Abstract
Emerging evidence suggests that the Notch/Delta-like ligand 4 (DLL4) pathway may offer important new targets for anti-angiogenesis approaches. In this study, we investigated the clinical and biological significance of DLL4 in ovarian cancer. DLL4 was overexpressed in 72% of tumors examined where it was an independent predictor of poor survival. Patients with tumors responding to anti-VEGF therapy had lower levels of DLL4 than patients with stable or progressive disease. Under hypoxic conditions, VEGF increased DLL4 expression in the tumor vasculature. Immobilized DLL4 also downregulated VEGFR2 expression in endothelial cells directly through methylation of the VEGFR2 promoter. RNAi-mediated silencing of DLL4 in ovarian tumor cells and tumor-associated endothelial cells inhibited cell growth and angiogenesis, accompanied by induction of hypoxia in the tumor microenvironment. Combining DLL4-targeted siRNA with bevacizumab resulted in greater inhibition of tumor growth, compared to control or treatment with bevacizumab alone. Together, our findings establish that DLL4 plays a functionally important role in both the tumor and endothelial compartments of ovarian cancer, and that targeting DLL4 in combination with anti-VEGF treatment might improve outcomes of ovarian cancer treatment.
Keywords: RNA interference, Dll4, chitosan, ovarian carcinoma, angiogenesis
Introduction
Ovarian cancer remains the leading cause of death from a gynecologic malignancy among women in the United States. Although tumor-reductive surgery and taxane- and platinum-based chemotherapy regimens are effective treatments for primary disease in the majority of patients with this cancer, recurrence is common and often leads to death. New therapeutic agents are needed to improve survival rates and, eventually, to cure this deadly disease.
The progressive growth of primary tumor and metastasis is dependent on angiogenesis. Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, plays a pivotal role in developmental, physiological, and pathological neovascularization (1-3). Targeting the tumor vasculature is a particularly attractive strategy because of the presumed genetic stability of endothelial cells (4). The recent success of anti-angiogenic therapy with bevacizumab in solid tumors, including ovarian cancer, has confirmed the clinical viability of this approach (5). Despite initial responses, however, most patients eventually experience tumor progression resulting in their death, mainly due to development of drug resistance. The precise molecular mechanisms underlying clinical resistance to anti-VEGF therapies are not well understood. Drug resistance can arise because of alterations in pharmacokinetics, cancer cell–specific abnormalities, and/or alterations in the tumor microenvironment (6-8). Therefore, additional targets for anti-angiogenesis strategies are urgently needed.
The Notch signaling pathway has recently been implicated in tumor angiogenesis, including vessel maturation, pericyte recruitment, branching and cell differentiation, proliferation, survival, and apoptosis. In mammalian cells, this pathway comprises five transmembrane Notch ligands (Jagged 1, Jagged 2, and Delta-like ligands [Dll] 1, 3, and 4) and four Notch receptors (Notch 1-4). Ligand receptor binding leads to cleavage via intramembrane proteolysis by γ-secretase and subsequent translocation from the cell membrane to the nucleus. The Notch intracellular domain interacts with transcription factors to regulate transcription of the basic helix-loop-helix proteins hairy/enhancer of split (HES) and HES-related proteins (HEY).
Dll4 is an endothelium-specific ligand expressed at sites of vascular development and angiogenesis. Dll4 expression has previously been shown to be upregulated within the vasculature of breast, renal, and bladder cancers (9). It has been suggested that VEGF induces Dll4/Notch signaling while Dll4/Notch signaling modulates the VEGF pathway, that Dll4 and VEGF merge to be the “yin and yang” of angiogenesis (10, 11, 12). Recently, some studies have reported that Dll4 blockade inhibits tumor growth by inducing nonproductive angiogenesis manifested by increased vascular density and decreased perfusion in tumors. The precise mechanism of inhibition has not been elucidated, however, and the biological significance of Dll4 in ovarian cancer, especially VEGF-resistant ovarian cancer, is not well understood.
In this study, we examined the clinical, functional, and biological significance of Dll4 in ovarian cancer angiogenesis and tumor progression by using therapeutically relevant Dll4 silencing. Our findings indicate that Dll4 is a potential target for anti-angiogenic therapeutic strategies.
Materials and Methods
Human ovarian cancer specimens
Following approval by the Institutional Review Board, 84 paraffin-embedded epithelial ovarian cancer specimens with available clinical outcome data and confirmed diagnosis by a board-certified gynecologic pathologist were obtained from the Gynecologic Oncology tumor bank of The University of Texas M. D. Anderson Cancer Center. All 84 cases were diagnosed between 1986 and 2003 following primary cytoreductive surgery. Slides of tumor samples were obtained for Dll4 expression analysis. Clinical variables obtained for correlative analyses included age at diagnosis, tumor stage and grade, and vital status of patients relative to disease-specific survival at the time of chart review. An additional 24 paraffin-embedded epithelial ovarian cancer specimens from patients who were treated with an anti-VEGF agent (aflibercept or bevacizumab) were obtained from the Gynecologic Oncology tumor bank.
