Abstract
People with Rett syndrome (RTT) have breathing instability in addition to other neuropathological manifestations. The breathing disturbances contribute to the high incidence of unexplained death and abnormal brain development. However, the cellular mechanisms underlying the breathing abnormalities remain unclear. To test the hypothesis that the central CO2 chemoreception in these people is disrupted, we studied the CO2 chemosensitivity in a mouse model of RTT. The Mecp2-null mice showed a selective loss of their respiratory response to 1–3% CO2 (mild hypercapnia), whereas they displayed more regular breathing in response to 6–9% CO2 (severe hypercapnia). The defect was alleviated with the NE uptake blocker desipramine (10 mg·kg−1·day−1 ip, for 5–7 days). Consistent with the in vivo observations, in vitro studies in brain slices indicated that CO2 chemosensitivity of locus coeruleus (LC) neurons was impaired in Mecp2-null mice. Two major neuronal pH-sensitive Kir currents that resembled homomeric Kir4.1 and heteromeric Ki4.1/Kir5.1 channels were identified in the LC neurons. The screening of Kir channels with real-time PCR indicated the overexpression of Kir4.1 in the LC region of Mecp2-null mice. In a heterologous expression system, an overexpression of Kir4.1 resulted in a reduction in the pH sensitivity of the heteromeric Kir4.1-Kir5.1 channels. Given that Kir4.1 and Kir5.1 subunits are also expressed in brain stem respiration-related areas, the Kir4.1 overexpression may not allow CO2 to be detected until hypercapnia becomes severe, leading to periodical hyper- and hypoventilation in Mecp2-null mice and, perhaps, in people with RTT as well.
Keywords: Rett syndrome, breathing, noradrenergic neurons, CO2 chemosensitivity, brain stem
rett syndrome (RTT), one of the autism spectrum disorders, is caused by defects in the X-linked gene encoding methyl-CpG-binding protein 2 (MeCP2), affecting 1 in ∼10,000 live births of females (9). People with RTT have breathing abnormalities such as episodic breath-holding, apnea, respiratory irregularity, apneusis, Valsalva breathing, and air swallowing, most of which are recapitulated in Mecp2-null mice (29). These breathing disturbances may underscore the high incidence of sudden unexplained death and contribute to the abnormal brain development (24, 42). Recent studies indicate that the abnormalities of brain stem monoamine systems, brain-derived neurotrophic factor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptors, and post-inspiratory phase of breathing activity contribute to the etiology of life-threatened breathing disorders in Mecp2-null mice (30, 44, 50, 53). However, the neurobiological mechanisms underlying such breathing disorders are still unclear.
The breathing instability of Mecp2-null mice is independent of peripheral O2 chemoreceptors, and seems to be of central origin (6). In the central nervous system, breathing activity is automatically regulated by central CO2 chemoreceptors that provide the brain stem respiration-regulatory networks with continuous information of CO2 levels in the blood stream. Disruption in the central CO2 chemoreception can lead to abnormal respiratory activity, a mechanism that may underlie the breathing disorders of RTT patients.
Monoamine neurotransmitters are involved in the control of breathing rhythm activity and CO2 chemosensitivity. Abnormalities in monoamine systems account for severe breathing disorders, such as sudden infant death syndrome and congenital central hypoventilation syndrome (16, 36). Deficient norepinephrine (NE) and/or its metabolites in central nervous system have been found in RTT patients as well as Mecp2-null mice (19, 62), contributing to the incidence of breathing arrhythmia (50). The most prominent group of NE-ergic neurons is located in the locus coeruleus (LC) (4). The LC neurons are CO2 chemosensitive, in addition to regulating behavioral state (14). Therefore, we chose this group of neurons to test the hypothesis that the central CO2 chemosensitivity is disrupted in Mecp2-null mice involving NE-ergic neurons in the LC.
MATERIALS AND METHODS
Generation of Mecp2-null mice.
Mecp2-null mice of strain B6.129P2 (C)-Mecp2tm1.1Bird, developed by Dr. Adrian Bird (17), was used as a mouse model for Rett syndrome in this study. To produce Mecp2-null mice (Mecp2−/Y), heterozygous female mice of Mecp2-knockout (Mecp2+/−) purchased from The Jackson Laboratory (Bar Harbor, ME) were crossed with either C57BL/6 or homozygous Prp57 males. The latter was generously provided by Dr. Anthony N. van den Pol (Department of Neurosurgery, Yale University School of Medicine, New Haven, CT). Genotypes of male F1 generation were identified by following protocols of the Jackson Laboratory. Only male offspring were used in the following studies. All animal procedures were conducted in compliance with the regulation of the Institutional Animal Care and Use Committee of the Georgia State University.
Plethysmograph recording of mouse breathing activity.
The breathing activity of unrestrained mice of 3–8 wk was measured without anesthesia. We could not narrow down the age range because of the availability of the mutant animals. The individual animals were kept in a plethysmograph chamber (∼40 ml) connected to an empty reference chamber for at least 30 min for adaptation followed by a 15-min recording under air condition with a flow rate of 50 ml/min. To test the CO2 sensitivity of the animals, the chamber was sequentially flushed with graded CO2 in concentrations of 1, 2, 3, 6, and 9%. A 30-min air washout was given for recovery after hypercapnia. The time-course study of CO2 response was performed with 3% CO2 for 30 min. Mouse breathing activity was barometrically recorded continuously by measuring the pressure changes between the animal chamber and a reference chamber with a signal transducer. The signal was amplified and collected with Axoscope 9 software. Data were stored in the computer for later analysis with Clampfit 9 software.
Ventilation parameters including breathing frequency (f), inspiration amplitude (AI), inspiration time (TI), and breathing interval (IB) were measured directly from the plethysmograph records. Inspiration (I; an analog of tidal volume) = TI × f; total respiration (RTOT; an analog of minute ventilation) was calculated with the equation RTOT = AI × TI × f. The variability of frequency was calculated as the division of standard deviation (SD) by arithmetic mean of IB. The variability of inspiration amplitude is calculated similarly. All of the SD and mean values were measured from at least 100 successive breathing cycles.
Western blots.
