Here, we report the impact of substitution for the α-70Val residue of Azotobacter vinelandii nitrogenase molybdenum-iron (MoFe) protein on the so-called “hi-CO” CO bound complex as monitored in real-time by stopped-flow infra-red (SF-IR) spectroscopy. Nitrogenase is a bacterial metalloenzyme system whose physiological function is to catalyze the reduction of dinitrogen to ammonia [1, 2] with a concomitant reduction of 2H+ to H2 and hydrolysis of MgATP. X-ray crystallography on MoFe nitrogenase reveals the active-site FeMo-cofactor to be an unprecedented [Fe7S9MoX:homocitrate] cluster [3, 4], figure 1. However, simple inspection of this structure does not provide an obvious location for substrate binding or any indication of the subsequent mechanism for substrate reduction. Mechanisms focused on substrate binding to either Mo and Fe sites have been proposed as have combination approaches that involve migration of substrate-derived moieties between metal atoms during reduction [5–8].
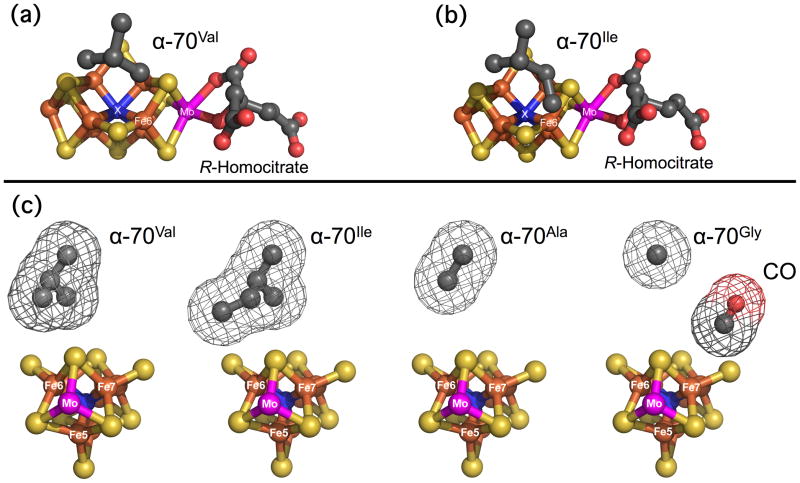
Figure 1.
FeMo-co showing the position and potential steric influence of the α-70 residue over the Fe 6 atom on the Fe 2, 3, 6, 7 face. (a) Side view of α-70Val. (b) Side view of α-70Ile. (c) End view of (left to right) α-70Val, α-70Ile, α-70Ala, α-70Gly. The wireframe indicates the van der Waals radii of the sidechains. A CO molecule is included for size comparison. Fe, rust; Mo, magneta; S, yellow; C, dark gray; O, red; X, blue. Structures built from PDB files: 1M1N.pdb [3] and 3K1A.pdb [13]
A series of recent studies have revealed the importance of substrate interactions at the 4Fe-4S face of FeMo-co defined by Fe atoms 2, 3, 6, and 7. These have analyzed the impact of modulating the steric influence of the uncharged α-70Val residue, which lies over Fe6, figure 1 [6]. Reducing the size of the sidechain by substitution with alanine or glycine allows the reduction of significantly larger alkyne substrates such as propargyl alcohol [9–11] while increasing its size by substitution with isoleucine severely restricts the reduction of nitrogenous and alkyne substrates while leaving the reactivity towards proton reduction unaltered [12, 13]. The clear implication of these results, and especially those from α-70Ile, is to implicate the Fe 2-3-6-7 face as the region for initial substrate binding.
CO is a valuable probe of the ligand binding properties of the FeMo-co active site. The molecule is a potent non-competitive inhibitor of the enzyme’s ability to reduce dinitrogen and other multiply bonded molecules [14, 15], although recent work has shown that for the vanadium enzyme it can be slow substrate [16]. It does not, however, inhibit enzyme turnover and MgATP hydrolysis, and the electron flux through the enzyme is redirected to increasing the rate of reduction of 2H+ to H2.
