Abstract
Autism and autism spectrum disorder (ASD) typically arise from a mixture of environmental influences and multiple genetic alterations. In some rare cases, such as Timothy syndrome (TS), a specific mutation in a single gene can be sufficient to generate autism or ASD in most patients, potentially offering insights into the etiology of autism in general. Both variants of TS (the milder TS1 and the more severe TS2) arise from missense mutations in alternatively spliced exons that cause the same G406R replacement in the CaV1.2 L-type calcium channel. We generated a TS2-like mouse but found that heterozygous (and homozygous) animals were not viable. However, heterozygous TS2 mice that were allowed to keep an inverted neomycin cassette (TS2-neo) survived through adulthood. We attribute the survival to lowering of expression of the G406R L-type channel via transcriptional interference, blunting deleterious effects of mutant L-type channel overactivity, and addressed potential effects of altered gene dosage by studying CaV1.2 knockout heterozygotes. Here we present a thorough behavioral phenotyping of the TS2-neo mouse, capitalizing on this unique opportunity to use the TS mutation to model ASD in mice. Along with normal general health, activity, and anxiety level, TS2-neo mice showed markedly restricted, repetitive, and perseverative behavior, altered social behavior, altered ultrasonic vocalization, and enhanced tone-cued and contextual memory following fear conditioning. Our results suggest that when TS mutant channels are expressed at levels low enough to avoid fatality, they are sufficient to cause multiple, distinct behavioral abnormalities, in line with the core aspects of ASD.
Keywords: channelopathy, CACNA1C, mouse model of psychiatric disease, sociability, communication
Autism and autism spectrum disorder (ASD) are characterized by the concomitant occurrence of impaired social interaction; restricted, perseverative, and stereotypical behavior; and abnormal communication skills (1). However, the etiology remains largely unknown, in large part because most cases of ASD arise from a mixture of multiple environmental and multiple genetic influences (2), making it difficult to forge causal links to behavior. In the face of such complexity, insights might be gleaned from simple forms of ASD, generated by single, highly penetrant mutations. Timothy syndrome (TS), is a rare disorder strongly associated with autism or ASD (penetrance ∼75%; P = 1.2 × 10−8). Other symptoms of TS include long QT syndrome, webbed fingers and toes, dysmorphic facial features, and immunodeficiency (3). Importantly, Splawski et al. (3) traced the disease to a single nucleotide mutation in the gene encoding the pore-forming subunit of an L-type calcium channel (CaV1.2). This sporadic glycine-to-arginine mutation is located at position 406 in exon 8A [Splawski's terminology (3), VNDAV-coding exon, low (∼20%) expression in brain and heart]. If a Gly-to-Arg mutation occurs in the more highly (∼80%) expressed (4) alternative exon 8 (MQDAM-coding exon, 5′ of exon 8A) it causes a more severe variant of TS (TS2). In heterologous expression systems, the TS1 and TS2 mutations sharply reduce channel inactivation (3, 5), possibly inducing heightened Ca2+ influx as a contributing factor to the multisystem defects in vivo.
We generated a TS2-like mouse but found that neither heterozygous nor homozygous mice survived to weaning, perhaps related to the predominant expression of exon 8 in brain and heart and a lethally high level of mutated channels. However, heterozygous mice that were allowed to keep an inverted neomycin cassette in exon 8A (TS2-neo) survived through adulthood. This might be due to lowered expression of the G406R L-type channel via transcriptional interference, blunting deleterious effects of the mutation. Capitalizing on the survival of the TS2-neo heterozygotes, we conducted an extensive behavioral analysis. These animals exhibited impaired social interaction and vocalization, and restricted and repetitive/perseverative behavior, key features reminiscent of ASD.
Results
Normal General Health, Anxiety Level, and Diurnal Rhythm of TS2-Neo Mice.
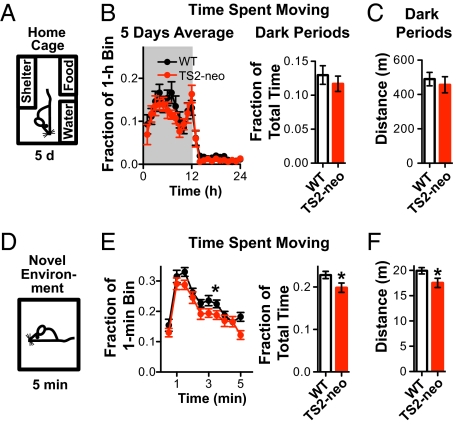
The heterozygote TS2-neo mice, hereafter referred to simply as TS2-neo mice, were constructed at a commercial facility and routinely grew to adulthood, breeding well without intervention (SI Materials and Methods). Attempts to remove the Neo cassette proved unsuccessful: all 5 pups positive for removal of the Neo cassette (out of 56 total) were stillborn. Clonal analysis of TS2-neo heterozygous mice revealed a low expression of the mutated channel, suggesting transcriptional interference from the neomycin cassette (SI Materials and Methods). In an array of control tests, TS2-neo male mice scored normally for general physical characteristics, motor abilities, and reflexes (Table S1). The pattern of locomotor activity was tested in a home-cage environment over 5 d with 12 h light/dark cycles (Fig. 1A). TS2-neo mice, like WT mice, displayed high activity during the dark periods and low activity during light periods (Fig. 1B and Fig. S1B). No significant differences were found between genotypes during either the dark or light periods for multiple activity parameters, including average time spent moving and distance moved (Fig. 1 B and C and Fig. S1C).