All animals used in this and other in vitro studies were anesthetized with the inhalation of 4% isoflurane (Baxter Healthcare, Deerfield, IL) and decapitated. Pontine sections containing LC (300 μm) were obtained from 3- to 6-wk-old mice. LC region ∼1.5 mm in diameter was dissected free from surrounding tissues with 15-G Luer-Stub Adapters (Becton Dickinson & Co, Franklin Lakes, NJ). Whole cell lysates were obtained from micropunched LC nucleus with RAPI buffer (Sigma-Aldrich, St. Louis, MO). Protein concentrations were estimated using BCA protein assay reagent (Pierce, Rockford, IL). For electrophoresis in 10% SDS-PAGE, 10 and 30 μg of proteins were used to detect Kir4.1 and Kir5.1 signals, respectively. Proteins were electrophoretically transferred to nitrocellulose membranes and subjected to Western blot analysis. The membranes were blocked for 1 h with 5% milk and incubated overnight at 4°C with polyclonal rabbit anti-rat Kir4.1 (1:400, Alomone Labs, Jerusalem, Israel), rabbit anti-rat Kir5.1 (1:200, Alomone), and mouse monoclonal β-actin (1:4,000, Sigma) antibodies, respectively. The HRP-conjugate goat anti-rabbit and goat anti-mouse secondary antibodies were used to visualize blotting signal. Membranes were exposed to film (Hy Blot CL; Denville, Metuchen, NJ) with a chemiluminescent detection system (Pierce). The photograph was scanned to and processed with Photoshop software. The histogram of immunoblot was obtained to quantify the immunobloting signals with the ImageJ software (NIH). Both Kir4.1 and Kir5.1 signals were normalized to β-actin controls.
Brain slice preparation.
As described previously (60), pontine slices containing LC were obtained from GFP-expressing Mecp2−/Y mice and their Mecp2+/Y (wild type) littermates at 3–4 wk of age. In brief, the brain stem was rapidly removed and sliced in oxygenated ice-cold sucrose-artificial cerebrospinal fluid (sucrose-ACSF) in which NaCl was replaced with 200 mM sucrose. The transverse pontine sections (300 μm) were then collected in standard ACSF oxygenated with 95% O2-5% CO2, containing (in mM) 124 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 D-glucose at pH 7.40. The slices were allowed to recover at 33°C for 1 h and then kept at room temperature for up to 6 h until recording. Since it is very difficult to record neuronal activity in brain slices from mice older than 4 wk, the experiments were done in mice that were 3–4 wk old. The data obtained from these younger mice remain valid since the breathing irregularity has been found in 3-wk-old mice in a recent study (52).
Acute dissociation of LC neurons.
The LC micropunches obtained from brain slices of 3- to 6-wk-old mice were digested for 50–70 min with 20 IU/ml papain and 0.005% DNase (Papain dissociation system; Worthington Biochemical, Lakewood, NJ) in HEPES buffer bubbled with O2 at 35°C. The HEPES buffer contained (in mM) 140 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 25 d-glucose, and 10 HEPES at pH 7.40. Tissues were then washed twice with HEPES-buffer containing 10 mg/ml ovomucoid protease inhibitor and 10 mg/ml bovine serum albumin, and then kept in oxygenated HEPES-buffer on ice until use. Before recording, LC neurons were dissociated in HEPES buffer by gentle triturating with fire-polished Pasteur pipettes. The dissociated cells were then plated in 35-mm Petri dishes and kept at room temperature for 20 min before patching.
Heterologous expression of Kir4.1/Kir5.1 in human embryonic kidney cells.
The human embryonic kidney cell line (HEK293; American Type Culture Collection, Rockville, MD) was maintained as monolayer in the DMEM with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2 in the atmosphere. Rat brain Kir4.1 (GenBank no. X83585) and Kir5.1 (GenBank no. X83581) cDNA was generously provided by Dr. Adelman (Oregon Health Science University) (7). The cDNAs were subcloned in a CMV promoter-driven eukaryotic expression vector, pcDNA3.1 (Invitrogen, Carlsbad, CA). The Kir4.1 (0.8 μg) was transfected into HEK293 cells cultured in a 30-mm dish with lipofectamine 2000 (Invitrogen) for 6–8 h. The co-expression of Kir4.1/Kir5.1 at various ratios (2:1, 1.5:1, and 1:1) was obtained by co-transfecting with 0.4, 0.53, and 0.8 μg of Kir5.1 cDNA, respectively. The positively transfected cells were readily identified with GFP expression by adding 0.4 μg of green fluorescent protein (GFP) cDNA (pEGFP-N2; Clontech, Palo Alto, CA) in the above transfection mixture. After transfection, cells were dissociated with 0.25% Trypsin and placed on 5 × 5 mm cover slips. The culture was then maintained for 16–48 h before experiments.
Electrophysiology.
Whole cell current clamp was performed on the GFP-expressing LC neurons in brain slice as described previously (60). Before experiment, slices used for recording were transferred to a recording chamber that was perfused with oxygenated ACSF at a rate of 3 ml/min and maintained at 35 ± 2°C. Hypercapnia was produced by bubbling ACSF with 6.5 or 8% CO2 (balanced with oxygen). Individual LC neurons were visualized with a ×40 water-immersion lens and a near-infrared CCD camera. Patch electrodes were pulled with a tip resistance of ∼3–5 MΩ. The intracellular solution contained (in mM) 130 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, and 0.4 EGTA, with pH adjusted to 7.30. Spontaneous firing activities were amplified using an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA) digitized at 2 kHz and recorded by Clampex 8.2 (Molecular Devices) data acquisition system. Only neurons that had initial resting potentials of less than −40 mV and action potential amplitude of >80 mV were accepted as healthy cells and further studied. Data were analyzed using Clampfit 9.0 software (Molecular Devices) and presented as means ± SE. Firing activities of LC neurons in response to hypercapnia were normalized to their basal activities at 5% CO2 and shown as a percentage. With injection of hyperpolarizing currents, input resistance of cells was measured and calculated as the ratio of the changes in membrane potentials to injected currents.
Inside-out patch clamp was performed on HEK293 cells and acutely dissociated LC neurons at room temperature (∼24°C). As described previously (60), macroscopic Kir currents were recorded with symmetric high K+ in bath (intracellular) and pipette (extracellular) solutions, containing (in mM) 40 KCl, 75 potassium gluconate, 5 potassium fluoride, 0.1 sodium vanadate, 10 potassium pyrophosphate, 5 EGTA 1, 0.2 ADP, 10 PIPES, and 10 glucose at pH 7.4 (FVPP solution). To elicit inward rectification, 0.1 mM spermine was added to the bath solution. Various pHi levels were adjusted using gluconic acid or N-methyl-d-glucamine. Giant inside-out patches were obtained using recording pipettes of 1–2 MΩ to study macroscopic currents. The pHi current relationship was expressed with the Hill equation: I/Imax = 1/[1 + (pH/ pKa)h], where Imax is maximum current, and pKa is the pH level at midpoint current inhibition.