Immunohistochemical staining
Immunohistochemical staining for Dll4 (1:200 dilution) was quantified by two investigators in a blinded fashion on the basis of percentage of positively stained tumor cells and staining intensity. The Dll4 antibody, which reacts with both mouse and human Dll4, was obtained from Rockland Immunochemicals for Research (Gilbertsville, PA). For the negative control, we used PBS instead of primary antibody. Dll4 expression in the endothelial cell compartment was defined as more than 25% of cells staining positive. The endothelial Dll4 score was dichotomized, with a positive score consisting of the presence of staining with moderate or strong intensity. In short, an overall score (OS 0-3) was generated based on the percentage of positively stained cells plus the intensity of staining (13).
Cell lines and culture
A2780, SKOV3ip, OVCAR3, IGROV, and 2774 human epithelial ovarian cancer cells and pericyte-like cells (10T1/2) were maintained as described previously (14). The derivation and characterization of murine ovarian endothelial cells (MOEC) have been described previously (15). Human umbilical vein endothelial cells (HUVEC) were purchased from Cambrex (Walkersville, MD) and maintained with heparin and gentamicin/amphotericin B.
Dll4 gene silencing in MOEC and A2780 ovarian cancer cells
Nonsilencing control small interfering RNA (siRNA) and human and mouse Dll4 siRNA sequences were obtained from Sigma-Aldrich (St. Louis, MO). The nonsilencing siRNA did not share sequence homology with any known human mRNA (based on a BLAST search). The sequences are included in supplementary Table 3. For in vitro transfection studies, N-TER transfection kit (Sigma-Aldrich, St. Louis, MO) was used per the manufacturer’s guidelines.
Reverse transcriptase polymerase chain reaction
Relative expression of Jag 1, Jag2, Dll1, Dll3, Dll4, Notch1, Notch2, Notch3, and Notch4 in cells representing ovarian cancer (HeyA8, SKOV3ip1, OVAR3, A2774, IGROV), endothelium (HUVEC, MOEC), and pericyte-like (10T1/2) and nontransformed ovarian surface epithelial cells (HIO180) was determined by reverse transcriptase polymerase chain reaction (RT-PCR). Each RT-PCR reaction used 5 μg total RNA isolated from treated cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). Primer sequences, size of PCR products, and annealing temperature are given in supplementary Tables S1and S2. Real time quantitative RT-PCR was performed in an ABI 7500 Sequence Detection system (Applied Biosystems, Austin, TX). The SensiMix™ SYBR Low-ROX Kit was used (Bioline USA Inc, MA, USA).The relative quantification (RQ) was calculated by 2−ΔΔCT.
Western Blot Analysis
Western blot analysis was performed as previously reported (16,17).
DNA extraction and methylation analysis
DNA was extracted from MOEC using standard phenol-chloroform methods. MOEC were treated with immobilized Dll4 (1, 10, or 20 μg/mL). Other MOEC were treated with the demethylating agent azacytidine (AZA; 25 μM), immobilized Dll4 (10 μg/mL), or a combination of the two for 48 hours. Methylation status was determined by methylation-specific PCR using a methylation kit (EZ-96 gold; Zymo Research, Orange, CA). MethPrimer software was used for prediction of the CpG island of VEGF receptor 2 (VEGFR2; NM-002253.2) and design of methylation-specific primers. The sequences of primers for methylated VEGFR2 at the promoter region were TGTTTTTA-GATGCGATTTGTCGTTC (forward) and AAAATAAAAACTCCCTACGTCCGAC (reverse); for unmethlyated VEGFR2 promoter, TTTTTAGATGTGATTTGTTGTTTGG (forward) and AAAATAAAAACTCCCTACATCCAAC (reverse). The PCR conditions were 94°C for 5 minutes with hot start, then 94°C for 45 seconds, 58°C and 60°C for 45 seconds, and 72°C for 45 seconds, repeated for 40 cycles. Image analysis (Scion Image for Windows) was used for semiquantitative measurement of methylated and unmethylated VEGFR2. Methylated VEGFR2 was normalized by comparison with unmethylated VEGFR2. The experiments were repeated three times.
Cell proliferation and migration
Cells were seeded in 12-well plates at 8 × 104 cells/well in replicates of two. After 48 hours, cell growth was arrested, and specific mediators were added to untreated cells. Proliferation was assessed by the BrdU proliferation kit (BD Biosciences, San Jose, CA). The membrane invasion culture system chamber was used to measure the in vitro migration ability of cells, as previously described by our group (16,17).
Animals
Female athymic nude mice (NCr-nu) were purchased from the Animal Production Area of the National Cancer Institute–Frederick Cancer Research and Development Center (Frederick, MD). The animals were kept under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in agreement with current regulations and standards of the United States Department of Health and Human Services, the United States Department of Agriculture, and the National Institutes of Health. Mice used in these experiments were aged 8–12 weeks. Tissue specimens were fixed with either formalin or optimum cutting temperature (OCT; Miles, Inc., Elkhart, IN) or were snap frozen.