The binding chemistry of CO to MoFe nitrogenase is known to be complex and dependent on the partial pressure or concentration of CO present [17, 18]. Electron paramagnetic resonance (EPR) and electron-nuclear double resonance (ENDOR) studies have identified three bound states: under limiting [CO] conditions (< 0.08 atm in the gas phase or < 1:1 [CO]:[FeMo-co] in solution) the “lo-CO” state forms, which is proposed to comprise a single CO bound to the FeMo-cofactor, while under excess [CO] or under a high partial pressure of the gas, two EPR signals are observed, termed “hi-CO” and “hi(5)-CO”. Each signal is proposed to comprise at least two CO molecules bound to separate sites on the cofactor [17, 18].
Significant insight into CO binding to nitrogenase is possible by SF-IR spectroscopy, which has the enormous advantage of being a real-time room temperature technique [19–21]. Under lo-CO conditions, SF-IR measurements on both Klebsiella pneumoniae and A. vinelandii MoFe enzymes show a transient υ(C≡O) band at 1904 cm−1 which corresponds to a single CO terminally bound to a metal site, which converts on the minute timescale to an infrared (IR) band at 1715 cm−1 which presumably arises from a bridged or protonated bound CO species [20, 21]. By contrast, under hi-CO conditions complex spectra are seen with υ(C≡O) bands at 1960, 1936, 1906 and 1880 cm−1, all of which most likely arise from terminally bound CO species at more than one metal site [19, 20]. Further insight has been gained from spectroelectrochemical IR studies of the isolated FeMo-cofactor in N-methyl formamide (NMF:FeMo-co), which also reveals lo-CO and hi-CO behavior. The lo-CO state has redox dependent υ(C≡O) bands at 1835 and 1808 cm−1 that are assigned to bridged CO groups, while hi-CO has bands at 1885 and 1920 cm−1; assigned to terminally bound CO on Fe and Mo respectively [22].
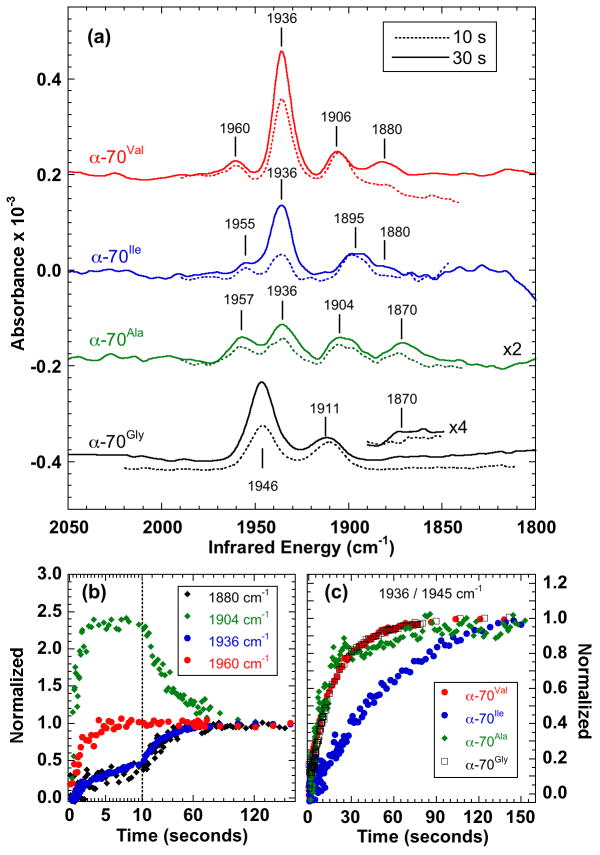
Here we use SF-IR to examine the sensitivity of the hi-CO complex to steric changes in the uncharged α-70 sidechain. The results for A. vinelandii wild-type MoFe protein (α-70Val) together with those from the α-70Gly, α-70Ala and α-70Ile variants are summarized in figure 2 and table 1. These data were all generated by reacting a 1:4 molar ratio of MoFe:Fe proteins with a buffered solution of MgATP saturated with CO, giving 50 μM FeMo-cofactor centers and 0.5 mM CO after mixing.
Figure 2.
Transient IR spectra of the A. vinelandii MoFe nitrogenase hi-CO complex showing the effect of varying the size of the α-70 sidechain. (a) Spectra averaged (solid line) 25 – 35 s and (broken line) 6 – 14 s after mixing. (b) Time courses for wild-type (α-70Val) measured at 1880, 1904, 1936 and 1960 cm−1, (c) Comparison of time courses at 1936 cm−1 (α-70Val, α-70Ile, α-70Ala) and 1945 cm−1 (α-70Gly). Intensities in (b) and (c) are normalized at 150 s.
Table 1.