Statistical analysis.
For comparison of respiratory parameters at certain CO2 levels between null and WT mice, two-tailed t-test was applied. For comparison of effects of serial graded CO2 elevation, one-way ANOVA was used. The intergroup differences in Western blot and neuronal response to hypercapnia were analyzed with two-tailed Student's t-test unless one-tailed test was mentioned. Paired t-test was used for intra-group comparisons in neuronal responses to hypercapnia and Ba2+. The statistical significance was taken at P < 0.05 for all tests.
RESULTS
Systemic sensitivity to the low arterial Pco2 range was selectively disrupted in Mecp2-knockout mice.
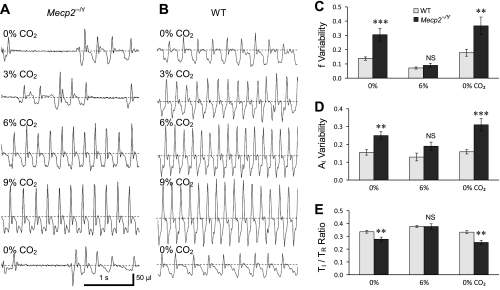
To demonstrate whether systemic CO2 sensitivity is normal, breathing activity was studied in unanesthetized Mecp2−/Y mice on a C57BL/6 background using the plethysmograph method with various levels of CO2. Consistent with a previous report (50), the Mecp2−/Y mice displayed alternating periods of fast and slow breathing with frequent gasping, apneas, and variable amplitude of inspiration (Fig. 1A) with atmospheric air breathing. The f and AI was measured, and their variabilities were quantitatively analyzed in WT and Mecp2−/Y mice. With air breathing, the f and AI variability were ∼0.3 and 0.25, respectively, in Mecp2−/Y mice, whereas they were 0.12 and 0.15 in the WT (P < 0.001, n = 14 and P < 0.01, n = 14, respectively; Fig. 1, A–D). Such breathing variations were markedly suppressed with the inhalation of 6% CO2 (severe hypercapnia). During the CO2 exposure, no statistical difference in breathing variations indeed was found between Mecp2−/Y and WT mice (Fig. 1, C and D). The CO2 exposure also affected the ratio of inspiratory time (TI) vs. the time for each cycle of breath (TR). With air breathing, the TI-to-TR ratio was significantly lower in the Mecp2−/Y mice than in the WT mice (P < 0.01, n = 14; Fig. 1E). The difference was totally eliminated during 6% CO2 exposure. Therefore, the breathing activity of Mecp2−/Y mice is still subject to the regulation at elevated CO2.
Fig. 1.
Modulation of breathing activity by CO2. A and B: breathing activity was measured using in vivo plethysmograph. When the plethysmograph chamber was ventilated with air (40 ml/min), the Mecp2−/Y mouse displayed typical alternating periods of fast and slow respiratory activity with gasping and apnea (A). The inspiration amplitude (AI; downdraft) was also unstable and irregular in contrast to the wild-type (WT) mouse (B). With 3% CO2, breathing frequency (f) increased obviously in the WT but not in the Mecp2−/Y mouse. With 6 and 9% CO2, the breathing pattern became stable in both groups with clear increases in f and AI. Respiratory activity of the Mecp2−/Y mouse returned to the irregular pattern after air breathing was resumed. C and D: the variability of f and AI was analyzed in seven pairs of mice with >100 breaths in each measurement and shown as standard deviation divided by arithmetic means. The f and AI variabilities were much greater in the Mecp2−/Y mice than in the WT mice (**P > 0.01; ***P > 0.001). Data are shown as means ± SE. The difference in f and AI variabilities between the WT and Mecp2−/Y mice was abolished with 6% CO2 (P > 0.05). E: with atmospheric air breathing, the inspiratory time (TI)-to-time for each cycle of breath (TR) ratio was significantly lower in the Mecp2−/Y mice than in the WT mice. The difference was totally eliminated during 6% CO2 breathing.
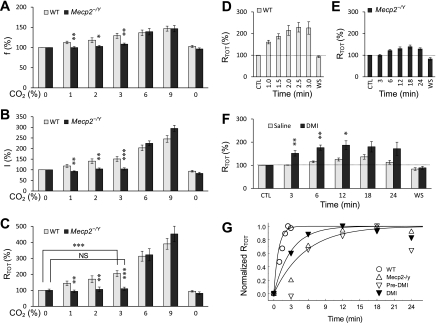
In contrast to the remarkable effect of high CO2, the breathing activity of the Mecp2−/Y mice barely responded to mild hypercapnia (1–3% CO2). No evident increase in f, I, and RTOT was detected with a 3-min exposure to 1, 2, or 3% CO2 in Mecp2−/Y mice, whereas all of these parameters were clearly augmented in the WT mice (Fig. 2, A–C). With a prolonged period of exposure to 3% CO2, the RTOT increased slightly to ∼30% of the WT level, whereas such a small change took place in 18 min (Fig. 2, D and E).
Fig. 2.
Defects in breathing responses to moderate hypercapnia in Mecp2−/Y mice. A–C: the f, I, and total respiration (RTOT) were analyzed with various levels of CO2 breathing in WT and Mecp2−/Y mice. The Mecp2−/Y mice failed to respond to 1–3% CO2 in all three measures (*P < 0.05; **P < 0.01; ***P < 0.001), although these respiratory parameters appeared normal with 6 and 9% CO2. No significant difference (NS) in RTOT was found between 3% CO2 and air breathing at the baseline in the Mecp2−/Y mice (n = 8 and 12 for WT and Mecp2−/Y mice, respectively). Note that the baseline level was counted as 100% for each mouse. D: with an exposure to 3% CO2, the RTOT of WT mice started to increase in the first minute of the exposure and reached the plateau level at 2.5 min (n = 11 animals). E: in contrast, it took nearly 20 min for the Mecp2−/Y mice to reach the maximum RTOT (n = 13 mice) during the 3% CO2 exposure, whereas at the peak level the RTOT remained 59% lower than that of the WT. F: the RTOT response to 3% CO2 was significantly improved after DIM treatment (n = 8 for each group). G: time constants were measured with the data in D–F. Only mean values were shown with error bars omitted. They are 0.8 min for WT mice (n = 11), 6 min for the Mecp2−/Y (n = 13), and 3 min for the Mecp2−/Y after desipramine (DMI) treatment (n = 8).
