Abstract
Aims
Methadone maintenance treatment has been made available in China in response to the rapid spread of HIV, but high rates of dropout and relapse are problematic. The aim of this study was to apply and test if a contingency management (or motivational incentives) intervention can improve treatment retention and reduce drug use.
Design
Random assignment to usual care with (n=160) or without (n=159) incentives during a 12-week trial. Incentives participants earned draws for a chance to win prizes on two separate tracks targeting opiate-negative urine sample or consecutive attendance; the number of draws increased with continuous abstinence or attendance.
Setting
Community-based methadone maintenance clinics in Shanghai and Kunming.
Participants
The sample was 23.8 % female, mean age was 38, mean years of drug use was 9.4, and 57.8 % had injected drugs in the past 30 days.
Measurements
Treatment retention and negative drug urine.
Findings
Relative to the treatment-as-usual (control) group, better retention was observed among the Incentives group in Kunming (44% vs. 75%), but no difference was found in Shanghai (90% vs. 86%). Submission of negative urine samples was more common among the Incentive group than the usual care (74% vs. 68% in Shanghai, 27% vs. 18% in Kunming), as was the longest duration of sustained abstinence (7.7 wks vs. 6.5 in Shanghai, 2.5 vs. 1.6 in Kunming). The average total prize amount was 371 Yuan (or $55) per participant (527 for Shanghai vs. 216 in Kunming).
Conclusions
Contingency management improves treatment retention and drug abstinence in methadone maintenance treatment clinics in China, although there can be considerable site differences in magnitude of effects.
INTRODUCTION
China faces the challenge of dual epidemics of drug use and HIV/AIDS [1]. Drug abuse in China increased dramatically in the 1980s and spread nationwide; drug addiction and related problems are now among China’s greatest challenges. The cumulative number of registered drug users in China increased from 70,000 in 1990 to 1.3 million in 2009, of which 980,000 were registered as opiate users [2]. The actual number of drug addicts is estimated to be more than 3.5 million. At least 75%–85% of registered drug addicts use opiates, 50%–70% of whom inject the drug. In 2007, approximately 700,000 people in China were HIV-positive [3]. Unprotected sex and injection drug use are two major contributors to new HIV cases [4]. Thus, intravenous opiate use represents a major contributor to HIV transmission in China [5–7].
Primarily to address the HIV problem, China recently implemented methadone maintenance treatment (MMT). China’s first eight MMT clinics were established in 2004 [1] and by 2009 China had opened more than 680 clinics serving some 242,000 patients nationwide [8]. This rapid expansion signals that the Chinese government understands the critical link between drug use and control of HIV/AIDS. Nevertheless, most clinics in China do not offer counseling or other behavioral interventions [7] and many face high dropout and relapse rates [9].
To address issues of treatment retention and opiate use in China’s MMT clinics, we adapted an intervention developed in the United States and tested its effectiveness in improving treatment retention and outcomes. The motivational incentives approach, a form of contingency management, applies well-established psychological principles of reinforcement and punishment to change targeted behavior (e.g., increase attendance, decrease illicit drug use) by manipulating the contingent delivery of salient reinforcers, occurring as a consequence of performance [10,11]. Contingency management is an effective evidence-based practice [12–14] and has been shown to improve retention and increase abstinence with various substance-abusing populations [11]. The U.S. National Drug Abuse Treatment Clinical Trials Network (CTN) further tested the motivational incentives intervention with lower-cost incentives using the “fishbowl” method (variable magnitude of reinforcement) [15,16]. Study results demonstrated that this intervention effectively increased stimulant abstinence in community-based MMT clinics [16].
The present study adapted the CTN motivational incentives intervention for use in Chinese MMT settings and experimentally tested its effectiveness in improving treatment retention and drug use among MMT patients. The study was conducted in two sites: (1) Kunming, the capital city of Yunnan province, borders the infamous Golden Triangle and has the most problems with drug trafficking and abuse, including high HIV rates. Currently there are 8 MMT clinics in Kunming serving approximately 2,900 patients. (2) Shanghai, the most populous and developed urban city in China, has 13 million permanent residents and 4 million transient residents. There are 14 MMT clinics in Shanghai providing services for about 2,700 patients but the HIV rate is very low. The two sites also differ considerably in living standards (e.g., per capita gross domestic product is about 27,140 Yuan [$3,992 US] in Kunming versus 88,398 Yuan [$13,000 US] in Shanghai). Unique to Shanghai, a non-governmental social work services organization exists to help drug users recover in the community following release from compulsory drug treatment centers [17]. All illicit drug users are monitored and contacted weekly by a social worker and are tested regularly for drug use for three years.
To adapt a motivational incentives intervention for Chinese settings, we conducted formative research to solicit feedback and suggestions from local providers and patients on the feasibility and acceptability of the research protocols. Participants expressed enthusiasm for the study and provided constructive suggestions for finalizing protocols. The senior investigators provided training before trial implementation.
The present study assessed the effectiveness of motivational incentives procedures in China by comparing a Motivational Incentives approach along with standard MMT to standard MMT alone for the treatment of opiate-dependent patients. The incentive procedure utilized a variable magnitude of reinforcement designed to enhance MMT retention and to reduce drug use among MMT patients. We hypothesized that the intervention would be effective and would produce improvements in treatment retention and opiate use.