Fig. 1.
TS2-neo mice showed normal diurnal rhythm and locomotor activity but decreased locomotion in a novel environment. (A) Basic activity monitored over 5 d in a home-cage with shelter and water/food. n (each genotype) = 12. (B) Time spent moving: average of five 24-h dark/light cycles (gray/white background, respectively) (Left) and pooled data for five dark periods (Right) reveal no differences between genotypes. (C) Distance moved: TS2-neo mice traveled the same average distance as WT littermates during five dark cycles. (D) Monitoring of ambulatory activity over 5 min in activity chamber. n (each genotype) = 31. (E) Time spent moving was slightly but significantly smaller in TS2-neo mice (P = 0.03 for genotype effect, ANOVA) (Left); likewise for cumulative time spent moving (P = 0.03, Student's t test) (Right). (F) Distance moved: TS2-neo mice traveled a slightly but significantly smaller distance (P = 0.04, Student's t test).
To test locomotor activity in a novel environment (NE), mice were put into a novel arena (activity chamber) for 5 min in the dark (Fig. 1D). The mutant mice spent a smaller fraction of time moving [time course: F(1,540) = 4.68, P = 0.03; cumulative: P = 0.03], displayed fewer ambulatory movements (P = 0.03), and traveled a smaller distance than WT mice (P = 0.04) (Fig. 1 E and F and Fig. S1D) without differences in other parameters of exploratory activity (Fig. S1 D and E). Furthermore, thigmotaxis (wall hugging), often considered as an index for anxiety, was not significantly different between genotypes [F(1,540) = 0.007, P = 0.93] (Fig. S1F). Similarly, no thigmotaxis could be detected in the open field test (P = 0.33) (Fig. S1H).
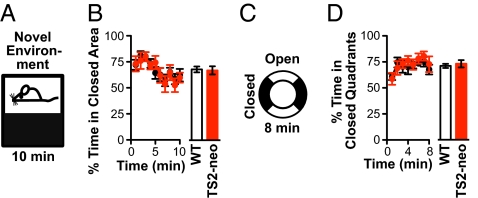
To test whether increased anxiety causes the decreased activity in a NE, we performed two more tests, starting with the light/dark box (Fig. 2A). The fraction of time spent in the dark area of the light/dark box is generally believed to be a measure of anxiety. The responsiveness of this test was initially validated with WT mice (C57BL/6J) preexposed to predator odor (Fig. S2A). The introduction of the TS2-neo modification had no significant effect on the fractional time mice spent in the dark area [time course: F(1,207) = 0.004, P > 0.8; cumulative: P > 0.8] (Fig. 2B). TS2-neo mice did display less overall activity in the NE (P < 0.02) (Fig. S2B), as in Fig. 1F.
Fig. 2.
TS2-neo mice exhibited no sign of increased anxiety in light/dark box and elevated zero maze. (A) Light/dark box: mice were exposed to an activity chamber with dark and bright sides for 10 min. (B) Percentage of time spent in dark side. No significant difference between TS2-neo and WT mice for time course (P > 0.8 for genotype effect, ANOVA) (Left) or cumulative time (P > 0.8, Student's t test) (Right). n (WT) = 13, n (TS2-neo) = 12. (C) Elevated zero maze: mice were exposed to elevated, annular platform with two closed and two open quadrants for 8 min. n (WT) = 9, n (TS2-neo) = 13. (D) No significant difference between TS2-neo and WT littermates for time course (P > 0.6 for genotype effect, ANOVA) (Left) and cumulative time spent in closed quadrants (P > 0.2, Mann–Whitney u test) (Right).
Anxiety was probed further in the elevated zero maze, an annular platform with two closed and two open quadrants (Fig. 2C). We first validated the test by showing that preference for closed quadrants, the index of anxiety, was lessened by diazepam treatment without indications of sedation (Fig. S2 C and D). TS2-neo mice were not significantly different from WT littermates in their preference for the closed quadrants [time course: F(1,140) = 0.20, P > 0.6; cumulative: P > 0.2] or in the number of open quadrants entries (P > 0.7, Fig. 2D and Fig. S2E).