The aberrant CO2 sensitivity of Mecp2-null mice was significantly improved by desipramine.
Desipramine (DMI), a selective NE uptake inhibitor, can improve the breathing rhythm of Mecp2−/Y mice by increasing the NE content (38, 59). If the defective CO2 response is related to the breathing arrhythmia, DMI may also affect the CO2 response of Mecp2−/Y mice. Therefore, we examined the CO2 response in the presence of DMI (10 mg·kg−1·day−1 ip, for 5–7 consecutive days before the experiment) in 4-wk-old Mecp2−/Y mice. Compared with the control group that received the same volume of saline injection during the same period, the RTOT of Mecp2−/Y mice increased significantly (by ∼50%) in the first 3 min of 3% CO2 exposure. At the maximum, the RTOT reached ∼70% of the WT level (Fig. 2F). The time constant (τ) for the CO2 response was 6 min for the untreated group and the experimental group before treatment. With DMI treatment, τ was shortened to 3 min (Fig. 2G). The improvement of the CO2 responses after DMI treatment indicates that the impairment of the NE system plays a role in the defect of central CO2 chemosensitivity of Mecp2−/Y mice.
LC neurons of Mecp2-null mice lacked chemosensitivity to mild hypercapnia.
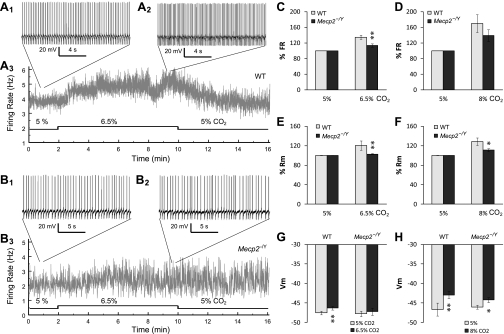
NE-ergic neurons in the locus coeruleus (LC) produce >70% of NE in central nervous system and are defective in Mecp2−/Y mice (39, 40, 45, 61). Since these cells play a role in the development of normal respiratory rhythm in neonatal mice (48, 49) and are intrinsically CO2/pH sensitive (22, 34), it is very likely that their intrinsic CO2/pH sensitivity may be altered. To test this possibility, we examined neuronal response to CO2 in brain stem slices obtained from Mecp2−/Y and Mecp2+/Y (WT) Prp57 mice with GFP expression in LC neurons. The basal breathing pattern and CO2 sensitivity of these hybrid mice showed no detectable differences from those with the C57BL/6 genetic background (see supplemental figure S1 available online at the Am J Physiol-Cell Physiol website).
Most LC neurons displayed spontaneous firing activity under normal CO2 (5%) condition. The baseline firing rate (FR) in the WT mice (3.1 ± 0.4 Hz; n = 36) was slightly lower than in the Mecp2-null mice (4.3 ± 0.6 Hz, n = 21; P = 0.049, one-tailed t-test). The FR was compared between the two groups of mice after normalization to basal firing rate. When the CO2 level was raised by 1.5% (6.5%), the FR increased by 34.8 ± 5.6% (from 3.3 ± 0.6 to 4.3 ± 0.7 Hz, n = 12; P < 0.001, paired t-test) in WT neurons (Fig. 3, A and C) but by only 13.6 ± 4.4% (from 3.9 ± 0.8 to 4.4 ± 0.9 Hz, n = 12; P < 0.05, paired t-test) in Mecp2−/Y cells (Fig. 3, B and C). The latter was significantly lower than the former (P < 0.01, n = 24). With a 3% rise in CO2 (8%), the FR increased by 69.9 ± 22.7% (from 3.0 ± 0.4 to 4.9 ± 0.8 Hz, n = 24; P < 0.01, paired t-test) in WT and 39.2 ± 15.2% (from 4.9 ± 0.7 to 6.6 ± 1.2 Hz, n = 9; P < 0.05, paired t-test) in Mecp2−/Y neurons, respectively (Fig. 3D). There was no significant difference in the percentage changes in FR between WT and mutant cells (P > 0.05, n = 33) with more severe hypercapnia (8%).
Fig. 3.
CO2 response of LC neurons. A: whole cell current clamp was made in a WT LC neuron in the brain stem slice. At baseline (5% CO2), the LC neuron fired spontaneously with basal firing rate 3.8 Hz (A1). Firing activity increased to 5.3 Hz when the cell was exposed to 6.5% CO2 (A2). A3: the instant frequency plot showed a clear increase in firing rate during 6.5% CO2 exposure. B: a representative recording from a Mecp2−/Y neuron. B1: in an LC neuron from a Mecp2−/Y mouse, baseline firing activity was 2.2 Hz. B2: the firing activity changed barely to 2.3 Hz in response to 6.5% CO2. B3: the instant frequency plot of the cell indicated a subtle increase in firing rate during hypercapnia. C and D: neuronal firing activity increased in response to 6.5 and 8% CO2. Such a cellular response to 6.5% CO2 was significantly reduced in LC neurons of Mecp2−/Y mice than in the WT. E and F: hypercapnia caused significant increases in input resistance (Rm). The effect was much smaller in Mecp2−/Y neurons than in the WT. G and H: membrane potential (Vm) response to hypercapnia. 6.5% CO2 caused clear depolarization in only WT, whereas depolarization with 8% CO2 was found in both WT and Mecp2−/Y cells (paired t-test) Significant difference: *P < 0.05; **P < 0.01. Data were obtained from 36 WT and 21 Mecp2−/Y LC neurons.