Assignment of observed IR bands
| α-70 | Lo-CO | Slow Hi-CO | Fast Hi-CO | |
|---|---|---|---|---|
| variant | (cm−1) | (cm−1) | (cm−1) | |
| Val[a] | 1906 | 1936 | 1880 | 1960 |
|
| ||||
| Ile | 1895 | 1936 | 1880 | 1957 |
|
| ||||
| Ala | 1904 | 1936 | 1870 | 1955 |
| Gly | 1911 | 1946 | 1870 | n/o[b] |
Wild-type
not observed or not resolved.
The spectrum of the wild-type (α-70Val) complex (figure 2a – top) is consistent with those previously reported [19, 20]. The time courses, figure 2b, allow us to assign the spectrum to a mixture of 2 distinct hi-CO forms and the lo-CO complex. The first hi-CO form comprises the intense band at 1936 cm−1 and the weaker band at 1880 cm−1. These bands share the same time course and so it is reasonable to assign them to the same species. This species forms slowly, reaching a maximum intensity at ~150 s before slowly decaying (not shown) so we term it “slow hi-CO”. The second hi-CO form is characterized in this region by a single observed band at 1960 cm−1. We term this “fast hi-CO” as it initially appears quickly, within 10 s, and decays more slowly than the 1936 cm−1 band. The band at 1906 cm−1 mostly likely corresponds to the lo-CO complex and reaches maximum intensity within 8 seconds before decaying slowly to about 50% maximum size. Interestingly, the time course of the lo-CO decay is similar to the time course of formation of the slow hi-CO species suggesting that the slow hi-CO species may in part result from further CO binding to the 1906 cm−1 lo-CO species.
Changing the size of the uncharged α-70Val isopropyl sidechain modifies the energies and intensities of the IR bands, figure 2, however, there is consistently a visual correspondence with the wild-type spectrum, suggesting that both the 2 hi-CO species and the lo-CO band are still present. Band energies and assignments are presented in table 1. Substitution with a methyl group in α-70Ala reduces all the band intensities and shifts their energies. Interestingly, the intense band at 1936 cm−1 is substantially attenuated so that it now has similar intensity to the other bands. Similarly, increasing sidechain size to isobutyl in α-70Ile also weakens the spectrum and again shifts the bands. Of particular interest is the α-70Gly variant where the sidechain is replaced by a hydrogen atom thereby eliminating any steric effect. In this case, not only are all the slow hi-CO and lo-CO bands shifted in energy, but unlike the other variants, they are also broadened; for example, the bandwidth of the large slow hi-CO band increases from 10.5 to 15.5 cm−1 (FWHM). The fast hi-CO band is not observed although it is possible that it is obscured by the intense 1946 cm−1 band.
The apparent correlation of the hi-CO bands in the variant spectra with those in the wild-type spectrum is confirmed through the similarity in their time-dependence. When observed, the fast hi-CO band close to 1960 cm−1 is essentially formed within 10 s in each case. Similarly, in each variant, the 2 slow hi-CO bands have the same time dependence, figure 2a. The kinetics of formation of the slow hi-CO species are of particular interest. As shown in figure 2c, the formation time-course for the wild-type, the α-70Gly and the α-70Ala variants are virtually identical, but for α-70Ile it is significantly slower. This can be rationalized in terms of the larger sidechain impeding access to the CO binding site.
The principal conclusion that can be drawn from these data is that the Fe 2-3-6-7 face of the FeMo-cofactor is likely responsible for binding all the CO ligands giving rise to the observed IR bands. This arises from observation that the α-70 sidechain exerts steric control on the energies, conformation freedom and/or the formation kinetics on each of the 4 observed bands in at least one variant. The potential steric influence of this residue on the Fe 2-3-6-7 face is clear from figure 1. However, the data do not indicate a change in overall pattern of CO binding; it is clear that the slow hi-CO and the lo-CO species are present in all the spectra in figure 2, while the fast hi-CO is observed in 3 of them and could well be present in them all. This is consistent with a model where CO molecules are bound to the same metal sites in each variant protein, but that the CO ligands are clustered about, and sterically influenced by, the α-70 aliphatic sidechain. Changing the sidechain size changes the angles and freedom of movement of the metal-CO bonds, which in turn impacts the energies and intensities of the υ(C≡O) stretching vibrations. Increasing the sidechain size in α-70Ile impedes binding at one site at least. The α-70Gly spectrum is particularly interesting, as the observed broadening of the IR bands is consistent with the bound CO groups being conformationally less constrained by the protein environment when the α-70 sidechain is reduced to a hydrogen atom.
