p21Kip1 (a negative growth regulator)22. These data suggest that the lack of growth inhibition by RACK1 siRNA in the presence of the inhibitors was not due to “non-specific” interference with anti-proliferative mechanisms.
Figure 4.
Bar graph depicts effects of RACK1 siRNA versus negative control siRNA (Control) in preglomerular vascular smooth muscle cells on cell number under high serum (2.5%) conditions in the absence (panel A) and presence of xestospongin C (5 μM; panel B), 2-aminoethoxydiphenyl borate (2-APB, 100 μM; panel C), cyclopiazonic acid (5 μM; panel D) or calmdiazolium (1 μM; panel E). Cell numbers for RACK1 siRNA-treated are expressed as a % of the average cell number for corresponding Control cells (A: 209,375; B: 176,042; C: 118,229; D: 135,417; E 145,833). Values represent means ± SEM, and p-values are from unpaired Student’s t-test.
Unlike 2-chloroadenosine (which increases the negative growth regulator p27Kip1), RACK1 siRNA caused a compensatory decrease in the expression of the negative growth regulators p21Cip1/Waf1 and p27Kip1 (Figure S1; please see http://hyper.ahajournals.org). RACK1 siRNA did not affect the positive growth regulators cyclin D1/2 or ppRb (Figure S1; please see http://hyper.ahajournals.org).
GMCs, like PGVSMCs, are contractile cells phenotypically similar to PGVSMCs, and like PGVSMCs are involved in the pathophysiology of glomerulosclerosis. Therefore, we anticipated that RACK1 knockdown would have the same effects in GMCs as observed in PGVSMCs. As shown in Figure S2 (please see http://hyper.ahajournals.org), RACK1 siRNA significantly decreased RACK1 mRNA expression in GMCs by 64% (p<0.0001) and decreased RACK1 protein expression in GMCs by 74% (p=0.0036). Consistent with our expectations, RACK1 siRNA reduced GMC proliferation by 45% (p<0.0001) (Figure S2; please see http://hyper.ahajournals.org).
DISCUSSION
The research field regarding the role of RACK1 in regulating cell proliferation is in its infancy, and only a small number of publications in this potentially important area exist. Moreover, even within the small number of available reports, the findings are contradictory. This heterogeneity of results is not surprising, however, given that RACK1 functions as a scaffolding protein and that the number of binding partners interacting with RACK1 is remarkably large5. Predictably, RACK1 should inhibit or promote cell growth depending on the precise expression levels of pro-growth versus anti-growth RACK1 binding partners in the cell, which would in turn depend on the type of cell under consideration and on the culture conditions. For example, in human colon carcinoma cells in which Src tyrosine kinase activity is elevated, RACK1 inhibits cell proliferation by binding to and inhibiting Src activity, thus inhibiting progression of the cell cycle at the G1 and mitotic checkpoints23, 24. In human pulmonary artery smooth muscle cells in which type II bone morphogenetic protein receptor is critical for inhibiting cell proliferation, RACK1 reduces cell growth by binding to the type II bone morphogenetic protein receptor and enabling Smad-mediated signaling25. In NIH 3T3 cells that express high levels of type 1 insulin-like growth factor receptors (IGF-IRs), RACK1 binds to IGF-IRs and alters signaling such that over-expression of RACK1 reduces IGF-IR-induced cell growth yet increases IGF-IR-induced cell spreading and formation of stress fibers and focal adhesions26.
In contrast to the aforementioned negative effects of RACK1 on cell proliferation, in other cell types RACK1 promotes cell growth. For example, in human ovarian cancer cell lines, RACK1 siRNA decreases stimulated colony formation27, and this effect of RACK1 is most likely due to RACK1-mediated scaffolding of STAT3 to the insulin receptor and IGF-IR. Interestingly, the African trypanosome trypanosoma brucei expresses a RACK1 homologue called TRACK, and depletion of TRACK using RNA interference blocks the onset and progression of cytokinesis in this protozoan to the point that inducing TRACK RNA interference in infected mice eliminates parasites from peripheral blood within three days post-infection28.