METHODS
Study Participants
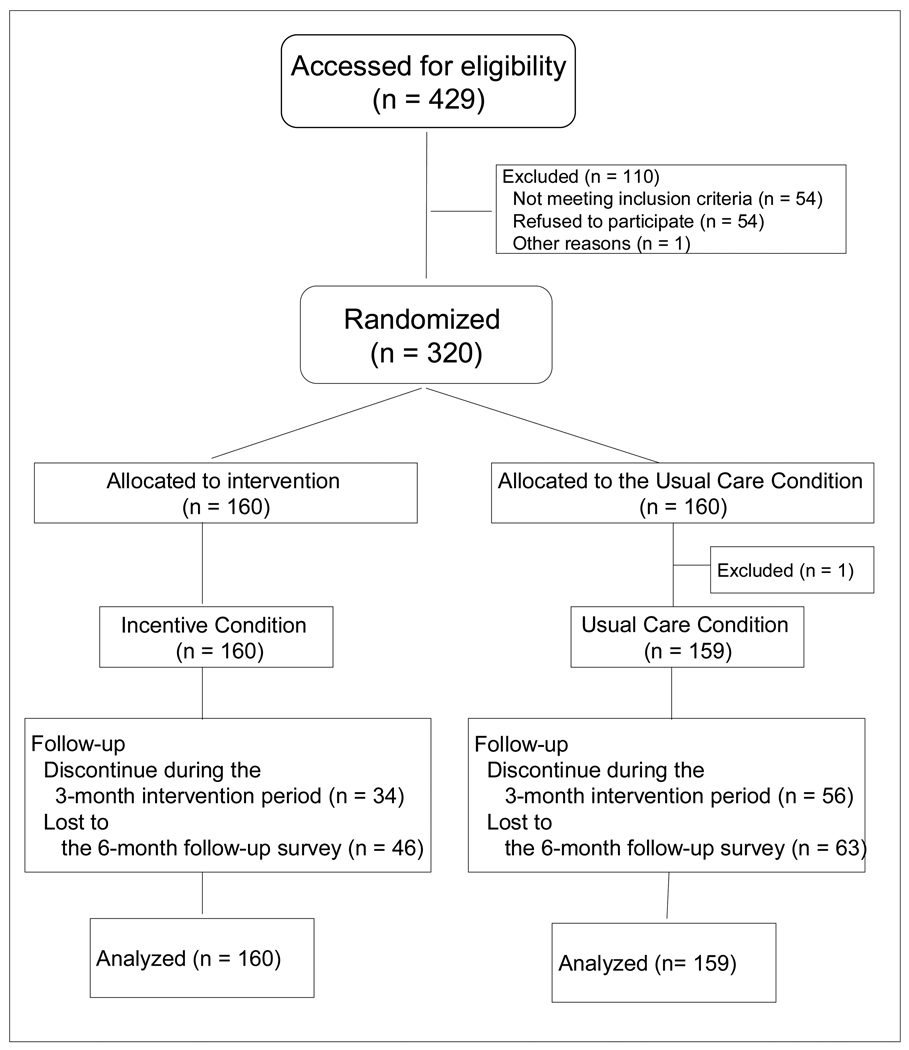
A total of 319 patients were enrolled in the study (159 from Shanghai, 160 from Kunming). Consecutive admissions to the five participating clinics (3 in Shanghai, 2 in Kunming) during 2009–2010 were invited to participate. Overall, of 429 participants invited, 320 agreed to participate and provided signed informed consent. Using a computer-generated randomization sheet, participants were randomly assigned to the Incentive group (n=160) or the Usual Care group (n=160) (Figure 1). Clinic staff conducted randomization, enrollment, and assignment to intervention groups. One participant randomly assigned to the Usual Care group was mistakenly given the Incentive condition, and thus was dropped from analysis. The study was approved by institutional review boards in Kunming, Shanghai, and UCLA, and was overseen by a Data Safety Monitoring Board.
Figure 1.
Flow diagram of participants through the trial
Study Design and Procedures
A two-group random assignment design was utilized. Participants were assigned to receive usual care or usual care plus a Motivational Incentives intervention. The intervention lasted 12 weeks, with follow-up interviews scheduled at 1, 3, and 6 months after study enrollment.
Usual care treatment
Those assigned to usual care received standard MMT procedures for 12 weeks. Usual Care MMT included a physical exam, weekly urine testing for opiates, and daily methadone ingestion under supervision (after initial dosage adjustment and stabilization). No counseling sessions were offered, except that in Shanghai social workers maintained contact with patients outside MMT.
Incentive procedures
In addition to usual care services, the Motivational Incentives group was given the opportunity to receive tangible incentives contingent on targeted behaviors. Incentives were provided for 2 target behaviors on 2 independent reinforcement “tracks:” (1) methadone ingestion observed, (2) submission of drug-negative urine specimens. When a participant tested negative for opiates or had visited the clinic and ingested methadone in the previous 3 days, s/he could make draws. A computerized “fishbowl” method was used to determine each incentive draw amount. Half of draws said “good job” and resulted in an “encouragement” incentive award (1 Yuan; $0.15 US), 30% resulted in a small incentive (5 Yuan; $0.74 US), 15% in a medium incentive (10 Yuan; $1.47 US), and 5% in a large incentive (20 Yuan; $2.94 US). Procedures were intended to prevent potential tampering and ensure the proper number, probability distribution, and amount. The number of times that participants could make computerized draws was based on the submission of a drug-free urine sample and/or attendance compliance. The results of the draw procedure, the appropriate incentive, and the incentive(s) selected were all recorded automatically. The intervention was delivered by the clinical staff (methadone prescribers or nurses).