An alternative hypothesis is that modifying the α-70 sidechain causes the FeMo-cofactor to move within the protein pocket with a concomitant impact on CO binding elsewhere on the cofactor. This is largely excluded by the recent crystal structure of α-70Ile, which shows close overall structural agreement with that of wild-type α-70Val with near identity in the positions of most of the amino acids in the FeMo-cofactor binding pocket [13].
There are a number of secondary conclusions. First, as noted previously [19, 20], the energies of all the bands observed in figure 2 indicate that they most likely comprise ν(C≡O) stretches from terminally bound metal-CO species. It is possible that the 1880 cm−1 band arises from a CO bridging two metal sites through the C atom as ν(C=O) stretches from such complexes have been observed to occur up to 1898 cm−1 [23]. We consider this unlikely, however, as bridging CO species in other iron-sulfur systems exhibit ν(C=O) at significantly lower energies; FeFe hydrogenase, for example, has bridging ν(C=O) bands between 1850 – 1800 cm−1 [24]. However, we cannot exclude the possibility of additional bridging CO or even protonated formyl groups as these could produce bands below the 1800 cm−1 limit of our measurements.
Second, the sensitivity of all the observed ν(C≡O) bands to α-70 substitution confirms that they all arise from CO bound to the active site FeMo-co. Finally, it is implicit from the principal conclusion that it is unlikely that the observed CO is bound to Fe atoms 4 or 5. The possibility that a CO may be bound to the Mo is also unlikely, however this is more difficult to rigorously exclude, as the Mo is adjacent to the Fe 2-3-6-7 face and its associated α-70 residue, and it has been proposed that CO can bind to the Mo in NMF:FeMo-co with ν(C≡O) energies similar to those in table 1 [22].
These data comprise the first spectroscopic observations of the impact of varying the α-70 residue on the physical properties of ligands bound to FeMo-cofactor. This turn localizes the likely binding sites of the CO ligands to the Fe 2-3-6-7 face of the FeMo-cofactor, confirming the importance of both this region of the cofactor and the α-70 sidechain. This work also shows the value of CO as a probe of nitrogenase mechanism as CO inhibition clearly involves a dynamic and complex chemistry at the FeMo-co active site. The hi-CO state is of particular use as it comprises multiple CO molecules bound to more than one metal site and this means it can explore the array of available high and low affinity binding sites on FeMo-co. A complete understanding of nitrogenase-CO chemistry will undoubtedly give substantial insight into the mechanism of this intriguing enzyme system.
Experimental Section
Spectroscopic quantities of wild-type and variant MoFe nitrogenase were prepared as previously described [11]. FTIR spectra were measured using a modified Bruker IFS/66s FTIR spectrometer interfaced to a home-built stopped-flow drive system with the sample cuvette and drive system maintained inside an anaerobic chamber (O2 < 1.1 ppm) as described elsewhere [20]. The IR cuvette was thermostated at 25°C. For these measurements, one side of the drive system was loaded with the protein mixture with the other containing a buffered solution of MgATP saturated with CO. IR spectra were collected between 2200 – 1800 cm−1 only because a narrow band optical filter was used to enhance sensitivity. Spectra were measured at 4 cm−1 resolution. The IR cuvette path length was calibrated at 47.6 μm. Appropriate corrections were made for water vapor contamination. The α-70Ala and α-70Ile spectra in figure 2a required arbitrary background corrections to make them flat.
Supplementary Material
Footnotes
This work was funded by NIH GM-65440 (SPC), GM-59087 (LCS and DRD), NSF CHE-0745353 (SPC), and the DOE Office of Biological and Environmental Research (SPC). The authors would like to thank Ms Celestine Grady-Smith for experimental assistance.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the authors.
Contributor Information
Dr. Zhi-Yong Yang, Department of Chemistry and Biochemistry, UMC 0300 Old Main Hill, Utah State University, Logan, UT 84322, USA
Prof. Lance C. Seefeldt, Department of Chemistry and Biochemistry, UMC 0300 Old Main Hill, Utah State University, Logan, UT 84322, USA
Prof. Dennis R. Dean, Department of Biochemistry, Virginia Polytechnic Institute and State University, 110 Fralin Hall, Blacksburg, VA 24061, USA
Prof. Stephen P. Cramer, Department of Applied Science, University of California, Davis, One Shields Avenue, Davis, CA 95616 USA. Advanced Biological and Environmental X-ray Facility, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, MS 6R2100, Berkeley, CA 94720, USA
Dr. Simon J. George, Department of Applied Science, University of California, Davis, One Shields Avenue, Davis, CA 95616 USA. Advanced Biological and Environmental X-ray Facility, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, MS 6R2100, Berkeley, CA 94720, USA.