The results described thus far indicated that TS2-neo animals were not significantly impaired with regard to general health, home-cage activity, or anxiety. They appeared slightly less active than WT in a NE. These findings provide a backdrop for the striking behavioral differences reported below.
Decreased Approach Behavior to a Novel Environment.
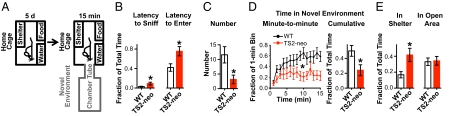
To test aversion to novelty, mice were exposed to a new paradigm that called for a choice between the home-cage and a NE. After habituation to the home-cage for 5 d, we attached an annex to the home-cage, consisting of a tube leading to a second chamber (Fig. 3A), and monitored behavior for 15 min. TS2-neo mice showed a greater latency to sniff at the tube (P = 0.02) and to enter it (P = 0.03) (Fig. 3B and Movie S1) and entered the NE less often (P = 0.01) (Fig. 3C). Furthermore, TS2-neo mice spent less than half as much time in the NE [time course: F(1,308) = 6.37, P = 0.02; cumulative: P = 0.02] (Fig. 3D) but remained a correspondingly longer time in the shelter, not in the open area of the home-cage (P = 0.01) (Fig. 3E). The genotypes did not differ in shelter time if no annex was attached (Fig. S1C).
Fig. 3.
TS2-neo mice showed decreased approach behavior to a novel environment. (A) After a 5-d exposure to home-cage environment with a shelter and water/food, NE (additional tube and chamber) was attached for 15 min. (B) TS2-neo mice showed twofold increases in latency to initiate contact with NE (P = 0.02, Mann–Whitney u test) (Left) and to enter it (P = 0.03, Mann–Whitney u test) (Right) and entered it significantly fewer times (C, P = 0.01, Student's t test). (D) A minute-to-minute comparison of time spent in NE showed significant genotype effect (P = 0.02, ANOVA) (Left). TS2-neo mice spent less than half as much time in NE than WT (P = 0.02, Student's t test) (Right) but a correspondingly longer time in the shelter (E, Left, P = 0.01, Student's t test). (E, Right) No genotype difference for time spent in the open area of the home-cage. n (each genotype) = 12.
Evidence for Repetitive and Perseverative Behavior in Marble Burying Task, Morris Water Maze, and Water Y-maze.
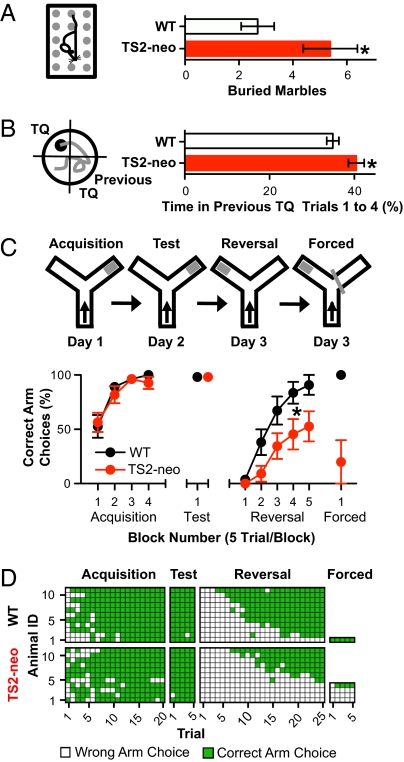
Repetitive, restricted, and perseverative behavior, of the ASD triad, may be further subdivided into “lower order” repetitive sensory-motor behaviors and “higher order” insistence on sameness (6). Corresponding traits were examined with a variety of assays, starting with the marble burying test, wherein mice are scored for the number of marbles they bury from the top of bedding (Fig. 4A). Increased marble burying is thought to be an index for repetitive/perseverative behavior and compulsion, as supported by animal models of ASD and experiments with serotonin reuptake inhibitors used to treat the condition (7, 8). Marble burying is also inhibited by calcium channel antagonists (9). TS2-neo mice buried 5.4 ± 1.0 marbles over a 30-min period, twofold more than their WT littermates (2.7 ± 0.6 marbles; P = 0.03).
Fig. 4.
Repetitive marble burying and perseveration in searching for previous escape platform location in MWM and water Y-maze. (A) Marble burying: TS2-neo mice buried twice as many marbles as WT littermates during a 30-min exposure to 20 marbles (P = 0.03, Student's t test). n (WT) = 23, n (TS2-neo) = 23. (B) MWM: during the first four trials of reversal learning, TS-neo mice spent significantly more time in previous target quadrant than WT (P < 0.03, Student's t test). n (WT) = 27, n (TS2-neo) = 29. (C) Water Y-maze: percentage of correct arm choices per trial block during acquisition (day 1 of experiment), test (day 2), reversal (day 3), and forced training (day 3). During reversal training, TS2-neo mice made significantly fewer correct choices than control mice (P = 0.02 for genotype effect, ANOVA). n (WT) = 11, n (TS2-neo) = 11. (D) Color-coded graph depicting individual trial outcomes in water Y-maze (white/green for wrong/correct arm choices, respectively). Forced training (incorrect arm blocked): three TS2-neo mice never made correct choice, whereas the WT mouse entered correct arm from first trial onward.