Escalating schedule of draws
Patients earned at least one draw for meeting each target behavior (e.g., negative urine sample, 3 days of attendance). The number of draws allowed at each target behavior escalated with consecutive weeks of meeting the criteria. Specifically, an additional draw was added for each consecutive week that the participant tested negative or was treatment compliant. Missed attendance or positive samples resulted in a reset to 1 in number of draws for the next incentive opportunity. The escalating schedule is designed to sustain long periods of attendance and abstinence and, as such, the highest rates of reinforcement were scheduled late in the protocol for continuously attending and abstinent participants [18,19]. To counteract discouragement from the low reinforcement rate expected early in the escalating draw protocol, a single 20 Yuan bonus incentive was given after two consecutive weeks of attendance or opiate abstinence.
Study Measures
Baseline data were collected with the Addiction Severity Index (ASI) [20,21] which has been validated in Chinese settings [22]. Clinical records included daily methadone dose and weekly urine test results over the intervention period. Draws and incentive awards were recorded by the computerized incentive program. The ASI was repeated at 3 follow-up interviews scheduled 1, 3, and 6 months after study enrollment.
Outcome measures
Treatment retention
Treatment retention was defined as number of days that elapsed between the first and last methadone uptake during the 12-week intervention period.
Opiate use
Urine test results were examined in several ways: (1) percent of opiate negative urine samples at each week, (2) overall percentage of opiate positive samples, (3) total number of opiate negative samples submitted by each participant, and (4) longest duration of sustained abstinence, defined as the most consecutive weeks of opiate negative samples. Self-reported ASI data were used to assess drug use at follow-up. Specifically, the mean numbers of days in which participants reported using opiates during the preceding month were contrasted between study groups.
Data Analysis
Group comparisons (demographic measures and outcomes) were made using 2×2 ANOVA for main effects of incentive condition, study site, and their interaction. Treatment retention was compared across groups using a Cox proportional hazard model. An event was considered to have occurred when a patient dropped out of treatment; therefore, it was defined as the last methadone uptake if it occurred before week 12. Data were censored at day 84 of the study if a patient continued to attend the clinic and take methadone. Results are reported using hazard ratios and 95% confidence intervals (CI). For binary variables that repeated over time (e.g., whether submitted samples tested negative for opiates), analysis was conducted using generalized estimating equations. For continuous variables that repeated over time (e.g., number of days using in the past 30 days, methadone dose), the analysis was conducted using a mixed-effects model.
RESULTS
Baseline Characteristics
Baseline patient characteristics are provided in Table 1. No differences were observed in patient characteristics (23% female, mean age of 38 years, 9 years of education, 37% married, and 44% employed) and opiate use (almost all patients reported using opiate in the 30 days prior to treatment entry) across conditions.
Table 1.
Demographics and Drug Use at Baseline
| Shanghai (n=159) | Kunming (n=160) | Total (n=319) | ||||
|---|---|---|---|---|---|---|
| Incentive Group (n=80) |
Usual Care Group (n=79) |
Incentive Group (n=80) |
Usual Care Group (n=80) |
Incentive Group (n=160) |
Usual Care Group (n=159) |
|
| Female, % | 18.8 | 25.3 | 27.5 | 23.8 | 23.1 | 24.5 |
| Mean age (SD)b | 40.2 (7.9) | 39.5 (7.9) | 36.2 (7.9) | 36.3 (7.7) | 38.2 (8.1) | 37.9 (7.9) |
| Less than high school, %b | 52.5 | 60.8 | 82.5 | 74.7 | 67.5 | 67.7 |
| Marital status, % | ||||||
| Married | 31.3 | 41.7 | 35.0 | 40.5 | 33.1 | 41.1 |
| Previously married | 22.5 | 8.9 | 13.8 | 21.5 | 18.1 | 15.2 |
| Never married | 46.3 | 49.4 | 51.3 | 38.0 | 48.8 | 43.7 |
| Employed, %b | 38.8 | 27.9 | 55.0 | 55.7 | 46.9 | 41.8 |
| Arrest history, %b | 26.3 | 25.3 | 50.0 | 52.5 | 38.1 | 39.0 |
| Prior alcohol/drug treatment-lifetime, %b | 82.5 | 92.4 | 71.3 | 67.5 | 76.9 | 80.0 |
| Drug use history | ||||||
| Age of first use (SD)b | 29.5 (7.6) | 26.8 (7.8) | 25.2 (6.0) | 25.6 (6.9) | 27.3 (8.1) | 26.2 (7.4) |
| Years of use (SD)b | 7.7 (4.2) | 9.3 (5.2) | 9.8 (6.2) | 10.7 (7.4) | 8.8 (5.4) | 10.1 (6.4) |
| Injection in past 30 days, % | 60.5 | 65.8 | 55.3 | 49.3 | 57.9 | 57.6 |
significant main effect of group (p<.05);
significant main effect of site (p<.05);
significant interaction effect (p<.05)
There were differences observed across sites. Shanghai patients were older, had more education, and fewer were employed. Also, Shanghai patients had fewer arrests, more prior treatment, and initiated opiate use at an older age. Because of considerable differences across sites, we report outcomes separately by site and for the total sample.