Similar analysis was performed for input resistance (Rm) and membrane potentials. As for Rm, WT neurons displayed much greater response to hypercapnia (both 6.5 and 8% CO2) than Mecp2−/Y cells (P < 0.01, n = 9 and P < 0.05, n = 15, respectively; Fig. 3, E and F). The increases of 20.6 ± 9.7% (from 422.1 ± 40.6 to 508.2 ± 60.7 MΩ; P < 0.05, n = 5) and 28.7 ± 9.7% (from 443.1 ± 40.7 to 553.2 ± 41.4 MΩ; P < 0.001, n = 11) in Rm were observed in WT with 6.5 and 8% CO2, respectively, whereas they were 2.7 ± 1.2% (from 459.7 ± 71.7 to 473.5 ± 77.2 MΩ; P > 0.05, n = 4, paired t-test) and 11.0 ± 2.9% (from 453.3 ± 55.1 to 498.3 ± 45.8 MΩ; P < 0.05, n = 4, paired t-test). Meanwhile, WT neurons showed clear depolarization with the exposures to 6.5 and 8% CO2 (from −47.6 ± 0.5 to −46.3 ± 0.4 mV; P < 0.01, n = 18, paired t-test; and from −46.7 ± 1.7 to −42.9 ± 0.9 mV, P < 0.01, n = 18, paired t-test), whereas only modest depolarization (from −46.1 ± 0.5 to −44.2 ± 0.7 mV, P < 0.05, n = 7, paired t-test) with 8% CO2 was seen in Mecp2−/Y neurons, and no significant depolarization (from −47.8 ± 0.7 to −47.2 ± 0.9 mV, P > 0.05, n = 10, paired t-test) occurred with 6.5% CO2 (Fig. 3, G and H). The changes in FR, Rm, and membrane potentials in the WT neurons were largely eliminated in the presence of 200 μM Ba2+, an inward rectifier K+ (Kir) channel blocker (see supplemental figure S2 available online at the Am J Physiol-Cell Physiol website), suggesting the involvement of Kir channels.
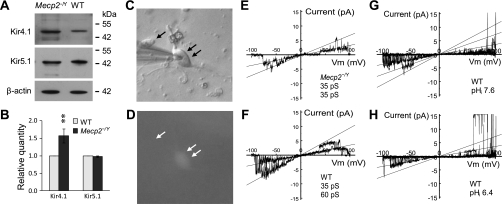
Overexpression of Kir4.1 in the defective response of LC neurons to intracellular acidic pH.
We have shown that LC neurons in the Mecp2−/Y mice displayed stronger inward rectification than those in the WT, which is associated with the overly expressed Kir4.1 mRNAs in LC neurons of the Mecp2−/Y mice (60). The overexpression of Kir4.1 subunits but not Kir5.1 was confirmed in the present study with the Western analysis (Fig. 4, A and B). It is known that the Kir4.1 can form both homomeric channels by itself and heteromeric channels with Kir5.1 (46), and these two types of Kir channels have clearly different pH/CO2 sensitivity (11, 32, 57). The Kir4.1 overexpression may have two adverse consequences. One is the reduction in the pH sensitivity of LC neurons by raising the ratio of homomeric Kir4.1 channels to the heteromeric Kir4.1/Kir5.1 channels when Kir4.1 outnumbers Kir5.1. Alternatively, more heteromeric channels may be assembled leading to hyperpolarization and a decrease in membrane excitability in Mecp2−/Y cells when the Kir5.1 is also overexpressed together with Kir4.1. To test these possibilities, we studied K+ currents in acutely dissociated LC neurons that were identified with endogenous GFP expression (Fig. 4, C and D). The sensitivity of the currents to intracellular pH (pHi) was further studied only if they were K+ selective, showed inward rectification, and were inhibited by the Kir channel blocker Ba2+ (see supplemental figure 3, A and B, available online at the Am J Physiol-Cell Physiol website).
Fig. 4.
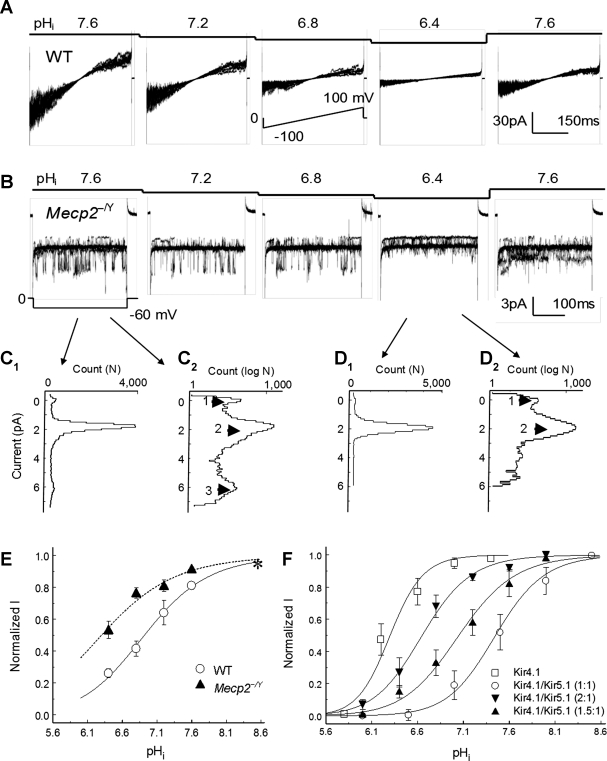
Inward rectifier K+ (Kir) 4.1 and Kir4.1/Kir5.1 channels in LC neurons. A and B: Western analysis indicated that both Kir4.1 and Kir5.1 were expressed in the LC region. The expression level of Kir4.1 was ∼50% higher in the Mecp2−/Y mice than in the WT, whereas there was no evident change in the Kir5.1 expression. Note that quantitation of the Western blot was done by normalizing the photo-intensities to that of β-actin (B; **P < 0.01; n = 5 pairs for each group). C and D: neurons dissociated freshly from LC. The presence of GFP fluorescence is found in one cell (double-arrow) but not the other (arrow). The recording pipette is on the left (C). E and F: K+ currents were studied in inside-out patches obtained from the acutely dissociated LC neurons. With a ramp command potential from −100 to 100 mV at the holding potential of 0 mV, two major Kir channels with inward rectification were identified with the unitary conductance 32pS (E) and 60pS (F), respectively. G and H: single channel conductance was not affected by pHi 6.4.
Two major inward rectifying K+ currents were identified in both WT and mutant cells. Their single-channel conductance was 32.0 ± 0.4 pS (n = 20) and 59.9 ± 1.1 pS (n = 12), respectively (Fig. 4, E and F), nearly identical to the homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels expressed in human embryonic kidney (HEK) cells (see supplemental figure 3, D and E, available online at the Am J Physiol-Cell Physiol website). Also similar to the Kir4.1 and Kir4.1/Kir5.1 channels in the heterologous expression system was the intracellular acid-induced channel inhibition by selective suppression of the open-state probability without affecting the single-channel conductance (Fig. 4, G and H).