Spatial reference memory acquisition and retrieval were tested in a classic hidden platform Morris water maze (MWM). Both genotypes performed equally well in learning the location of the platform and recalling that location after platform removal, with no detectable difference in escape latency, in time spent in the target quadrant, or in other parameters (Fig. S3 A and B). A difference emerged when the hidden escape platform was moved to a new location to test reversal learning. TS2-neo mice spent significantly more time searching in the previous location (P < 0.03 for trials 1–4, Fig. 4B; P < 0.04 for trials 1–8) (Fig. S3C) without significant differences in overall motor performance (Fig. S3D). Thus, TS2-neo mice performed normally for spatial reference memory acquisition and retrieval, but showed mild signs of perseveration during reversal learning.
A new cohort of mice was tested in a water Y-maze, a test that called for a simple left/right decision (8) (Fig. 4C). TS2-neo mice and WT littermates were equally capable of finding and recalling a submerged escape platform in one of two target arms but exhibited a significant deficit in reversal learning after the platform was switched to the other target arm [F(1,80) = 6.197, P = 0.02] (Fig. 4C). Indeed, four TS2-neo mice and one WT mouse never entered the correct target arm during reversal learning even after 25 trials (Fig. 4D). Subjecting these animals to blockade of the incorrect arm in an attempt to coax the choice of the correct arm, led to correct choices by the WT animal. However, three out of four TS2-neo mice never entered the correct arm even after 5 further trials (Fig. 4D). In many trials, the TS2-neo mice pushed their noses against the partition blocking the incorrect arm before returning to the origin for another attempt (Movie S2), thus displaying a striking perseverative phenotype. The results from water Y-maze, marble burying, and MWM tasks provide consistent evidence in support of increased repetitive and perseverative behavior in TS2-neo mice.
Altered Social Behavior in an Automated Social Home-Cage Assay.
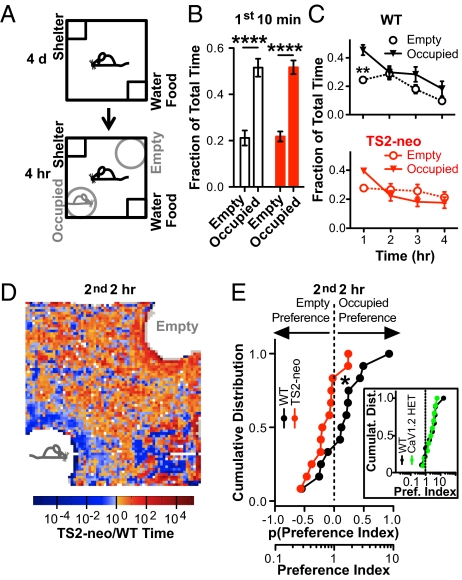
Social behavior, so profoundly altered in ASD, is often tested in mice with the classical three-chamber test (10). In this 10-min test, both TS2-neo and WT mice met the conventionally defined criteria for sociability by exhibiting relatively greater interest in a corral harboring a mouse than an empty corral (corral preference: WT, P < 0.0001; TS2-neo, P < 0.0001 and chamber preference: WT, P < 0.0001; TS2-neo, P < 0.05) (Fig. S4A). Interest in the occupied corral might be driven by transitory interest in a moving object rather than sociability per se. We prolonged the test to 4 h to allow such interest to habituate (Fig. 5A). Testing was performed in the dark period in a home-cage. An infrared camera tracked the behavior in absence of an experimenter. In the initial 10 min, both genotypes spent significantly more time in proximity of the occupied corral than close to the empty corral (P < 0.0001) (Fig. 5B). WT mice maintained this initial trend over all 4 h, whereas the TS2-neo mice lost this preference after the first hour, and, if anything, showed the opposite difference over the remaining time (Fig. 5C). To analyze the behavior after the initial habituation, we focused on the performance during the last 2 h. An intensity map depicts the ratio of TS2-neo vs. WT time in pseudocolor (Fig. 5D). Pixels surrounding the empty corral are largely red, indicating a longer dwell time of TS2-neo animals, whereas pixels near the stranger-occupied corral are largely blue, indicating shorter dwell time of TS2-neo mice. To analyze the preference for the occupied corral in individual animals, we determined a preference index (ratio of time close to occupied vs. empty corral). Thus, sociability is reflected by a preference index greater than unity. Collectively, TS2-neo mice showed significantly less preference for the occupied corral than WT mice (Fig. 5E) (P = 0.04). Whereas 8 of 12 WT mice preferred the occupied corral, 10 of 12 TS2-neo mice preferred the unoccupied corral (Fig. 5E). Again, no differences were found for total time spent near the corrals, time spent moving, or distance moved (Fig. S4 C–E). Overall, the home-cage assay for social behavior revealed a distinct deficit in the TS2-neo mice with regard to social preference.