Treatment Retention
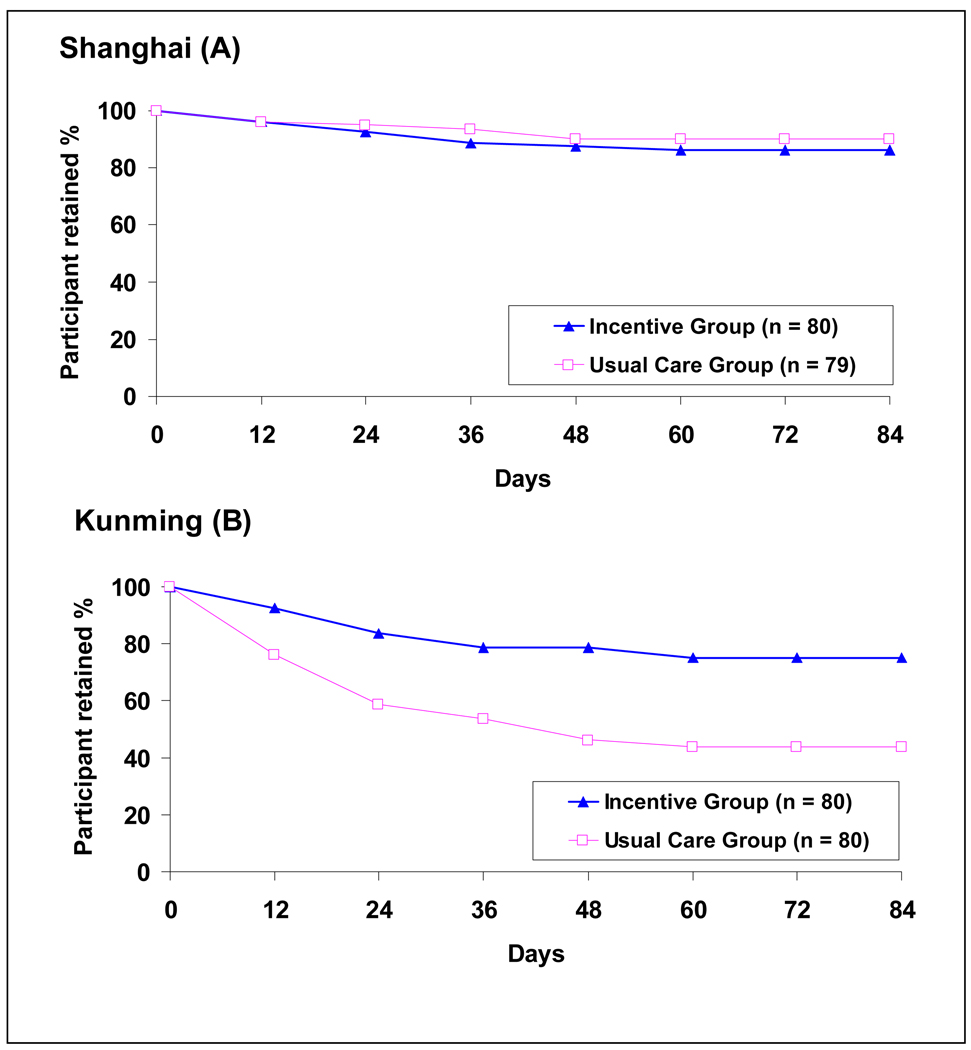
At the end of the 12-week trial, 81% of the Incentive group versus 67% of the Usual Care group remained in treatment (p < .05) (Table 2a). However, the difference was greater in Kunming (75% vs. 44%, p< .05) than in Shanghai (86% vs. 90%). On average, Shanghai participants in both conditions spent 11 weeks in treatment during the intervention period. In contrast, Kunming participants in the Incentive group spent 9.8 weeks in treatment, which was significantly longer than the 6.7 weeks of the Usual Care group.
Table 2.
Treatment Retention and Drug Use
| Shanghai (n=159) | Kunming (n=160) | Total (n=319) | ||||
|---|---|---|---|---|---|---|
| Incentive Group (n=80) |
Usual Care Group (n=79) |
Incentive Group (n=80) |
Usual Care Group (n=80) |
Incentive Group (n=160) |
Usual Care Group (n=159) |
|
| (a) Treatment retention and completion | ||||||
| Weeks in treatment, mean (SD)a, b, c | 10.8 (3.1) | 11.1 (2.8) | 9.8 (3.9) | 6.7 (4.9) | 10.3 (3.6) | 8.9 (4.6) |
| Percentage of participants completing 12 weeks treatment, %a, b, c | 86.3 | 89.9 | 75.0 | 43.8 | 80.6 | 66.7 |
| (b) Drug use | ||||||
| Longest duration of sustained abstinence (weeks)a, b | 7.7 (4.2) | 6.5 (3.8) | 2.5 (3.4) | 1.6 (3.1) | 5.1 (4.6) | 4.1 (4.2) |
| Total # of opiate negative sample submitted, mean (SD)b | 8.1 (4.0) | 7.7 (3.5) | 2.9 (3.6) | 1.9 (3.4) | 5.5 (4.6) | 4.8 (4.5) |
| Percentage of negative samples among total samples, %b | 67.5 | 64.0 | 23.8 | 15.8 | 45.5 | 39.8 |
| Percentage of negative samples among submitted samples, %a, b | 73.5 | 68.3 | 27.4 | 18.5 | 50.3 | 43.2 |
significant main effect of group (p<.05);
significant main effect of site (p<.05);
significant interaction effect (p<.05)
Retention over time was virtually identical for the 2 groups in Shanghai, but the pattern in Kunming was different with the decline in the Usual Care group significantly more than that in the Incentive group (Figure 2). Cox model results showed that time to dropout (survival curves) was significantly different by incentive condition, study site, and their interaction. Compared to patients in the Usual Care group, patients in the Incentive group were less likely to dropout (HR=0.471, p=0.0009). Compared to Kunming patients, Shanghai patients were less likely to dropout (HR=0.245, p<0.001). Of the 4 groups (incentive condition by site), the Usual Care group in Kunming was most likely to dropout and was more than twice as likely to dropout as the Incentive group in Kunming (HR=0.343, p<0.001). The two groups in Shanghai demonstrated no differences from each other and both were significantly less likely to dropout (Usual Care group with HR=0.145, p<0.0001; Incentive group with HR=0.178, p<0.0001) than the Usual Care group in Kunming.