Orthotopic implantation of tumor cells
To produce tumors in nude mice, subconfluent cultures of A2780 and SKOV3ip1 cells were lifted with trypsin, mixed with medium containing 10% fetal bovine serum, subjected to centrifugation at 1000 rpm for 5 minutes, and washed in PBS. Mice were injected intraperitoneally (i.p.) with one cell type at a concentration of 2 × 106 cells/0.2 ml for A2780 cells or 1 × 106 cells/0.2 ml for SKOV3ip1cells. Mice were killed 40 to 50 days after tumor cell injection. We have used the intraperitoneal (i.p.) injection model for therapeutic studies since it reflects a typical pattern of ovarian cancer spread in patients with recurrent disease(16, 18).
Treatment and data collection
For systemic delivery of siRNA into both tumor cells and tumor-associated vasculature, we developed and characterized chitosan (CH) nanoparticles and demonstrated that nanoparticles with a 3:1 chitosan:triphosphate ratio (CH3) showed the greatest (75%) incorporation efficiency (16). For all subsequent experiments, therefore, we used the siRNA/CH3 nanoparticles because of their small size, slight positive charge, and high efficiency in incorporating siRNA. To assess tumor growth for long-term therapy experiments, A2780 and SKOV3ip1 cells were injected i.p.; 7 days later, mice were randomized into eight groups (n = 10/group) and underwent the following treatments including bevacizumab, i.p, twice per week or human/murine Dll4siRNA, i.v. twice per week: (1) control siRNA 150 μg/kg; (2) mouse Dll4 siRNA 150 μg/kg; (3) human Dll4 siRNA 150 μg/kg; (4) mouse Dll4 siRNA plus human Dll4 siRNA; (5) bevacizumab 6.25mg/kg; (6) bevacizumab plus mouse Dll4 siRNA; (7) bevacizumab plus human Dll4 siRNA; or (8) bevacizumab plus human Dll4 siRNA and mouse Dll4 siRNA. Mice were killed after 4–6 weeks of therapy, when animals in the control group became moribund. Fifteen minutes before sacrifice, the mice were i.v. injected with 100μl of Hypoxyprobe ™-1 (pimonidazole HCl, 100mg/kg, NPI, Inc, Burlington, MA) through the tail vein, At the time of death, mouse weight, tumor weight, number of nodules, and distribution of tumors were recorded. The individuals who performed the necropsies, tumor collections, and tissue processing were blinded to the treatment group assignments.
Immunofluorescence double staining for CD31 and desmin
Sections were fixed in cold acetone for 15 minutes, blocked with protein blocker for 30 minutes, and then incubated with anti-CD31 antibody (1:500; BD Pharmingen, San Diego, CA) overnight at 4°C, after which they were incubated with Alexa 594-conjugated anti-rat antibody (1:1000; Invitrogen, Eugene, OR) for 1 hour. After being washed with PBS, samples were incubated with anti-desmin (1:400; DakoCytomation, Glostrup, Denmark) antibody for 1 hour and then with Alexa 488-conjugated anti-rabbit antibody (1:1000; Invitrogen) for 1 hour. Samples were then counterstained with Hoechst 33342 for 2 minutes and mounted.
Immunohistochemical staining
Paraffin-embedded tissues were used for detection of proliferating cell nuclear antigen (PCNA, i.e., cell proliferation) and pimonidazole (i.e., hypoxic area). Sections were deparaffinized, rehydrated, and transferred to PBS. After antigen retrieval with citrate buffer (pH 6.0), the sections were blocked with 3% hydrogen peroxide in methanol and protein blocker at room temperature. The sections were then incubated with the monoclonal mouse anti-PCNA PC10 antibody (1:50; DAKO) or Hypoxyprobe-1-Mab 1 (1:50; Natural Pharmacia International, Inc., Burlington, MA) overnight at 4°C. After being washed with PBS, sections were incubated with horseradish peroxidase (HRP)–conjugated rat anti-mouse IgG2a (1:100; Serotec, Harlan Bioproducts for Science, Inc., Madison, WI) for PCNA staining or anti-fluorescein isothiocyanate HRP IgG (1:500; Natural Pharmacia International, Inc.) for 1 hour. CD31 staining was performed on frozen sections. Sections were fixed in cold acetone for 15 minutes, washed with PBS, blocked with protein blocker (4% fish gel), and then incubated with rat monoclonal anti-mouse CD31 (1:800, PharMingen, San Diego, CA) overnight at 4°C. They were then washed with PBS and incubated with HRP-conjugated goat anti-rat IgG (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour. Reactive tissues were visualized by staining with 3,3′-diaminobenzidine (Research Genetics, Huntsville, AL) and counterstaining with Gil’s hematoxylin (BioGenex Laboratories, San Ramon, CA).
Quantification of microvessel density, PCNA, pericyte coverage, and hypoxic area
For quantification, five samples from each group were examined. To quantify microvessel density (MVD) for each sample, the microvessels within five randomly selected 0.159-mm2 fields at ×200 were counted. A single microvessel was defined as a discrete cluster or single cell stained positive for CD31 (CD31+). To quantify PCNA expression, the percentage of positive cells was determined in five random 0.159-mm2 fields at ×200 magnification. For pericyte coverage, the percentage of vessels with at least 50% coverage of associated desmin-positive cells was determined in five random 0.159-mm2 fields at ×200 magnification. Hypoxic area was measured using image J at magnification ×20. The percentage of hypoxic area was determined by subtracting areas of necrosis from total pimonidazole-positive areas and then normalizing by whole section areas.