The existence of both Kir4.1- and Kir4.1/Kir5.1-like currents indicates the excess of Kir4.1 over Kir5.1 in LC neurons, thus supporting the first possibility that the increased Kir4.1 levels may lead to the reduction of pH sensitivity of Mecp2−/Y cells. Exposures of the intracellular side of patch membranes to acidic perfusates led to a concentration-dependent inhibition of these Kir channels (Fig. 5, A and B). Notably, the 60pS channel was more sensitive to acidic pHi than the 32pS channel (Fig. 5, B–D). When the pHi current relationship was described with the Hill equation, these Kir currents showed the maximum activity at pH 8.6, decreased in the current amplitude by 20–40% at pHi 7.1, and were further inhibited at pHi 6.8 (Fig. 5C). The pKa value was 6.38 ± 0.08 (n = 10) for Mecp2−/Y neurons, which was significantly reduced (P < 0.01) compared with that for WT (6.95 ± 0.10, n = 8) (Fig. 5C). To prove that this shift of the titration curve in Mecp2−/Y cells is a result of excessive homomeric Kir4.1 channels over heteromeric Kir4.1/Kir5.1 channels, we expressed Kir4.1 and Kir5.1 in HEK cells in various ratios (Fig. 5D). Clear shifts in the titration curves were observed with each addition of the Kir4.1 (Fig. 5D; see supplemental figure 4 available online at the Am J Physiol-Cell Physiol website), indicating that an overexpression of Kir4.1 indeed can cause a decrease in the pH sensitivity of Kir currents.
Fig. 5.
Reduced pH sensitivity of Kir currents in Mecp2−/Y neurons. A: step decreases in intracellular pH (pHi) in the perfusate produced concentration-dependent inhibition of inward rectifying K+ currents in a WT neuron. B: in a Mecp2−/Y cell, inward K+ currents were tested with various pHi levels with command potential of −60 mV at the holding potential of 0 mV. The 66pS current was completely inhibited at pHi 6.4, whereas the 33pS current was only partially inhibited. Note that eight superimposed traces are shown in each panel of A and B. C: all-point histograms in conventional (C1) and log scale (C2). The data analysis was based on the record at pHi 7.6 from B. Three peaks are seen at 0, 2, and 5.8 pA, respectively. With a hyperpolarizing −60-mV pulse, the unitary conductance is 33pS between arrowheads 1 and 2, and 63pS between arrowheads 2 and 3. D: similar data analysis was done with record at pHi 6.4. Only the 33pS current is seen. E: the pHi current relationship was described with Hill equation. Compared with the K+ currents in WT neurons (pKa 7.0), these currents in Mecp2−/Y neurons were less sensitive to acidic pH with pKa 6.4. F: Kir4.1 and Kir5.1 were expressed in HEK cells with various combinations. Serial titration curves were produced for the Kir4.1/Kir5.1 currents (see supplemental figure 4 available online at the Am J Physiol-Cell Physiol website). The pKa was 7.5 ± 0.2 (n = 5) for the Kir4.1/Kir5.1 channel in a 1:1 ratio, 7.1 ± 0.2 (n = 4) in a 1.5:1 ratio, and 6.6 ± 0.1 (n = 5) in a 2:1 ratio, respectively. The pKa was 6.3 ± 0.1 (n = 4) for Kir4.1 alone.
DISCUSSIONS
Various abnormalities in neuronal morphological, biochemical, and electrophysiological properties of Mecp2−/Y mice can potentially contribute to the breathing irregularities commonly seen in RTT patients (39, 45, 50). Our findings suggest that central CO2 chemosensitivity is impaired in Mecp2−/Y mice providing a new insight into the etiology of the life-threatening breathing disorders. The rhythmic activity of breathing relies on CO2, although it can be generated in vitro in the absence of any peripheral sensory input (15). Both respiratory frequency and amplitude are augmented with hypercapnia and inhibited with hypocapnia. Our in vivo studies have shown that such a feedback regulation that works smoothly in proportion to arterial Pco2 (PaCO2) levels in WT animals appears to be disrupted in Mecp2-null mice specifically at mild hypercapnic levels (<3%). In contrast, the mouse breathing response to more severe hypercapnia (>3%) appears normal, consistent with a previous report (51). It has been reported that human subjects with Cheyne-Stokes respiration and emphysema display reduced CO2 sensitivity (2, 43). Compared with normal groups, CO2 inhalation in these patients only evokes much weaker ventilation response, resulting in a moderate accumulation of arterial CO2. Their PaCO2 level rises by 5 Torr with 3% CO2, comparable to that in normal subjects with inhalation of 5% CO2, whereas 5% CO2 inhalation induces an increase in PaCO2 level by ∼10 Torr. It seems that the impaired respiratory response to low CO2 (≤3%), at least in humans, may not lead to significant hypercapnia, which could be also true in Mecp2-null mice. Consistently, hypercapnia is not commonly found in RTT patients with blood gas analysis (41), although hypercapnia and acidosis usually indicate the severity of RTT phenotypes (13). Therefore, unlike congenital central hypoventilation syndrome, which causes the early death of infants due to the severe impairment of hypercapnic drive on respiration (3), the defect in Mecp2-null mice does not seem to be as lethal but can lead to the sporadic and interrupted feedback regulation of respiratory rhythm by PaCO2. In other words, CO2 could be detected only when hypercapnia is developed, thus resulting in periodical hypo- and hyperventilation in these animals, which is consistent with our hypothesis that the selective disruption of systemic CO2 sensitivity handicaps the central CO2 chemosensitivity, contributing to breathing arrhythmia in Mecp2-null mice.