Fig. 5.
TS2-neo mice displayed decreased preference for a social object in an automated social home-cage assay. (A) After a 4-d exposure to a home-cage with shelter and water/food, empty and occupied corrals were presented in opposite corners. (B) During initial 10 min, both genotypes preferred to stay close to occupied corral than near empty corral (P < 0.0001 for corral effect, ANOVA). (C) Whereas WT mice displayed significant preference for the occupied vs. the empty corral during the first full hour (P < 0.01, Bonferroni post hoc test), and a trend for similar preference over the last 3 h, TS2-neo mice showed a trend for preference for the occupied corral during the first full hour and opposite preference for the last 3 h. (D) Intensity map of last 2 h, depicting spatial distribution of TS2-neo:WT time ratio (red/blue for increased/decreased TS2-neo dwell time, respectively). (E) Cumulative distribution of preference index for last 2 h. TS2-neo mice showed significantly less preference for the occupied corral than WT mice (P = 0.04, Student's t test). n (each genotype) = 12. (Inset) CaV1.2+/− mice show the same preference for the occupied corral as WT. n (WT) = 9, n (CaV1.2+/−) = 10.
We further explored social memory in a six-trial social memory test (Fig. S4H). Mice repeatedly exposed to the same ovariectomized female intruder (OEF) for four consecutive trials were then presented with a novel OEF in the fifth trial. Both mutant and WT mice showed significant habituation to the same OEF (WT, P < 0.01; TS2-neo, P < 0.001), and dishabituation to the novel OEF (P < 0.01). Following a suggestion from R. Paylor (Baylor College of Medicine, Houston), we extended the test by adding a sixth trial with the same OEF, wherein both genotypes displayed significantly reduced interest (WT, P < 0.01; TS2-neo, P < 0.0001). By these criteria, TS2-neo mice appear unimpaired with regard to social memory.
Contrasts with Behavior of CaV1.2 Knockout Heterozygote Animals.
The results thus far demonstrate robust phenotypes consistent with two ASD core traits. At this stage, we addressed potential complications arising from the neomycin cassette left in exon 8A, which reduced expression of mutant exon 8, most likely by transcriptional interference (SI Materials and Methods), and introduced a stop codon in exon 8A, normally much less prevalent in brain than exon 8. Both actions would attenuate the expression of the modified CaV1.2 allele. Therefore, we performed control experiments in heterozygote knockout mice (CaV1.2+/−) (Fig. S5). CaV1.2+/− mice were severely hypoactive compared with control littermates, both in their home-cage and in a NE (activity chamber), and exhibited significantly increased anxiety, as shown by increased thigmotaxis (Fig. S5 A–G). Regarding restricted and repetitive behavior, CaV1.2+/− mice performed normally both in the annex and marble burying tests (Fig. S5 H–K). In the automated social home-cage assay, the preference of CaV1.2+/− mice for the occupied corral was no different from that of the WT littermates (Fig. 4, Inset and Fig. S5 L–O). Taken together, these observations indicated that TS2-neo mice have a phenotype that is distinct from CaV1.2+/− mice, suggesting that gene dosage effects do not account for the TS2-neo phenotype. We returned to the TS2-neo animals for further tests of behaviors relevant to other core features of ASD.
Altered Ultrasonic Vocalization.
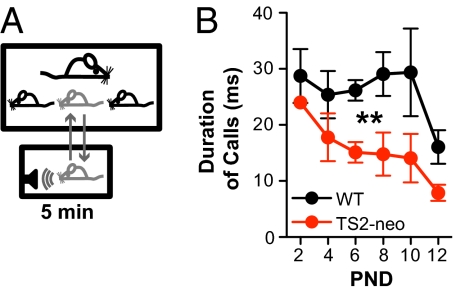
We attempted to assess communication in TS2-neo mice by studying ultrasonic vocalization (USV) patterns of pups separated from their dam and litter (11) (Fig. 6A). USVs are thought to mediate communication because pup USV calls induce pup approach and retrieval by the dam (11), even if presented in playback (12). Testing for USVs of pups began on postnatal day (PND) 2 and was repeated on alternate days until PND 12. Calls in response to the separation paradigm were significantly shorter in duration for TS2-neo mice compared with WT littermates, roughly twofold briefer at PND 6, 8, and 10 [F(1,40) = 13.02, P = 0.007] (Fig. 6B). On the other hand, no significant differences were found in body weight or in other aspects of calls such as number, peak frequency, and peak amplitude (Fig. S6). The shorter duration of calls suggests that communication is altered in TS2-neo mice.