Figure 2.
Treatment retention for Shanghai (A) and Kunming (B); See text for Cox modeling results
Drug Use
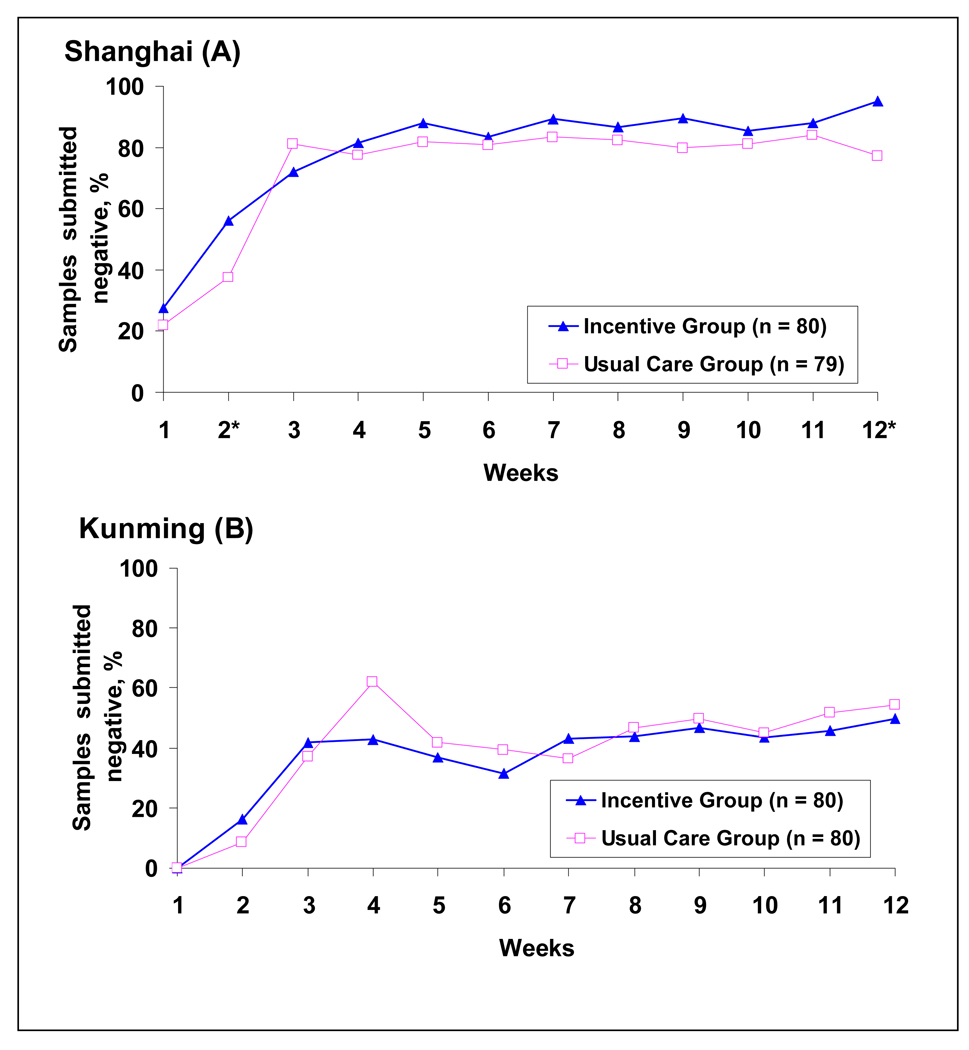
The mean (SD) consecutive weeks with detected abstinence was 7.7 (4.2) for Incentive participants vs. 6.5 (3.8) for Usual Care participants in Shanghai, and it was 2.5 (3.4) vs. 1.6 (3.1) in Kunming (Table 2b). The percentage of negative samples was also higher in the Incentive group than the Usual Care group in both sites.
Figure 3 shows the percentage of opiate negative samples during the 12-week protocol. In both groups and for both sites, percentage of negative urine results increased over the first few study visits, but with a few exceptions there were no differences between the two conditions at each time point. At the conclusion of the intervention (week 12), significantly more negative urine results were found in the Incentive group in Shanghai.
Figure 3.