Defects of multiple monoamine systems are found in RTT patients as well as Mecp2-null mice, whereas the LC NE-ergic system is a major site affected by MeCP2 dysfunction (28, 39, 40, 45, 60, 61). DMI, a NE uptake blocker that increases NE concentration in synaptic cleft, has recently been demonstrated to rescue the neurochemical phenotype in brain stem NE-ergic neurons and to stabilize the breathing rhythm (38, 59) by certain unknown mechanisms. The early observations on respiratory effects of DMI are mostly done in patients with primary depression. The hypercapnia is commonly found in these patients, and their PaCO2 levels are positively related to the depression score. DMI, as a traditional antidepressant, thus has been proposed to potentially alleviate the hypercapnia (12). In fact, the administration of DMI does not affect the resting PaCO2 in both depressive and normal groups, indicating that dysfunction of NE system may not be the origin of hypercapnia in depressive cases (12, 31). Notably, in the present study, we showed for the first time that DMI improves the systemic CO2 chemosensitivity in Mecp2-null mice. Since DMI neither affects the resting breathing activity in WT animals (59) nor induces respiratory alkalosis in normal human groups (31), the effect of DMI found in the present study supports the idea that MeCP2 deficiency-induced NE dysfunction is associated with the breathing disturbance, including the impaired CO2 drive to central breathing activity (25, 34, 40). However, we have noticed that the effect of DMI observed in this study is somehow limited. Whether it results from the low potency or efficacy of DMI is still an open question. In addition, defects in several other neurotransmitter systems and neurotrophic factors, such as serotonin, glutamate, GABA, and BDNF have also been known to contribute to their breathing phenotypes (23), and the drugs targeting at these systems also help to improve breathing function in Mecp2-null mice (1, 25, 30). It is possible that the combination of multiple pharmaceuticals could be more effective to cure severe breathing disorders in RTT, as proposed previously (23).
The importance of LC neurons in central CO2 chemoreception has been well recognized. Elam et al. (14) proposed that the LC was a central CO2 chemoreceptor. Consistent with systemic ventilation responses, elevation in CO2 levels linearly increased the firing frequency of LC neurons in rats (14). Compared with other brain stem regions, LC contains the highest proportion of CO2-sensitive neurons. More than 80% of LC neurons are activated by hypercapnia or acidic pH (35). Local acidification of the LC region raises discharge frequency of phrenic nerves in cats (10), accounting for ∼30% of the overall response to hypercapnia. Because of their widespread projections to other brain stem nuclei related to respiratory control (4), LC neurons may affect breathing activity by 1) the direct activation of pre-Bötzinger complex neurons that are critical for respiratory pattern generation via α1 adrenoceptors (18) and/or 2) interactions between the LC and other CO2 sensitive sites, such as the midline raphé nuclei, the retrotrapezoid nucleus, and A1 and A2 groups of NE-ergic neurons (21). Chemical lesion of the majority of LC NE-ergic neurons by microinjecting 6-hydroxydopamine (6-OHDA) significantly reduces the CO2-induced respiratory response (5, 26). Similarly, defective CO2 chemosensitivity of Mecp2−/Y LC neurons may affect neuronal activity of critical respiratory nuclei in the brain stem and hinder CO2-driven regulatory mechanisms, thus contributing to the alternated hyper- and hypoventilation.
The identification of CO2-sensing molecules remains a central issue in unraveling the mechanisms underlying central CO2 chemoreception. Although various pH-sensitive channels have been proposed as putative CO2 sensors (21), direct evidence is still needed to support a key role of these molecules. Among them, heteromeric Kir4.1/Kir5.1 channels appear to be an ideal candidate for CO2/H+ sensing, not only because its pKa 7.45 is exactly at the physiological pH level but also because their channel activity determines the resting membrane potentials (13). Although the expression of both Kir4.1 and Kir5.1 mRNAs has been observed in LC and other CO2 chemosensitive sites (56), most studies suggest that Kir4.1 and Kir4.1/Kir5.1 channels are prominently expressed in the glial cells of the CNS (8, 58). Therefore, the identification of functional Kir4.1 and Kir4.1/Kir5.1-like currents in the present study provides functional evidence for the neuronal expression of Kir4.1 and/or Kir5.1 subunits in CO2 chemosensitive neurons, at least in the LC region, which enhances our understanding of the impact of these highly CO2-sensitive molecules on central CO2 chemoreception. Interestingly, a recent study has shown that the genetic disruption of Kir5.1 impairs LC neuronal PaCO2/pH sensitivity (55).
Several lines of evidence suggest that the overexpression of Kir4.1 channel underlies the defect: 1) stronger inward rectification was found in Mecp2−/Y cells (60); 2) both Kir4.1- and Kir4.1/Kir5.1-like currents are present in LC neurons; 3) the Kir4.1 expression at the protein and mRNA levels is upregulated in the LC nucleus of Mecp2−/Y mice without changing the Kir5.1 expression; 4) the Kir4.1 channel has lower sensitivity to CO2/pH than the Kir4.1/Kir5.1; 5) excessive expression of Kir4.1 reduces the pH sensitivity of Kir currents in HEK cells. As a member of Kir family, Kir4.1 is highly selective for K+ and plays a role in the regulation of membrane potentials and cellular excitability (20). It can form homomeric and heteromeric channels with Kir5.1 with distinct biophysical characteristics and pH sensitivities (33, 37, 57). Such properties have been shown to allow cells to change membrane potentials at different pHi levels in heterologous expression systems, depending on whether the Kir4.1 or Kir4.1/Kir5.1 channel is expressed (11) and how they are co-expressed in different ratios as shown in the present study. Apparently, the latter mechanism is altered with the deficiency of MeCP2, although how the MeCP2 affects the Kir4.1 expression is still unclear. As a universal transcriptional modulator, MeCP2 may have a direct effect on the expression of Kir4.1. Furthermore, the erratic expression of Kir4.1 channels may not be limited to LC neurons but rather commonly exist in multiple CO2-chemosensitive nuclei in the brain stem. Thus the drugs aimed at restricting the function of Kir4.1 or enhancing the activity of Kir4.1/Kir5.1 channels may potentially help the recovery of normal CO2 drive to respiration in mutant mice. The putative candidates could be neuromodulators that regulate the Kir4.1/Kir5.1 channels but not Kir4.1 channels through the PKC signaling pathway (37), such as substance P, 5-HT, and thyrotropin-releasing hormone. In addition to the Kir4.1, we cannot rule out the involvement of other pH-sensitive molecules. However, the downregulation of other Kir channels with low pH sensitivity as suggested in our recent study (60) is unlikely to contribute to the defective CO2 response of Mecp2−/Y neurons.