Fig. 6.
TS2-neo pups emit shorter ultrasonic vocalization calls. (A) Starting at PND 2, pups were separated from their dam and litter and their USVs were recorded for 5 min. (B) The duration of calls was significantly decreased in TS2-neo mice compared with WT littermates (P = 0.007 for genotype effect, ANOVA). n (WT) = 6, n (TS2-neo) = 4.
Increased Persistence of Tone-Cued and Contextual Fear Memory.
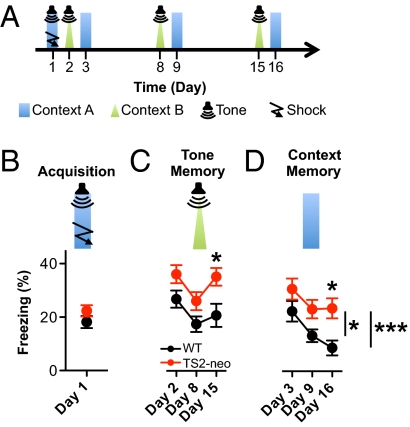
Having found that TS2-neo mice performed normally in place learning and memory (Fig. S3 A and B and Fig. 4C), we went on to assess associative learning and memory. We used a well-known fear conditioning paradigm wherein mice learned to fear emotionally neutral conditional stimuli (context A and tone) through their pairing with an aversive, unconditional stimulus (foot shock). Mice were then scored for a fear response called freezing (Fig. 7A). Memory acquisition was no different between TS2-neo and WT littermates (P = 0.2) (Fig. 7B). Memory for association with tone, assessed by presenting the same tone within a different context (context B), was significantly enhanced in TS2-neo mice (ANOVA, P < 0.05 by post hoc test) (Fig. 7C, day 15). Likewise, memory for association with context, measured by placing mice back into context A without any tone, was significantly increased in TS2-neo mice (ANOVA, P < 0.05 by post hoc test) (Fig. 7D, day 16). The decline of freezing, whether due to extinction or decay, was significant only in WT (day 3 vs. d 9, P < 0.05; day 3 vs. d 16, P < 0.001) but not in TS2-neo mice (Fig. 7D). These effects were not due to (i) increased baseline freezing, as freezing was virtually absent in both genotypes before the first tone-shock presentation (Fig. S7A), (ii) altered reaction to an auditory stimulus, as TS2-neo mice did not differ in freezing from WT mice during the first presentation of the tone (Fig. S7I), or (iii) altered reaction to aversive stimuli, as assessed in the hot plate test (Fig. S7M). Evidently, the TS mutation leads to enhanced persistence of both tone and context memory.
Fig. 7.
TS2-neo mice exhibit increased persistence of tone-cued and contextual fear memory. (A) Protocol: on day 1 (acquisition), mice were exposed to five tone-shock pairings in context A. On day 2 (tone memory), mice were exposed to the tone in a different context (context B), and on day 3 (context memory) to the same context (context A) without tone. Long-term tone memory and context memory were retested on days 8 and 15 or 9 and 16, respectively. (B) Acquisition: TS2-neo mice froze at the same level as WT littermates (P = 0.2, Student's t test). (C) Tone memory: TS2-neo froze significantly more on day 15 (P < 0.02 for genotype effect, ANOVA; day 15: P < 0.05, Bonferroni post hoc test). (D) Context memory: TS2-neo froze significantly more on day 16 (P < 0.02 for genotype effect, ANOVA; day 16: P < 0.05, Bonferroni post hoc test). The decrease in freezing was significant in WT (P < 0.0001 for day effect, ANOVA; day 3 vs. day 9: P < 0.05; day 3 vs. day 16: P < 0.001, Bonferroni post hoc test) but not TS2-neo mice. n (WT) = 10, n (TS2-neo) = 11.
Discussion
TS is caused by a single G406R mutation in CaV1.2 channels and generates ASD in humans with a 75% penetrance. In mice, the mutation-bearing channel causes death at high levels of expression, but leaves basic functioning largely unaffected at lower levels (reduced to one-fourth in TS2-neo animals, SI Materials and Methods). TS2-neo mice exhibited distinct traits strikingly reminiscent of the entire core triad of ASD: repetitive/perseverative behavior, impaired social behavior, and impaired communication. TS2-neo mice also displayed enhanced fear memory, reminiscent of persistent memories (13) or abnormal fear associated with ASD (14).
Normal Basic Behavior.