Statistical analysis
Differences in continuous variables such as mean body weight, tumor weight, MVD and vessel maturation, tumor cell proliferation, and intratumoral hypoxia were analyzed using the Mann-Whitney rank sum test. Statistical analyses were performed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL). A two-tailed p<0.05 was considered statistically significant. Kaplan-Meier survival plots were generated and comparisons between survival curves were made using the log-rank statistic.
Results
Clinical significance of Dll4 in ovarian cancers
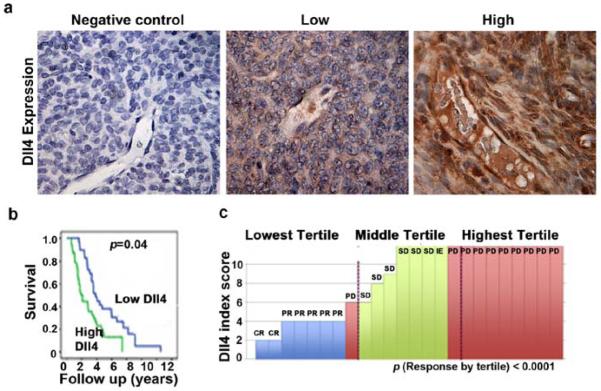
Before investigating the biological significance of Dll4, we examined its clinical significance in ovarian carcinoma by using immunohistochemical peroxidase staining in 84 tumor samples (Fig. 1a). The mean age of patients was 59.3 years (range, 36-88); 83% of tumors were of serous histology and 91% of high-grade histology. Eighty-eight percent of patients had advanced stage disease and 59% had associated ascites. Dll4 was expressed in both tumor and endothelial compartments of ovarian tumors and its expression was not related to stage, grade, or extent of cytoreduction. Patients with high endothelial Dll4 expression had a median survival of 2.07 years, compared to 3.86 years (p<0.004; Fig. 1b) for those with low expression. Multivariate analysis using a Cox proportional hazard model revealed that suboptimal cytoreduction (p=0.04) and endothelial Dll4 overexpression (p<0.01) were independent predictors of poor survival.
Figure 1. Dll4 expression in human ovarian carcinoma.
(a) Representative images of human tumors with low and high Dll4 expression based on immunohistochemical staining. (b) Kaplan-Meier curves for disease-specific mortality of patients whose ovarian tumors expressed high or low levels of Dll4. The log-rank test (two-sided) was used to compare differences between the two groups. Patients whose endothelial highly overexpressed Dll4 had a median survival of 2.07 years, while those whose tumor expressed a low Dll4 level had a median survival of 3.86 years (p<0.004). Multivariate analysis using a Cox proportional hazard model revealed that suboptimal cytoreduction (p=0.04) and Dll4 overexpression (p<0.01) were independent predictors of poor survival. (c) Dll4 index scores were based on Dll4 immunostains of 24 available tumor samples obtained at initial surgery prior to any treatment. Dll4 expression may be related to response to anti-VEGF therapy. Patients whose disease responded to aflibercept/bevacizumab had lower levels of Dll4 than those who had stable or progressive disease. Pictures in panel were taken at original magnification ×200.
Given Dll4’s role in ovarian cancer progression and tumor angiogenesis, we next examined whether Dll4 expression is related to response to anti-VEGF therapy. Dll4 immunostaining was performed on 24 available tumor samples from patients treated with a regimen that included either aflibercept or bevacizumab. Patients whose disease responded to the anti-VEGF treatment had lower levels of Dll4 than those who had stable or progressive disease (Fig.1c). This finding further supports the role of Dll4 in modulating response to VEGF-targeted therapies.
Effect of VEGF and hypoxia on Dll4 levels in ovarian cancer and endothelial cells
On the basis of the clinical observations already described, we next examined the functional and biological roles of Dll4 in ovarian cancer angiogenesis and tumor progression. First, we tested the expression of Notch ligands and receptors in ovarian cancer, endothelial, and pericyte-like cells by RT-PCR (Fig. 2a) and Dll4 expression in vivo SKOV3ip1 and A2780 models by Western blot analysis (Fig.2b). All Notch receptors (1-4) were expressed in MOEC and 10T1/2 pericyte-like cells and Notch receptors 1 and 4 were expressed in HUVEC cells. Dll3 and Dll4 were expressed in the A2780 ovarian cancer cells. Jag 1 and Jag2 were frequently expressed in most of the cells tested. Since the effects of Dll4 could affect Notch signaling on endothelial cells directly or on tumor cells indirectly, we tested whether VEGF and/or hypoxia exposure could result in increased Dll4 levels in ovarian cancer and endothelial cells. We treated MOECs with recombinant VEGF165 (100 ng/mL; Peprotech, Rocky Hill, NJ) for 48 hours, and examined Dll4 levels using real time quantitative RT-PCR. There was a two fold increase in Dll4 in response to VEGF and bFGF treatment under hypoxic conditions (Fig. 2c, d), but not under normoxic conditions (data not shown), which is consistent with previous reports (9). These results suggest that there may be a counter- regulatory mechanism within the tumor microenvironment, which may function to reduce responsiveness to VEGF-targeted agents.