References
- 1.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Peters JW, Szilagyi RK. Curr Opin Chem Biol. 2006;10:101. doi: 10.1016/j.cbpa.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 4.Rees DC, Tezcan FA, Haynes CA, Walton MY, Andrade S, Einsle O, Howard JB. Philos Trans Roy Soc Lon A. 2005;363:971. doi: 10.1098/rsta.2004.1539. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi RY, Seefeldt LC. Crit Rev Biochem Mol Biol. 2003;38:351. doi: 10.1080/10409230391036766. [DOI] [PubMed] [Google Scholar]
- 6.Seefeldt LC, Hoffman BM, Dean DR. Annu Rev Biochem. 2009;78:701. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC, Hoffman BM. J Am Chem Soc. 2010;132:2526. doi: 10.1021/ja910613m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seefeldt LC, Dance IG, Dean DR. Biochemistry. 2004;43:1401. doi: 10.1021/bi036038g. [DOI] [PubMed] [Google Scholar]
- 9.Benton PMC, Laryukhin M, Mayer SM, Hoffman BM, Dean DR, Seefeldt LC. Biochemistry. 2003;42:9102. doi: 10.1021/bi034595x. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi RY, Dos Santos PC, Niehaus WG, Dance IG, Dean DR, Seefeldt LC. J Biol Chem. 2004;279:34770. doi: 10.1074/jbc.M403194200. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos PC, Mayer SM, Barney BM, Seefeldt LC, Dean DR. J Inorg Biochem. 2007;101:1642. doi: 10.1016/j.jinorgbio.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barney BM, Igarashi RY, Dos Santos PC, Dean DR, Seefeldt LC. J Biol Chem. 2004;279:53621. doi: 10.1074/jbc.M410247200. [DOI] [PubMed] [Google Scholar]
- 13.Sarma R, Barney BM, Keable S, Dean DR, Seefeldt LC, Peters JW. J Inorg Biochem. 2010;104:385. doi: 10.1016/j.jinorgbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang JC, Chen CH, Burris RH. Biochim Biophys Acta. 1973;292:256. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- 15.Pham DN, Burgess BK. Biochemistry. 1993;32:13725. doi: 10.1021/bi00212a043. [DOI] [PubMed] [Google Scholar]
- 16.Lee CC, Hu Y, Ribbe MW. Science. 2010;329:642. doi: 10.1126/science.1191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HI, Cameron LM, Hales BJ, Hoffman BM. J Am Chem Soc. 1997;119:10121. [Google Scholar]
- 18.Maskos Z, Fisher K, Sorlie M, Newton WE, Hales BJ. J Biol Inorg Chem. 2005;10:394. doi: 10.1007/s00775-005-0648-2. [DOI] [PubMed] [Google Scholar]
- 19.George SJ, Ashby GA, Wharton CW, Thorneley RNF. J Am Chem Soc. 1997;119:6450. [Google Scholar]
- 20.Thorneley RNF, George SJ. In: Prokaryotic nitrogen fixation: a model system for analysis of a biological process. Triplett EW, editor. Horizon Scientific Press; Wymondham, UK: 2000. p. 81. [Google Scholar]
- 21.Tolland JD, Thorneley RNF. Biochemistry. 2005;44:9520. doi: 10.1021/bi050453m. [DOI] [PubMed] [Google Scholar]
- 22.Pickett CJ, Vincent KA, Ibrahim SK, Gormal CA, Smith BE, Best SP. Chem Eur J. 2003;9:76. doi: 10.1002/chem.200390033. [DOI] [PubMed] [Google Scholar]
- 23.Adams DM. Metal-Ligand and Related Vibrations. Edward Arnold; London: 1967. [Google Scholar]
- 24.Roseboom W, De Lacey AL, Fernandez VM, Hatchikian EC, Albracht SPJ. J Biol Inorg Chem. 2006;11:102. doi: 10.1007/s00775-005-0040-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.