Control tests suggested that the behavioral phenotype of TS2-neo mice was not caused by factors other than the TS mutation in brain. Although CaV1.2 is expressed in multiple organs, and the TS mutation leads to prominent cardiac disease (3, 4), TS2-neo mice were normal with regard to general health, basic sensorimotor functioning, locomotor activity, and diurnal rhythm. There were no signs of sensorimotor deficits or heart failure even under the high stress of the MWM.
The neomycin cassette left in exon 8A represents a potential confounding factor; although it allowed viability, it would be expected to lower the level of CaV1.2 expression overall. However, CaV1.2+/− animals exhibit behavior inconsistent with that of the TS2-neo mice (Fig. S6), suggesting that the phenotype in TS2-neo mice cannot be attributed to lowered expression of the channel.
Anxiety is a common comorbid symptom in ASD, although not a member of the core triad (1). We assessed the potential importance of anxiety for the behavior of TS2-neo mice by performing standard tests for anxiety, using an activity chamber, open field, light/dark box, and elevated zero maze. In each case, TS2-neo mice did not show an overt anxiety phenotype.
Repetitive, Restricted, and Perseverative Behavior.
The doubling of marbles buried in the marble burying test, an assay for repetitive and perseverative behavior, was telling because this test was shown not to be confounded by anxiety, fear, or novelty (7). We also examined the animals’ resistance to changes in environment, a higher order insistence on sameness. In the NE of the activity chamber, TS2-neo mice were slightly hypoactive without thigmotaxis, suggesting aversion to novelty but not increased anxiety. Aversion to novelty was also suggested when mice were confronted with a clear choice between a familiar home-cage and an unfamiliar annex, whereas TS2-neo mice showed normal approach–avoidance behavior when given a choice of two novel areas (light/dark box and elevated zero maze). Altered exploratory drive or increased inhibition did not seem causally involved because TS2-neo mice showed normal exploratory activity in a home-cage setting. Further evidence for insistence on sameness was found in MWM and water Y-maze. Mice exhibited a mild persistence in seeking an outdated platform location in the MWM and a persistence in repeatedly attempting to enter the wrong arm of the Y-maze, even when physically blocked. These tests provide striking, convergent evidence for both aspects of repetitive, restricted, and perseverative behavior in the mutant animals. It would be interesting to determine whether this behavior arises from defects in corticostriatal circuits, implicated in human ASD and mouse models of ASD and obsessive-compulsive disorder (15).
Altered Social Behavior.
Social behavior was tested in a newly devised automated social assay, incorporated within a home-cage environment. We mostly focused on ongoing social behavior after a habituation period when fractional time spent near occupied and empty corrals had fallen off. TS2-neo mice showed significantly less preference for the occupied corral than their WT littermates, a clear alteration of social behavior. It would be interesting to test other mouse models of ASD that have not displayed altered sociability in the three-chamber assay (16, 17). In addition, TS2-neo mice would be a candidate to test for excitation/inhibition (E/I) imbalance in the medial prefrontal cortex (18).
Altered Ultrasonic Vocalization.
In the pup separation test, TS2-neo pups emitted ultrasonic calls lasting half their normal length, without significant reduction in number. This attenuation in vocalization is reminiscent of reduced communication in ASD. Fewer calls have been reported for pups lacking oxytocin, oxytocin receptor, neuroligin-4, and FoxP2 (11, 19). On the other hand, pups of the MALTT mouse, the chromosome 15q11-13 duplication model, and the inbred strain BTBR T+tf/J display increased levels of vocalization (20).
Enhanced Fear Memory.
During fear memory acquisition, TS2-neo mice froze with the same frequency as WT mice, suggesting normal associative fear learning. However, mutant mice maintained a higher level of freezing than WT littermates when presented with either tone or context alone. This, together with findings from various anxiety tests, supports the notion that TS2-neo mice exhibit increased fear memory but no overt anxiety. Another gene-induced mouse model of ASD, the FKBP12-deficient mouse, shows normal anxiety but enhanced fear memory for context (but not tone) (8), whereas the valproic acid-induced rat model shows increased anxiety along with enhanced tone and context fear memory (14). Taken together, the behavior in various rodent models, including TS2-neo mice, is reminiscent of abnormal fear (14) and persistent memories (13) in humans.
Comparison with Other ASD Mouse Models, Possible Mechanisms, and Future Directions.
With some possible exceptions (20, 21), genes associated with ASD can be roughly divided into two major groups (22), a synaptic group involved in synaptogenesis and possibly excitation/inhibition imbalance, and a gene expression group that participates in control of transcription or translation. Mouse models for human disease genes in the first group (NLGN3/4, SHANK2, SHANK3, DLGAP2, and NRXN1) recapitulate various aspects of ASD. The Neuroligin-3 R451C knockin mouse exhibits impaired social interactions (23, but see ref. 17), the neurexin-1α knockout mouse shows repetitive grooming but no apparent changes in social behavior (22), and ultrasonic vocalization is affected by NLGN4 deletion (22). The Shank3e4-9 mouse recapitulates all three ASD core traits (24). Synaptic inhibition and E/I balance falls under regulation by oxytoxin signaling (25), which has been implicated in ASD (2). Oxytocin receptor knockout mice also display the major aspects of ASD (25).