Percentage of negative urine among submitted samples across 12 weeks for Shanghai (A) and Kunming (B); *p< .05; See text for GEE modeling results
The Generalized Estimating Equation (GEE) was applied to examine trajectories of negative urine test results across 12 weeks by incentive condition and site. The results showed: (1) negative urine results increased across 12 weeks (slope=0.915, p<0.0001; quadratic=-0.056, p<0.0001); (2) negative urines at week 1 (intercept) were significantly different by site (p<0.0001), but not by condition (p=0.27). In contrast to Shanghai, Kunming had a lower percentage (1.34 lower) of negative urine test results at week 1; (3) slope of trajectories was significant by site (slope diff=−0.27, p=0.02), but was not significant by condition (p=0.43). In contrast to Shanghai, Kunming had a lower acceleration over time (0.915 vs. 0.645).
Self-reported opiate use in the past 30 days considerably decreased from almost daily use at baseline to 4.2 days (Incentive group) or 5.4 days (Usual Care group) at 1-month follow-up (Table 3). Group differences at the 3- and 6-month follow-up were not significant. To assess changes over time, mixed-effects model results showed: (1) days of opiate use significantly decreased. There was a significant decrease from intake to 1-month follow-up, and the deceleration rate decreased thereafter (slope=−13.01 p<0.0001; quadratic=1.60, p<0.0001); (2) days of opiate use at intake (intercept) were significantly different by site (p<0.001), but not by condition (p=0.505). In contrast to Shanghai, Kunming had more days (4.56 higher) of opiate use at intake; and (3) the slope of trajectories differed significantly by site (slope diff=0.589, p=0.0014), but was not different by condition (p=0.399). Patients in Kunming had less deceleration than those in Shanghai (−13.01 vs. −12.43) over time.
Table 3.
Self-Reported Opiate Use at Baseline and Follow-ups
| Shanghai (n=159) | Kunming (n=160) | Total (n=319) | ||||
|---|---|---|---|---|---|---|
| Incentive Group (n=80) |
Usual Care Group (n=79) |
Incentive Group (n=80) |
Usual Care Group (n=80) |
Incentive Group (n=160) |
Usual Care Group (n=159) |
|
| Opiate use in past 30 days, mean (SD) | ||||||
| Baseline | 26.5 (7.1) | 24.7 (9.3) | 27.4 (6.5) | 26.8 (7.5) | 27.0 (6.8) | 25.8 (8.5) |
| 1 month FUb, c | 0.9 (3.7) | 2.3 (6.8) | 8.1 (9.9) | 11.8 (10.6) | 4.2 (8.1) | 5.4 (9.4) |
| 3 month FUb | 1.3 (5.7) | 1.0 (4.5) | 8.2 (9.9) | 8.6 (9.2) | 4.1 (8.4) | 3.3 (7.2) |
| 6 month FUb | 0.3 (1.7) | 0.2 (1.0) | 9.3 (9.0) | 8.7 (10.1) | 4.2 (7.5) | 3.1 (7.1) |
significant main effect of group (p<.05);
significant main effect of site (p<.05);
significant interaction effect (p<.05)
See text for mixed-effects modeling results
Methadone Dose
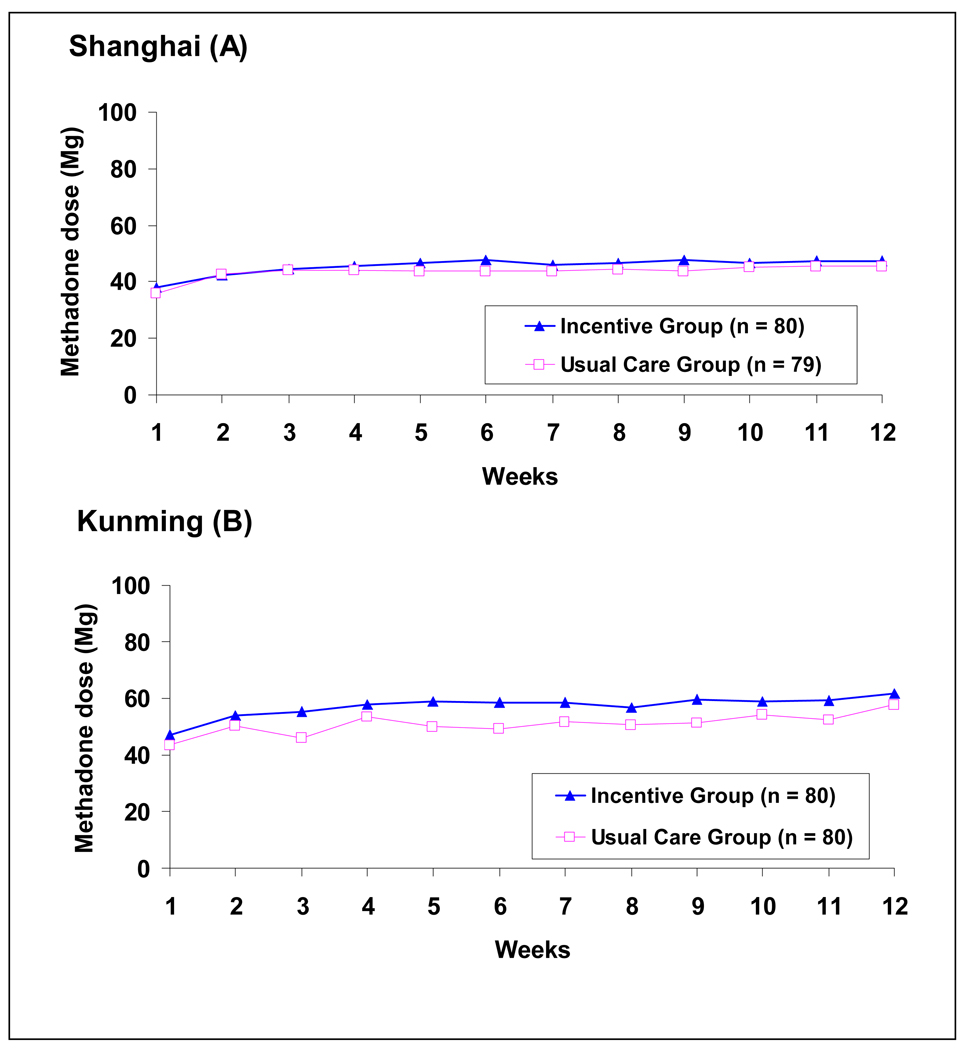
The mean methadone dose at stabilization was about 46mg in Shanghai and 55mg in Kunming, but no variation was found across conditions (Figure 4). Dose was not associated with retention (r=.11) or with the total number of negative urine samples (r=.03).