Taken together, the demonstration of the selective disruption of central CO2 chemosensitivity at a particular PaCO2 range, its correlation with the functionality of CO2-sensing neuron (LC cells used as model), and the pH-sensitive Kir4.1 channel in the present study may help us to understand the etiology of breathing disorders in Mecp2−/Y mice as well as the central CO2 chemoreception in WT animals. However, several questions are still open: Do the presynaptic inputs and other neuromodulators affect the CO2 sensitivity of LC neurons through GABA, glutamate, serotonin, and adrenergic receptor? Are other CO2 chemosensitive neurons in the brain stem, such as serotonergic neurons in brain stem raphé nuclei (54), glutamatergic neurons in the retrotrapezoid nucleus (27), and other groups of NE-ergic neurons in the pons and medulla (47), also involved? Therefore, more investigations are needed. Nonetheless, the demonstration of the defect of K+ channels in LC neurons seems to be a remarkable step toward understanding the breathing instability in RTT.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (1R01NS073875 and 1R21HD060959) and the International Rett Syndrome Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Anthony N. van den Pol in the Department of Neurosurgery at Yale University School Medicine for the gift of Prp57 transgenic mice.
REFERENCES
- 1. Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 107: 18208–18213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander JK, West JR, Wood JA, Richards DW. Analysis of the respiratory response to carbon dioxide inhalation in varying clinical states of hypercapnia, anoxia, and acid-base derangement. J Clin Invest 34: 511–532, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol 168: 125–132, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch 455: 1119–1128, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol 104: 198–204, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels 2: 183–191, 1994 [PubMed] [Google Scholar]
- 8. Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10: 33–44, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron 56: 422–437, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Cui N, Giwa LR, Xu H, Rojas A, Abdulkadir L, Jiang C. Modulation of the heteromeric Kir4.1-Kir51 channels by Pco2 at physiological levels. J Cell Physiol 189: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Damas-Mora J, Souster L, Jenner FA. Diminished hypercapnic drive in endogenous or severe depression. J Psychosom Res 26: 237–245, 1982 [DOI] [PubMed] [Google Scholar]
- 13. De Felice C, Ciccoli L, Leoncini S, Signorini C, Rossi M, Vannuccini L, Guazzi G, Latini G, Comporti M, Valacchi G, Hayek J. Systemic oxidative stress in classic Rett syndrome. Free Radic Biol Med 47: 440–448, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res 222: 373–381, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaultier C. Genes and genetics in respiratory control. Paediatr Respir Rev 5: 166–172, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Hakuno H, Oyamada Y, Murai M, Ito Y, Yamaguchi K. Effects of inactivation and stimulation of locus coeruleus on respiratory activity of neonatal rat. Respir Physiol Neurobiol 140: 9–18, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neurosci Lett 386: 14–17, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol 505: 267–282, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol 145: 115–126, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing locus ceruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol 105: 1301–1311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 168: 101–108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerr AM, Armstrong DD, Prescott RJ, Doyle D, Kearney DL. Rett syndrome: analysis of deaths in the British survey. Eur Child Adolesc Psychiatry 6, Suppl 1: 71–74, 1997 [PubMed] [Google Scholar]
- 25. Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 30: 5303–5310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol 605: 371–376, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Nomura Y, Segawa M, Higurashi M. Rett syndrome: an early catecholamine and indolamine deficient disorder? Brain Dev 7: 334–341, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Ogier M, Katz DM. Breathing dysfunction in Rett syndrome: understanding epigenetic regulation of the respiratory network. Respir Physiol Neurobiol 164: 55–63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci 27: 10912–10917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pentel P, Benowitz N. Efficacy and mechanism of action of sodium bicarbonate in the treatment of desipramine toxicity in rats. J Pharmacol Exp Ther 230: 12–19, 1984 [PubMed] [Google Scholar]
- 32. Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol 532: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J 15: 2980–2987, 1996 [PMC free article] [PubMed] [Google Scholar]
- 34. Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Rojas A, Su J, Yang L, Lee M, Cui N, Zhang X, Fountain D, Jiang C. Modulation of the heteromeric Kir4.1-Kir5.1 channel by multiple neurotransmitters via Galphaq-coupled receptors. J Cell Physiol 214: 84–95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci 25: 1915–1922, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Roux JC, Panayotis N, Dura E, Villard L. Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice. J Neurosci Res 88: 1500–1509, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, HylK, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA 106: 21966–21971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schluter B, Aguigah G, Buschatz D, Trowitzsch E, Aksu F. Polysomnographic recordings of respiratory disturbances in Rett syndrome. J Sleep Res 4: 203–207, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH, Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child 63: 1039–1048, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, Kryger MH. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep 17: 61–68, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol 579: 863–876, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J Neurosci 29: 12187–12195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol 525: 587–592, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viemari JC. Noradrenergic modulation of the respiratory neural network. Respir Physiol Neurobiol 164: 123–130, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci 24: 928–937, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viemari JC, Maussion G, Bevengut M, Burnet H, Pequignot JM, Nepote V, Pachnis V, Simonneau M, Hilaire G. Ret deficiency in mice impairs the development of A5 and A6 neurons and the functional maturation of the respiratory rhythm. Eur J Neurosci 22: 2403–2412, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci 25: 11521–11530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol 168: 109–118, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Voituron N, Zanella S, Menuet C, Lajard AM, Dutschmann M, Hilaire G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respir Physiol Neurobiol 170: 173–182, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci 26: 10911–10915, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104: 3042–3052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol 197: 179–191, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1, Kir5.1 by hypercapnia and intracellular acidosis. J Physiol 524: 725–735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamamoto Y, Ishikawa R, Omoe K, Yoshikawa N, Yamaguchi-Yamada M, Taniguchi K. Immunohistochemical distribution of inwardly rectifying K+ channels in the medulla oblongata of the rat. J Vet Med Sci 70: 265–271, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Zanella S, Mebarek S, Lajard AM, Picard N, Dutschmann M, Hilaire G. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir Physiol Neurobiol 160: 116–121, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C. Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. Am J Physiol Cell Physiol 298: C635–C646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang X, Su J, Rojas A, Jiang C. Pontine norepinephrine defects in Mecp2-null mice involve deficient expression of dopamine beta-hydroxylase but not a loss of catecholaminergic neurons. Biochem Biophys Res Commun 394: 285–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zoghbi HY, Percy AK, Glaze DG, Butler IJ, Riccardi VM. Reduction of biogenic amine levels in the Rett syndrome. N Engl J Med 313: 921–924, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.