Representatives of the second gene group include modifiers of the mTOR pathway (TSC1/TSC2, NF1, and PTEN) (22). Mice deficient in brain Fkbp12, a protein that modulates the mTOR pathway, show increased repetitive/perseverative behavior and enhanced contextual fear memory, along with increased late-phase long-term potentiation and altered translational control (8). Mice with truncations in Mecp2, a model of Rett syndrome, a disorder with aspects of autism, appear to recapitulate the ASD triad (22).
CaV1.2 channels must be regarded as full-fledged members of both synaptic and gene expression groups. L-type channels not only provide Ca2+ signals at postsynaptic structures to drive synaptic plasticity (26), but also help control local translation (27) and global transcription (28). Given the strategic roles of CaV1.2, and the high penetrance of the TS mutation in causing autism, it is both striking and reassuring that we were able to observe a full triad of autism-related behaviors in our mice. The critical importance of CaV1.2 channels may also be reflected in its involvement in other psychiatric diseases, like bipolar disorder and, potentially, substance abuse and dependence (29). Our behavioral approaches set the stage for future studies to explore altered brain circuits underlying the behavioral traits described here, and to determine whether the various behaviors can be modified by L-type channel blockers, either by early intervention or acute treatment.
Materials and Methods
See SI Materials and Methods for remaining behavioral tests, construction of TS2-neo mice, and general experimental conditions.
Annex Test.
One day after the home-cage activity test, a tube was attached to the home-cage that established a connection to a novel chamber. Approach behavior was scored from video recordings.
Automated Social Home-Cage Assay.
After a 4-d habituation to a home-cage with a shelter and water/food in opposite corners, mice were further exposed for 4 h to an occupied (C57BL/6J male mouse) and an empty corral. Proximity to the corrals (<5 cm) was tracked with an infrared camera and tracking software.
Statistics.
All data are presented as average ± SEM. Statistical significance was tested by using a two-tailed Student's t test or two-way ANOVA, except where indicated. See Table S2 for a list of statistical tests used for each dataset.
Supplementary Material
Acknowledgments
We thank M. Priestley, N. Saw, Ch. Tun, A. Encarnacion, L. Coutellier, and B. Agredano for excellent technical assistance; R.W.T. laboratory members for suggestions and discussions; A. Mitra for helpful comments on the manuscript; and L. Jan and D. Young for excellent guidance in ultrasonic vocalization experiments. CaV1.2+/− mice were kindly provided by R. Dolmetsch and G. Panagiotakos. This study was supported by Swiss National Science Foundation Grant PBBEA-121061 (to P.L.B.), National Institute of Neurological Disorders and Stroke P30 Center Core Grant NS069375, the Simons Foundation and the Burnett Family Fund (to R.W.T.), and National Institute for Mental Health National Research Service Award F31MH084430 (to S.F.O) and R21HL088058 (to R.L.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112667108/-/DCSupplemental.
References
- 1.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Splawski I, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8096, discussion 8086–8088. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci USA. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albelda N, Joel D. Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.04.006. 10.1016/j.neubiorev.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Hoeffer CA, et al. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egashira N, et al. Calcium-channel antagonists inhibit marble-burying behavior in mice. J Pharmacol Sci. 2008;108:140–143. doi: 10.1254/jphs.08160sc. [DOI] [PubMed] [Google Scholar]
- 10.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 11.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu A, et al. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 2007;1163:91–99. doi: 10.1016/j.brainres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JR. A review of Savant Syndrome and its possible relationship to epilepsy. Epilepsy Behav. 2010;17:147–152. doi: 10.1016/j.yebeh.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Markram H, Rinaldi T, Markram K. The intense world syndrome—an alternative hypothesis for autism. Front Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peça J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawley JN, et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011 doi: 10.1038/nature10360. 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton SM, et al. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakatani J, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ey E, Leblond CS, Bourgeron T. Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- 23.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sala M, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Morgan SL, Teyler TJ. VDCCs and NMDARs underlie two forms of LTP in CA1 hippocampus in vivo. J Neurophysiol. 1999;82:736–740. doi: 10.1152/jn.1999.82.2.736. [DOI] [PubMed] [Google Scholar]
- 27.Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- 28.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 29.Casamassima F, et al. L-type calcium channels and psychiatric disorders: A brief review. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1373–1390. doi: 10.1002/ajmg.b.31122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.