Figure 4.
Average daily methadone dose (Mg) over 12 weeks; See text for mixed-effects modeling results
The mixed-effects model assessing dose levels over 12 weeks showed: (1) daily dose increased across 12 weeks (slope=1.74 p<0.0001; quadratic=−0.089, p=0.0009); (2) daily dose at week 1 (intercept) was significantly different by site (p=0.0016), but was not different by condition (p=0.266). In contrast to Shanghai, Kunming had a higher daily dose (7.75 higher) at week 1; and (3) the slope of trajectories was not significant by study site (p=0.31) or by condition (p=0.74).
Incentives Earned
Participants assigned to the incentive condition earned an average of 26.0 draws for abstinence and 40.9 draws for attendance. These draws resulted in a mean (SD) of 31.5 (31.8) Good Job, 20.5 (21.0) Small, 12.0 (12.8) Medium, and 3.7 (4.3) Large prizes per participant. The average total cost of the incentive procedure was 371 (334) Yuan per participant (527 in Shanghai vs. 216 in Kunming, p < .05), or less than 5 Yuan per participant per day.
DISCUSSION
Using contingent incentives to target attendance and opiate-negative urines, the present study demonstrated overall improved treatment retention and abstinence among patients in Chinese MMT, although there were considerable site differences. Most incentive effects were observed in Kunming, where the Incentive group performed better than the Usual Care group in treatment retention and completion (75% vs. 44% completed the 12-week treatment). Retention rates in Shanghai were high (about 88%), and patients in the Incentive group demonstrated a long duration of sustained abstinence (7.7 vs. 6.5 weeks) and high percentages of negative samples (74% vs. 68%). Taken together, these results demonstrate the effectiveness of motivational incentives targeted to treatment attendance and opiate abstinence when implemented in community MMT programs in China. The study contributes to the empirical evidence for the use of contingency management to reinforce target behaviors [16].
Prior studies using contingency management among MMT patients typically targeted substances other than opiates, such as cocaine or alcohol. Studies that applied contingency management to opiate abstinence have focused on patients who continued opiate use during MMT. One study maintaining patients at a methadone dose of 100mg [23] found no differences during the intervention regardless of whether the incentive targeted cocaine abstinence or both cocaine and opiate abstinence, but opiate abstinence was greater in the opiate-cocaine group post-intervention. Another study [24] contrasted methadone dose increases and abstinence reinforcement for treatment of continued opiate use during methadone maintenance. This study found that contingent vouchers and increasing methadone dose significantly increased opiate abstinence during the intervention, but did not dramatically enhance effects when combined. The baseline dose in this study was 50mg and could have reached 70mg. Standard treatment in all of these studies included counseling sessions as are routinely practiced in community treatment.
MMT in China is at an early stage of diffusion. As is the case in other countries new to MMT, service providers and patients in China had concerns that methadone is “yet another drug” and often requested reduced dosages to avoid methadone addiction [25]. Another unique aspect of MMT in China is that MMT staff members are medical doctors and nurses and the main service is to dispense methadone. Most MMT programs do not have staff specifically trained to deliver psychosocial interventions. Within this context, the present study still demonstrated dramatic decreases in opiate use after treatment entry across study conditions and sites, which is consistent with a large body of international research showing the efficacy of MMT for the treatment of opiate addiction. Reduced opiate use reduces needle sharing and may also reduce the likelihood of risky sex associated with using drugs, all contributing to China’s goal of establishing MMT to reduce HIV [26]. Nevertheless, there are areas for improvement. Higher dosages and provision of psychosocial support could decrease both treatment dropout and heroin use. Given the current general low-dose practice, behavioral interventions may be particularly useful to maintain patients in treatment, as was shown in Kunming. Although Shanghai patients demonstrated very good treatment attendance and high retention rates regardless of study condition, contingent incentives did improve sustained abstinence. Typical of incentive approaches (as well as other therapies), post-treatment outcomes did not differ between conditions.