Figure 2. VEGF increases Dll4 expression in endothelial cells.
(a) RT-PCR analysis of Notch ligands and receptors in non-transformed ovarian epithelial (HIO180), ovarian cancer (HeyA8, SKOV3ip1, OVCAR3, A2774, A2780, IGROV), endothelial (HUVEC, MOEC), and pericyte-like (10T1/2) cells. (b) Western blot analysis of Dll4 expression in the tissues obtained from SKOV3ip1 and A2780 ovarian cancer models. (c, d) RT-PCR and real time RT-PCR analysis of Dll4 expression in MOECs after exposure to VEGF or basic fibroblast growth factor (bFGF) for 48 hours under hypoxic (1% O2) conditions.
Effect of Dll4 expression on function of ovarian cancer and endothelial cells
To determine the functional role of Dll4 in MOEC and ovarian cancer cells, we treated MOECs with immobilized Dll4 as an agonist for 48 or 72 hours and tested the expression of VEGFR2 by RT-PCR. Immobilized Dll4 downregulated VEGFR2 at 72 hours (Fig. 3a, and 3b), supporting the existence of a negative feedback effect of Dll4 on VEGFR2 expression, which in turn might reduce sensitivity to anti-VEGF treatment. At the cellular level, we investigated whether Dll4 affects proliferation and migration of MOECs. Treatment with VEGF or Dll4 increased MOECs proliferation by 3.6- and 2.6-fold, respectively, compared with controls. However, treatment with Dll4 inhibited VEGF-induced proliferation by 1.5-fold compared to treatment with VEGF alone (Supplementary Fig. 1a; p>0.05). The effect of immobilized Dll4 on proliferation of the A2780 cells was also investigated and there was a slight increase in proliferation (Supplementary Fig. 1d). Furthermore, silencing Dll4 expression with Dll4 siRNA inhibited proliferation of A2780 ovarian cancer cells by 2.1-fold compared with the control and Dll4 was silenced by >80% in the A2780 cells (Supplementary Fig. 1b). We also investigated the effects of treatment with immobilized Dll4 for 48 hours on cell migration. Immobilized Dll4 increased the migration of MOECs by 2.7-fold compared to untreated cells (p<0.05), but had no significant effect on VEGF-induced migration (Supplementary Fig. 1c).
Figure 3. Effect of immobilized Dll4 on VEGFR2 expression.
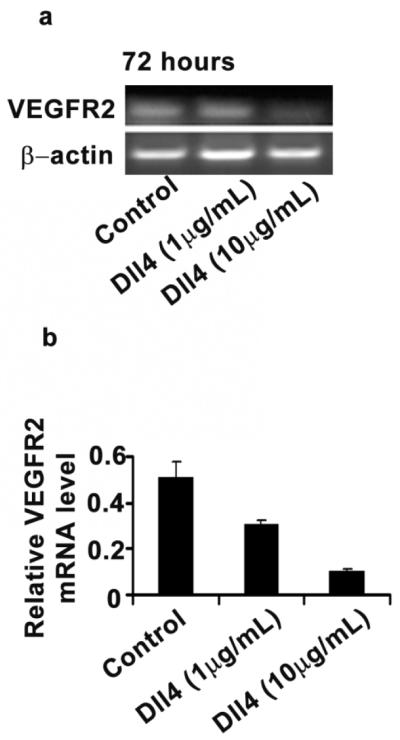
(a) RT-PCR analysis of VEGFR2 expression in MOECs after exposure to immobilized Dll4 (10 μg/mL) for 48 or 72 hours. (b) Semiquantitative measurement of VEGFR2 expression in MOECs. The results represent the mean ± SEM.
Dll4-mediated downregulation of VEGFR2 through promoter methylation
To identify potential mechanisms underlying the decrease in VEGFR2 expression following Dll4 exposure under hypoxic conditions, we considered whether the effects could be epigenetic in nature. We examined whether there is an increase in VEGFR2 promoter methylation in MOECs following exposure to immobilized Dll4 as an agonist under hypoxic conditions. Methylation-specific PCR showed that Dll4 blockade can induce VEGFR2 methylation in MOECs in a dose-dependent manner (Fig. 4a, b). Furthermore, methylation-specific PCR showed that methylation of VEGFR2 was increased by 1.7-fold in MOECs treated with Dll4 and decreased by 2.4 fold in MOECs treated with demethylating agent AZA and immobilized Dll4, compared to that in Dll4 treated MOECs (Fig.4c,d).
Figure 4. Dll4-mediated downregulation of VEGFR through promoter methylation.
(a, b) Methylation-specific PCR analysis of methylation status of VEGFR2 in MOECs after exposure to immobilized mouse Dll4 (1, 10, or 20 μg/mL) for 48 hours. (c, d) Methylation-specific PCR analysis of methylation status of VEGFR2 in MOECs after exposure to AZA (25 μM), immobilized Dll4 (10 μg/mL), or a combination of the two for 48 hours. M: methylated; UM: unmethylated.