The results of the present study support the conclusion that RACK1 is a positive modulator of proliferation of PGVSMCs. The evidence for this conclusion is that RACK1 is highly expressed in PGVSMCs and reduction of RACK1 expression inhibits PGVSMC proliferation. The role of RACK1 as a positive modulator of proliferation in PGVSMCs is independent of whether the cells are grown under low or high serum conditions. This is important because the level of growth factors in the medium would be expected to alter the cellular expression of RACK1 binding partners, which could potentially profoundly alter the role of RACK1 in regulating growth. The fact that RACK1 siRNA inhibits PGVSMC growth similarly in cells cultured under both low and high serum conditions indicates that in PGVSMCs RACK1 is likely to positively modulate cell proliferation under a variety of physiological and pathophysiological settings.
We also cloned rat RACK1 and attempted to over-express RACK1 in PGVSMCs using a plasmid-based vector. However, the basal level of expression of RACK1 was so high in PGVSMCs that we were unable to increase RACK1 expression (assessed by Western blotting) over high basal levels. Nonetheless, we did observe a 40% increase in proliferation of PGVSMCs by transfecting cells with a RACK1 plasmid, and this response was blocked by xestospongin C and cyclopiazonic acid. Given the high basal expression of RACK1 in PGVSMCs, increasing RACK1 further by over-expression would not be expected to inform the physiological roles of endogenous RACK1 in PGVSMCs.
The mechanism by which RACK 1 regulates cell growth most likely involves modulation of calcium signaling. The evidence for this conclusion is: 1) Increased cytosolic calcium levels stimulate proliferation of vascular smooth muscle cells8–13 and other contractile cells, for example mesangial cells29, 30; 2) Studies by Patterson et al.6 demonstrate that in some cell types RACK1 physiologically binds IP3Rs and modulates calcium release by augmenting IP3R binding affinity for IP3; 3) In PGVSMCs, IP3R and RACK1 also physically interact; 4) In PGVMSCs, RACK1 siRNA decreases basal (steady state) levels of calcium; and 5) In PGVSMCs, inhibition of IP3Rs (with xestospongin C or 2-APB) or depletion of SR calcium stores (with cyclopiazonic acid) or inhibition of calmodulin (with calmidazolium) decreases proliferation and blocks any further effects of RACK1 siRNA. These results are consistent with the hypothesis that RACK1 influences the proliferation of PGVSMCs via the IP3R/calcium/calmodulin pathway.
Calmodulin binds calcium, and the calcium-calmodulin complex binds to and alters the activity of many downstream effector proteins. Thus calmodulin is a critically import signal transduction component in calcium signaling. Our study is the first to show that RACK1 not only interacts with IP3Rs in PGVSMCs, but also binds to calmodulin. Although not investigated here, it is conceivable that RACK1 modulates the activity of calmodulin, for example by positioning calmodulin near IP3Rs for efficient calcium loading or near downstream effector molecules for efficient modulation of their activity. Thus the regulation by RACK1 of the IP3R/calcium/calmodulin pathway may involve multiple sites and mechanisms of action.
Recently we discovered that adenosine A2B receptors inhibit growth of vascular smooth muscle cells by up-regulating the expression of p27Kip1, a cell-signaling factor that inhibits the actions of cyclin-dependent kinases and thus restricts cell proliferation by impairing progression through the cell cycle22. To test whether RACK1 siRNA triggers a similar mechanism, we examined in the current study the effects of RACK1 siRNA on the expression of not only p27Kip1 but also p21Cip1/Waf1, another inhibitor of cyclin-dependent kinases. Our results show that RACK1 siRNA down-regulates, rather than up-regulates, the expression of both p27Kip1 and p21Cip1/Waf1. Since down-regulation of p27Kip1 and p21Cip1/Waf1 would be expected to increase, rather than decrease, cell proliferation, it appears that the change in expression of p27Kip1 and p21Cip1/Waf1 is a compensatory response to the inhibitory effects of RACK1 siRNA. We do not observe any effects of RACK1 siRNA on the expression of cyclin D1/2 or hyperphosphorylated retinoblastoma protein, which rules out an involvement of these key cell cycle regulators in the growth-modulatory effects of RACK1 in PGVSMCs.
The present study addresses the role of RACK1 as a regulator of microvascular smooth muscle cell proliferation. As with PGVSMCs, RACK1 also promotes the proliferation of GMCs. This is not surprising because GMCs are phenotypically similar to PGVSMCs and can be viewed as the “contractile smooth muscle cell” of the glomerular vessels. In preliminary studies, we also examined the effects of RACK1 on proliferation of macrovascular smooth muscle cells including rat and human aortic and human coronary artery vascular smooth muscle cells. Importantly, in macrovascular smooth muscle cells, RACK1 siRNA did not inhibit cell proliferation yet did reduce RACK1 mRNA expression. These data suggest that the proliferative effects of RACK1 may be restricted to smooth muscle cells from resistance arteries.