There were significant site differences with Shanghai demonstrating fewer intervention effects but overall better outcomes than Kunming. Methadone dosages were similarly low in both sites (about 46mg in Shanghai and 55mg in Kunming), but patient populations varied considerably as reflected in the many differences in baseline characteristics. Social workers in Shanghai may have played an important role promoting more favorable patient outcomes overall. Shanghai’s relatively higher economic status may have also reduced the attractiveness of study incentives (e.g., of the 54 patients that refused participation, 53 were from Shanghai). Currently, all patients in China pay 10 Yuan per day for methadone, which could be a barrier for Kunming patients who are generally poorer. Future studies should explore if varying incentive amounts for populations of different socio-economic status may optimize outcomes for respective patient populations. Because intervention effects vary by site, replication of findings and inclusion of more sites would increase confidence in intervention effectiveness. Furthermore, the incentives earned by Kunming participants were more for attendance than for abstinence. Future studies should examine how incentive schedules could be altered to improve substance use outcomes while still maintaining attendance. As for other study limitations, despite the effects of the intervention on retention, attrition remained high (particularly in Kunming) which reduced power for some analyses. Similar studies have not been conducted in China previously to inform power calculation. Thus, the sample size for the present study was determined by considering several U.S. contingency management studies as well as resources available to the study. The study sample size had sufficient power (80%) for primary outcomes, e.g., an effect size of 0.31 for a continuous measure (e.g., treatment retention) with alpha at 0.05. Future studies taking attrition into consideration may find that a larger sample size, (e.g., 400 participants) will detect a smaller effect size of 0.28 in testing the intervention effect in ANOVA analyses.
Overall, methadone-maintained patients in China can benefit from reinforcement of attendance and abstinence. A small investment (371 Yuan per person; less than 5 Yuan per day) can produce sustained abstinence and longer retention. The observed site differences also suggest that incentives are useful for solving a problem (poor retention, high drug use rates) in some areas (e.g., Kunming), but may not be worthwhile when applied to other areas. Given the current widespread endorsement of low-dose practices among patients and providers in China, a behavioral intervention reinforcing attendance and abstinence should be considered, particularly in areas with few resources and social support.
ACKNOWLEDGEMENTS
The authors thank the support of methadone providers and the participation of patients in China.
This work was supported in part by Grant # R21DA025385, P30DA016383 & K05DA017648 (PI: Hser), from the National Institute on Drug Abuse (NIDA).
Footnotes
DECLARATION OF INTEREST
NIDA played no direct role in the design or conduct of the study or in the collection, management, analysis, and interpretation of the data and did not review or approve this manuscript. Dr. Hser had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Sullivan SG, Wu Z. Rapid scale up of harm reduction in China. Int J Drug Policy. 2007;18:118–128. doi: 10.1016/j.drugpo.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 2.China National Narcotics Control Commission (NNCC) Beijing, China: Office of NNCC; 2010. Annual National Narcotic Control Report 2009. [Google Scholar]
- 3.UNAIDS. Geneva: Joint United Nations Programme on HIV/AIDS; 2007. Reducing HIV Stigma and Discrimination: A Critical Part of National AIDS Programmes. [Google Scholar]
- 4.China National Narcotics Control Commission (NNCC) Beijing, China: Office of NNCC; 2009. Report of the International Narcotics Control Board for 2009. [Google Scholar]
- 5.Yin L, Qin G, Qian HZ, Zhu Y, Hu W, Zhang L, et al. Continued spread of HIV among injecting drug users in southern Sichuan Province, China. Harm Reduct J. 2007;4:6. doi: 10.1186/1477-7517-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–690. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill B, Okie S. China and HIV - a window of opportunity. N Engl J Med. 2007;356:1801–1885. doi: 10.1056/NEJMp078010. [DOI] [PubMed] [Google Scholar]
- 8.Yin W, Hao Y, Sun X, Gong X, Li F, Li J, et al. Scaling up the national methadone maintenance treatment program in China: Achievements and challenges. International Journal of Epidemiology. 2010;39(2):29–37. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian HZ, Schumacher JE, Chen HT, Ruan YH. Injection drug use and HIV/AIDS in China: review of current situation, prevention and policy implications. Harm Reduct J. 2006;3:4. doi: 10.1186/1477-7517-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 11.Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psycho. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- 12.Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute on Drug Abuse. Washington, DC: U.S: Government Printing Office; 1999. Principles of Drug Addiction Treatment: A Research Based Guide. (NIH Publication No. 99–4180) [Google Scholar]
- 15.Silverman K, Roll JM, Higgins ST. Introduction to the special issue on the behavior analysis and treatment of drug addiction. J Appl Behav Anal. 2008;41:471–480. doi: 10.1901/jaba.2008.41-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 17.Xu L. Shanghai social work development. China Social Welfare. 2007;1:15–17. [Google Scholar]
- 18.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 19.Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. J Appl Behav Anal. 1996;29:495–504. doi: 10.1901/jaba.1996.29-495. quiz 504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 21.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Li X, Hao W, Wang Z, Zhang M, Xu D, et al. A preliminary study of the reliability and validity of the Addiction Severity Index. J Chin Med Res. 2004;4:679–680. [Google Scholar]
- 23.Preston KL, Ghitza UE, Schmittner JP, Schroeder JR, Epstein DH. Randomized trial comparing two treatment strategies using prize-based reinforcement of abstinence in cocaine and opiate users. J Appl Behav Anal. 2008;41:551–563. doi: 10.1901/jaba.2008.41-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Arch Gen Psychiatry. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Jiang HF, Peng CY, Zhang R, Zhao M, Li JH, et al. Methadone maintenance treatment in China: perceptions and challenges from the perspectives of service provider and patients. Under Review. 2010 doi: 10.1093/pubmed/fds079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghitza UE, Epstein DH, Preston KL. Contingency management reduces injection-related HIV risk behaviors in heroin and cocaine using outpatients. Addict Behav. 2008;33:593–604. doi: 10.1016/j.addbeh.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]