In vivo Dll4 gene silencing
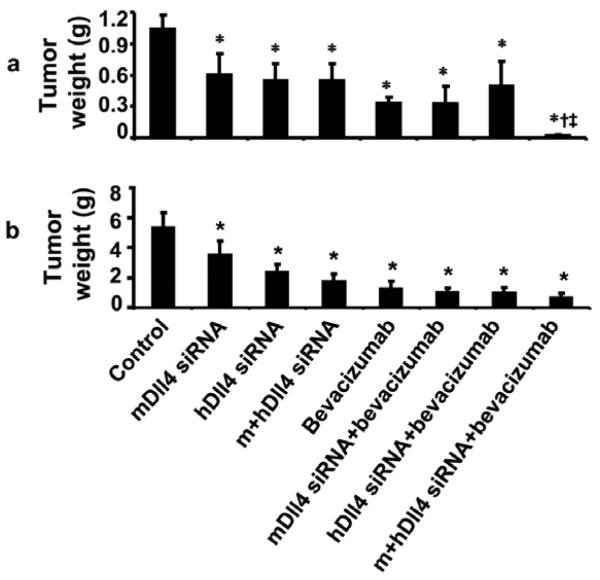
On the basis of our in vitro findings, we next asked whether Dll4 silencing in combination with anti-VEGF treatment in vivo would affect tumor growth and angiogenesis. Moreover, to address the biological significance of tumor versus endothelial cell Dll4 expression, we used human (tumor) and mouse (endothelial) Dll4 siRNA in the A2780 and SKOV3ip1 models of ovarian cancer. We also examined Dll4 protein expression in vivo in the SKOV3ip1 and A2780 models (Fig.2b). Dll4 was expressed in the tissues from both SKOV3ip1 and A2780 ovarian cancer models, which might be reflective of induction under hypoxic conditions. Seven days after tumor cell injection, mice were randomized into eight treatment groups. Single treatment with mouse or human Dll4 siRNA significantly inhibited tumor growth in comparison to treatment with control siRNA. In the A2780 model, treatment with mouse or human Dll4 siRNA reduced tumor weight by 35% and 56%, respectively (p<0.05 for both, Figure 5a). In the same model, treatment with a combination of mouse and human Dll4 siRNA inhibited tumor growth to a greater degree than either siRNA alone, but the difference was not statistically significant. Bevacizumab alone reduced tumor weight by 76% compared to control (p<0.05). Neither mouse nor human Dll4 siRNA alone significantly enhanced the tumor-inhibiting effect of bevacizumab. However, the combination of mouse and human Dll4 siRNA plus bevacizumab resulted in the greatest inhibition of tumor growth, reducing tumor weight by 87% (p<0.05) compared to control, and by 46% compared to bevacizumab alone. The effects of Dll4 siRNA were similar in the SKOV3ip1 tumor model (Fig. 5b). We also examined the effects of treatment on the number of tumor nodules and body weight of mice. The number of tumor nodules was significantly decreased following treatment in the bevacizumab alone, bevacizumab combined with mDll4 or hDll4 alone and bevacizumab combined with mDll4 and hDll4 siRNA in SKOV3ip1 model, in comparison to control (p<0.05, data not shown). In the A2780 model, the tumor nodule numbers were significantly decreased in the hDll4 siRNA treated group, compared to control. However, there was no significant difference in the number of nodules between the hDll4 treated, and mDll4/hDll4 siRNA treated groups. There was no significant difference in the mean mouse weight between any of the different treatment groups (data not shown). These data suggest that Dll4 plays a functionally significant role in both the tumor and endothelial compartments.
Figure 5. In vivo Dll4 silencing: DLL4 gene silencing in vivo in the A2780 (a) and SKOV3ip1 (b) models of ovarian cancer.
Mice were randomly allocated to eight groups (n=10 mice per group) and underwent treatment as follows: (1) control siRNA; (2) mouse Dll4 siRNA; (3) human Dll4 siRNA; (4) mouse Dll4 siRNA plus human Dll4 siRNA; (5) bevacizumab; (6) bevacizumab plus mouse Dll4 siRNA; (7) bevacizumab plus human Dll4 siRNA; (8) bevacizumab plus human Dll4 siRNA and mouse Dll4 siRNA. Mice were euthanized when animals appeared moribund because of significant tumor burden (4 to 5 weeks after cell injection depending on the cell line). *p< 0.05, in compared with control group; †‡ p<0.01, in compared with bevacizumab treatment group.
Effect of Dll4 blockade on microvessel density, vessel maturation, tumor cell proliferation, and intratumor hypoxia
To identify potential mechanisms underlying the efficacy of Dll4 and VEGF blockade, we first examined their potential effects on angiogenesis. As shown in Figure 6a, MVD was significantly increased in the group treated with mouse Dll4 siRNA, while it was significantly decreased in the group treated with human Dll4 siRNA (P<0.05 vs. control for both). The combination of mouse and human Dll4 siRNA reduced MVD in comparison to control (p<0.05). MVD was decreased by bevacizumab monotherapy compared to control (p<0.05) and decreased even further when the anti-VEGF agent was combined with mouse and human Dll4 siRNA (p<0.05).