Because RACK1 is involved in calcium signaling in microvascular smooth muscle cells and because calcium mediates smooth muscle cell contraction, it is conceivable that RACK1 also regulates microvascular tone and arterial blood pressure, and this hypothesis is under investigation. Interestingly, in preliminary studies, we detected RACK1 protein in the membrane, cytosolic, nuclear and cytoskeletal fractions of PGVSMCs, and the expression levels were 2.5-fold higher in the membrane fraction of cells derived from spontaneously hypertensive rats versus normotensive Wistar-Kyoto rats. This finding is consistent with the fact that genetically hypertensive rats have increased renovascular tone and PGVSMCs from SHR proliferate more rapidly in culture.
Perspective
Despite the growing awareness of the importance of RACK1 in cell biology4, 5, the roles of RACK1 in regulating the function, structure and proliferation of renal microvascular smooth muscle cells are unknown. Indeed, as far as we know, this is the first report of the role of RACK1 in regulating the biology of microvascular smooth muscle cells from any source. Importantly, the present study indicates that RACK1 promotes proliferation of the preglomerular renal microvascular smooth muscle cell and its phenotypically-related cousin, the glomerular mesangial cell. Proliferation of renal preglomerular microvascular smooth muscle cells would be expected to increase resistance to blood flow and therefore could reduce renal blood flow and kidney function and increase systemic blood pressure. Moreover, proliferation of both preglomerular microvascular and glomerular mesangial cells are involved in the pathophysiology of hypertensive and diabetic renal diseases as well as in other types of nephropathies. Therefore, RACK1 inhibitors may prove to be a new class of cardiorenal drugs.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grants DK068575, HL069846, and DK079307.
Footnotes
DISCLOSURES
None.
References
- 1.McGill JB. Improving microvascular outcomes in patients with diabetes through management of hypertension. Postgraduate Medicine. 2009;121:89–101. doi: 10.3810/pgm.2009.03.1980. [DOI] [PubMed] [Google Scholar]
- 2.Marin R, Gorostidi M, Fernandez-Vega F, Alvarez-Navascues R. Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney International - Supplement. 2005:S52–56. doi: 10.1111/j.1523-1755.2005.09910.x. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD, Inscho EW. Adaptations of the renal microcirculation to hypertension. Microcirculation. 2002;9:315–328. doi: 10.1038/sj.mn.7800145. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Spiegelberg BD, Lin F, Dell EJ, Hamm HE. Interaction of Gbetagamma with RACK1 and other WD40 repeat proteins. Journal of Molecular & Cellular Cardiology. 2004;37:399–406. doi: 10.1016/j.yjmcc.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Sklan EH, Podoly E, Soreq H. RACK1 has the nerve to act: structure meets function in the nervous system. Progress in Neurobiology. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isacson CK, Lu Q, Karas RH, Cox DH. RACK1 is a BKCa channel binding protein. American Journal of Physiology - Cell Physiology. 2007;292:C1459–1466. doi: 10.1152/ajpcell.00322.2006. [DOI] [PubMed] [Google Scholar]
- 8.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre A-M, Hulot J-S. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2009;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molostvov G, Fletcher S, Bland R, Zehnder D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cellular Physiology & Biochemistry. 2008;22:413–422. doi: 10.1159/000185484. [DOI] [PubMed] [Google Scholar]
- 10.David KC, Scott RH, Nixon GF. Advanced glycation endproducts induce a proliferative response in vascular smooth muscle cells via altered calcium signaling. Biochemical Pharmacology. 2008;76:1110–1120. doi: 10.1016/j.bcp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. American Journal of Physiology - Cell Physiology. 2008;295:C779–790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobbert P, Schluter H, Schultheiss HP, Reusch HP. Diadenosine polyphosphates Ap3A and Ap4A, but not Ap5A or Ap6A, induce proliferation of vascular smooth muscle cells. Biochemical Pharmacology. 2008;75:1966–1973. doi: 10.1016/j.bcp.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 13.House SJ, Ginnan RG, Armstrong SE, Singer HA. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. American Journal of Physiology - Cell Physiology. 2007;292:C2276–2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mokkapatti R, Vyas SJ, Romero GG, Mi Z, Inoue T, Dubey RK, Gillespie DG, Stout AK, Jackson EK. Modulation by angiotensin II of isoproterenol-induced cAMP production in preglomerular microvascular smooth muscle cells from normotensive and genetically hypertensive rats. J Pharmacol Exp Ther. 1998;287:223–231. [PubMed] [Google Scholar]
- 15.Inoue T, Mi Z, Gillespie DG, Jackson EK. Cyclooxygenase inhibition reveals synergistic action of vasoconstrictors on mesangial cell growth. European Journal of Pharmacology. 1998;361:285–291. doi: 10.1016/s0014-2999(98)00720-1. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Izumi M, Hori M, Kobayashi M, Ozaki H, Karaki H. Xestospongin C, a selective and membrane-permeable inhibitor of IP(3) receptor, attenuates the positive inotropic effect of alpha-adrenergic stimulation in guinea-pig papillary muscle. British Journal of Pharmacology. 2000;130:650–654. doi: 10.1038/sj.bjp.0703358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soulsby MD, Wojcikiewicz RJH. 2-Aminoethoxydiphenyl borate inhibits inositol 1,4,5-trisphosphate receptor function, ubiquitination and downregulation, but acts with variable characteristics in different cell types. Cell Calcium. 2002;32:175–181. doi: 10.1016/s0143416002001525. [DOI] [PubMed] [Google Scholar]
- 18.Bilmen JG, Michelangeli F. Inhibition of the type 1 inositol 1,4,5-trisphosphate receptor by 2-aminoethoxydiphenylborate. Cellular Signalling. 2002;14:955–960. doi: 10.1016/s0898-6568(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Kato Y, Adachi M, Agata N, Tanaka H, Shigenobu K. Effects of cyclopiazonic acid on rat myocardium: inhibition of calcium uptake into sarcoplasmic reticulum. Journal of Pharmacology & Experimental Therapeutics. 1995;272:1095–1100. [PubMed] [Google Scholar]
- 20.Johnson JD, Wittenauer LA. A fluorescent calmodulin that reports the binding of hydrophobic inhibitory ligands. Biochemical Journal. 1983;211:473–479. doi: 10.1042/bj2110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circulation Research. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Fingerle J, Gillespie DG, Jackson EK. Adenosine Inhibits Injury-Induced Neointimal Formation and Human Coronary Artery Smooth Muscle Cell Growth: Role of A(2B)Adenosine Receptor-Mediated Skp-2 Downregulation and p27(Kip1) Upregulation. Hypertension. 2009;54:E94–E95. [Google Scholar]
- 23.Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene. 2007;26:2914–2924. doi: 10.1038/sj.onc.1210091. [DOI] [PubMed] [Google Scholar]
- 24.Mamidipudi V, Zhang J, Lee KC, Cartwright CA. RACK1 regulates G1/S progression by suppressing Src kinase activity. Molecular & Cellular Biology. 2004;24:6788–6798. doi: 10.1128/MCB.24.15.6788-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, Eickelberg O. Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation. 2007;115:2957–2968. doi: 10.1161/CIRCULATIONAHA.106.670026. [DOI] [PubMed] [Google Scholar]
- 26.Hermanto U, Zong CS, Li W, Wang L-H. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Molecular & Cellular Biology. 2002;22:2345–2365. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Zong CS, Hermanto U, Lopez-Bergami P, Ronai Ze, Wang L-H. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Molecular & Cellular Biology. 2006;26:413–424. doi: 10.1128/MCB.26.2.413-424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothberg KG, Burdette DL, Pfannstiel J, Jetton N, Singh R, Ruben L. The RACK1 homologue from Trypanosoma brucei is required for the onset and progression of cytokinesis. Journal of Biological Chemistry. 2006;281:9781–9790. doi: 10.1074/jbc.M600133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahara A, Tsukada J, Tomura Y, Momose K, Suzuki T, Yatsu T, Shibasaki M. Effects of YM218, a nonpeptide vasopressin V(1A) receptor-selective antagonist, on vasopressin-induced growth responses in human mesangial cells. European Journal of Pharmacology. 2006;538:32–38. doi: 10.1016/j.ejphar.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 30.Kwak JO, Kwak J, Kim HW, Oh KJ, Kim YT, Jung SM, Cha SH. The extracellular calcium sensing receptor is expressed in mouse mesangial cells and modulates cell proliferation. Experimental & Molecular Medicine. 2005;37:457–465. doi: 10.1038/emm.2005.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.