Figure 6.
Effects of silencing human and murine Dll4 or human Dll4 or mouse plus human Dll4 on biological endpoints, including (a) MVD (CD31); (b) pericyte coverage (desmin); (c) cell proliferation (PCNA); and (d) hypoxia pimonidazole. Tumors harvested following 3 to 4 weeks of therapy were stained for CD31 (red) and desmin (green). All pictures were taken at original magnification ×200. The bars in the graphs correspond sequentially to the labeled columns of images at left. Error bars represent SEM. *p<0.01; §p<0.001.
It has been reported that Dll4 plays an important role in pericyte recruitment (19). We examined the extent of pericyte coverage using dual immunofluorescence staining for desmin (a pericyte marker) and CD31. As shown in Figure 6b, pericyte coverage was significantly decreased in the group treated with mouse Dll4 siRNA (P<0.05) vs. control, but was not altered in other treatment groups. To determine the effect of Dll4 blockade on tumor cell proliferation, we performed immunohistochemical staining for PCNA. As shown in Figure 6c, PCNA was significantly decreased in groups treated with mouse or human Dll4 alone or in combination (P<0.05 in all), compared to control. Proliferation was decreased by bevacizumab monotherapy, bevacizumab combined with mouse, or human Dll4 siRNA, or both mouse and human Dll4 siRNA compared to control (p<0.05), and decreased even further when it was combined with mouse and human Dll4 siRNA (p<0.05 vs. bevacizumab).
Because it has been suggested that blockage of Dll4 inhibits tumor growth and produces nonproductive angiogenesis, which is accompanied by increased intratumoral hypoxia (11), we measured viable hypoxic areas by staining tumor sections with pimonidazole. As shown in Figure 6d, tumors (A2780 model) from groups treated with Dll4 siRNA showed significant increases in intratumoral hypoxia. The proportion of hypoxic area increased 2.3-, 1.8-, and 2.5- fold compared to control (p<0.05 in all vs. control) in groups treated with mouse, human, and combination Dll4 siRNA. Bevacizumab monotherapy also induced hypoxia in the A2780 model in comparison to control, but greater intratumoral hypoxia was seen in the combination treatment groups of bevacizumab with mouse Dll4 siRNA, human Dll4 siRNA, and mouse plus human Dll4 siRNA, respectively (p<0.05 in all vs. bevacizumab).
Discussion
The key findings of this study are that Dll4 overexpression was significantly associated with worse overall patient survival and was a predictor of response to anti-VEGF treatment. Moreover, immobilized Dll4 downregulated VEGFR2 expression in endothelial cells directly through methylation of VEGFR2 at its promoter region. Silencing Dll4 in tumor cells and in tumor-associated endothelial cells resulted in inhibition of ovarian cancer growth and deregulation of angiogenesis, accompanied by induction of hypoxia in the tumor microenvironment. Our data suggest that Dll4 plays an important role in ovarian cancer and that targeting Dll4 in combination with anti-VEGF treatment might hold promise for improving the efficacy of ovarian cancer treatment.
Current experimental evidence suggests that resistance to anti-VEGF therapies may be related to activation and/or upregulation of alternative proangiogenic signaling pathways within the tumor (e.g., VEGF, fibroblast growth factors 1 and 2, ephrins A1 and A2, angiopoietin 1, platelet-derived growth factor A, and interleukin-8); to recruitment of vascular progenitor cells and proangiogenic monocytes from the bone marrow, such as tumor-associated macrophages, immature monocytes, VEGFR1+ hemangiocytes, CXCR4+ bone marrow–derived cells, and CD45+ and CD11b+ myeloid cells within the tumor; to increased pericyte coverage of the tumor vasculature, serving to support its integrity and attenuate the necessity for VEGF-mediated survival signaling; and to activation and enhancement of invasion and metastasis to provide access to normal tissue vasculature without obligate neovascularization (20). The tumor vasculature is complex and the process of angiogenesis in cancers relies on many factors that are not fully understood (21, 22). We show for the first time that Dll4 plays a functionally important role in both the tumor and endothelial compartments of ovarian cancer and expression of endothelial Dll4 was a predictor of response to anti-VEGF treatment, which is consistent with a recent correlative study linked to a clinical trial in patients with metastatic breast cancer treated with capecitabine or capecitabine plus bevacizumab (23).
Whether activated Dll4/Notch signaling has an effect on proliferation of endothelial cells is not fully understood. Some studies have reported that blockage of the Notch signaling pathway was associated with enhanced angiogenic sprouting and branching, resulting in marked increase in tumor vessel density, but decrease in vessel function (11, 12). We show here that MVD was significantly increased by blockage of mouse Dll4, which is consistent with other reports (11). MVD was decreased, however, by silencing tumor Dll4 in the tumor cells can overcome effect of silencing endothelial Dll4 on increasing tumor vessel density.
Dll4 and VEGF merge to be the yin and yang of angiogenesis (24, 25). VEGF-induced Dll4/Notch signaling can induce a negative feedback loop in which VEGF induces Dll4, which in turn activates Notch in neighboring endothelial cells to suppress VEGFR2 levels. Decreased expression of VEGFR2 by activated Notch/Dll4 signaling may be explained by epigenetic modification of the VEGFR2 gene. Our in vitro findings support the suggestion that Dll4 can suppress VEGFR2 expression in endothelial cells. Prior to our work, the mechanism by which Dll4 suppresses VEGFR2 expression in endothelial cells was not known. It has been suggested that the Notch intracellular domains of Notch receptors and Notch-targeted genes (Hes1 and Hey1) might be involved in downregulation of VEGFR2, possibly through binding on the VEGFR2 promoter region, especially at GC-rich regions. We show here, for the first time, that Dll4 can directly increase VEGFR2 methylation levels in endothelial cells, which in turn might inhibit response to VEGF. We searched our previous microarray analysis of tumor endothelial cells for genes that could potentially regulate methylation (26). EZH2 (16,26) and DNMT were among the genes that were overexpressed in tumor endothelial cells. We also examined whether DNMT is required for VEGFR2 methylation in MOECs, and our findings suggest that AZA at least partially inhibits VEGFR2 methylation induced by immobilized Dll4, and that DNMT might be involved in VEGFR2 methylation after activation of Dll4/Notch signaling. Since it has been suggested that there is cross-talk between Notch and the bone morphogenetic protein/transforming growth factor beta, JAK-STAT, Ras, and hypoxia-inducible factor signaling pathways to enhance activation of Hey/Hes expression (27,28), we cannot rule out the possibility that other coactivators and repressors are also involved in this process. The exact mechanisms of VEGFR2 regional methylation need to be investigated further.
In summary, our data indicate that Dll4 plays a functionally important role in both the tumor and endothelial compartments of ovarian cancer and dual targeting the Dll4/Notch pathway and VEGF signaling pathway may lead to new therapies.
Supplementary Material
Acknowledgments
The authors thank Kathryn Hale in the Department of Scientific Publications for reviewing the manuscript.
Portions of this work were supported by the NIH (CA 110793, 109298, P50 CA083639, P50 CA098258, CA128797, RC2GM092599, U54 CA 151668), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC073399, OC093146, BC085265), NSC-96-3111-B, the Zarrow Foundation, the Marcus Foundation, the Betty Anne Asche Murray Distinguished Professorship, NCI institutional Core Grant CA16672 and the Laura and John Arnold Foundation.AMN and RLS were supported by NCI-DHHS-NIH T32 Training Grant (T32 CA101642). MMKS was supported by the NIH/NICHD WRHR Grant (HD050128) and the GCF Ovarian Cancer Research Grant. MCH and LYL were supported by the #NSC 97-3111-B-039. WH was partially supported by GCF/Florence & Marshall Schwid Ovarian Cancer Award and Phi Beta Psi Sorority.
References
- 1.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996b;380(6573):439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 2.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 3.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80(1):98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol. 2007;25(20):2902–8. doi: 10.1200/JCO.2007.12.1509. [DOI] [PubMed] [Google Scholar]
- 6.Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13(6):345–8. doi: 10.1097/PPO.0b013e31815a7b69. [DOI] [PubMed] [Google Scholar]
- 7.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11(6):219–30. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25(8):911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 9.Patel NS, Dobbie MS, Rochester M, Steers G, Poulsom R, LeMonnier K, et al. Up-regulation of endothelial delta-like 4 expression correlates with vessel maturation in bladder cancer. Clin Cancer Res. 2006;12(16):4836–44. doi: 10.1158/1078-0432.CCR-06-0285. [DOI] [PubMed] [Google Scholar]
- 10.Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis? Trends Mol Med. 2007;13(9):389–95. doi: 10.1016/j.molmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 12.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67(23):11244–53. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 13.Lin YG, Han L, Merritt W, Landen CN, Deavers MT, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109(2):332–340. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 14.Halder J, Landen CN, Jr., Lutgendorf SK, Li Y, Jennings NB, Fan D, et al. Focal adhesion kinase silencing augments docetaxel-mediated apoptosis in ovarian cancer cells. Clin Cancer Res. 2005;11(24 Pt 1):8829–36. doi: 10.1158/1078-0432.CCR-05-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63(11):2971–6. [PubMed] [Google Scholar]
- 16.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18(2):185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165(4):1087–95. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 19.Schadler KL, Zweidler-McKay PA, Guan H, Kleinerman ES. Delta-like ligand 4 plays a critical role in pericyte/vascular smooth muscle cell formation during vasculogenesis and tumor vessel expansion in Ewing’s sarcoma. Clin Cancer Res. 2010;16(3):848–56. doi: 10.1158/1078-0432.CCR-09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Jubb AM, Miller KD, Rugo HS, Harris AL, Chen D, Reimann JD, Cobleigh MA, Schmidt M, Langmuir VK, Hillan KJ, Chen DS, Koeppen H. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17(2):372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 25.Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer. 2008;99(8):1204–9. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67(4):1757–68. doi: 10.1158/0008-5472.CAN-06-3700. [DOI] [PubMed] [Google Scholar]
- 27.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102(6):637–52. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 28.Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11(1):41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.